Abstract
Vaccinations are crucial to fighting SARS-CoV-2, and high coverage rates can in most countries probably only be achieved with the involvement of primary care physicians (PCPs). We aimed to explore how SARS-CoV-2 vaccination payment schemes in 43 countries differ with regard to the (i) type of payment scheme, (ii) amount paid, (iii) degree of bundling, and (iv) use of pay-for-performance elements.
We collected information on payments and health system characteristics, such as PCP income and employment status, in all EU and OECD countries over time. We regressed the payment amount on the income of PCPs for countries with activity-dependent schemes using a linear regression (OLS), and we interpreted the residuals of this regression as a vaccination payment index.
The majority of countries (30/43) had chosen payment schemes that reward PCPs for the activity they perform. Seventeen countries paid less per vaccination than the income-adjusted average, whereas 13 countries paid more. Twelve countries used pay-for-performance elements.
Keywords: Payment schemes, COVID-19, Primary care physicians, Reimbursement, Tariff schemes
1. Introduction
The pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is one of the greatest public health challenges in recent history [1]. Vaccines are a core strategy to reduce the transmission of SARS-CoV-2 and the severity of disease resulting from SARS-CoV-2 infection [2], [3], [4]. Several vaccines have been available to the public since late 2020 [5]. Their success at the population level, however, depends largely on whether countries can quickly achieve high vaccination coverage rates. Equitable distribution of and easy access to vaccines both among and within countries are crucial to avoid severe COVID-19 and to slow the development of potentially dangerous variants, for “no one is safe until everyone is safe” [6], [7], [8].
Primary care physicians (PCPs) can play a vital part in improving access to vaccines against SARS-CoV-2 and their equitable distribution within individual nations [9]. Indeed, in most jurisdictions, PCPs provide lower-barrier care than that offered by specialists [10,11] and have a closer relationship to their patients than do many other providers and can therefore build trust in the vaccine and help overcome vaccine hesitancy [12]. A number of countries have explored options to mobilise PCPs for this purpose. Germany, for example, included PCPs in its vaccination campaign in April 2021 and managed to double its daily vaccination rate within a day [13]. That same month, the US Department of Health and Human Services and the Centers for Disease Control and Prevention recommended that the jurisdictions should increase the proportion of vaccines allocated to primary care providers, with at least 60% of that proportion going to providers in socially vulnerable communities [11].
Payments to PCPs can serve as a policy instrument to increase the access to and the equitable distribution of vaccinations [8]. They compensate PCPs for providing services and set incentives for how they provide care. When designing a payment scheme, policy-makers must decide on several design elements in order to align these incentives with their vaccination strategy. We identify four intervention points policy-makers have when designing a payment scheme, them being the (i) type of scheme, (ii) amount paid, (iii) degree of bundling, and (iv) pay-for-performance elements. When choosing a scheme, policy-makers can largely decide between two types: those that are activity-independent and those that are activity-dependent. The former pay providers independently of the number of services they perform, i.e., PCPs receive a fixed amount irrespective of the number of vaccinations they administer. Instead, they receive a monthly salary or a fixed amount based on the number of people enrolled in their region or on their list (capitation). As part of such schemes, payers and PCPs usually negotiate a minimum performance level, e.g., a certain number of vaccinations to be administered in a given amount of time. In contrast, activity-dependent payment schemes pay providers in a manner that is dependent on the services they perform, i.e., PCPs receive a payment per service (fee-for-service). In such schemes, more vaccinations translate into higher total payments. Activity-dependent schemes vary by the amount paid per service, and the degree of bundling. Policy-makers have to set a price that is sufficiently high to incentivise PCPs to prioritize vaccinations against SARS-CoV-2 over other duties, but low enough not to crowd out the provision of other essential services. In addition, they have to decide between one average price, that comprises all vaccination-related services, or a combination of several payments, i.e. the degree of bundling. The disaggregation into several payments offers policy-makers more intervention points to reflect differences in complexity, and to prioritize some services over others. For example, policy-makers can decide to pay a higher price for specific services such as time-consuming consultations. However, this might go at the expense of a higher administrative burden, more fragmented care, and up-coding. Activity-independent and activity-dependent schemes can be combined with one another, e.g., policy-makers can decide to offer PCPs a combination of capitation and fee-for-service. In addition, policy-makers can add pay-for-performance elements on top of the standard payment, i.e., additional payments that are conditional on meeting pre-defined quality criteria, such as outcomes or volumes.
Although the effects of general payment schemes and pay-for-performance elements on the provision of various types of care and other types of vaccination have already been discussed in the literature [14], [15], [16], [17], [18], [19], [20], there is no comprehensive overview of the payment schemes used to compensate PCPs for administering vaccines against SARS-CoV-2 or of how these schemes correspond to health system characteristics and support or hinder equity and access to vaccines. In our study, we aimed to explore the heterogeneity in SARS-CoV-2 vaccination payment schemes among 43 countries in terms of the (i) type of scheme, (ii) amount paid, (iii) degree of bundling, and (iv) pay-for-performance elements.
