Abstract
Background and objective
Few head‐to‐head evaluations of immune responses to different vaccines have been reported.
Methods
Surrogate virus neutralization test (sVNT) antibody levels of adults receiving either two doses of BNT162b2 (n = 366) or CoronaVac (n = 360) vaccines in Hong Kong were determined. An age‐matched subgroup (BNT162b2 [n = 49] vs. CoronaVac [n = 49]) was tested for plaque reduction neutralization (PRNT) and spike‐binding antibody and T‐cell reactivity in peripheral blood mononuclear cells.
Results
One month after the second dose of vaccine, BNT162b2 elicited significantly higher PRNT50, PRNT90, sVNT, spike receptor binding, spike N‐terminal domain binding, spike S2 domain binding, spike FcR binding and antibody avidity levels than CoronaVac. The geometric mean PRNT50 titres in those vaccinated with BNT162b2 and CoronaVac vaccines were 251.6 and 69.45, while PRNT90 titres were 98.91 and 16.57, respectively. All of those vaccinated with BNT162b2 and 45 (91.8%) of 49 vaccinated with CoronaVac achieved the 50% protection threshold for PRNT90. Allowing for an expected seven‐fold waning of antibody titres over 6 months for those receiving CoronaVac, only 16.3% would meet the 50% protection threshold versus 79.6% of BNT162b2 vaccinees. Age was negatively correlated with PRNT90 antibody titres. Both vaccines induced SARS‐CoV‐2‐specific CD4+ and CD8+ T‐cell responses at 1 month post‐vaccination but CoronaVac elicited significantly higher structural protein‐specific CD4+ and CD8+ T‐cell responses.
Conclusion
Vaccination with BNT162b2 induces stronger humoral responses than CoronaVac. CoronaVac induces higher CD4+ and CD8+ T‐cell responses to the structural protein than BNT162b2.
Keywords: Biontech, BNT162b2, CoronaVac, coronavirus disease, COVID‐19, immunogenicity, SARS‐CoV‐2, Sinovac
Through the head‐to‐head comparison, vaccination with BNT162b2 induces significantly higher levels of SARS‐CoV‐2‐specific binding and neutralizing antibody responses compared to CoronaVac. CoronaVac induces higher CD4+ and CD8+ T‐cell responses to the structural protein than BNT162b2.

See related Editorial
INTRODUCTION
SARS‐CoV‐2, the cause of COVID‐19, emerged in late 2019 leading to a devastating pandemic. 1 , 2 Over 240 million COVID‐19 infections including 4.9 million deaths have been reported to the World Health Organization as of 25 October 2021. 3 Many COVID‐19 vaccines were rapidly developed, evaluated and deployed with over 4 billion doses of COVID‐19 vaccines administered worldwide so far. 3 These include inactivated whole virus, lipid nanoparticle‐encapsulated mRNA, adenovirus‐vectored and protein sub‐unit vaccines. Virus neutralizing antibodies correlate with protection from re‐infection following natural infection as well as vaccination 4 , 5 and in experimental animal models. 6 T‐cell immune responses are consistently elicited after natural infection and correlate with reduced disease severity in humans and reduced viral loads in non‐human primates. 7 , 8 , 9 , 10 , 11
The safety, immunogenicity and efficacy of these vaccines have been evaluated in separate clinical trials, 12 , 13 , 14 but there are few ‘head‐to‐head’ comparisons of different vaccines. Here, we compare the humoral and cellular responses from vaccinees who received either BNT162b2 or CoronaVac.
METHODS
Cohort study design and participants
Healthy adults aged between 18 and 79 years were recruited in Hong Kong SAR, China, at vaccination centres of the Chinese University of Hong Kong Medical Centre, Prince of Wales Hospital and Kowloon Bay Vaccination Station between 10 March and 31 August 2021. Those with previous COVID‐19 infection were excluded. The participants received two doses of either BNT162b2 (Comirnaty [BioNTech]) vaccine (21 days of interval between the two doses) or CoronaVac (Sinovac) vaccine (28 days of interval between the two doses), according to the manufacturer's recommendations. Demographic information was collected from each participant prior to vaccination (Table S1 in the Supporting Information). Ten millilitres of heparinized blood was collected from each donor before vaccination and at 1 month after receiving the second vaccine dose.
Immunological assays
Humoral and cellular immune responses were examined to compare the levels of SARS‐CoV‐2‐neutralizing, ‐binding and FcR+‐binding antibody in plasma and T‐cell reactivity in peripheral blood mononuclear cells (PBMC) between the two vaccine groups. Details of specimen storage and processing and the serological methods used in ELISA for spike receptor binding domain (RBD), spike N‐terminal domain (NTD) binding, S2 and N protein antibodies, ELISA for FcγRIIIa‐binding and antibody avidity, surrogate virus neutralization test (sVNT), plaque reduction neutralization test (PRNT) and SARS‐CoV‐2‐specific T cells by intracellular cytokine staining are provided in Appendix S1 in the Supporting Information.
Statistical analysis
Methods used for statistical analysis are provided in Appendix S1 in the Supporting Information.
