Abstract
Brain-derived neurotrophic factor (BDNF) is implicated in the pathology of major depression and influences the inflammatory response. Prolonged immune system activation can cause depression symptoms, and individuals with low BDNF expression may be vulnerable to inflammation-induced depression. We tested the hypothesis that BDNF deficient mice are vulnerable to the induction of depressive-like behavior following peripheral immune challenge. BDNF heterozygous (BDNF+/−) or wild-type (BDNF+/+) littermate mice were injected intraperitoneally (i.p.) with endotoxin (lipopolysaccharide, LPS) to trigger an acute pro-inflammatory response. After resolution of the acute sickness response, central expression of inflammatory genes, kynurenine metabolites, and depressive-like behaviors across multiple dimensions (symptoms) were measured. BDNF+/− mice displayed an exaggerated neuroinflammatory response following peripheral immune challenge. Pro-inflammatory cytokines interleukin-1β (IL-1β), tumor necrosis factor α (TNFα) and interleukin-6 (IL-6) were overexpressed in BDNF+/− mice relative to BDNF+/+ littermate control mice. While behavioral despair and anxiety-like behavior was not different between genotypes, LPS-induced anhedonia-like behavior was significantly more pronounced in BDNF+/− mice relative to BDNF+/+ mice. The kynurenine pathway mediates the many depressive-like behavioral effects of peripheral LPS, and similar to pro-inflammatory cytokine gene expression, indoleamine 2,3-dioxygenase (IDO) expression and kynurenine metabolism was exaggerated in BDNF+/− mice. Genetic BDNF deficiency results in a dysregulated neuroinflammatory and metabolic response to peripheral immune challenge and in a specific vulnerability to the development of inflammation-induced anhedonia.
Keywords: BDNF, neuroinflammation, anhedonia, kynurenine, depressive-like behavior, LPS
1. Introduction
Though there is a widely recognized correlation between inflammation and depression, mounting evidence suggests that pro-inflammatory processes may directly contribute to the pathogenesis of depression and the development of symptoms (Miller and Raison, 2016). Experimental data in healthy human volunteers and preclinical rodent models have demonstrated that peripheral endotoxin challenge results in depression-related symptoms and an associated inflammatory response (Eisenberger et al., 2010; Grigoleit et al., 2011; Inagaki et al., 2012; Jain et al., 2001; Lacosta et al., 1999; Yirmiya, 1996). Interferon α (IFNα) immunotherapy also precipitates depression symptom development, associated with increased pro-inflammatory markers and altered kynurenine metabolism(Raison et al., 2010; Walker et al., 2013; Wichers et al., 2005). Kynurenine metabolic alterations have been associated both with mood disorders and inflammation, providing a potential mechanism underlying inflammation-related depression (Parrott and O’Connor, 2015; Schwarcz et al., 2012). Despite the evidence implicating immune activation as a causative factor in the pathogenesis of depression, not all patients develop the same symptoms in response to inflammatory conditions (Evans et al., 2005). Therefore, vulnerability factors likely play a critical role in determining the “depressogenic liability” of inflammation. Identifying and understanding these factors is paramount to cultivating more efficacious therapeutic strategies for patients at risk for developing inflammation-associated depression.
Brain-derived neurotrophic factor (BDNF) is a well characterized depression vulnerability factor. BDNF expression is often decreased in depressed patients, increases in parallel to successful antidepressant treatment in patients, and a functional BDNF single nucleotide polymorphism (Val66Met) that reduces the release of BDNF from neurons has been associated with depression and depressive symptoms (Chen et al., 2001; Duric and Duman, 2013; Schumacher et al., 2005; Sen et al., 2008). In the brain, BDNF supports survival of existing neurons in addition to facilitating proliferation, differentiation and synaptogenesis of newborn neurons (D’Sa and Duman, 2002; Gonzalez et al., 2016). Studies suggest that the BDNF system may act as a negative regulator of pro-inflammatory responses. In vitro, BDNF application suppressed primary microglia pro-inflammatory responses (Neumann et al., 1998), and in vivo, BDNF artificial up-regulation was anti-inflammatory and even neuroprotective during neuroinflammation (Chen et al., 2015; Jiang et al., 2011). Conversely, pro-inflammatory cytokines suppress BDNF levels both in vitro and in vivo during neuroinflammation (Lapchak et al., 1993; Schulte-Herbrüggen et al., 2005; Tong et al., 2008). Together, these results suggest that BDNF is a negative regulator of pro-inflammatory cytokine expression and can in-turn be downregulated by their up-regulation. During IFNα immunotherapy, both the Val66Met BDNF polymorphism genotype and lower baseline BDNF levels were associated with increased scores on the Montgomery-Asberg Depression Rating Scale (MADRS) (Lotrich et al., 2013), providing evidence that BDNF levels may be an indicator of a ‘depressogenic liability” to inflammation. However, whether disruption of the BDNF system engenders a vulnerability to the development of preclinical inflammation-induced depressive-like behaviors and the resulting neuroinflammatory response has not been directly investigated.
To understand this potential gene (BDNF) x environment (peripheral inflammation) interaction, we conducted a series of experiments using the well characterized preclinical model of LPS-induced depressive-like behavior. In human volunteers and in rodents, acute peripheral administration of endotoxin (LPS) precipitates the development of increased anhedonia, anxiety-like behavior, behavioral despair, and impaired cognitive function (Eisenberger et al., 2010; Grigoleit et al., 2011; Inagaki et al., 2012; Konsman et al., 2000; Lacosta et al., 1999). In this study, the behavioral and neuroinflammatory response to intraperitoneal (i.p.) LPS challenge was measured in male BDNF deficient (BDNF+/−) mice or their wild-type (BDNF+/+) littermates. These data are the first to indicate that basal BDNF expression may negatively regulate the development of anhedonia (sucrose preference), locomotor activity and components of the central neuroinflammatory response following peripheral immune challenge. Within the context of inflammation, a functional disruption of BDNF expression is indeed a likely vulnerability factor for the development of a specific subset of depression symptoms.
2. Methods and Materials
2.1. Animals:
All animal care and use was carried out in accord with the Guide for the Care and Use of Laboratory Animals, 8th edition (NRC) and approved by the Institutional Animal Care and Use Committee at The University of Texas Health Science Center at San Antonio. Male BDNF heterozygous (BDNF+/−) mice with an approximate 50% reduction in BDNF transcripts (Ernfors et al., 1994) and 57%−65% expression level of BDNF protein within discrete brain regions (Chourbaji et al., 2004) and wild-type littermate control (BDNF+/+) mice were obtained from an in-house breeding colony consisting of C57BL/6J female and B6.129S4-Bdnftm1Jae/J male breeding pairs (Jackson Laboratory, Bar Harbor, ME; stock# 000664 and 002266 respectively). BDNF+/− exhibit baseline brain monoamine levels and emotional behavior that is not different that WT littermate controls (Chourbaji et al., 2004) The genotype of each mouse was determined by a standard PCR reaction described in detail by Jackson Laboratory (https://www2.jax.org/protocolsdb/f?p=116:2:0::NO:2:P2_MASTER_PROTOCOL_ID,P2_JRS_CODE:339,002266). Male mice 10–16 weeks of age (adults, n=20–23 per treatment group) were used in all experiments during which all mice were individually housed in standard shoebox cages to facilitate individual mouse measurement of sucrose preference and maintain continuity with our previously published data (Parrott et al., 2016; Salazar et al., 2012). All cages were equipped with microisolator tops and ad libitumfood and water access. Two weeks prior to testing, mice were maintained on a modified 12:12 h reverse light cycle (lights on at 23:00–11:00). Only male mice were used in the current study to maintain continuity with our previous behavioral studies in LPS challenged mice. It is noteworthy that when female mice were tested in pilot studies, several baseline phenotypic differences were observed that will be the subject of follow-up investigation.
2.2. Treatment:
Endotoxin (LPS) isolated from E. coli (L-3129, serotype 0127:B8) was purchased from Sigma (St. Louis, MO), and dissolved in sterile 0.9% saline on the morning of injections. LPS, at a dose of 0.83mg/kg, or saline (vehicle) was administered (i.p.) to BDNF+/+ or BDNF+/− mice. Injections were performed within the first hour of the dark cycle (11:00–12:00), 24hr prior to behavioral assessments also performed within the first hour of the dark cycle.
2.3. General Health:
The general health and vitality of the mice were monitored by veterinary technicians or research staff by daily visual inspection. At least one week prior to treatment, mice were gently handled by the experimenter to minimize stress associated with the environmental aspects of behavioral testing. Additionally, body and food weights were recorded daily during the experiment to track the physiological response to treatment.
2.4. Locomotor Function and Open Field Test:
Exploratory locomotor activity (eLMA) was assessed, at the indicated times (5 minutes prior to, 2h following and 24h following injections), by placing mice in a dimly lit (~5 lux) open field (OF) chamber for five minutes and measuring the total horizontal distance traveled (Ethovision XT 7.1, Noldus, Leesburg, VA). The chamber was cleaned with 70% ethanol after each individual test. In addition to measuring eLMA in the OF, the duration of time spent in the central area was measured as an index of anxiety-like behavior, as previously described (Salazar et al., 2012).
2.5. Forced Swim Test:
The duration of immobility in the forced swim test (FST) was measured during the first two hours of the dark light cycle (24h following injections), similar to previously described (O’Connor et al., 2009). Water temperature was maintained at 24 ± 0.5 °C and changed after each mouse. Immobility during a six-minute test was determined using the mobility function of Ethovision XT (7.1) tracking software.
