Abstract
A common bridge between a linear cytoplasmic signal and broad nuclear regulation is the family of MAP kinases which can translocate to the nucleus upon activation by the cytoplasmic signal. One pathway which functions to activate the ERK family of MAP kinases is the Ras signaling pathway which functions at multiple times and locations during the development of Caenorhabditis elegans including the development of the excretory cell, germ cells, male tail, and vulva. It has been most extensively characterized during the development of the vulva which is formed from the vulval precursor cells (VPCs), a set of six equivalent, epithelial cells designated P3.p – P8.p. Although LIN-1 appears to be a primary target of ERK MAP kinase during vulval development, it is likely that other developmentally important molecules are also regulated by ERK-mediated phosphorylation. The identification of physiological substrates of MAP kinases has been aided by the identification of docking site domains in substrate proteins that contribute to high-affinity interactions with kinases.
Our laboratory has identified the C. elegans protein, T08D10.1/NFYA-1, as a potential ERK MAP kinase substrate in this manner, and we have initiated a characterization of its role during Ras-mediated development. T08D10.1 possesses significant homology to the CCAAT-box DNA-binding domain of the vertebrate nuclear transcription factor-Y, alpha (NF-YA) family of proteins. NF-Y proteins act as part of a complex to regulate the transcription of a large number of genes, in particular, genes that function in the G1/S cell cycle transition. T08D10.1/NFYA-1 is predicted to code for a protein containing multiple potential phosphorylation sites for ERK MAP kinase and a D-domain docking site. We demonstrate through biochemical analysis of purified NFYA-1 protein that it can act in vitro as a high affinity substrate for activated ERK MAP kinase. Growth factor activation of the Ras pathway in a tissue culture system has negligible effect on the protein’s transactivation potential, however, the DNA-binding activity of the protein is reduced after treatment with activated ERK-MAP kinase. We demonstrate through mutant analysis that nfya-1 acts to inhibit vulval development and functions downstream or in parallel to let-60/ras. Both the NF-Y complex and the Ras signaling pathway play a fundamental role in cell proliferation and oncogenesis and the connection between the two is an important insight into the mechanisms of cell fate specification and cellular response.
Keywords: Nfya-1, C. elegans, vulval development, Ras signaling
1. Introduction
Signaling pathways interact with each other to regulate a distinct set of genes in order to influence the developmental fate of a cell. While the core of many signaling pathways is relatively linear in the cytoplasm, the set of nuclear factors activated by these pathways is likely tailored to a particular development goal [1].
A common bridge between a linear cytoplasmic signal and broad nuclear regulation is the family of MAP kinases which can translocate to the nucleus upon activation by the cytoplasmic signal [2]. One pathway which functions to activate the ERK family of MAP kinases is the Ras signaling pathway which functions at multiple times and locations during the development of Caenorhabditis elegans including the development of the excretory cell, germ cells, male tail, and vulva (Figure 1). It has been most extensively characterized during the development of the vulva which is formed from the vulval precursor cells (VPCs), a set of six equivalent, epithelial cells designated P3.p – P8.p [3–6]. During wild-type vulval development, a Ras-mediated signal originating from the anchor cell of the gonad causes the P6.p VPC to adopt the 1° cell fate characterized by three equal cell divisions resulting in 8 descendants (Figure 1a). P5.p and P7.p adopt the 2° vulval cell fate and divide to form 7 descendants in response to a lateral lin-12/Notch mediated signal from P6.p [7]. The 22 vulval cells descendants of P5.p, P6.p and P7.p form a symmetrical cone that creates an opening between the gonad and environment through which eggs can be laid and sperm can enter. P3.p, P4.p, and P8.p adopt non-vulval fates in wild-type animals and divide once before fusing with the hypodermis, however, these cells have the potential to adopt 1° or 2° fates. Ectopic Ras signaling can lead to the adoption of 1° cell fates by multiple VPCs and ectopic 2° fates, and these extra cell divisions result in a Multivulval (Muv) or Protruding Vulval (Pvl) phenotype (Figure 1d). These phenotypes can be visualized under the dissecting scope with the Muv phenotype characterized by multiple ventral protrusions or a mislocated ventral protrusion while the Pvl phenotype is characterized by a centrally located ventral protrusion. In contrast, insufficient Ras-mediated signaling may lead to a Vulvaless (Vul) phenotype resulting in the inability to lay eggs. The Ras-mediated anchor cell signal serves to inactivate the ETS transcription factor, LIN-1, which inhibits the 1° cell fate in the vulval precursor cells. ERK MAP kinase will efficiently phosphorylate LIN-1 in vitro, and in vivo phosphorylation of LIN-1 in P6.p likely leads to its inactivation thereby relieving 1° cell fate inhibition [8,9]. Although LIN-1 appears to be a primary target of ERK MAP kinase during vulval development, it is likely that other developmentally important molecules are also regulated by ERK-mediated phosphorylation. A few other potential ERK substrates have been identified in C. elegans including DDX-19 and GSK-3 [10]. The identification of physiological substrates of MAP kinases has been aided by the identification of docking site domains in substrate proteins [11,12]. In particular, three distinct docking sites, the D-domain, the FXF domain, and the Rsk domain have been demonstrated to mediate high affinity interactions with MAP kinases [13]. The D-domain and Rsk domain mediate interactions with ERK and JNK family of MAP kinases while the FXF docking site specifically interacts with ERK. The characterization of these docking sites has proven useful in the verification of presumed MAP kinase substrates, and the identification of novel substrates [14,15].
