Abstract
Background:
We conducted a systematic review with meta-analysis to estimate rates and trends of colectomy in patients with ulcerative colitis (UC), and of primary and re-resection in patients with Crohn’s disease (CD), focusing on contemporary risks.
Methods:
Through a systematic review till September 3, 2019, we identified population-based cohort studies that reported patient-level cumulative risk of surgery in patients with UC and CD. We evaluated overall and contemporary risk (after 2000) of surgery and analyzed time trends through mixed-effects meta-regression.
Results:
In patients with UC (26 studies), overall 1-, 5-, and 10-year risk of colectomy was 4.0% (95% CI,3.3–5.0), 8.8% (7.7–10.0) and 13.3% (11.3–15.5), respectively, with decline in risk over time (p<0.001). Corresponding contemporary risks were 2.8% (2.0–3.9), 7.0% (5.7–8.6) and 9.6% (6.3–14.2), respectively. In patients with CD (22 studies), overall 1-, 5-, and 10-year risk of surgery was 18.7% (15.0–23.0), 28.0% (24.0–32.4) and 39.5% (33.3–46.2), respectively, with decline in risk over time (p<0.001). Corresponding contemporary risks were 12.3% (10.8–14.0), 18.0% (15.4–21.0) and 26.2% (23.4–29.4), respectively. On meta-analysis of 8 studies in patients with CD with prior resection, cumulative risk of second resection 5- and 10-years after first resection was 17.7% (13.5–22.9) and 31.3% (24.1–39.6), respectively.
Conclusion:
Patient-level risks of surgery have declined significantly over time, with 5-year cumulative risk of surgery of 7.0% in UC, and 18.0% in CD, in contemporary cohorts. This decline may be related early detection and/or better treatment.
Funding:
National Institute of Health/ NIDDK (K23DK117058)
Keywords: Natural history, disease modification, inflammatory bowel diseases, resection, tumor necrosis factor
INTRODUCTION
The global incidence and prevalence of inflammatory bowel disease (IBD) is rising.1 By 2030, the disease is estimated to affect 1% of individuals in the Western World. IBD is characterized by a lifelong unpredictable relapsing-remitting course, leading to substantial morbidity, diminished quality of life and healthcare resource utilization.2 Approximately 80% patients require hospitalization, with 25% being readmitted within 30–90 days of admission.3, 4 A prior meta-analysis suggested that approximately 1/3rd of patients with Crohn’s disease (CD) require surgery within 5 years of diagnosis, with the number rising to nearly 50% by 10 years of diagnosis.5 Similarly, approximately one in six patients with ulcerative colitis (UC) undergo colectomy within 10 years of diagnosis. However, the number of cohorts reporting contemporary surgical risk in patients diagnosed in the 21st century was very small in this meta-analysis. Over the last two decades, several therapeutic measures have improved disease outcomes including: (1) earlier diagnosis, (2) changes in approach to management of IBD with targeted use of disease-modifying immunosuppressive therapy, (3) introduction and increasing uptake of biologic agents like tumor necrosis factor-α antagonists, and (4) earlier detection and endoscopic management of colorectal neoplasia.6–8 Accordingly, several studies have variably shown a decline in risk of surgery over the last two decades.9–11
To better understand surgical risk of IBD in contemporary cohorts, we performed a systematic review with meta-analysis to analyze the cumulative 1-, 5-, and 10-year risk of major abdominal surgery (and repeat surgery in patients with CD) in patients with UC and CD, in population-based inception cohorts.
METHODS
We performed this systematic review based on an a priori protocol and reported according to the guidelines as prescribed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).12
Study Selection
We included population-based cohort studies in patients with incident UC and/or CD, reporting the cumulative risk of major abdominal surgery since time of diagnosis, with at least 1-year minimum follow-up. Population-based studies were identified as those that investigated the entire population in a defined geographical area in a defined time period, used appropriate sampling techniques to infer risk for the entire population, or used national registries capturing nearly the entire population in a region (>90%). For inclusion, these studies were required to report number of patients with incident UC or CD (or number of patients with incident CD with initial abdominal surgery to analyze risk of repeat surgery), calendar year of cohort recruitment, and cumulative risk of surgery estimated by Kaplan-Meier methodology. When multiple studies reported surgical risk from the same cohort, the most comprehensive study reporting from non-overlapping times were included.
We excluded studies that: (1) reported only overall annual surgical rates without patient-level cumulative risk of surgery, (2) reported risk of surgery in patients with IBD, without distinguishing CD or UC, (3) were not population-based (single- or multi-center referral studies, or clinical trials), or (4) reported incidence rate of surgery without cumulative risk.
Search Strategy, Data Extraction and Risk of Bias Assessment
Details of the search strategy, data extraction and risk of bias assessment are reported in the Supplementary Appendix.
Outcomes
The primary outcome was the cumulative 1-, 5- and 10-year risk of major abdominal surgery in patients with UC (defined as colectomy with or without an ileal pouch anal anastomosis) and CD (intestinal resection in patients with CD), and 5- and 10-year risk of repeat major abdominal surgery in patients with CD with initial intestinal resection. While most studies reported cumulative risk at 1-, 5- and 10-year, there were some instances where different cumulative risk at different years were reported. As such, we grouped 1–3y risks as 1-year risk; 4–6y risks as 5-year risk; and 7–10y risk as 10-year risk.
Subsequently, to estimate contemporary risks of surgery in patients diagnosed with IBD in the 21st century, we performed an analysis of cohorts in which the majority of patients were diagnosed after 2000 (>90% cohort).
Statistical Analysis
The pooled risk of major abdominal surgery and 95% confidence intervals (CIs) at 1, 5, and 10 years for both UC and CD, and 5- and 10-year risk of repeat abdominal surgery in patients with CD with prior resection, was estimated using a random effects model.13 To estimate 95% CI for individual study estimates from Kaplan-Meier curves, we assumed complete follow-up of the entire cohort. For time-trend analyses, to assess changes in surgical risk over time, the start year of inclusion of patients with incident UC and CD was included as a continuous variable in a meta-regression model of all studies.5 When the slope of the surgery incidences fit by the mixed-effect model had an associated p<0.05, we concluded that the incidence of surgery was changing significantly over time.
Heterogeneity between studies was assessed using the inconsistency index (I2) with values >50% suggesting significant heterogeneity.14 We anticipated high statistical heterogeneity as a meta-analysis of cumulative incidence, and took measures to address this in the design stage (strict study inclusion/exclusion criteria) and analysis. We performed subgroup analyses based on geographical location (North America vs. Europe vs. other geographical locations) and age of cohort, with a p-value for differences between subgroups of <0.10 being considered statistically significant. We also conducted mixed effects meta-regression based on population composition (proportion of males) and disease characteristics (UC: proportion of patients with extensive colitis [E3 on Montreal classification]; CD: proportion of patients with ileum-dominant CD [L1/L3 on Montreal classification], proportion of patients with penetrating and/or fibrostenotic behavior [B2/B3 on Montreal classification]); specific data on age at cohort entry was not consistently reported. Sensitivity analyses were performed (1) excluding conference proceedings, and (2) for estimating 10y risk of surgery, by excluding studies in which median population follow-up since diagnosis was <5y or not reported. Publication bias was assessed quantitatively using Egger’s regression test (publication bias considered present if p≤0.10), and qualitatively, by visual inspection of funnel plots.15 All analyses were performed using Comprehensive Meta-Analysis (CMA) software, version 2 (Biostat, Englewood, NJ).
