Abstract
Background:
Cancer cachexia is a wasting syndrome associated with functional impairment and reduced survival that impacts up to 50% of patients with gastrointestinal cancers. However, data are limited on the prevalence and clinical significance of cachexia in patients with hepatocellular carcinoma (HCC).
Methods:
We performed a retrospective cohort study of patients diagnosed with HCC at two U.S. health systems between 2008 – 2018. Patient weights were recorded six months prior and at HCC diagnosis. Cachexia was defined as >5% weight loss (or >2% weight loss if BMI <20 kg/m2) and precachexia defined as 2–5% weight loss. We used multivariable logistic regression models to identify correlates of cachexia and multivariable Cox proportional hazard models to identify factors associated with overall survival.
Results:
Of 604 patients with HCC, 201 (33.3%) had precachexia and 143 (23.7%) had cachexia at diagnosis, including 19.0%, 23.5%, 34.7%, and 34.0% of patients with BCLC stages 0/A, B, C, and D, respectively. Patients with cachexia were less likely to receive HCC treatment (OR 0.38, 95%CI 0.21 – 0.71) and had worse survival than those with precachexia or stable weight (11.3 vs 20.4 vs 23.5 months, respectively, p<0.001). Cachexia remained independently associated with worse survival (HR 1.43, 95%CI 1.11 – 1.84) after adjusting for age, sex, race/ethnicity, Child Pugh class, AFP, BCLC stage and HCC treatment.
Conclusions:
Nearly 1 in 4 patients with HCC present with cachexia, including many with compensated cirrhosis or early-stage tumors. The presence of cancer-associated weight loss appears to be an early and independent predictor of worse outcomes in patients with HCC.
Keywords: cachexia, wasting, body mass index, liver cancer
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fastest rising cause of cancer-related death in the U.S. It is unique among malignancies, in that the majority of cases occur in the background of a diseased organ, the cirrhotic liver.1, 2 The most commonly used staging system in HCC -- the Barcelona Clinic Liver Cancer (BCLC) staging system – includes not only the patient’s tumor burden but also their degree of liver dysfunction and performance status, measured using the Eastern Cooperative Oncology Group (ECOG) scale. Prior studies have demonstrated the impact of sarcopenia and functional status on survival in HCC3–5. In parallel, frailty has emerged as an important predictor of prognosis in patients with cirrhosis (including those without HCC) 6–8, and objective measures of performance status and frailty are increasingly being utilized in this population for prognostication and therapeutic decision-making, including liver transplant eligibility.6
Cancer cachexia is a related but distinct clinical syndrome of wasting, characterized by the ongoing loss of adipose tissue and skeletal muscle mass, leading to progressive functional impairment.9 The mechanism of cachexia is multifactorial and not merely related to caloric deficiency, but in part driven by systemic inflammation and tumor-secreted factors leading to abnormal metabolism.10–13 It is considered to exist along a continuum with three clinical phases (precachexia, cachexia, and refractory cachexia) and is objectively defined based on percentage of total body weight lost (i.e., patients with >5% weight loss [or >2% weight loss for patients with BMI <20 mg/kg2] over a 6 month period are classified as having cachexia, per international consensus definition).9 Cachexia is common, occurring in approximately 50% of patients with cancer11, 14, 15, including those with other gastrointestinal malignancies (e.g., pancreatic16, esophageal17, colon cancers15). It is associated with impaired physical function18, poorer quality of life, lower rates of treatment response, and worse overall survival.15, 16 As cachexia in patients with cancer is often underrecognized and undertreated, earlier recognition and management of this syndrome may lead to improved quality of life and survival outcomes.
While cachexia has been shown to be an important prognostic indicator in other cancers15, its prevalence and clinical significance in patients with HCC remain unknown. To our knowledge, no prior studies have applied the international consensus definition of cancer cachexia to a well-characterized patient population with cirrhosis and HCC. Therefore, we aimed to comprehensively evaluate the prevalence, correlates and prognostic impact of cachexia in patients with HCC.
METHODS
Study Cohort
We identified consecutive patients diagnosed with HCC between January 2008 and December 2018 at two large U.S. health systems, the University of Texas (UT) Southwestern Medical Center and Parkland Health and Hospital System, using our prospectively maintained institutional database. All HCC diagnoses were confirmed on chart review using the AASLD guidelines, i.e. characteristic imaging findings (LI-RADS 5) or histology.19 We excluded patients lacking weight data at time of HCC diagnosis (+/− 1 month), as well as patients with detectable ascites on imaging 6 months prior to HCC diagnosis. Patients without characteristic imaging or histology confirming HCC diagnosis, patients in whom the date of initial HCC diagnosis could not be ascertained from review of the electronic medical record (EMR), and patients who received HCC treatment at an outside facility prior to presentation at one of the study sites were also excluded. This study was approved by the Institutional Review Board of the UT Southwestern Medical Center.
Data Collection
We abstracted relevant demographic, laboratory, and imaging data at time of HCC diagnosis from the EMR. The date of HCC diagnosis was defined as date at which lesions met HCC criteria per AASLD guidelines.19 Demographic factors including age, race, ethnicity, and gender were obtained through self-report from each patient at initial clinic visit.20, 21 Insurance status was recorded from the EMR and categorized as private insurance, Medicaid, Medicare, other (including Parkland’s subsidy program for medical care) or no insurance. Clinical variables of interest included ECOG performance status abstracted from clinical notes, presence of cirrhosis (defined by: 1) histopathology indicating F4 disease; 2) serum or radiologic markers of fibrosis consistent with cirrhosis; 3) imaging with nodular liver and signs of portal hypertension; or 4) platelet count less than 150,000 in the setting of chronic liver disease), etiology of cirrhosis (classified as hepatitis C virus, hepatitis B virus, alcohol-related liver disease, and nonalcoholic fatty liver disease)22, and degree of liver dysfunction (Child Pugh score). The presence of encephalopathy and/or ascites at time of HCC diagnosis was classified as none, mild/controlled, or severe/uncontrolled. Laboratory data at diagnosis included total bilirubin, international normalized ratio, albumin, platelet count, creatinine, and alpha fetoprotein (AFP).
Tumor characteristics were determined via imaging, and all tumors were staged using the BCLC staging system.23 All HCC-directed treatments were recorded; if a patient received multiple HCC treatments, the treatments were ranked as “most definitive” as described previously,20 i.e., liver transplantation > surgical resection > local ablation (e.g. radiofrequency or microwave ablation) > stereotactic body radiation therapy (SBRT) > transarterial chemoembolization (TACE) or transarterial radioembolization (TARE) > systemic therapy > best supportive care (BSC). Curative treatment was defined as liver transplantation, surgical resection, or local ablative therapy. Survival was calculated from date of HCC diagnosis to date of death, with patients censored at last known follow-up or liver transplantation.
