Abstract
Carfilzomib (CFZ) is an FDA-approved proteasome inhibitor with antineoplastic properties against various cancers, yet its short blood retention time after intravenous injection (< 30 min) makes clinical applications limited to multiple myeloma. We previously developed ternary polypeptide nanoparticles (tPNPs) as a new nanoparticle formulation of CFZ to overcome these limitations. The formulation was prepared by polyion complexation between poly(ethylene glycol)-poly(L-glutamate) block copolymers (PEG-PLE) and CFZ-cyclodextrin (CD) inclusion complexes, where CDs were positively charged with 7 primary amines attached while PEG-PLE carried 100 carboxyl groups per polymer chain. Although tPNPs greatly improved biostability of CFZ, CFZ-loaded tPNPs (CFZ-tPNPs) still showed burst drug release and mediocre drug retention under physiological conditions. To address these issues, organic acids are tested as stabilizers in this study to improve particle stability and drug retention for tPNPs. Charge densities in the core of CFZ-tPNPs were optimized with selected organic acids such as citric acid (CA) and lactic acid (LA) at varying mixing ratios. Organic acids successfully maintained small particle size suitable for intravenous injection and drug delivery (diameters < 60 nm), improved CFZ solubility (> 1 mg/mL), allowed for lyophilization and easy reconstitution in various buffers, enhanced drug retention (> 60% post 24 h incubation), and suppressed burst drug release in the first 6 h following solubilization. These results demonstrate that organic acid stabilized tPNPs are useful as an injection formulation of CFZ, which may expand the utility of the proteasome inhibitor.
Keywords: drug delivery, formulations, carfilzomib, cyclodextrin, polypeptide, complexation
INTRODUCTION
Carfilzomib (CFZ) is a second generation proteasome inhibitor approved by the FDA in 2012 for the treatment of relapsed or refractory multiple myeloma1–2. The tetrapeptide epoxyketone induces apoptosis by inhibiting proteasomes that otherwise would degrade many intracellular proteins in cancer cells for survival3. The FDA-approved proteasome inhibitor is used as a formulation (known as Kyprolis®), which includes CFZ as an active pharmaceutical ingredient along with other chemical substances such as sulfobutylether beta-cyclodextrin, citric acid, and sodium hydroxide for pH adjustment4. The cyclodextrin (CD) in Kyprolis plays an important role in improving aqueous solubility of hydrophobic CFZ by forming inclusion complexes with the drug because carfilzomib is practically insoluble in aqueous solutions (10 μM in PBS)5. However, the current CFZ formulation requires 50 times more CD to dissolve CFZ (typically 3,000 mg of CD is needed for 60 mg of CFZ), which poses a major limitation of CD-based CFZ formulations causing toxicity at high concentrations. The CD-CFZ formulations also have difficulties in controlling the release of CFZ, which is significant to achieve safe and effective chemotherapy using the proteasome inhibitor in the clinic6.
To address these issues, we previously developed ternary polypeptide nanoparticles (tPNPs) as a new injection formulation for CFZ7. tPNPs are prepared by entrapping inclusion complexes of CFZ and heptakis(6-amino-6-deoxy)-β-cyclodextrin(hepta-hydrochloride) (HaβCD) in a self-assembling polyion complexes made of poly(ethylene glycol)-conjugated anionic polypeptides such as polyglutamate (Figure 1). CFZ-loaded tPNPs (CFZ-tPNPs) improved entrapment and aqueous solubility of CFZ while extending proteasome inhibition in cancer cells for greater efficacy than free drug and current CD-based formulations7. Despite these exciting results, CFZ-tPNPs still need further improvement in suppressing initial burst drug release and extending drug release half-life in an early stage of treatment (approximately 3 h). One possible solution to these problems is to stabilize the core of tPNPs without hampering drug release from the nanoparticles for fast yet prolonged drug responses. We hypothesized that organic acids stabilize polyionic complexes of HaβCD and tPNPs and extend drug release half-life for CFZ while suppressing burst drug release. This study focuses on determining the effects of organic acids commonly used in drug formulation design, such as citric acid (CA) and lactic acid (LA), on drug entrapment, drug release profiles, and overall particle stability of CFZ-tPNPs.
Figure 1.
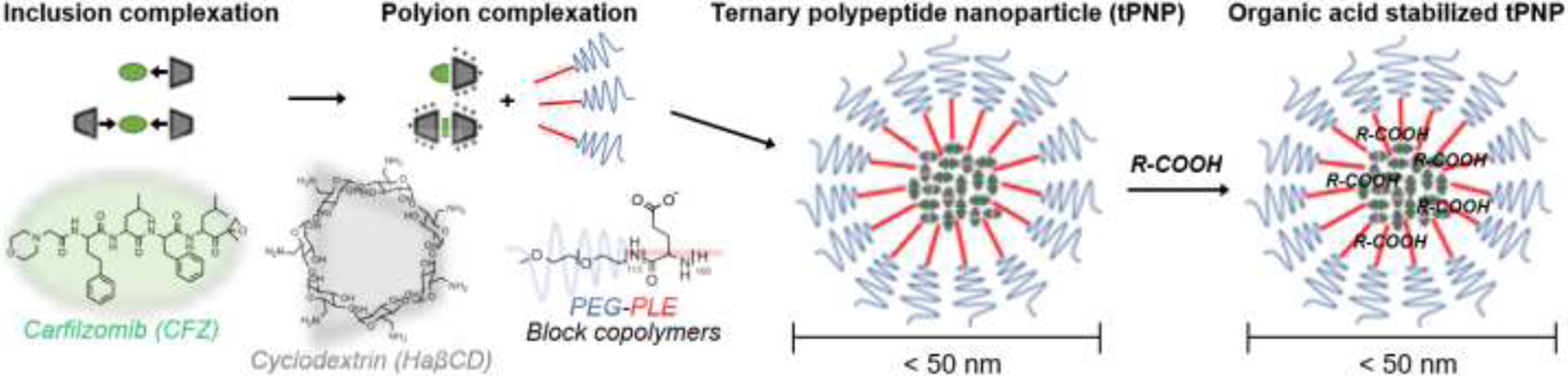
Preparation of organic acid stabilized tPNPs for improved particle stability and drug retention
We aimed the minimum drug entrapment to be greater than 10 percentage by weight (wt%) for an active pharmaceutical ingredient (i.e. CFZ) with respect to other ingredients in the drug formulation (polymer, CD, and organic acids), which is 5 times greater than the current FDA-approved injection formulation of CFZ4. CFZ is a proteasome inhibitor that selectively causes cytotoxic effects on targeted cancer cells, and the drug is less likely to damage non-cancer cells that have low response to proteasome inhibition compared to proliferation cancer cells8–10. Thus, we anticipate that high drug entrapment of CFZ-tPNPs is beneficial to increase drug accumulation in targeted sites in the body such as tumor tissues with reduced toxicity to patients.
