Abstract
Some herbivorous insects possess the ability to synthesize phytohormones and are considered to use them for manipulating their host plants, but how these insects acquired the ability remains unclear. We investigated endogenous levels of auxin (IAA) and cytokinins (iP and tZ), including their ribosides (iPR and tZR), in various terrestrial arthropod taxa. Surprisingly, IAA was detected in all arthropods analysed. In contrast, tZ and/or tZR was detected only in some taxa. Endogenous levels of IAA were not significantly different among groups with different feeding habits, but gall inducers possessed significantly higher levels of iPR, tZ and tZR. Ancestral state reconstruction of the ability to synthesize tZ and tZR revealed that the trait has only been acquired in taxa containing gall inducers. Our results strongly suggest critical role of the cytokinin synthetic ability in the evolution of gall-inducing habit and IAA has some function in arthropods.
Subject terms: Entomology, Phylogenetics, Plant hormones
Introduction
Insects are the most speciose taxa in terrestrial ecosystems1–3. In terms of feeding habit, almost half of extant insect species are phytophagous, meaning they consume living plant material4. Based on the oldest insect fossils and recent molecular studies, insects are estimated to have evolved on the earth at roughly the same time as plants3,5,6. It should be noted that phytophagy is uncommon in terrestrial arthropods other than Acari and Insecta. This suggests that the evolution of phytophagy was an important event in the adaptive radiation and diversification of extant insects.
Among phytophagous insects, some taxa have evolved the ability to induce galls on their host plants and can manipulate host plant metabolism and morphogenesis in highly sophisticated ways7–9. Phytohormones, particularly auxin (indole-3-acetic acid, IAA) and cytokinins, have been reported to play an important role in gall induction by insects10–12. A study has demonstrated that the larvae of a gall-inducing sawfly Pontania sp. (Hymenoptera: Tenthredinidae) possess the enzymatic activity necessary to synthesize IAA within their body10. In addition, this sawfly probably has the ability to synthesize trans-zeatin riboside (tZR), a riboside form of the bioactive cytokinin trans-zeatin (tZ). The sawfly larvae that exit galls in the autumn possess only low amounts of tZR, whereas the female adults that emerge in spring, after overwintering in the soil, exhibit an extraordinarily high concentration of tZR10. Such high concentrations of auxin and cytokinins (or their ribosides) have been detected in some gall-inducing insects, including a cecidomyiid (Diptera)13 and a psyllid (Hemiptera)14, suggesting that those phytohormones are associated with gall-inducing insects or with galls in various insect taxa.
Surprisingly, the ability to synthesize auxin has been found not only in gall-inducing insects but also in other insects, such as the silkworm, Bombyx mori (Lepidoptera), the western honeybee, Apis mellifera (Hymenoptera), the common fruit fly, Drosophila melanogaster (Diptera), and even in non-phytophagous species including the housefly, Musca domestica (Diptera)15,16. Most recently, the biosynthetic pathway of IAA was clarified in B. mori and Pontania sp., and some key enzymes involved in the IAA synthesis were identified17–19.
On the basis of the above, we hypothesized that insects evolved the ability to synthesize auxin or cytokinin prior to the evolution of phytophagy or gall induction. To explore the evolutionary origins of the ability to synthesize auxin and cytokinins in insects, we comprehensively investigated endogenous levels of these phytohormones in various terrestrial arthropods. We then clarified that endogenous IAA was present in all major groups of terrestrial arthropods, including spiders, mites, crustaceans, millipedes and insects. In contrast, we revealed that only some taxa containing gall inducers seem to have acquired the ability to synthesize tZ or tZR in insects. Based on these results, we discuss the possible involvement of the acquisition of an ability to synthesize phytohormones in the evolution of phytophagous and gall-inducing habits in insects.
Methods
Preparation of samples and quantification of phytohormones
Various terrestrial arthropods were either sampled from laboratory-reared strains or were collected from the field by direct capture or net-sweeping for flying adults. Detailed collection data are shown in Table S1. During the sampling, we took measures to diminish contamination with plant-derived phytohormones, as follows: living samples (especially herbivores) were starved for two days, or developmental stages not directly associated with living plants (hatchlings, pupae etc.) were used. Each sample consisted of one individual (if they weighed ca. ≥ 5 mg), while for tiny species, several to hundreds of individuals were combined per sample to give 5–10 mg fresh weight (FW). As far as the situation allowed, three replications were prepared for each species. Samples were weighed and frozen as soon as they had been prepared.
The concentration of indole-3-acetic acid (IAA) and cytokinins, such as isopentenyladenine (iP) and trans-zeatin (tZ), and their ribosides, isopentenyladenosine (iPR) and trans-zeatin riboside (tZR), were measured using whole body extracts, according to a previously described method using stable isotope-labelled internal standards14.