Definition of Cancer Cachexia and Assessment of HCC-Associated Weight Loss
Two authors (N.D. and S.M.) manually recorded patient weights at HCC diagnosis and 6 months prior to HCC diagnosis; weights were included if available +/− 1 month within the date of interest. In both health systems, weight is routinely recorded in the EMR at each clinic visit and during all hospital stays. Each patient’s height was also recorded for calculation of body mass index (BMI). The presence of ascites on imaging and/or use of diuretics was captured at the same time points. Weight loss was defined as the percentage of total body weight lost and classified according to previously validated, international consensus definitions. Cachexia was defined as >5% weight loss (or weight loss >2% in patients with BMI <20 kg/m2) over a 6 month period, whereas precachexia was defined as minimal unintentional weight loss not meeting the criteria for cachexia (i.e., <5% weight loss).9
Statistical Analysis
Demographic, clinical, and tumor characteristics are reported using descriptive statistics, stratified by presence of cachexia. Categorical variables were compared using Chi-square and Fisher’s exact tests, while continuous variables were compared using the Kruskal-Wallis test. We used logistic regression models to identify correlates of cachexia. Univariable and multivariable Cox proportional hazard models were utilized to identify factors associated with overall survival. Log-rank tests were used to compare survival distributions between groups. Multivariable models were adjusted for factors determined a priori to be associated with HCC prognosis (e.g., Child Pugh score) and those significant in univariate analyses (p< 0.20). All multivariable analyses were 2-sided and performed with a 5% significance level. Statistical analysis was performed utilizing Stata 16.1 (StataCorp, College Station, TX).
RESULTS
Patient Characteristics
Clinical characteristics of the 604 patients at time of HCC diagnosis are summarized in Table 1. Median age was 60.9 years, 72.2% were male, and over half (56.2%) had Child Pugh class A cirrhosis. The cohort was diverse with regard to race/ethnicity (32.1% White, 34.1% Black, 29.0% Hispanic) and tumor stage (58.6% BCLC stage A, 16.3% B, 16.8% C, and 8.3% stage D).
Table 1.
Patient and tumor characteristics of the entire cohort, stratified by presence of cancer cachexia at time of HCC diagnosis (n=604)
| Variable | Cachexia (n=143) | Pre-cachexia (n=201) | Stable weight/weight gain (n=260) | p-value |
|---|---|---|---|---|
| Age, mean (SD) | 60.8 (9.9) | 62.0 (8.9) | 60.3 (8.6) | 0.13 |
|
| ||||
| Male sex, n (%) | 102 (71.3) | 156 (77.6) | 178 (68.4) | 0.09 |
|
| ||||
| Race/ethnicity, n (%) | 0.62 | |||
| White | 44 (30.8) | 68 (33.8) | 82 (31.5) | |
| Black | 49 (34.3) | 66 (32.8) | 91 (35.0) | |
| Hispanic | 44 (30.8) | 59 (29.4) | 72 (27.7) | |
| Asian | 3 (2.1) | 8 (4.0) | 9 (3.5) | |
| Other | 3 (2.1) | 0 (0.0) | 6 (2.3) | |
|
| ||||
| Body Mass Index (BMI), median (IQR) | 25.4 (21.9 – 29.5) | 28.3 (24.5 – 32.4) | 28.5 (25.4 – 32.9) | <0.001 |
|
| ||||
| BMI category | <0.001 | |||
| < 25 kg/m2 | 66 (46.2) | 59 (29.5) | 49 (18.9) | |
| 25 – 30 kg/m2 | 48 (33.6) | 71 (35.5) | 107 (41.2) | |
| > 30 kg/m2 | 29 (20.3) | 70 (35.0) | 104 (40.0) | |
|
| ||||
| Hospital site, n (%) | 0.54 | |||
| Parkland | 101 (71.1) | 140 (69.7) | 193 (74.2) | |
| UT Southwestern | 41 (28.9) | 61 (30.4) | 67 (25.8) | |
|
| ||||
| Insurance status, n (%) | 0.56 | |||
| Medicare | 24 (16.9) | 36 (18.1) | 58 (22.4) | |
| Medicaid | 56 (39.4) | 73 (36.7) | 102 (39.9) | |
| Other | 38 (26.8) | 44 (22.1) | 59 (22.8) | |
| Private | 15 (10.6) | 30 (15.1) | 26 (10.0) | |
| Uninsured | 9 (6.3) | 16 (8.0) | 15 (5.4) | |
|
| ||||
| Liver Disease etiology, n (%) | 0.07 | |||
| HCV | 84 (58.7) | 131 (65.2) | 164 (63.1) | |
| NAFLD | 19 (13.3) | 35 (17.4) | 31 (11.9) | |
| Alcohol | 23 (16.1) | 20 (9.6) | 44 (16.9) | |
| HBV | 8 (5.6) | 11 (5.5) | 16 (6.2) | |
| Other/unknown | 9 (6.3) | 3 (1.5) | 4 (1.5) | |
|
| ||||