In this study, drug release profiles were analyzed to determine drug release half-life (t1/2) in the initial stage and later stage (t1/2, fast and t1/2, slow), which provide information on burst and sustained drug release, respectively. Small molecule drugs are generally cleared from the body quickly through renal filtration or hepatic degradation. The blood retention half-life of CFZ is between 4–39 minutes when injected intravenously using a CD formulation6. Our previous study showed that tPNPs extend t1/2, fast of CFZ up to 3 h, and thus tPNPs are expected to extend the blood retention half-life and duration of action for CFZ by protecting the proteasome inhibitor in the body. We expect that tPNPs stabilized by organic acids may further suppress the drug fast release half-life due to improved particle stability. CA and LA were selected as organic acids for testing the half-life in simulation because the toxicity profiles of these acids as excipients are reported minimal11–14. Particle stability is assessed in pH-controlled buffer solutions mimicking in vivo conditions by measuring particle size before and after freeze drying of drug-loaded nanoparticles. We also monitored the particle stability in the presence and absence of drug to better investigate interactions between organic acids and drugs inside the nanoparticle. We determined particle stability after lyophilization of CFZ-tPNPs because freeze drying is important for long storage of drug-loaded nanoparticles for clinical applications15–16. Freeze-dried CFZ-tPNPs were then reconstituted in a buffer solution to determine drug concentrations. All data was obtained from triplicate experiments and reported as mean values with standard deviations.
MATERIALS AND METHOD
Materials
Carfilzomib (free base, >99%) was purchased from LC Laboratories (Woburn, MA). Poly(ethylene glycol)-block-poly(L-glutamic acid sodium salt) with 5 kDa PEG and 100 repeating units of glutamate (PEG-PLE) was purchased from Alamanda Polymers (Huntsville, AL). Heptakis (6-deoxy-6-amino)-beta-cyclodextrin heptahydrochloride (HaβCD) was purchased from Cyclolab R&D Laboratory Ltd. (Budapest, Hungary). Citric acid was purchased from EM Science (Gibbstown, NJ). LA was purchased from Spectrum (Gardena, CA). Slide-A-Lyzer MINI Dialysis Units 20,000 MWCO and HPLC grade water were purchased from Thermo Fisher Scientific (Rockford, IL).
Nanoparticle preparation
Nanoparticles were prepared by a modified nanoprecipitation method. For empty tPNPs, the starting procedure was as follows. HaβCD (4 mg) and PEG-PLE (2 mg) both dissolved in deionized water at a concentration of 10 mg/mL were mixed in a 1.5 mL conical tube. The resulting mixture was sonicated in a bath sonicator to form drug free tPNPs. For drug-loaded nanoparticles, 5 different CFZ/tPNPs were prepared following the method we previously reported7. Briefly, CFZ and HaβCD were dissolved in ethanol and water respectively, and the solutions were mixed in a 50 mL round bottom flask to form inclusion complexes at a drug to cyclodextrin mass ratio of 1:4. Organic acids (CA and LA) were added to the flask to stabilize the complexes. The mixing ratio of CA and LA was determined to neutralize the net charge in the nanoparticle core. The mixed solution was evaporated using a rotary evaporator under reduced pressure at 60°C to remove ethanol. PEG-PLE (2 mg) was then added to the flask and sonicated for 2 minutes to form self-assembled CFZ/tPNPs. CFZ/tPNPs were transferred into 1.5 mL conical tubes and freeze-dried. Our previous study showed that a 5% trehalose solution used as cryoprotectant performed best in maintaining the physiochemical properties of CFZ/tPNPs after freeze-drying compared to before7. Briefly, a 5% (w/v) trehalose solution was added to CFZ/tPNPs followed by flash freezing in dry ice (−78.5°C) for 4 h. Frozen CFZ/tPNPs was lyophilized using a Labconco FreeZone freeze drying system. The samples were lyophilized overnight at a primary drying temperature of −50°C and vacuum pressure of 0.02 mbar, followed by secondary drying phase of 25°C for 24 hours and maximum vacuum. Freeze-dried CFZ/tPNPs were weighed and stored in a −20°C freezer until use to extend the pharmaceutical stability of the formulation during storage.