2. Methods
We selected all member states of the European Union (EU) and the organisation for Economic Co-Operation and Development (OECD). Two authors independently extracted information on the vaccination payment rates, the services covered by the payment, and additional information from official documents at the national, state and regional levels published by governments, health insurers, and other stakeholders, and two additional authors validated the results. We focused on payments to PCPs, such as general practitioners and family physicians. We did not consider the procurement costs of the vaccine itself. We report all payments in US$ converted at the official interbank exchange rate from June 14, 2021 [21,22]. We contacted experts from academia with a publication record in health policy analysis, and from payment authorities in the respective countries to validate and confirm our data, and completed our information where necessary. Subsequently, we collected information on the employment status of PCPs, as well as on the manner in which they are generally paid and their average salary, from the OECD Health System Characteristics Survey [23], the Health Systems and Policy Monitor of the European Observatory on Health Systems and Policies [24], and the International Profiles of Health Care System of the Commonwealth Fund [25]. We validated and completed our data with additional information from national statistical offices. Data were collected first between May 17 and July 16, 2021, and were then updated between September 20 and 26, 2021 to track changes over time (see Appendix 1).
Next, we regressed the payments for the first vaccination on the income of PCPs for countries with activity-dependent schemes and plotted the results with 95% confidence intervals. We interpreted the residuals of our linear regression (OLS) as a vaccination payment index (for regression results, please see Appendix 2). This approach accounts for differences in PCP income to adjust for the strength of the set incentives. For example, $20 per vaccination may have a conceivably stronger effect in a country with an average PCP income of $50,000 than in a country with $150,000. The residuals in our index represent the difference between the income-adjusted average across all countries and the effective payment by country. A country with a negative index value pays less, and a country with a positive index value pays more compared to the income-adjusted average of all countries.
3. Results
We collected information from 43 OECD and EU countries. Twenty-seven were member states of the EU, 38 of the OECD, and 19 of both organisations. Table 1 gives information on the employment status of PCPs, their average annual income in US$, the manner in which they are normally paid, and the manner in which they are paid for providing SARS-CoV-2 vaccinations. Table 2 gives information on payment rates for the first and second vaccination, and the services that were included. Additional information, e.g., on changes over time and pay-for-performance elements are presented in Appendix 1. We observed differences in the way countries paid for vaccinations in four domains: (i) the payment scheme, (ii) the amount paid per vaccination, (iii) the degree of bundling, and (iv) the use of pay-for-performance elements.
Table 1.
Overview of average annual PCP income (in US$), as well as general payment and vaccination payment schemes in 43 EU and OECD countries.
| Country | Employment status |
Average annual PCP income [US$] | Dominant mode of payment |
Vaccination payment scheme |
||||
|---|---|---|---|---|---|---|---|---|
| Publicly employed | Self-employed | Salary | Capitation | Fee-for-service | Activity-independent | Activity-dependent | ||
| Australia | X | 113,720 | X | X | ||||
| Austria | X | 152,759 | X | X | ||||
| Belgium | X | 141,844 | X | X | ||||
| Bulgaria | X | 26,743 | X | X | ||||
| Canada (British Columbia) | X | 175,124 | X | X | ||||
| Canada (Ontario) | 172,185 | |||||||
| Chile | X | 53,226 | X | X | ||||
| Colombia | X | – | X | X | ||||
| Costa Rica | X | 39,428 | X | X | ||||
| Croatia | X | – | X | X | ||||
| Cyprus | X | 66,616 | X | X | ||||
| Czech Republic | X | 42,591 | X | X | ||||
| Denmark | X | 187,965 | X | X | ||||
| Estonia | X | 57,367 | X | X | ||||
| Finland | X | 92,652 | X | X | ||||
| France | X | 136,623 | X | X | ||||
| Germany | X | 228,311 | X | X | ||||
| Greece | X | 30,929 | X | X | ||||
| Hungary | X | 26,564 | X | X | ||||
| Iceland | X | 184,028 | X | X | ||||
| Ireland | X | 158,890 | X | X | ||||
| Israel | X | 85,727 | X | X | ||||
| Italy | X | 121,120 | X | X | ||||
| Japan | X | 112,319 | X | X | ||||
| Korea (Republic of) | X | 146,087 | X | X | ||||
| Latvia | X | 23,535 | X | X | ||||
| Lithuania | X | 21,307 | X | X | ||||
| Luxembourg | X | 204,912 | X | X | ||||
| Malta | X | – | X | X | ||||
| Mexico | X | 22,178 | X | X | ||||
| Netherlands | X | 137,229 | X | X | ||||
| New Zealand | X | 145,595 | X | X | ||||
| Norway | X | 104,696 | X | X | ||||
| Poland | X | 31,498 | X | X | ||||
| Portugal | X | 56,021 | X | X | ||||
| Romania | X | 38,538 | X1 | X1 | X | |||
| Slovak Republic | X | 34,316 | X1 | X1 | X | |||
| Slovenia | X | 66,085 | X | X | ||||
| Spain | X | 84,281 | X | X | ||||
| Sweden | X | 114,036 | X | X | ||||
| Switzerland | X | 294,096 | X | X | ||||
| Turkey | X | 20,994 | X | X | ||||
| UK (England) | X | 159,899 | X | X | ||||
| UK (Northern Ireland) | 131,698 | |||||||
| UK (Scotland) | 131,275 | |||||||
| UK (Wales) | 140,722 | |||||||
| United States (Medicare) | X | 237,000 | X | X | ||||
| Total | 17 | 26 | – | 13 | 10 | 23 | 13 | 30 |
Note: PCP=Primary Care Physician, 1: Similar share of capitation and fee-for-services payments.