| Cirrhosis, n (%) | 136 (95.1) | 179 (89.5) | 244 (93.9) | 0.16 |
|
| ||||
| Child Pugh, n (%) | <0.001 | |||
| A | 59 (41.8) | 133 (66.8) | 145 (55.8) | |
| B | 65 (46.1) | 53 (26.6) | 96 (36.9) | |
| C | 17 (12.1) | 13 (6.5) | 19 (7.3) | |
|
| ||||
| ECOG performance status, n (%) | 0.004 | |||
| 0 | 83 (60.1) | 132 (67.4) | 172 (67.2) | |
| 1 | 28 (20.3) | 53 (27.0) | 58 (22.7) | |
| 2 | 21 (15.2) | 10 (5.1) | 23 (9.0) | |
| 3 or 4 | 6 (4.4) | 1 (0.5) | 3 (1.2) | |
|
| ||||
| Ascites, n (%) | <0.001 | |||
| None | 61 (42.7) | 140 (69.7) | 164 (63.1) | |
| Mild/controlled | 71 (49.7) | 51 (25.4) | 82 (31.5) | |
| Severe/uncontrolled | 11 (7.7) | 10 (5.0) | 14 (5.4) | |
|
| ||||
| Hepatic encephalopathy, n (%) | 0.02 | |||
| None | 100 (69.9) | 172 (85.6) | 206 (79.2) | |
| Mild/controlled | 39 (27.3) | 28 (13.9) | 52 (20.0) | |
| Severe/uncontrolled | 3 (2.1) | 1 (0.5) | 2 (0.8) | |
|
| ||||
| Platelet count (109/L), median (IQR) | 130 (77 – 184) | 125 (82 – 191) | 104 (68– 156) | |
|
| ||||
| AFP (ng/mL), median (IQR) | 38 (5 – 699) | 22 (6 – 166) | 16 (5 – 188) | |
|
| ||||
| AFP (ng/mL), n (%) | 0.02 | |||
| <20 | 65 (45.5) | 93 (46.2) | 137 (52.7) | |
| 20–200 | 30 (21.0) | 61 (30.4) | 73 (28.1) | |
| >200 | 48 (33.6) | 47 (23.4) | 50 (19.2) | |
|
| ||||
| Number of tumors at diagnosis, n (%) | 0.53 | |||
| 1 | 89 (64.0) | 117 (58.2) | 168 (65.1) | |
| 2 | 24 (17.3) | 39 (19.4) | 42 (16.3) | |
| 3 or more | 9 (6.5) | 24 (11.9) | 25 (9.7) | |
| Infiltrative and/or innumerable | 17 (12.2) | 21 (10.5) | 23 (8.9) | |
|
| ||||
| Largest tumor diameter (cm) | <0.001 | |||
| <2 cm | 20 (14.9) | 37 (19.0) | 58 (23.1) | |
| 2–5 cm | 62 (46.3) | 109 (55.9) | 148 (59.0) | |
| >5 cm | 52 (38.8) | 49 (25.1) | 45 (17.9) | |
|
| ||||
| Infiltrative-type tumor, n (%) | 22 (16.4) | 27 (13.7) | 24 (9.5) | 0.12 |
|
| ||||
| Extrahepatic metastases, n (%) | 10 (7.9) | 6 (3.0) | 8 (3.1) | 0.11 |
|
| ||||
| BCLC stage at diagnosis | 0.007 | |||
| 0/A | 67 (47.2) | 121 (60.2) | 165 (63.7) | |
| B | 23 (16.2) | 34 (16.9) | 41 (15.8) | |
| C | 35 (24.6) | 35 (17.4) | 31 (12.0) | |
| D | 17 (12.0) | 11 (5.5) | 22 (8.49) | |
|
| ||||
| Received any HCC treatment (%) | 91 (63.6) | 171 (85.1) | 221 (85.0) | |
|
| ||||
| Most definitive HCC treatment | <0.001 | |||
| Resection | 15 (10.6) | 48 (23.9) | 42 (16.3) | |
| Ablation | 17 (12.1) | 32 (15.9) | 58 (22.5) | |
| OLT | 14 (9.9) | 17 (8.5) | 30 (11.6) | |
| TACE/TARE/SBRT | 33 (23.4) | 53 (26.4) | 79 (30.6) | |
| Systemic therapy | 12 (8.5) | 21 (10.5) | 12 (4.6) | |
| None/BSC | 50 (35.5) | 30 (14.9) | 37 (14.3) | |
<5% missing data for all variables unless otherwise specified
AFP – alpha-fetoprotein, BCLC - Barcelona Clinic Liver Cancer; HBV – hepatitis B virus; HCC - hepatocellular carcinoma; HCV – hepatitis C virus; IQR – Interquartile range; NAFLD – nonalcoholic fatty liver disease; SD – standard deviation
Prevalence and Correlates of Cachexia
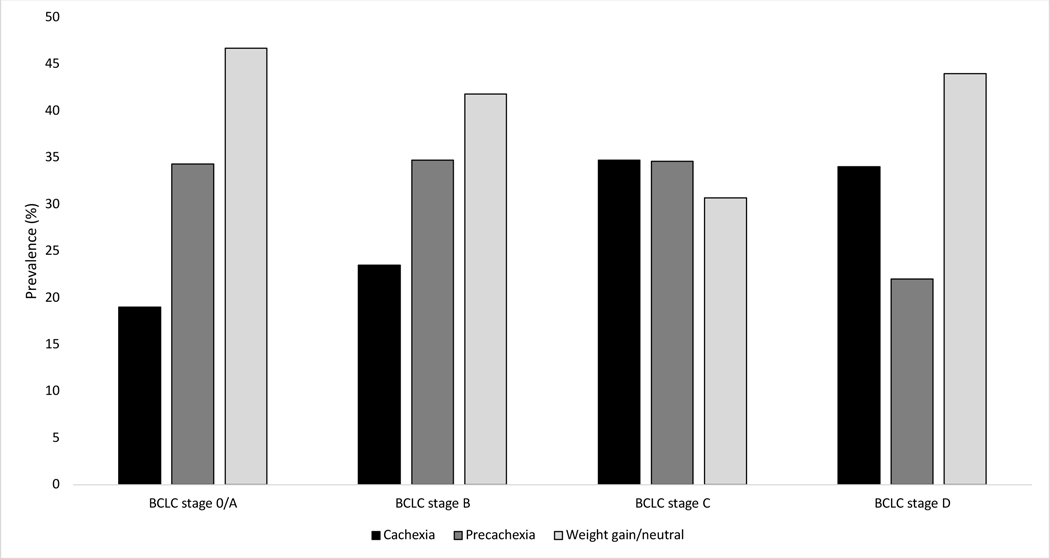
At time of HCC diagnosis, 143 (23.7%) patients had cachexia, including 19.0%, 23.5%, 34.7%, and 34.0% of patients with BCLC stage 0/A, B, C, and D HCC, respectively (Figure 1). Precachexia was observed in 201 (33.3%) patients, and 260 (43.0%) patients had either stable or increased weight. As expected, BMI at HCC diagnosis was lower in patients with cachexia compared to those with pre-cachexia or stable weight, (median BMI 25.4, 28.3, and 28.5, respectively). The proportion of patients with cachexia was similar by sex (p=0.09), race/ethnicity (p=0.62) and age (p=0.42). In univariable analyses (Table 2), pre-treatment cachexia was associated with ECOG performance status ≥ 2, Child Pugh class B/C cirrhosis, presence of ascites, presence of encephalopathy, AFP >200 ng/mL, tumor size >5 cm, and BCLC stage C or D disease. The presence of pre-treatment cachexia remained significantly associated with Child Pugh class B/C cirrhosis and tumor size >5 cm on multivariable analyses.
Figure 1.
Prevalence of cachexia and pre-cachexia in patients with HCC, stratified by BCLC tumor stage
Table 2.