2.6. Sucrose Preference Test:
Sucrose preference (SP) was measured as previously described using a 2-bottle testing paradigm with a 1% sucrose solution and water (Salazar et al., 2012). Training (with both bottles) began three days prior to treatment and testing continued for 24 hours after treatment. Preference was calculated as (sucrose intake)/(water intake + sucrose intake)*100.
2.7. Tissue Sample Collection:
Following behavior at 24h post saline or LPS injections, mice were euthanized and blood was collected for analysis of plasma. Following heparinized-saline perfusion, brain tissues were collected, immediately frozen in liquid nitrogen and stored at −80°C with plasma samples. Prior to analysis, brain tissue was homogenized (mortar-pestle) on dry ice and divided evenly into two samples for mRNA and metabolite analysis (described below).
2.8. RNA Isolation and Real-Time RT-PCR:
To determine the impact of treatment and genotype on central mRNA expression changes, brain tissue samples were analyzed using real-time RT-PCR as previously described (Heisler and O’Connor, 2015). Real-time RT-PCR was performed over 40 cycles using a CFX384™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA) and pre-validated Taqman® Gene Expression Assays (Life Technologies) for both target and housekeeping control genes: Gapdh (GAPDH, Mm99999915_g1), Itgam (CD11b, Mm00434455_m1), Il1b (IL-1β, Mm01336189_m1), Tnfa (TNFα, Mm00443258_m1), Il6 (IL-6, Mm00446190_m1), Il10 (IL-10, Mm0043614_m1), Ido1 (IDO, Mm00492586_m1). Data are expressed as relative fold change (Target ΔmRNA) using the 2-ΔΔCt calculation method and GAPDH as the housekeeping gene as previously described (O’Connor et al., 2005).
2.9. Luminex® 26-Plex Immunoassay:
Plasma samples were assayed for cytokine and chemokine protein levels using a mouse Affymetrix ProcartaPlex 26-plex cytokine and chemokine panel (eBioscience Inc, San Diego, CA). The panels were analyzed using a Luminex® Flexmap 3D System (Millipore, Billerica, MA) and conducted by the Bioanalytics and Single-Cell Core Facility at the University of Texas Health Science Center at San Antonio. Targets included in the immunoassay include: IFN gamma; IL-12p70; IL-13; IL-1 beta; IL-2; IL-4; IL-5; IL-6; TNF alpha; GM-CSF; IL-18; IL-10; IL-17A; IL-22; IL-23; IL-27; IL-9; GRO alpha; IP-10; MCP-1; MCP-3; MIP-1 alpha; MIP-1 beta; MIP-2; RANTES; Eotaxin. Only those analytes exhibiting either a main effect (treatment, genotype) or interaction are shown, while unchanged analytes are not shown.
2.10. High Performance Liquid Chromatography:
Plasma samples were additionally analyzed for tryptophan and kynurenine using high performance liquid chromatography (HPLC) with electrochemical detection as previously described (Heisler and O’Connor, 2015). Following preparation, samples were analyzed using a BAS 502 isocratic liquid chromatographic system (Bioanalytical Systems, West Lafayette, IN) with an Ag/AgCl reference electrode and 3 mm glassy carbon electrode at +950 mV.
2.11. Liquid Chromatography/Mass Spectrometry:
Brain tissue samples were also prepared for liquid chromatography/mass spectrometry (LC/MS) as previously described (Lawson et al., 2013; Walker et al., 2013) and analyzed for kynurenine metabolites, including tryptophan, serotonin, 5-hydroxyindoleacetic acid, kynurenine, 3-hydroxykynurenine, kynurenic acid, 3-hydroxyanthranilic acid, xanthurenic acid, quinolinic acid, picolinic acid. Following preparation, samples were analyzed on a Q Exactive mass spectrometer (Thermo Fisher Scientific, Waltham, MA) with on-line separation by a Dionex UltiMate 3000 HPLC system (Thermo Fisher Scientific) and the data collected was analyzed using Thermo Xcalibur 2.2 software (Thermo Fisher Scientific) in the Mass Spectrometry Core Facility at the University of Texas Health Science Center at San Antonio. The chromatographic peaks for quinolinic acid were collected during a second run of the same samples using selective ion monitoring to improve sensitivity. 3-hydroxyindoleacetic acid was not reliably detected and picolinic acid was not changed under any experimental conditions, so those data are not shown.
2.12. Experimental Design and Statistical Analysis:
The experiments were arranged in a factorial design with 2 factors (genotype x treatment), and testing was performed in a complete randomized blocks. All data were analyzed using SigmaPlot 12.0 statistical software (Systat Software Inc., San Jose, CA) and represent group means ± standard error of the mean (SEM). A two- or three-way analysis of variance (ANOVA) was conducted, following a single pass Chauvenet’s test to identify outliers (Taylor, 1997) as previously described (Heisler and O’Connor, 2015). When the ANOVA analysis identified a significant interaction, the data were further analyzed (post-hoc) with the Holm-Sidak correction for pairwise multiple comparisons to identify significant between-group differences. Significant main effects (p<0.05, genotype or treatment) and multiplicity adjusted P values with family-wise alpha threshold of 0.01 for between-group comparisons between saline- and LPS-treated groups within the same genotype (BDNF+/+ or BDNF+/−) are denoted as such (*) i.e. (*p<0.05–0.01 **p<0.05–0.01 ***p<0.05–0.01). Likewise, significant comparisons (p<0.05) between similarly-treated (saline or LPS) BDNF+/+ and BDNF+/− groups are denoted as such (+) i.e. (+p<0.05–0.01 ++ p<0.05–0.01 +++ p<0.05–0.01).
3. Results
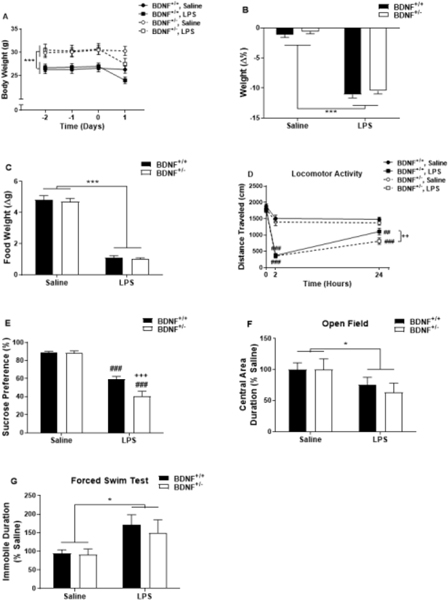
3.1. BDNF deficient mice are more susceptible to the locomotor reducing effects of peripherally administered lipopolysaccharide.
Whether BDNF plays a similar pertinent role in inflammation-induced depression remains unknown (Duman, 2009; Taliaz et al., 2011). Body weight, food weight and locomotor activity of BDNF+/− and BDNF+/+ mice were recorded prior to and following treatment to assess the behavioral sickness response to LPS. While BDNF+/− mice were significantly heavier than BDNF+/+ mice (Figure 1A, genotype, F1,328=61.16, p<0.001) at the time of treatment, Figure 1B indicates that the LPS-induced reduction in body weight 24h post-LPS was similar between BDNF+/− and BDNF+/+ mice (treatment, F1,82=455.19, p<0.001). Paralleling the observed reduction in body weight, both genotypes exhibited a similar diminished food intake (Figure 1C) during the 24h period following LPS treatment (treatment, F1,75=301.20, p<0.001).
Figure 1.
BDNF deficient mice have a similar LPS-induced sickness response as WT mice but are more susceptible to the locomotor reducing effects and sucrose preference behavioral response of LPS. (A and B) Body weight was recorded prior to and following peripheral LPS administration. LPS induced a reduction in body weight in the 24h following injections in both genotypes (BDNF+/+ and BDNF+/−). (C) Food weight was also recorded, and a reduction was induced in both genotypes following LPS treatment. (D) Locomotor activity (distance traveled in an open field) was recorded for 5 minutes prior to, 2h- and 24h-post LPS treatment. Data represent sample means ± SEM and were analyzed using a two- or three-way ANOVA followed by the Holm-Sidak method for pairwise multiple comparisons. (E) After training, BDNF+/− and BDNF+/+ mice were subjected to a two-bottle (1% sucrose and water) preference test for 24h following injections. Following LPS administration, activity in the open field (OF) was assessed over a five minute period. Duration spent in the center of the arena was assessed (F, represented as % saline). (F) Duration spent immobile (represented as % saline) was assessed in the forced swim test (FST) at 24h post injections in BDNF+/− and BDNF+/+ mice. n = 5–23 mice/group.
* = main effect (treatment or genotype)
# = post-hoc comparison between saline and LPS within the same genotype (BDNF+/+ or BDNF+/−)
+ = post-hoc comparison between BDNF+/+ and BDNF+/− with the same treatment (saline or LPS)
*,+,# p<0.05–0.01 **,++,## p<0.01–0.001 ***,+++,###p<0.001
LPS induced a time dependent reduction in eLMA assessed in the OF (Figure 1D, time x treatment, F2,160=30.51, p<0.001) prior to, 2h and 24h following treatment. To better understand the effect of genotype and treatment on eLMA, each time point was analyzed individually. Prior to treatment, there was no difference between the genotypes in eLMA and both genotypes experienced a similar reduction in eLMA at 2h post-LPS (treatment, F1,40=169.27, p<0.001). Interestingly, there was main effect of both treatment (F1,72=31.17, p<0.001) and genotype (F1,72=5.95, p=0.017) on eLMA at 24h following treatment. Post-hoc analysis revealed that LPS decreased eLMA at 24h in both genotypes (p=0.002 vs BDNF+/+-saline; p<0.001 vs BDNF+/− saline). However, eLMA of LPS-treated BDNF+/− mice was significantly reduced compared to similarly treated BDNF+/+ mice (p=0.018).