Reconstruction of ancestral traits
Ancestral state reconstructions were performed using maximum-likelihood reconstructions analysis and the Markov k-state one-parameter (Mk1) model in Mesquite 3.619. The maximum-likelihood analyses find the ancestral states (the internal nodes) that maximize the probability that the observed character states (the terminal nodes) would evolve under a stochastic model of evolution21,22. The Mk1 model assumes that any character change is terminal probable23. The tree topology follows the latest phylogenetic tree inferring the relationships among insect orders and their ancestral terrestrial arthropods based on 1478 protein-coding genes3. The ability of synthesizing phytohormones in the major taxa of the phylogeny was categorized in the analyses based on our data (Table S2) as follows: (0) absent, (1) present, (2) present and absent. Taxa in which either the bioactive phytohormone or its riboside form was not detected, and the mean concentration of the other form was less than 1.0 ng/g FW were treated as contaminated and were considered lacking the ability to synthesize phytohormones.
Comparisons of endogenous phytohormone levels among taxa and feeding habits
Endogenous phytohormone concentrations were analysed among taxa (Apterous insects/Paleoptera, Polyneoptera, Condylognatha and Holometabola) and feeding habit (gall inducers, non-galling herbivores feeding on fresh plant parts, and feeding guilds other than herbivores) using generalized linear models (GLM) with a Gaussian distribution. Categorization of feeding habits were based either on literatures24–26. The categorization of taxa and feeding habits is summarized in Table S2. Treatment means were compared using Tukey’s HSD test. In cases when interaction effects between taxa and feeding habit were significant, phytohormone concentrations were analysed among feeding habit within each higher taxon (Condylognatha and Holometabola). All statistical analyses were performed using R ver. 4.0.327.
Results
A certain concentration of IAA was detected in all terrestrial arthropods, including spiders, mites, crustaceans and a millipede, that were analysed in this study (Table S2). The IAA concentrations were extraordinarily high comparing to the concentrations in general plant tissues in some taxa, such as mites, the millipede, an Archaeognatha (bristletail), and a sawfly (> 1000 ng/g FW). Even in the other species, the concentration of IAA was relatively high (237.9 ± 61.8 ng/g FW on average ± SE in all taxa).