Figure 1: Vulval Development in C. elegans.
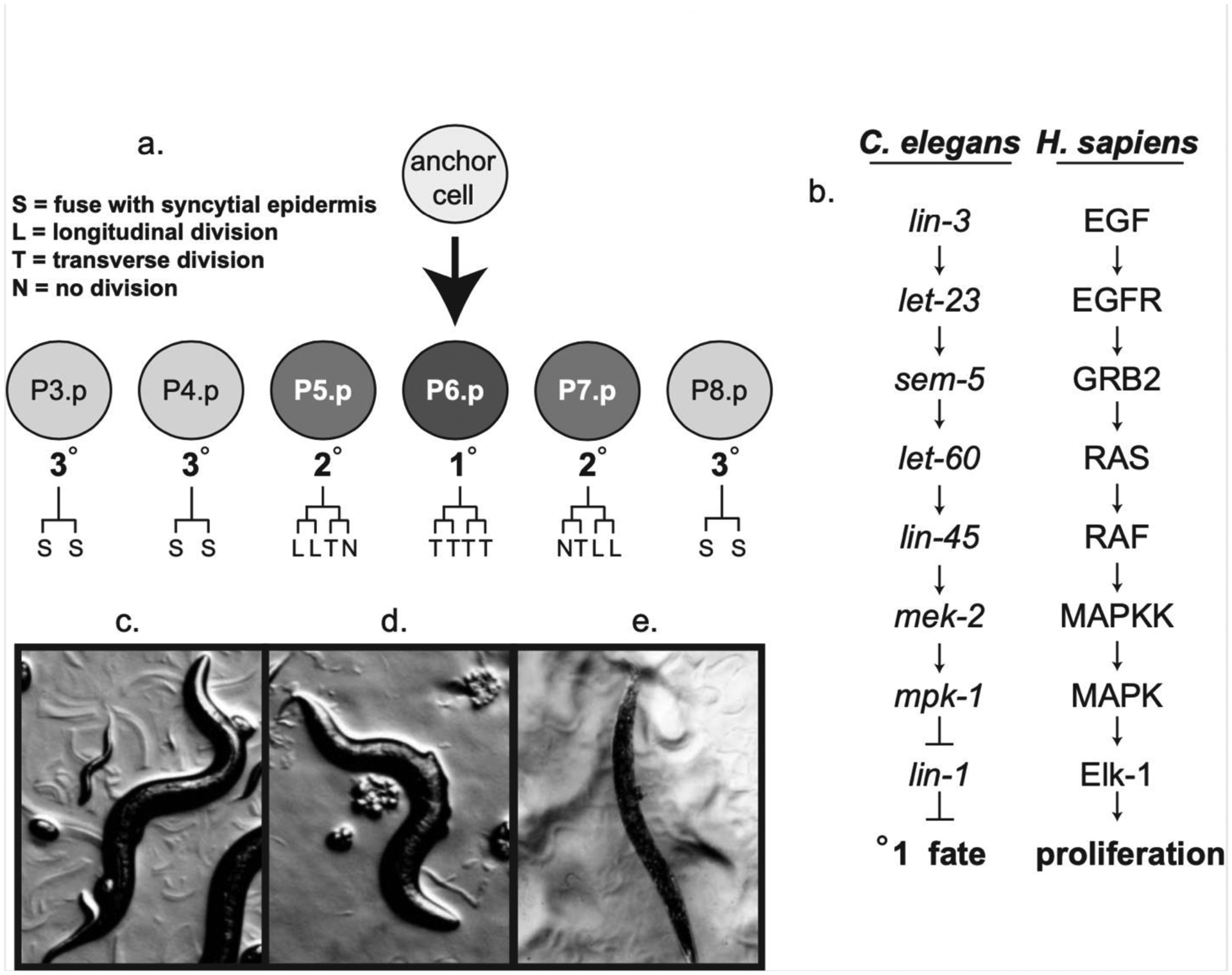
Figure 1a: Ras signaling promotes the 1° vulval cell fate in the P6.p vulval precursor cell. Terminal cell fates (S, L, T, N) as defined in the figure.
Figure 1b: C. elegans genes of the Ras signaling pathway and their H. sapiens homologs.
Figure 1c: Phenotype of wild-type adult C. elegans.
Figure 1d: Phenotype of lin-1(n383) loss-of-function adult multivulval (Muv) hermaphrodites.
Figure 1e: Phenotype of lin-1(n1761) gain-of-function adult vulvaless (Vul) hermaphrodites.
Our laboratory has identified the C. elegans protein, T08D10.1/NFYA-1, as a potential ERK MAP kinase substrate in this manner, and this paper provides an initial characterization of its role during Ras-mediated development.