RESULTS
Adult volunteers (age range 18–79 years) were recruited between 10 March and 31 August 2021; 366 of whom received two doses of BNT162b2 and 360 received two doses of CoronaVac. The mean (SD) age of the two groups were 45.01 (13.16) and 51.78 (9.92) years, respectively (p < 0.0001). Demographic information is summarized in Table S1 in the Supporting Information. We used sVNT, which has high overall concordance to the PRNT, 15 , 16 to examine the level of SARS‐CoV‐2‐specific neutralizing antibody from the plasma samples collected before and at 1 month after the second dose of vaccination. The plasma samples from all vaccinees before vaccination were negative in sVNT (data not shown). The mean % inhibition in the sVNT in post‐vaccination plasma for BNT1626 and CoronaVac was 93.63% (SD 7.92) and 52.11% (SD 22.06), respectively (p < 0.0001) (Figure 1A).
FIGURE 1.
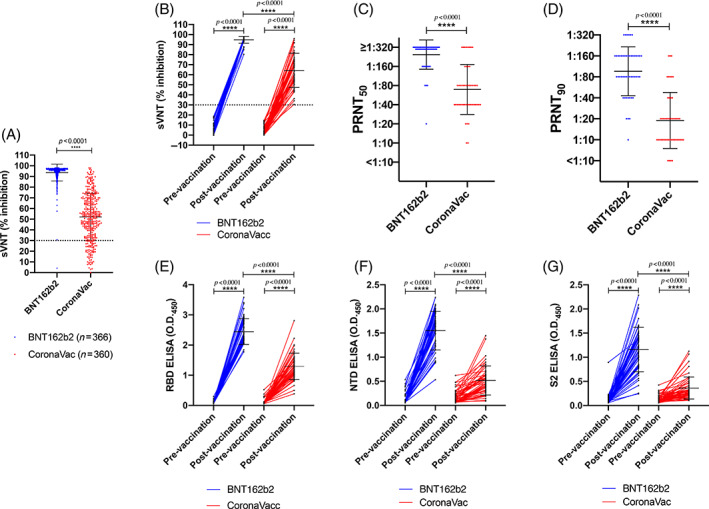
Antibody responses of individuals before and after BNT162b2 or CoronaVac vaccination. The percentage of inhibition was detected by surrogate virus neutralization test (sVNT) from the plasma collected from adult individuals who received two doses of BNT162b2 (n = 366) or CoronaVac (n = 360) (A). Various antibody responses were further determined from the plasma from an age‐matched subgroup of (A) (49 vs. 49). (B) The percentage of inhibition from the plasma of pre‐vaccination and 1 month after two doses of vaccination was tested by sVNT. The dashed line at 30% indicates the negative threshold of the sVNT. Comparison of the (C) PRNT50 (plaque reduction neutralization test) and (D) PRNT90 from the plasma collected at 1 month after two doses of vaccination between the BNT162b2 and CoronaVac groups. The levels of (E) receptor binding domain‐specific (F) N‐terminal domain‐specific and (G) S2‐specific IgG antibodies from the plasma of pre‐vaccination and 1 month after two doses of vaccination were tested by ELISA. ****p < 0.0001
From the data mentioned above, we estimated that a sample size of 49 for each vaccine group provided statistical power higher than 80% with 95% confidence level to discern differences between the two vaccines. To avoid age‐related bias, we randomly 49 selected age‐matched samples from BNT162b2 or CoronaVac vaccinees for more detailed analysis. The demographic, underlying co‐morbidities and other potential risk factors are shown in Table 1. The mean % inhibition in the sVNT in post‐vaccination plasma for BNT1626 and CoronaVac was 94.8% (SD 3.45) and 63.9% (SD 16.72), respectively (p = 7.71 × 10−17) (Figure 1B).
TABLE 1.
Comparison of characteristics between the two vaccine groups (n = 98)
| Total (n = 98) | BNT162b2 (n = 49) | CoronaVac (n = 49) | p value a | |
|---|---|---|---|---|
| Age, mean (SD) | 51.4 (8.3) | 51.5 (8.3) | 51.3 (8.3) | 0.913 b |
| Gender | 0.106 | |||
| Female | 49 (50.0) | 20 (40.8) | 29 (59.2) | |
| Male | 49 (50.0) | 29 (59.2) | 20 (40.8) | |
| Smoker | 0.242 | |||
| No | 95 (97.9) | 49 (100) | 46 (95.8) | |
| Yes | 2 (2.1) | 0 (0) | 2 (4.2) | |
| Alcohol | 0.581 c | |||
| Never | 61 (62.9) | 29 (59.2) | 32 (66.7) | |
| Sometimes | 36 (37.1) | 20 (40.8) | 16 (33.3) | |
| Always | 0 (0) | 0 (0) | 0 (0) | |
| Cardiovascular disease | 0.999 | |||
| No | 93 (94.9) | 47 (95.9) | 46 (93.9) | |
| Yes | 5 (5.1) | 2 (4.1) | 3 (6.1) | |
| Diabetes mellitus | 0.268 | |||
| No | 90 (91.8) | 43 (87.8) | 47 (95.9) | |
| Yes | 8 (8.2) | 6 (12.2) | 2 (4.1) | |
| Exercise | 0.761 | |||
| No | 48 (49.5) | 23 (46.9) | 25 (52.1) | |
| Yes | 49 (50.5) | 26 (53.1) | 23 (47.9) | |
| Vaccination history | ||||
| Influenza | 0.027 | |||
| No | 20 (20.6) | 15 (30.6) | 5 (10.4) | |
| Yes | 77 (79.4) | 34 (69.4) | 43 (89.6) | |
| Hepatitis A/B | 0.157 | |||
| No | 46 (46.9) | 19 (38.8) | 27 (55.1) | |
| Yes | 52 (53.1) | 30 (61.2) | 22 (44.9) | |
| Mumps | 0.522 | |||
| No | 87 (88.8) | 42 (85.7) | 45 (91.8) | |
| Yes | 11 (11.2) | 7 (14.3) | 4 (8.2) | |
| PCV | 0.999 | |||
| No | 89 (90.8) | 45 (91.8) | 44 (89.8) | |
| Yes | 9 (9.2) | 4 (8.2) | 5 (10.2) | |
| Rabies | 0.617 | |||
| No | 94 (95.9) | 46 (93.9) | 48 (98) | |
| Yes | 4 (4.1) | 3 (6.1) | 1 (2) | |
| Typhoid | 0.436 | |||
| No | 91 (92.9) | 44 (89.8) | 47 (95.9) | |
| Yes | 7 (7.1) | 5 (10.2) | 2 (4.1) | |
| Haemorrhagic fever | 0.242 | |||
| No | 95 (96.9) | 46 (93.9) | 49 (100) | |
| Yes | 3 (3.1) | 3 (6.1) | 0 (0) |
Abbreviation: PCV, pneumococcal conjugate vaccine.
