Abstract
Background.
Racemic (R,S) ketamine is a glutamatergic drug with potent and rapid acting antidepressant effects. An intranasal formulation of (S)-ketamine was recently approved by the US Food and Drug Administration (FDA) to be used in individuals with treatment-resistant-depression (TRD). There is no data directly comparing outcomes on depression or other co-morbidities between these two formulations of ketamine. However, recent meta-analyses have suggested that IV racemic ketamine may be more potent than IN-(S)-ketamine.
Methods.
We retrospectively analyzed clinical outcomes in 15 Veterans with comorbid treatment resistant depression (TRD) and post-traumatic-stress-disorder (PTSD) who underwent ketamine treatment at the VA San Diego Neuromodulation Clinic. All Veterans included in this analysis were given at least 6 intranasal (IN)-(S)-ketamine treatments prior to switching to treatment with IV racemic ketamine.
Results.
Veterans receiving ketamine treatment (including both IN-(S)-ketamine and IV-(R,S)-ketamine), showed significant reductions in both the Patient Health Questionnaire-9 (PHQ-9), a self-report scale measuring depression symptoms (rm ANOVA F(14,42) = 12.6, p < 0.0001) and in the PTSD- Checklist for DSM-5 (PCL-5), a self-report scale measuring PSTD symptoms (rm ANOVA F(13,39) = 5.9, p = 0.006). Post-hoc testing revealed that PHQ-9 scores were reduced by an average of 2.4 +/− 1.2 compared to baseline after (S)-ketamine treatments (p=0.18) and by an average of 5.6 +/−1 after IV ketamine treatments (p=.0003) compared to pre-treatment baseline scores. PCL-5 scores were reduced by an average of 4.3 +/− 3.3 after IN (S)-ketamine treatments (p = 0.6) and 11.8 +/− 3.5 after IV ketamine treatments (p = 0.02) compared to pre-treatment base-line scores.
Conclusions.
This work suggests that off-label IV (R,S)-ketamine could be considered a reasonable next step in patients who do not respond adequately to the FDA-approved IN (S)-ketamine. Further double-blinded, randomized-controlled-trials are warranted to assess whether IV racemic ketamine is more effective than IN-(S)-ketamine.
Keywords: ketamine, Depression, (S)-ketamine
Introduction:
Many randomized controlled trials have now shown that racemic (R,S) ketamine has rapid antidepressant effects, with significant improvements in symptoms typically observed after the first treatment1 2–5. These antidepressant effects are thought to be mediated by antagonism to the NMDA receptor6. As the (S) enantiomer is about 2–4x more potent than the R enantiomer in modulating NMDA currents7,8, it was theorized that a lower dosage of (S)-ketamine might show similar efficacy to a slightly higher dosage of racemic ketamine. Based on this theory, and the fact that intranasal formulations generally have ~40–50% bioavailability compared to IV dosing9,10, an intranasal formulation of (S)-ketamine (trade name Spravato) was developed and demonstrated to have clinically significant anti-depressant effects for treatment-resistant depression (TRD)11,12, leading to FDA approval.
At present, it is unclear whether IV racemic ketamine is more effective than IN (S)-ketamine for treatment-resistant-depression. IV racemic ketamine has several theoretical advantages over IN (S)-ketamine. First, IV racemic ketamine may lead to a higher effective dosage of ketamine. IV ketamine is prescribed off-label, dosed by weight up to 1mg/kg. By contrast, IN-(S)-ketamine uses a fixed dosing protocol14–16 with stringent limits on the maximum dosage achievable. Moreover, there is likely significant variability in terms of the actual bioavailability of intranasal ketamine across patients (i.e., spraying incorrectly, structural issues in nasal passages, etc. that limits absorption) compared to IV ketamine. Second, a metabolite of (R)-ketamine (hydroxynorketamine) has been postulated to have a unique mechanism of antidepressant action via modulation of AMPA receptors. The analogous metabolite of (S)-ketamine does not seem to share that action17,18. Thus, IV racemic ketamine may be more effective than IN (S)-ketamine due to the R-enantiomer or due to, on average, a higher effective dose (even when both are dosed according to recommended guidelines). There have been no head-to-head or cross-over studies that compare effects between the two formulations. However, a recent meta-analysis comparing effect sizes across various studies have suggested that the effect size of racemic IV ketamine (dosage ranging from 0.5mg/kg to 1mg/kg) may be about twice as strong as those observed from intranasal (IN) (S)-ketamine13.
In this paper, we retrospectively analyzed patient outcomes from 15 Veterans treated at the San Diego Veterans Affairs Medical Center (SDVAMC) who were first given antidepressant treatment with intranasal (S)-ketamine (IN-(S)-ketamine) and were then switched to IV racemic ketamine. All Veterans in this study received at least 6 doses of intranasal (S)-ketamine prior to the switch. They had all been diagnosed with treatment resistant depression (TRD), defined by a lack of response to at least two antidepressant trials prior to being referred for IN (S)-ketamine treatment, as well as post-traumatic-stress disorder (PTSD). In this study we analyzed changes in depression symptoms using the PHQ-9 self-report scale and changes in PTSD symptoms using the PCL-5 self-report scale, both gathered as part of routine clinical care. We hypothesized that IV ketamine would show a larger effect in clinical symptoms then IN (S)-ketamine.