The mean concentrations (± SE) of cytokinins were 8.5 ± 3.0, 22.1 ± 4.4, 12.1 ± 3.6 and 7.3 ± 1.9 ng/g FW for iP, iPR, tZ and tZR, respectively, which was also relatively high. In contrast to IAA, cytokinins were not detected in some taxa of insects (Table S1). Notably, concentrations of 1.0 ng/g FW or more of tZ and tZR were not detected in any taxa belonging to Myriapoda, apterous insects, Paleoptera, or Polyneoptera, although the Polyneoptera include phytophagous orders such as Orthoptera and Phasmatodea. In addition, heteropterans, which also include phytophagous taxa, generally possessed very low levels of tZ and tZR. Gall inducers, such as Gynaikothrips uzeli, Tetraneura nigriabdominalis, Pontania sp. and Rhopalomyia yomogicola, had relatively high concentrations of tZ and tZR. In other arthropods, mites possessed extraordinary high concentrations of iPR (Table S2).
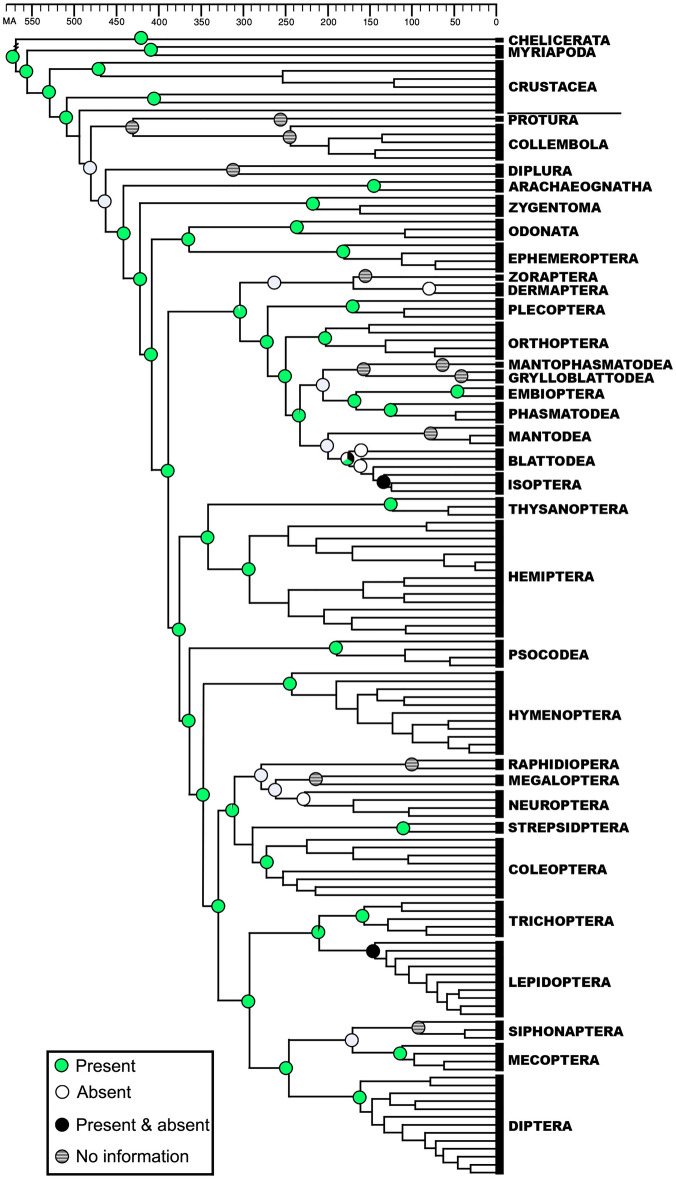
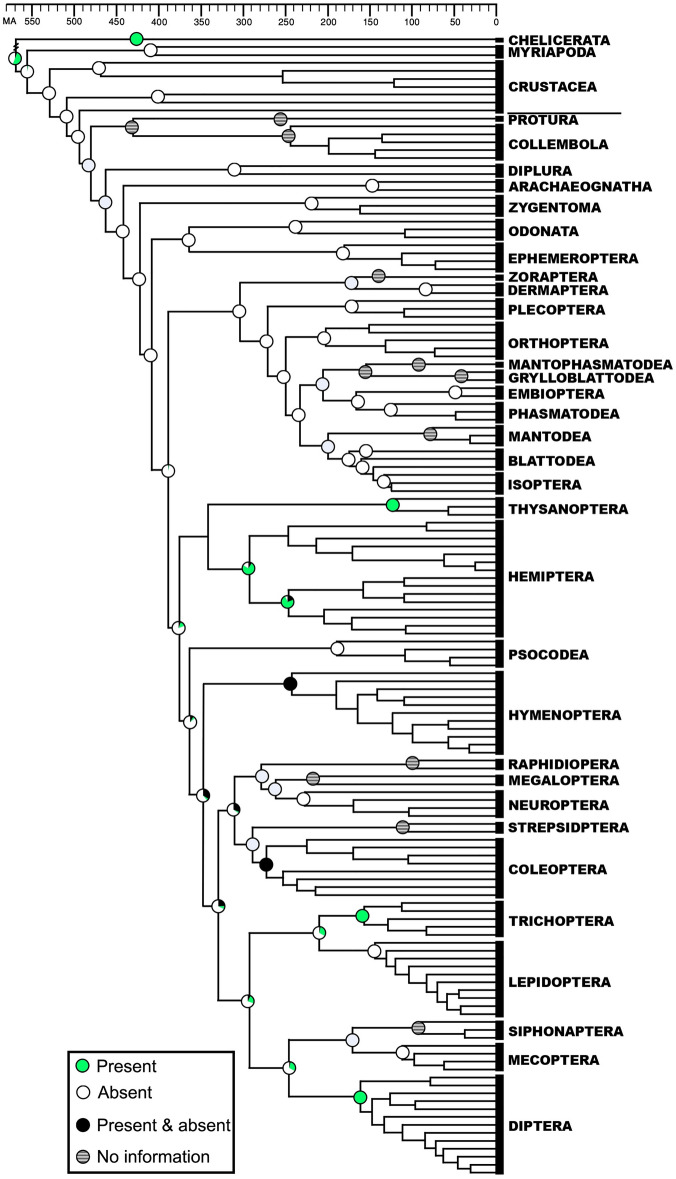
The reconstruction of ancestral traits led us to deduce that inferred common ancestor of insects possessed the ability to synthesize iP (or iPR), but some taxa have since lost this ability (Fig. 1). In contrast, the common ancestor of insects was inferred not to have had the ability to synthesize tZ (or tZR), and the ability appears to have been acquired independently in Condylognatha and Holometabola around 300 MA (Fig. 2).
Figure 1.
Ancestral state reconstruction of the ability to synthesize isopentenyladenine (iP) and isopentenyladenosine (iPR) in terrestrial arthropods. Circles in nodes represent the percentage of the probability of the reconstructed character state. The time-calibrated phylogenetic tree is modified from (3). Time Scale is in millions of years before present.
Figure 2.
Ancestral state reconstruction of the ability to synthesize trans-zeatin (tZ) and trans-zeatin riboside (tZR) in terrestrial arthropods. Circles in nodes represent the percentage of the probability of the reconstructed character state. The time-calibrated phylogenetic tree is modified from (3). Time Scale is in millions of years before present.