Unless stated, Fisher's exact test was used to test the null hypothesis of independence between the two categorical variables and vaccine group (BNT162b2 vs. CoronaVac).
Student's t‐test.
Chi‐square test.
PRNT is the gold‐standard method to evaluate virus neutralization. All vaccinees developed detectable PRNT50 antibody titres. BNT162b2 vaccinees had significantly higher geometric mean titres (GMT) of 251.60 (±1 SD, range from 147 to 432) compared to 69.45 (±1 SD, range from 28 to 172) for the CoronaVac group (p = 1.24 × 10−9) (Figure 1C). The corresponding PRNT90 antibody responses for BNT162b2 (GMT 98.91, ±1 SD, range from 44 to 221) were significantly higher than for CoronaVac vaccines (GMT 16.57, ±1 SD, range from 5 to 54) (p = 5.82 × 10−10) (Figure 1D). It has been suggested that the neutralizing antibody titre associated with protection from re‐infection in 50% of individuals is 20% of the mean convalescent antibody levels. 5 Using reverse transcription (RT)‐PCR‐confirmed convalescent symptomatic patients sera, 16 using the same PRNT methods, we estimated that this 20% convalescent antibody titre threshold for 50% protection from re‐infection for PRNT90 was 1:8.75 (95% CI 1:6.6–1:11.6) (Figure S1 in the Supporting Information). Thus, we estimated that all 49 vaccinated with BNT162b2 and 45 (91.8%) of 49 of those vaccinated with CoronaVac achieved the 50% protection threshold 1 month after the second dose of vaccination. Neutralizing antibody levels reportedly fell by 7.3‐fold within 6 months of CoronaVac immunization. 17 Thus, we estimate that only eight (16.3%) of 49 CoronaVac vaccines would retain protective levels of neutralizing antibody 6 months post‐vaccination while 79.6% of BNT162b2 vaccinees would do so, assuming comparable waning of antibody in BNT162b2 vaccinees.
Adjusting for age, gender and history of other vaccine uptake, subjects who received CoronaVac had a significantly lower PRNT90 value compared to those who received BNT162b2 (Table S2 in the Supporting Information). Stratifying regression analysis by choice of vaccine, older age was independently associated with lower PRNT90 values with either vaccine (Table S3 in the Supporting Information).
Antibodies against different domains of the spike can facilitate protection. 18 , 19 , 20 Thus, we further tested the levels of IgG antibodies specifically binding to the RBD, NTD and S2 domains of the spike protein. While both vaccines elicited significant increases in these antibodies as detected by ELISA, BNT162b2 vaccine elicited significantly higher antibody levels to all three antigens (Figure 1E–G). As expected from a whole virus vaccine which also contains the virus N protein, 59.2% (29/49) and 40.8% (20/49) of post‐vaccination plasma from CoronaVac group were positive in ELISA against the full length (aa 44–419) and C‐terminal domain of the N protein (Figure S2 in the Supporting Information). One plasma sample from a BNT162b2 vaccinee had high ELISA‐binding antibody to N protein (but not to C‐terminal domain of N) in post‐vaccination plasma, but was negative for other viral proteins (ORF8, data not shown), and therefore may represent cross‐reactivity in the N antibody assay, perhaps with other seasonal coronaviruses.
Antibodies that bind to Fc receptors on effector cells such as natural killer (NK) cells may stimulate antibody‐dependent cell cytotoxicity (ADCC) upon FcgRIIIa binding of virus–antibody complexes leading to downstream signalling. Antigen‐specific FcgRIIIa binding significantly corelates with NK cell function of degranulation, killing and cytokine production. 21 Furthermore, increased ADCC function is associated with mRNA 22 and protein 23 vaccination and reduced morbidity during infection, 24 , 25 and is therefore regarded as one of the indicators of immune protection. We found that recipients from both BNT162b2 or CoronaVac elicit increases of FcγRIIIa‐binding spike antibodies after two doses of vaccination (Figure 2A). However, the antibody level from the BNT162b2 group was significantly higher than the CoronaVac group. CoronaVac recipients had higher levels of FcγRIIIa‐binding antibodies to N than the BNT162b2 group after vaccination (p < 0.0001) as expected, but CoronaVac N‐FcγRIIIa responses were still significantly lower than COVID‐19 cases (Figure 2B). FcγRIIIa binding is more likely to result in downstream signalling leading to effector function if antibodies have high avidity. 26 S‐specific FcγRIIIa as well as S‐IgG avidity were significantly lower in CoronaVac recipients than BNT162b2 recipients or COVID‐19 convalescent patients (Figure 2C,D). The average avidity of N‐specific FcγRIIIa from CoronaVac recipients was comparable to the patients with COVID‐19 (Figure 2E).