3.2. BDNF deficiency confers susceptibility to the development of sucrose preference behavior following peripheral immune challenge with lipopolysaccharide.
Previous studies from our lab and others have demonstrated characteristic depressive- and anxiety-like behaviors that are precipitated by LPS treatment (Henry et al., 2008; O’Connor et al., 2009; Salazar et al., 2012). Therefore, to determine whether BDNF mediates the development of these behaviors in response to peripheral immune challenge, BDNF+/− and BDNF+/+ mice were tested in various behavioral paradigms 24h post injections. To assess inflammation-induced changes in behavior, mice were tested for sucrose preference in a twobottle testing paradigm (Figure 1E). The sucrose preference of BDNF+/− mice and BDNF+/+ mice were statistically identical following saline treatment (88.58 ± 2.4 vs 88.76 ± 1.6). Peripheral immune challenge with LPS significantly reduced sucrose preference in both genotypes (treatment, F1,42=130.56, p<0.001), and there was a significant treatment by genotype interaction (F1,42=7.41, p=0.009). Post-hoc analysis revealed that the LPS-induced reduction in sucrose preference was more pronounced in BDNF+/− mice than in BDNF+/+ mice (p<0.001). To determine whether a BDNF deficiency leaves mice vulnerable to additional behavioral effects of peripheral LPS, the duration of time spent within the central area of the OF was measured 24h post treatment. Figure 1F shows that LPS reduced the OF central area duration of both BDNF+/− and BDNF+/+ mice (treatment, F1,71=4.67, p=0.034), however, there was not a genotype x treatment interaction. Finally, to determine whether BDNF contributes to the development of behavior in the FST in response to peripheral immune challenge, mice were tested 24h following treatment. Consistent with previous findings, Figure 1G indicates that LPS triggered an increase in the duration of immobility during the FST in both BDNF+/− and BDNF+/+ mice (treatment, F1,23=7.71, p=0.011). However, there was not a significant treatment x genotype interaction.
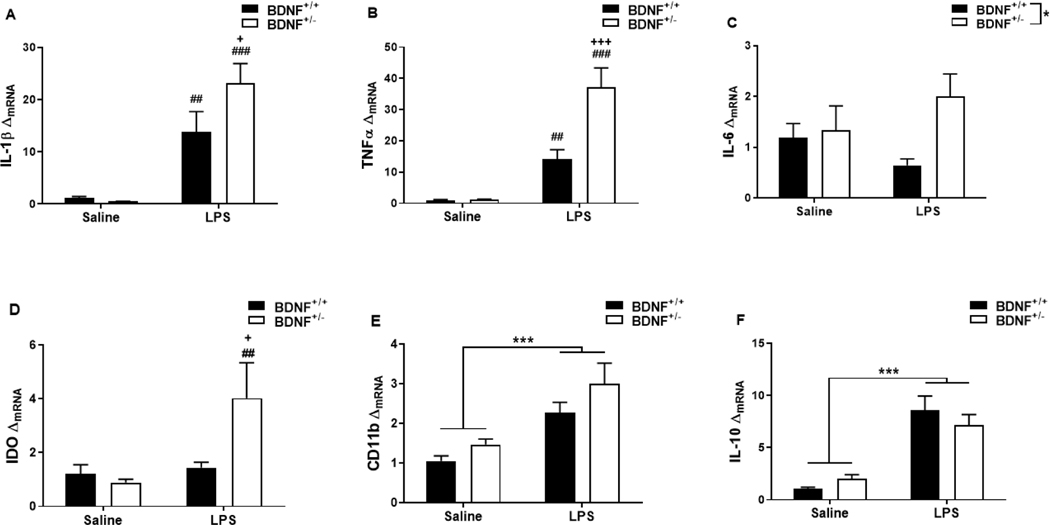
3.3. Peripheral lipopolysaccharide induces an exaggerated central pro-inflammatory cytokine response in BDNF deficient mice.
Activation of microglia and up-regulation of cytokine expression within the brain are essential processes in the depressive-like behavioral response to peripheral immune challenge (Henry et al., 2008; O’Connor et al., 2009). To determine whether these processes were dysregulated in LPS-treated BDNF+/− mice, steady-state mRNA expression of IL-1β, TNFα, IL-6, IDO, CD11b and IL-10 was measured in whole brain samples 24h after treatment. LPS treatment increased mRNA expression of IL-1β (Figure 2A) and there was a trend in the genotype x treatment interaction (F1,25=10.34, p=0.004). LPS treatment resulted in a greater elevation in IL-1β mRNA in BDNF+/− mice than BDNF+/+ mice (p=0.028). The same genotype x treatment effect on the expression pattern was observed for mRNA of pro-inflammatory cytokine TNFα (Figure 2B, F1,23=8.25, p=0.009). BDNF+/− mice also had higher expression of TNFα mRNA than BDNF+/+ mice following LPS treatment (p<0.001). Finally, there was also a genotype x treatment effect on mRNA expression of pro-inflammatory cytokine IL-6 (Figure 2C, F1,25=5.79, p=0.024). Treatment with LPS also resulted in greater up-regulation of IL-6 mRNA in BDNF+/− mice than BDNF+/+ mice (p=0.003). As IDO has also been demonstrated as a mediator of LPS-induced depressive-like behaviors (Heisler and O’Connor, 2015; O’Connor et al., 2009; Salazar et al., 2012), IDO mRNA was measured to extend the characterization of pro-inflammatory expression markers. Interestingly, there was only a main effect of treatment (F1,21=9.69, p=0.005) on IDO expression (Figure 2D). Peripheral immune challenge with LPS caused an up-regulation of CD11b mRNA (Figure 2E, treatment, F1,23=19.82, p<0.001). However, the CD11b mRNA response was not different between BDNF+/− and BDNF+/+ mice. Finally, figure 2F indicates that LPS treatment resulted in a robust up-regulation of IL-10 mRNA expression in both BDNF+/− and BDNF+/+ mice (F1,23=56.30, p<0.001), but there was no difference in the response between genotypes.
Figure 2.
Peripheral LPS induces an exaggerated central pro-inflammatory cytokine response in BDNF deficient mice. Whole brain tissues collected 24h following treatment were used to analyze central mRNA in response to peripheral LPS administration. Panels represent mRNA fold changes (ΔmRNA) of (A) interleukin-1β (IL-1β), (B) tumor necrosis factor α (TNFα), (C) interleukin-6 (IL-6), (D) indoleamine 2,3-dioxygenase (IDO), (E) interleukin-10 (IL-10) and (F) CD11b. Data represent sample means ± SEM. n = 5–8 samples/group.
* = main effect (treatment or genotype)
# = post-hoc comparison between saline and LPS within the same genotype (BDNF+/+ or BDNF+/−)
+ = post-hoc comparison between BDNF+/+ and BDNF+/− with the same treatment (saline or LPS)
*,+,# p<0.05–0.01 **,++,## p<0.01–0.001 ***,+++,###p<0.001
To determine whether this exaggerated pro-inflammatory response occurred specifically within the brain, we analyzed plasma cytokine and chemokine protein levels (Table 1) 24h post LPS treatment. Of the 21 cytokine and chemokine protein targets that were detectable using the Luminex® immunoassay, 15 were elevated by LPS treatment (Table 1). Only plasma interleukin-18 levels exhibited an interaction between genotype and treatment (Table 1, F1,27=5.82, p=0.023), while the remainder of the targets were unaffected by genotype.
Table 1.
Peripheral inflammation induces plasma cytokines and chemokines. Plasma was analyzed using a Luminex® 26-plex immunoassay panel for cytokines and chemokines. Peripheral LPS treatment elevated all of the detectable cytokine or chemokine targets assessed at 24h post treatment, regardless of genotype, except for exotoxin, IL-9, IL-13, IL-27, IL-23 and IL-17A. Data represent sample means (SEM) and were analyzed using a two-ANOVA followed by the Holm-Sidak method for pairwise multiple comparisons. n = 6–9 samples/group.
