Abstract
We conducted a double-blind, placebo-controlled, parallel, dose-escalation trial to evaluate the pharmacokinetics and safety of single, oral doses of amprenavir (141W94; formerly VX-478), a potent inhibitor of human immunodeficiency virus (HIV) type 1 protease, administered as hard gelatin capsules in 12 HIV-infected subjects. The doses of amprenavir evaluated were 150, 300, 600, 900, and 1,200 mg. Amprenavir was rapidly absorbed, with the time to maximum concentration occurring within 1 to 2 h after dosing. On the basis of power model analysis, the increase in the maximum concentration of amprenavir in plasma (Cmax) was less than dose proportional, and the increase in the area under the concentration-time curve from time zero to infinity (AUC0–∞) was greater than dose proportional; mean slopes (with 90% confidence intervals) were 1.25 (1.16 to 1.35) and 0.78 (0.78 to 0.86) for AUC0–∞ and Cmax, respectively. Amprenavir was eliminated slowly, with a terminal-phase half-life of 8 h. A second study was conducted to determine the bioavailability of the hard gelatin capsule relative to that of a subsequently developed soft gelatin capsule. The capsules were bioequivalent in terms of AUC0–∞ but not in terms of Cmax; geometric-least-squares means ratios (with 90% confidence intervals) were 1.03 (0.92 to 1.14) and 1.25 (1.03 to 1.53) for AUC0–∞ and Cmax, respectively. Administration of soft gelatin capsules of amprenavir with a high-fat breakfast resulted in a 14% decrease in the mean AUC0–∞ (from 9.58 to 8.26 μg · h/ml), which is not likely to be clinically significant. The most common adverse events related to amprenavir were headache, nausea, and hypesthesia. Amprenavir appears to be safe and well tolerated over the dose range of 150 to 1200 mg. On the basis of the present single-dose studies, amprenavir is an HIV protease inhibitor with favorable absorption and clearance pharmacokinetics that are only minimally affected by administration with food.
The clinical use of inhibitors of the human immunodeficiency virus (HIV) type 1 (HIV-1) protease enzyme represents a major advance in the treatment of HIV disease. HIV and AIDS surveillance efforts show that an overall, abrupt decline in opportunistic infections and deaths due to AIDS occurred in 1996 and that the decline resulted specifically from treatment advances (4). Protease inhibitors are now recommended as part of a combination of multiple antiretroviral agents for treatment of HIV infection: as initial therapy for recently infected individuals, as therapy for chronically infected individuals (symptomatic and asymptomatic), and as therapy for persons with AIDS (2, 3). Despite gains in the management of HIV-related disease, treatment failure remains a considerable problem. A number of factors can lead to treatment failure with the currently available protease inhibitors (1, 6, 7), including incomplete viral suppression and suboptimal drug exposure. Thus, new protease inhibitors that have potent antiviral activity and pharmacokinetic properties are urgently needed.
The protease inhibitor amprenavir (141W94) is an N,N-disubstituted hydroxyethylamino sulfonamide that was originally synthesized by a structure-based drug design process (10). Amprenavir has a molecular mass of 506 Da and is relatively soluble in phosphate-buffered saline (0.19 mg/ml at pH 6.8) (10, 13). Amprenavir is 90% bound to plasma proteins, and as is the case with other protease inhibitors, the high-affinity plasma protein for amprenavir is α1-acid glycoprotein (11). In vitro and in vivo studies have shown that amprenavir is primarily metabolized by the 3A4 isozyme of the hepatic cytochrome P-450 system (CYP3A4) and that amprenavir inhibits CYP3A4 to a degree comparable to those exhibited by indinavir and nelfinavir (20).
In a number of cell culture systems, amprenavir was shown to have a high level of antiviral activity (13, 14, 18). In cell culture systems with medium containing 10% fetal calf serum, the mean 50% inhibitory concentration (IC50) of amprenavir for the laboratory HIV-1IIIB strain in human peripheral blood lymphocytes is 0.08 μM (or 0.04 μg/ml), and the IC50 for clinical isolates of HIV is 0.012 μM (or 0.006 μg/ml) (18). Amprenavir showed good oral bioavailability in several animal species (13). We have studied and report here on the pharmacokinetics of single doses of amprenavir as a hard gelatin capsule formulation (Glaxo Wellcome protocol PROA1001) in HIV-infected subjects. Because this formulation was found to be unstable at room temperature and required refrigeration, a soft gelatin capsule formulation was developed. We therefore sought to establish the relative bioavailabilities of the two formulations and examine the effect of food on the absorption of amprenavir in the soft gelatin capsule (Glaxo Wellcome protocol PROA1004).