Table 2.
Overview of activity-dependent payment of PCPs for COVID-19 vaccinations in EU and OECD countries (in US$).
| Country | 1st vaccination | 2nd vaccination | Consultation | Data administration |
|---|---|---|---|---|
| Australia | 23.96 | 18.87 | included | included |
| Austria | 30.28 | 24.22 | included | included |
| Bulgaria | 7.99 | 7.99 | included | included |
| Canada (British Columbia) | 11.51 | 11.51 | included | included |
| Canada (Ontario) | 10.68 | 10.68 | 4.60 | included |
| Cyprus | 12.11 | 12.11 | included | included |
| Czech Republic | 12.75 | 12.75 | included | included |
| Denmark | 23.82 | 23.82 | included | included |
| Estonia | 6.42 | 6.42 | included | included |
| Finland | 12.11 | 12.11 | included | included |
| France | 30.28 | 30.28 | included | 6.54 |
| Germany | 24.22 | 24.22 | included | included |
| Greece | 12.11 | 12.11 | included | included |
| Ireland | 30.28 | 30.28 | 12.11 | included |
| Italy1 | 7.46 | 7.46 | included | 3.03 |
| Japan | 3.19 | 3.19 | 14.03 | 1.64 |
| Korea (Republic of) | 17.18 | 17.18 | included | included |
| Latvia | 11.05 | 11.05 | included | included |
| Lithuania | 4.08 | 4.08 | included | included |
| Luxembourg | 14.47 | 14.47 | included | included |
| Netherlands | 25.44 | 25.44 | 2.42 | included |
| New Zealand | 25.75 | 25.75 | included | included |
| Norway | 26.43 | 26.43 | included | included |
| Poland | 16.43 | 16.43 | included | included |
| Romania | 9.85 | 9.85 | included | included |
| Slovak Republic | 12.11 | 12.11 | included | included |
| Slovenia | 16.96 | 16.96 | included | included |
| Sweden | 33.00 | 33.00 | included | included |
| Switzerland | 27.25 | 27.25 | included | included |
| UK (England) | 17.74 | 17.74 | included | included |
| UK (Northern Ireland) | 17.74 | 17.74 | included | included |
| UK (Scotland) | 17.74 | 17.74 | included | included |
| UK (Wales) | 17.74 | 17.74 | included | included |
| United States (Medicare) | 40 | 40 | included | included |
Note:1: Italy pays an additional US$ 1.82 per vaccination for personal protective equipment. Further details on the SARS-CoV-2 vaccination payment schemes are presented in Appendix 1. A list of the share of vaccinations administered per population can be found in Appendix 3. A comprehensive list of sources can be found in Appendix 4.
3.1. Payment schemes
The design of a country's SARS-CoV-2 vaccination payment scheme appears to be dependent on the dominant form of payment for PCPs in the country (see Table 1). Twenty-two of the 23 countries in our sample that typically use fee-for-service payments for PCPs and in which PCPs are usually self-employed used activity-dependent schemes to pay for SARS-CoV-2 vaccinations. Only Belgium used an activity-independent scheme and paid PCPs on an hourly basis for their work in vaccination centres. Of the 13 countries that usually pay PCPs a salary and in which PCPs are public employees, 10 chose an activity-independent scheme for SARS-CoV-2 vaccination payments. Only Finland, Greece, and Slovenia used activity-dependent schemes. Of the 10 countries that usually pay PCPs mainly by capitation, eight used activity-dependent schemes to pay for SARS-CoV-2 vaccinations, whereas Croatia and Turkey used activity-independent schemes to do so.
3.2. Payment per vaccination
Countries differed with regard to the amount they paid per vaccination. Countries with activity-independent payments such as Spain and Portugal did not offer any additional payments for performing vaccinations. PCPs received their usual salary, which covered this service. Payments in countries with activity-dependent schemes ranged from $4.08 per vaccination in Lithuania to $42.39 per vaccination in Ireland. All countries paid the same rate for each of the vaccinations, with the exception of Austria and Australia, which paid 20% less for the second vaccination.