2. Results
2.1. T08D10.1 interacts with ERK MAP kinase and is a homolog of the NFYA-1 protein
The C. elegans protein, T08D10.1 was initially identified as a potential physiological substrate of ERK MAP kinase through a two-hybrid screen conducted to identify protein-protein interactions occurring during C. elegans vulval development [16]. Inspection of the 27 reported ERK MAP kinase/MPK-1 interacting proteins revealed two genes that coded for recognizable docking sites – MEK-2, the C. elegans MAP kinase kinase homolog, and T08D10.1. T08D10.1 is predicted to code for a protein containing nine canonical phosphorylation sites for ERK MAP kinase and a potential D-domain ERK/JNK MAP kinase docking site. At the amino acid level, T08D10.1 possesses significant homology to the vertebrate nuclear transcription factor-Y, alpha (NF-YA) family of proteins with the highest level of conservation present in the CCAAT-box DNA-binding domain (Figure 2). NF-Y proteins act as part of a multi-subunit complex to regulate the transcription of a large number of genes, in particular, genes that function in the G1/S cell cycle transition [17]. T08D10.1/NFYA-1 has been demonstrated by Deng et al. to regulate expression of the egl-5 gene and bind to a CCAAT box consensus sequence as a complex with the C. elegans NFYB-1 and NFYC-1 homologs [18]. Milton et al. demonstrated a repressive role of the Nfy transcription factor on tbx-2 expression in C. elegans [19]. Based on this information, T08D10.1 is likely to act as a CCAAT-box binding transcription factor that is regulated by ERK MAP kinase phosphorylation during C. elegans development.
Figure 2: Full-length amino acid protein sequence alignment of C. elegans predicted proteinT08D10.1 to Mouse and Human NFYA-1 proteins.

Amino acid identities are outlined in black and chemical similarities are outlined in gray. Consensus ERK-MAP kinase phosphorylation sites are indicated with pins (S/T P). The CAAT-box DNA-binding domain is indicated in the C-terminal end of the aligned sequences. The start and end of the deletion points for the nfya-1(ok1174) deletion allele are indicated with arrows. The consensus D-domain ERK-MAP kinase docking site is indicated ψ1–3X3–7Φ-X-Φ substrate sequence motif, which is shared by diverse MAPK substrates (where ψ, X, Φ denote positively charged, intervening, and hydrophobic residues, respectively).
2.2. NFYA-1 acts as an in vitro substrate for ERK MAP kinase
In order to determine if the C. elegans NFYA-1 protein could act as a substrate of ERK MAP kinase, in vitro kinase assays were performed. Bacterially expressed NFYA-1 proteins were purified using a maltose-binding-protein tag and incubated with active ERK MAP kinase in the presence of labeled ATP. Wild-type NFYA-1 protein was observed to act as a high-affinity substrate for ERK MAP kinase in these assays with an average Km of 3.33 × 10−2 μM (Figure 3a, 2b). This Km value is comparable to that of well-documented substrates of ERK MAP kinase such as C. elegans LIN-1 (Km = 1.8 × 10−1 μM) and provides confirmation that the C. elegans NFYA-1 protein interacts with the Ras pathway as a substrate of ERK MAP kinase [11]. Myelin basic protein was used as a positive control for these experiments and resulted in a calculated Km value of 8.45 μM which is similar to previously described [11]. Surprisingly, mutation of the D-domain did not alter the measured affinity between enzyme and substrate, however the observed Vmax was reduced by three-fold. This suggests that the D-domain is not mediating binding between the ERK MAP kinase and NFYA-1, however, it may be positioning the enzyme to allow for efficient targeting of phosphoacceptor sites in a manner similar to the targeting of the activating Ser383 in Elk-1 by the FXFP ERK/MAP kinase docking site [12].
Figure 3: Kinase Assays of Nfy complex proteins.

Figure 3a: Maltose-binding-protein (MBP):Nfya-1 (full-length cDNA) or Myelin Basic Protein (New England Biolabs) was incubated with activated Erk2 (New England Biolabs) in the presence of 32PATP-for kinetic analysis showing incorporated 32P measured by filter binding and scintillation counting (counts per minute, CPM) using increasing concentrations of substrate. Values are the average of two samples; a bar indicates the range. Figure 3b shows a Lineweaver-Burke plot of the data. To determine Vmax, we calculated total phosphate incorporated using the measured CPM and the specific activity of the [32P]ATP, and factored in the assay time and the amount of Erk2. Relative acceptor ratio (RAR) is Vmax/ Km; values were normalized by assigning a value of 1.0 to myelin basic protein. Figure 3c and 3d show the kinase data for NFYB-1 and NFYC-1, demonstrating their inability to act as effective substrates for ERK MAP kinase in vitro.
2.3. Nfyb-1 and Nfyc-1 do not function as in vitro substrates for ERK MAP kinase
NFYA-1 has been reported to interact with Nfyb-1 and Nfyc-1 in a protein complex that regulates DNA-binding and transactivation of target genes [17]. Loss of C. elegans Nfyb-1 has been recently shown to negatively affect mitochondrial function and longevity through alteration of prosaposin expression [20]. Although Nfyb-1 and Nfyc-1 possesses three and two potential ERK phosphoacceptor sites (S/TP) respectively, no identifiable docking sites are present, and in vitro analysis demonstrates that neither protein functions as a substrate of ERK MAP kinase (Figure 2c, 2d).
