Abstract
Background
Parasitism is a relationship where one, the parasite, harms the host or lives at the expense of the host. Intestinal parasites (protozoa and STHs-soil-transmitted helminths) cause gastrointestinal tract infection in humans and animals. Intestinal parasitic infections (IPIs) predominate the tropics and subtropics and affect poor countries, where school children suffer the most. To prevent and control these infections, local risk factors must first be identified. The aim of the study was to determine the prevalence of intestinal parasitic infections and associated risk factors among school children in Jaragedo town schools, South Gondar Zone of Ethiopia.
Methods
A cross-sectional study was conducted from October 2018 to April 30, 2019, involving 396 students from one elementary and one secondary school. Stratified simple random sampling method was used. A questionnaire was prepared to collect sociodemographic and socioeconomic data of the study subjects. Stool samples were collected and examined using formalin-ether concentration technique. Data were analyzed using SAS software version 9.4. Descriptive statistics were used to give a clear picture of population characteristics. Logistic regression was also used to determine the relationship between dependent variables (primary infection) with independent (explanatory) variables using SAS software.
Results
Results showed that the overall prevalence of intestinal parasitic infections was 65.4%. E. histolytica was the most prevalent intestinal parasite (12–14%) followed by G. lamblia (8–9%); other parasites could not infect more than 5% of the study subjects. Generally, parasitism did not vary between the sexes. The logistic regression analysis showed that grade, level of students, water source, habit of consuming raw meat, and level of income had a strong effect on intestinal parasitic infection (P < 0.05). Other explanatory variables were not significant (P > 0.05). High prevalence of parasites indicates improper disposal of waste, low socioeconomic level, low living standard, and poor water quality.
Conclusion
Therefore, short-term and long-term intervention strategies are required to minimize rates of infection.
1. Introduction
Parasitism is the relationship in which one of the participants, the parasite, harms or lives at the expense of the host. Parasites may cause mechanical injury, dig into the skin or other tissues of its host, stimulate an inflammatory or immune responses, or simply rob the host of nutrition. Most parasites inflict a combination of those conditions on their hosts [1].
Intestinal parasites infect the gastrointestinal tract and affect health [2]. Parasitic protozoa are single cell microorganisms that possess one and rarely two nuclei. The two most prevalent intestinal protozoa among children are E. histolytica and G. lamblia. E. histolytica/dispar infects 10% of the worlds' population, while the prevalence may rise as high as 50% in many countries [3]. The prevalence of giardiasis was 2 to 7% in developed and 20 to 30% in developing countries. The disparity in prevalence emanates from factors such as geographical area, the urban or rural setting of the society, age, and socioeconomic conditions [4]. Parasitic helminths include nematodes (round worms), trematodes (flukes), and cestodes (tapeworms). The transmission is by contact with water, food, or soil contaminated with infected human feces; these infections cause anemia, vitamin A deficiency, stunted growth, malnutrition, intestinal obstruction, and impaired mental development. The global prevalence of soil-transmitted helminths is high. Recent estimates show that 1472 million people have round worms, 1298 million have hookworms, and 1049 million have whipworm infection [5].
Soil transmitted helminths and protozoan intestinal infections are common among preschool and school-age children. Children tend to play on soil, suck fingers, and defecate in open fields. Maternal awareness also has its own impact on the prevalence. In Ethiopia, poor sanitation and poor personal hygiene and lack of potable water contribute to the high prevalence of parasitism and the associated morbidity and mortality [6]. Playing with soil, sucking fingers, defecation in open fields, lack of potable water, lack of latrines, contact with animals, poor waste disposal system, walking barefoot, poverty, and occupation of family members all contribute to the high parasitism level. Soil transmitted helminths and protozoan intestinal infections are common diseases in Ethiopia. In Jaragedo town, South Gondar zone, Ethiopia, diarrheal cases are common, but the status of school children with respect to helminths and protozoan intestinal infections is unknown, and the current study was conducted to address the gap.
2. Materials and Methods
2.1. Description of Study Area
The study was conducted in Andabet district, Jaragedo town, South Gondar zone, Amhara region, Ethiopia. Andabet district covers an area of about 8000 ha, and it is located 150 km from Bahir Dar, the regional capital, and 91 km from Debre Tabor, the zonal capital. The total population is 154,797 (51.2% male and 48.8% female). The area receives an annual rainfall of 673.2–1538 mm, temperatures range from 11–25.5°C, and altitudes vary between 1500 and 2300 m above sea level.
The geographical location of Andabet district is 11°10′ to 11°30′ North latitude and 37°45′ to 38°00′ East longitude. In Andabet district, 19 rural and 2 urban kebele administrations are found, one of which is Jaragedo kebele. Jaragedo town has a population of 8894 (5257 males and 3637 females), and it is located 12 km from Andabet town. One-half (50%) of the residents are farmers, 15% merchants, 3% weavers, and 2% others. And 95% of the town dwellers are Orthodox Christian, and 5% are Muslims (Jaragedo community administration, Pers. Comm., 2010). About 66% of the population had access to pipe water.
2.2. Study Design
A cross-sectional study was conducted from October 2018 to April 30, 2019, to determine the prevalence of soil-transmitted helminths and protozoan intestinal infections and associated risk factors among school children of Jaragedo primary school and Jaragedo secondary school.
2.3. Sample Size Determination and Sampling Technique
2.3.1. Sample Size Determination Technique
A total of 403 (202 females and 201 males) were included in the study. Out of these, 102 male and 101 female students were from the Jaragedo primary school and 100 male and 100 females from Jaragedo secondary school. Fifty-percent prevalence rate was used in the sample size calculation because there was no published report in the study area. The minimum sample size “n” required was determined using the statistical formula [7].
| (1) |
where n = required sample size, Z = standard value of 1.96, p = prevalence rate, and m = margin of error of 0.05. To minimize errors arising from nonresponse, 5% of the sample was added to the initial estimate. Seven students were excluded from the study because of insufficient data; the total sample size was, therefore, 396 students.
2.3.2. Sampling Technique
Students were stratified according to grade level (1–4, grades 5–8, and 9–10) to select target groups. Quota was allocated for each stratum, grade level, and section. Finally, a systematic random sampling technique was used by using the classroom roster as a sample frame.
2.4. Inclusion and Exclusion Criteria
All children registered in both schools and who agreed and signed the consent were included in the study. Students who were treated for intestinal parasites in the last two months and those whose parents were unwilling were excluded.
2.5. Data Collection
A structured questionnaire was prepared to collect sociodemographic and socioeconomic data of the study subjects (including risk factors). The questionnaire was first written in English and then translated into Amharic. Students and parents filled the questionnaire. Fingernails of children were inspected. Accuracy and completeness of the filled questionnaire were verified at the end of each day. Immediately after that, each child was given a dry, clean, and leak proof stool cup labeled with the identification number and an applicator stick. Students were advised to fill the stool cup with their own fresh stool about the size of a bean (approximately 3 g) and bring them to the researcher. Stool samples were preserved in 8 ml of 10% formalin solution and transported to Jaragedo Health Center for parasitological examination. A formalin-ether concentration technique was employed to examine the collected samples.