Correlates of pre-treatment cachexia (at time of HCC diagnosis)
| Univariate | Multivariable (n=563) | |||
|---|---|---|---|---|
|
| ||||
| Variable | OR | 95% CI | aOR | 95% CI |
| Female sex | 1.06 | 0.70 – 1.60 | 1.15 | 0.69 – 1.92 |
|
| ||||
| Age | 1.01 | 0.99 – 1.03 | 1.01 | 0.98 – 1.03 |
|
| ||||
| Race/ethnicity | ||||
| White | Ref | Ref | Ref | Ref |
| Black | 1.06 | 0.67 – 1.69 | 0.75 | 0.42 – 1.32 |
| Hispanic | 1.15 | 0.71 – 1.85 | 1.08 | 0.61 – 1.88 |
| Asian | 0.60 | 0.17 – 2.15 | 0.41 | 0.08 – 2.19 |
|
| ||||
| Health system | ||||
| Safety-net | Ref | Ref | ||
| University | 1.06 | 0.70 – 1.60 | ||
|
| ||||
| Insurance status | ||||
| Medicare | Ref | Ref | ||
| Medicaid | 0.80 | 0.47 – 1.37 | ||
| Other/Parkland | 1.15 | 0.71 – 1.86 | ||
| Private | 0.84 | 0.44 – 1.59 | ||
| None | 0.94 | 0.42 – 2.09 | ||
|
| ||||
| Liver disease etiology | ||||
| HCV | Ref | Ref | Ref | Ref |
| Alcohol | 1.26 | 0.74 – 2.15 | 0.86 | 0.44 – 1.70 |
| NASH | 1.01 | 0.57 – 1.78 | 0.57 | 0.27 – 1.21 |
| HBV | 1.04 | 0.46 – 2.38 | 1.60 | 0.64 – 4.05 |
| Other | 4.51 | 1.63 – 12.48 | 4.32 | 1.41 – 13.24 |
|
| ||||
| Liver disease etiology | ||||
| Viral | Ref | Ref | ||
| Nonviral | 1.28 | 0.86 – 1.91 | ||
|
| ||||
| Child Pugh class | ||||
| A | Ref | Ref | Ref | Ref |
| B | 2.06 | 1.37 – 3.08 | 2.08 | 1.28 – 3.38 |
| C | 2.50 | 1.30 – 4.80 | 2.39 | 1.10 – 5.18 |
|
| ||||
| Ascites | ||||
| None | Ref | Ref | ||
| Controlled | 2.66 | 1.79 – 3.96 | ||
| Uncontrolled | 2.28 | 1.06 – 4.91 | ||
|
| ||||
| Hepatic encephalopathy | ||||
| None | Ref | Ref | ||
| Controlled | 1.84 | 1.18 – 2.87 | ||
| Uncontrolled | 3.78 | 0.75 – 19.01 | ||
|
| ||||
| BCLC stage at diagnosis | ||||
| 0/A | Ref | Ref | ||
| B | 1.31 | 0.76 – 2.24 | ||
| C | 2.26 | 1.39 – 3.69 | ||
| D | 2.20 | 1.16 – 4.18 | ||
|
| ||||
| AFP (ng/mL), n (%) | ||||
| <20 | Ref | Ref | Ref | Ref |
| 20–200 | 0.79 | 0.49 – 1.28 | 0.69 | 0.40 – 1.19 |
| >200 | 1.75 | 1.13 – 2.72 | 1.14 | 0.65 – 2.02 |
|
| ||||
| Tumor number | 1 | |||
| 1 | Ref | Ref | Ref | Ref |
| 2 | 0.95 | 0.57 – 1.59 | 0.83 | 0.47 – 1.46 |
| 3 or more | 0.59 | 0.28 – 1.24 | 0.43 | 0.19 – 0.98 |
| TNTC/infiltrative | 1.24 | 0.67 – 2.27 | 0.45 | 0.18 – 1.1 |
|
| ||||
| Maximum tumor diameter | ||||
| <2 cm | Ref | Ref | Ref | Ref |
| 2–5 cm | 1.15 | 0.66 – 2.00 | 1.23 | 0.68 – 2.23 |
| >5 cm | 2.63 | 1.46 – 4.74 | 3.20 | 1.56 – 6.56 |
|
| ||||
| ECOG performance status | ||||
| 0 | Ref | Ref | Ref | Ref |
| 1 | 0.92 | 0.57 – 1.49 | 0.67 | 0.38 – 1.17 |
| 2 | 2.33 | 1.28 – 4.24 | 1.76 | 0.84 – 3.70 |
| 3 | 5.49 | 1.52 – 19.92 | 1.76 | 0.34 – 9.11 |
AFP – alpha-fetoprotein; CI – confidence interval; HBV – hepatitis B virus; HCV – hepatitis C virus; NASH – nonalcoholic steatohepatitis; OR – odds ratio, TNTC – too numerous to count
Among the subset of patients (n=335) with preserved liver function (Child Pugh A) and performance status (ECOG 0–1), cachexia was present in 17.3% of patients – including 12.7%, 25.4%, and 30.4% of patients with BCLC stage 0/A, B, and C HCC, respectively. Similarly, when we examined the subset of patients with no prior history of ascites or diuretic use (n=363), cachexia was present in 16.7% of patients – including 11.1%, 24.2%, 25.0% and 66.7% of patients with BCLC stage 0/A, B, C, and D HCC, respectively.
Cachexia and HCC Treatment Receipt
Patients with cachexia were less likely to receive HCC treatment compared to those without cachexia (OR 0.30, 95% CI 0.19 – 0.50). Results were consistent in multivariable analyses after adjusting for age, sex, race/ethnicity, Child Pugh class and BCLC tumor stage (OR 0.38, 95% CI 0.21 – 0.71). Similarly, patients with cachexia were less likely to receive curative HCC treatment (OR 0.48, 95% CI 0.31 – 0.73) compared to those without cachexia, however this relationship was no longer statistically significant in multivariable analyses (OR 0.71, 95%CI 0.42 – 1.19). Of note, results were similar in both analyses when we adjusted for MELD score, ascites, and encephalopathy in the multivariable models rather than Child Pugh class.
When patients with precachexia were compared to those with stable weight, there was no significant difference in overall treatment receipt (OR 0.99, 95% CI 0.52 – 1.89) or curative treatment receipt (OR 0.98, 95% CI 0.62 – 1.55) in multivariable analyses.