2.4. ERK MAP kinase activity does not significantly alter the transactivation potential of NFYA-1
Deng, et al. have demonstrated that the CAAT-box region of NFYA-1 possesses DNA-binding capability in the presence of NFYb-1 and NFYc-1, however we were interested in whether the NFYA-1 protein functions to enhance or inhibit transcription and whether this activity is regulated by Ras signaling [18]. In order to probe the transactivation potential of NFYA-1, a construct was generated that contained the GAL4 DNA-binding domain in place of the CCAAT-box domain of nfya-1. This construct was cotransfected into embryonic fibroblast NIH/3T3 cells with a luciferase reporter gene driven by a promoter containing a TATA box and upstream-activating-sequences (UAS). The regulatory domain of NFYA-1 is not sufficient to activate transcription of the luciferase reporter gene, and exhibited a lower activity than that of the GAL4 DNA-binding domain alone (Figure 4). The transactivation potential of NFYA-1 was slightly increased in the presence of FGF-induced RAS signaling, however, the level of reporter transcription was similar to that induced by the GAL4 DNA binding domain. As a positive control, the C-terminal regulatory domain of human transactivating protein Elk-1, a well characterized substrate of ERK MAP kinase, was used in this assay. The regulatory domain of Elk-1 demonstrated significant transactivation potential in the absence of Ras signaling, and this activity was significantly increased upon addition of FGF as previously reported [21,12,22]. While the regulatory domain of NFYA-1 did not show significant transactivation, the small amount of baseline expression generated by the TATA box containing reporter was observed to decrease in the presence of NFYA-1. In order to determine whether NFYA-1 functions as a transcriptional repressor, a luciferase reporter driven by the thymidine kinase (TK) promoter containing UAS elements was used. The baseline expression of this reporter in the presence of the GAL4 DNA-binding domain is higher than that of the TATA-box reporter, and this activity appears to be slightly inhibited by NFYA-1. NFYA-1 dependent inhibition was relieved upon activation of Ras signaling by FGF, and the level of transcription returned to a level slightly higher than the baseline expression, however, overall changes in transactivation potential are minimal.
Figure 4: Transactivation potential of NFYA-1.

The transactivation domain of NFYA-1 was tested in MC3T3 cells. Cells were transiently transfected with expression plasmids containing the GAL4 DNA-binding domain. GAL4dbd is the GAL4dbd without a transactivation domain, Elk-1 contains the regulatory C-terminus of Elk-1 fused to the GAL4dbd, and NFYA-1 represents a contruct with the CCAAT-box replaced with the GAL4 DNA-binding domain. In some samples (+FGF), fibroblast growth factor was used to stimulate ERK. In other samples, ERK was stimulated by cotransfection of plasmid expressing a constitutively active MAP kinase kinase, MEK (+MEK). Relative Firefly Luciferase measurements are normalized to control Renilla Luciferase values for each transfection.
2.5. ERK MAP kinase activity reduces the DNA-binding activity of NFYA-1
Electrophoretic mobility shift assays were conducted using a canonical CCAAT box DNA binding element as a probe for analysis of in vitro binding of the NFY complex (Figure 5). DNA binding is observed in the presence of purified NFYA-1, NFYB-1, and NFYC-1, and this binding appears to have some sequence specificity as introduction of an unlabeled CCAAT box competitor probe abrogated observable binding to the labeled probe, while a competitor probe with the CCAAT element mutated was not able to effectively compete against the labeled CCAAT box probe confirming results by Deng, et al [18]. Interestingly, in the final lane, we have demonstrated that phosphorylation of NFYA-1 by activated ERK MAP kinase prevented the protein-DNA complex from forming. While the effect of phosphorylation on the in vitro transactivation potential assay was minimal, phosphorylation of NFYA-1 may alter the function of the NFY complex by preventing efficient binding of the NFY proteins in a complex or by subsequent inhibition of DNA binding by the NFY protein complex.
Figure 5: DNA-binding function of the Nfy complex.

Electrophoretic mobility shift assays (EMSA) were conducted to investigate the CCAAT-box binding function of the Nfy complex. Bacterially expressed and purified maltose-binding-protein (MBP) fusion proteins representing the NFYA-1 (a), NFYB-1 (b), and NFYC-1 (c) proteins were incubated with a 32P-dATP radioactively labeled dsDNA containing the consensus CCAAT-box DNA binding element (a/b/c). Shifted probe indicates a low-mobility DNA-protein complex. Unlabeled dsDNA competitors were introduced to the binding reaction to reveal the specificity of the binding complex. Unlabeled wild-type competitor (+CAAT) served to eliminate the signal while introduction of the mutant competitor (+mut) was insufficient to eliminate the shifted complex signal. Pretreatment of NFYA-1 with activated ERK2 MAP kinase (a-P) prevented efficient complex formation.
2.6. Loss of nfya-1 activity promotes vulval cell divisions
To investigate how nfya-1 activity affects Ras signaling during C. elegans vulval development, we decreased nfya-1 function using knockout mutant animals and RNA interference (RNAi). Animals with decreased nfya-1 activity due to mutation or RNAi were analyzed for vulval phenotypes under the dissecting scope (Muv, Pvl, or Vul) to identify changes in Ras signaling. A deletion mutant, nfya-1(ok1174), missing the DNA-binding domain, the putative D-domain docking site, and 8 of 9 phosphoacceptor sites was obtained from a consortium and phenotypically analyzed (Figure 2). Nfya-1(ok1174) hermaphrodites were found to have a low penetrance (14–35%) protruding vulva (Pvl) phenotype, with very rare observation of Muv animals (Figure 6a,b). The Pvl phenotype results from increased vulval cell divisions and has been observed as a phenotype in many vulval genes documented to interact with Ras-pathway components [23]. Although, knockout animals exhibited a Pvl phenotype, knockdown of nfya-1 in wild-type animals using RNAi did not reveal an observable phenotype.
Figure 6: Loss-of-function nfya-1 phenotypes.