Methods:
Study Design:
This retrospective chart review and analysis was approved as an IRB-exemption by the VA San Diego Medical Center IRB committee. We conducted a chart review of Veterans who were referred to the VA San Diego neuromodulation program between the dates of Jan 2020 to March 2021. For this chart review, we included all Veterans who were treated in our clinic between the above dates, received at least six IN (S)-ketamine treatments and then received at least two IV ketamine treatments. Fifteen Veterans met that inclusion criteria. One Veteran was excluded because the Veteran stopped treatment after only 1 IV ketamine treatment and we had no follow-up PHQ-9 scores.
Treatment:
Eligibility criteria for IN-(S)-ketamine in our clinic required a PHQ-9 score of at least 15 at the time of consultation, a failure to respond to at least two antidepressants and a recommendation by a clinician. Veterans were not eligible for IN-(S)-ketamine treatments if they had a history of psychosis, medical contraindications to ketamine treatment, or a history of ketamine abuse. IN-(S)-ketamine dosing in our clinic starts with twice/week treatments for 4 weeks (typically a total of 8 treatments) during the induction phase. Further treatments are offered after discussion with clinician based on initial treatment response. Indications for offering IV (R,S) ketamine treatment to Veterans varied and decisions were based on clinical discussions between the treating provider and Veteran. We did not perform a detailed review of this clinical decision-making, though generally individuals were referred based on a lack of optimal response to IN (S)-ketamine. Eight of the 15 in this analysis individuals had received IV or IM ketamine for depression prior to receiving ketamine treatment at the San Diego Neurostimulation clinic; thus these individuals had had prior exposure. Additionally, there was considerable variability in how quickly the 15 individuals were switched from IN (S)-ketamine to IV (R,S) ketamine. Five Veterans received only 6 IN (S)-ketamine dosages prior to switching. In the remainder, the number of IN (S)-ketamine treatments ranged from 8–30 prior to the switch to IV ketamine. IV ketamine was typically offered at a frequency of twice/week for at least the first 3 weeks, followed by flexible frequency as determined by the clinician.
Dosing in our clinic occurs in the following manner. (S)-ketamine treatments are always started at 56 mg on the first day of treatment, with flexible dosing increases/decreases on subsequent treatments based on tolerability and efficacy. All Veterans in this study were titrated up to the maximum dose (84mg) for at least 3 sessions prior to switching to IV (R,S)-ketamine. IV (R,S)-ketamine dosing started at 0.5 mg/kg, was increased to 0.75mg/kg at the second treatment and then up to 1mg/kg for the third treatment. All subsequent sessions continued at the 1mg/kg dosing.
Clinical Assessments:
Veterans were administered a Patient Health Questionnaire-9 (PHQ-9) to track depression and a PTSD Checklist for DSM5 (PCL-5) to track PTSD symptoms prior to each treatment. The PHQ-9 is a 9-question survey with score ranges from 0 to 27. Scores of 5–9 in the mild depression range; 10–14 in the moderate depression range; 15–19 in the moderately-severe depression range and scores 20 and above in the severe depression range. The PCL-5 is a 20 question self-report survey with scores ranging from 0 to 80. A score of 33 is often used as a reasonable cut-off for symptoms above a clinical threshold, with scores above 60 considered “severe”.
We also performed a chart review to gather auxiliary data related to severity of symptoms and co-morbidities (listed in Table 1). The data included age, gender, years in mental health treatment (based on first mental health treatment note in the VA records), number of suicide attempts and number of hospitalizations. We reviewed medical record diagnosis codes, along with the initial consultation note for our clinic to determine diagnostic history and co-morbidities such as Unipolar versus Bipolar Depression, history of treatment-resistance, diagnosis of PTSD, chronic pain diagnosis, and a history of alcohol, tobacco and substance use disorders. We also reviewed treatment history including past trials of (S)-ketamine/IV ketamine, ECT and history of adequate/inadequate antidepressant trials (as defined by the MGH Antidepressant Treatment Response Questionnaire). A full description of the Veterans included in this case series is included in Table 1. We did not systematically collect or review chart data on side effects of the ketamine treatments possibly reported by patients to clinicians, thus we did not include any information on this in the manuscript, as we thought that reporting incomplete data might bias towards reporting a lower side-effect rate than were actually experienced.