| Genotype | Main Effects | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|
| Protein Target (pg/mL) | BDNF+/+ | BDNF+/− | Treatment | Genotype | Treatment × Genotype | |||
| Saline | LPS | Saline | LPS | p-value | p-value | |||
| Cytokines | IL-10 | 0.34 (0.2) | 9.23 (2.5) | 0.15 (0.1) | 9.58 (2.7) | p<0.001 | n.s. | n.s. |
| IL-1 β | 0.20 (0.l) | 0.91 (01) | 0.080 (0.05) | 1.15 (0.3) | p< 0.001 | n.s. | n.s. | |
| IL-5 | 4.50 (0.7) | 26.66 (9.5) | 3.91 (1.3) | 14.72 (5.3) | p<0.01 | n.s. | n.s. | |
| IL-6 | 8.62 (2.0) | 161.90 (76.2) | 4.43 (11) | 147.31 (72.0) | p<0.01 | n.s. | n.s. | |
| IL-22 | 17.65 (3.4) | 89.04 (28.7) | 23.24 (5.5) | 272.23 (137.4) | p<0.01 | n.s. | n.s. | |
| TNFα | 1.99 (0.2) | 5.73 (1–1) | 1.85 (0.2) | 7.79 (2.1) | p<0.001 | n.s. | n.s. | |
| IL-18 | 247.07 (38.8) | 1679.50 (331.8)*** | 170.25 (43.0) | 2805.17 (428.4)### +++ | p<0.001 | p<0.05 | p<0.05 | |
| Chemokines | IP-10 | 12.74 (13) | 79.82 (8.0) | 14.41 (1.5) | 93.96 (14.5) | p<0.001 | n.s. | n.s. |
| GROα | 9.08 (5.8) | 31.23 (12.9) | 7.35 (3.0) | 36.35 (10.6) | p<0.01 | n.s. | n.s. | |
| RANTES | 40.82 (10.7) | 619.38 (150.1) | 56.52 (13.6) | 552.39 (209.4) | p< 0.001 | n.s. | n.s. | |
| MIP-1α | 1.21 (0.3) | 5.75 (0–9) | 1.99 (0.8) | 5.33 (0.9) | p<0.001 | n.s. | n.s. | |
| MCP-3 | 47.04 (6.3) | 537.43 (124) | 54.01 (3.2) | 562.74 (23.6) | p<0.001 | n.s. | n.s. | |
| MCP-1 | 19.27 (2.5) | 248.2 (49.7) | 18.01 (1.9) | 379.02 (82.2) | p<0.001 | n.s. | n.s. | |
| MIP-2 | 6.86 (0.4) | 21.55 (3.2) | 7.30 (0.5) | 24.60 (4.7) | p<0.001 | n.s. | n.s. | |
| MIP-1 β | 0.25 (0.08) | 4.97 (12) | 0.42 (0.1) | 3.91 (0.8) | p<0.001 | n.s. | n.s. | |
= main effect (treatment or genotype)
= post-hoc comparison between saline and LPS within the same genotype (BDNF+/+ or BDNF+/−)
= post-hoc comparison between BDNF+/+ and BDNF+/− with the same treatment (saline or LPS)
p<0.05–0.01
p<0.01–0.001
p<0.001
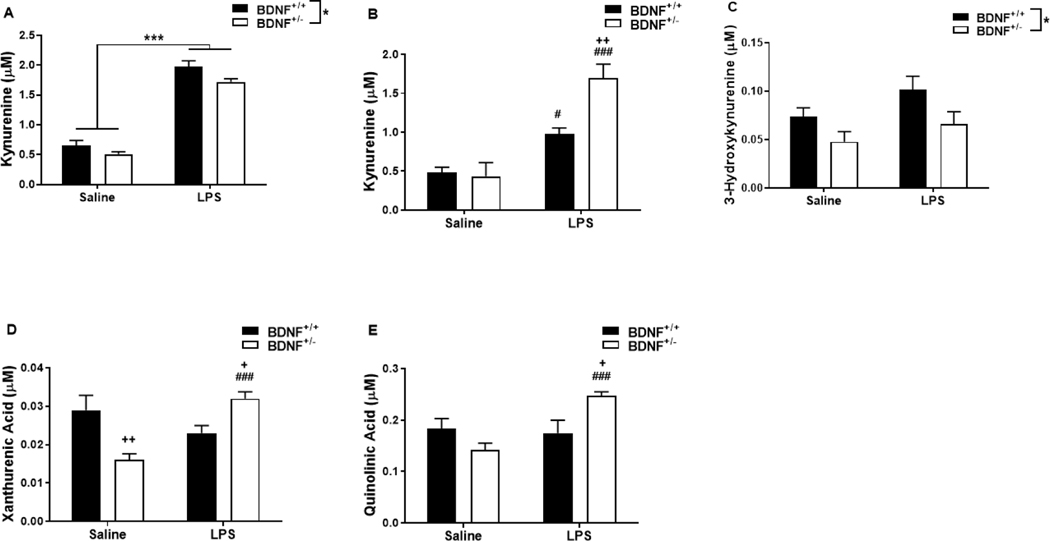
3.4. Peripheral lipopolysaccharide challenge up-regulates central tryptophan metabolism along kynurenine pathway in BDNF deficient mice.
To determine the impact of BDNF expression levels on LPS-induced central tryptophan metabolism, plasma and brain tissue samples were analyzed for kynurenine metabolites 24h following treatment. The concentration of plasma tryptophan (Table 2) was unaffected by treatment and was not different between BDNF+/− and BDNF+/+ mice. While BDNF+/− mice had a lower concentration of plasma kynurenine (Figure 3A, genotype, F1,23=6.47, p=0.018), LPS treatment resulted in a similar increase in both genotypes (treatment, F1,23=261.22, p<0.001). However, there was no genotype x treatment interaction in the concentration of plasma kynurenine. The plasma kynurenine/tryptophan ratio (Table 2), used as an indicator of IDO activity, was elevated by LPS treatment (F1,25=57.91, p<0.001) but was not different between the two genotypes.
Table 2.
Peripheral LPS impacts peripheral and central tryptophan metabolism. Plasma and whole brain tissues collected 24h post-treatment were used to analyze the kynurenine pathway of tryptophan metabolism in response to peripheral immune stimulation. Tryptophan and kynurenine/tryptophan ratio were measured in the plasma (see Figure 3A for plasma kynurenine). Tryptophan, kynurenine/tryptophan ratio, and kynurenic acid (KA) were evaluated in the brain (see Figures 4B, 4C and 4E for brain kynurenine, 3-HK and QA respectively). Data represent sample means (SEM) and were analyzed using a two-ANOVA followed by the Holm-Sidak method for pairwise multiple comparisons. n = 5–8 samples/group.
| Genotype | Main Effects | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|
| BDNF+/+ | BDNF+/− | Treatment | Genotype | Treatment × Genotype | ||||
| Metabolites (nM) | Saline | LPS | Saline | LPS | p-value | p-value | ||
| Plasma | Tryptophan | 53.55 (6.6) | 58.02 (5.4) | 51.82 (4.3) | 50.27 (5.4) | n.s. | n.s. | n.s. |
| Kynurenine/ Tryptophan Ratio | 0.013 (0.0009) | 0.034 (0.002) | 0.012 (0.001) | 0.038 (0.006) | p<0.001 | n.s. | n.s. | |
| Brain | Tryptophan | 46.45 (5.1) | 45.54 (3.4) | 42.95 (1.9) | 55.29 (3.8) | n.s. | n.s. | n.s. |
| Kynurenine/ Tryptophan Ratio | 0.0078 (0.001) | 0.019 (0.0008) | 0.011 (0.004) | 0.032 (0.004) | p<0.001 | p<0.05 | n.s. | |
| KA | 0.46 (0.05) | 0.30 (0.09)* | 0.25 (0.04f | 0.38 (0.03) | n.s. | n.s. | p<0.05 | |
= main effect (treatment or genotype)
= post-hoc comparison between saline and LPS within the same genotype (BDNF+/+ or BDNF+/−)
= post-hoc comparison between BDNF+/+ and BDNF+/− with the same treatment (saline or LPS)
p<0.05–0.01
p<0.01–0.001
p<0.001
Figure 3.
Peripheral LPS challenge up-regulates neurotoxic kynurenine metabolism in BDNF deficient mice. Plasma and whole brain tissues collected 24h after saline or LPS injections and were used to analyze kynurenine pathway metabolites in response to peripheral immune stimulation. Panels represent concentrations (μM) of (A) plasma kynurenine, (B) brain kynurenine, (C) brain 3-hydroxykynurenine (3-HK), (D) brain xanthurenic acid (XA) and (E) brain quinolinic acid (QA). Data represent sample means ± SEM and were analyzed using a two-ANOVA followed by the Holm-Sidak method for pairwise multiple comparisons. n = 5–8 samples/group.
* = main effect (treatment or genotype)
# = post-hoc comparison between saline and LPS within the same genotype (BDNF+/+ or BDNF+/−)
+ = post-hoc comparison between BDNF+/+ and BDNF+/− with the same treatment (saline or LPS)
*,+,# p<0.05–0.01 **,++,## p<0.01–0.001 ***,+++,###p<0.001
As in the plasma, there was no impact of genotype or treatment on the concentration of central tryptophan (Table 2). In contrast to plasma levels, there was a genotype x treatment interaction in the concentration of central kynurenine (Figure 3B, F1,21=8.01, p=0.01). Post-hoc analysis revealed that the central kynurenine concentration increased following LPS treatment in both BDNF+/+ mice (p=0.016 vs saline) and BDNF+/− mice (p<0.001 vs saline), however, it is significantly higher in BDNF+/− mice (p=0.003 vs BDNF+/+-LPS). There was a main effect of both genotype (F1,22=5.63, p=0.027) and treatment (F1,22=25.60, p<0.001) in the central kynurenine/tryptophan ratio (Table 2), though there was no interaction.