Figure 6a: Nfya-1(ok1174) animals possess a large deletion of 80% of the coding sequence of the gene including the predicted DNA-binding domain, MAP kinase docking site and 9 out 10 phosphoacceptor sites. Homozygous animals were cultured and compared to wild-type, N2 animals at various temperatures. Mutant animal populations possessed a partially penetrant protruding vulva (Pvl) phenotype compared to wild-type animals. Figure 6b: Arrows indicate protrusions on the ventral side of a representative animal under Nomarski optics. Figure 6c: Vulval mutant animals were fed E. coli expressing nfya-1 dsRNA or control bacteria without expression plasmid. Let-60 is the C. elegans Ras homolog, and the gain-of-function let-60(n1046) mutation produces a partially penetrant multi-vulval (Muv) phenotype. The polycistronic lin-15 locus encodes two negative regulators of Ras signaling which act epistatic to lin-3, and EGF receptor homolog, and homozygous lin-15(n765) animals exhibit a strong Muv phenotype [24]. Mutant animals scored for Muv phenotypes and p-value comparisons are reported between the RNAi and control animals.
In order to provide a more sensitive genetic background to observe alterations in Ras signaling, RNAi experiments were repeated in Ras-pathway mutant animals that exhibit partially-penetrant Muv phenotypes. Reduction of nfya-1 activity via RNAi may increase or decrease the penetrance of Muv phenotypes in these mutant genetic backgrounds, if nfya-1 functions to regulate Ras-signaling. As seen in Figure 6c, a gain-of-function mutant of the C. elegans Ras gene, let-60(n1046), exhibits a partially penetrant Muv phenotype of 24% at 15°C and 50% at 20°C. In the presence of nfya-1 dsRNA, vulval development was enhanced in let-60(n1046) animals to 38% Muv at 15°C and 70% Muv at 20°C (p = 3.6 × 10−4 at 15°C; p = 8.3 × 10−8 at 20°C). This result indicates one of the biological functions of nfya-1 is to suppress vulval development, and the loss of activity via RNAi enhances vulval cell divisions. To confirm this result, RNAi of nfya-1 was conducted in a temperature sensitive mutant of the Ras pathway component lin-15. Lin-15 negatively regulates Ras signaling at an upstream level, likely through repression of the expression of the anchor cell signal, Lin-3/EGF. Interestingly, lin-15 codes for two distinct genes, A and B, and a loss-of-function mutation of both is required to observe a partially penetrant Muv phenotype due to increase of anchor cell function [24]. The lin-15(n765) mutation causes a loss-of-function of lin-15A and lin-15B resulting in an observable Muv phenotype at 15°C of 82% and at 20°C of 72%. The combination of this mutant with RNAi of nfya-1 results in only slight changes in the Muv phenotype at 15°C (p = 0.035) and a non-significant increase from 72% Muv to 86% Muv at 20°C (p = 0.11). These results indicate that loss of nyfa-1 activity enhances ectopic cell divisions caused by hyperactivity of Ras, however, significant enhancement is not observed in combination with mutation of lin-15. Enhancement of vulval development can be observed in a sensitized Ras gain-of-function mutant background exhibiting increased Ras signaling, and these results are consistent with the hypothesis that NFYA-1 functions as a substrate of mpk-1/ERK MAP kinase downstream of lin-15 and let-60/Ras.
To determine if the enhancement of Muv phenotype observed is dependent on hyperactive Ras, RNAi experiments were performed on a mutant in the C. elegans Notch gene, lin-12(n137). Lin-12 functions to specify the 2° cell fate during vulval development as well as repressing the Ras pathway at the level of MAP kinase through activation of the lip-1 MAP kinase phosphatase [25]. Loss-of-function mutation of lin-12 results in a Muv phenotype and the lin-12(n137) mutant was maintained in a him-5(e1467) background which leads to a high incidence of males in the progeny of an unmated hermaphrodite. RNAi of nfya-1 enhanced the lin-12(n137) ; him-5(e1467) Muv phenotype from 88% to 100%, in a manner similar to the enhancement of the Ras/let-60 Muv phenotype. This indicates that nfya-1 is normally acting to inhibit vulval development and is functioning downstream or in parallel to the Ras/let-60 and ERK MAP kinase/mpk-1 gene.
3. Discussion and Conclusions
We have provided evidence connecting Ras signaling and NFY-1 protein complex activity. This connection acts through activated ERK MAP kinase which phosphorylates NFYA-1 leading to a decrease in DNA-binding activity of the complex. We have demonstrated that nfya-1 functions is necessary for proper vulval development, and the role of the NFY complex may be to regulate gene expression necessary for proper 1° cell fate divisions and perhaps secondary signaling to VPCs destined for 2° or non-vulval cell fates. Nfya-1 functions to inhibit vulval cell fates and reduction of nfya-1 function is sufficient to observe Pvl phenotypes in knockout animals and RNAi enhances vulval cell divisions in Ras gain-of-function mutants.