RESULTS
A total 5138 unique studies were identified using our search strategy. Of these, 137 full text articles were reviewed, and 44 studies were included in quantitative synthesis, reporting on 26 cohorts of patients with UC,11, 16–40 22 cohorts of patients with CD,9, 11, 17, 21, 23, 27, 30–32, 39, 41–52 and 8 cohorts of patients with CD with prior surgery.9, 10, 45, 53–57 Ninety three studies were excluded with detailed reasons reported in eFigure 1.
Cumulative Risk of Colectomy in Patients with Ulcerative Colitis
Table 1 details the characteristics of 26 studies in patients with UC.11, 16–40 These studies included patients diagnosed between 1962 to 2016, with sample sizes ranging from 41 to 35,782 patients with UC; the largest study was a Danish nationwide register-based study.11 Six studies reported only on pediatric-onset UC. Fourteen studies reported data from patients in the biologic era, whereas 13 studies reported on surgical risks in the pre-biologic era. Study-level risk-of-bias assessment demonstrated unclear risk of bias, specifically for cohort attrition and reasons for loss to follow-up (Appendix Table 2).
Table 1.
Characteristics of included studies on colectomy risk in Ulcerative Colitis
| Benchimol et al,16 2010 | Canada | Ontario Province | 1994 – 2004 | - | Pediatric | - | - | 3 |
| Charpentier et al,17 2014 | France | EPIMAD | 1988 – 2006 | 474 | Elderly | M (62), F (38) | E1 (29), E2 (45), E3 (26) | 1, 5, and 10 |
| Chhaya et al,18 2015 | UK | CPRD | 1989 – 2009 | 8673 | All ages | M (52), F (48) | - | 1, 5, and 10 |
| Chow et al,19 2009 | China | Prince of Wales Hospital | 1985 – 2006 | 172 | All ages | M (52), F (48) | E1 (28), E2 (30), E3 (42) | 1 and 10 |
| Eriksson et al,20 2017 | Sweden | Orebro University Hospital | 1963 – 2005 | 835 | All ages | - | - | 10 |
| Gheorge et al,21 2004 | Romania | 18 secondary and tertiary centers | 2002 – 2003 | 163 | Adults | M (56), F (44) | E1 (22), E2 (54), E3 (24) | 1 |
| Gower-Rousseau et al,22 2014 | France | EPIMAD | 1988 – 2004 | 159 | Pediatric | M (42.1), F (57.9) | E1 (25), E2 (38), E3 (37) | 1, 5, and 10 |
| Guasch et al,23 2018 | Spain | ENEIDA | 1995 – 2000, 2007 – 2012 |
8028 | All ages | - | - | 1 and 5 |
| Lakatos et al,24 2011 | Hungary | Veszprem Province | 2002 – 2006 | 220 | All ages | M (56.8), F (43.2) | E1 (27), E2 (51), E3 (22) | 1 and 5 |
| Langholz et al,25 1997 | Denmark | Copenhagen | 1962 – 1987 | 80 | Pediatric | M (53), F (47) | E1 (25), E2 (43), E3 (29) | 1, 5 and 10 |
| Leijonmarck et al,26 1990 | Sweden | Stockholm County | 1955 – 1984 | 1586 | All Ages | - | - | 1, 5 and 10 |
| Lirhus et al,27 2018 | Norway | Norwegian Patient Registry | 2010 – 2012 | 5428 | All Ages | M (52.8), F (47.2) | - | 3 |
| Lund et al,28 2019 | Denmark | DNPR | 1977 – 2016 | 4449 | Pediatric | - | - | 5 |
| Malaty et al,29 2013 | USA | Single Pediatric Center | 1989 – 2003 | 112 | Pediatric | M (45), F (55) | E1 (25), E2 (40), E3 (35) | 1 and 5 |
| Nguyen et al,30 2017 | Canada | Ontario Province | 1999 – 2008 | 12233 | Adult | M (48.7), F (51.3) | - | 5 and 10 |
| Niewiadomski et al,31 2015 | Australia | Barwon Area | 2007 – 2008, 2010 – 2013 |
96 | All ages | - | - | 1 and 5 |
| Nordenvall et al,32 2018 | Sweden | Swedish Patient Register | 2002 – 2014 | 2295 | Pediatric | M (54), F (46) | - | 3 |
| O’Keefe et al,33 1989a | South Africa | Cape Town | 1970 – 1979 | 91 (5y), 61 (10y) |
All ages | - | - | 5 and 10 |
| Parragi et al,34 2018 | Switzerland | SIBDCS | 2006 – 2015 | 1245 | All ages | M (54.6), F (45.4) | E1 (20), E2 (32), E3 (38) | 5 and 10 |
| Probert et al,35 1993 | England | Leicestershire | 1972 – 1989 | 691 | All ages | - | - | 5 and 10 |
| Ronnblom et al,36 2016 | Sweden | Uppsala Healthcare Region | 2005 – 2009 | 524 | All ages | M (55.2), F (44.8) | E1 (32), E2 (31), E3 (31) | 1 and 5 |
| Rungoe et al,11 2014 | Denmark | DNPR | 1979 – 2011 | 35782 | All ages | M (47), F (53) | - | 1, 5, and 9 |
| Samuel et al,37 2013 | USA | Olmsted County | 1970 – 2004 | 369 | All ages | M (58), F (42) | E1 (29), E2 (37), E3 (32) | 1, 5, and 10 |
| Solberg et al,38 2009 | Norway | IBSEN | 1990 – 1993 | 519 | All ages | M (51.4), F (48.6) | E1 (33), E2 (35), E3 (32) | 1, 5, and 10 |
| Spizzo et al,39 2016 | Australia | GECCO | 2007 – 2015 | 41 | All ages | - | - | 4 |
| Targowinik et al,40 2012 | Canada | UMIBDED | 1984 – 2008 | 3752 | All Ages | M (48), F (52) | - | 1, 5, and 10 |
M: Male, F: Female, E1: Proctitis, E2: Left-sided Colitis, E3: Pancolitis
On meta-analysis, the cumulative risk of colectomy 1-, 5-, and 10-years after diagnosis was 4.0% (95% CI, 3.3–5.0), 8.8% (95% CI, 7.7–10.0) and 13.3% (95% CI, 11.3–15.5), respectively, with considerable heterogeneity (I2=95–98%) (Figures 1A–C). Time-trend mixed-effects meta-regression showed progressive decline in the 1-, 5- and 10-year risk of colectomy (p<0.01). In contemporary cohorts of patients diagnosed with UC in the 21st century, the cumulative risk of colectomy 1-, 5-, and 10-years after diagnosis was 2.8% (95% CI, 2.0–3.9) [42% lower than in patients diagnosed in prior decades, p=0.01], 7.0% (95% CI, 5.7–8.6) [26% lower than in patients diagnosed in prior decades, p=0.04] and 9.6% (95% CI, 6.3–14.2) [37% lower than in patients diagnosed in prior decades, p=0.04], respectively (eFigures 2A–C).