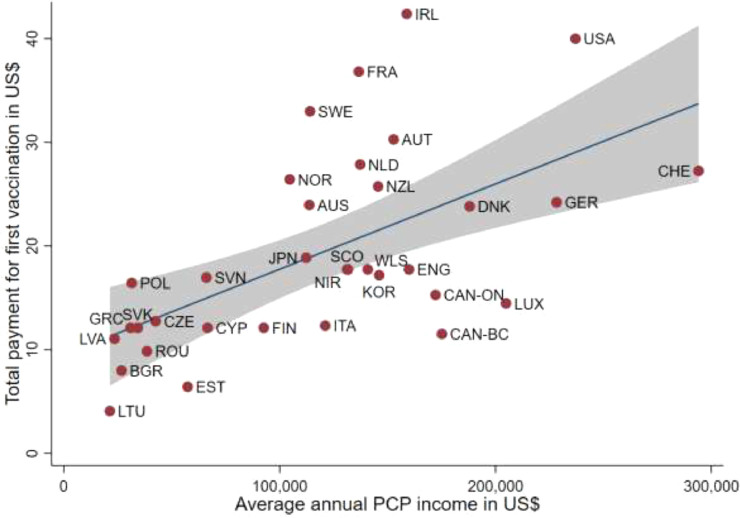
The amount paid per vaccination positively correlated with the average annual income of PCPs in countries that used an activity-dependent payment scheme (Fig. 1 ). In Eastern European countries, the average PCP income was lower than that in other EU and OECD countries. There, the payment per vaccination ranged from $4.08 in Lithuania to $16.96 in Slovenia. In countries where PCPs had an annual average income between $100,000 and $200,000, the payment per vaccination ranged from slightly below $20 to about $35. In Germany, Switzerland and the United States (Medicare), the average annual PCP income exceeded $200,000. However, whereas Germany and Switzerland offered rather low payment levels that were similar to those in their neighbouring countries, the United States paid $40 per vaccination, which was the second highest payment after Ireland ($42.39).
Fig. 1.
Total payment for first vaccination against SARS-CoV-2 in relation to average annual PCP income (in US$).
Description: This graph displays the relationship between the average annual PCP income (in US$) (x-axis) and the total payment for the first vaccination against SARS-CoV-2 (in US$) (y-axis). Each dot represents one country/jurisdiction. The blue line is the regression line between the annual PCP income and total payment. It shows a positive relationship between annual PCP income and total payment. The gray area displays the 95%-confidence interval.
Note: This figure includes only countries using activity-dependent payments. AUS = Australia, AUT = Austria, BGR = Bulgaria, CAN-BC = Canada (British-Columbia), CAN—ON = Canada (Ontario), CHE = Switzerland, CZE = Czech Republic, CYP = Cyprus, DNK = Denmark, ENG = England, EST = Estonia, FIN = Finland; FRA = France, GER = Germany, GRC = Greece, IRL = Ireland, ITA = Italy, JPN = Japan, KOR = Korea (Republic), LTU = Lithuania, LVA = Latvia, LUX = Luxembourg, NIR = Northern Ireland, NLD = The Netherlands, NOR = Norway, NZL = New Zealand, POL = Poland, ROU = Romania, SCO = Scotland, SVK = Slovak Republic, SVN = Slovenia, SWE = Sweden, USA = United States (Medicare), WLS = Wales.
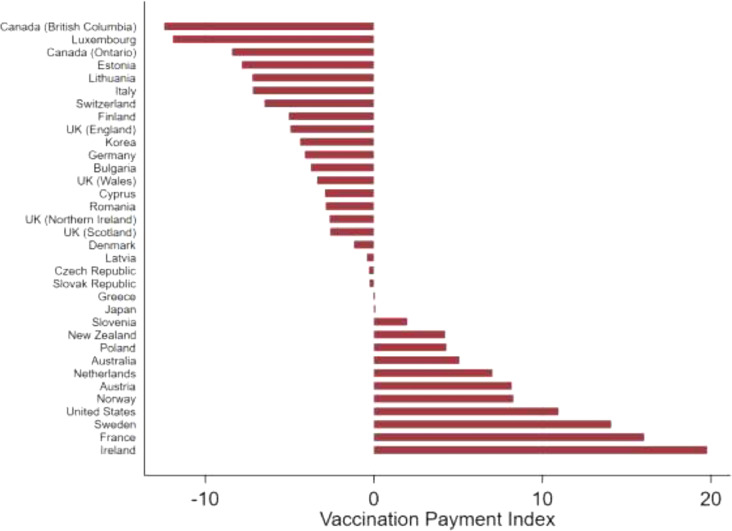
Our vaccination payment index is presented in Fig. 2 . Seventeen countries (21 jurisdictions) paid less per vaccination than the country-specific income adjusted average (negative index value), whereas 13 countries paid more (positive index value). We observed high heterogeneity in this measure, with Canada (British Columbia) paying $12.43 less and Ireland paying $19.79 more than the income-adjusted average per vaccination. Country experts confirmed the validity of the raw data. A comparison of the vaccination index and the regression plot shows high face validity.
Fig. 2.
Vaccination payment index in countries with activity-dependent payment schemes.
Description: This figure displays the vaccination payment index, i.e. the difference between the amount a country pays for the first vaccination against SARS-CoV-2, and its income-adjusted average payment in ascending order. A negative value means that a country pays less for the first vaccination than the income-adjusted average payment, a positive value means that a country pays more for the first vaccination than the income-adjusted average payment.