To ascertain the influence of BDNF expression on inflammation-induced neurotoxic kynurenine metabolism, downstream metabolites (KA, 3-HK, XA, QA) were assessed in brain tissue samples. There was a genotype x treatment interaction in the concentration of central KA (Table 1, F1,20=7.17, p=0.014) in which the baseline KA concentration in BDNF+/− mice was less than in BDNF+/+ mice (p=0.010) and LPS treatment decreased central KA in BDNF+/+ mice (p=0.038 vs saline). On the opposing branch of kynurenine metabolism, central 3-HK concentration (Figure 3C) was only impacted by a genotype main effect (F1,21=7.27, p=0.014). Downstream of 3-HK, there was a significant genotype x treatment interaction in the concentration of both central XA (Figure 3D, F1,22=15.40, p<0.001) and central QA (Figure 3E, F1,22=10.07, p=0.004). Post-hoc analysis revealed that central XA concentration was decreased in saline-treated BDNF+/−mice (p=0.003 vs BDNF+/+-saline) and significantly elevated in LPS-treated BDNF+/− mice compared to both saline-treated BDNF+/− (p<0.001) and LPS-treated BDNF+/+ (p=0.036) mice. Similarly, central QA concentration was significantly increased in BDNF+/− mice following LPS treatment compared to both saline-treated BDNF+/− (p<0.001) and LPS-treated BDNF+/+ (p=0.012) mice.
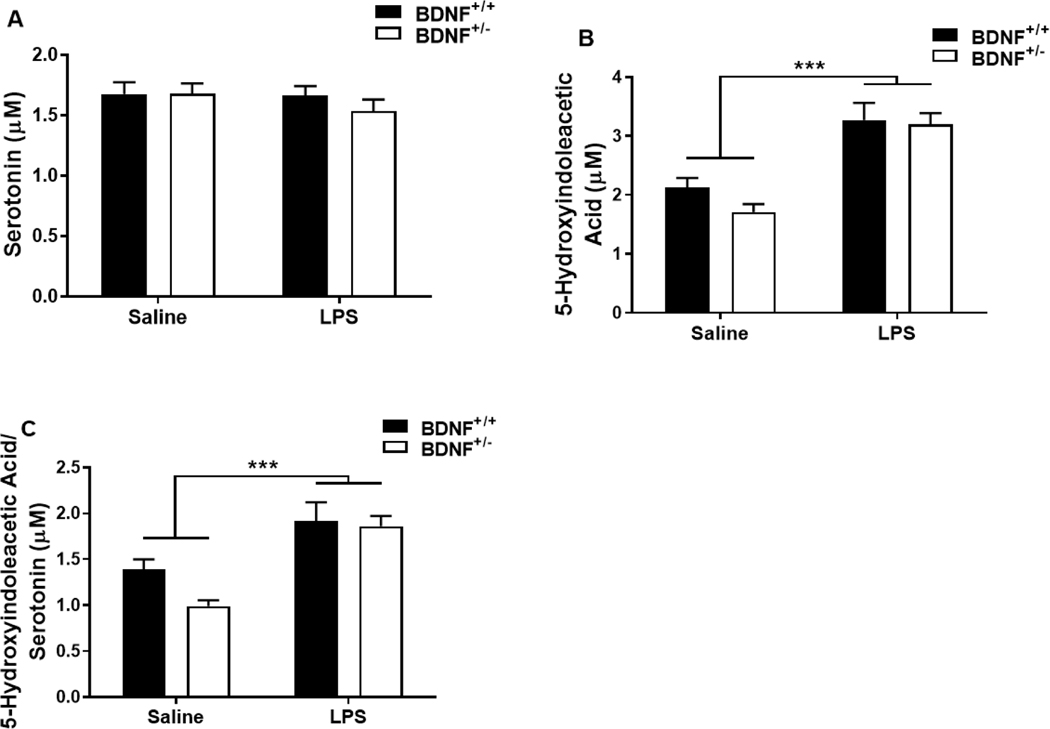
3.5. Peripheral inflammation induced by lipopolysaccharide results in changes in serotonin turnover independent of BDNF deficiency.
To confirm the impact of peripheral inflammation on serotonin turnover in BDNF deficient mice, serotonin and 5-hydroxyindoleacetic acid (5-HIAA) were assessed in brain tissue samples 24h after treatment. Central serotonin concentration (Figure 4A) remained unchanged by LPS treatment and was not different between genotypes. The concentration of central 5-HIAA (Figure 4B) increased in response to LPS treatment (F1,21=45.15, p<0.001), but was not affected by genotype. Serotonin turnover or the 5-HIAA/serotonin ratio (Figure 4C) also significantly higher following LPS treatment (F1,23=33.91, p<0.001) and unaffected by genotype.
Figure 4.
Central serotonin turnover is impacted similarly by peripheral LPS treatment between BDNF heterozygous and control mice. Whole brain tissues collected 24h post injections were analyzed for serotonin metabolites to determine the impact of peripheral inflammation. Panels represent brain concentrations (μM) of (A) serotonin, (B) 5-hydroxyindoleacetic acid (5-HIAA) and (C) 5-HIAA/serotonin ratio. Data represent sample means ± SEM. n = 5–8 samples/group.
* = main effect (treatment or genotype)
*p<0.05–0.01 **p<0.01–0.001 ***p<0.001
4. Discussion
The objective of this study was to determine the consequences of a BDNF deficiency on inflammation-induced depressive-like behaviors and neuroinflammation. Both genotypes (BDNF+/− and BDNF+/+ mice) had a similar physiological sickness response to peripheral inflammation stimulated by lipopolysaccharide (LPS). BDNF+/− mice had a greater reduction in exploratory locomotor activity (eLMA). Both genotypes exhibited a depressive-like behavioral response in the forced swim test (FST) and anxiety-like response in the open field (OF) test. Consistent with our previous reports, these data demonstrate that peripheral inflammation results in a reduction in OF central area duration (Salazar et al., 2012) and increased immobility in the FST (J M Parrott et al., 2016) however, BDNF+/− mice are not more susceptible to the decreased duration spent in the center of the arena as a result of peripheral LPS treatment. Interestingly, BDNF+/− mice had a greater anhedonia-like response in the sucrose preference (SP) test than BDNF+/+ mice following LPS treatment. This was accompanied by an exaggerated increase in central pro-inflammatory cytokine expression, kynurenine, xanthurenic acid (XA), and quinolinic acid (QA) in LPS-treated BDNF+/− mice. Together, these data indicate that BDNF+/− mice specifically have an exaggerated central pro-inflammatory cytokine response following peripheral LPS. Thus, BDNF may exert functional negative regulatory control of pro-inflammatory cytokine expression within the brain in response to peripheral immune inflammatory signals.
The physiological sickness response to LPS, evaluated by body weight and food intake assessed in the 24h period following treatment, was not different between BDNF+/− and BDNF+/+ mice (Figures 1B and 2C). These parameters were not impacted by an initial genotype difference in body weight, as the BDNF+/− mice were heavier than BDNF+/+ mice (Figure 1A), similar to a previously observed trend (Shirayama et al., 2002). Acute sickness behavior can also be assessed by eLMA in the OF at 2h post LPS, which was reduced in both BDNF+/− and BDNF+/+ mice (Figure 1D). 24h following LPS treatment, eLMA was still diminished in both genotypes, though it was significantly lower in BDNF+/− mice than in BDNF+/+ mice (Figure 1D). It is interesting to note that although eLMA was different between genotypes following LPS treatment at 24h (Figure 1D), immobility during the FST was not (Figure 1G). These data suggest that reduced eLMA 24h following LPS injections does not simply reflect a general motor impairment. Previous data from our lab has also noted this divergence between eLMA and other behavioral assays with a necessary motor component following LPS treatment at 24h (Salazar et al., 2012). These previous data suggest that the disconnect between eLMA and other motor-dependent behaviors assessed 24h after peripheral immune challenge may represent a distinct behavior with unique neurobiological substrates. The exaggerated reduction in 24h eLMA of the LPS-treated BDNF+/− mice could be driven by the observed amplification in central IL-1β and TNFα expression (Figures 2A and 2B) that occurs in parallel in these mice. While a full time-course analysis of cytokine expression was not conducted in this study, it is well established that pro-inflammatory cytokines IL-1β and TNFα mediate the development of peripheral inflammation-induced sickness behavior (Bluthé et al., 2000, 1994; Konsman et al., 2002). As the acute 2h eLMA and physiological sickness response (reduction in body weight or food intake) was not different between genotypes, our data suggest that the negative regulation of central pro-inflammatory cytokine expression within BDNF+/− mice is impaired.