Particle size measurement
The Zetasizer Nano ZS (Malvern, UK) was used to obtain particle characteristics. This instrument measures molecular size, molecular weight, particle size and zeta potential using dynamic light scattering (DLS). DLS was used for characterization of the nanoparticle in its liquid phase17. The freeze-dried tPNPs were reconstituted in PBS (10mM, pH 7.4) to a final concentration of 2 mg/mL to achieve this liquid phase. The suspension was exposed to an electromagnetic wave causing the light beam to scatter. From this scattering, a diffusion coefficient of the particles is determined with an equation f = kBT/6πηRH to find the hydrostatic radius. The range of hydrostatic radius detected by DLS is between 0.3 nm to 10 μm, while parameters on the Zetasizer were set to 173° backscatter and 25°C temperature. The reconstituted sample was placed in a DTS 1070 cuvette for particle size analysis. Each sample was measured in triplicate and data was recorded as mean values and standard deviation.
Drug loading quantification
Freeze-dried CFZ/tPNP were reconstituted in saline. High performance liquid chromatography (HPLC) was used to determine the amount of CFZ in the nanoparticle. The mobile phase of the HPLC was an isocratic mixture of acetonitrile and water (ACN:water = 55:45, 1% formic acid, 0.5 mL/min, Agent Eclipse XDB-C18 column). Drug peaks were integrated at a wavelength of 210 nm and elution time was around 3.4 minutes. A calibration curve of known concentrations of CFZ (0.03 – 1 mg/ml) was used to determine the amount of CFZ loaded in the freeze-dried tPNPs. Encapsulation efficiency was calculated as the percentage of drug encapsulated to the drug added in preparation. Drug loading efficiency was defined as the percentage of drug by weight of drug encapsulated to the weight of tPNP. All samples were measured in triplicate and data were summarized by mean ± standard deviation.
Effects of buffers on particle stability and drug release kinetics
Freeze-dried CFZ/tPNPs were reconstituted in different buffers and the dissolved nanoparticles were first observed visually to see how fast they aggregated over time. Buffers used for stability assessment were tris buffered saline (TBS), phosphate buffered saline (PBS), normal saline (NS) and citrate buffer (CB). Particle size was also measured with DLS as described above to determine stability in these buffers. In parallel, drug release kinetics were analyzed for CFZ/tPNPs by using the dynamic dialysis method18. Briefly, freshly prepared tPNPs were reconstituted in potassium biphthalate sodium hydroxide buffer (pH 7.4, 5 mM) to a concentration of 10 mg/mL. Reconstituted CFZ/tPNPs were transferred to Thermo Scientific™ Slide-A-Lyzer™ MINI Dialysis Cups (20,000 MWCO) and placed in buffer (37°C, pH 7.4) to be dialyzed under sink conditions. At predetermined timepoints of 0, 0.5, 1, 3, 6, 24, 48, 72 h, samples were collected from the dialysis cups and drug remaining was determined by HPLC method described above. Drug remaining for each time point was normalized to the value obtained at t = 0 h. Drug release profiles were obtained by fitting the curve to an exponential decay using GraphPad Prism 9 software.
RESULTS
Preparation of CFZ/tPNPs using organic acids
Light scattering analysis of particle size showed tPNPs with less than 60 nm in diameter for all formulations with a size range between 33 nm and 55 nm after freeze drying and reconstitution. The average size of empty tPNPs was 46.1 ± 4.0 nm. CFZ precipitated after ethanol was evaporated without the inclusion of small molecule organic acids in our preparation. Therefore, organic acids were added to the formulations to prevent precipitation of CFZ from solution and enhance particle stability. Several organic acids were tested in preliminary studies, such as CA, LA, malic acid, maleic acid, succinic acid, and tartaric acid. Among these acids, CA, LA and tartaric acid were successful in preventing precipitation during ethanol evaporation. However, tartaric acid required extensive sonication after freeze-drying to facilitate complete dissolution of particles. tPNPs prepared using CA and LA prevented precipitation of CFZ and dissolved readily with vortexing. The incorporation of CA and LA did not adversely affect particle size distribution for all combinations. Average particle size after the addition of these organic acids and CFZ was 41.1 ± 8.7nm. The distribution was unimodal with a Gaussian profile. Figure 2 shows the particle size distribution for tPNPs with various combinations of CA and LA.
Figure 2.

Particle size distribution of nanoparticles
Effect of buffers on particle stability
CFZ/tPNPs were observed to see how fast they aggregated after reconstitution in various buffers. Tris buffer was unable to dissolve CFZ/tPNPs as indicated in its very large particle size (Figure 3). This is due to the amine groups in tris buffer disrupting the polyionic complex between HaβCD and PEG-PLE and breaking up the nanoparticles. PBS completely dissolved CFZ/tPNPs as no particles were seen in tube, but CFZ/tPNPs dissolved in PBS began to aggregate within 1 h after reconstitution and vortexing. Sonication was employed to enhance particle stability in PBS, but aggregation still occurred within 1 h. Citrate buffer and normal saline also completely dissolved CFZ/tPNPs. CFZ/tPNPs dissolved in citrate buffer and normal saline remained in solution for over 24 h.
Figure 3.

Particle stability in various buffers such as tris buffered saline (TBS), phosphate buffered saline (PBS), normal saline (NS) and citrate buffer (CB).
Drug loading quantification
HPLC analysis revealed that CFZ was successfully entrapped in organic acid stabilized tPNPs. The calibration curve from known concentrations of CFZ was used to determine drug concentrations. CFZ/tPNPs for all combinations of CA and LA showed a drug loading average above 1 mg/mL indicating a high encapsulation efficiency. Drug loading efficiency of all CFZ/tPNPs was about 13 wt%. Physiochemical properties of tPNPs are summarized in Table 1.
Table 1.