Cachexia and Overall Survival
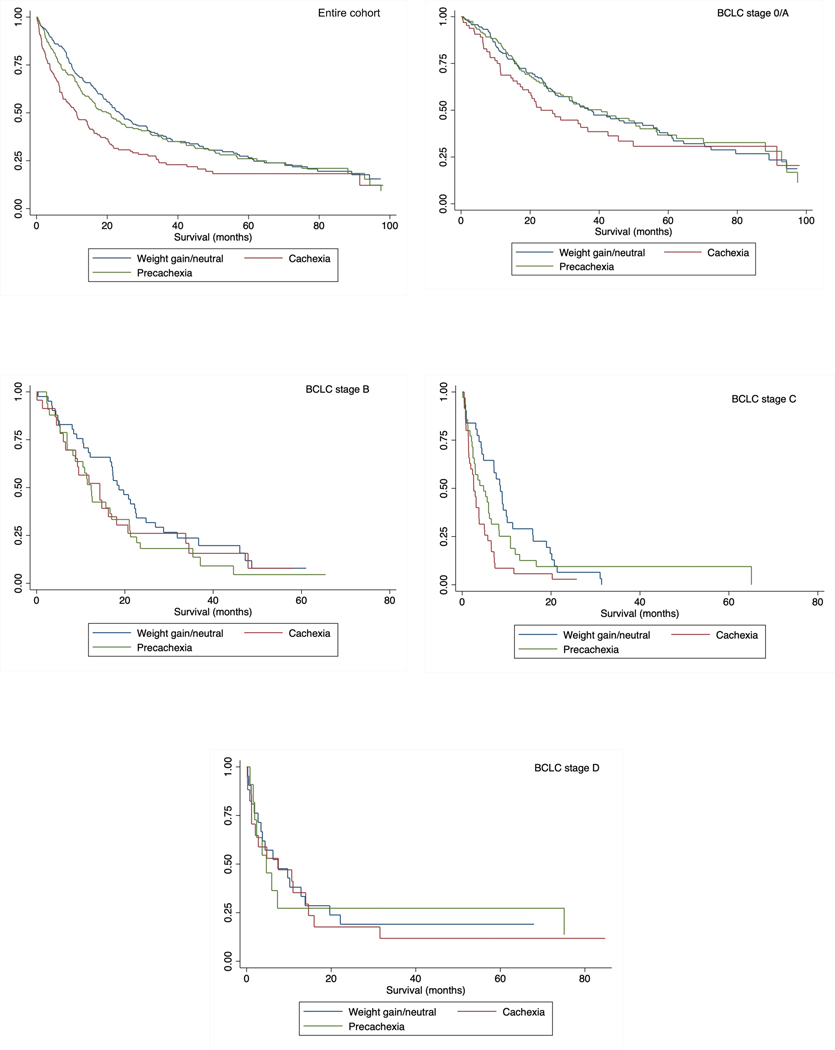
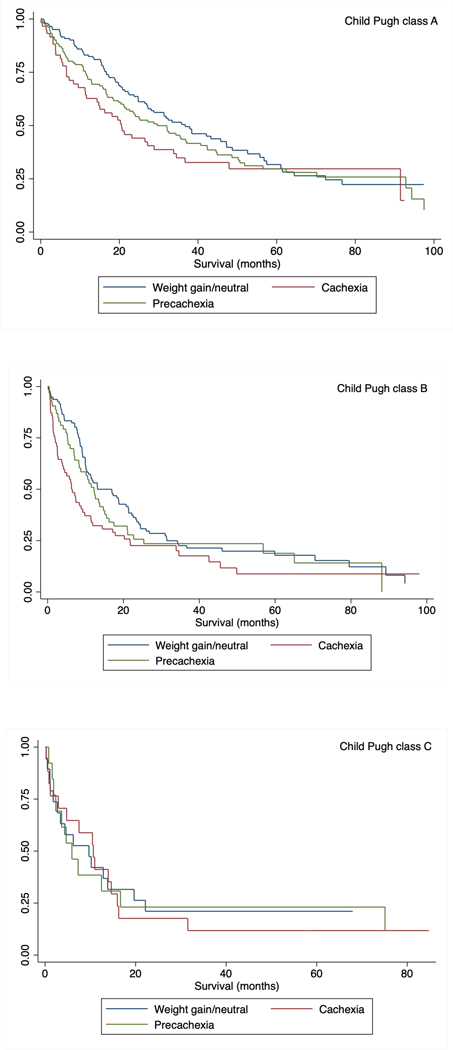
Median overall survival for all patients was 19.7 months (interquartile range [IQR] 7.1 – 57.5 months). Overall survival, stratified by presence of cachexia, is shown in Figure 2. Patients with cachexia had median overall survival of 11.3 months (IQR 3.7 – 34.5 months) compared to 20.4 months (IQR 6.9 – 62.4 months) and 23.5 months (9.9 – 61.5 months) in patients with pre-cachexia and those with stable weight, respectively (p<0.001). Survival differences between patients with cachexia, precachexia, and stable weight were consistent across BCLC stages and Child Pugh classes (Figures 2 and 3), as well as by subgroups of sex, race/ethnicity, BMI category, and most definitive HCC treatment type (Supplemental Table 1). In multivariable analyses, after adjusting for age, sex, race, Child Pugh class, AFP, BCLC tumor stage and HCC treatment received, cachexia (HR 1.43, 95% CI 1.11 – 1.84) and precachexia (HR 1.30, 95% CI 1.02 – 1.65) were both independently associated with worse overall survival (Table 3). Again, results were similar in multivariable analyses when we adjusted for MELD score, ascites, and encephalopathy rather than Child Pugh class.
Figure 2.
Overall survival among patients with HCC with cachexia, precachexia, and those who had weight gain/weight neutral in in a) the entire cohort, b) BCLC stage 0/A, c) BCLC stage B, d) BCLC stage C, and e) BCLC stage D HCC
Figure 3.
Overall survival, stratified by presence of cachexia, among patients with HCC and a) Child Pugh class A cirrhosis, b) Child Pugh class B cirrhosis, c) Child Pugh class C cirrhosis
Table 3.