To assess conventional inflammation-induced depressive-like behaviors, anhedonia (SP), behavioral despair (FST), and anxiety-like behavior (OF) were analyzed 24h following treatment. Though LPS treatment resulted in anxiety-like behavior in the OF (Figure 1F) and behavioral despair in the FST (Figure 1G), there was no difference between the response in BDNF+/− or BDNF+/+ mice. However, BDNF+/− mice exhibited a more pronounced anhedonia-like behavior following LPS treatment as compared to BDNF+/+ mice. Together, these data indicate that a BDNF deficiency prompts a state of vulnerability specifically to inflammation-induced anhedonia-like behavior, but a more extensive timecourse and phenotypic battery is needed to determine the full duration and nature of this interaction. Preclinical investigations have determined that disruptions to the BDNF system, whether positive or negative, in a brain region-dependent manner result in varying behavioral changes following depressogenic stimulus (stress) (Shirayama et al., 2015). Furthermore, antidepressant treatment or stress results in region-dependent changes in central BDNF expression (Ma et al., 2011; Zhang et al., 2015). Though central BDNF expression changes have been measured in multiple brain regions following LPS treatment (Hou et al., 2016; Schnydrig et al., 2007; Wei et al., 2015; Zhu et al., 2015) these same changes have not been established in BDNF+/− mice. Therefore, it is feasible that low BDNF expression at the time of immune system activation disrupts neuroinflammatory responses in a regionally distinct manner, particularly as several reports indicate that peripheral immune activation with LPS itself lowers BDNF expression (Frühauf-Perez et al., 2018; Sun et al., 2019). While only whole brain analysis was performed and only depressive-like behaviors were measured in the current study, it is well accepted that distinct behavioral domains are regulated by regionally discrete neurocircuits within the brain (Leppänen, 2006; McKinnon et al., 2009; Smoski et al., 2009). Additional behavioral/cognitive testing and discrete brain region analysis may shed additional light onto the regional relevance of the BDNF +/− x LPS interaction. Differential susceptibility to the effects of low BDNF expression within these discrete brain regions would then engender a behavior-specific vulnerability of BDNF+/− mice to peripheral inflammation, perhaps as is suggested in the behavioral data here. The notion that some, but not all, behavioral and neurocognitive phenotypes altered by LPS interact with BDNF would be consistent with the findings of Greenwood et. al. In their study, inescapable stress, rather than BDNF +/− genotype, was employed to reduce BDNF levels by about 50%, while exercise or bilateral injection of fluoxetine into the dentate gyrus was administered to restore BDNF levels. Learned helplessness behavior was found to be independent of BDNF levels (Greenwood et al., 2007). While our current study also suggests selectivity in the role of BDNF in the behavioral and neuroinflammatory response to LPS, a few notable differences from the Greenwood et. al. study should be noted. Mice were the model system in our study, while they used rats, and our study utilized a BDNF-reducing challenge on the backdrop of BDNF deficiency as opposed to a treatment (exercise) to mitigate BDNF deficiency. The role of BDNF in the antidepressant response has been extensively investigated, while the role of this critical neurotrophic factor in the neuroinflammatory response has been understudied.
The neuroinflammatory response, measured in part by mRNA in the brain 24h following treatment, revealed that there is an exaggerated increase in pro-inflammatory cytokine expression (IL-1β, TNFα) in BDNF+/− mice. Additionally, LPS-associated neuroinflammation also induces the up-regulation of IDO expression (O’Connor et al., 2009). As with the pro-inflammatory cytokines, IDO expression was similarly elevated in LPS-treated BDNF+/− mice. These data indicate that BDNF expression levels may play a role in negatively regulating the progression of the LPS-stimulated neuroimmune response which could underlie the vulnerability of BDNF+/− mice to LPS-induced specific behaviors, although a more complete time course will be required to understand the kinetics of the BDNF +/− x LPS interaction more completely. Remarkably, central IL-6 mRNA expression, which was also assessed following treatment, was not different between genotypes. Previously published data demonstrate that IL-1β and TNFα mRNA have similar temporal expression profiles and remain elevated while IL-6 mRNA expression returns to saline levels following peripheral LPS treatment (André et al., 2008). Analysis of mRNA expression earlier than 24h following LPS treatment could reveal a similar contribution of IL-6 mRNA to the exaggerated pro-inflammatory response observed in BDNF+/− mice.
IL-10 is the prototypical anti-inflammatory cytokine known to negatively regulate LPS-induced neuroimmune responses, and it often co-expresses with IL-1β, IL-6 and TNFα (Ledeboer et al., 2000), and CD11b expression is a general indication of microglia/perivascular macrophage activation (Buttini et al., 1996; Roy et al., 2008). Both IL-10 and CD-11b expression (Figures 2E and 2F) increased in response to LPS, similar to previously reported effects (Durez et al., 1993; Tanaka et al., 2006), there was not a difference between BDNF+/− and BDNF+/+ mice. This suggests that BDNF deficiency may specifically potentiate IL-1β and TNFα, both of which could elevate IDO expression during neuroinflammation. Measurement of a more detailed time course and comprehensive neuroimmune panel will be necessary to confirm other mediators are not involved.
When IDO expression is up-regulated during neuroinflammation, central kynurenine metabolism is also elevated (Heyes et al., 1988; Walker et al., 2013). Pro-inflammatory cytokines up-regulate indoleamine 2,3-dioxygenase (IDO) expression, the rate-limiting enzyme in kynurenine metabolism (André et al., 2008), and induce IDO-dependent depressive-like behaviors associated with central neurotoxic kynurenine metabolism (Heisler and O’Connor, 2015; Henry et al., 2008; O’Connor et al., 2009; Salazar et al., 2012; Walker et al., 2013). Studies suggest LPS induces depressive-like behaviors through an increase in neurotoxic kynurenine metabolites, specifically QA (Heisler and O’Connor, 2015; J M Parrott et al., 2016; Jennifer M Parrott et al., 2016; Walker et al., 2013). Previous studies have demonstrated that BDNF+/− mice have a marked increase in 3-HK, a neurotoxic metabolite that generates reactive oxygen species, following chronic mild stress. However, the effects of peripheral inflammation on kynurenine metabolism have not yet been explored in this genotype (Dugan et al., 2015; Parrott and O’Connor, 2015). Here, we show that following LPS treatment, peripheral and central kynurenine concentration increases in both genotypes (Figures 3A and 3B), however, only central, not peripheral, kynurenine metabolism was exaggerated in BDNF+/− mice. The disconnect between central and peripheral kynurenine metabolism provides further evidence of specific neuroinflammatory consequences as opposed to a general non-specific potentiation of the immune response. Downstream of kynurenine, 3-HK (Figure 3C), was not elevated by LPS treatment which has been observed previously (Walker et al., 2013). 3-HK can be metabolized further to two end products, XA and QA (Figures 3D and 3E), both of which are significantly increased following LPS in BDNF+/− mice relative to BDNF+/+ mice. Both XA and QA can modulate glutamate neurotransmission, XA as an agonist of metabotropic glutamate receptors (mGluRs) and QA as an agonist of the N-methyl-D-aspartate receptor (NMDAR) (Parrott and O’Connor, 2015). Consistent with this notion, a recent study demonstrated that LPS-induced anhedonia-like behavior and behavioral despair was dependent on NMDAR activation (Walker et al., 2013). Our behavioral data suggest that vulnerability of BDNF+/− mice to LPS-induced depressive-like behaviors is likely the result of exaggerated pro-inflammatory cytokine expression leading to neurotoxic kynurenine metabolism in a region dependent manner. These data demonstrate that peripheral inflammation results in the elevation of central and peripheral kynurenine production, however, LPS-treated BDNF+/− mice experience an amplified response specifically in the production of central kynurenine. However, limitation of our current data is that the neuroinflammatory response was analyzed at the whole brain level. To better understand the mechanistic relationship between BDNF expression, pro-inflammatory cytokines and kynurenine metabolism, future studies investigating discrete brain regions will need to be performed.
Under basal conditions, further metabolism of kynurenine results in the production of kynurenic acid (KA) and during neuroinflammation, kynurenine is metabolized to 3-hydroxykynurenine (3-HK). 3-Hydroxyanthranilic acid (3-HAA) and xanthurenic acid (XA) are produced from the metabolism of 3-HK and 3-HAA is metabolized to quinolinic acid (QA). This branch of the kynurenine pathway is considered neurotoxic and is thought to contribute to the development of inflammation-induced depression-related behaviors (Parrott and O’Connor, 2015; Raison et al., 2010; Walker et al., 2013). While numerous studies have implicated oxidative (neurotoxic) kynurenine metabolism as a pathogenic feature of neuroinflammation and neurodegenerative disease, our recent study is the first to probe the direct effects of kynurenine metabolites on microglia activity (Garrison et al., 2018). The predominant hypothesis is centered on the notion that excess QA generated during neuroinflammation potentiates glutamate excitotoxicity. In vitro studies by Kaindl et. al. revealed that activation of NMDARs on cultured microglia resulted in proinflammatory activation (Kaindl et al., 2012), while another in vitro revealed an anti-inflammatory effect of Arylhydrocarbon receptor activation (Kimura et al., 2009). In both aforementioned cases, known receptor targets of kynurenine metabolites were demonstrated to have regulatory effects on proinflammatory activity of immune cells, but in neither case was a kynurenine metabolite used as the agonist. Our data indicated that inhibition of the rate-limiting enzyme, KMO, of this branch of kynurenine metabolism attenuated microglial pro-inflammatory activity, suggesting a direct role for these metabolites in regulating the central inflammatory response (Garrison et al., 2018). The precise relationships between the BDNF system, neuroinflammation and kynurenine metabolism remain poorly understood; however, convergent lines of evidence support the conclusion that BDNF expression very likely plays an important role in mediating the neuroimmune response to peripheral immune activation.
The metabolism of tryptophan to kynurenine is important for inflammation-induced behavior changes, but tryptophan can also be metabolized to serotonin, a neurotransmitter also implicated in the pathology of depression. Previous studies have independently demonstrated that peripheral inflammation increases serotonin turnover in wild-type mice (O’Connor et al., 2009) and that BDNF deficient mice maintain normal serotonin turnover as adults (Chourbaji et al., 2004). Our data in Figure 4 indicate that the serotonin turnover increases in the brain following peripheral inflammation, however, the response is not different between BDNF+/− and BDNF+/+ mice. Prior studies have also demonstrated that peripheral inflammation increases the expression of the serotonin transporter (SERT) (Dunn et al., 1999). Assessment of central SERT mRNA revealed that was no impact of treatment or genotype on expression (data not shown). Together, these data suggest that the serotoninergic system is not likely involved in the differential phenotype of BDNF+/−.