3.3. Bundling of services
Countries with activity-dependent payment schemes used different degrees of bundling. In 25 countries, such as the UK (England), Germany, Switzerland, and the United States (Medicare), the payment covered all vaccination-related services, such as preparation of the vaccine and patient consultation, the vaccination itself, and administrative services. Seven countries offered separate payments for the vaccination, for patient-related tasks such as consultations (e.g., Ontario), for the preparation of the vaccine (e.g., Denmark), and for administrative services such as data collection (e.g., France, Ireland, and the Netherlands). Two countries (Canada (British Columbia) and Australia) made higher payments if consultations exceeded 10 min to adjust for differences in time intensity. Five countries (six jurisdictions) made payments for additional services, such as actively identifying patients eligible for vaccination in Norway and Canada (Ontario). Finally, four countries reduced payments if the vaccination was provided in combination with other services. This was the case in Australia, Canada (British Columbia), and France. Appendix 1 details payment modifications.
3.4. Pay-for-performance
Twenty countries (21 jurisdictions) used pay-for-performance elements as part of their vaccination payment scheme (Table 3 ). They did so for four purposes. First, eight countries introduced financial incentives to increase workforce capacities, for example by offering financial incentives to retired professionals and medical students, as well as higher payments for administering vaccines outside of regular office hours. Second, four countries (Australia, Japan, the Slovak Republic, and the UK (Wales)) sought to increase the activity of PCPs by offering additional payments for meeting vaccination targets. Third, thirteen countries offered higher payments to increase access to vaccinations, for example by offering incentives for administering vaccines at patients’ homes or in nursing homes, or by offering higher payments to PCPs in rural areas (e.g., Australia). Fourth, one country (Australia) experimented with incentives to increase the completion of vaccine series by providing an add-on payment of $7.72 for each fully vaccinated patient.
Table 3.
Overview of countries using pay-for-performance elements to achieve specific goals.
| Work force capacity | Provider activity | Provider mobility | Vaccination outcome | |
|---|---|---|---|---|
| Australia | X | X | X | X |
| Czech Republic | X | |||
| Denmark | X | |||
| Estonia | X | |||
| France | X | X | ||
| Germany | X | |||
| Greece | X | |||
| Italy | X | |||
| Japan | X | X | ||
| Latvia | X | |||
| Lithuania | X | |||
| Netherlands | X | |||
| New Zealand | X | |||
| Norway | X | |||
| Poland | X | |||
| Romania | X | |||
| Slovak Republic | X | |||
| Slovenia | X | |||
| UK – England | X | (X) | ||
| UK – Wales | X | |||
| United States – (Medicare) | X | (X) | ||
| Total | 8 | 4 | 13 | 1 |
“X” indicates presence of pay-for-performance scheme, brackets indicate that the country retired the policy. Note: We only included pay-for-performance elements that were introduced to pay for vaccinations against SARS-CoV-2.
3.5. Changes in vaccination payment schemes
Twelve countries adapted their vaccination payment schemes to respond to declining demand, and to improve access and coverage. Between the introduction of the various vaccination campaigns and the end of our analysis in September 2021, we observed major changes in the type of payment schemes used, the payment amount, and the design of pay-for-performance elements. For example, the National Health Service (NHS) in England made payments to PCPs conditional on completing both vaccinations, but dropped this requirement after a short period. Greece and Luxembourg launched a completely new activity-dependent scheme to reimburse office-based PCPs in August 2021. The United States increased its payment from $16.94 for the first and $28.39 for the second vaccination to $40 per vaccination in March 2021. Later, the country introduced additional payments of $35 per vaccination to compensate PCPs for at-home vaccinations in June and expanded this part of the scheme in late August 2021. Lithuania increased its payments from $3.57 in March to $4.08 in May 2021. Estonia and Lithuania added additional payments for vaccinations performed after hours in May and August 2021, respectively. Australia introduced a bonus of $777.66 for vaccinating 50 long-term care workers and an additional payment of $15.43 per every additional vaccination of a long-term care worker in June 2021. In regions with an overall vaccination rate below the national average, the Slovak Republic started offering, in August 2021, an additional $2.42 per patient for practices that had a vaccination rate 15% higher than the average vaccination rate in their region. In regions with an overall vaccination rate above the national average, an additional payment per patient was also used, but was lower and amounted to $1.21. A comprehensive list of the changes can be found in Appendix 1.
4. Discussion
We have provided an overview of the payment schemes chosen by 43 EU and OECD countries to compensate PCPs for administering vaccinations against SARS-CoV-2, and of how these schemes correspond to characteristics of each health system. In doing so, we also explored the heterogeneity among these schemes and created a vaccination payment index to inform how much countries pay PCPs for vaccinations compared to other countries.