Correlates of overall survival
| Univariate | Multivariable (n=588) | |||
|---|---|---|---|---|
|
| ||||
| Variable | HR | 95% CI | aHR | 95% CI |
| Female sex | 0.93 | 0.75 – 1.15 | 0.95 | 0.76 – 1.19 |
|
| ||||
| Age | 1.01 | 0.996 – 1.02 | 1.01 | 1.00 – 1.02 |
|
| ||||
| Race/ethnicity | ||||
| White | Ref | Ref | Ref | Ref |
| Black | 1.08 | 0.86 – 1.36 | 1.01 | 0.79 – 1.28 |
| Hispanic | 1.01 | 0.79 – 1.28 | 0.67 | 0.52 – 0.87 |
| Asian | 0.67 | 0.37 – 1.21 | 0.85 | 0.46 – 1.55 |
|
| ||||
| Liver disease etiology | ||||
| HCV | Ref | Ref | ||
| Alcohol | 1.09 | 0.83 – 1.43 | ||
| NASH | 1.13 | 0.86 – 1.49 | ||
| HBV | 0.92 | 0.61 – 1.40 | ||
| Other | 0.76 | 0.41 – 1.43 | ||
|
| ||||
| Child Pugh | ||||
| A | Ref | Ref | Ref | Ref |
| B | 1.93 | 1.58 – 2.35 | 1.66 | 1.33 – 2.06 |
| C | 2.04 | 1.45 – 2.85 | 0.71 | 0.35 – 1.46 |
|
| ||||
| Ascites | ||||
| None | Ref | Ref | ||
| Controlled | 1.60 | 1.31 – 1.96 | ||
| Uncontrolled | 2.07 | 1.41 – 3.03 | ||
|
| ||||
| Hepatic encephalopathy | ||||
| None | Ref | Ref | ||
| Controlled | 1.16 | 0.92 – 1.46 | ||
| Uncontrolled | 2.19 | 0.98 – 4.91 | ||
|
| ||||
| AFP (ng/mL), n (%) | ||||
| <20 | Ref | Ref | Ref | Ref |
| 20–200 | 1.26 | 1.00 – 1.59 | 1.29 | 1.01 – 1.65 |
| >200 | 2.95 | 2.35 – 3.71 | 1.83 | 1.41 – 2.37 |
|
| ||||
| BCLC stage at diagnosis | ||||
| 0/A | Ref | Ref | Ref | Ref |
| B | 2.29 | 1.77 – 2.95 | 1.76 | 1.34 – 2.31 |
| C | 5.98 | 4.63 – 7.71 | 3.88 | 2.75 – 5.47 |
| D | 2.47 | 1.76 – 3.46 | 2.94 | 1.43 – 6.03 |
|
| ||||
| Tumor number | ||||
| 1 | Ref | Ref | ||
| 2 | 1.36 | 1.06 – 1.76 | ||
| 3 or more | 1.87 | 1.37 – 2.55 | ||
| TNTC/infiltrative | 5.04 | 3.75 – 6.76 | ||
|
| ||||
| Maximum tumor diameter | ||||
| <2 cm | Ref | Ref | ||
| 2–5 cm | 1.49 | 1.12 – 1.98 | ||
| >5 cm | 4.19 | 3.08 – 5.72 | ||
|
| ||||
| Most Definitive HCC Treatment | ||||
| Surgical | Ref | Ref | Ref | Ref |
| Locoregional | 3.73 | 2.79 – 5.00 | 4.10 | 3.00 – 5.59 |
| Systemic | 9.54 | 6.33 – 14.39 | 3.59 | 2.20 – 5.86 |
| None/BSC | 17.41 | 12.48 – 24.29 | 12.65 | 8.65 – 18.50 |
|
| ||||
| Weight change prior to HCC diagnosis | ||||
| None/weight gain | Ref | Ref | Ref | Ref |
| <5% weight loss | 1.10 | 0.88 – 1.36 | 1.30 | 1.02 – 1.65 |
| >5% weight loss | 1.56 | 1.23 – 1.97 | 1.43 | 1.11 – 1.84 |
AFP – alpha-fetoprotein; CI – confidence interval; HBV – hepatitis B virus; HCV – hepatitis C virus; NASH – nonalcoholic steatohepatitis; OR – odds ratio; TNTC – too numerous to count
DISCUSSION
Although cachexia has been identified as an important prognostic factor in several cancers, data are limited on its prevalence and clinical significance in HCC. In this study using clinically granular data from a large and diverse cohort, we found pre-treatment weight loss is prevalent, with 25% of patients experiencing cachexia and 33% of patients having precachexia. Cachexia and precachexia impacted patients across the spectrum of cirrhosis severity and HCC tumor stages, including some with compensated cirrhosis and early-stage tumors. Notably, 43% of patients with cachexia had BCLC stage 0/A tumors. Patients with cachexia were less likely to receive HCC treatment and had significantly worse survival compared to those without weight loss. Further, patients with precachexia had intermediate survival outcomes compared to those with cachexia and those without significant weight loss. To our knowledge, this is the first study to comprehensively describe the prevalence and clinical outcomes of cancer cachexia in a well characterized cohort of patients with cirrhosis and HCC.
Cachexia is multifactorial and thought to be driven by tumor- and inflammatory-secreted factors. The assessment of cachexia in HCC is particularly challenging as the tumor predominately arises in the background of cirrhosis, a disease which itself leads to muscle wasting (sarcopenia) and physical debility (i.e., frailty) in advanced stages. When assessed solely using weight measurements, cachexia may therefore be underrecognized in patients with decompensated cirrhosis that have developed ascites despite significant loss of muscle mass. Prior studies have evaluated the prognostic role of sarcopenia and body composition in patients with HCC by performing cross-sectional skeletal muscle measurements.5, 24 However, these studies are limited by one-time measurements, the extrapolation of imaging definitions of sarcopenia based on other cancers, and the difficulty distinguishing HCC-related sarcopenia from that related to the underlying cirrhosis. Alternatively, serum biomarkers are easier to follow longitudinally and may facilitate earlier detection of patients with cachexia.25, 26
The constucts of cachexia, malnutrition, and frailty are distinct but overlap in patients with cirrhosis and HCC. While all patients with cachexia are malnourished, not all patients that are malnourished experience cachexia.27, 28 When we performed subgroup analyses by neutrophil-lymphocyte ratio29 and Prognostic Nutritional Index (PNI), surrogates for nutritional and immunological status30, we found cachexia remained associated with worse survival among the subgroups of patients with low PNI and high NLR (Supplemental Table 1). As this was a retrospective study, we did not have data on patient-reported dietary intake and other inflammatory markers. Additionally, patients may have malnutrition and frailty related to the underlying cirrhosis24, 31, whereas cachexia is a cancer-related phenomenon. The high prevalence of cachexia among patients with Child Pugh class A cirrhosis in our study suggests cachexia is a distinct process as this population generally has minimal to no cirrhosis-related frailty.32 Further studies are needed to determine the association between objective measures of frailty (e.g., Liver Frailty Index33) and cachexia in patients with HCC.