The identification of NFYA-1 as an effector of Ras signaling in C. elegans provides novel insight into the connection between general signaling pathways and specific gene expression changes required for cell fate specification. Ras signaling is known to activate and promote nuclear translocation of ERK/MAP kinase, however, the delicate regulation of the appropriate team of substrates is important to understand as we determine the mechanisms necessary for organogenesis. While LIN-1 acts as a primary target for ERK MAP kinase during vulval development, NFYA-1 appears to act in a similar ancillary role. The Nfy-1 complex has been shown to primarily act as an inhibitor of gene expression, acting through histone assembly and through binding of transcriptional repressor proteins, and in C. elegans, the egl-5 gene has been shown to be regulated by nfya-1 [17]. Egl-5 is a member of a Hox gene cluster including lin-39 and ceh-13, and negative regulation by nfya-1 may be a mechanism to ensure proper vulval development. Lin-39 is a homeotic gene known to integrate cellular signaling pathways and activates the 2° cell fate in VPCs during vulval development [28]. Tbx-2 genes have been shown to function to specify cell fates during organogenesis in multicellular animals, and the C. elegans gene tbx-2 has been shown to be repressed by the Nfy-1 complex [19,29].
In animals, the NF-Y complex has been shown to regulate many genes involved in cell-cycle regulation, and has a clear role in cell proliferation and cancer development [30]. CCAAT-box elements are often over-represented in the promoters of genes that are over-expressed in many cancers, and upregulation of NF-Y target genes has been correlated with poor clinical outcomes [31]. While the NF-Y complex is relatively ubiquitous, the activation of the Ras signaling pathway is tightly controlled and specific. Phosphorylation of NFYA-1 by ERK MAP kinase connects the developmentally precise Ras signaling pathway with a global effector of cell proliferation and provides a mechanism by which linear cytoplasmic signals can result in broad nuclear regulation of gene expression. Both the NF-Y complex and the Ras signaling pathway play a fundamental role in cell proliferation and oncogenesis and the connection between the two is an important area for future research to determine how they rely on each other to elicit specific proliferative developmental fates and how mutation may lead to dysfunction of this connection.
Materials and Methods
Protein Production and MAP kinase assays
Nfya-1, nfyb-1, and nfyc-1 full-length cDNAs were obtained from Yuji Kohara, cloned into pBluescript, and confirmed by DNA sequencing. cDNAs were cloned into pMAL-KK-1 vectors (New England Biolabs) downstream of the maltose binding protein (MBP) region. MBP expression constructs were transformed into E. coli BL21 (DE3) and growth was performed in liquid LB for 2 h with shaking at 37 °C. This culture was cooled on ice, protein expression was induced upon the addition of isopropyl 1-thio-β-d-galactopyranoside to a final concentration of 0.1 mM and shaking at 15 °C for 8 h, and the bacteria was pelleted and stored at −80 °C. The MBP-Nfy proteins were purified using a standard procedure for purifying MBP fusion proteins [32]using a buffer consisting of 20 mM Tris, pH 7.5, 200 mM NaCl, 1 mM EDTA, 1 mM DTT. The resulting protein was frozen in liquid nitrogen and stored at −80°C.
Assays of ERK Activity
Purified, recombinant murine ERK2 (New England Biolabs) is produced in E. coli containing constitutively active MEK and, thus, is phosphorylated and active. Assays were performed as described previously [12]. A 50-μl reaction contained 100 μM ATP to which was added 0.79 μCi of [32P]ATP (4500 Ci/mmol) and 1 unit of ERK2. Reactions were terminated after 15 min at 30 °C. To quantify phosphorylation, we measured radioactive protein bound to phosphocellulose paper (P81, Whatman) using a scintillation counter. Purified myelin basic protein was purchased from Sigma.
Electrophoretic mobility shift assay:
Double-stranded DNA was created by annealing two single-stranded oligonucleotides. Wild-type oligonucleotides were 5’ AGTTGTAGGCCTCTGCTTCCTGACCAATCTACAGAATAGGCTCCGCCTTC and 5’-AGTTGAAGGCGGAGCCTATTCTGTAGATTGGTCAGGAAGCAGAGGCCTAC. Mutant oligonucleotides were 5’-AGTTGTAGGCCTCTGCTTCCTGACATGTCTACAGAATAGGCTCCGCCTTC and 5’-AGTTGAAGGCGGAGCCTATTCTGTAGACATGTCAGGAAGCAGAGGCCTAC [18]. DNA was radioactively labeled by using DNA polymerase I Klenow (New England Biolabs) to fill the 3’ single-stranded overhang with [α−32P]dATP. Radiolabeled DNA was purified by Qiaquick Nucleotide Removal Kit (Qiagen). We incubated ~30,000 cpm of radio- labeled DNA, various amounts of protein, and 1x reaction buffer (5 mm Tris-HCl, pH 7.5, 50 mm NaCl, 1 mm MgCl2, 0.5 mm EDTA, 5% glycerol) in a total volume of 12.5 ul for 20 min on ice or at room temperature. Samples were fractionated using a 6% polyacrylamide gel (30% acrylamide/ 0.8% bis-acrylamide) with 0.5xTBE [33]. Gels were dried and exposed to film or a Kodak PhosphorScreen (Molecular Dynamics, Sunnyvale, CA). The PhosphorScreen was scanned using a Molecular Imager FX (Bio-Rad, Richmond, CA) and analyzed with Quantity One 4.1.0 (Bio-Rad) software.