Physiochemical properties of CFZ/tPNPs
| Formulation | CA(%):LA(%) | Particle size (nm) | PDI | Drug loading (mg/mL) |
|---|---|---|---|---|
| Empty tPNPs | - | 46.1 ± 4.0 | 0.043 | - |
| 100:0 | 33.7 ± 3.3 | 0.182 | 1.16 ± 0.23 | |
| 75:25 | 38.1 ± 5.8 | 0.313 | 0.94 ± 0.18 | |
| tPNPs with CFZ | 50:50 | 55.8 ± 2.1 | 0.301 | 0.93 ± 0.05 |
| 25:75 | 41.9 ± 7.9 | 0.364 | 1.06 ± 0.06 | |
| 0:100 | 36.1 ± 3.0 | 0.356 | 0.89 ± 0.16 |
Drug Release
All tPNPs prepared showed a biphasic drug release profile with an initial burst followed by sustained release shown as t1/2, fast and t1/2, slow, respectively (Figure 4). t1/2, fast for initial burst release was evaluated within the first 1 h, followed by determining t1/2, slow for slow drug release for 72 h. Figure 4 indicates that pure organic acids (100% CA or 100%LA) had the shortest burst release. The longest burst release was with 75% CA and 25% LA. Results with the inset displayed that using a ratio of organic acids produced greater efficacy in burst release reduction compared to pure organic acids. As summarized in Table 2, the half-life slow reduced as a function of CA proportion in tPNPs. Fast drug release was suppressed in all formulations except tPNPs with 100% LA, while half-life slow was delayed with the type or ratio of organic acids. tPNPs prepared with 100% CA had the longest half-life slow, and the formulation with 100% LA showed the shortest half-life slow.
Figure 4.
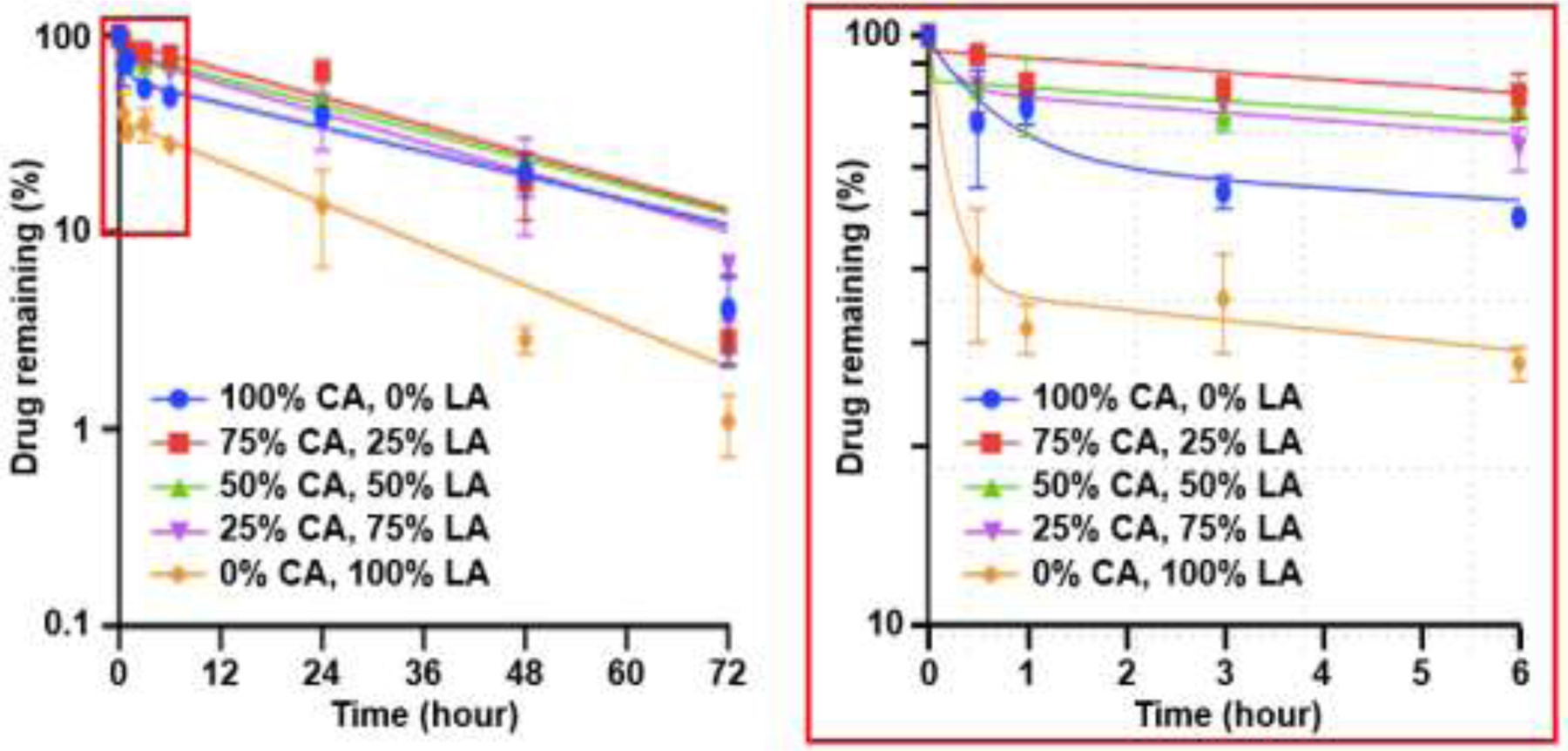
Drug release profiles for tPNPs with organic acids at various mixing ratios (enlarged view of inset for initial 6 h post incubation)
Table 2.