In GLM analyses, IAA concentrations were significantly different among feeding habit, but not among taxa (DF = 3, χ2 = 7.41, p = 0.060 for taxa; DF = 2, χ2 = 6.84, p = 0.033 for feeding habit; DF = 3, χ2 = 4.38; p = 0.224 for taxa × feeding habit) (Fig. 3); iP concentrations were not significantly different among both taxa and feeding habit (GLM; DF = 3, χ2 = 1.11; p = 0.78 for taxa; DF = 2, χ2 = 0.95, p = 0.621 for feeding habit; DF = 3, χ2 = 0.284, p = 0.963 for taxa × feeding habit) (Fig. 3); iPR concentrations were significantly different among both taxa and feeding habit and their interaction was also significant (GLM; DF = 3, χ2 = 34.46; p < 0.001 for taxa; DF = 2, χ2 = 52.18, p < 0.001 for feeding habit; DF = 3, χ2 = 47.712, p < 0.001 for taxa × feeding habit) (Fig. 4); in Condylognatha, gall inducers possessed significantly higher concentrations of iPR than non-galling herbivores and others, but no significant differences were detected among different feeding habits in Holometabola (Fig. 4); tZ concentrations were significantly different among feeding habit and interaction between taxa and feeding habit was significant (GLM; DF = 3, χ2 = 3.39; p = 0.336 for taxa; DF = 2, χ2 = 16.70, p < 0.001 for feeding habit; DF = 3, χ2 = 22.65, p < 0.001 for taxa × feeding habit) (Fig. 4); in contrast to iPR, no significant differences were detected in tZ concentrations among different feeding habits in Condylognatha, but gall inducers possessed significantly higher concentrations of tZ than non-galling herbivores and others in Holometabola (Fig. 4); tZR concentrations were not significantly different among both taxa and feeding habit (GLM; DF = 3, χ2 = 5.93; p = 0.115 for taxa; DF = 2, χ2 = 4.60, p = 0.100 for feeding habit; DF = 3, χ2 = 1.11, p = 0.774 for taxa × feeding habit).
Figure 3.
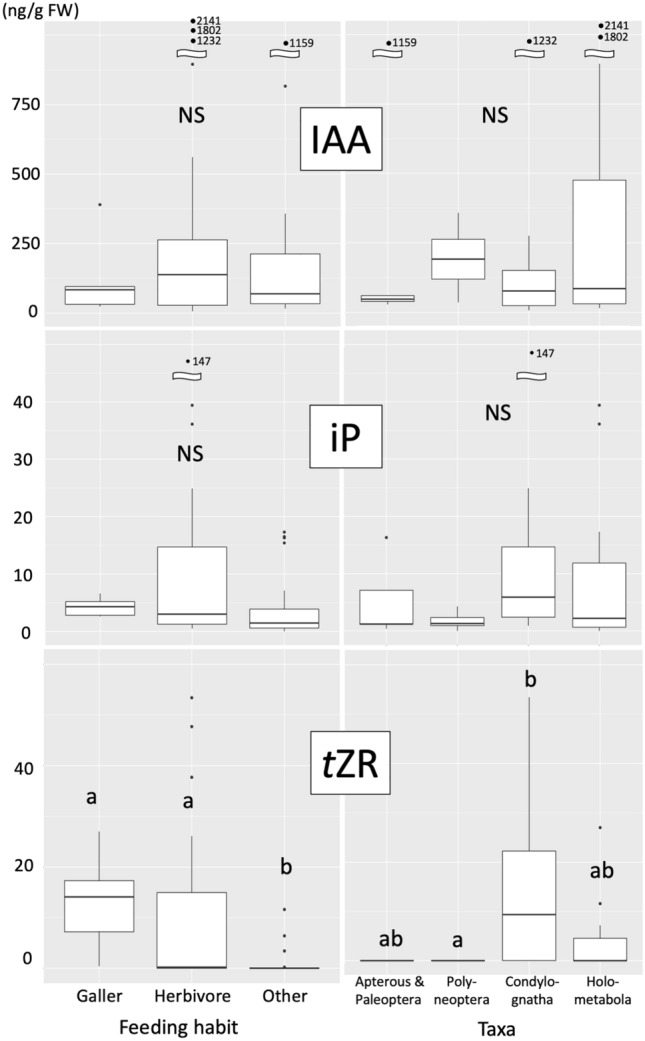
Comparison of endogenous concentrations of auxin (indole-3-acetic acid, IAA), isopentenyladenine (iP), and trans-zeatin riboside (tZR) among different taxa and feeding habits (gallers, non-galling herbivores and others) of terrestrial insects. The same letters above bars indicate no significant differences among groups (GLM).
Figure 4.
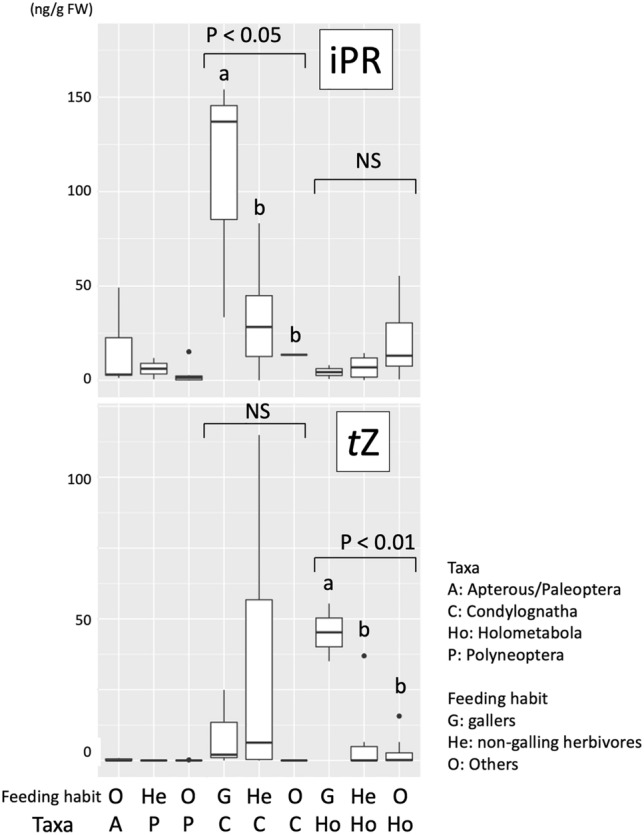
Comparison of endogenous concentrations of trans-zeatin (tZ), and isopentenyladenosine (iPR) among different feeding habits of insects. Because significant interaction effects between taxa and feeding habit were detected, concentrations were compared among different feeding guilds within Condylognatha and Holometabola, respectively.