2.6. Parasitological Examination
2.6.1. Formalin-Ether Concentration
A formalin-ether concentration technique was adopted. About 1 to 1.5 g of stool specimen was mixed in a centrifuge tube containing 10 ml formalin mixture and stirred until a suspension formed. Then, 3 ml of ether was added to the suspension and thoroughly mixed by putting a rubber stopper in the tube and then shaken for ten seconds. The tube was placed in a centrifuge for 2–3 minutes at 2000 revolutions per minute. Then, the tube was removed from the centrifuge, where 4 layers have been observed from the top to the bottom (top layer ether, 2nd layer fat debris, 3rd layer formalin, and bottom layer sediment). The first three layers were discarded. A small amount of residual fluid was flown back to the sediment, properly mixed with the sediment, and a drop of suspension was transferred to a clean slide and covered with a cover slip. Finally, the slide was examined at 10x and 40x objectives for the presence of intestinal parasites.
2.7. Data Analysis
Questionnaires and parasitological data were analyzed using SAS software version 9.4. Descriptive statistics were used to show population characteristics, i.e., age, sex, prevalence of intestinal protozoa, and soil-transmitted helminthic infections. Logistic regression was used to determine the relationship between dependent variables (primarily infections) with independent (explanatory) variables or risk factors by using SAS software. Parameter estimates were considered significant at α (alpha) = 0.05. Mother's level of education and hand washing habits of the study subjects before the meal were excluded from the logistic regression analysis because the sample size was not balanced; i.e., three study participants did not wash hands. Study subjects whose parents had college education were few, and this factor was excluded from the model.
2.8. Ethics Clearance
The study was conducted after research ethics approval was obtained from Bahir Dar University, Science College, Bahir Dar, Ethiopia. Letters were written to schools, to district Health Office, and to Jaragedo Health Center. Participants were briefed about the objectives of the study and that all personal information was treated strictly confidential. Consent letter was prepared and ratified with students and parents. Study participants confirmed positive for intestinal parasites were treated free of charge.
3. Results
3.1. Demographic Characteristics of Study Subjects
Four hundred and six kids were selected for the study. Seven of them (∼2%) were excluded because of lack of stool samples. A total of 396 students took part in the investigation; 197 (49.7%) were male, and 199 (50.3%) were female. About 40% of the students were 17 years or older; 55% resided in rural and the rest in urban areas; 22% were in the first cycle of elementary school (1–4 grades), 28% were in the second cycle of elementary school (5–8 grades), and 50% students were from Jaragedo secondary school.
3.2. Risk Factors Associated with Intestinal Parasitic Infections
3.2.1. Socioeconomic Risk Factors
Over 65% of the parents were illiterate, and most fathers were farmers. About half of the parents (∼48%) used both tap water and river water interchangeably; 38% of them used tap water alone. Over 70% of them belonged to the middle-income group; the rest were rich or poor. About 23% of the families involved had a family size less than 5, 60% had 5 to 6 members, and 20% above 7.
3.2.2. Behavioral Risk Factors
Over 90% of the study subjects washed hands before meal and after toilet use; more than 75% of them washed vegetables. Slightly more than half of the kids did not consume raw meat. Most of the students (∼85%) wore shoes always. Over 90% of families had their own toilet in close proximity, and more than 80% of them buried household waste. On direct observation, about 35% of the students had unclean nails. The details are given in Table 1.
Table 1.
Behavioral risk factors associated with intestinal parasitic infections.
| Risk factors | Options | Frequency (#) | Percent |
|---|---|---|---|
| Hand washing before meal | Yes | 393 | 99.2 |
| No | 3 | 0.8 | |
| Washing hands after toilet | Yes | 365 | 92.2 |
| No | 31 | 7.8 | |
| Do you wash vegetables? | Yes | 309 | 78.0 |
| No | 87 | 22.0 | |
| Do you eat raw meat? | Yes | 183 | 46.2 |
| No | 213 | 53.8 | |
| How often you shoe? | No at all | 25 | 6.3 |
| Some times | 28 | 7.1 | |
| Always | 343 | 86.6 | |
| Way of disposing waste | Burry | 326 | 82.3 |
| Open space | 70 | 17.7 | |
| Latrine type of families | Private | 366 | 92.4 |
| Common | 12 | 3.0 | |
| Open space | 18 | 4.6 | |
| Students' fingernail during interview | Clean | 258 | 65.2 |
| Not clean | 138 | 34.8 |
3.3. Prevalence of Intestinal Parasitic Infections
Overall prevalence of intestinal parasitic infections was 65.4%. E. histolytica was the most prevalent intestinal parasite (12–14%) followed by G. lamblia (8–9%); other parasites, the ones that could not be grouped into any of the six species, infected less than 5% of the study subjects. Parasitism did not vary between the sexes (a difference of only about 2%) (Figure 1).
Figure 1.
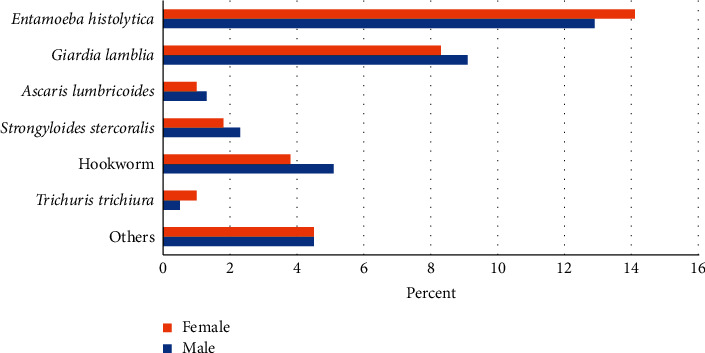
Prevalence of single intestinal parasites among school children in Jaragedo primary and secondary schools, northwest Ethiopia.
As the odds ratios show, the prevalence of intestinal parasitic infections was high for students who were 15-16 years old, grades 9-10, rural dwellers, illiterate family heads, families who practiced farming, very poor families, river water users, and students with six family members (Table 2). Kids who did not wash hands before the meal and who did not wear shoes were all found infected. The odds of being positive for intestinal parasitic infections were about twice higher among rural dwellers compared to urban dwellers. Students who did not wash their hands after use of the toilet had two times higher odds ratio having these infections compared to students who washed hands. Students from very poor families were 5 times more likely to be infected compared to kids from rich families; those who did not use latrines had two-fold chance of being affected compared to children who used latrines. Increasing level of education of fathers helped reduce infection compared to fathers without education. River water users were 7.5 times more likely to be infected compared to tap water users. The odds of being positive for intestinal parasitic infections were two times higher among kids in 15–16 age category compared to kids in 7–10 age categories. Secondary school grade levels were 2.5 times more likely to be infected compared to elementary school grade levels.