Kinetic parameters of CFZ release from tPNPs
| Formulation | CA(%):LA(%) | Kfast(h−1) | Kslow(h−1) | t1/2, fast (h) | t1/2, slow (h) |
|---|---|---|---|---|---|
| tPNPs with CFZ | 100:0 | 1.54 | 0.0239 | 0.45±0.11 | 29.06 ± 6.18 |
| 75:25 | 4.09 | 0.0249 | 0.15±0.05 | 27.88 ± 4.34 | |
| 50:50 | 4.23 | 0.0275 | 0.18±0.10 | 26.29 ± 5.61 | |
| 25:75 | 4.46 | 0.0325 | 0.16±0.09 | 24.10 ± 3.81 | |
| 0:100 | 5.36 | 0.0402 | 0.13±0.07 | 17.26 ± 3.90 |
Effect of mixing ratio on drug remaining after 24h post incubation.
The percent of drug remaining at 24 h was greater for tPNPs with mixed CA:LA than tPNPs with single acid. tPNPs with higher CA% contained more CFZ. The plateau of the tPNP with 75%CA:25%LA contained the highest amount of CFZ after 24 h, with 50%CA:50%LA and the 25%CA/75%LA formulations following respectively (Figure 5). Nanoparticles containing single organic acids reduced the initial burst release but did not have as much drug remaining after 24 h. tPNPs containing 100% CA and 100% LA contained ~35% and ~57% respectively at 24 h. Although the burst release was lengthened, single organic acid tPNPs contained less than the ratioed organic acid tPNPs which contained between ~80–95% of drug after 1 h.
Figure 5.

Drug entrapment assessment (% of CFZ remaining at t = 24 h)
DISCUSSION
CFZ was approved by the FDA for the treatment of multiple myeloma due to the higher efficacy and lower toxicity than the first in class proteasome inhibitor Bortezomib19. Because of its success in multiple myeloma patients, CFZ has been further studied for the treatment of solid tumors and metastatic diseases. Despite promising preclinical data, CFZ achieved limited success in solid tumor treatment in the clinic potentially due to pharmaceutical limitations such as low solubility, poor biostability, and short release half-life, which lowered therapeutic outcomes20–22. Previous studies suggest that nanoparticle formulations can improve therapeutic efficacy of small molecule drugs by fine-tuning the in vivo drug release rates23–29, which would better control tumor accumulation, body clearance, and toxicity of various anticancer drugs30–35. Unfortunately, few injection formulations are currently available that can safely increase drug retention in the body to maximize therapeutic potential and overcome clinical limitations of CFZ.
We previously reported that nanoparticle injection formulations made of tPNPs have great potential to address these problems because they can improve water solubility, biostability, and tumor accumulation of hydrophobic drugs including CFZ7, 36–40. In this study, we confirmed that organic acid stabilized CFZ/tPNPs can prevent rapid degradation of the epoxyketone pharmacophore of CFZ and degradation of its tetrapeptide chain, which is beneficial to increase therapeutic efficacy of CFZ. We particularly assessed the potential utility of tPNPs to improve the entrapment and drug release half-life of CFZ.
tPNPs were designed by supramolecular interactions, comprising the formation of inclusion complex between CFZ and HaβCD followed by ionic interactions between amine groups of HaβCD and carboxylic groups of polyglutamate. These hydroxy acids enhance complexation of hydrophobic drugs with CDs compared to just binary complexation of drugs and CDs. This is because these acids modify the intramolecular hydrogen bond system involving the secondary hydrogen groups of CDs. Additionally, the hydroxy groups through hydrogen bonding allows the CD to have a more favorable interaction with surrounding water molecules thereby increasing solubility41. By utilizing organic acids such as CA and LA, we aimed to improve particle stability and extend drug release half-life of CFZ from tPNP. In some studies, CA has been shown to be effective in stabilizing nanoformulations42–45. Three carboxylic acid groups of CA can stabilize the core of nanoparticles by surface adsorption and steric stabilization43. LA has previously garnered some attention in biomedical research due to its involvement in cellular metabolism in the human body, and its ability to mitigate adverse reactions46.
Our findings indicate that CFZ/tPNPs for all combinations of CA and LA maintained a ~50 nm particle size and inclusion of these additives did not adversely change particle size compared to drug-free tPNPs. Additionally, there was no significant difference in particle size across all combinations of CA and LA. This shows efficient entrapment of CFZ within the core of HaβCD and stabilization with the hydroxy groups of the organic acids. It must be noted that there was an increase in PDI for tPNPs where LA was included. The increase in PDI may be attributed to formation of PNPs becoming less stable as LA replaced CA, which have distinctive molecular structures and geometries in the nanoparticle core. Particle size is an important factor for nanoparticle biodistribution and circulation time in the body which determines therapeutic efficacy47–48. Maintaining particle size below 50 nm is important because reports have shown that large nanoparticles (> 150 nm) are susceptible to plasma protein adsorption and opsonization leading to uptake by the liver35, 49. On the other hand, sub 50 nm particles have been shown to have reduced blood clearance by macrophages50–51.
Our results demonstrated a high drug loading of CFZ in tPNPs. All CFZ/tPNPs showed a drug loading approximately 1 mg/mL regardless of the CA to LA ratio. This translates to a very high encapsulation efficiency. Many nanoparticle systems have low to moderate encapsulation efficiency which limits the concentration of cytotoxic agents accumulating in tumors. CFZ/tPNPs have the potential to improve the accumulation of CFZ in solid tumors. The small size also allows it to have longer circulating time to offload its payload all together enhancing therapeutic efficacy.