Table 1:
Patient Information
| Mean +/− STD | |
|---|---|
| Gender | 7F, 8M |
| Age | 49.1 +/− 11.3 years |
| Treatment Severity / Refractoriness | |
| # adequate antidepressants | 2.7 +/− 0.8 |
| Duration of illness | 12.5 +/− 7.2 years |
| Hospitalizations | 0.5 +/− 0.8 |
| Suicide Attempts | 0.3 +/− 0.6 |
| History of ECT | 8 (53%) |
| History of rTMS | 7 (47%) |
| History of Ketamine | 8 (53%) |
| Pre-treatment PHQ-9 | 21.9 +/− 2.7 |
| Pre-treatment PCL-5 | 57.1 +/− 11.3 |
| Co-Morbid Diagnoses | |
| Bipolar Spectrum | 6 (40%) |
| Chronic Pain | 11 (73%) |
| Tobacco | 3 (20%) |
| Marijuana | 3 (20%) |
| Alcohol | 4 (27%) |
Data Analyses:
We conducted four main analyses to understand the relative efficacy of IV ketamine. A repeated-measures ANOVA (rmANOVA) was performed on both PCL-5 and PHQ-9 at 4 key time points: 1) prior to starting (S)-ketamine (pre-IN); 2) prior to the last (S)-ketamine treatment (last-IN); 3) prior to the first IV ketamine treatment (pre-IV); 4) prior to the 6th-IV treatment (6th-IV). The 6th IV treatment was chosen as a final end-point as that is a common induction-period end-point used in formal ketamine trials. Three individuals (out of the 15 total) stopped IV ketamine treatment prior to the 6th treatment. To account for the missing data in those individuals we used a last-observation carried-forward (LOCF) method, i.e. we carried forward the score from their last treatment prior to stopping to subsequent data points. We chose a repeated-measures ANOVA model with a LOCF approach in order to perform a more conservative estimate for the effects over time. We also tested a mixed-effects model as well (implemented within Prism) in which the last-data point is not carried forward and effects are modeled with some missing data. The results from these two models were largely similar and hence we chose to present results using a LOCF approach analyzed with a rmANOVA model. A Greenhouse-Geisser correction was used to correct p-values for the rmANOVA and post-hoc Tukey’s HSD test was used to estimate significant difference between time-points. We next performed a paired t-test to measure whether the change in PCL-5/PHQ-9 scores that occurred with IN (S)-ketamine was significantly smaller than that which occurred after IV (R,S)-ketamine (both compared to base-line/pre-treatment levels). To determine approximately how quickly these effects emerged, we performed a rmANOVA across the first 6 IV (R,S)-ketamine treatment time-points, (Greenhouse-Geisser correction and post-hoc Dunnett’s test was used to control for multiple comparison tests compared to base-line). Finally, to assess the longevity of these effects, we performed a paired t-test comparing the 6th IV ketamine treatment with the very last IV-ketamine treatment in each patient. Normality of score distributions was confirmed prior to all t-test/ANOVA comparisons. Statistical analyses were conducted using SPSS (IBM) and Prism (GraphPad). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results:
Table 1 describes the specific clinical characteristics of the 15 Veterans included in this analysis. Notably, all of these Veterans had co-morbid depression and PTSD.
Dosages:
All Veterans in this analysis were transitioned to the highest dose of intranasal (IN) (S)-ketamine (81mg) after their first treatment. IV ketamine was dosed based on weight. Veterans were started at 0.5mg/kg, transitioned to 0.75mg/kg for their second IV treatment and then to 1mg/kg for their third IV treatment. The mean adjusted weight across Veterans was 88.6 +/− 4.8kg (mean/SEM), and thus the average IV ketamine dose was 88.6 +/− 4.8mg (mean/SEM) by treatment 3. The effective (S)-ketamine dose would likely be no higher than 40.5 mg (given estimates of ~ 50% bioavailability of IN (S)-ketamine9,10, which is roughly comparable to the 44mg of (S)-ketamine patients were given on average with IV. However, the IV racemic ketamine formulate, at the dose listed also would provide an additional 44mg of (R)-ketamine.