Table 2.
Prevalence of intestinal parasitic infections among school children in relation to explanatory variables.
| Characteristics | Options | Examined | Positive | Negative | Ratio | Odds ratio |
|---|---|---|---|---|---|---|
| Sex | Male | 197 | 130 | 67 | 1.94 | 1.05 |
| Female | 199 | 129 | 70 | 1.84 | 1 | |
| Age (years) | 7–10 | 60 | 36 | 30 | 1.2 | 1.14 |
| 11–14 | 93 | 44 | 49 | 0.89 | 1 | |
| 15–16 | 84 | 60 | 24 | 2.5 | 2.8 | |
| ≥17 | 159 | 119 | 140 | 0.85 | 0.95 | |
| Residence | Rural | 221 | 159 | 62 | 2.56 | 1.92 |
| Urban | 175 | 100 | 75 | 1.33 | 1 | |
| Education (grade level) | 1–4 | 88 | 50 | 38 | 1.3 | 0.8 |
| 5–8 | 109 | 56 | 53 | 1.05 | 1 | |
| 9–10 | 199 | 153 | 46 | 3.32 | 3.16 | |
| Father's education level | Illiterate | 269 | 185 | 84 | 2.2 | 2.75 |
| Church | 31 | 20 | 11 | 1.8 | 2.25 | |
| Primary | 74 | 44 | 30 | 1.5 | 1.87 | |
| Secondary | 13 | 6 | 7 | 0.85 | 1.06 | |
| College and above | 9 | 4 | 5 | 0.8 | 1 | |
| Father's occupation | Farming | 290 | 198 | 92 | 2.15 | 1.59 |
| Nonfarming | 106 | 61 | 45 | 1.35 | 1 | |
| Water source | Tap | 150 | 74 | 76 | 0.97 | 1 |
| River | 58 | 51 | 7 | 7.28 | 7.5 | |
| Both | 188 | 134 | 54 | 2.48 | 2.5 | |
| Wealth status | Very poor | 11 | 10 | 1 | 10 | 5.2 |
| Poor | 32 | 19 | 13 | 1.46 | 0.7 | |
| Middle income | 295 | 192 | 103 | 1.86 | 0.97 | |
| Rich | 58 | 38 | 20 | 1.9 | 1 | |
| Family size (#) | ≥7 | 75 | 45 | 30 | 1.5 | 0.78 |
| 6 | 115 | 82 | 33 | 2.48 | 1.3 | |
| 5 | 116 | 73 | 43 | 1.6 | 0.84 | |
| <5 | 90 | 59 | 31 | 1.9 | 1 | |
| Hand washing after toilet | Yes | 365 | 235 | 130 | 1.8 | 1 |
| No | 31 | 24 | 7 | 3.42 | 1.9 | |
| Washing vegetables | Yes | 309 | 207 | 102 | 2.03 | 1 |
| No | 87 | 52 | 35 | 1.48 | 0.73 | |
| Eating raw meat | Yes | 183 | 111 | 72 | 1.54 | 0.67 |
| No | 213 | 148 | 65 | 2.27 | 1 | |
| Shoe wearing habit | Not at all | 25 | 25 | 0 | — | — |
| Sometimes | 28 | 25 | 3 | 8.33 | 5.33 | |
| Always | 343 | 209 | 134 | 1.55 | 1 | |
| Waste disposal | Buried | 326 | 208 | 118 | 1.76 | 1 |
| Open space | 70 | 51 | 20 | 2.55 | 1.44 | |
| Type of latrine used by the family | Private | 366 | 236 | 130 | 1.8 | 1 |
| Common | 12 | 9 | 3 | 3 | 1.66 | |
| Open space | 18 | 14 | 4 | 3.5 | 1.94 | |
| Cleanliness of fingernails | Clean | 258 | 162 | 96 | 1.68 | 1 |
| Not clean | 138 | 97 | 41 | 2.36 | 1.4 |
A large majority of the students were found infected by a single parasite (61%), and only 4% had double infection. Those who consumed unwashed vegetables, whose nails were not clean, who disposed waste in open fields, and who had no latrine were found more likely to be infected.
3.4. Logistic Regression Analysis
3.4.1. Protozoan Infections
About 44% of the subjects were found positive for E. histolytica/dispar or G. lamblia or both. According to the results of the logistic regression analysis, father's level of education, consumption of raw meat, source of water, and level of income had a strong effect on protozoan infection (P < 0.05) (Table 3). Other explanatory variables were not statistically significant (P > 0.05).
Table 3.
Relationship between intestinal protozoan parasitic infections and explanatory variables among school children in Jaragedo town, South Gondar Zone, Ethiopia.
| Term | Parameter estimate | Standard error | χ 2 | P value |
|---|---|---|---|---|
| Intercept | 0.317 | 0.491 | 0.42 | 0.518 |
| Age (11 to 14) | 0.433 | 0.284 | 2.32 | 0.127 |
| Age (15 to 16) | −0.309 | 0.300 | 1.07 | 0.301 |
| Age (17 and above) | 0.320 | 0.367 | 0.76 | 0.382 |
| Sex (female) | 0.056 | 0.117 | 0.23 | 0.629 |
| Residence (rural) | 0.123 | 0.152 | 0.66 | 0.417 |
| Grade (1 to 4) | 0.542 | 0.415 | 1.70 | 0.191 |
| Grade (5 to 8) | 0.190 | 0.242 | 0.61 | 0.433 |
| Father education level (Church education) | −0.329 | 0.439 | 0.56 | 0.452 |
| Father education level (college and above) | 1.119 | 0.927 | 1.46 | 0.227 |
| Father education level ( illiterate) | −0.679 | 0.325 | 4.35 | 0.036 |
| Father education level (primary education) | −0.036 | 0.354 | 0.01 | 0.917 |
| Father's occupation (farming) | 0.120 | 0.167 | 0.52 | 0.471 |
| Hand washing after toile (no) | −0.166 | 0.244 | 0.46 | 0.496 |
| Eating unwashed vegetable (no) | 0.149 | 0.154 | 0.93 | 0.334 |
| Eating raw meat (no) | −0.347 | 0.128 | 7.33 | 0.006 |
| Shoe wearing (always) | −0.421 | 0.249 | 2.85 | 0.091 |
| Shoe wearing (no at all) | −0.088 | 0.354 | 0.06 | 0.803 |
| Water source (both tap and river) | −0.295 | 0.174 | 2.87 | 0.090 |
| Water source (river) | −0.875 | 0.254 | 11.84 | <0.0001 |
| Family economic status (middle-income) | 0.484 | 0.255 | 3.60 | 0.057 |
| Family economic status (poor) | 0.453 | 0.377 | 1.44 | 0.229 |
| Family economic status (rich) | 0.179 | 0.322 | 0.31 | 0.578 |
| Family size (5 members) | 0.324 | 0.200 | 2.62 | 0.105 |
| Family size (6 members) | −0.270 | 0.192 | 1.97 | 0.160 |
| Family size (7 and above) | −0.121 | 0.225 | 0.29 | 0.589 |
| Waste disposal system ( burry underground) | 0.281 | 0.176 | 2.56 | 0.109 |
| Toilet type (common pit latrine) | 0.098 | 0.562 | 0.03 | 0.860 |
| Toilet type (open space) | −0.170 | 0.462 | 0.14 | 0.711 |
| Nail cleanness (not trimmed and not clean) | −0.030 | 0.131 | 0.05 | 0.815 |
3.4.2. E. histolytica/Dispar
E. histolytica/dispar was the leading parasite identified among the school children under investigation. According to logistic regression analysis father's occupation, water source, family size, and consumption of raw meat had a strong effect on E. histolytica/dispar infection (P < 0.05) (Table 4). Other explanatory variables remained statistically insignificant (P > 0.05).