We tested the stability of CFZ/tPNPs in different buffers. tPNPs were formed by the self-assembling process through ionic interactions between amine groups and carboxylic acid groups. CFZ/tPNPs dissolved in TBS showed poor stability as indicated by particle size. This is due to the competition between the amine group of TBS and the amine group on the surface of HaβCD, which disrupts the formation of ionic interaction needed for tPNPs. PBS performed better than TBS, but we observed aggregation of CFZ/tPNPs within 3 h of reconstitution in buffer. This is likely due to counter ions present in PBS slowly infiltrating and disrupting ionic interactions in tPNPs. NS and CB performed optimally in stabilizing tPNPs and remained stable over 24 h. Drug release profiles are important predictors of the stability and usefulness of drug delivery systems and how they modulate drug concentrations in vivo52. We aimed to improve the half-life of CFZ release from tPNPs. CA alone showed best performance in producing a sustained release of CFZ with a half-life of almost 30 h. This is a significant improvement over the current commercial CFZ formulation which has a half-life less than 1 h. Slow half-life reduced with increasing proportion of LA, and tPNPs made with 100% LA had the shortest slow half-life. However, CA and LA seemed to have a synergistic effect in reducing burst release. Using CA and LA at a 3 to1 ratio appeared to suppress the burst release 3-fold better than 100% CA. In addition, it has a comparable slow half-life (~ 28 h) to 100% CA. This 3 to 1 combination of CA to LA also showed the highest amount of drug remaining after 24 h, although the exact reason is unclear. One speculation is that four carboxylic acid groups in the combination might have improved ternary complexation in the nanoparticle core in comparison to the three on citric acid and the one on lactic acid. This indicates that the presence of LA reduces the amount of drug released in early time frame (< 24 h), leaving much of the drug available for sustained release. With 100% CA, a significant amount of the drug is lost due to burst release. With 100% LA burst release is mitigated, but the rapid release in the sustained phase reduces the amount of drug remaining after 24 h. Drug remaining after 24 h reduces as the proportion of CA in the formulation decreases. These results suggest that preparing CFZ/tPNPs with this particular combination of organic acids produced the most stable formulation.
CONCLUSION
tPNPs stabilized by CA and LA in mixture were found to improve the entrapment and drug release half-life for CFZ, which has few injection formulations for maximum therapeutic outcomes in vivo. The organic acid stabilized tPNPs formed sub-60 nm particles with > 1 mg/ml CFZ. The type and mixing ratio of organic acids appeared to mainly affect tPNP stability and drug release kinetics rather than charge density, which suggests that chemical structures of organic acids and drugs entrapped in the core of nanoparticle may play a major role in modulating entrapment and release of CFZ from tPNP. These findings are significant because organic acid-stabilized tPNPs having small particle size with high drug loading may expand the in vivo utility of CFZ and potentially other proteasome inhibitors from treating blood cancers to solid cancers.
ACKNOWLEDGEMENT
This work was partially supported by the National Institutes of Health grant (R01 AG073122) to KK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Herndon TM; Deisseroth A; Kaminskas E; Kane RC; Koti KM; Rothmann MD; Habtemariam B; Bullock J; Bray JD; Hawes J, US Food and Drug Administration approval: carfilzomib for the treatment of multiple myeloma. Clinical cancer research 2013, 19 (17), 4559–4563. [DOI] [PubMed] [Google Scholar]
- 2.Dimopoulos MA; Roussou M; Gavriatopoulou M; Psimenou E; Ziogas D; Eleutherakis-Papaiakovou E; Fotiou D; Migkou M; Kanellias N; Panagiotidis I, Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood advances 2017, 1 (7), 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.J Kuhn D; Z Orlowski R; C Bjorklund C, Second generation proteasome inhibitors: carfilzomib and immunoproteasome-specific inhibitors (IPSIs). Current cancer drug targets 2011, 11 (3), 285–295. [DOI] [PubMed] [Google Scholar]
- 4.Lewis E; Shwonek P; Dalziel S; Jumaa M, Cyclodextrin complexation methods for formulating peptide proteasome inhibitors. Google Patents: 2018. [Google Scholar]
- 5.Ashley JD; Stefanick JF; Schroeder VA; Suckow MA; Alves NJ; Suzuki R; Kikuchi S; Hideshima T; Anderson KC; Kiziltepe T, Liposomal carfilzomib nanoparticles effectively target multiple myeloma cells and demonstrate enhanced efficacy in vivo. Journal of Controlled Release 2014, 196, 113–121. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z; Yang J; Kirk C; Fang Y; Alsina M; Badros A; Papadopoulos K; Wong A; Woo T; Bomba D, Clinical pharmacokinetics, metabolism, and drug-drug interaction of carfilzomib. Drug Metabolism and Disposition 2013, 41 (1), 230–237. [DOI] [PubMed] [Google Scholar]
- 7.Agbana P; Lee MJ; Rychahou P; Kim K-B; Bae Y, Ternary Polypeptide Nanoparticles with Improved Encapsulation, Sustained Release, and Enhanced In Vitro Efficacy of Carfilzomib. Pharmaceutical Research 2020, 37 (11), 1–12. [DOI] [PubMed] [Google Scholar]
- 8.Jagannath S; Vij R; Stewart AK; Trudel S; Jakubowiak AJ; Reiman T; Somlo G; Bahlis N; Lonial S; Kunkel LA, An open-label single-arm pilot phase II study (PX-171–003-A0) of low-dose, single-agent carfilzomib in patients with relapsed and refractory multiple myeloma. Clinical Lymphoma Myeloma and Leukemia 2012, 12 (5), 310–318. [DOI] [PubMed] [Google Scholar]