Figure 1.
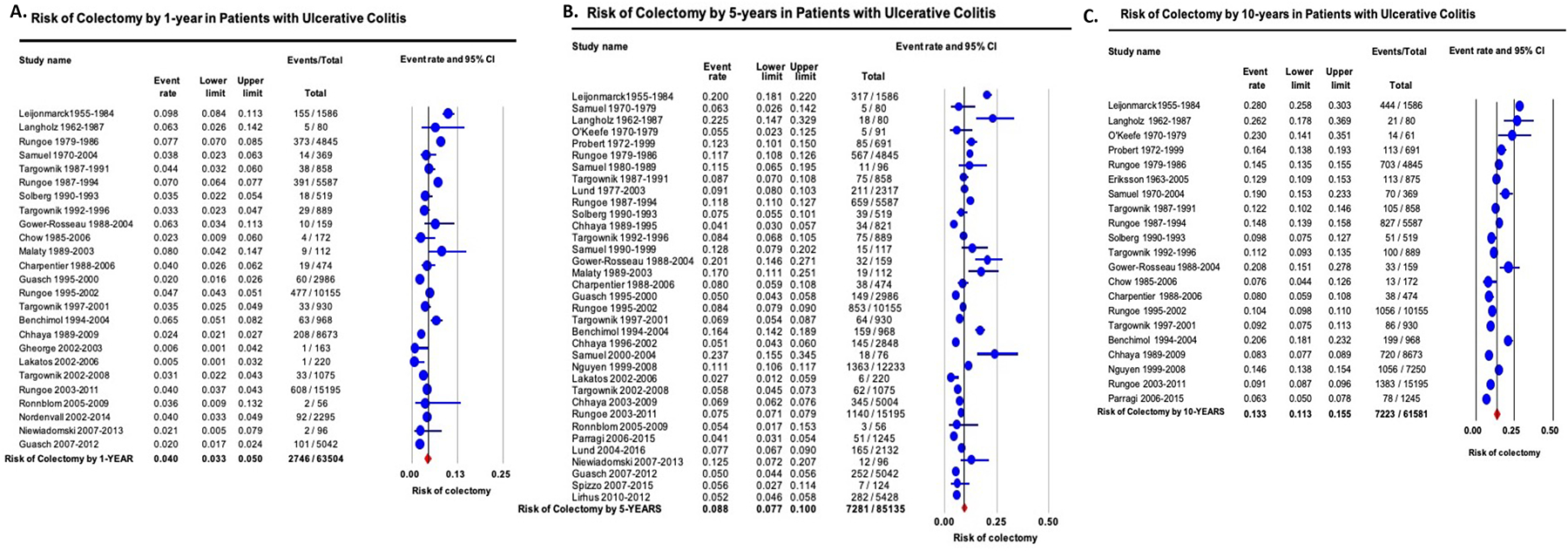
Cumulative risk of colectomy in patients with ulcerative colitis by (A) 1-year, (B) 5-years and (C) 10-years after diagnosis. Studies are arranged in order of mid-year of cohort recruitment.
On subgroup analyses, no significant differences were observed in 1-, 5- and 10-year risk of colectomy based upon geographical location (Appendix Table 3). On meta-regression, study-level sex distribution and disease extent did not affect risk of surgery (Appendix Table 4). Cumulative risk of 1-, 5- and 10-year risk of colectomy in patients with pediatric-onset UC was 5.7% (95% CI, 4.2–7.8), 14.1% (95% CI, 10.0–19.6) and 21.0% (95% CI, 18.8–23.4), respectively. Sensitivity analyses after exclusion of studies published only in abstract form, and cumulative 10y risk of surgery after excluding studies with <5y follow-up, did not significantly change estimates (data not shown).
Cumulative Risk of First Major Abdominal Surgery in Patients with Crohn’s Disease
Table 2 details the characteristics of 22 studies in patients with CD reporting on risk of first surgery.9, 11, 17, 21, 23, 27, 30–32, 39, 41–52 These studies included patients diagnosed between 1955 to 2015, with sample sizes ranging from 53 to 13,185 patients with CD; the largest study was a Danish nationwide register-based study.11 Two studies were conducted exclusively in pediatric-onset CD. Thirteen studies each reported data from patients in the biologic and pre-biologic era.
Table 2.
Characteristics of included studies on primary resection risk in Crohn’s Disease
| Bernell et al,41 2000 | Sweden | Stockholm County | 1955 – 1989 | 1936 | All ages | M (47) F (53) |
- | - | 1, 5, and 10 |
| Burr et al,9 2019 | UK | Research One | 1994 – 2013 | 3059 | All ages | M (47) F (53) |
- | - | 1, 5, and 10 |
| Charpentier et al,17 2014 | France | EPIMAD | 1988 – 2006 | 367 | Elderly | M (38) F (62) |
- | B1 (78), B2 (17), B3 (5) | 1, 5, and 10 |
| Chatu et al,42 2014 | UK | CPRD | 1989 – 2010 | 5640 | All ages | M (42) F (57) |
- | - | 5 |
| Gheorge et al,21 2004 | Romania | 18 secondary and tertiary centers | 2002 – 2003 | 85 | Adults | M (57) F (43) |
L1 (15), L2 (52), L3 (32), L4 (2) | - | 1 |
| Guasch et al,23 2018 | Spain | ENEIDA | 1990 – 1995, 2007 – 2012 | 7496 | All ages | - | - | - | 1 and 5 |
| Jeuring et al,43 2015 | Netherlands | IBD-SL | 1991 – 2011 | 1159 | All ages | - | - | - | 1, 5, and 10 |
| Lakatos et al,44 2012 | Hungary | Veszprem Province | 1977 – 2008 | 506 | All ages | M (49.6) F (50.4) |
L1 (32.8), L2 (36.0), L3 (30.6), L4 (0.6) | B1 (56.9), B2 (19.8), B3 (23.3) | 1, 5, and 10 |
| Lirhus et al,27 2018 | Norway | Norwegian Patient Registry | 2010 – 2012 | 2829 | All Ages | M (47.5) F (52.5) |
- | - | 3 |
| Nguyen et al,30 2017 | Canada | Ontario Province | 1999 – 2008 | 8985 | Adult | M (44.3) F (55.7) |
- | - | 5 and 10 |
| Niewiadomski et al,31 2015 | Australia | Barwon Area | 2007 – 2008, 2010 – 2013 |
146 | All ages | - | - | - | 1 and 5 |
| Nordenvall et al,32 2018 | Sweden | Swedish Patient Register | 2002 – 2014 | 2174 | Pediatric | M (58) F (42) |
- | - | 3 |
| O’Keefe et al,45 1989 b | South Africa | Cape Town | 1970 – 1979 | 72 (5y), 53 (10y) |
All ages | - | - | - | 5 and 10 |
| Pandey et al,46 2015 | Singapore | 8 hospitals | 1970 – 2013 | 430 | All ages | M (61.8) F (38.2) |
L1 (27.7), L2 (27.7), L3 (41.9), L4 (17.9) |
B1 (78.1), B2 (14.0), B3 (7.9) | 5 and 10 |
| Peneau et al,47 2012 | France | EPIMAD | 1988 – 2004 | 538 | Pediatric | - | - | - | 1, 5, and 10 |