Table 4.
Relationship between explanatory variables and Entamoeba histolytica/dispar infection among school children in Jaragedo town, South Gondar Zone, Ethiopia.
| Term | Parameter estimate | Standard error | χ 2 | P value |
|---|---|---|---|---|
| Intercept | 0.490 | 0.496 | 0.98 | 0.3229 |
| Age (11 to 14) | 0.330 | 0.310 | 1.13 | 0.2877 |
| Age (15 to 16) | −0.141 | 0.330 | 0.18 | 0.6680 |
| Age (17 and above) | 0.082 | 0.391 | 0.04 | 0.8340 |
| Sex (female) | −0.017 | 0.125 | 0.02 | 0.8864 |
| Residence (rural) | 0.164 | 0.163 | 1.01 | 0.3140 |
| Grade (1 to 4) | 0.194 | 0.455 | 0.18 | 0.6690 |
| Grade (5 to 8) | 0.167 | 0.263 | 0.41 | 0.5238 |
| Father education level(Church education) | 0.122 | 0.466 | 0.07 | 0.7938 |
| Father education level (college and above) | 0.779 | 0.938 | 0.69 | 0.4064 |
| Father education level (illiterate) | −0.287 | 0.340 | 0.71 | 0.3981 |
| Father education level (primary education) | −0.127 | 0.370 | 0.12 | 0.7296 |
| Father's occupation (farming) | 0.136 | 0.180 | 0.57 | 0.4485 |
| Hand washing after toile (no) | −0.535 | 0.240 | 4.95 | 0.0262 |
| Eating unwashed vegetable (no) | 0.148 | 0.166 | 0.80 | 0.3722 |
| Eating raw meat (no) | −0.411 | 0.137 | 8.96 | 0.0028 |
| Shoe wearing (always) | −0.081 | 0.257 | 0.10 | 0.7523 |
| Shoe wearing (no at all) | 0.044 | 0.378 | 0.01 | 0.9068 |
| Water source (both tap and river) | −0.443 | 0.181 | 5.99 | 0.0144 |
| Water source (river) | −0.418 | 0.251 | 2.76 | 0.0967 |
| Family economic status (middle income) | 0.421 | 0.258 | 2.66 | 0.1026 |
| Family economic status (poor) | 0.382 | 0.383 | 1.00 | 0.3178 |
| Family economic status (rich) | 0.418 | 0.342 | 1.49 | 0.2217 |
| Family size (5 members) | 0.339 | 0.218 | 2.42 | 0.1199 |
| Family size (6 members) | −0.451 | 0.201 | 5.04 | 0.0248 |
| Family size (7 and above) | 0.076 | 0.246 | 0.10 | 0.7553 |
| Waste disposal system ( burry underground) | 0.142 | 0.180 | 0.62 | 0.4302 |
| Toilet type (common pit latrine) | 0.162 | 0.561 | 0.08 | 0.7727 |
| Toilet type (open space) | −0.257 | 0.464 | 0.31 | 0.5799 |
| Nail cleanness (not clean) | −0.020 | 0.140 | 0.02 | 0.8835 |
3.4.3. G. lamblia
G. lamblia was the second most prevalent intestinal parasite. Logistic regression analysis showed that water source was the only explanatory variable that is significantly associated with G. lamblia infection (P=0.0059) (Table 5). Other variables were not significant (P > 0.05).
Table 5.
Relationship between explanatory variables and G. lamblia infection among school children in Jaragedo town, South Gondar Zone, Ethiopia.
| Term | Parameter estimate | Standard error | χ 2 | P value |
|---|---|---|---|---|
| Intercept | 3.945 | 6.589 | 0.36 | 0.5494 |
| Age (11 to 14) | 0.310 | 0.392 | 0.63 | 0.4287 |
| Age (15 to 16) | −0.26039 | 0.397 | 0.43 | 0.5118 |
| Age (17 and above) | 0.190 | 0.468 | 0.16 | 0.6846 |
| Sex (female) | 0.094 | 0.146 | 0.42 | 0.5183 |
| Residence (rural) | −0.024 | 0.194 | 0.02 | 0.8998 |
| Grade (1 to 4) | 0.625 | 0.606 | 1.06 | 0.3027 |
| Grade (5 to 8) | 0.023 | 0.341 | 0.00 | 0.9445 |
| Father education level (Church education) | −2.188 | 6.578 | 0.11 | 0.7394 |
| Father education level (college and above) | 5.353 | 26.24 | 0.04 | 0.8384 |
| Father education level (illiterate) | −1.927 | 6.569 | 0.09 | 0.7692 |
| Father education level (primary education) | −0.868 | 6.577 | 0.02 | 0.8950 |
| Father's occupation (farming) | −0.020 | 0.235 | 0.01 | 0.9289 |
| Hand washing after toile (no) | 0.354 | 0.315 | 1.26 | 0.2609 |
| Eating unwashed vegetable (no) | 0.058 | 0.191 | 0.09 | 0.7593 |
| Eating raw meat (no) | 0.065 | 0.156 | 0.17 | 0.6760 |
| Shoe wearing (always) | −0.580 | 0.374 | 2.40 | 0.1210 |
| Shoe wearing (no at all) | −0.248 | 0.491 | 0.26 | 0.6131 |
| Water source (both tap and river) | 0.089 | 0.203 | 0.19 | 0.6607 |
| Water source (river) | −0.743 | 0.269 | 7.59 | 0.0059 |
| Family economic status (middle income) | 0.286 | 0.308 | 0.86 | 0.3531 |
| Family economic status (poor) | 0.383 | 0.472 | 0.66 | 0.4167 |
| Family economic status (rich) | −0.362 | 0.382 | 0.90 | 0.3418 |
| Family size (5 members) | 0.122 | 0.242 | 0.25 | 0.6137 |
| Family size (6 members) | 0.231 | 0.243 | 0.90 | 0.3424 |
| Family size (7 and above) | −0.263 | 0.277 | 0.90 | 0.3430 |
| Waste disposal system ( burry underground) | 0.177 | 0.206 | 0.74 | 0.3904 |
| Toilet type (common pit latrine) | 0.488 | 0.839 | 0.34 | 0.5605 |
| Toilet type (open space) | −0.480 | 0.608 | 0.62 | 0.4299 |
| Nail cleanness (not clean) | −0.031 | 0.157 | 0.04 | 0.8399 |
3.4.4. Soil Transmitted Helminths
Out of the total study participants, 16.7% confirmed positive for soil-transmitted helminthic parasites. From the logistic regression analysis, age of study subjects, habit of shoe wearing, water source, waste disposal system of families, latrine type, and accessibility had a strong effect on soil-transmitted helminthic infection (Table 6).