Reporter gene assays in mammalian cultured cells
The MC3T3 mouse fibroblast cell line was grown in alpha minimum essential medium (a-MEM) with 10% fetal calf serum and transfected as described previously by Fantz et al. with (1) the pFR-LUC reporter plasmid (Stratagene) and the pHRG-LUC1 Renilla luciferase control reporter (Promega), (2) an expression plasmid that encodes GAL4 DNA-binding domain (G4) alone or fused to a construct of nfya-1 with the G4 replacing the CCAAT-box, or Elk-1 (pFA-Elk, Stratagene), and (3) and in some cases the constitutively active MEK plasmid (25ng/well) (pFC-MEK1 Stratagene) [12]. Cells were treated with 5% glycerol 1 day after transfection, incubated an additional 2 days, and harvested for cell lysates; luciferase activity was quantified using the dual-luciferase system of reagents (Promega). Cells were exposed to 50 mg/ml recombinant basic fibroblast growth factor (bFGF) (Sigma) for 20 hr beginning 2 days post-transfection. Site-directed mutagenesis was used to generate mut. D (K280A, K283A, L287A, L289A). Reporter luciferase measurements were normalized for transfection efficiency using the corresponding control Renilla luciferase measurement.
Phenotypic analysis:
Alleles were obtained from the CGC or the C. elegans Reverse Genetics Core Facility at the University of British Columbia (nfya-1(ok1174)). Pvul phenotypes were analyzed using standard culture techniques on NGM plates seeded with the E.coli OP50 strain. RNAi feeding analysis was performed using standard techniques on solid media using the E. coli HT115(DE3) strain and induced with 1mM IPTG [34]. RNAi strains were obtained from OpenBiosystems [35].
Highlights.
The NFYA-1 transcription factor acts as a substrate of ERK MAP kinase
Nfya-1 mutation affects vulval in Caenorhabditis elegans
NFYA-1 binds to a CAAT-box element
The DNA-binding activity of NFYA-1 is mediated by Ras signaling
Acknowledgements
Kerry Kornfeld, David Jacobs
Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440) and the C. elegans Reverse Genetics Core Facility at the University of British Columbia. The Kohara lab provided cDNAs, and the Hope Lab provided GFP strains. We thank Dave Jacobs from the Kornfeld lab for background research and advice.
Funding
This work was supported by the National Institutes of Health [grant number 1R15GM081832–01]; and the Agnes Scott College Julia Gary Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Credit Author Statement:
Segen Aklilu: Investigation, Methodology Michelle Krakowiak: Investigation, Methodology Abena Frempong: Investigation, Methodology Katherine Wilson: Investigation, Methodology Visualization Christy Powers: Investigation, Methodology Douglas Fantz: Conceptualization, Supervision, Funding Acquisition, Writing
References
- [1].Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. [DOI] [PubMed] [Google Scholar]
- [2].Schaeffer HJ, Weber MJ. Mitogen-Activated Protein Kinases: Specific Messages from Ubiquitous Messengers. Mol Cell Biol. 1999;19:2435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kornfeld K Vulval development in Caenorhabditis elegans. Trends Genet. 1997;13:55–61. [DOI] [PubMed] [Google Scholar]
- [4].Sternberg PW, Han M. Genetics of RAS signaling in C. elegans. Trends Genet. 1998;14:466–73. [DOI] [PubMed] [Google Scholar]
- [5].Schmid T, Hajnal A. Signal transduction during C. elegans vulval development: a NeverEnding story. Curr Opin Genet Dev. 2015;32:1–9. [DOI] [PubMed] [Google Scholar]
- [6].Sternberg PW. (2005). WormBook Jun 25:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Underwood RS, Deng Y, Greenwald. Integration of EGFR and LIN-12/Notch Signaling by LIN-1/Elk1, the Cdk8 Kinase Module, and SUR-2/Med23 in Vulval Precursor Cell Fate Patterning in Caenorhabditis elegans. Genetics. 2017;207:1473–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Beitel GJ, Tuck S, Greenwald I, Horvitz HR. The Caenorhabditis elegans gene lin-1 encodes an ETS-domain protein and defines a branch of the vulval induction pathway. Gen Dev. 1995;9:3149–62. [DOI] [PubMed] [Google Scholar]
- [9].Jacobs D, Glossip D, Xing H, Muslin AJ, Kornfeld K. Multiple docking sites on substrate proteins form a modular system that mediates recognition by ERK MAP kinase. Genes Dev. 1999;13:163–75. [PMC free article] [PubMed] [Google Scholar]
- [10].Arur S, Ohmachi M, Nayak S, Hayes M, Miranda A, Hay A, Golden A, Schedl T. Multiple ERK substrates execute single biological processes in Caenorhabditis elegans germ-line development. PNAS. 2009;106:4776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jacobs D, Beitel GJ, Clark SG, Horvitz, HR, Kornfeld K. Gain-of-function mutations in the Caenorhabditis elegans lin-1 ETS gene identify a C-terminal regulatory domain phosphorylated by ERK MAP kinase. Genetics. 1998;149:1809–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fantz DA, Jacobs D, Glossip D, Kornfeld K. Docking sites on substrate proteins direct extracellular signal-regulated kinase to phosphorylate specific residues. J. Biol Chem 2001;276:27256–65. [DOI] [PubMed] [Google Scholar]