Depression Outcomes
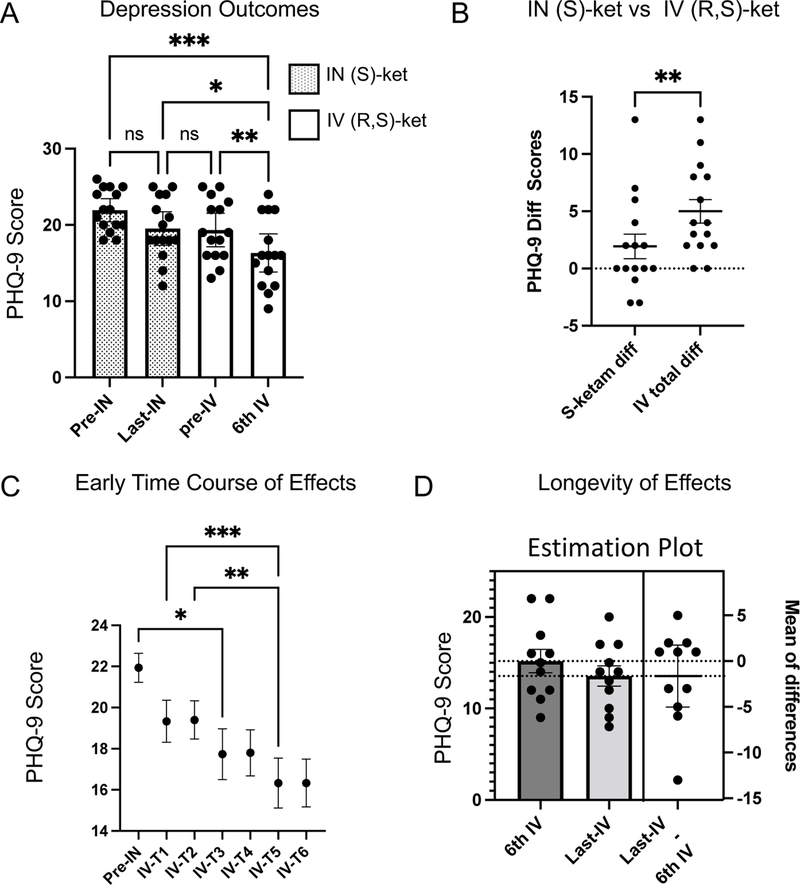
We first performed a repeated-measures ANOVA analysis of PHQ-9 scores at 4 distinct time-points: 1): prior to first intranasal (S)-ketamine (pre-IN-(S)-ketamine), which represents the base-line symptom scores prior to any treatment; 2) prior to their last (S)-ketamine treatment (last-IN-(S)-ketamine); 3) prior to their first IV ketamine dose (pre-IV-(R,S)-ketamine) and 4) prior to their 6th IV ketamine dose (6th-IV-(R,S)-ketamine) (Fig 1A). We found a significant effect of treatment (F(3,42)=12.6, p<0.0001, eta2 = 0.47)). Total pre-IV ketamine PHQ-9 scores were reduced from 21.9 +/− 0.7 (mean/SEM) to 16.3 +/− 1.2 (mean/SEM) at the end of 6 IV ketamine treatments. This reflects a category change in their depression from severe to moderately severe on average. Post-hoc testing revealed that PHQ-9 scores were not significantly different between the first and last (S)-ketamine treatments (mean difference of 2.4 +/− 1.12 (SEM), Tukey’s post-hoc HSD, adjusted p = 0.19). By contrast, there was a significant reduction in PHQ-9 scores after the 6th IV treatment time-point (mean difference of 5.6 +/− 1 (SEM), p = 0.0003 compared to base-line pre-IN time point). The difference in reduction following IV-(R,S)-ketamine was significantly greater than that observed with IN-(S)-ketamine alone (Fig 1B, paired t-test, t(14)=3.6, p = 0.003). To better compare effects between IV and IN ketamine, we calculated the relative effect size for IV ketamine compared to IN-(S)-ketamine using the following approach: the mean difference from pre-IN to last-IN was used as our control and the mean difference from pre-IN to 6th IV was used as the treatment group, divided by the pooled STD. This resulted in a standardized (bias-corrected) Hedge’s g = 0.9, a large effect size for IV (R,S) ketamine compared to IN (S)-ketamine in these Veterans. Nine Veterans showed a reduction of at least 6 points (a commonly used metric of partial response) after IV-(R,S)-ketamine treatments.
Fig 1: Treatment Outcomes for Depression After Switch From Intranasal (S)-Ketamine to Intravenous (R,S)-Ketamine.
A. We analyzed PHQ-9 scores at four time-points of interest: immediately prior to the first dose of intranasal (S)-ketamine (pre-IN); immediately prior to the last-dose of intranasal (S)-ketamine (last-IN); immediately prior to the first dose of intravenous (R,S)-ketamine (pre-IV); immediately prior to the 6th dose of intravenous (R,S)-ketamine (6th-IV). There was a significant reduction in PHQ-9 scores only after switching to IV(R,S)-ketamine. B. To compare effects of IN (S)-ketamine and IV (R,S)-ketamine, we performed a paired t-test in the reduction in PHQ-9 between the two treatments. Reduction after IV (R,S) ketamine was significantly greater than from (S)-ketamine. C. Repeated-measures ANOVA demonstrated a significant effect of IV ketamine treatment emerged by the 3rd treatment, with continued significant reductions in depression symptoms between treatment 3 and treatment 5. D. Estimation plot demonstrates stability in PHQ-9 scores with continued treatment. Error bars represent SEM. *p<0.05; **p<−.01, ***p<0.001
Next, we performed a repeated-measures ANOVA over the first 6 IV ketamine treatments to identify when significant effects emerged (Fig 1C). Significant improvements (F(6,84)=13, p<0.0001) from the pre-treatment baseline started at IV ketamine treatment 3 (mean difference of 4.2 +/− 1.2 (SEM), post-hoc Tukey’s HSD, p<0.05, compared with the pre-IN time point). This suggests that the clinical benefits of the IV ketamine treatment were observed after individuals transitioned to the 0.75mg/kg dosing. Eleven Veterans continued to receive IV (R,S) ketamine treatments after the initial six treatments noted above. We had varying levels of longer-term follow-up data for these Veterans, with a mean/STD of 38 +/− 18 treatments (range of 7 to 68). To probe the longevity of IV ketamine effects we performed a paired t-test between the 6th and last IV treatment across patients. In these Veterans the mean PHQ-9 score remained stable, with a mean score of 15.2 +/− 1.3 (SEM) at the 6th IV treatment and a mean score of 13.6 +/− 1.1 (SEM) by the last IV ketamine treatment (Fig 1D, t(10)=1, p = 0.3).