FIGURE 2.
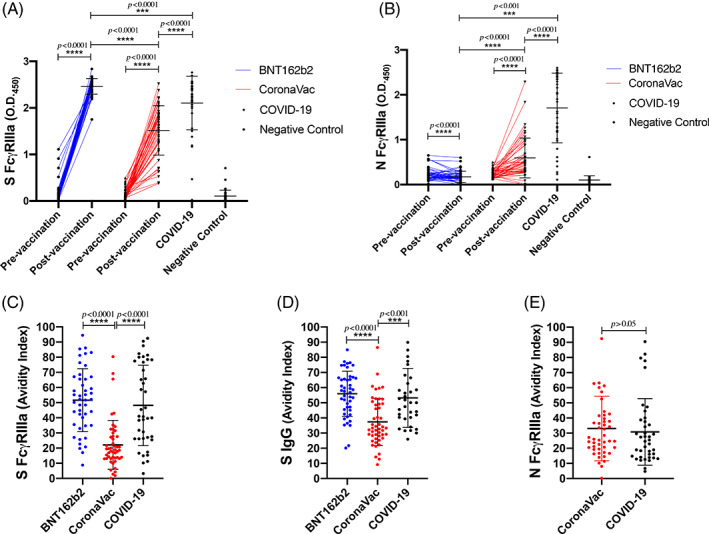
FcγRIIIa‐binding antibodies and IgG avidity in the BNT162b2 and CoronaVac groups. The levels of FcγRIIIa‐binding antibodies and their avidity were detected from the plasma collected from adult individuals who received two doses of BNT162b2 (n = 49) or CoronaVac (n = 49). Recovered COVID‐19 cases (n = 34, timepoint 56 ± 17 days post infection [mean ± SD]) and healthy adults negative for SARS‐CoV‐2 (n = 40) served as positive and negative controls, respectively. The levels of (A) FcγRIIIa‐binding S antibodies and (B) FcγRIIIa‐binding N antibodies were tested from the plasma collected before and at 1 month after two doses of vaccination. The avidity indexes of (C) S FcγRIIIa, (D) S IgG and (E) N FcγRIIIa were determined as the proportion of antibodies remaining after 3× washes with 8 M urea compared to the total FcγRIIIa‐binding antibodies to each protein. ***p < 0.001; ****p < 0.0001
We collected PBMCs from the same subgroup where possible, making a cohort of 25 vaccinees who received two doses of BNT162b2 and 30 with two doses of CoronaVac. Overlapping peptides of the structural proteins including S, N, Envelope and Matrix (SNEM) or S only pools were used to induce specific T‐cell responses in the PBMCs. The structural and S‐specific CD4+ and CD8+ T‐cell responses were quantified by flow cytometry (Figure S3 in the Supporting Information) from paired samples at pre‐ and post‐vaccination of CoronaVac and BNT162b2, while RT‐PCR‐confirmed COVID‐19 patients served as positive controls. The average magnitude of the post‐vaccination CD4+ and CD8+ T‐cell responses after stimulation of SNEM peptides was significantly higher in CoronaVac group compared to those who received BNT162b2 (Figure 3A,B). The magnitude of the post‐vaccination CD4+ T‐cell response after the stimulation of S‐only peptides was also significantly higher in CoronaVac group but their CD8+ T‐cell responses were comparable to the BNT162b2 group (Figure S4A,B in the Supporting Information). Overall, the proportion of subjects who had detectable post‐vaccination T‐cell responses (% of IFNγ+ [interferon γ] cell is higher than 0.001), termed ‘responders’, was higher in CoronaVac recipients than BNT162b2 recipients for either SNEM peptides or S peptides alone (Table 2).
FIGURE 3.
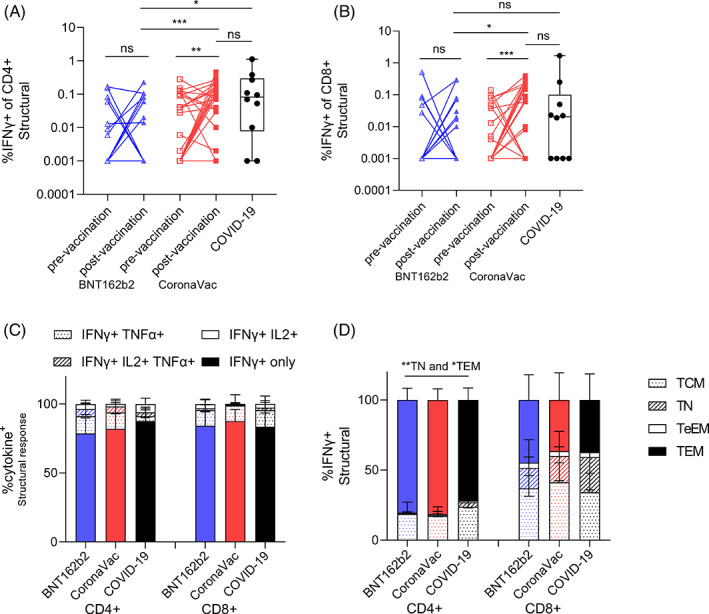
T‐cell responses post vaccination are comparable between BNT162b2 and CoronaVac. PBMCs from pre‐ (Day 0) and post‐vaccination (Day 30 after the second dose) of BNT162b2 mRNA (pre‐vaccination n = 25, post‐vaccination n = 25) and CoronaVac (pre‐vaccination n = 30, post‐vaccination n = 30) and recovered COVID‐19 cases (n = 10, timepoint 59 ± 20 days post infection [mean ± SD]) were stimulated with pooled structural (S, N, Envelope and Matrix [SNEM]) peptides or a dimethyl sulphoxide (DMSO) control. The percentage of (A) interferon γ (IFNγ)+ CD4+ and (B) IFNγ+ CD8+ T cells was measured by flow cytometry. Dotted lines represent the limit of detection following DMSO background subtraction (IFNγ of CD4+ = 0.001, IFNγ of CD8+ = 0.001). (C) The proportion of IFNγ producing IL‐2 and TNF‐α CD4+ and CD8+ T cells post vaccination. (D) The phenotype (by CCR7 and CD45RA) of IFNγ responses for T effector memory (TEM), central memory (TCM), terminal effector memory (TeEM) or naïve (TN) CD4+ and CD8+ T cells post vaccination. Bars represent the mean values, and error bars represent SD. Statistical significance was determined by paired t‐test between pre‐ versus post‐vaccination timepoint samples, and Kruskal–Wallis test for multiple comparisons between vaccines, and COVID‐19 patients. *p < 0.05; **p < 0.01; ***p < 0.001
TABLE 2.