The data from this study are the first to provide direct evidence that a BDNF deficiency causes vulnerability to specific aspects of peripheral inflammation-induced neuroinflammation and depressive-like behavior. BDNF may act as a negative regulator of central pro-inflammatory cytokine expression in response to peripheral inflammation, although this hypothesis will need to be more extensively investigated. Additionally, BDNF may play an important role in mediating tryptophan metabolism during pro-inflammatory conditions. Future studies are necessary to determine if central XA or QA kynurenine metabolites are directly responsible for the behavioral vulnerabilities that develop in BDNF+/− mice following peripheral inflammation. As a BDNF deficiency due to genetic polymorphism or chronic inflammatory conditions is prevalent in the human population, understanding the implications of gene x immune activation interactions on neuroinflammatory and behavioral responses is essential. Our initial data provide a framework for future investigations into the pathogenic mechanisms underlying vulnerability associated with BDNF deficiency.
Highlights.
BDNF+/− mice are more vulnerable to inflammation-induced lethargy
BDNF+/− mice are also more susceptible to inflammation-induced anhedonia
LPS induces exaggerated central pro-inflammatory cytokines in BDNF+/− mice
Central kynurenine metabolism is significantly elevated in BDNF+/− mice after LPS
Acknowledgements:
A special thanks to Susan T. Weintraub, PhD and Xiaoli Gao, PhD at the University of Texas Health Science Center at San Antonio Mass Spectrometry Core Facilities for their assistance with LC/MS method design, sample preparation, sample analysis, and data collection. Mass spectrometry analyses were conducted on instrumentation obtained with funding from the National Institutes of Health (1S10OD016417-01 to STW). Additional thanks to the University of Texas Health Science Center at San Antonio Bioanalytics and Single-Cell Core Facility for sample analysis and data collection.
This research was supported by Merit Review Award I01BX003195 from the from the U.S. Department of Veterans Affairs Biomedical Laboratory Research and Development Service (to JOC) and funding from the National Institute of Mental Health (R01MH090127 and P30MH089868 to JOC; 1F31MH102070-01A1 to JP) and the National Center for Advancing Translational Studies (UL1TR001120 to JOC, Translational Sciences Training Award TL1 TR002647 to GAP). The content is the sole the responsibility of the authors and does not necessarily represent the views of the U.S. Department of Veterans Affairs, United States Government, National Center for Advancing Translational Studies, or the National Institutes of Health.
Footnotes
Declaration of Competing Interest
The authors declare no real or perceived conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- André C, O’Connor JC, Kelley KW, Lestage J, Dantzer R, Castanon N, 2008. Spatio-temporal differences in the profile of murine brain expression of proinflammatory cytokines and indoleamine 2,3-dioxygenase in response to peripheral lipopolysaccharide administration. J. Neuroimmunol 10.1016/j.jneuroim.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthé RM, Layé S, Michaud B, Combe C, Dantzer R, Parnet P, 2000. Role of interleukin-1β and tumour necrosis factor-α in lipopolysaccharide-induced sickness behaviour: A study with interleukin-1 type I receptor-deficient mice. Eur. J. Neurosci 10.1046/j.1460-9568.2000.01348.x [DOI] [PubMed] [Google Scholar]
- Bluthé RM, Pawlowski M, Suarez S, Parnet P, Pittman Q, Kelley KW, Dantzer R, 1994. Synergy between tumor necrosis factor α and interleukin-1 in the induction of sickness behavior in mice. Psychoneuroendocrinology. 10.1016/0306-4530(94)90009-4 [DOI] [PubMed] [Google Scholar]
- Buttini M, Limonta S, Boddeke HWGM, 1996. Peripheral administration of lipopolysaccharide induces activation of microglial cells in rat brain. Neurochem. Int 10.1016/0197-0186(95)00141-7 [DOI] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT, 2001. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol. Psychiatry 10.1016/S0006-3223(01)01083-6 [DOI] [PubMed] [Google Scholar]
- Chen HH, Zhang N, Li WY, Fang MR, Zhang H, Fang YS, Ding MX, Fu XY, 2015. Overexpression of brain-derived neurotrophic factor in the hippocampus protects against post-stroke depression. Neural Regen. Res 10.4103/1673-5374.165510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourbaji S, Hellweg R, Brandis D, Zörner B, Zacher C, Lang UE, Henn FA, Hörtnagl H, Gass P, 2004. Mice with reduced brain-derived neurotrophic factor expression show decreased choline acetyltransferase activity, but regular brain monoamine levels and unaltered emotional behavior. Mol. Brain Res 10.1016/j.molbrainres.2003.11.002 [DOI] [PubMed] [Google Scholar]
- D’Sa C, Duman RS, 2002. Antidepressants and neuroplasticity. Bipolar Disord. 10.1034/j.1399-5618.2002.01203.x [DOI] [PubMed] [Google Scholar]
- Dugan AM, Parrott JM, Redus L, Hensler JG, O’Connor JC, 2015. Low-Level Stress Induces Production of Neuroprotective Factors in Wild-Type but Not BDNF+/− Mice: Interleukin-10 and Kynurenic Acid. Int. J. Neuropsychopharmacol 19, pyv089. 10.1093/ijnp/pyv089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, 2009. Neuronal damage and protection in the pathophysiology and treatment of psychiatric illness: Stress and depression. Dialogues Clin. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Wang J, Ando T, 1999. Effects of cytokines on cerebral neurotransmission: Comparison with the effects of stress, in: Advances in Experimental Medicine and Biology. 10.1007/978-0-585-37970-8_8 [DOI] [PubMed] [Google Scholar]
- Durez P, Abramowicz D, Gérard C, Mechelen M. Van, Amraoui Z, Dubois C, Leo O, Velu T, Goldman M, 1993. In vivo induction of interleukin 10 by anti-CD3 monoclonal antibody or bacterial lipopolysaccharide: Differential modulation by cyclosporin A. J. Exp. Med 10.1084/jem.177.2.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V, Duman RS, 2013. Depression and treatment response: Dynamic interplay of signaling pathways and altered neural processes. Cell. Mol. Life Sci 10.1007/s00018-012-1020-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR, 2010. Inflammation-induced anhedonia: Endotoxin reduces ventral striatum responses to reward. Biol. Psychiatry 68, 748–754. 10.1016/j.biopsych.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R, 1994. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 10.1038/368147a0 [DOI] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KRR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O’Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Valvo WJ, 2005. Mood disorders in the medically ill: Scientific review and recommendations. Biol. Psychiatry 10.1016/j.biopsych.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Frühauf-Perez PK, Temp FR, Pillat MM, Signor C, Wendel AL, Ulrich H, Mello CF, Rubin MA, 2018. Spermine protects from LPS-induced memory deficit via BDNF and TrkB activation. Neurobiol. Learn. Mem 10.1016/j.nlm.2018.02.012 [DOI] [PubMed] [Google Scholar]
- Garrison AM, Parrott JM, Tuñon A, Delgado J, Redus L, 2018. Kynurenine pathway metabolic balance influences microglia activity: Targeting kynurenine monooxygenase to dampen neuroin fl ammation. Psychoneuroendocrinology 94, 1–10. 10.1016/j.psyneuen.2018.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Moya-Alvarado G, Gonzalez-Billaut C, Bronfman FC, 2016. Cellular and molecular mechanisms regulating neuronal growth by brain-derived neurotrophic factor. Cytoskeleton. 10.1002/cm.21312 [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Foley TE, Thompson RS, Fleshner M, 2007. Learned helplessness is independent of levels of brain-derived neurotrophic factor in the hippocampus. Neuroscience. 10.1016/j.neuroscience.2006.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoleit JS, Kullmann JS, Wolf OT, Hammes F, Wegner A, Jablonowski S, Engler H, Gizewski E, Oberbeck R, Schedlowski M, 2011. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One. 10.1371/journal.pone.0028330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler JM, O’Connor JC, 2015. Indoleamine 2,3-dioxygenase-dependent neurotoxic kynurenine metabolism mediates inflammation-induced deficit in recognition memory. Brain. Behav. Immun 10.1016/j.bbi.2015.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP, 2008. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J. Neuroinflammation 10.1186/1742-2094-5-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes MP, Kim P, Markey SP, 1988. Systemic Lipopolysaccharide and Pokeweed Mitogen Increase Quinolinic Acid Content of Mouse Cerebral Cortex. J. Neurochem 10.1111/j.1471-4159.1988.tb01183.x [DOI] [PubMed] [Google Scholar]
- Hou Y, Xie G, Liu X, Li G, Jia C, Xu J, Wang B, 2016. Minocycline protects against lipopolysaccharide-induced cognitive impairment in mice. Psychopharmacology (Berl). 10.1007/s00213-015-4169-6 [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI, 2012. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. 10.1016/j.neuroimage.2011.10.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain NK, Kulkarni SK, Singh A, 2001. Lipopolysaccharide-mediated immobility in mice: Reversal by cyclooxygenase enzyme inhibitors. Methods Find. Exp. Clin. Pharmacol 10.1358/mf.2001.23.8.662131 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Wei N, Lu T, Zhu J, Xu G, Liu X, 2011. Intranasal brain-derived neurotrophic factor protects brain from ischemic insult via modulating local inflammation in rats. Neuroscience. 10.1016/j.neuroscience.2010.10.054 [DOI] [PubMed] [Google Scholar]