PTSD Outcomes
We next examined changes in PCL-5 scores using the same analytic approach as above. All 15 of these Veterans had a diagnosis of PTSD, though one patient had missing data on PCL-5 scores during (S)-ketamine administration and was thus excluded from further analysis. A repeated measures ANOVA analysis on the PCL-5 scores over the four key time-points (pre-IN-(S)-ketamine, last-IN-(S)-ketamine, pre-IV-(R,S)-ketamine and 6th IV-(R,S)-ketamine) showed a significant effect of treatment (Fig 2A, F(3,39) = 5.9, p=0.006, partial eta2 = 0.31). Post-hoc testing showed a 4.3 +/− 3.4 change (Tukey’s HSD, p = 0.6) in PCL-5 scores after IN (S)-ketamine treatments. However, there was a 11.8 +/− 3.5 (mean/SEM) point difference after comparing 6th IV ketamine treatment to the pre-IN-(S)-ketamine score (Tukey’s HSD, p=0.03), from 58.9 +/− 2.9 (mean/SEM) prior to any treatment down to 47.1 +/− 4.2 (mean/SEM) after IV (R,S)- ketamine treatments. The change observed after switching to IV (R,S) ketamine was significantly greater compared to that observed with IN (S)-ketamine alone (Fig 2B, t(13)=3.2, p=0.007). A 10 point reduction in PCL-5 scores has been used as a marker of clinically meaningful change in symptoms. After IN-(S)-ketamine treatment only 2/14 Veterans showed this level of change; only those same 2 Veterans had scores below the clinical cut-off after treatment. After IV ketamine treatment, 8 Veterans showed at least a 10 point reduction in PCL-5 scores, though only 3 dropped below the 33 point cut-off indicating remission of symptoms.
Fig 2: Treatment Outcomes for PTSD After Switch From Intranasal (S)-Ketamine to Intravenous (R,S)-Ketamine.
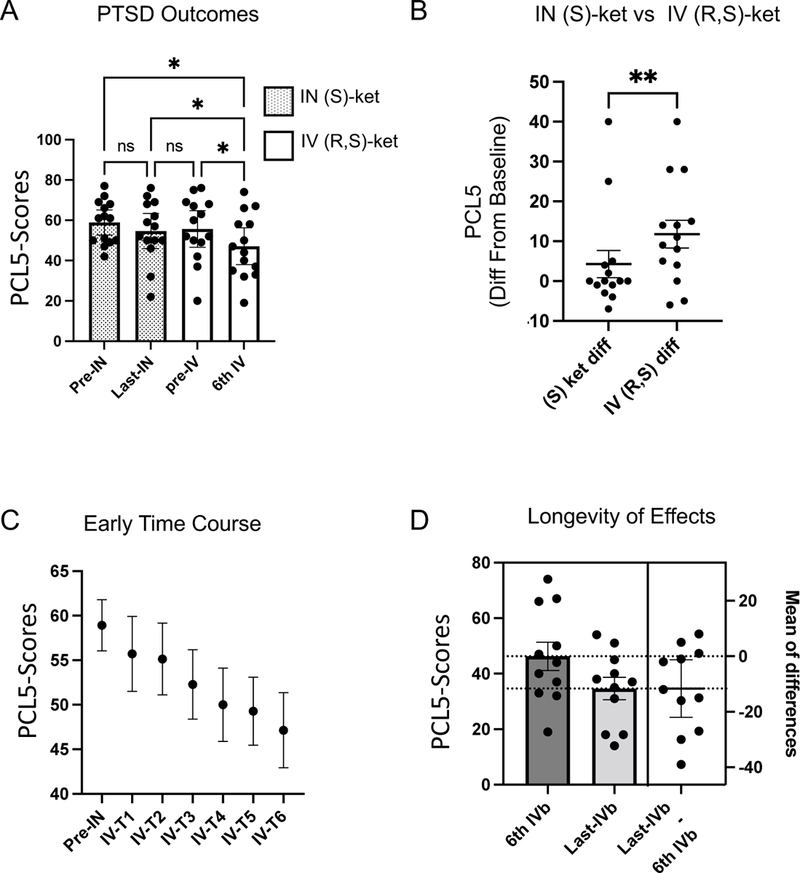
A. We analyzed PCL-5 scores at four time-points of interest: immediately prior to the first dose of intranasal (S)-ketamine (pre-IN); immediately prior to the last-dose of intranasal (S)-ketamine (last-IN); immediately prior to the first dose of intravenous (R,S)-ketamine (pre-IV); immediately prior to the 6th dose of intravenous (R,S)-ketamine (6th-IV) There was a significant reduction in PCL-5scores driven by a change after switching to IV(R,S)-ketamine (p<0.05). B. Paired t-test was used to compare reductions in PCL-5 following (S)-ketamine, vs reductions following IV ketamine. IV (R,S) ketamine was significantly more effective (p<0.01). C. Repeated-measures ANOVA demonstrated a linear trend with treatment. D. Estimation plot demonstrates significant reductions in PCL-5 scores with continued treatment past the initial 6. Error bars represent SEM. *p<0.05; **p<−.01, ***p<0.001
Post-traumatic-stress-disorder is often broken into four major clusters of symptoms. This includes Cluster B (re-experiencing symptom), Cluster C (avoidance symptoms), Cluster D (mood and cognition symptoms) and Cluster E (hyper-arousal symptoms). This is particularly relevant because Cluster D (mood symptoms) in particular might capture a lot of the overlap with depressive symptomatology, whereas Cluster B and Cluster E are more specific to PTSD.