Structural and spike‐specific T‐cell responses assessed by intracellular staining for IFNγ
| Pre‐vaccination | Post‐vaccination | |||||||
|---|---|---|---|---|---|---|---|---|
| % (n) | Responder | Non‐responder | % IFNγ+ of T cells | Responder | Non‐responder | % IFNγ+ of T cells | % IFNγ+ of T‐cells fold change | |
| Structural protein | ||||||||
| CD4+ T cells | BNT162b2 | 28% (7) | 72% (18) | 0.02032 ± 0.04548 | 32% (8) | 68% (17) | 0.0288 ± 0.054 | 13.40 ± 27.59 |
| CoronaVac | 50% (15) | 50% (15) | 0.0431 ± 0.06474 | 83.3% (25) | 16.7% (5) | 0.11217 ± 0.10786 | 72.39 ± 112.04 | |
| CD8+ T cells | BNT162b2 | 20% (5) | 80% (20) | 0.03081 ± 0.10046 | 32% (8) | 68% (17) | 0.03246 ± 0.07995 | 21.05 ± 60.39 |
| CoronaVac | 33.3% (10) | 66.7% (20) | 0.0189 ± 0.03491 | 63.3% (19) | 36.7% (11) | 0.1104 ± 0.1167 | 77.20 ± 103.89 | |
| Spike protein | ||||||||
| CD4+ T cells | BNT162b2 | 52% (13) | 48% (12) | 0.05808 ± 0.1085 | 44% (11) | 56% (14) | 0.04656 ± 0.07888 | 25.01 ± 51.82 |
| CoronaVac | 60% (18) | 40% (12) | 0.0597 ± 0.08131 | 73.3% (22) | 26.7% (8) | 0.0875 ± 0.08939 | 37.65 ± 64.64 | |
| CD8+ T cells | BNT162b2 | 36% (9) | 64% (16) | 0.04053 ± 0.0729 | 36% (9) | 64% (16) | 0.09492 ± 0.19278 | 39.83 ± 80.84 |
| CoronaVac | 46.7% (14) | 53.3% (16) | 0.0286 ± 0.07923 | 50% (15) | 50% (15) | 0.0744 ± 0.10961 | 47.94 ± 87.52 | |
Abbreviation: IFNγ, interferon γ.
Post‐vaccination polyfunctional cytokine production, including TNF‐α and IL‐2 by T cells in the vaccine responders, was equivalent between the two vaccine types and convalescent COVID‐19 samples after the stimulation of either the SNEM or S‐only peptides (Figures 3C and S4C in the Supporting Information). The memory phenotype of IFNγ+ CD4+ T cells in both vaccine groups showed significantly higher percentage of T effector memory (TEM) (p < 0.05) and lower T naïve (TN) (p < 0.01) than the recovered COVID‐19 patients (Figure 3D). Interestingly, S‐specific CD4+ T‐cell responses memory phenotypes further showed TEM > TCM (T central memory) in the CoronaVac group compared to BNT126b2, which may impact recall at infection and long‐term cellular memory (Figure S4D in the Supporting Information).
DISCUSSION
Using a cohort of RT‐PCR‐confirmed convalescent sera from COVID‐19 patients and the assays used in the present study, we estimated that the 20% convalescent antibody titre threshold for 50% protection from re‐infection for PRNT90 was 1:8.75 (95% CI 1:6.6–1:11.6). 5 , 16 We conclude that all those vaccinated with BNT162b2 and 91.8% of those vaccinated with CoronaVac achieved the 50% protection threshold 1 month after the second dose of vaccination. One month post‐second dose likely represents the peak antibody response, beyond which antibody titres are likely to wane. It was reported that neutralizing antibody titres wane by 7.3‐fold within 6 months of CoronaVac vaccination, 17 but comparable data are not available for BNT126b2 vaccination. If we adjust for a 7.3‐fold waning of antibody titres for both vaccines, we estimate that only eight (16.3%) of 49 receiving CoronaVac vaccines meet the protective threshold while 39 (79.6%) of 49 of those receiving BNT162b2 do so 6 months post‐vaccination. This difference in immunogenicity may explain reported difference in vaccine efficacy between the two vaccines. 27 , 28 , 29 , 30 Many Phase 3 studies assessed protection within 1–3 months after the second dose of vaccine and may not reflect the impact of waning immunity. Furthermore, some of the virus variants (e.g., B.1.351 Beta variant and P1 Gamma variant) circulating in parts of the world lead to an eight‐ to 10‐fold reduction in neutralizing titres and this is likely to further compromise protection afforded by CoronaVac vaccines with its weaker immunogenicity. A third dose of CoronaVac vaccine appears to boost antibody levels 17 and our data suggest that these may be needed for older CoronaVac recipients.