| Peyrin-Biroulet et al,48 2012 | USA | Olmsted County | 1970 – 2004 | 310 | All ages | M (50.3) F (49.7) |
L1 (31.2), L2 (33.1), L3 (33.4), L4 (2.3) | B1 (81.3), B2 (4.6), B3 (14.1) | 5 and 10 |
| Rabilloud et al,49 2016 | France | Brittany Area | 1994–1997 | 272 | All ages | - | - | - | 1, 5, and 10 |
| Ramadas et al,50 2010 | UK | Cardiff | 1986 – 2003 | 341 | All ages | M (38) F (62) |
- | - | 1 and 5 |
| Rungoe et al,11 2014 | Denmark | DNPR | 1979 – 2011 | 13185 | Adult | M (41) F (59) |
- | - | 1, 5, and 9 |
| Solberg et al,51 2007 | Norway | IBSEN | 1990 – 1993 | 237 | All ages | M (50.2) F (49.8) |
L1 (27), L2 (48.5), L3 (22.8), L4 (1.7) | B1 (62.0), B2 (27.0), B3 (11.0) | 1, 5, and 10 |
| Spizzo et al,39 2016 | Australia | GECCO | 2007 – 2015 | 62 | All ages | - | - | - | 4 |
| Zhulina et al,52 2016 | Sweden | Orebro University Hospital | 1963 – 2005 | 472 | All ages | M (47) F (53) |
L1 (40.5), L2 (33.9), L3 (23.7), L4 (1.7) | B1 (63.5), B2 (19.5), B3 (16.7) | 1 and 5 |
M: Male, F: Female, L1: Ileal, L2: Colonic, L3: Ileocolonic, L4: Upper GI Tract, B1: Inflammatory, B2: Fibrostenotic, B3: Penetrating
On meta-analysis, the cumulative risk of first major abdominal surgery 1-, 5-, and 10-years after diagnosis was 18.7% (15.0–23.0), 28.0% (24.0–32.4) and 39.5% (33.3–46.2), respectively, with considerable heterogeneity (I2=95–98%) (Figures 2A–C). Time-trend mixed-effects meta-regression showed progressive decline in 1-, 5- and 10-year risk of first major abdominal surgery (p<0.001). In contemporary cohorts of patients diagnosed with CD in the 21st century, cumulative risk of first major abdominal surgery 1-, 5-, and 10-years after diagnosis was 12.3% (10.8–14.0) [48% lower than in patients diagnosed in prior decades, p<0.01], 18.0% (15.4–21.0) [50% lower than in patients diagnosed in prior decades, p<0.01] and 26.2% (23.4–29.4) [44% lower than in patients diagnosed in prior decades, p<0.01], respectively, respectively (eFigures 3A–C).
Figure 2.
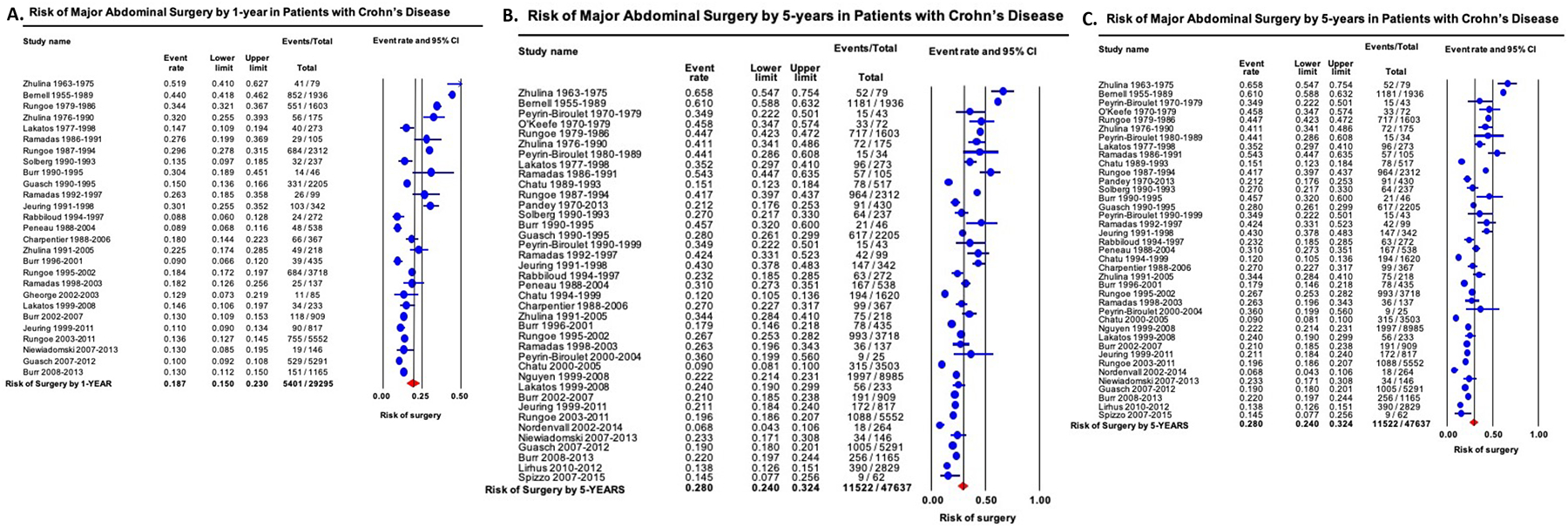
Cumulative risk of major abdominal surgery in patients with Crohn’s disease by (A) 1-year, (B) 5-years and (C) 10-years after diagnosis. Studies are arranged in order of mid-year of cohort recruitment.
On subgroup analyses, no significant differences were observed in 1-, 5- and 10-year risk of major abdominal surgery based upon geographical location (Appendix Table 3). On meta-regression, study-level sex distribution, disease extent and behavior did not affect risk of surgery, except a higher 1y risk of surgery in studies with higher proportion of patients with ileum-dominant CD (Appendix Table 4).Cumulative risk of 1-, 5- and 10-year risk of major abdominal surgery in patients with pediatric-onset CD was 8.9% (95% CI, 6.8–11.6), 15.5% (95% CI, 3.0–52.2) and 44.1% (95% CI, 39.9–48.3), respectively. Sensitivity analyses after exclusion of studies published only in abstract form, and cumulative 10y risk of surgery after excluding studies with <5y follow-up, did not significantly change estimates (data not shown).
Cumulative Risk of Re-Resection in Patients with Crohn’s Disease with Prior Surgery
Appendix Table 5 details the characteristics of 8 studies in patients with CD with prior resection. reporting on risk of re-resection.9, 10, 45, 53–57 These studies included patients diagnosed between 1982 to 2016, with sample sizes ranging from 130 to 8,172 patients with CD with prior resection. Two studies were conducted exclusively in pediatric-onset CD. Three studies each reported data from patients in the biologic and pre-biologic era.