To better understand whether symptom clusters responded equally to IV-(R,S)-ketamine treatments, we analyzed changes in these symptom sub-domain scores (extracted from the PCL-5 according to convention). We used a paired t-test to compare symptom cluster scores pre-treatment (pre-IN-S-ketamine) with scores measured at the 6th IV ketamine treatment. We observed a significant reduction on cluster B (t(13) = 2.4, p=0.03), Cluster D (t(13) = 2.5, p=0.026) and Cluster E (t(13)=3.1, p=0.009). We did not observe a significant change in Cluster C symptoms (t(13)=1.5, p = 0.15).
To understand the time-course of response, we next performed a repeated-measures ANOVA across the first 6 IV treatments. We found a significant reduction in PCL-5 scores across treatments (Fig 2C, F(6,78) = 6, p = 0.003), though post-hoc Tukey’s HSD test did not reveal a significant difference between pre-treatment and at any IV-ketamine time-point (post-hoc Tukey’s HSD adjusted p = 0.056 comparing base-line scores with IV ketamine treatment 6). Finally, we performed an analysis of PCL-5 scores during the maintenance period after the initial 6 IV ketamine treatments. Interestingly (and differing from what we observed with PHQ-9 scores noted above), we observed a significant reduction in PCL-5 scores comparing from the 6th IV (R,S)-ketamine treatment and the final treatment point in each Veteran (Fig 2D, paired t-test t(10)=2.5, p =0.03). Scores changed from 46.3 +/− 5 (mean/SEM) at treatment 6 down to 34.6 +/− 4 (mean/SEM) at Veteran’s last measured IV ketamine treatment time. At that last time-point 10 Veterans achieved at least a 10 point reduction in PCL-5 scores, although only 4 were below the 33 point cut-off indicating remission of symptoms.
Discussion
This retrospective case series was focused on whether IV-(R,S)-ketamine would show any additional effect on either depression or PTSD symptoms in Veterans who did not adequately respond to intranasal (S)-ketamine treatments. In this specific cohort, we found that IV-(R,S)-ketamine was associated with a significant improvement in both depression and PTSD symptoms in Veterans who had a suboptimal response to intranasal (S)-ketamine. This data suggests that a trial of IV racemic ketamine could be considered as a reasonable next-step for individuals who do not respond to the highest dosages of intranasal (S)-ketamine.
There are several aspects of these results worthy of further discussion. First, the anti-depressant response that we observed after Veterans transitioned to IV (R,S)-ketamine treatments occurred relatively rapidly (within 2 treatments) and were then stable. By contrast, the effects on PTSD symptoms seemed to take longer and continued even during the switch to maintenance (weekly or less frequent) dosing. These temporal differences may be related to distinct differences in brain circuits and action of ketamine, but may also simply relate to how, after depression symptoms improve, other behavioral/life-style changes might occur that slowly improve functioning across broader aspects of mental health, including those measured on the PCL-5.