The average magnitude of post‐vaccination responses was higher in CoronaVac subjects for structural and S‐specific T‐cell responses. As it is likely that T‐cell responses are important in limiting severity and fatal outcomes, 7 , 8 , 9 , 10 , 11 both vaccines may be effective in preventing such adverse outcomes of COVID‐19. This is consistent with high levels of protection against hospitalization and death in CoronaVac vaccines observed in Chile and Turkey despite lower antibody neutralization titres than BNT162b2. 30 , 31 In animal models of re‐infection, spike‐specific CD8+ T‐cell responses can compensate for inadequate antibody responses 24 and are also highly cross‐reactive to different variants of concern. 32 Therefore, SARS‐CoV‐2‐specific T cells may provide an additional contribution to the immune correlate of protection. An advantage of inactivated vaccines is that they also contain additional viral antigens, such as the highly abundant and immunogenic N protein which may also elicit T‐cell immunity 33 and contributed to the higher responses in CoronaVac subjects here. Sampling at earlier timepoints (Days 7–14) post vaccination may reveal differences in response magnitude given the phenotypic expansion of S‐specific TEM CD4+ T cells by the BNT162b2 vaccine which may also have greater recall potential at infection. 34 Longitudinal follow‐up is thus necessary to confirm the long‐term T‐cell responses between the two vaccines.
Proline mutations used in BNT162b2 vaccine to stabilize the spike protein in its pre‐fusion state 35 and the serial passages in Vero cells and inactivation procedures used in the CoronaVac vaccine 36 contribute to the differences in neutralizing antibody responses elicited by the two vaccines.
Preliminary results from another study have recently reported a marked difference of immunogenicity between BNT162b2 and CoronaVac in healthcare workers. 37 However, the median age of the two groups were 37 and 47 years, respectively, in that study while our subgroup was designed to be age matched. Moreover, the ADCC and T‐cell responses were not examined in their cohort.
There were some limitations in our study. The choice of vaccine was not randomized and there might be a selection bias in those opting for each vaccine. Our study only focused on investigating the immunogenicity at 1 month after the two doses of vaccination. The durability of immune responses needs to be monitored, and indeed, this cohort will be followed up to address this question in the coming years. Our estimates to adjust for antibody waning were based on reports on CoronaVac; however, comparable data for BNT162b2 were lacking. Thus, our assumption of comparable rates of antibody waning for the two vaccines may not be correct. As our primary study endpoint was neuralization titres after vaccination, we did not collect plasma prior to the second dose of vaccine to assess the effect of the first dose of the vaccine, or acute phase responses, where earlier responses may account for the final post‐vaccination differences. Similar comparisons between vaccines in teenagers and older adults will be needed.
In conclusion, our data showed that vaccination with BNT162b2 induces stronger humoral responses than CoronaVac. However, CoronaVac induces higher CD4+ and CD8+ T‐cell responses to the structural protein than BNT162b2.
CONFLICT OF INTEREST
The study was partly supported by Fast Grant ##2161 (Emergent Ventures to Gaya K. Amerasinghe) and NIH grants (P01AI120943 and R01AI123926 to Gaya K. Amerasinghe; R01AI107056 to Daisy W. Leung).
AUTHOR CONTRIBUTION
Chris Ka Pun Mok: Conceptualization (lead); data curation (equal); formal analysis (equal); funding acquisition (lead); investigation (lead); methodology (equal); project administration (equal); supervision (lead); writing – original draft (lead); writing – review and editing (lead). Carolyn A. Cohen: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Samuel M. S. Cheng: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal). Chunke Chen: Data curation (equal); investigation (equal). Kin‐On Kwok: Formal analysis (equal); methodology (equal). Karen Yiu: Project administration (equal). Tat‐On Chan: Formal analysis (equal); methodology (supporting). Maireid Bull: Investigation (supporting); methodology (supporting). Kwun Cheung Ling: Investigation (supporting). Zixi Dai: Investigation (supporting). Susanna S. Ng: Funding acquisition (supporting). Grace Chung‐Yan Lui: Funding acquisition (supporting). Chao Wu: Resources (supporting). Gaya K. Amerasinghe: Resources (supporting). Daisy W. Leung: Resources (supporting). Samuel Yeung Shan Wong: Funding acquisition (supporting); supervision (equal). Sophie A. Valkenburg: Conceptualization (lead); data curation (equal); formal analysis (equal); methodology (equal); supervision (equal); writing – original draft (equal); writing – review and editing (equal). David S. Hui: Conceptualization (lead); formal analysis (equal); funding acquisition (equal); supervision (lead); writing – original draft (lead); writing – review and editing (lead). Malik Peiris: Conceptualization (lead); formal analysis (equal); funding acquisition (equal); supervision (lead); writing – original draft (lead); writing – review and editing (lead). All authors critically reviewed and commented on the manuscript.