- 9.Jakubowiak AJ; Dytfeld D; Griffith KA; Lebovic D; Vesole DH; Jagannath S; Al-Zoubi A; Anderson T; Nordgren B; Detweiler-Short K, A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood, The Journal of the American Society of Hematology 2012, 120 (9), 1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vij R; Wang M; Kaufman JL; Lonial S; Jakubowiak AJ; Stewart AK; Kukreti V; Jagannath S; McDonagh KT; Alsina M, An open-label, single-arm, phase 2 (PX-171–004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood, The Journal of the American Society of Hematology 2012, 119 (24), 5661–5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Fernandez MJ; Tabary N; Chai F; Cazaux F; Blanchemain N; Flament M-P; Martel B, New multifunctional pharmaceutical excipient in tablet formulation based on citric acid-cyclodextrin polymer. International journal of pharmaceutics 2016, 511 (2), 913–920. [DOI] [PubMed] [Google Scholar]
- 12.Metz JK; Scharnowske L; Hans F; Schnur S; Knoth K; Zimmer H; Limberger M; Groß H; Lehr CM; Hittinger M, Safety assessment of excipients (SAFE) for orally inhaled drug products. 2020. [DOI] [PubMed]
- 13.Rayaprolu BM; Strawser JJ; Anyarambhatla G, Excipients in parenteral formulations: selection considerations and effective utilization with small molecules and biologics. Drug development and industrial pharmacy 2018, 44 (10), 1565–1571. [DOI] [PubMed] [Google Scholar]
- 14.Pifferi G; Restani P, The safety of pharmaceutical excipients. Il Farmaco 2003, 58 (8), 541–550. [DOI] [PubMed] [Google Scholar]
- 15.Wang L; Ma Y; Gu Y; Liu Y; Zhao J; Yan B; Wang Y, Cryoprotectant choice and analyses of freeze-drying drug suspension of nanoparticles with functional stabilisers. Journal of microencapsulation 2018, 35 (3), 241–248. [DOI] [PubMed] [Google Scholar]
- 16.Alihosseini F; Ghaffari S; Dabirsiaghi AR; Haghighat S, Freeze-drying of ampicillin solid lipid nanoparticles using mannitol as cryoprotectant. Brazilian journal of pharmaceutical sciences 2015, 51, 797–802. [Google Scholar]
- 17.Lim J; Yeap SP; Che HX; Low SC, Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale research letters 2013, 8 (1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponta A; Fugit KD; Anderson BD; Bae Y, Release, partitioning, and conjugation stability of doxorubicin in polymer micelles determined by mechanistic modeling. Pharmaceutical research 2015, 32 (5), 1752–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart AK; Rajkumar SV; Dimopoulos MA; Masszi T; Špička I; Oriol A; Hájek R; Rosiñol L; Siegel DS; Mihaylov GG, Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. New England Journal of Medicine 2015, 372 (2), 142–152. [DOI] [PubMed] [Google Scholar]
- 20.Yang W; Monroe J; Zhang Y; George D; Bremer E; Li H, Proteasome inhibition induces both pro-and anti-cell death pathways in prostate cancer cells. Cancer letters 2006, 243 (2), 217–227. [DOI] [PubMed] [Google Scholar]
- 21.Ao L; Wu Y; Kim D; Jang ER; Kim K; Lee D.-m.; Kim KB; Lee W, Development of peptide-based reversing agents for p-glycoprotein-mediated resistance to carfilzomib. Molecular pharmaceutics 2012, 9 (8), 2197–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papadopoulos KP; Burris HA; Gordon M; Lee P; Sausville EA; Rosen PJ; Patnaik A; Cutler RE; Wang Z; Lee S, A phase I/II study of carfilzomib 2–10-min infusion in patients with advanced solid tumors. Cancer chemotherapy and pharmacology 2013, 72 (4), 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parveen S; Misra R; Sahoo SK, Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine: Nanotechnology, Biology and Medicine 2012, 8 (2), 147–166. [DOI] [PubMed] [Google Scholar]
- 24.Singh R; Lillard JW Jr, Nanoparticle-based targeted drug delivery. Experimental and molecular pathology 2009, 86 (3), 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mody N; Tekade RK; Mehra NK; Chopdey P; Jain NK, Dendrimer, liposomes, carbon nanotubes and PLGA nanoparticles: one platform assessment of drug delivery potential. Aaps Pharmscitech 2014, 15 (2), 388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parsian M; Unsoy G; Mutlu P; Yalcin S; Tezcaner A; Gunduz U, Loading of Gemcitabine on chitosan magnetic nanoparticles increases the anti-cancer efficacy of the drug. European journal of pharmacology 2016, 784, 121–128. [DOI] [PubMed] [Google Scholar]
- 27.Feng C; Yuan X; Chu K; Zhang H; Ji W; Rui M, Preparation and optimization of poly (lactic acid) nanoparticles loaded with fisetin to improve anti-cancer therapy. International journal of biological macromolecules 2019, 125, 700–710. [DOI] [PubMed] [Google Scholar]
- 28.Sahana D; Mittal G; Bhardwaj V; Kumar MR, PLGA nanoparticles for oral delivery of hydrophobic drugs: influence of organic solvent on nanoparticle formation and release behavior in vitro and in vivo using estradiol as a model drug. Journal of pharmaceutical sciences 2008, 97 (4), 1530–1542. [DOI] [PubMed] [Google Scholar]
- 29.Yang T; Sheng H-H; Feng N-P; Wei H; Wang Z-T; Wang C-H, Preparation of andrographolide-loaded solid lipid nanoparticles and their in vitro and in vivo evaluations: characteristics, release, absorption, transports, pharmacokinetics, and antihyperlipidemic activity. Journal of pharmaceutical sciences 2013, 102 (12), 4414–4425. [DOI] [PubMed] [Google Scholar]