Preliminary data from the dose-escalation and bioequivalence studies were reported previously (16, 19).
MATERIALS AND METHODS
Study population.
In both the dose-escalation study and the relative bioavailability study, the major inclusion criteria were as follows: age of 18 to 55 years, body weight of 50 to 90 kg, and documented HIV-1 infection (by an enzyme-linked immunosorbent assay positive for HIV-1, as confirmed by Western blotting, a blood culture positive for HIV-1, or a serum antigen test positive for HIV-1). Prior antiretroviral therapy was permitted. All subjects had to give written informed consent to participate in the trial. All subjects were monitored for clinical adverse experiences and/or abnormal laboratory test findings throughout the study period, including the follow-up evaluation.
(i) Dose-escalation study.
Eighteen HIV-positive subjects who met all study entry criteria were randomly assigned to either the treatment or the placebo group. Subjects were excluded from the trial if they had conditions that would interfere with drug absorption (e.g., chronic diarrhea), a history of pancreatitis or hepatitis within the previous 3 years, abnormal laboratory test results within 14 days before enrollment, including anemia (hemoglobin concentrations, <11.0 g/dl for men and <10.0 g/dl for women), neutropenia (neutrophil count, <1,500 cells/mm3), thrombocytopenia (platelet count, <100,000/mm3), elevated liver function test results (aspartate aminotransferase [AST; serum glutamic oxalacetic transaminase] or alanine aminotransferase [ALT; serum glutamic pyruvic transaminase] levels more than two times the upper limit of normal), renal function impairment (creatinine clearance, ≤50 ml/min), or severe debility from HIV-related disease or its treatment, as judged by the investigator. Subjects were also excluded from trial participation if concomitant medication could not be withheld for 48 h (or 24 h for antiretroviral therapy) prior to dosing and during the five dosing periods, if subjects were receiving other investigational treatments, if they had current alcohol or illicit drug use which would interfere with compliance with the dosing schedule and protocol evaluations, or were pregnant or breast-feeding.
(ii) Relative bioavailability study.
Eighteen HIV-infected subjects who met all study entry criteria were randomly assigned to one of six treatment sequences. The major exclusion criteria were similar to those for the dose-escalation study, with the following exceptions. A history of pancreatitis or hepatitis within the previous 3 years did not exclude potential subjects from participating in the relative bioavailability study. The timing and laboratory test values were slightly different, such that subjects were excluded from the trial if they had abnormal laboratory test results within 21 days prior to dosing on their first dosing period, including anemia (hemoglobin concentrations, <11.0 g/dl for men and <10.0 g/dl for women), neutropenia (neutrophil count, <1,000 cells/mm3), thrombocytopenia (platelet count, <75,000/mm3), elevated liver function test results (AST or ALT levels more than two times the upper limit of normal), and renal function impairment (creatinine clearance, ≤40 ml/min). Additional exclusion criteria stipulated that subjects were excluded from the trial if they had AIDS, including a CD4+ cell count of ≤200 cells/mm3, were taking drugs known to influence the metabolism or distribution of other drugs (e.g., inducers or inhibitors of the P-450 cytochrome system), or were receiving medications that could not be withheld for 48 h (or 24 h for antiretroviral agents and prophylactic agents for opportunistic infections) prior to dosing and during the three dosing periods.
Study design.
The dose-escalation study and the relative bioavailability study were conducted at a single center (a different center for each study), and the study protocol was approved by the institutional review board affiliated with each study site.
(i) Dose-escalation study.
The dose-escalation study was designed as a double-blind, placebo-controlled, parallel, dose-escalation trial to evaluate the pharmacokinetics and safety of single oral doses of amprenavir. Subjects were randomly assigned, in a 2:1 ratio, to receive either five single oral doses of amprenavir or matching placebo doses over a 5-week period. Amprenavir was administered orally as 150-mg, hard gelatin capsules with 200 ml of water. The doses of amprenavir evaluated were 150, 300, 600, 900, and 1,200 mg. These doses were selected on the basis of the results of multiple-dose (30-day) studies with rats. The 1,200-mg dose was less than 1/5 of the lowest no-effect dose (100 mg/kg/day) and the 150-mg dose was less than 1/46 of the lowest no-effect dose in these preclinical toxicology studies (8a). To ensure blinding, both groups received the same number of capsules. Each dose administration was separated by at least 6 days. Dosing periods were defined as 48 h prior to and 24 h after dosing. Subjects fasted at least 8 h before and 4 h after dosing. The consumption of alcoholic beverages and foods was prohibited for 48 h prior to dosing and during each dosing period. Coffee, tea, and other xanthine-containing beverages and foods were prohibited on the day of dosing. Subjects were admitted to the clinical research unit of the study site on the evening prior to dosing to ensure a true overnight fast and compliance with the protocol restrictions.