Table 6.
Relationship between explanatory variables and soil-transmitted helminthic infections among school children in Jaragedo town, South Gondar Zone, Ethiopia.
| Term | Parameter estimate | Standard error | χ 2 | P value |
|---|---|---|---|---|
| Intercept | 0.388 | 0.599 | 0.42 | 0.5172 |
| Age (11 to 14) | −0.769 | 0.467 | 2.71 | 0.0994 |
| Age (15 to 16) | 0.308 | 0.499 | 0.38 | 0.5376 |
| Age (17 and above) | 1.120 | 0.544 | 4.23 | 0.0397 |
| Sex (female) | 0.050 | 0.173 | 0.09 | 0.7702 |
| Residence (rural) | −0.377 | 0.220 | 2.93 | 0.0870 |
| Grade (1 to 4) | 0.514 | 0.600 | 0.73 | 0.3919 |
| Grade (5 to 8) | 0.450 | 0.364 | 1.53 | 0.2156 |
| Father education level (Church education) | −0.211 | 0.581 | 0.13 | 0.7155 |
| Father education level (college and above) | −0.903 | 0.984 | 0.84 | 0.3591 |
| Father education level (illiterate) | 0.703 | 0.429 | 2.68 | 0.1016 |
| Father education level (primary education) | −0.331 | 0.445 | 0.56 | 0.4560 |
| Father's occupation (farming) | −0.236 | 0.262 | 0.81 | 0.3680 |
| Hand washing after toile (no) | −0.127 | 0.344 | 0.14 | 0.7104 |
| Eating unwashed vegetable (no) | 0.146 | 0.226 | 0.42 | 0.5166 |
| Eating raw meat (no) | 0.125 | 0.184 | 0.46 | 0.4959 |
| Shoe wearing (always) | 2.276 | 0.316 | 51.67 | <0.0001 |
| Shoe wearing (no at all) | −1.588 | 0.402 | 15.61 | <0.0001 |
| Water source (both tap and river) | 0.218 | 0.243 | 0.81 | 0.3691 |
| Water source (river) | −0.706 | 0.328 | 4.63 | 0.0314 |
| Family economic status (middle income) | −0.146 | 0.360 | 0.17 | 0.6836 |
| Family economic status (poor) | 0.039 | 0.560 | 0.01 | 0.9431 |
| Family economic status (rich) | −0.463 | 0.460 | 1.01 | 0.3142 |
| Family size (5 members) | −0.057 | 0.290 | 0.04 | 0.8425 |
| Family size (6 members) | −0.126 | 0.285 | 0.20 | 0.6582 |
| Family size (7 and above) | 0.351 | 0.365 | 0.92 | 0.3364 |
| Waste disposal system (burry underground) | −0.658 | 0.302 | 4.72 | 0.0298 |
| Toilet type (common pit latrine) | −1.167 | 0.582 | 4.02 | 0.0450 |
| Toilet type (open space) | 1.194 | 0.651 | 3.37 | 0.0666 |
| Nail cleanness (not clean) | −0.304 | 0.192 | 2.50 | 0.1135 |
3.4.5. Ascaris lumbricoides, Strongyloides stercoralis, and Trichuris trichiura Infections
A. lumbricoides, S. stercoralis, and T. trichiura occurred rarely. They were, therefore, less important in the current study.
3.4.6. Hookworm Infection
Hookworm infection was the third most prevalent species of parasites among school kids in Jaragedo town. The logistic regression showed significant association with wearing shoes, water source, and access to latrine (P < 0.05).
3.4.7. Other Intestinal Parasites
During the parasitological examination, other intestinal parasites that could not be grouped to any of the six parasites were found in the stool samples in addition to the six species. Their overall prevalence was 9.1%. The logistic regression analysis showed that only water source had a strong effect on these other intestinal parasitic infections (P < 0.05).
3.4.8. All Intestinal Parasites
The overall prevalence of intestinal parasitic infections in the current investigation was 65.7% including all gastrointestinal parasitic species. The logistic regression analysis showed that grade level of students, water source, habit of consuming raw meat, and level of income were significantly associated with all intestinal parasites taken together.
4. Discussion
The overall prevalence of intestinal parasitic infection among school children of Jaragedo town was 65%, which corroborates previous reports in Tigray, Ethiopia (61%) [8], South Africa (65%) [9], Bahir Dar, Ethiopia [10], Sao Tome and Principe (65%) [11], and Borena, Ethiopia (59%) [6].
However, the present prevalence rate was far lower than the results from southwestern Ethiopia (83%) [12], Delgi area of the Amhara region, Ethiopia (78%) [13], and Sudan (84%) [14].
Still, others have reported lower rates than the present figures. Some report a 51% prevalence rate in Adigrat town of Ethiopia [15], 53% in Nigeria [16], 40% in India [17], 28% in Arba Minch town, Ethiopia [18], 44% in Tilili, Ethiopia [19], and 40% in Gamo area, South Ethiopia [20], all studied on children.
In the present study, the overall prevalence of intestinal protozoa infections (E. histolytica/dispar and G. lamblia) was 44%. This corroborates a study conducted in Mekelle city of Ethiopia among patients with watery diarrhea (45%) [21], Tanzania (48.7%) [22], and Merhabete of Ethiopia (47%) [23]. Other studies reported lower protozoan infections such as Wollo, Ethiopia (11%) [24], Nepal (18.5%) [25], Gerbe Guracha, Ethiopia (17%) [26], and southern Thailand (4.6%) [27].
Contributing factors for the present study could be consuming unwashed vegetables, reluctance to wash hands before meal and after the toilet, accessibility of latrine, eating raw meat, and dirty fingernails.
Soil transmitted infections in the present study gave an overall prevalence rate of 16.7%, which corroborated previous studies: Gelemso, western Hararghe (17.1%) [28], and Gerbe Guracha, Ethiopia (15%) [29]. All of these other reports were conducted on children.
Soil transmitted infections were low in other reports such as Babile town, Ethiopia (<1%) [30], Enderta, Ethiopia (7%) [31], Sao Tome and Principe (11%) [11], and Ambo town, Ethiopia (13%) [32]. The present study gave a lower prevalence of soil-transmitted helminth infections than reports from other areas in Ethiopia such as Jima zone around Gilgel Gibe Dam (44%) among the general community [33], and Jima Arjo (46.7%) among school children [34]. Differences could emanate from variations in the habit of wearing shoes, sources of drinking water, climate, socioeconomic status, habit of clipping and cleaning fingernails, habit of eating raw/unwashed vegetables, waste disposal systems, parasitological methods, and other unknown/undiscovered risk factors.