Discussion
In this study, we determined that terrestrial arthropods possess relatively high concentrations of endogenous IAA compared with the concentration of IAA in most plant tissues, i.e., approximately 20 and 24 ng/g FW in Arabidopisis28 and in Oryza sativa29, respectively. This phenomenon cannot be explained by sequestration of phytohormones in insect bodies, because not only herbivores but also non-herbivores, even species which do not consume plant materials throughout their life similarly possess certain amounts of phytohormones. In addition, the amounts of phytohormones detected in the insect bodies are two or three orders of magnitude higher than those in ordinal plant tissues. As mentioned earlier, a gall-inducing sawfly (Pontania sp.) and some other insect species were experimentally proved to possess the enzymatic activity to synthesize IAA within their body10. Although we did not perform an analysis of the ability to synthesize IAA, it is clear that the common ancestor of insects, as well as that of other terrestrial arthropods, possessed endogenous auxin. This suggests the possibility that the auxin synthetic ability has been retained in terrestrial arthropods for a long period. To date, studies of the associations between phytohormones and insects have focused on gall inducers and their host-manipulating mechanisms12. In fact, we believe that no researchers have either expected or predicted the presence of phytohormones in such a wide range of terrestrial arthropods, including not only phytophagous insects but also other insect species, in addition to spiders, millipedes and crustaceans. This is surprising and challenges the notion that phytohormones are substances primarily involved in plant signalling. Moreover, we found that the endogenous concentrations of IAA were generally high in terrestrial arthropods, which strongly suggests that IAA and/or enzymes responsible for its synthesis have specific functions in arthropods. In D. melanogaster, the function of the gene most homologous (Aldox89A) to the aldehyde oxidase of B. mori is unknown, but the activity of this enzyme increases at pupation and midway through the pupal stage30. Comparative studies and functional analyses will be necessary in the future, but we detected IAA in both males and females, as well as in various developmental stages, implying that IAA and/or related enzymes play particular roles throughout the lifecycle stages. Although we did not detect any significant relationships between endogenous IAA levels and feeding habits, at least in some gall inducers IAA is involved in their gall induction31,32. For example, fundatrices of the aphid Tetraneura nigriabdominalis (Hemiptera), which are responsible for gall induction, appear to actively synthesize IAA during the gall induction initiation process33. In our current study, we analysed IAA levels using specimens not directly related to plants, even for herbivores and gall inducers, so the basic level of IAA may be similar among different feeding guilds, and some gall inducers and herbivores may enhance their IAA levels as needed.
The mean concentrations of cytokinins were also somewhat high in comparison with the concentrations of iP, iPR, tZ and tZR in most plant tissues: ca. 0.15, 5.0, 0.4 and 5.0 ng/g FW, respectively, in Arabidopsis34 and 0.18, 0.4, 0.16 and 0.7 ng/g FW, respectively, in O. sativa29. Although a recent study showed the widespread distribution of cytokinins in insects35, the investigation was restricted to herbivorous insects belonging to Condylognatha and Holometabola and did not discuss differences between iP- and tZ-type cytokinins. Our analysis suggested that the common ancestor of insects also possessed the ability to synthesize iP (or iPR) but, unlike with IAA, this ability seems to have been lost in some taxa. The common ancestor of insects was also thought not to possess the ability to synthesize tZ (and tZR). Insects seem to possess genes for tRNA isopentenyltransferase (tRNA-IPT) but not for adenylate-IPT; therefore, they may have acquired the highly effective ability to synthesize cytokinin via prenylation of tRNA. Further studies are needed to clarify the hydroxylation mechanism required to produce tZ/tZR. In leaf-mining moths, which induce ‘green island effects’ by the prolongation of cell life in plant tissue surrounding their mines on shed leaves, the endosymbiont Wolbachia seems to play a critical role in the production of cytokinins in these insects’ bodies36,37. Although information relating to endosymbiotic bacteria in our study is limited, it is estimated that almost 40% of insects possess Wolbachia endosymbionts38. So, in some insects, endogenous cytokinins may be derived from Wolbachia or other symbionts. However, a recent study suggested that specific bacterial symbionts are not involved in gall induction by insects39. Moreover, in the gall-inducing sawfly Pontania sp., which contains very high quantities of tZ and tZR10, studies involving both de novo RNA-seq using rRNA-depleted mRNA and de novo draft genome sequencing detected only a single insect-derived IPT gene (Suzuki et al., unpublished data). So, these insects are likely to have the ability to synthesize cytokinins without any assistance from symbionts.