- [13].Bardwell AJ, Frankson E, Bardwell L. Selectivity of docking sites in MAPK kinases. J Biol Chem. 2009;284:13165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Unal EB, Uhlitz F, Bluthgen N. A compendium of ERK targets. FEBS Letters. 2017;591:2607–15. [DOI] [PubMed] [Google Scholar]
- [15].Seger R and Krebs EG. The MAPK signaling cascade. FASEB J. 1996;9:726–35. [PubMed] [Google Scholar]
- [16].Walhout AJM, Sordella R, Lu X, Hartley JL, Temple GF, Brasch MA, Thierry-Mieg N, Vidal M. Protein Interaction Mapping in C. elegans Using Protein Involved in Vulval Development. Science. 2000;287:116–22. [DOI] [PubMed] [Google Scholar]
- [17].Yamaguchi M, Saheb Ali Md, Yoshioka Y, Linh Ly L, Yoshida H. NF-Y in invertebrates. BBA – Gene Regulatory Mech. 2017;1860:630–5. [DOI] [PubMed] [Google Scholar]
- [18].Deng H, Sun Y, Zhang Y, Luo X, Hou W, Yan L, Chen Y, Tian E, Han J, Zhang H. Transcription factor NFY globally represses the expression of the C. elegans Hox gene Abdominal-B homolog egl-5. Dev Bio. 2007;308:583–92. [DOI] [PubMed] [Google Scholar]
- [19].Milton AC, Packard AV, Clary L, Okkema PG. The NF-Y complex negatively regulates Caenorhabditis elegans tbx-2 expression. Dev Biol. 2013;382:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tharyan RG, Annibal A, Schiffer I, Laboy R, Atanassov I, Weber AL, Gerisch B, Antebi A. NFYB-1 regulates mitochondrial function and longevity via lysosomal prosaposin. Nature Met. 2020;2:387–96. [DOI] [PubMed] [Google Scholar]
- [21].Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb MH, Shaw PE. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO. 1995;15:951–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Leight ER, Murphy JT, Fantz DA, Pepin D, Schneider DL, Ratliff TM, Mohammad DH, Herman MA, Kornfeld K. Conversion of the LIN-1 ETS protein of Caenorhabditis elegans from a SUMOylated transcriptional repressor to a phosphorylated transcriptional activator. Genetics. 2015;199:761–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Eisenmann DM, Kim SK. Protruding Vulva Mutants Identify Novel Loci and Wnt Signaling Factors That Function During Caenorhabditis elegans Vulval Development. Genetics. 2000;156:1097–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fay DS, Yochem J. The SynMuv genes of Caenorhabditis elegans in vulval development and beyond. Dev Biol. 2007;306:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tiensuu T, Larsen MK, Vernersson E, Tuck S. lin-1 has both positive and negative functions in specifying multiple cell fates induced by Ras/MAP kinase signaling in C. elegans. Dev Biol. 2005;286:338–51. [DOI] [PubMed] [Google Scholar]
- [26].Hope IA, Stevens J, Garner A, Hayes J, Cheo DL, Brasch MA, Vidal M. Feasibility of Genome-Scale Construction of Promoter::Reporter Gene Fusions for Expression in Caenorhabditis elegans Using a MultiSite Gateway Recombination System. Genome Res. 2004;14:2070–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dupuy D, Bertin N, Hidalgo CA, Venkatesan K, Tu D, Lee D, Rosenberg J, Svrzikapa N, Blanc A, Carnec A, Carvunis AR, Pulak R, Shingles J, Reece-Hoyes J, Hunt-Newbury R, Viveiros R, Mohler WA, Tasan M, Roth FP, Le Peuch C, Hope IA, Johnsen R, Moerman DG, Barabási AL, Baillie D, Vidal M. Genome-scale analysis of in vivo spatiotemporal promoter activity in Caenorhabditis elegans. Nat Biotechnol. 2007;25:663–8. [DOI] [PubMed] [Google Scholar]
- [28].Clark SG, Chisholm AD, Horvitz HR. Control of cell fates in the central body region of C. elegans by the homeobox gene lin-39. Cell. 1993;17:43–55. [DOI] [PubMed] [Google Scholar]
- [29].Milton AC, Okkema PG. Caenorhabditis elegans TBX-2 Directly Regulates Its Own Expression in a Negative Autoregulatory Loop. G3. 2015;5:1177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gurtner A, Manni I, Piaggio G. NF-Y in cancer: Impact on cell transformation of a gene essential for proliferation. Biochem Biophys Acta. 2017;1860:604–16. [DOI] [PubMed] [Google Scholar]
- [31].Yi G, Zhao H, Wang L, Wang Y, Guo X, Xu B. The animal nuclear factor Y: an enigmatic and important heterotrimeric transcription factor. Am J Cancer Res. 2018;8:1106–25. [PMC free article] [PubMed] [Google Scholar]
- [32].Mercer KB, Miller RK, Tinley TL, Sheth S, Qadota H, Benian GM. Caenorhabditis elegans UNC-96 is a new component of M-lines that interacts with UNC-98 and paramyosin and is required in adult muscle for assembly and/or maintenance of thick filaments. Mol. Biol. Cell 2006;17:3832–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sambrook J, Fritsch EE, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- [34].Ahringer J, Wnt signaling (June 25, 2005), WormBook, ed. The C. elegans Research Community, WormBook, doi/ 10.1895/wormbook.1.7.1, http://www.wormbook.org. [DOI] [Google Scholar]
- [35].Rual JF, Ceron J, Koreth J, Hao T, Nicot AS, HIrozane-Kishikawa T, Vandenhaute J, Orkin SH, Hill DE, van den Heuvel S, Vidal M. Toward Improving Caenorhabditis elegans Phenome Mapping With an ORFeome-Based RNAi Library. Genome Res. 2004;14:2162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