Most prior clinical studies evaluating cancer cachexia have focused on its prevalence among patients with advanced stage tumors.16, 34 However, we found cachexia and precachexia were prevalent across all HCC stages, including in patients with early stage tumors. Gannavarapu and colleagues previously demonstrated the survival impact of cachexia and precachexia varied across the spectrum of solid tumor types.35 Cachexia is highly prevalent in traditionally aggressive cancers (e.g., pancreas, small cell lung cancer) affecting up to 80% of patients; there are fewer data comparing prevalence of cachexia within a cancer type.36, 37 While HCC is generally considered to be an aggressive cancer, its growth patterns are heterogeneous, with up to 1 in 4 tumors exhibiting indolent behavior.38, 39 It is possible that the presence of pre-treatment cachexia may be a marker of more aggressive cancer phenotype, regardless of tumor burden, or that more aggressive tumors secrete certain humoral factors that result in significant weight loss.10
Notably, we observed that cachexia appeared to play an important role in survival in patients with Child Pugh A cirrhosis, whereas there was no significant difference in survival between the cachexia and non-cachexia groups among those with Child Pugh class C cirrhosis. Patients with more advanced liver disease (i.e., Child Pugh class B or C cirrhosis) have a significant competing risk of mortality that is unrelated to their HCC, as the median overall survival of patients with decompensated cirrhosis is approximately 2 years.40 This scenario is in stark contrast to patients with compensated (i.e., Child Pugh class A) cirrhosis and HCC, in whom the competing risk of liver-related mortality is significantly lower. This finding is consistent with the idea that the prognostic value of cachexia as a biomarker in HCC is distinct from the concept of physical frailty related to cirrhosis and liver dysfunction itself. These data suggest early recognition of minimal weight loss and appropriate interventions may improve survival.
Management of cancer cachexia is intrinsically complex due to its multifactorial nature, and has traditionally focused on nutritional supplementation, appetite modulators and aggressive treatment of the underlying tumor itself.41 More recently, preclinical models have identified several promising therapeutic targets (e.g., IL-6, IL-1, TNF-alpha, COX-2, TLRs) and various clinical trials of unimodal and multimodal interventions for cachexia prevention and treatment are ongoing.42, 43 Early identification of cachexia may lead to better outcomes, as advanced (i.e., refractory) cachexia is considered terminal. This would be of particular importance in patients with HCC given the presence of concomitant cirrhosis, with the goal to begin therapy prior to development of hepatic decompensation.
Though our study has several strengths including its large sample size and well characterized cohort with granular information on degree of liver dysfunction and longitudinal weight assessments, it has a few limitations. First, this was a retrospective cohort study, so there is inherent potential for selection bias and unmeasured confounders. Second, while we used international consensus definition of cachexia, it is based on a dichotomous cutoff of 5% total body weight. Third, in patients with cirrhosis in particular, the presence of ascites and/or diuretic use may result in measurement bias due to the inaccurate assessment and/or fluctuation of patient weights. However, we are likely underestimating the true prevalence of cachexia in our study, as ascites would result in “artificial” weight gain. Further, after we excluded patients with any history of ascites or diuretic use, cachexia was still present in 16.7% of patients, and after we excluded patients with uncontrolled ascites in sensitivity analyses (n=30), results were unchanged (data not shown). Fourth, given the retrospective nature of this study we were unable to evaluate intentional vs unintentional weight loss; however, large fluctuations in weight from intentional weight loss are likely to be uncommon in 6 months prior to HCC diagnosis. We also lacked granular information on patient-reported dietary intake and serum inflammatory markers. Finally, though trends were consistent across various subgroups, our study was underpowered to detect statistically significant differences in survival in some of these smaller groups. However, we believe these limitations are outweighed by the granular clinical data included in our analyses; to our knowledge, this is the first study to adjust for severity liver dysfunction and treatment receipt in survival analyses.
In conclusion, we found 1 in 4 patients with HCC present with cachexia, which appears to be an important prognostic factor in HCC. Given its association with survival, early recognition and treatment of cancer-associated wasting is a potential novel intervention target for reducing mortality in patients with HCC.
Supplementary Material
What You Need to Know.
Background
Cancer cachexia is a wasting syndrome that adversely impacts up to 50% of patients with various types of cancer. Little is known about the prevalence and significance of cachexia in patients with hepatocellular carcinoma (HCC).
Findings
1 in 4 patients with HCC present with cachexia at time of cancer diagnosis, including nearly half of patients with early stage tumors. Compared to patients who did not lose weight, patients with cachexia were less likely to receive HCC treatment and had worse survival.
Implications for Patient Care
Early recognition and treatment of cancer cachexia may lead to improved quality of life and survival in patients with HCC.
Acknowledgments
Grant Support: Dr Singal is supported by National Institute of Health R01 CA222900 and R01 MD12565. Dr Marrero is supported by National Institute of Health R01 CA237659. Dr Infante is supported by Burroughs Wellcome Fund Career Awards for Medical Scientists (1019692); American Gastroenterological Association Scholar Award (2019AGARSA3); American Cancer Society grant (133889-RSG-19–195-01-TBE); Cancer Prevention and Research Institute of Texas (RP200170); and the V Foundation Scholar Award (V2019–014). Dr Rich is supported by the American College of Gastroenterology Junior Faculty Development Award and the Texas Health Resources Clinical Scholar Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- BCLC
Barcelona Clinic Liver Cancer
- HR
hazard ratio
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- NAFLD
nonalcoholic fatty liver disease
- OR
odds ratio
Footnotes
Conflicts of Interest: Jorge Marrero has served as a consultant for Glycotest and received research funding from AstraZeneca. Amit Singal has been on advisory boards and served as a consultant for Wako Diagnostics, Roche, Exact Sciences, Glycotest, GRAIL, Bayer, Eisai, Exelixis, BMS, AstraZeneca, Genentech, and TARGET-RWE. Pfizer, Inc. is currently supporting a collaborative project with the laboratory of Rodney Infante that is independent of all data presented in this manuscript. The other authors have no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clinical Gastroenterology and Hepatology 2020;18:2650–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clinical Gastroenterology and Hepatology 2016;14:124–131. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harimoto N, Shirabe K, Yamashita YI, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg 2013;100:1523–30. [DOI] [PubMed] [Google Scholar]