In cytokinins, we found that gall inducers and non-galling herbivores possessed higher concentrations of tZR than other feeding guilds. These results imply that herbivorous insects may use cytokinins when they feed on their host plants for some purposes (e.g., disturbance of antiherbivore defense). A recent study demonstrated that saliva of the fall armyworm Spodoptera frugiperda contains some phytohormones, such as jasmonic acid, salicylic acid and abscisic acid, and non-protein components40. These phytohormones are suggested to modulate plant defensive responses40. Furthermore, gallers possessed higher concentrations of iPR in Condylognatha and tZ in Holometabola. In addition, major phytophagous orders, including Orthoptera, Phasmatodea and Heteroptera, largely lack endogenous tZ and tZR and, notably, the gall-inducing habit has not evolved in these taxa41. These imply that phytohormones mainly used by Condylognatha and Holometabola are somewhat different from each other. Moreover, the acquisition of the ability to synthesize tZ seem to have played a critical role in the evolution of the gall-inducing habit in Holometabola. Gall inducers are well known to use these phytohormones in manipulating plant tissue10,12. In Arabidopsis, tZ-type cytokinins are distributed mainly in xylem sap and transported from the roots to the aboveground parts, while iP-type cytokinins are distributed in phloem sap and transported in the opposite direction42. In crown gall formation caused by Agrobacterium, tZ-type cytokinins were shown to be more effective at gall induction than iP-type cytokinins43,44, possibly because of the lower affinity of tZ than that of iP for cytokinin oxidase, an enzyme responsible for the degradation of cytokinins43. In our previous study using a gall-inducing leafhopper, Cicadulina bipunctata, and susceptible and resistant varieties of maize, a significant increase in tZ (and not IAA and iP) was detected only in the susceptible variety of maize on which galls were induced11. The tZ concentrations are slightly high in gallers and non-galling herbivores belonging to Condylognatha and this is contrastive to the fundamental lack of this phytohormones in other feeding guilds as well as in Polyneoptera, Paleoptera, and apterous insects. Further studies are needed to clarify the importance of tZ type cytokinin in gall induction by insects. High concentrations of cytokinins were also detected in mites, which also include herbivores and gall inducers. The cytokinin synthetic ability of mites and its involvement of phytophagy and gall induction is an interesting future study subject.
In conclusion, in this study we showed that a broad range of terrestrial arthropods possess relatively high concentrations of IAA within their bodies. It is suggested that the common ancestor of insects possessed the ability to synthesize IAA and iP (or iPR). In contrast, the ability to synthesize tZ and/or tZR was inferred to have been acquired in just a few insect taxa, including gall inducers. Specifically, gall inducers possessed a higher concentration of some cytokinins than other feeding guilds, a strong indication of the importance and involvement of these phytohormones in the evolution of gall-inducing habits among terrestrial arthropods.
Supplementary Information
Acknowledgements
We thank M. Ryuda and T. Matsumura for their kind help and valuable information on Drosophila melanogaster. We also thank T. Saito, T. Sobagaki, A. Kita, Y. Mochioka, Y. Nakabayashi, Y. So, and other members of the Laboratory of Systems Ecology for their kind support in collecting materials. This work was partly supported by JSPS KAKENHI Grants no. JP17H03947 and JP20K21316.
Author contributions
M.T. and Y.S. conceived and designed the study. M.T., Y.S., S.F., H.M., S. A-F. and A. K. E. performed the experiments. M.T., Y.S. and A.K.E. analyzed data. M.T. and Y.S. wrote the main manuscript text, A. K. E. prepared Figs. 1–2, M.T. prepared the other figures. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-08558-6.