In our sample, more than two thirds of the countries (30 countries) chose an activity-dependent scheme to pay PCPs for providing SARS-CoV-2 vaccinations. Research has shown that activity-dependent payment schemes lead to a larger increase in the volume of medical services provided compared to activity-independent schemes [26], but at the expense of poorer cost control and the potential overprovision of care. If a country's priority is to roll out vaccinations very quickly, opting for an incentive structure that incentivises high rates of activity over cost control seems like a reasonable approach.
Another interesting observation from our data is that most countries designed their vaccination payment scheme by expanding their general payment scheme. By doing so, they could make use of existing administrative structures while helping ensure acceptability among payers and PCPs, who were already familiar with these structures and how to navigate them. In the case of activity-dependent schemes, countries can expand upon the general payment schemes simply by defining new payments. In the case of activity-independent schemes, however, countries may choose either to oblige PCPs to provide vaccinations as part of their normal activities or to motivate PCPs to increase their activity by adding activity-dependent payments. The latter approach could be observed in countries such as Greece and Cyprus, both of which set monetary incentives to increase the volume of vaccinations provided, thus prioritizing higher vaccination rates over the additional effort of introducing a new scheme.
Furthermore, despite adjusting for income disparities to account for the different economic strength of each country in our sample, we found a high degree of heterogeneity in the amount countries paid per vaccination. According to our vaccination payment index, 13 countries paid substantially more than the income-adjusted average, whereas 17 countries (21 jurisdictions) paid less. In general, higher payments for health services have been found to result in higher levels of activity. This being said, the optimal payment for vaccinations remains unknown, and policy-makers, particularly those in countries with high levels of vaccination hesitancy, face the challenge of finding the point of diminishing returns. Given the uncertainty about appropriate payment rates, some countries in our sample used comparable services, such as the seasonal flu vaccination, as a benchmark. Often, however, they opted for a somewhat higher payment in order to set an incentive to prioritize vaccinations against SARS-CoV-2 over these benchmark services, and to reflect additional complexities, such as the storage and handling of the messenger RNA (mRNA) vaccines.
Vaccinations involve numerous steps and services beyond the “jab” itself, ranging from consultations to build trust in the vaccine to administrative tasks, such as data recording. The countries in our sample took this into account in various ways. We found that most countries that used an activity-dependent scheme did so by bundling payments, i.e., paying an average price to cover all vaccination-related services. A core advantage of bundled payments is their decreased administrative burden. At the same time, bundling offers fewer opportunities to account for cost differences that might arise, for example, from complex consultations or other non-standard situations. PCPs confronted with these might therefore perceive bundled payments as unfair, particularly if they are serving populations with a larger proportion of elderly or disadvantaged individuals. This, in turn, might lead to the preferential treatment of simpler over complex cases, leading to further health disparities [27]. In countries with stark regional differences in health and wealth, policy-makers that choose bundled payments may therefore wish to consider having the bundled payments differ in amount according to region and based on some measure of social deprivation.
Interestingly, about half of the countries in our sample introduced pay-for-performance elements to their SARS-CoV-2 vaccination payment schemes. These might provide specific incentives to increase workforce capacity, provider activity and provider mobility, or even vaccination coverage in terms of improved access, the equitable distribution of vaccines within a country, and vaccination rates. Although evidence on the general effects of pay-for-performance in health care remains mixed [28], there is some indication that it might be an effective tool to increase vaccination rates [19]. While evidence base is too weak to make any conclusive assessments in this regard, even a minimal effect is probably more cost-effective in the current pandemic context than no effect. Policy-makers should therefore experiment with this tool, perhaps in rapid pilot programmes, and rigorously evaluate the effects.
Lastly, we found that a number of countries adjusted their payment schemes over the short period since the start of their vaccination campaigns. More specifically, we observed (i) an increased use of pay-for-performance elements, (ii) a move from activity-independent to activity-dependent payment schemes, and (iii) an increase in the amount paid per vaccination. Most adjustments to the payment schemes involved adding pay-for-performance elements to increase the mobility of PCPs, their working hours and their activity. Overall, these adjustments probably reflect both the high degree of uncertainty that policy-makers faced when designing vaccination payment schemes during the pandemic and the need to response to changes in demand for vaccination as their countries achieved coverage rates of 60% and higher. As population demand for vaccination continues to decline in most countries, we expect more policy-makers to modify their payment schemes in the near future. Countries may decide to lower payments and re-integrate them into the general payment scheme for PCPs, or they may increase payments and introduce additional pay-for-performance elements to respond to declining demand. Adjustments to payment schemes will certainly continue, as the challenges of the pandemic are constantly changing and many schemes are still young and partially in the experimental stage. Due to the unpredictability of the pandemic and, indeed, societies’ response to it, it seems important for SARS-CoV-2 vaccination payment schemes to be designed in a flexible way so that they can be rapidly adapted if needed.
Our paper has two important limitations. First, we focused on PCPs in office-based practices, but in several countries, a substantial share of vaccinations against SARS-CoV-2 have been administered in other settings, especially mass vaccination centres. Second, we did not consider the costs of administering such schemes. However, bureaucratic effort to implement some designs might be high and is very dependent on the countries’ institutions.