- 30.Mattu C; Brachi G; Menichetti L; Flori A; Armanetti P; Ranzato E; Martinotti S; Nizzero S; Ferrari M; Ciardelli G, Alternating block copolymer-based nanoparticles as tools to modulate the loading of multiple chemotherapeutics and imaging probes. Acta biomaterialia 2018, 80, 341–351. [DOI] [PubMed] [Google Scholar]
- 31.Yallapu MM; Gupta BK; Jaggi M; Chauhan SC, Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. Journal of colloid and interface science 2010, 351 (1), 19–29. [DOI] [PubMed] [Google Scholar]
- 32.Van Vlerken LE; Amiji MM, Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert opinion on drug delivery 2006, 3 (2), 205–216. [DOI] [PubMed] [Google Scholar]
- 33.Yadav KS; Chuttani K; Mishra AK; Sawant KK, Effect of size on the biodistribution and blood clearance of etoposide-loaded PLGA nanoparticles. PDA J Pharm Sci Technol 2011, 65 (2), 131–139. [PubMed] [Google Scholar]
- 34.Zhao J; Qin Z; Wu J; Li L; Jin Q; Ji J, Zwitterionic stealth peptide-protected gold nanoparticles enable long circulation without the accelerated blood clearance phenomenon. Biomaterials science 2018, 6 (1), 200–206. [DOI] [PubMed] [Google Scholar]
- 35.Hoshyar N; Gray S; Han H; Bao G, The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11 (6), 673–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reichel D; Lee MJ; Lee W; Kim KB; Bae Y, Tethered polymer nanoassemblies for sustained carfilzomib release and prolonged suppression of proteasome activity. Therapeutic delivery 2016, 7 (10), 665–681. [DOI] [PubMed] [Google Scholar]
- 37.Park JE; Chun S-E; Reichel D; Min JS; Lee S-C; Han S; Ryoo G; Oh Y; Park S-H; Ryu H-M, Polymer micelle formulation for the proteasome inhibitor drug carfilzomib: Anticancer efficacy and pharmacokinetic studies in mice. PloS one 2017, 12 (3), e0173247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ao L; Reichel D; Hu D; Jeong H; Kim KB; Bae Y; Lee W, Polymer micelle formulations of proteasome inhibitor carfilzomib for improved metabolic stability and anticancer efficacy in human multiple myeloma and lung cancer cell lines. Journal of Pharmacology and Experimental Therapeutics 2015, 355 (2), 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee MJ; Bhattarai D; Yoo J; Miller Z; Park JE; Lee S; Lee W; Driscoll JJ; Kim KB, Development of novel epoxyketone-based proteasome inhibitors as a strategy to overcome cancer resistance to carfilzomib and bortezomib. Journal of medicinal chemistry 2019, 62 (9), 4444–4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ao L; Reichel D; Hu D; Jeong HY; Kim KB; Lee W; Bae Y, Nanoformulations of Carfilzomib for Improved Metabolic Stability and Anti-Cancer Efficacy. The FASEB Journal 2015, 29, 620.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Redenti E; Szente L; Szejtli J, Drug/cyclodextrin/hydroxy acid multicomponent systems. Properties and pharmaceutical applications. Journal of pharmaceutical sciences 2000, 89 (1), 1–8. [DOI] [PubMed] [Google Scholar]
- 42.De Sousa ME; Fernandez van Raap MB; Rivas PC; Mendoza Zelis P; Girardin P; Pasquevich GA; Alessandrini JL; Muraca D; Sánchez FH, Stability and relaxation mechanisms of citric acid coated magnetite nanoparticles for magnetic hyperthermia. The Journal of Physical Chemistry C 2013, 117 (10), 5436–5445. [Google Scholar]
- 43.Shinohara S; Eom N; Teh E-J; Tamada K; Parsons D; Craig VS, The Role of citric acid in the stabilization of nanoparticles and colloidal particles in the environment: measurement of surface forces between hafnium oxide surfaces in the presence of citric acid. Langmuir 2018, 34 (8), 2595–2605. [DOI] [PubMed] [Google Scholar]
- 44.Dheyab MA; Aziz AA; Jameel MS; Noqta OA; Khaniabadi PM; Mehrdel B, Simple rapid stabilization method through citric acid modification for magnetite nanoparticles. Scientific reports 2020, 10 (1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miskeen S; Hong JS; Choi H-D; Kim J-Y, Fabrication of citric acid-modified starch nanoparticles to improve their thermal stability and hydrophobicity. Carbohydrate Polymers 2021, 253, 117242. [DOI] [PubMed] [Google Scholar]
- 46.Casalini T; Rossi F; Castrovinci A; Perale G, A perspective on polylactic acid-based polymers use for nanoparticles synthesis and applications. Frontiers in bioengineering and biotechnology 2019, 7, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aggarwal P; Hall JB; McLeland CB; Dobrovolskaia MA; McNeil SE, Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Advanced drug delivery reviews 2009, 61 (6), 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He C; Hu Y; Yin L; Tang C; Yin C, Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31 (13), 3657–3666. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y-N; Poon W; Tavares AJ; McGilvray ID; Chan WC, Nanoparticle–liver interactions: cellular uptake and hepatobiliary elimination. Journal of controlled release 2016, 240, 332–348. [DOI] [PubMed] [Google Scholar]
- 50.Walkey CD; Olsen JB; Guo H; Emili A; Chan WC, Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. Journal of the American Chemical Society 2012, 134 (4), 2139–2147. [DOI] [PubMed] [Google Scholar]
- 51.Niidome T; Yamagata M; Okamoto Y; Akiyama Y; Takahashi H; Kawano T; Katayama Y; Niidome Y, PEG-modified gold nanorods with a stealth character for in vivo applications. Journal of Controlled Release 2006, 114 (3), 343–347. [DOI] [PubMed] [Google Scholar]
- 52.Huang X; Brazel CS, On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. Journal of controlled release 2001, 73 (2–3), 121–136. [DOI] [PubMed] [Google Scholar]