On meta-analysis, the cumulative risk of re-resection 5- and 10-years after first resection was 17.7% (95% CI, 13.5–22.9) and 31.3% (95% CI, 24.1–39.6), respectively, with considerable heterogeneity (I2=97–98%) (Figures 3A–B). Time-trend mixed-effects meta-regression did not show a significant decline in risk of re-resection (5- and 10-year re-resection, p=0.21 and p=0.16, respectively). In contemporary cohorts of patients diagnosed with CD in the 21st century, the cumulative risk of re-resection in patients with CD with prior resection 5-, and 10-years after diagnosis was 14.8% (95% CI, 11.0–19.7) and 25.5% (95% CI, 11.9–46.6), respectively. Cumulative risk of re-resection in patients with pediatric-onset CD with prior resection 5- and 10-year after diagnosis was 21.4% (95% CI, 14.5–30.4) and 33.6% (95% CI, 27.5–40.2), respectively.
Figure 3.
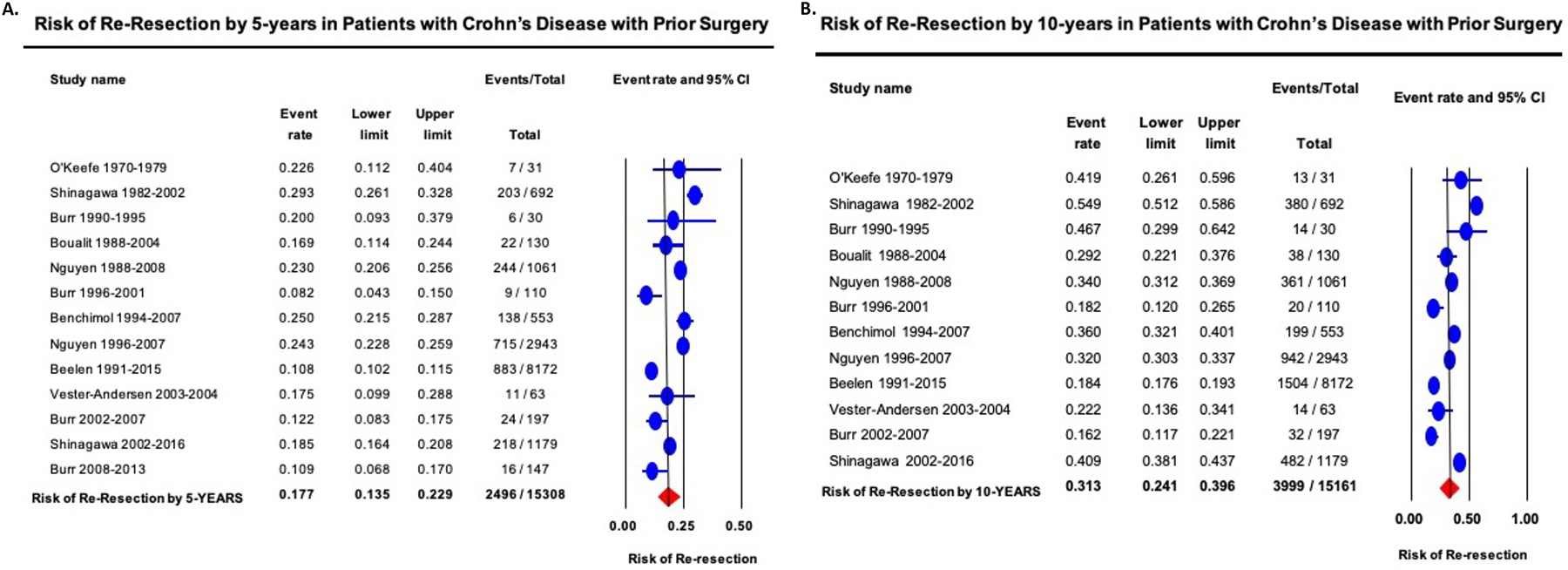
Cumulative risk of re-resection in patients with Crohn’s disease with prior surgery by (A) 5-years and (B) 10-years after first surgery. Studies are arranged in order of mid-year of cohort recruitment.
Due to considerable heterogeneity for all analyses, statistical assessment of publication bias was not performed.
DISCUSSION
In this systematic review of 44 population-based cohort studies, we estimated cumulative risk of surgery in patients with UC and CD and observed that short- and long-term risk of surgery was 25–50% lower in patients diagnosed with IBD in the last two decades than prior decades. In contrast to prior estimates, contemporary 5-year risk of major abdominal surgery is 7.0% in UC, and 17.8% in CD, in 21st century. These risks were comparable in patients in North America and Europe, while contemporary data from other parts of the world are evolving. Overall, these findings confirm declining trends in risk of surgery which may be related to disease-modifying effect of contemporary management approach in patients with IBD.
Our systematic review updates a prior comprehensive review on risk of surgery in patients with IBD that was published in 2013.5 In that review, Frolkis and colleagues included 30 population-based studies in patients with IBD, largely diagnosed before 2007 and followed up to 2011. The number of cohorts reporting contemporary surgical risk in patients diagnosed after 2000 was very small – 3 and 0 cohorts reporting 5- and 10y risk of surgery in UC, and 2 and 0 cohorts reporting 5- and 10y risk of surgery in CD. They inferred progressively lower 5y risk of surgery in patients with CD, but not UC over four decades. The authors speculated that a lower burden of surgery would be observed for patients diagnosed with IBD in the 21st century but could not provide reliable estimates of contemporary surgical risks due to paucity of studies. With an updated literature search, based on 13 and 12 cohorts of patients with UC and CD, respectively, diagnosed after 2000, we have observed a significantly lower 5- and 10y risks of major abdominal surgery in patients diagnosed at the beginning of the 21st century, than those observed in the 20th century.
Several population-based studies have attempted to examine surgical trends in IBD, over the last decade. However, most studies have examined annual rates of IBD-related surgery amongst patients with prevalent IBD, rather than evaluating individual patient-level cumulative risk of surgery in incident cases. These studies have generally demonstrated decline in annual rates of emergent surgeries in patients with IBD over time. Using administrative claims data from Ontario between 2003–14, Rahman and colleagues observed an approximately 40% decline in resection surgeries in patients with CD between 2003 to 2014, with a corresponding 33% increase in risk of outpatient, non-resection, surgeries for CD (related to perianal fistulae and stricture dilations) over the same time period.58 Ma and colleagues similarly observed a 3.5% annual decline in rates of surgery in patients with CD between 2002 to 2010, driven primarily by a 10.1% annual decline in rates of emergent surgery, offset by a 3.7% annual increase in rates of elective surgeries.59 Based upon an administrative claims study, Barnes and colleagues observed similar trends in risk of colectomy in patients with UC.60 They observed a significant 46% decline in risk of colectomy between 2007 to 2016, with a 4.5-fold increase in use of biologic therapy in the same time period. Kayal and colleagues observed 7.4% annual decline in risk of emergent colectomy in patients with UC, without a significant change in risk of elective ileal pouch anal anastomosis surgeries.61 While these studies are helpful in informing the overall burden of IBD-related surgeries to the health system, they do not provide patient-level risk estimates that are critical for prognostication for both patient care and development of risk-based treatment algorithms.