From the six species of intestinal parasites, the leading one was E. histolytica/dispar with a 27% prevalence rate, a report corroborating reports from other regions of Ethiopia such as North Gondar (27%) [35], Berhe et al. (24.7%) [21]. Rituparna et al. [17] reported the overall prevalence of 24% in India. Others reported much lower rates than the current one: Dessie, Ethiopia (6.5%) [36], Nigeria (10.5%) [16], Gerbe Guracha, Ethiopia (9.4%) community-based study [26], Arba Minch town, Ethiopia (12.9%) [18], and Adigrat town, Ethiopia (4.5%) [15]. A study from Haramaya, Ethiopia, reported a 47% infection rate among asymptomatic food handlers working at Haramaya University Cafe [37].
In the present study, the second leading intestinal parasite was G. lamblia (17.5%), which corroborated results from Tanzania (16.4%) [22] and Uganda (16.0%) [38]. It was much lower than reports from Delgi, North Gondar, Ethiopia (16.4%) [35], Turkey (47.9%) [39], Sudan (46.9%) [14], and Nepal (40.5%) [17].
On the other hand, the prevalence of G. lamblia was 3% in Taif, Saudi Arabia [40], 9.4% in Gondar, Ethiopia, among kids under the age of five [41], 2.3% in Adigrat town, Ethiopia [15], 4.2% in Arba Minch town, Ethiopia [18], and 9.6% in Addis Ababa, Ethiopia, among street dwellers [42, 43].
Hookworm prevalence was 8.8% in the current study. Other reports on hookworm include an estimated Ethiopian national prevalence of 16% [44] and 47% in Sub-Saharan Africa [45]. Lower prevalence of hookworm includes 2.2% in Chuahit, North Gondar, Ethiopia [46].
The overall prevalence of S. stercoralis (4.0%) in the current study was higher than 0.21% reported from Enderta area of Ethiopia [31] and 8.6% from Mozambique [47].
The overall prevalence of A. lumbricoides in this study was 2.3%, which was similar to other reports such as 3.7% in Slovakia [48]. Another study conducted in Qatar among newly arrived immigrants revealed that the overall prevalence was 1.8% [49]. In contrast, the prevalence of A. lumbricoides infection was reported to be 29% in the highlands, 35% in temperate areas, and 38% in the lowlands of Ethiopia [50], 22.1% in Jima [34], and 29% in South Africa [9].
The lowest encountered intestinal parasite was T. trichiura (1.5%). This was consistent with the report from Qatar (1.4%) [49], Nepal (1.3%) [51], Chuahit, North Gondar, Ethiopia (1.7%) [46], Nigeria (1.7%) [52], and Merhabete, Ethiopia (1.4%) [28]. Higher rates than the present one include reports from Addis Ababa (22.8%) on street dwellers [42], and Nigeria (16.0%) among elementary school children [53].
In the current study, logistic regression showed that sexes were not associated with intestinal parasites. Hailegebriel [10] has reported similar results before from Bahir Dar area of Ethiopia. In Iran, on the other hand, male gender was significantly associated with intestinal parasites [54]. Using river water was significantly associated with these parasites, as was reported before in Ethiopia from North Gondar [35] and from Arba Minch town [18]. Tap water is safer as it may be chlorinated, while river water gets contaminated with animals and humans. Income was another predisposing factor, as poverty may lead to an unhygienic environment, lack of clean water, improper clothing, and poor nutrition [39]. Raw meat consumption also significantly contributed in a similar manner reported before [35].
5. Conclusion
Intestinal parasitic infections were confirmed as common health problems among the Jaragego town school-age children in Ethiopia. Entamoeba histolytica/dispar, G. lamblia, and hookworm infections were the most common intestinal parasites. High prevalence of these parasites indicates poor disposal of waste, poverty, contaminated water supply, consuming raw meat, etc. Deworming programs, sanitation of the school environment, latrines for males and females, improving water supply, and education of the general community should be launched.
Acknowledgments
The authors would like to thank the students and their parents who volunteered to participate and the two schools for their permission to conduct the study in their school compound. The research fund was obtained through a local scholarship fund by Bahir Dar University, Ethiopia. The writing of this paper was the responsibility of the authors, and the funder was not involved.
Data Availability
Data are available from the corresponding author upon request.
Consent
All parties including authors agree to its publication in the current form.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
The senior author (MW) identified the existing gap in knowledge, conceived the research idea, formulated the design, participated in field and laboratory work, analyzed the data and helped interpret the findings, and eventually wrote the paper. The second author (SG) wrote the proposal, conducted the field and lab work, collected data, and wrote the draft results.
References
- 1.Schmidt G. D., Roberts L. S. Foundations of Parasitology . 8th. Boston, MA, USA: McGraw-Hill; 2009. Introduction to parasitology; p. p. 4. [Google Scholar]
- 2.Haque R. Human intestinal parasites. Journal of Health, Population, and Nutrition . 2007;25(4):387–391. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2754014/ [PMC free article] [PubMed] [Google Scholar]
- 3.Bethony J., Brooker S., Albonico M., et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. The Lancet . 2006;367(9521):1521–1532. doi: 10.1016/s0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 4.Rayan P., Verghese S., McDonnell P. N. Geographical location and age affect the incidence of parasitic infestations in school children. Indian Journal of Pathology & Microbiology . 2010;53(3):498–502. doi: 10.4103/0377-4929.68292. [DOI] [PubMed] [Google Scholar]
- 5.Chan M. S., Medley G. F., Jamison D., Bundy D. A. P. The evaluation of potential global morbidity attributable to intestinal nematode infections. Parasitology . 1994;109(3):373–387. doi: 10.1017/s0031182000078410. [DOI] [PubMed] [Google Scholar]
- 6.Yimam A. Addis Ababa, Ethiopia: Addis Ababa University; 2016. Intestinal parasitic infections among school age children in Mekane Selam Health Center, Borena, North East Ethiopia. M.Sc. Thesis. [Google Scholar]
- 7.Naing L., Winn T., Rusli B. N. Practical issues in calculating the sample size for the prevalence studies. Archives of Orofacial Sciences . 2006;1:9–14. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.504.2129&rep=rep1&type=pdf . [Google Scholar]
- 8.Kidane E., Menkir S., Kebede A., Desta M. Prevalence of intestinal parasitic infections and their associations with anthropometric measurements of school children in selected primary schools, Wukro town, eastern Tigray, Ethiopia. International Journal of Current Microbiology and Applied Sciences . 2014;3(3):11–29. [Google Scholar]