References
- 1.Mora C, Tittensor DP, Adl S, Simpson GB, Worm B. How many species are there on Earth and in the ocean. PLoS Biol. 2011;9:e1001127. doi: 10.1371/journal.pbio.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costello MJ, Wilson S, Houlding B. Predicting total global species richness using rates of species description and estimates of taxonomic effort. Syst. Biol. 2012;61:871–883. doi: 10.1093/sysbio/syr080. [DOI] [PubMed] [Google Scholar]
- 3.Misof B, Liu S, Meusemann K, Peters RS, Donath A, Mayer C, et al. Phylogenomics resolves the timing and pattern of insect evolution. Science. 2014;346:763–767. doi: 10.1126/science.1257570. [DOI] [PubMed] [Google Scholar]
- 4.Price PW, Denno RF, Eubanks MD, Finke DL, Kaplan I. Insect ecology: behavior, populations and communities. Cambridge University Press; 2011. [Google Scholar]
- 5.Kevan PG, Chaloner WG, Savile D. Interrelationships or early terrestrial arthropods and plants. Palaeontology. 1975;18:391–417. [Google Scholar]
- 6.Engel MS, Grimaldi DA. New light shed on the oldest insect. Nature. 2004;427:627–630. doi: 10.1038/nature02291. [DOI] [PubMed] [Google Scholar]
- 7.Stone GN, Schönrogge K. The adaptive significance of insect gall morphology. TRENDS Ecol. Evolut. 2003;18:512–522. doi: 10.1016/S0169-5347(03)00247-7. [DOI] [Google Scholar]
- 8.Shorthouse JD, Wool D, Raman A. Gall-inducing insects: nature’s most sophisticated herbivores. Basic Appl. Ecol. 2005;6:407–411. doi: 10.1016/j.baae.2005.07.001. [DOI] [Google Scholar]
- 9.Giron D, Huguet E, Stone GN, Body M. Insect-induced effects on plants and possible effectors used by galling and leaf-mining insects to manipulate their host-plant. J. Insect Physiol. 2016;84:70–89. doi: 10.1016/j.jinsphys.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi H, Tanaka H, Hasegawa M, Tokuda M, Asami T, Suzuki Y. Phytohormones and willow gall induction by a gall-inducing sawfly. New Phytol. 2012;196:586–595. doi: 10.1111/j.1469-8137.2012.04264.x. [DOI] [PubMed] [Google Scholar]
- 11.Tokuda M, Jikumaru Y, Matsukura K, Takebayashi Y, Kumashiro S, Matsumura M, Kamiya Y. Phytohormones related to host plant manipulation by a gall-inducing leafhopper. PLoS ONE. 2013;8:e62350. doi: 10.1371/journal.pone.0062350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tooker JF, Helms AM. Phytohormone dynamics associated with gall insects, their potential role in the evolution of the gall-inducing habit. J. Chem. Ecol. 2014;40:742–753. doi: 10.1007/s10886-014-0457-6. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y, Okada K, Asami T, Suzuki Y. Phytohormones in Japanese mugwort gall induction by a gall-inducing gall midge. Biosci. Biotechnol. Biochem. 2013;77:1942–1948. doi: 10.1271/bbb.130406. [DOI] [PubMed] [Google Scholar]
- 14.Kai S, Kumashiro S, Adachi S, Suzuki Y, Shiomi Y, Matsunaga K, Gyoutoku N, Asami T, Tokuda M. Life history of Stenopsylla nigricornis (Hemiptera: Psylloidea: Triozidae) and phytohormones involved in its gall induction. Arthropod-Plant Interact. 2017;11:99–108. doi: 10.1007/s11829-016-9470-8. [DOI] [Google Scholar]
- 15.Suzuki H, Yokokura J, Ito T, Arai R, Yokoyama C, Toshima H, Nagarta S, Asami T, Suzuki Y. Biosynthetic pathway of the phytohormone auxin in insects and screening of its inhibitors. Insect Biochem. Mol. Biol. 2014;53:66–72. doi: 10.1016/j.ibmb.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama C, Takei M, Kouzuma Y, Nagata S, Suzuki Y. Novel tryptophan metabolic pathways in auxin biosynthesis in silkworm. J. Insect Physiol. 2017;101:91–96. doi: 10.1016/j.jinsphys.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama C, Takei M, Kouzuma Y, Nagata S, Suzuki Y. Novel tryptophan metabolic pathways in auxin biosynthesis in silkworm. J. Insect Physiol. 2019;101:91–96. doi: 10.1016/j.jinsphys.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Takei M, Kogure S, Yokoyama C, Kouzuma Y, Suzuki Y. Identification of an aldehyde oxidase involved in indole-3-acetic acid synthesis in Bombyx mori silk gland. Biosci. Biotechnol. Biochem. 2019;83:129–136. doi: 10.1080/09168451.2018.1525275. [DOI] [PubMed] [Google Scholar]
- 19.Miyata U, Arakawa K, Takei M, Asami T, Asanbou K, Toshima H, Suzuki Y. Identification of an aromatic aldehyde synthase involved in indole-3-acetic acid biosynthesis in the galling sawfly (Pontania sp.) and screening of an inhibitor. Insect Biochem. Mol. Biol. 2021;137:103639. doi: 10.1016/j.ibmb.2021.103639. [DOI] [PubMed] [Google Scholar]
- 20.Maddison, W. P., & Maddison, D. R. Mesquite, a modular system for evolutionary analysis. Version 3.6 (http://www.mesquiteproject.org) (2019)
- 21.Schluter D, Price T, Mooers AØ, Ludwig D. Likelihood of ancestor states in adaptive radiation. Evolution. 1997;51:1699–1711. doi: 10.1111/j.1558-5646.1997.tb05095.x. [DOI] [PubMed] [Google Scholar]
- 22.Pagel M. The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Syst. Biol. 1999;48:612–622. doi: 10.1080/106351599260184. [DOI] [Google Scholar]