- Kaindl AM, Degos V, Peineau S, Gouadon E, Chhor V, Loron G, Le Charpentier T, Josserand J, Ali C, Vivien D, Collingridge GL, Lombet A, Issa L, Rene F, Loeffler JP, Kavelaars A, Verney C, Mantz J, Gressens P, 2012. Activation of microglial N-methyl-D-aspartate receptors triggers inflammation and neuronal cell death in the developing and mature brain. Ann. Neurol 10.1002/ana.23626 [DOI] [PubMed] [Google Scholar]
- Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T, 2009. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J. Exp. Med 10.1084/jem.20090560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsman JP, Luheshi GN, Bluthé RM, Dantzer R, 2000. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur. J. Neurosci 10.1046/j.0953-816X.2000.01319.x [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R, 2002. Cytokine-induced sickness behaviour: Mechanisms and implications. Trends Neurosci. 10.1016/S0166-2236(00)02088-9 [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H, 1999. Behavioral and neurochemical consequences of lipopolysaccharide in mice: Anxiogenic-like effects. Brain Res. 10.1016/S0006-8993(98)01288-8 [DOI] [PubMed] [Google Scholar]
- Lapchak P, Araujo DM, Hefti F, 1993. Systemic interleukin-1 beta decreases brain-derived neurotrophic factor messenger RNA expression in the rat hippocampal formation. Neuroscience. https://doi.org/0306-4522(93)90196-M [pii] [DOI] [PubMed] [Google Scholar]
- Lawson MA, Parrott JM, McCusker RH, Dantzer R, Kelley KW, O’Connor JC, 2013. Intracerebroventricular administration of lipopolysaccharide induces indoleamine-2,3-dioxygenase-dependent depression-like behaviors. J. Neuroinflammation 10.1186/1742-2094-10-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeboer A, Brevé JJP, Poole S, Tilders FJH, Van Dam AM, 2000. Interleukin-10, interleukin-4, and transforming growth factor-β differentially regulate lipopolysaccharide-induced production of pro-inflammatory cytokines and nitric oxide in co-cultures of rat astroglial and microglial cells. Glia. [DOI] [PubMed] [Google Scholar]
- Leppänen JM, 2006. Emotional information processing in mood disorders: A review of behavioral and neuroimaging findings. Curr. Opin. Psychiatry 10.1097/01.yco.0000191500.46411.00 [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Albusaysi S, Ferrell RE, 2013. Brain-derived neurotrophic factor serum levels and genotype: Association with depression during interferon-α treatment. Neuropsychopharmacology. 10.1038/npp.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Wang D-D, Zhang T-Y, Yu H, Wang Y, Huang S-H, Lee FS, Chen Z-Y, 2011. Region-Specific Involvement of BDNF Secretion and Synthesis in Conditioned Taste Aversion Memory Formation. J. Neurosci 31, 2079–2090. 10.1523/JNEUROSCI.5348-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM, 2009. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J. Psychiatry Neurosci [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2016. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Misgeld T, Matsumuro K, Wekerle H, 1998. Neurotrophins inhibit major histocompatibility class II inducibility of microglia: Involvement of the p75 neurotrophin receptor. Proc. Natl. Acad. Sci. U. S. A 10.1073/pnas.95.10.5779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R, 2009. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 10.1038/sj.mp.4002148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Satpathy A, Hartman ME, Horvath EM, Kelley KW, Dantzer R, Johnson RW, Freund GG, 2005. IL-1β-Mediated Innate Immunity Is Amplified in the db/db Mouse Model of Type 2 Diabetes. J. Immunol 174, 4991–4997. 10.4049/jimmunol.174.8.4991 [DOI] [PubMed] [Google Scholar]
- Parrott JM, O’Connor JC, 2015. Kynurenine 3-monooxygenase: An influential mediator of neuropathology. Front. Psychiatry 6, 1–17. 10.3389/fpsyt.2015.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott, Jennifer M, Redus L, O’Connor JC, 2016. Kynurenine metabolic balance is disrupted in the hippocampus following peripheral lipopolysaccharide challenge. J. Neuroinflammation 13, 124. 10.1186/s12974-016-0590-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott JM, Redus L, Santana-Coelho D, Morales J, Gao X, O’Connor JC, 2016. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl. Psychiatry 6, e918. 10.1038/tp.2016.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott JM, Redus L, Santana-Coelho D, Morales J, Gao X, O’Connor JC, 2016. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl. Psychiatry 6, e918. 10.1038/tp.2016.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Ph D, Kelley KW, Marcus A, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH, 2010. CSF Concentrations of Brain Tryptophan and Kynurenines during Immune Stimulation with IFN-alpha: Relationship to CNS Immune Responses and Depression. October 15, 393–403. 10.1038/mp.2009.116.CSF [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Jana A, Yatish K, Freidt MB, Fung YK, Martinson JA, Pahan K, 2008. Reactive oxygen species up-regulate CD11b in microglia via nitric oxide: Implications for neurodegenerative diseases. Free Radic. Biol. Med 10.1016/j.freeradbiomed.2008.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar A, Gonzalez-Rivera BL, Redus L, Parrott JM, O’Connor JC, 2012. Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Horm. Behav 62, 202–209. 10.1016/j.yhbeh.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnydrig S, Korner L, Landweer S, Ernst B, Walker G, Otten U, Kunz D, 2007. Peripheral lipopolysaccharide administration transiently affects expression of brain-derived neurotrophic factor, corticotropin and proopiomelanocortin in mouse brain. Neurosci. Lett 10.1016/j.neulet.2007.09.067 [DOI] [PubMed] [Google Scholar]
- Schulte-Herbrüggen O, Nassenstein C, Lommatzsch M, Quarcoo D, Renz H, Braun A, 2005. Tumor necrosis factor-α and interleukin-6 regulate secretion of brain-derived neurotrophic factor in human monocytes. J. Neuroimmunol 10.1016/j.jneuroim.2004.10.026 [DOI] [PubMed] [Google Scholar]
- Schumacher J, Jamra RA, Becker T, Ohlraun S, Klopp N, Binder EB, Schulze TG, Deschner M, Schmäl C, Höfels S, Zobel A, Illig T, Propping P, Holsboer F, Rietschel M, Nöthen MM, Cichon S, 2005. Evidence for a relationship between genetic variants at the brain-derived neurotrophic factor (BDNF) locus and major depression. Biol. Psychiatry 10.1016/j.biopsych.2005.04.006 [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ, 2012. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci 10.1038/nrn3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G, 2008. Serum Brain-Derived Neurotrophic Factor, Depression, and Antidepressant Medications: Meta-Analyses and Implications. Biol. Psychiatry 10.1016/j.biopsych.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC-H, Nakagawa S, Russell DS, Duman RS, 2002. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci 22, 3251–61. https://doi.org/20026292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Yang C, Zhang J. chun, Ren Q, Yao W, Hashimoto K, 2015. Alterations in brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in the brain regions of a learned helplessness rat model and the antidepressant effects of a TrkB agonist and antagonist. Eur. Neuropsychopharmacol 10.1016/j.euroneuro.2015.09.002 [DOI] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, Dichter GS, 2009. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J. Affect. Disord 10.1016/j.jad.2009.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XY, Zheng T, Yang X, Liu L, Gao SS, Xu HB, Song YT, Tong K, Yang L, Gao Y, Wu T, Hao JR, Lu C, Ma T, Gao C, 2019. HDAC2 hyperexpression alters hippocampal neuronal transcription and microglial activity in neuroinflammation-induced cognitive dysfunction. J. Neuroinflammation 10.1186/s12974-019-1640-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaz D, Loya A, Gersner R, Haramati S, Chen A, Zangen A, 2011. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J. Neurosci 10.1523/JNEUROSCI.5725-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Ide M, Shibutani T, Ohtaki H, Numazawa S, Shioda S, Yoshida T, 2006. Lipopolysaccharide-induced microglial activation induces learning and memory deficits without neuronal cell death in rats. J. Neurosci. Res 10.1002/jnr.20752 [DOI] [PubMed] [Google Scholar]
- Taylor JR, 1997. Chapter 3. Propagation of Uncertainties, in: An Introduction to Error Analysis: The Study of Uncertainties in Physical Measurements. [Google Scholar]
- Tong L, Balazs R, Soiampornkul R, Thangnipon W, Cotman CW, 2008. Interleukin-1β impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol. Aging 10.1016/j.neurobiolaging.2007.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, Kelley KW, Dantzer R, 2013. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 38, 1609–1616. 10.1038/npp.2013.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P, Liu Q, Li D, Zheng Q, Zhou J, Li J, 2015. Acute nicotine treatment attenuates lipopolysaccharide-induced cognitive dysfunction by increasing BDNF expression and inhibiting neuroinflammation in the rat hippocampus. Neurosci. Lett 10.1016/j.neulet.2015.08.008 [DOI] [PubMed] [Google Scholar]
- Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpé S, Maes M, 2005. IDO and interferon-α-induced depressive symptoms: A shift in hypothesis from tryptophan depletion to neurotoxicity. Mol. Psychiatry 10.1038/sj.mp.4001600 [DOI] [PubMed] [Google Scholar]
- Yirmiya R, 1996. Endotoxin produces a depressive-like episode in rats. Brain Res. 10.1016/0006-8993(95)01415-2 [DOI] [PubMed] [Google Scholar]
- Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, Han M, Hashimoto K, 2015. Comparison of ketamine, 7,8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl). 232, 4325–4335. 10.1007/s00213-015-4062-3 [DOI] [PubMed] [Google Scholar]
- Zhu L, Wei T, Gao J, Chang X, He H, Miao M, Yan T, 2015. Salidroside attenuates lipopolysaccharide (LPS) induced serum cytokines and depressive-like behavior in mice. Neurosci. Lett 10.1016/j.neulet.2015.08.025 [DOI] [PubMed] [Google Scholar]