- 9.Nxasana N., Baba K., Bhat V., Vasaikar S. Prevalence of intestinal parasites in primary school children of Mthatha, Eastern Cape Province, South Africa. Annals of Medical and Health Sciences Research . 2013;3(4):511–516. doi: 10.4103/2141-9248.122064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hailegebriel T. Prevalence of intestinal parasitic infection and associated risk factors among students at Dona Berber Primary School, Bahir Dar, Ethiopia. BMC Infectious Diseases . 2017;17(362):1–8. doi: 10.1186/s12879-017-2466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao C.-W., Fu C.-J., Kao C.-Y., et al. Prevalence of intestinal parasitic infections among school children in capital areas of the Democratic Republic of São Tomé and Príncipe, West Africa. African Health Sciences . 2016;16(3):690–697. doi: 10.4314/ahs.v16i3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mengistu A., Gebre-Selassie S., Kassa T. Prevalence of Intestinal infections among urban dwellers in southwest Ethiopia. The Ethiopian Journal of Health Development . 2007;21(1):12–17. doi: 10.4314/ejhd.v21i1.10026. [DOI] [Google Scholar]
- 13.Alamir M., Awoke W., Feleke A. Intestinal parasites infection and associated factors among school children in Dagi primary school, Amhara National Regional State, Ethiopia. Health . 2013;05(10):1697–1701. doi: 10.4236/health.2013.510228. [DOI] [Google Scholar]
- 14.Siddig H. S., Mohammed I. A., Mohammed M. N., Bashir A. M. Prevalence of intestinal parasites among selected group of primary school children in Alhag Yousif area, Khartoum, Sudan. International Journal of Medical Research & Health Sciences . 2017;6(8):125–131. [Google Scholar]
- 15.Dinku S. M. Prevalence of intestinal parasitic infections and associated risk factors among school children in Adigrat town, northern Ethiopia. International Journal of Emerging Trends in Science and Technology . 2017;4(1):4943–4948. [Google Scholar]
- 16.Odo G. E., Agwu J. E., Ekeh F. N., et al. Prevalence of intestinal parasites among school children in Uzo-Uwani local government area of Enugu State. International Journal of Research Studies in Microbiology and Biotechnology . 2016;2(2):7–14. doi: 10.20431/2454-9428.0202002. [DOI] [Google Scholar]
- 17.Rituparna B., Bhattacharya P., Paul U. K., Bandyopadhyay A. Prevalence of intestinal parasites in a tertiary care hospital in Rural Bihar. International Journal of Scientific Study . 2017;4(12):89–93. doi: 10.17354/ijss/2017/104. [DOI] [Google Scholar]
- 18.Haftu D., Deyessa N., Agedew E. Prevalence and determinant factors of intestinal parasites among school children in Arba Minch town, southern Ethiopia. American Journal of Health Research . 2014;2(5):247–254. doi: 10.11648/j.ajhr.20140205.15. [DOI] [Google Scholar]
- 19.Alamneh A., Endalkachew N. Prevalence of gastrointestinal helminthic infections and associated risk factors among schoolchildren in Tilili town, northwest Ethiopia. Asian Pacific Journal of Tropical Medicine . 2014;7(7):525–530. doi: 10.1016/S1995-7645(14)60088-2. [DOI] [PubMed] [Google Scholar]
- 20.Wegayehu T., Tsalla T., Seifu B., Teklu T. Prevalence of intestinal parasitic infections among highland and lowland dwellers in Gamo area, South Ethiopia. BMC Public Health . 2013;13(1):p. 151. doi: 10.1186/1471-2458-13-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berhe B., Bugssa G., Bayisa S., Alemu M. Foodborne intestinal protozoan infection and associated factors among patients with watery diarrhea in Northern Ethiopia; a cross-sectional study. Journal of Health, Population, and Nutrition . 2018;37(5):1–7. doi: 10.1186/s41043-018-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Speich B., Marti H., Ame S. M., et al. Prevalence of intestinal protozoa infection among school-aged children on Pemba Island, Tanzania, and effect of single-dose albendazole, nitazoxanide and albendazole-nitazoxanide. Parasites & Vectors . 2013;6(3):p. 3. doi: 10.1186/1756-3305-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demisew G. Dire Dawa, Ethiopia: Haramaya University; 2015. Prevalence of intestinal protozoan parasitic infections and associated risk factors among school children in Goranda village, Merhabete District, Central Ethiopia. M.Sc. Thesis. [Google Scholar]
- 24.Haji M. Addis Ababa, Ethiopia: Addis Ababa University; 2016. Status of sanitary practices and amebiasis and giardiasis among patients visiting Haik Health Center, South Wollo, Northeast Ethiopia. M.Sc. Thesis. [Google Scholar]
- 25.Sah R. B., Paudel I. S., Baral R., Poudel P., Jha N., Pokharel P. K. A study of prevalence of intestinal protozoan infections and associated risk factors among the school children of Itahari, Eastern Region of Nepal. Journal of Chitwan Medical College . 2013;3(3):32–36. doi: 10.3126/jcmc.v3i1.8463. [DOI] [Google Scholar]
- 26.Negase A. Dire Dawa, Ethiopia: Haramaya University; 2014. Prevalence of intestinal protozoan parasites among primary school children and drinking water sources in Gerbe Guracha town, Kuyu woreda, North Shoa, Oromia, Ethiopia. M.Sc. Thesis. [Google Scholar]
- 27.Punsawad C., Phasuk N., Bunratsami S., Thongtup K., Siripakonuaong N., Nongnaul S. Prevalence of intestinal parasitic infection and associated risk factors among village health volunteers in rural communities of southern Thailand. BMC Public Health . 2017;17(1):564–572. doi: 10.1186/s12889-017-4486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bikila G. L. Dire Dawa, Ethiopia: Haramaya University; 2014. Prevalence, intensity and risk factors of soil–transmitted helminth parasitic infections among Biftu Primary School Children, Gelemso Town, Western Hararghe, Ethiopia. M.Sc. Thesis. [Google Scholar]
- 29.Gemeda L. Dire Dawa, Ethiopia: Haramaya University; 2014. Prevalence and intensity of soil–transmitted helminths parasitic infections and associated risk factors among primary school children in Gerbe Guracha Town, North Shewa, Ethiopia. M.Sc. Thesis. [Google Scholar]
- 30.Tefera E., Mohammed J., Mitiku H. Intestinal helminthic infections among elementary students of Babile town, Eastern Ethiopia. The Pan African Medical Journal . 2015;20:50–59. doi: 10.11604/pamj.2015.20.50.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashenafi T., Tadesse D., Zewdneh T. Infection prevalence of intestinal helminths and associated risk factors among schoolchildren in selected kebeles of Enderta district, Tigray, Northern Ethiopia. Journal of Parasitology and Vector Biology . 2014;6(11):166–173. doi: 10.5897/jpvb2014.0173. [DOI] [Google Scholar]