It is noteworthy that no significant antidepressant effects were observed after the starting IV dosage (0.5mg/kg), which is the dose most consistently used in clinical trials. Instead, we observed a significant change in symptoms only after a slightly higher dose (0.75 mg/kg). This suggests that individuals who did not respond to IN-S-ketamine may require slightly higher than average dosing of ketamine. Prior clinical trials of intranasal (S)-ketamine12 have indicated there is a dose-dependent effect at currently approved dosages and do not preclude that a higher than approved dose might offer even greater benefits. The IV (R,S)-ketamine dosages used in Veterans starting at their third treatment (1mg/kg), resulted in an average dose of 89mg of (R,S)-ketamine, or 45mg of (S)-ketamine and 45mg of (R)-ketamine. Recent estimates of the bio-availability of intranasal (S)-ketamine are around 50% of IV ketamine 10, suggesting that individuals receiving the highest dose of IN-(S)-ketamine may have at most an effective dose of ~ 40.5mg of (S)-ketamine. Thus, while the effective (S)-ketamine dosage is similar between the IN and IV formulations used in these Veterans, the additional supply of (R)-ketamine in the IV-(R,S)-ketamine formulation results in an overall higher effective dose in these Veterans. Prior data has not revealed clear dose-dependent effects of ketamine on average15,16, but it certainly seems plausible, as with many drugs, that some individuals may require higher dosages to achieve an efficacious response. A suboptimal response to intranasal (S)-ketamine might thus automatically select for Veterans requiring a higher dosage, which is then revealed when they are switched over to IV-ketamine therapy. A higher dosage of ketamine may result in greater activation either on NMDA receptors or on mu-opioid receptors (MOR) 19,20, both of which have been implicated in the antidepressant efficacy of ketamine (though these findings remain debated21–23).
In addition to a simple dose-response effect, it is also possible that the added benefit of IV ketamine may be related to the presence of the (R)-enantiomer in the IV racemic ketamine formulation we used. There continues to be debate on whether NMDA antagonism is either the primary (or only) mechanism of antidepressant action26. Some other NMDA antagonists, like memantine, do not seem to produce the same level of robust antidepressant effects, suggesting possibly a different mechanism of action27,28. Relevant to this study, recent data from pre-clinical rodent models have suggested that that a metabolite of (R)-ketamine, hydroxy-nor-ketamine, may produce antidepressant effects that are distinct from those produced by (S)-ketamine18,29,30, via AMPA-receptor modulation instead of NMDA receptor modulation17,18. There is limited clinical data at present on the potency of hydroxy-nor-ketamine in humans. Thus, further research will be required to understand dose-related effects of ketamine and whether there are meaningful differences in the clinical response for (R) vs (S) ketamine that could explain results noted here.
A simple interpretation of our data is that IV (R,S)-ketamine is more effective than IN-S-ketamine. However, there are important caveats to these results that may limit our ability to make that interpretation. First, and most importantly, we did not randomize or blind Veterans ahead of time. To be able to truly interpret a cross-over-design, most prospectively designed studies will randomize individuals to the order of treatments. Without this randomization, it is possible that we are observing effects related to time on treatment outcome; or some other form of biased selection of individuals. Second, both the clinicians and the Veterans undergoing treatment might have had a much larger placebo-related expectation that IV-ketamine formulations would be superior to IN-ketamine. This expectation could be driven by the differences in treatment itself (i.e. the act of placing an IV might drive a stronger placebo response than a nasal spray, for example). In addition, 8/15 Veterans in this analysis had prior exposure to IM or IV ketamine and in those Veterans that prior exposure, the exposure might have also resulted in a stronger placebo response to the IV ketamine formulation, or even a “nocebo” response to the IN ketamine formulation. Finally, though we did not measure this, it is possible that IV ketamine induced a greater degree of dissociation, which leads to a greater placebo response subsequently. Additionally, this case-series reflects a relatively small and very specific sample of patients that may not generalize well. Thus, to truly understand comparative efficacy of IV-R,S-ketamine vs. IN-S-ketamine, a double-blinded randomized controlled trial comparing the two treatment options would be needed. Due to all of these limitations, we cannot easily conclude from our study that IV ketamine is more effective than intranasal (S)-ketamine on average – but we do believe it provides initial data to warrant an RCT directly comparing the two, with a hypothesis that IV ketamine, at doses up to 1mg/kg, may be more effective than IN (S)-ketamine.
In sum, we found some evidence that, in the right individuals, IV (R,S)-ketamine significantly improves depression and PTSD symptoms more than could be achieved with intranasal (S)-ketamine alone. Our results argue that in individuals who do not get a meaningful response to IN (S)-ketamine (< 3 pt change in symptom scores, for example), IV racemic (R,S) ketamine could be a reasonable option. These effects further suggest either that some individuals may require either a higher dose of ketamine than can be achieved from the intranasal formulation at present or that the addition of (R)-ketamine provides an additional mechanism of action beyond that of (S)-ketamine alone. Confirmation will require further research in properly designed and controlled trials.
Funding and Acknowledgements:
This work was supported by awards from the Department of Veterans Affairs, Veterans Health Administration (Career Development Award: 7IK2BX003308 to D.S.R.), and funding from the Center of Excellence for Stress and Mental Health (CESAMH) at the San Diego VA Medical Center, as well as from the National Institute of Mental Health (K23 MH119375-01 to E.E.L.)
Footnotes
Conflict of Interest:
The authors declare no conflicts of interest.