The exact factors at play contributing to decrease in risk of surgery in patients with IBD are unclear, though the causes are likely multifactorial and merit further assessment. Whilst reduction in surgical rates has been associated with the parallel increase in use of biologic agents, their exact contribution is hard to quantify. Through claims-based analyses in Ontario, Murthy and colleagues determined that introduction of infliximab may not have resulted in substantial decline in risk of CD- and UC-related surgeries, despite high market penetration in patients with CD.62 They attributed these findings to “misguided use of infliximab in CD patients and underuse of infliximab in UC”. Other factors such as early diagnosis due to increased patient and provider awareness and improved diagnostics may allow timely introduction of disease-modifying therapy decreasing risk of early surgery. Clinical monitoring and algorithmic treatment escalation have also been shown to decrease the risk of surgery and disease-related complications in the patients with CD.8, 63 The population-wide, patient-level impact evolving treat-to-target strategies remains to be seen and will be better examined in the coming decade when there is penetration into routine clinical practice. Finally, with better disease control, risk of dysplasia in patients with long-standing UC is decreasing; with advanced endoscopic imaging and therapeutic modalities, several neoplastic lesions, which previously warranted colectomy, are now being managed endoscopically, which is also likely contributing to lower risk of colectomy in patients with UC.64
Despite the merits and strengths of our synthesis, there are important limitations. First, considerable heterogeneity was observed in most analyses. However, it is important to note that the implications and interpretation of a statistical measure as I2 is not the same for studies of incidence and prevalence, as for comparative observational or interventional studies. High statistical heterogeneity is often observed in these analyses and could not be explained despite subgroup and sensitivity analyses, and meta-regression. We tried to minimize conceptual heterogeneity through strict inclusion and exclusion criteria. Other factors including differences in diagnostic evaluation and evolving treatment paradigms and access in different populations may account for unexplained heterogeneity. Second, as noted earlier, we were unable to examine factors that may have contributed to a decline in risk of surgery. There was limited information on use of disease-modifying therapy in these cohorts. Moreover, the potential impact of newer non-TNF-directed biologics, and the practice of cycling through multiple biologics prior to surgery, could not be assessed in these population-based cohorts. Future individual patient-level syntheses are required to comprehensively understand the multitude of factors that may contribute to declining surgical risks. Third, the number of studies examining rates of re-resection in patients with CD was limited, and hence, time-trend analysis was underpowered. Future studies are needed to quantify risk of repeat surgery better. Fourth, studies did not distinguish between types of surgery, including emergent and elective surgeries, resection vs. non-resection surgeries and indications for surgery (for example, medically refractory UC vs. colorectal neoplasia). Risk of surgery for perianal CD was not well-reported. Finally, in pooling cumulative risks, we assumed complete follow-up of cohort which may bias findings particularly for 10y risks of surgery; however, our estimates for long-term risk of surgery were unchanged when limiting to studies with >5y follow-up. Future studies with individual patient-level syntheses of risks, or alternative statistical approaches such as bootstrapping, may provide more reliable estimates.
In conclusion, based on a systematic review of 44 population-based cohorts, we provided robust contemporary cumulative risks of first major abdominal surgery in patients with UC and CD (and repeat surgery in patients with CD) diagnosed in the 21st century. Contemporary cumulative 5-year risk of surgery of 7.0% in UC, and 17.8% in CD is substantially lower than those observed in patients diagnosed in the 20th century. Factors contributing meaningfully to these decreased risks, and cost-effectiveness of those strategies, merit further evaluation, including the impact of newer biologics and treat-to-target strategies, to promote value-based care.
Supplementary Material
eFigure 1. Study selection flowchart
eFigure 2. (A) 1-year, (B) 5-years and (C) 10-year cumulative risk of colectomy in patients with ulcerative colitis, diagnosed in the 21st century vs. 20th century.
eFigure 3. (A) 1-year, (B) 5-years and (C) 10-year cumulative risk of major abdominal surgery in patients with Crohn’s disease, diagnosed in the 21st century vs. 20th century.
Disclosures:
Dr. Dulai is supported by the American Gastroenterological Association Career Development Award. Dr. Sandborn is supported in part by NIDDK-funded San Diego Digestive Diseases Research Center (P30 DK120515). Dr. Singh is supported by NIH/NIDDK (K23DK117058), ACG Junior Faculty Development Award (#144271) and the Crohn’s and Colitis Foundation Career Development Award (#404614).
Conflicts of Interest:
Lester Tsai – None to declare
Christopher Ma – consulting fees from AbbVie, Janssen, Takeda, Pfizer, Roche, Robarts Clinical Trials Inc.; speaker’s fees from AbbVie, Janssen, Takeda, and Pfizer.
Parambir S. Dulai – research support from Takeda, Pfizer, Abbvie, Janssen, Polymedco, ALPCO, Buhlmann, Prometheus, and consulting fees from Takeda, Pfizer, Abbvie and Janssen.
Larry J. Prokop – None to declare
Samuel Eisenstein – Consulting fees from Auris Health, inc
Sonia L. Ramamoorthy – None to declare
Brian G. Feagan – received grant/research support from Millennium Pharmaceuticals, Merck, Tillotts Pharma AG, AbbVie, Novartis Pharmaceuticals, Centocor Inc., Elan/Biogen, UCB Pharma, Bristol-Myers Squibb, Genentech, ActoGenix, and Wyeth Pharmaceuticals Inc.; consulting fees from Millennium Pharmaceuticals, Merck, Centocor Inc., Elan/Biogen, Janssen-Ortho, Teva Pharmaceuticals, Bristol-Myers Squibb, Celgene, UCB Pharma, AbbVie, Astra Zeneca, Serono, Genentech, Tillotts Pharma AG, Unity Pharmaceuticals, Albireo Pharma, Given Imaging Inc., Salix Pharmaceuticals, Novonordisk, GSK, Actogenix, Prometheus Therapeutics and Diagnostics, Athersys, Axcan, Gilead, Pfizer, Shire, Wyeth, Zealand Pharma, Zyngenia, GiCare Pharma Inc., and Sigmoid Pharma; and speaker’s fees from UCB, AbbVie, and J&J/Janssen.
Vipul Jairath – consulting fees from AbbVie, Eli Lilly, GlaxoSmithKline, Arena pharmaceuticals, Genetech, Pendopharm, Sandoz, Merck, Takeda, Janssen, Robarts Clinical Trials, Topivert, Celltrion; speaker’s fees from Takeda, Janssen, Shire, Ferring, Abbvie, Pfizer
William J. Sandborn – research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, Abbvie, Janssen, Takeda, Lilly, Celgene/Receptos,Pfizer, Prometheus Laboratories (now Prometheus Biosciences); consulting fees from Abbvie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pfizer, Progenity, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust, HART), Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, Tigenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals; and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Progenity, Ritter Pharmaceuticals, Ventyx Biosciences, Vimalan Biosciences. Spouse: Opthotech - consultant, stock options; Progenity - consultant, stock; Oppilan Pharma - employee, stock options; Escalier Biosciences - employee, stock options; Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories) - employee, stock options; Ventyx Biosciences – employee, stock options; Vimalan Biosciences – employee, stock options.