In this paper, we focussed on payments, which serve as a means to compensate, support, and incentivise PCPs in providing their services [29]. Other researchers may want to expand on this study by investigating the intrinsic motivation of PCPs to perform vaccinations to contribute to the public good, or non-monetary incentives of PCPs, such as public rankings and feedback options [30]. Furthermore, additional factors might determine the success of vaccination campaigns beyond the payment amounts, such as the supply of vaccines, the willingness of different population groups to be vaccinated, and the country's pandemic strategy. Focusing on these two latter points would have gone beyond the scope of our research, but policy-makers must bear them in mind because without them (as could be seen in the slow rollout of many vaccination campaigns), even the best payment schemes will falter [31], [32], [33], [34], [35].
5. Conclusion
Vaccination payment schemes are policy instruments that can improve SARS-CoV-2 vaccination rates. Our overview of 43 countries and the design of their payment schemes, the payment amount, the degree of bundling, and the use of pay-for-performance elements provides important insights for policy-makers seeking to achieve high SARS-CoV-2 vaccination rates by enlisting the services of primary care physicians. Policy-makers may wish to make use of this evidence, determine which elements of other schemes might best be translated into their own national and regional contexts, and seek to adjust their payment schemes on an ongoing basis in response to population needs during this unpredictable pandemic.
CRediT authorship contribution statement
Ricarda Milstein: Conceptualization, Writing – review & editing, Visualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft. Kosta Shatrov: Conceptualization, Data curation, Methodology, Validation, Writing – original draft. Lea Miranda Schmutz: Writing – review & editing, Data curation, Validation. Carl Rudolf Blankart: Conceptualization, Writing – review & editing, Data curation, Validation, Methodology, Writing – original draft, Supervision.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgments
Acknowledgements
We thank Rowan Iskandar for his helpful comments on an earlier version of this paper.
Funding
The authors did not receive any grants or other funding in order to conduct this research.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.healthpol.2022.03.008.
Appendix. Supplementary materials
References
- 1.Haldane V., de Foo C., Abdalla S.M., Jung A.S., Tan M., Wu S., et al. Health systems resilience in managing the COVID-19 pandemic: lessons from 28 countries. Nat Med. 2021;27(6):964–980. doi: 10.1038/s41591-021-01381-y. [DOI] [PubMed] [Google Scholar]
- 2.Hall V.J., Foulkes S., Saei A., Andrews N., Oguti B., Charlett A., et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet North Am Ed. 2021;397(10286):1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore S., Hill E.M., Tildesley M.J., Dyson L., Keeling M.J. Vaccination and non-pharmaceutical interventions for COVID-19: a mathematical modelling study. Lancet Infect Dis. 2021;21(6):793–802. doi: 10.1016/S1473-3099(21)00143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Priesemann V., Brinkmann M.M., Ciesek S., Cuschieri S., Czypionka T., Giordano G., et al. Calling for pan-European commitment for rapid and sustained reduction in SARS-CoV-2 infections. Lancet North Am Ed. 2021;397(10269):92–93. doi: 10.1016/S0140-6736(20)32625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wouters O.J., Shadlen K.C., Salcher-Konrad M., Pollard A.J., Larson H.J., Teerawattananon Y., et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet North Am Ed. 2021;397(10278):1023–1034. doi: 10.1016/S0140-6736(21)00306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt H., Weintraub R., Williams M.A., Miller K., Buttenheim A., Sadecki E., et al. Equitable allocation of COVID-19 vaccines in the United States. Nat Med. 2021;27(7):1298–1307. doi: 10.1038/s41591-021-01379-6. [DOI] [PubMed] [Google Scholar]
- 7.The Lancet Infectious Diseases COVID-19 vaccine equity and booster doses. Lancet Infect Dis. 2021;21(9):1193. doi: 10.1016/S1473-3099(21)00486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doran T., Fullwood C., Kontopantelis E., Reeves D. Effect of financial incentives on inequalities in the delivery of primary clinical care in England: analysis of clinical activity indicators for the quality and outcomes framework. Lancet North Am Ed. 2008;372(9640):728–736. doi: 10.1016/S0140-6736(08)61123-X. [DOI] [PubMed] [Google Scholar]
- 9.Schaffer DeRoo S., Pudalov N.J., Fu L.Y. Planning for a COVID-19 vaccination program. JAMA. 2020;323(24):2458–2459. doi: 10.1001/jama.2020.8711. [DOI] [PubMed] [Google Scholar]
- 10.Ratzan S., Schneider E.C., Hatch H., Cacchione J. Missing the point — how primary care can overcome COVID-19 vaccine “Hesitancy. N Engl J Med. 2021;384(25):e100. doi: 10.1056/NEJMp2106137. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention . U.S. Department of Health and Human Services; Washington D.C: 2021. Expanding COVID-19 vaccine distribution to primary care providers to address disparities in immunization: guide for jurisdictions to increase COVID-19 vaccine distribution to primary care providers to address disparities in immunization. [Google Scholar]
- 12.Wilkinson E., Jetty A., Petterson S., Jabbarpour Y., Westfall J.M. Primary care's historic role in vaccination and potential role in COVID-19 immunization programs. Ann Family Med. 2021:2679. doi: 10.1370/afm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.German Federal Ministry of Health. Impfdashboard. Current vacincation status. [October 18, 2021]; Available from: https://impfdashboard.de/en/.