- 4.Hsu CY, Lee YH, Hsia CY, et al. Performance status in patients with hepatocellular carcinoma: determinants, prognostic impact, and ability to improve the Barcelona Clinic Liver Cancer system. Hepatology 2013;57:112–119. [DOI] [PubMed] [Google Scholar]
- 5.Meza-Junco J, Montano-Loza AJ, Baracos VE, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. Journal of clinical gastroenterology 2013;47:861–870. [DOI] [PubMed] [Google Scholar]
- 6.Lai JC, Rahimi RS, Verna EC, et al. Frailty Associated With Waitlist Mortality Independent of Ascites and Hepatic Encephalopathy in a Multicenter Study. Gastroenterology 2019;156:1675–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai JC, Segev DL, McCulloch CE, et al. Physical frailty after liver transplantation. American Journal of Transplantation 2018;18:1986–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017;66:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. The Lancet Oncology 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 10.Arora GK, Gupta A, Narayanan S, et al. Cachexia-associated adipose loss induced by tumor-secreted leukemia inhibitory factor is counterbalanced by decreased leptin. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 2009;89:381–410. [DOI] [PubMed] [Google Scholar]
- 12.Aoyagi T, Terracina KP, Raza A, et al. Cancer cachexia, mechanism and treatment. World journal of gastrointestinal oncology 2015;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora GK, Gupta A, Guo T, et al. Janus kinase inhibitors suppress cancer cachexia-associated anorexia and adipose wasting in mice. JCSM Rapid Communications 2020;3:115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau SK, Gannavarapu BS, Carter K, et al. Impact of Socioeconomic Status on Pretreatment Weight Loss and Survival in Non–Small-Cell Lung Cancer. Journal of oncology practice 2018;14:e211–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gannavarapu BS, Lau SK, Carter K, et al. Prevalence and survival impact of pretreatment cancer-associated weight loss: a tool for guiding early palliative care. Journal of oncology practice 2018;14:e238–e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachmann J, Heiligensetzer M, Krakowski-Roosen H, et al. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg 2008;12:1193–201. [DOI] [PubMed] [Google Scholar]
- 17.Shen S, Araujo JL, Altorki NK, et al. Variation by stage in the effects of prediagnosis weight loss on mortality in a prospective cohort of esophageal cancer patients. Dis Esophagus 2017;30:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moses AW, Slater C, Preston T, et al. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br J Cancer 2004;90:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 20.Rich NE, Murphy CC, Yopp AC, et al. Sex disparities in presentation and prognosis of 1110 patients with hepatocellular carcinoma. Alimentary Pharmacology & Therapeutics 2020;52:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rich NE, Hester C, Odewole M, et al. Racial and Ethnic Differences in Presentation and Outcomes of Hepatocellular Carcinoma. Clinical Gastroenterology and Hepatology 2019;17:551–559.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hester CA, Rich NE, Singal AG, et al. Comparative Analysis of Nonalcoholic Steatohepatitis–Versus Viral Hepatitis–and Alcohol-Related Liver Disease– Related Hepatocellular Carcinoma. Journal of the National Comprehensive Cancer Network 2019;17:322–329. [DOI] [PubMed] [Google Scholar]
- 23.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification, In Seminars in liver disease, © 1999 by Thieme Medical Publishers, Inc., 1999. [DOI] [PubMed] [Google Scholar]
- 24.Plauth M, Bernal W, Dasarathy S, et al. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr 2019;38:485–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mondello P, Lacquaniti A, Mondello S, et al. Emerging markers of cachexia predict survival in cancer patients. BMC Cancer 2014;14:828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephens NA, Skipworth RJE, Gallagher IJ, et al. Evaluating potential biomarkers of cachexia and survival in skeletal muscle of upper gastrointestinal cancer patients. Journal of Cachexia, Sarcopenia and Muscle 2015;6:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gingrich A, Volkert D, Kiesswetter E, et al. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatrics 2019;19:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berardi E, Madaro L, Lozanoska-Ochser B, et al. A Pound of Flesh: What Cachexia Is and What It Is Not. Diagnostics (Basel) 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rich NE, Parvathaneni A, Sen A, et al. High Neutrophil-Lymphocyte Ratio and Delta Neutrophil-Lymphocyte Ratio Are Associated with Increased Mortality in Patients with Hepatocellular Cancer. Dig Dis Sci 2021. [DOI] [PubMed] [Google Scholar]
- 30.Sun K, Chen S, Xu J, et al. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. Journal of Cancer Research and Clinical Oncology 2014;140:1537–1549. [DOI] [PubMed] [Google Scholar]
- 31.Lai JC, Tandon P, Bernal W, et al. Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021;n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tandon P, Montano-Loza AJ, Lai JC, et al. Sarcopenia and frailty in decompensated cirrhosis. Journal of Hepatology 2021;75:S147–S162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai JC, Covinsky KE, McCulloch CE, et al. The Liver Frailty Index Improves Mortality Prediction of the Subjective Clinician Assessment in Patients With Cirrhosis. Official journal of the American College of Gastroenterology | ACG 2018;113:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LeBlanc TW, Nipp RD, Rushing CN, et al. Correlation between the international consensus definition of the Cancer Anorexia-Cachexia Syndrome (CACS) and patient-centered outcomes in advanced non-small cell lung cancer. J Pain Symptom Manage 2015;49:680–9. [DOI] [PubMed] [Google Scholar]
- 35.Gannavarapu BS, Lau SKM, Carter K, et al. Prevalence and Survival Impact of Pretreatment Cancer-Associated Weight Loss: A Tool for Guiding Early Palliative Care. J Oncol Pract 2018;14:e238–e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun L, Quan XQ, Yu S. An Epidemiological Survey of Cachexia in Advanced Cancer Patients and Analysis on Its Diagnostic and Treatment Status. Nutr Cancer 2015;67:1056–62. [DOI] [PubMed] [Google Scholar]
- 37.Anker MS, Holcomb R, Muscaritoli M, et al. Orphan disease status of cancer cachexia in the USA and in the European Union: a systematic review. J Cachexia Sarcopenia Muscle 2019;10:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rich NE, John BV, Parikh ND, et al. Hepatocellular Carcinoma Demonstrates Heterogeneous Growth Patterns in a Multicenter Cohort of Patients With Cirrhosis. Hepatology 2020;72:1654–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nathani P, Gopal P, Rich N, et al. Hepatocellular carcinoma tumour volume doubling time: a systematic review and meta-analysis. Gut 2021;70:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of hepatology 2006;44:217–231. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki H, Asakawa A, Amitani H, et al. Cancer cachexia—pathophysiology and management. Journal of gastroenterology 2013;48:574–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muscaritoli M, Molfino A, Lucia S, et al. Cachexia: a preventable comorbidity of cancer. A TARGET approach. Critical reviews in oncology/hematology 2015;94:251–259. [DOI] [PubMed] [Google Scholar]
- 43.Argilés JM, Busquets S, López-Soriano FJ. Cytokines in the pathogenesis of cancer cachexia. Current Opinion in Clinical Nutrition & Metabolic Care 2003;6:401–406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.