References
- 1.Berman RM et al. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47, 351–354 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Murrough JW et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am. J. Psychiatry 170, 1134–1142 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matveychuk D et al. Ketamine as an antidepressant: overview of its mechanisms of action and potential predictive biomarkers. Ther. Adv. Psychopharmacol. 10, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarate CA et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63, 856–864 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Phillips JL et al. Single, Repeated, and Maintenance Ketamine Infusions for Treatment-Resistant Depression: A Randomized Controlled Trial. Am. J. Psychiatry 176, 401–409 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Autry AE et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475, 91–95 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulrich Zeilhofer H, Swandulla D, Geisslinger G & Brune K Differential effects of ketamine enantiomers on NMDA receptor currents in cultured neurons. Eur. J. Pharmacol. 213, 155–158 (1992). [DOI] [PubMed] [Google Scholar]
- 8.Yamakura T, Sakimura K & Shimoji K The Stereoselective Effects of Ketamine Isomers on Heteromeric N-Methyl-d-Aspartate Receptor Channels. Anesth. Analg. 91, 225–229 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Yanagihara Y et al. Plasma concentration profiles of ketamine and norketamine after administration of various ketamine preparations to healthy Japanese volunteers. Biopharm. Drug Dispos. 24, 37–43 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Perez-Ruixo C et al. Population Pharmacokinetics of Esketamine Nasal Spray and its Metabolite Noresketamine in Healthy Subjects and Patients with Treatment-Resistant Depression. Clin. Pharmacokinet. 60, 501–516 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Canuso CM et al. Efficacy and Safety of Intranasal Esketamine for the Rapid Reduction of Symptoms of Depression and Suicidality in Patients at Imminent Risk for Suicide: Results of a Double-Blind, Randomized, Placebo-Controlled Study. Am. J. Psychiatry 175, 620–630 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Daly EJ et al. Efficacy and Safety of Intranasal Esketamine Adjunctive to Oral Antidepressant Therapy in Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry 75, 139–148 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahji A, Vazquez GH & Zarate CA Comparative efficacy of racemic ketamine and esketamine for depression: A systematic review and meta-analysis. J. Affect. Disord. 278, 542–555 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavender E, Hirasawa-Fujita M & Domino EF Ketamine’s dose related multiple mechanisms of actions: Dissociative anesthetic to rapid antidepressant. Behav. Brain Res. 390, 112631 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Abdallah CG et al. Dose-Related Effects of Ketamine for Antidepressant-Resistant Symptoms of Posttraumatic Stress Disorder in Veterans and Active Duty Military: A Double-blind, Randomized, Placebo-Controlled Multi-Center Clinical Trial. medRxiv 2021.04.30.21256273 (2021) doi: 10.1101/2021.04.30.21256273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fava M et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol. Psychiatry 25, 1592–1603 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanos P et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533, 481–486 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukumoto K et al. Antidepressant Potential of (R)-Ketamine in Rodent Models: Comparison with (S)-Ketamine. J. Pharmacol. Exp. Ther. 361, 9–16 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Klein ME, Chandra J, Sheriff S & Malinow R Opioid system is necessary but not sufficient for antidepressive actions of ketamine in rodents. Proc. Natl. Acad. Sci. 117, 2656–2662 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams NR et al. Attenuation of Antidepressant Effects of Ketamine by Opioid Receptor Antagonism. Am. J. Psychiatry 175, 1205–1215 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon G, Petrakis IL & Krystal JH Association of Combined Naltrexone and Ketamine With Depressive Symptoms in a Case series of Patients With Depression and Alcohol Use Disorder. JAMA Psychiatry 76, 337–338 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marton T, Barnes DE, Wallace A & Woolley JD Concurrent Use of Buprenorphine, Methadone, or Naltrexone Does Not Inhibit Ketamine’s Antidepressant Activity. Biol. Psychiatry 85, e75–e76 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Zhang K & Hashimoto K Lack of Opioid System in the Antidepressant Actions of Ketamine. Biol. Psychiatry 85, e25–e27 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Hirota K et al. Stereoselective interaction of ketamine with recombinant mu, kappa, and delta opioid receptors expressed in Chinese hamster ovary cells. Anesthesiology 90, 174–182 (1999). [DOI] [PubMed] [Google Scholar]
- 25.Bonaventura J et al. Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability. Mol. Psychiatry 1–19 (2021) doi: 10.1038/s41380-021-01093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zorumski CF, Izumi Y & Mennerick S Ketamine: NMDA Receptors and Beyond. J. Neurosci. 36, 11158–11164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarate CA et al. A Double-Blind, Placebo-Controlled Study of Memantine in the Treatment of Major Depression. Am. J. Psychiatry 163, 153–155 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Gideons ES, Kavalali ET & Monteggia LM Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc. Natl. Acad. Sci. 111, 8649–8654 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang C et al. AMPA Receptor Activation–Independent Antidepressant Actions of Ketamine Metabolite (S)-Norketamine. Biol. Psychiatry 84, 591–600 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Li S & Hashimoto KR (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol. Biochem. Behav. 116, 137–141 (2014). [DOI] [PubMed] [Google Scholar]