- 14.OECD . Better ways to pay for health care. OECD Health Policy Studies. OECD Publishing; Paris: 2016. [Google Scholar]
- 15.Papanicolas I., Woskie L.R., Jha A.K. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024–1039. doi: 10.1001/jama.2018.1150. [DOI] [PubMed] [Google Scholar]
- 16.Waitzberg R., Gerkens S., Dimova A., Bryndová L., Vrangbæk K., Jervelund S.S., et al. Balancing financial incentives during COVID-19: a comparison of provider payment adjustments across 20 countries. Health Policy (New York) 2021 doi: 10.1016/j.healthpol.2021.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu T., Decker S.L., Chou S.-Y. Medicaid pay for performance programs and childhood immunization status. Am J Prev Med. 2016;50(5):S51–S57. doi: 10.1016/j.amepre.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doran T., Kontopantelis E., Reeves D., Sutton M., Ryan A.M. Setting performance targets in pay for performance programmes: what can we learn from QOF? BMJ Br Med J. 2014;348:g1595. doi: 10.1136/bmj.g1595. [DOI] [PubMed] [Google Scholar]
- 19.Benabbas R., Shan G., Akindutire O., Mehta N., Sinert R. The effect of pay-for-performance compensation model implementation on vaccination rate: a systematic review. Qual Manag Healthc. 2019;28(3) doi: 10.1097/QMH.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 20.Brekke K.R., Holmås T.H., Monstad K., Straume O.R. How does the type of remuneration affect physician behavior?: Fixed salary versus fee-for-service. Am J Health Econ. 2019;6(1):104–138. doi: 10.1086/706624. [DOI] [Google Scholar]
- 21.European Central Bank. Statistical data warehouse. Currency converter. [February 21, 2022]; Available from: https://sdw.ecb.europa.eu/curConverter.do.
- 22.OANDA. Currency converter; Available from: https://www.oanda.com/eu-en/.
- 23.OECD. Health system characteristics survey. [10/18/21]; Available from: https://qdd.oecd.org/subject.aspx?Subject=hsc.
- 24.European Observatory on Health Systems and Policies. The health systems and policy monitor. [10/18/21]; Available from: https://www.hspm.org/mainpage.aspx.
- 25.Tikkanen R., Osborn R., Mossialos E., Djordjevic A., Wharton G. The Commonwealth Fund; New York City: 2020. International profiles of health care systems. (editors) [Google Scholar]
- 26.Clemens J., Gottlieb J.D. Do physicians’ financial incentives affect medical treatment and patient health? Am Econ Rev. 2014;104(4):1320–1349. doi: 10.1257/aer.104.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mechanic R.E., Altman S.H. Payment reform options: episode payment is a good place to start. Health Aff. 2009;28(Supplement 2):w262–w271. doi: 10.1377/hlthaff.28.2.w262. [DOI] [PubMed] [Google Scholar]
- 28.Eijkenaar F., Emmert M., Scheppach M., Schöffski O. Effects of pay for performance in health care: a systematic review of systematic reviews. Health Policy (New York) 2013;110(2):115–130. doi: 10.1016/j.healthpol.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Casalino L.P. Balancing incentives: how should physicians be reimbursed? JAMA. 1992;267(3):403–405. doi: 10.1001/jama.1992.03480030081042. [DOI] [PubMed] [Google Scholar]
- 30.Niewoehner R.J., Staats B.R. Focusing provider attention: an empirical examination of incentives and feedback in flu vaccinations. Manage Sci. 2021 doi: 10.1287/mnsc.2021.4051. [DOI] [Google Scholar]
- 31.Walkey A.J., Law A., Bosch N.A. Lottery-based incentive in Ohio and COVID-19 vaccination rates. JAMA. 2021;326(8):766–767. doi: 10.1001/jama.2021.11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazarus J.V., Ratzan S.C., Palayew A., Gostin L.O., Larson H.J., Rabin K., et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27(2):225–228. doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Figueiredo A., Simas C., Karafillakis E., Paterson P., Larson H.J. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet North Am Ed. 2020;396(10255):898–908. doi: 10.1016/S0140-6736(20)31558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdul-Mutakabbir J.C., Casey S., Jews V., King A., Simmons K., Hogue M.D., et al. A three-tiered approach to address barriers to COVID-19 vaccine delivery in the Black community. Lancet Global Health. 2021;9(6):e749–e750. doi: 10.1016/S2214-109X(21)00099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forman R., Shah S., Jeurissen P., Jit M., Mossialos E. COVID-19 vaccine challenges: what have we learned so far and what remains to be done? Health Policy (New York) 2021;125(5):553–567. doi: 10.1016/j.healthpol.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.