- 32.Fikreslasie S., Asalif D., Yonas A., Yonas H. Soil transmitted helminthiasis and associated risk factors among elementary school children in Ambo town, western Ethiopia. BMC Public Health . 2017;17:p. 791. doi: 10.1186/s12889-017-4809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mekonnen Z., Suleman S., Biruksew A., Tefera T., Chelkeba L. Intestinal polyparasitism with special emphasis to soil-transmitted helminths among residents around Gilgel Gibe Dam, Southwest Ethiopia: a community based survey. BMC Public Health . 2016;16(1):p. 1185. doi: 10.1186/s12889-016-3859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gemeda G., Menkir S., Getachew Y. Soil-transmitted helminth infections and their associations with hemoglobin concentration and anthropometric measurements of school children in Jimma, Arjo Primary School children, Oromiya Region, western Ethiopia. International Journal of Information Science and Management . 2013;1(1):27–32. https://www.ijism.org/administrator/components/com_jresearch/files/publications/IJAIR_313_IJISM.pdf . [Google Scholar]
- 35.Ayalew A., Debebe T., Worku A. Prevalence and risk factors of intestinal parasites among Delgi school children, North Gondar, Ethiopia. Journal of Parasitology and Vector Biology . 2011;3(5):75–81. [Google Scholar]
- 36.Gebretsadik D., Metaferia Y., Seid A., Fenta G. M., Gedefie A. Prevalence of intestinal parasitic infection among children under 5 years of age at Dessie Referral Hospital: cross sectional study. BMC Research Notes . 2018;11(1):771–776. doi: 10.1186/s13104-018-3888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marami D., Hailu K., Tolera M. Prevalence and associated factors of intestinal parasitic infections among asymptomatic food handlers working at Haramaya University cafeterias, eastern Ethiopia. Annals of Occupational and Environmental Medicine . 2018;30(1):53–59. doi: 10.1186/s40557-018-0263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ntulume I., Tibyangye J., Aliero A., Banson B. Prevalence of Intestinal protozoan infections and the associated risk factors among children in Bushenyi district, western Uganda. International Journal of Tropical Disease & Health . 2017;23(2):1–9. doi: 10.9734/ijtdh/2017/33255. [DOI] [Google Scholar]
- 39.Yentur Doni N., Gürses G., Şimşek Z., Yıldız Zeyrek F. Prevalence and associated risk factors of intestinal parasites among children of farm workers in the southeastern Anatolian region of Turkey. Annals of Agricultural and Environmental Medicine . 2015;22(3):438–442. doi: 10.5604/12321966.1167709. [DOI] [PubMed] [Google Scholar]
- 40.Ismail K. A. Prevalence of intestinal parasitic infection among school children in Taif. Insights Biomedical . 2018;3(2):10–13. doi: 10.21767/2572-5610.100045. [DOI] [Google Scholar]
- 41.Aleka Y., Egziabher G., Tamir W., Birhane M., Alemu A. Prevalence and associated risk factors of intestinal parasitic infection among under five children in University of Gondar Hospital, Gondar, northwest Ethiopia. Biomedical Research and Therapy . 2015;2(8):347–353. doi: 10.7603/s40730-015-0020-2. [DOI] [Google Scholar]
- 42.Mekonnen B. Prevalence of intestinal parasitic infections and related risk factors among street dwellers in Addis Ababa, Ethiopia. International Journal of Tropical Diseases . 2014;2(2) doi: 10.4172/2329-891x.1000132. [DOI] [Google Scholar]
- 43.Mekonnen B., Erko B., Legesse M. Prevalence of intestinal parasitic infections and related risk factors among street dwellers in Addis Ababa, Ethiopia. Journal of Tropical Diseases . 2014;2(2):p. 1000132. doi: 10.4172/2329-891X.1000132. [DOI] [Google Scholar]
- 44.Tadesse Z., Hailemariam A., Kolaczinski J. H. Potential for integrated control of neglected tropical diseases in Ethiopia. Transactions of the Royal Society of Tropical Medicine and Hygiene . 2008;102(3):213–214. doi: 10.1016/j.trstmh.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Pullan R. L., Smith J. L., Jasrasaria R., Brooker S. J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites & Vectors . 2014;7(1):37–56. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alemu A., Tegegne Y., Damte D., Melku M. Schistosoma mansoni and soil-transmitted helminths among preschool-aged children in Chuahit, Dembia district, northwest Ethiopia: prevalence, intensity of infection and associated risk factors. BMC Public Health . 2016;16(1):422–430. doi: 10.1186/s12889-016-2864-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuamba I., Grau-Pujol B., Nhabomba A., Gutiérrez J., Lázaro C., Mejia R. Prevalence of gastrointestinal parasites in southern Mozambique using a novel multiparallel quantitative real-time PCR. BMJ Global Health . 2017;2(2):2–A61. doi: 10.1136/bmjgh-2016-000260.164. [DOI] [Google Scholar]
- 48.Dudlová A., Juriš P., Jurišová S., Jarčuška P., Krčméry V. Epidemiology and geographical distribution of gastrointestinal parasitic infection in humans in Slovakia. Institute of Parasitology . 2016;53(4):309–317. https://sciendo.com/pdf/10.1515/helmin-2016-0035 . [Google Scholar]
- 49.Abu-Madi M. A., Behnke J. M., Doiphode S. Intestinal parasitic infections among long-term-residents and settled immigrants in Qatar in the period 2005 to 2011. The American Journal of Tropical Medicine and Hygiene . 2013;88(6) doi: 10.4269/ajtmh.13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jemaneh L. Comparative prevalence of some common intestinal helminth infections in different altitudinal regions in Ethiopia. Ethiopian Medical Journal . 1998;36(1):1–8. https://pubmed.ncbi.nlm.nih.gov/10214442/ [PubMed] [Google Scholar]
- 51.Chandrashekhar T. S., Joshi H. S., Gurung M., Subba S. H., Rana M. S., Shivananda P. G. Prevalence and distribution of intestinal parasitic infestations among school children in Kaski District, western Nepal. Peer-Review Policy-Journal of Biomedical Science . 2005;4(1):78–82. doi: 10.4314/jmbr.v4i1.10672. [DOI] [Google Scholar]
- 52.Afolaranmi T. O., Hassan Z. I., Bello D. A., et al. Pattern of intestinal helminthiasis among under five in a rural community of Plateau State, Nigeria. E3 Journal of Scientific Research . 2015;3(1):15–20. http://www.e3journals.org . [Google Scholar]
- 53.Dada E. O. Prevalence of human intestinal helminth parasites among primary school children in Ipogun, Efordore Local Government area, Nigeria. Journal of Global Biosciences . 2016;5(1):3401–3407. https://www.mutagens.co.in/jgb/vol.05/1/050102.pdf . [Google Scholar]
- 54.Babakhani M., Safari R., Rajati F., Omidian doost A. Prevalence and risk factors associated with intestinal parasitic infections among school children in Gashky, West of Iran. International Journal of Pediatrics . 2017;5(7):5263–5273. doi: 10.22038/ijp.2017.23173.1949. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding author upon request.