Siddharth Singh – research grants from AbbVie, Janssen
REFERENCES
- 1.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;390:2769–2778. [DOI] [PubMed] [Google Scholar]
- 2.Coward S, Clement F, Benchimol EI, et al. Past and Future Burden of Inflammatory Bowel Diseases Based on Modeling of Population-Based Data. Gastroenterology 2019;156:1345–1353.e4. [DOI] [PubMed] [Google Scholar]
- 3.King JA, Underwood FE, Panaccione N, et al. Trends in hospitalisation rates for inflammatory bowel disease in western versus newly industrialised countries: a population-based study of countries in the Organisation for Economic Co-operation and Development. The Lancet Gastroenterology & Hepatology 2019;4:287–295. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen NH, Koola J, Dulai PS, et al. Rate of Risk Factors for and Interventions to Reduce Hospital Readmission in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frolkis AD, Dykeman J, Negron ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology 2013;145:996–1006. [DOI] [PubMed] [Google Scholar]
- 6.Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet 2017;389:1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres J, Mehandru S, Colombel JF, et al. Crohn’s disease. Lancet 2017;389:1741–1755. [DOI] [PubMed] [Google Scholar]
- 8.Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2018;390:2779–2789. [DOI] [PubMed] [Google Scholar]
- 9.Burr NE, Lord R, Hull MA, et al. Decreasing Risk of First and Subsequent Surgeries in Patients With Crohn’s Disease in England From 1994 through 2013. Clinical Gastroenterology & Hepatology 2019;17:2042–2049.e4. [DOI] [PubMed] [Google Scholar]
- 10.Shinagawa T, Hata K, Ikeuchi H, et al. Rate of Reoperation Decreased Significantly After Year 2002 in Patients with Crohn’s Disease. Clinical Gastroenterology & Hepatology 2019;20:20. [DOI] [PubMed] [Google Scholar]
- 11.Rungoe C, Langholz E, Andersson M, et al. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979–2011. Gut 2014;63:1607–16. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001;323:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benchimol E, Guttmann A, To T, et al. Changes in surgical and hospitalization rates in pediatric inflammatory bowel disease in Ontario, Canada (1994–2007). Inflammatory Bowel Diseases 2011;1):S10. [DOI] [PubMed] [Google Scholar]
- 17.Charpentier C, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut 2014;63:423–32. [DOI] [PubMed] [Google Scholar]
- 18.Chhaya V, Saxena S, Cecil E, et al. The impact of timing and duration of thiopurine treatment on colectomy in ulcerative colitis: a national population-based study of incident cases between 1989–2009. Alimentary Pharmacology & Therapeutics 2015;41:87–98. [DOI] [PubMed] [Google Scholar]
- 19.Chow DK, Leong RW, Tsoi KK, et al. Long-term follow-up of ulcerative colitis in the Chinese population. Am J Gastroenterol 2009;104:647–54. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson C, Cao Y, Rundquist S, et al. Changes in medical management and colectomy rates: a population-based cohort study on the epidemiology and natural history of ulcerative colitis in Orebro, Sweden, 1963–2010. Alimentary Pharmacology & Therapeutics 2017;46:748–757. [DOI] [PubMed] [Google Scholar]
- 21.Gheorghe C, Pascu O, Gheorghe L, et al. Epidemiology of inflammatory bowel disease in adults who refer to gastroenterology care in Romania: a multicentre study. Eur J Gastroenterol Hepatol 2004;16:1153–9. [DOI] [PubMed] [Google Scholar]
- 22.Gower-Rousseau C, Sarter H, Turck D, et al. Long-term outcome of paediatric-onset ulcerative colitis: Early years are shaping the future. Journal of Crohn’s and Colitis 2014;1):S32–S33. [Google Scholar]
- 23.Guasch M, Clos A, Ordas I, et al. The availability of anti-TNF agents is associated with reduced early surgical requirements in Crohn’s disease but not in ulcerative colitis. A nationwide study from the Eneida registry. Journal of Crohn’s and Colitis 2018;12 (Supplement 1):S301–S302. [Google Scholar]
- 24.Lakatos L, Kiss LS, David G, et al. Incidence, disease phenotype at diagnosis, and early disease course in inflammatory bowel diseases in Western Hungary, 2002–2006. Inflammatory Bowel Diseases 2011;17:2558–65. [DOI] [PubMed] [Google Scholar]
- 25.Langholz E, Munkholm P, Krasilnikoff PA, et al. Inflammatory bowel diseases with onset in childhood. Clinical features, morbidity, and mortality in a regional cohort. Scand J Gastroenterol 1997;32:139–47. [DOI] [PubMed] [Google Scholar]
- 26.Leijonmarck CE, Persson PG, Hellers G. Factors affecting colectomy rate in ulcerative colitis: an epidemiologic study. Gut 1990;31:329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lirhus SS, Hoivik ML, Moum B, et al. Regional differences in anti-TNF-alpha therapy and surgery in the treatment of inflammatory bowel disease patients: a Norwegian nationwide cohort study. Scandinavian Journal of Gastroenterology 2018;53:952–957. [DOI] [PubMed] [Google Scholar]
- 28.Lund K, Larsen MD, Knudsen T, et al. Anti-TNF-alpha therapy, use of corticosteroids, and colectomy among paediatric and adolescent patients with ulcerative colitis: A nationwide study. Journal of Crohn’s and Colitis 2019;13 (Supplement 1):S442–S443. [Google Scholar]
- 29.Malaty HM, Abraham BP, Mehta S, et al. The natural history of ulcerative colitis in a pediatric population: a follow-up population-based cohort study. Clinical & Experimental Gastroenterology 2013;6:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen GC, Bernstein CN, Benchimol EI. Risk of Surgery and Mortality in Elderly-onset Inflammatory Bowel Disease: A Population-based Cohort Study. Inflammatory Bowel Diseases 2017;23:218–223. [DOI] [PubMed] [Google Scholar]
- 31.Niewiadomski O, Studd C, Hair C, et al. Prospective population-based cohort of inflammatory bowel disease in the biologics era: Disease course and predictors of severity. Journal of Gastroenterology & Hepatology 2015;30:1346–53. [DOI] [PubMed] [Google Scholar]
- 32.Nordenvall C, Rosvall O, Bottai M, et al. Surgical Treatment in Childhood-onset Inflammatory Bowel Disease-A Nationwide Register-based Study of 4695 Incident Patients in Sweden 2002–2014. Journal of Crohn’s & colitis 2018;12:157–166. [DOI] [PubMed] [Google Scholar]
- 33.O’Keefe EA, Wright JP, Froggatt J, et al. Medium-term follow-up of ulcerative colitis in Cape Town. S Afr Med J 1989;76:142–5. [PubMed] [Google Scholar]
- 34.Parragi L, Fournier N, Zeitz J, et al. Colectomy Rates in Ulcerative Colitis are Low and Decreasing: 10-year Follow-up Data From the Swiss IBD Cohort Study. Journal of Crohn’s & colitis 2018;12:811–818. [DOI] [PubMed] [Google Scholar]
- 35.Probert CSJ JV, Bhakta P, Wicks TCB, Mayberry JF. How necessary is colectomy? An epidemiological study of the surgical management of ulcerative colitis amongst different ethnic groups in Leicestershire. Eur J Gastroenterol Hepatol 1993;5:17–20. [Google Scholar]