HUMAN ETHICS APPROVAL DECLARATION
The study was approved by the Joint Chinese University of Hong Kong‐New Territories East Cluster Clinical Research Ethics Committee (Ref No.: 2020.229) and all participants provided written consent.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
We acknowledge the technical support from Mr Huibin Lv and Mr Ho Lun Lai. We also thank Dr Fung Hong (Chinese University Medical Center), Dr Ken Tsang (Kowloon Bay community vaccination centre) and Dr Beatrice Cheng (Prince of Wales Hospital) for allowing us to recruit subjects for this study. The recombinant RBD and NTD proteins were kindly gifted by Prof Ian A. Wilson and Dr Meng Yuan. This project utilized an Invitrogen Attune flow cytometer funded by the Pasteur Foundation Asia which is a non‐profit organization.
Research funding: This research was supported by grants from the Health and Medical Research Fund Commissioned Research on the Novel Coronavirus Disease (COVID‐19), Hong Kong SAR (COVID1903003) (Chris Ka Pun Mok, Susanna S. Ng, Grace Chung‐Yan Lui, Malik Peiris and David S. Hui) (COVID‐190115 and COVID‐190126, Sophie A. Valkenburg); Guangdong Province International Scientific and Technological Cooperation Projects (2020A0505100063) (Chris Ka Pun Mok); the National Research Foundation of Korea (NRF) grant funded through the Korea Government (NRF‐2018M3A9H4055203) (Chris Ka Pun Mok); US National Institutes of Health (contract no. HHSN272201400006C) (Malik Peiris); National Natural Science Foundation of China (NSFC)/Research Grants Council (RGC) Joint Research Scheme (N_HKU737/18) (Chris Ka Pun Mok and Malik Peiris); and RGC theme‐based research scheme (T11‐712/19‐N and T11‐705/21‐N) (David S Hui). The research was partly supported by Fast Grant ##2161 (Emergent Ventures to Gaya K. Amerasinghe) and NIH grants (P01AI120943 and R01AI123926 to Gaya K. Amerasinghe; R01AI107056 to Daisy W. Leung). The sponsor of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Mok CKP, Cohen CA, Cheng SMS, Chen C, Kwok K‐O, Yiu K, et al. Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID‐19 vaccines in Hong Kong. Respirology. 2022;27:301–310. 10.1111/resp.14191
Chris Ka Pun Mok and Carolyn A. Cohen contributed equally to this study.
Malik Peiris and David S. Hui contributed equally to this study. Both had full access to all the study data and final responsibility for the published research.
Associate Editor: Diane Gray and Senior Editors: Philip Bardin and Paul Reynolds
Funding information Fast Grant, Grant/Award Number: 2161; National Natural Science Foundation of China (NSFC)/Research Grants Council (RGC) Joint Research Scheme, Grant/Award Numbers: T11‐705/21‐N, T11‐712/19‐N, N_HKU737/18; US National Institutes of Health, Grant/Award Numbers: R01AI107056, R01AI123926, P01AI120943, HHSN272201400006C; National Research Foundation of Korea (NRF), Grant/Award Number: NRF‐2018M3A9H4055203; Guangdong Province International Scientific and Technological Cooperation Projects, Grant/Award Number: 2020A0505100063; Health and Medical Research Fund Commissioned Research on the Novel Coronavirus Disease (COVID‐19), Hong Kong SAR, Grant/Award Numbers: COVID‐190126, COVID‐190115, COVID1903003; Pasteur Foundation Asia
See related Editorial
Contributor Information
Malik Peiris, Email: malik@hku.hk.
David S. Hui, Email: dschui@cuhk.edu.hk.
REFERENCES
- 1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization WHO website. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Access on 25/10/2021.
- 4. Addetia A, Crawford KHD, Dingens A, Zhu H, Roychoudhury P, Huang ML, et al. Neutralizing antibodies correlate with protection from SARS‐CoV‐2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol. 2020;58(11):e02107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27(7):1205–11. [DOI] [PubMed] [Google Scholar]
- 6. Corbett KS, Nason MC, Flach B, Gagne M, O'Connell S, Johnston TS, et al. Immune correlates of protection by mRNA‐1273 vaccine against SARS‐CoV‐2 in nonhuman primates. Science. 2021;373(6561):eabj0299. doi: 10.1126/science.abj0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodda LB, Netland J, Shehata L, Pruner KB, Morawski PA, Thouvenel CD, et al. Functional SARS‐CoV‐2‐specific immune memory persists after mild COVID‐19. Cell. 2021;184(1):169–183.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS‐CoV‐2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen‐specific adaptive immunity to SARS‐CoV‐2 in acute COVID‐19 and associations with age and disease severity. Cell. 2020;2183(4):996–1012.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tan AT, Linster M, Tan CW, Le Bert N, Chia WN, Kunasegaran K, et al. Early induction of functional SARS‐CoV‐2‐specific T cells associates with rapid viral clearance and mild disease in COVID‐19 patients. Cell Rep. 2021;34(6):108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muñoz‐Fontela C, Dowling WE, Funnell SGP, Gsell PS, Riveros‐Balta AX, Albrecht RA, et al. Animal models for COVID‐19. Nature. 2020;586(7830):509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pormohammad A, Zarei M, Ghorbani S, Mohammadi M, Razizadeh MH, Turner DL, et al. Efficacy and safety of COVID‐19 vaccines: a systematic review and meta‐analysis of randomized clinical trials. Vaccines (Basel). 2021;9(5):467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA‐based Covid‐19 vaccine candidates. N Engl J Med. 2020;383(25):2439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bueno SM, Abarca K, González PA, Gálvez NMS, Soto JA, Duarte LF, et al. Interim report: safety and immunogenicity of an inactivated vaccine against SARS‐CoV‐2 in healthy Chilean adults in a phase 3 clinical trial. medRxiv [Preprint] posted 1 April 2021. https://www.medrxiv.org/content/10.1101/2021.03.31.21254494v1.full-text [Google Scholar]