- 23.Lewis PO. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 2001;50:913–925. doi: 10.1080/106351501753462876. [DOI] [PubMed] [Google Scholar]
- 24.Hirashima Y, Morimoto K, Tadauchi O. A textbook of systematic entomology. Kawashima Shoten Publ. Co.; 1989. [Google Scholar]
- 25.Yukawa, J., & Masuda, H. Insect and Mite Galls of Japan in Colors. Zenkoku Nôson Kyôiku Kyôkai, Tokyo. (In Japanese with English explanations for color plates.) (1996)
- 26.The Japanese Society of Applied Entomology and Zoology. Major insect and other pests economic plants in Japan. Revised Edition. The Japanese Society of Applied Entomology and Zoology, Tokyo (2006)
- 27.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/ (2021)
- 28.Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, et al. The main auxin biosynthesis pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2011;108:18512–18517. doi: 10.1073/pnas.1108434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kojima M, Kamada-Nobusada T, Komatsu H, Takei K, Kuroha T, Mizutani M, et al. Highly sensitive and high-throughput analysis of plant hormones using MS-probe modification and liquid chromatography–tandem mass spectrometry: an application for hormone profiling in Oryza sativa. Plant Cell Physiol. 2009;50:1201–1214. doi: 10.1093/pcp/pcp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickinson WJ. A genetic locus affecting the developmental expresson of an enzyme in Drosophila melanogaster. Develop. Biol. 1975;42:131–140. doi: 10.1016/0012-1606(75)90319-X. [DOI] [PubMed] [Google Scholar]
- 31.Tooker JF, De Moraes CM. Feeding by a gall-inducing caterpillar species increases levels of indole-3-acetic and decreases abscisic acid in Solidago altissima stems. Arthropod Plant Interact. 2011;5:115–124. doi: 10.1007/s11829-010-9120-5. [DOI] [Google Scholar]
- 32.Tooker JF, De Moraes CM. Feeding by Hessian fly (Mayetiola destructor [Say]) larvae increases levels of fatty acids and indole-3-acetic acid, but not hormones involved in plant-defense signaling. J. Plant Growth Regul. 2011;30:158–165. doi: 10.1007/s00344-010-9177-5. [DOI] [Google Scholar]
- 33.Takei M, Yoshida S, Kawai T, Hasegawa M, Suzuki Y. Adaptive significance of gall formation for a gall-inducing aphids on Japanese elm trees. J. Insect Physiol. 2015;72:4351. doi: 10.1016/j.jinsphys.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Miyawaki K, Tarkowski P, Matsumoto-Kitano M, Kato T, Sato S, Tarkowska D, et al. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA. 2006;103:16598–16603. doi: 10.1073/pnas.0603522103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andreas P, Kisiala A, Emery RJN, De Clerk-Floate R, Tooker JF, Price PW, Miller DG, III, Cheng M-S, Connor EF. Cytokinins are abundant and widespread among insect species. Plants. 2020;9:208. doi: 10.3390/plants9020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaiser W, Huguet E, Casas J, Commin C, Giron D. Plant green-island phenotype induced by leaf-miners is mediated by bacterial symbionts. Proc. Roy. Soc. B. 2010;277:2311–2319. doi: 10.1098/rspb.2010.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giron D, Frago E, Glevarec G, Pieterse CMJ, Dicke M. Cytokinins as key regulators in plant–microbe–insect interactions, connecting plant growth and defence. Funct. Ecol. 2013;27:599–609. doi: 10.1111/1365-2435.12042. [DOI] [Google Scholar]
- 38.Zug R, Hammerstein P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE. 2012;7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammer TJ, De Clerk-Floate R, Tooker JF, Price PW, Miller DG, III, Connor EF. Are bacterial symbionts associated with gall induction in insects? Arthropod Plant Interact. 2021;15:1–12. doi: 10.1007/s11829-020-09800-6. [DOI] [Google Scholar]
- 40.Acevedo FE, Smith P, Peiffer M, Helms A, Tooker J, Felton GW. Phytohormones in fall armyworm saliva modulate defense responses in plants. J. Chem. Ecol. 2019;45:598–609. doi: 10.1007/s10886-019-01079-z. [DOI] [PubMed] [Google Scholar]
- 41.Raman, A., Schaefer, C. W., & Withers, T. M. Biology, Ecology, and Evolution of Gall-inducing Arthropods. Vols. I & II. Science Pub Inc, Enfield (2005)
- 42.Hirose N, Takei K, Kuroha T, Kamada-Nobusada T, Hayashi H, Sakakibara H. Regulation of cytokinin biosynthesis, compartmentalization and translocation. J. Exp. Bot. 2008;59:75–83. doi: 10.1093/jxb/erm157. [DOI] [PubMed] [Google Scholar]
- 43.Sakakibara H, Kasahara H, Ueda N, Kojima M, Takei K, Hishiyama S, et al. Agrobacterium tumefaciens increases cytokinin production in plastids by modifying the biosynthetic pathway in the host plant. Proc. Natl. Acad. Sci. USA. 2005;102:9972–9977. doi: 10.1073/pnas.0500793102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ueda N, Kojima M, Suzuki K, Sakakibara H. Agrobacterium tumefaciens tumor morphology root plastid localization and preferential usage of hydroxylated prenyl donor is important for efficient gall formation. Plant Physiol. 2012;159:1064–1072. doi: 10.1104/pp.112.198572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.