- 36.Ronnblom A, Holmstrom T, Tanghoj H, et al. Low colectomy rate five years after diagnosis of ulcerative colitis. Results from a prospective population-based cohort in Sweden (ICURE) diagnosed during 2005–2009. Scandinavian Journal of Gastroenterology 2016;51:1339–44. [DOI] [PubMed] [Google Scholar]
- 37.Samuel S, Ingle SB, Dhillon S, et al. Cumulative incidence and risk factors for hospitalization and surgery in a population-based cohort of ulcerative colitis. Inflammatory Bowel Diseases 2013;19:1858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solberg IC, Lygren I, Jahnsen J, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scandinavian Journal of Gastroenterology 2009;44:431–40. [DOI] [PubMed] [Google Scholar]
- 39.Spizzo P, Hair C, Beswick L, et al. A combined cohort IBD natural history study: High rates of immunomodulator and biologic use, low rates of intestinal surgery for Crohn’s disease. Journal of Gastroenterology and Hepatology (Australia) 2016;31 (Supplement 2):147. [Google Scholar]
- 40.Targownik LE, Singh H, Nugent Z, et al. The epidemiology of colectomy in ulcerative colitis: results from a population-based cohort. American Journal of Gastroenterology 2012;107:1228–35. [DOI] [PubMed] [Google Scholar]
- 41.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Annals of Surgery 2000;231:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chatu S, Saxena S, Subramanian V, et al. The impact of timing and duration of thiopurine treatment on first intestinal resection in Crohn’s disease: national UK population-based study 1989–2010. American Journal of Gastroenterology 2014;109:409–16. [DOI] [PubMed] [Google Scholar]
- 43.Jeuring S, Van Den Heuvel T, Zeegers M, et al. Hospitalisation and surgery risk in Crohn’s disease in the biological era - Results from the Dutch population-based IBD-SL cohort. Journal of Crohn’s and Colitis 2015;1):S210. [Google Scholar]
- 44.Lakatos PL, Golovics PA, David G, et al. Has there been a change in the natural history of Crohn’s disease? Surgical rates and medical management in a population-based inception cohort from Western Hungary between 1977–2009. American Journal of Gastroenterology 2012;107:579–88. [DOI] [PubMed] [Google Scholar]
- 45.O’Keefe EA, Wright JP, Froggatt J, et al. Medium-term follow-up of Crohn’s disease in Cape Town. S Afr Med J 1989;76:139–41. [PubMed] [Google Scholar]
- 46.Pandey A, Salazar E, Kong CSC, et al. Risk of Major Abdominal Surgery in an Asian Population-based Crohn’s Disease Cohort. Inflammatory Bowel Diseases 2015;21:2625–33. [DOI] [PubMed] [Google Scholar]
- 47.Peneau A, Salleron J, Fumery M, et al. Long-term outcome of paediatric-onset crohn’s disease: A population-based study. Gastroenterology 2012;1):S25. [Google Scholar]
- 48.Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, et al. Surgery in a population-based cohort of Crohn’s disease from Olmsted County, Minnesota (1970–2004). American Journal of Gastroenterology 2012;107:1693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rabilloud ML, Bretagne JF, Bajeux E, et al. Predictive factors of surgery and bowel damage during the course of Crohn’s disease: A population-based study. Journal of Crohn’s and Colitis 2016;10 (Supplement 1):S192. [Google Scholar]
- 50.Ramadas AV, Gunesh S, Thomas GAO, et al. Natural history of Crohn’s disease in a population-based cohort from Cardiff (1986–2003): a study of changes in medical treatment and surgical resection rates. Gut 2010;59:1200–6. [DOI] [PubMed] [Google Scholar]
- 51.Solberg IC, Vatn MH, Hoie O, et al. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clinical Gastroenterology & Hepatology 2007;5:1430–8. [DOI] [PubMed] [Google Scholar]
- 52.Zhulina Y, Udumyan R, Tysk C, et al. The changing face of Crohn’s disease: a population-based study of the natural history of Crohn’s disease in Orebro, Sweden 1963–2005. Scandinavian Journal of Gastroenterology 2016;51:304–13. [DOI] [PubMed] [Google Scholar]
- 53.Beelen EMJ, van der Woude CJ, Pierik MJ, et al. Decreasing Trends in Intestinal Resection and Re-Resection in Crohn’s Disease: A Nationwide Cohort Study. Annals of Surgery 2019;10:10. [DOI] [PubMed] [Google Scholar]
- 54.Benchimol E, Boualit M, Wong J, et al. Predictors of the need for second intestinal resection in children withCrohn’s disease. Inflammatory Bowel Diseases 2011;2):S6. [Google Scholar]
- 55.Boualit M, Salleron J, Turck D, et al. Long-term outcome after first intestinal resection in pediatric-onset Crohn’s disease: a population-based study. Inflammatory Bowel Diseases 2013;19:7–14. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen GC, Saibil F, Steinhart AH, et al. Postoperative healthcare utilization in crohn’s disease: The impact of specialist care. Gastroenterology 2012;1):S251. [DOI] [PubMed] [Google Scholar]
- 57.Vester-Andersen MK, Vind I, Prosberg MV, et al. Hospitalisation, surgical and medical recurrence rates in inflammatory bowel disease 2003–2011-a Danish population-based cohort study. Journal of Crohn’s & colitis 2014;8:1675–83. [DOI] [PubMed] [Google Scholar]
- 58.Rahman A, Jairath V, Feagan BG, et al. Declining hospitalisation and surgical intervention rates in patients with Crohn’s disease: a population-based cohort. Aliment Pharmacol Ther 2019;50:1086–1093. [DOI] [PubMed] [Google Scholar]
- 59.Ma C, Moran GW, Benchimol EI, et al. Surgical Rates for Crohn’s Disease are Decreasing: A Population-Based Time Trend Analysis and Validation Study. American Journal of Gastroenterology 2017;112:1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barnes EL, Herfarth HH, Kappelman M, et al. A Significant Decrease in the Rate of Colectomy for Ulcerative Colitis among Commercially Insured Patients in the United States between 2007–2016. Gastroenterology 2019;156 (6 Supplement 1):S-155–S-156. [Google Scholar]
- 61.Kayal M, Saha A, Poojary P, et al. Emergent colectomy rates decreased while elective ileal pouch rates were stable over time: a nationwide inpatient sample study. Int J Colorectal Dis 2019;34:1771–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murthy SK, Begum J, Benchimol EI, et al. Introduction of anti-TNF therapy has not yielded expected declines in hospitalisation and intestinal resection rates in inflammatory bowel diseases: a population-based interrupted time series study. Gut 2019;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khanna R, Bressler B, Levesque BG, et al. Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. Lancet 2015;386:1825–34. [DOI] [PubMed] [Google Scholar]
- 64.Hata K, Anzai H, Ikeuchi H, et al. Surveillance Colonoscopy for Ulcerative Colitis-Associated Colorectal Cancer Offers Better Overall Survival in Real-World Surgically Resected Cases. Am J Gastroenterol 2019;114:483–489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Study selection flowchart
eFigure 2. (A) 1-year, (B) 5-years and (C) 10-year cumulative risk of colectomy in patients with ulcerative colitis, diagnosed in the 21st century vs. 20th century.
eFigure 3. (A) 1-year, (B) 5-years and (C) 10-year cumulative risk of major abdominal surgery in patients with Crohn’s disease, diagnosed in the 21st century vs. 20th century.