- 15. Perera RAPM, Ko R, Tsang OTY, Hui DSC, Kwan MYM, Brackman CJ, et al. Evaluation of a SARS‐CoV‐2 surrogate virus neutralization test for detection of antibody in human, canine, cat, and hamster sera. J Clin Microbiol. 2021;59(2):e02504–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lau EHY, Hui DSC, Tsang OTY, Chan WH, Kwan MYK, Chiu SS, et al. Long‐term persistence of SARS‐CoV‐2 neutralizing antibody responses after infection and estimates of the duration of protection. SSRN [Preprint] posted 7 July 2021. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3881728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pan H, Wu Q, Zeng G, Yang J, Jiang D, Deng X, et al. Immunogenicity and safety of a third dose, and immune persistence of CoronaVac vaccine in healthy adults aged 18‐59years: interim results from a double‐blind, randomized, placebo‐controlled phase 2 clinical trial. medRxiv [Preprint] posted 25 July 2021. 10.1101/2021.07.23.21261026 [DOI] [Google Scholar]
- 18. Ju B, Zhang Q, Ge J, Wang R, Sun J, Ge X, et al. Human neutralizing antibodies elicited by SARS‐CoV‐2 infection. Nature. 2020;584(7819):115–9. [DOI] [PubMed] [Google Scholar]
- 19. Chi X, Yan R, Zhang J, Zhang G, Zhang Y, Hao M, et al. A neutralizing human antibody binds to the N‐terminal domain of the spike protein of SARS‐CoV‐2. Science. 2020;369(6504):650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andreano E, Nicastri E, Paciello I, Pileri P, Manganaro N, Piccini G, et al. Extremely potent human monoclonal antibodies from COVID‐19 convalescent patients. Cell. 2021;184(7):1821–1835.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McLean MR, Madhavi V, Wines BD, Hogarth PM, Chung AW, Kent SJ. Dimeric Fcγ receptor enzyme‐linked immunosorbent assay to study HIV‐specific antibodies: a new look into breadth of Fcγ receptor antibodies induced by the RV144 vaccine trial. J Immunol. 2017;199(2):816–26. [DOI] [PubMed] [Google Scholar]
- 22. Kaplonek P, Cizmeci D, Fischinger S, Collier AR, Suscovich T, Linde C, et al. Subtle immunological differences in mRNA‐1273 and BNT162b2 COVID‐19 vaccine induced Fc‐functional profiles. bioRxiv. [Preprint] posted 31 August 2021. https://www.biorxiv.org/content/10.1101/2021.08.31.458247v1.full [Google Scholar]
- 23. Gorman MJ, Patel N, Guebre‐Xabier M, Zhu AL, Atyeo C, Pullen KM, et al. Fab and Fc contribute to maximal protection against SARS‐CoV‐2 following NVX‐CoV2373 subunit vaccine with Matrix‐M vaccination. Cell Rep Med. 2021;2(9):100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, et al. Correlates of protection against SARS‐CoV‐2 in rhesus macaques. Nature. 2021;590(7847):630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Atyeo C, Fischinger S, Zohar T, Slein MD, Burke J, Loos C, et al. Distinct early serological signatures track with SARS‐CoV‐2 survival. Immunity. 2020;53(3):524–532.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu J, Mold C, Du Clos TW, Sun PD. Pentraxins and Fc receptor‐mediated immune responses. Front Immunol. 2018;9:2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS‐CoV‐2 infections and COVID‐19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Recommendation for an emergency use listing of CODI‐19 vaccine (Vero Cell), inactivated. Submitted by Sinovac. Available from: https://extranet.who.int/pqweb/sites/default/files/documents/SINOVAC_TAG_PEG_REPORT_EUL‐Final28june2021.pdf. Assessed on 21/11/2021
- 30. Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole‐virion SARS‐CoV‐2 vaccine (CoronaVac): interim results of a double‐blind, randomised, placebo‐controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, et al. Effectiveness of an inactivated SARS‐CoV‐2 vaccine in Chile. N Engl J Med. 2021;385:946–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Geers D, Shamier MC, Bogers S, den Hartog G, Gommers L, Nieuwkoop NN, et al. SARS‐CoV‐2 variants of concern partially escape humoral but not T‐cell responses in COVID‐19 convalescent donors and vaccinees. Sci Immunol. 2021;6:eabj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nguyen THO, Rowntree LC, Petersen J, Chua BY, Hensen L, Kedzierski L, et al. CD8+ T cells specific for an immunodominant SARS‐CoV‐2 nucleocapsid epitope display high naive precursor frequency and TCR promiscuity. Immunity. 2021;54:1066–1082.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J Exp Med. 2005;202:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in healthy adults aged 18‐59 years: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lim WW, Mak L, Leung GM, Cowling BJ, Peiris M. Comparative immunogenicity of mRNA and inactivated vaccines against COVID‐19. Lancet Microbe. 2021;2:e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