(ii) Relative bioavailability study.
The relative bioavailability study was designed as an open-label, randomized, single-dose, three-period crossover study to compare two formulations of amprenavir and to determine what effect, if any, food has on the pharmacokinetics of amprenavir. The dose of amprenavir evaluated was 600 mg, which was administered in four 150-mg capsules with 200 ml of water over three treatments. This dose was chosen because in the initial, dose-escalation study, it was well tolerated and yielded concentrations which were consistently above the limit of quantitation of the assay for the duration of the evaluation period. The three treatments, which each subject received in a random sequence, consisted of the following: four 150-mg hard gelatin capsules after an 8-h fast, four 150-mg soft gelatin capsules after an 8-h fast, and four 150-mg soft gelatin capsules after an 8-h fast and consumption of a standardized, high-calorie, high-fat breakfast. The breakfast consisted of 58 g of carbohydrate, 33 g of protein, and 67 g of fat. For the treatment in which subjects had a breakfast meal, the amprenavir dose was administered 20 min after the start of the meal. Each subject was randomly assigned to one of six treatment sequences (three subjects per treatment sequence), with each treatment being separated by an interval of 7 days (for all subjects and all treatments). Subjects fasted for at least 4 h after dosing. Restrictions on food and beverage consumption were similar to those for the dose-escalation study with the exception that water and tobacco products were prohibited 4 h pre- and postdosing.
Safety evaluation.
For both studies, safety and tolerability were evaluated by physical examination, vital sign determinations, electrocardiography, hematology (complete blood count with differential, mean corpuscular volume, hemoglobin, and platelet count), clinical chemistry studies (electrolytes, AST, ALT, total bilirubin, creatinine, albumin, glucose, alkaline phosphatase, and serum amylase), urinalysis (dipstick for protein and blood), and assessment of clinical adverse experiences. Physical examinations and electrocardiogram testing were performed only at the screening and follow-up visits unless they were otherwise warranted. All other measurements and recording of clinical adverse experiences were conducted at screening, during each dosing period, and at follow-up evaluations. In addition, a targeted medical history review (to ensure that the entry criteria continued to be met) was conducted during each dosing period. Hematology and clinical chemistry values were recorded on each treatment day; values not in the normal range were labeled as high or low with regard to a reference range (dose-escalation study) and as a change from the baseline (relative bioavailability study). For both studies, laboratory values above or below the reference range were considered clinically significant. The AIDS Clinical Trials Group toxicity grading scale was used to evaluate abnormal laboratory values (relative bioavailability study only).
Blood and urine collection.
Samples of both plasma and urine were collected for amprenavir concentration determinations in the dose-escalation study. Only plasma samples were collected in the relative bioavailability study. The concentration of amprenavir was determined from serial samples of blood and urine taken before and after administration of each dose. Plasma samples were collected immediately before drug administration (predosing, or 0 h) and then at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12, and 24 h postdosing. In the dose-escalation study, five urine samples were collected from each subject during each dosing period: 5 min before dosing and then at 0 to 4, 4 to 8, 8 to 12, and 12 to 24 h after dosing. All plasma and urine samples were stored frozen at −20°C until analysis.
Assay for amprenavir in plasma and urine samples.
Briefly, plasma amprenavir concentrations were determined by a reversed-phase high-performance liquid chromatographic method with fluorescence detection (excitation at 245 nm, emission at 340 nm for plasma and 380 nm for urine). Amprenavir was extracted from thawed plasma samples by either protein precipitation or a solid-phase extraction performed with a Waters MilliLab Workstation and C18 Sep-Pak cartridges. After extraction, a sample was injected into a Waters Symmetry C18 liquid chromatography column (3.9 by 125 mm) at 40°C. Samples were eluted off the column by using a mobile phase consisting of 45% acetonitrile (in water; 45:55 [vol/vol]) at a constant flow rate of 1.0 ml/min. Thawed urine samples (10 to 100 μl) were injected without extraction directly into the high-performance liquid chromatography column at 40°C as described above (dose-escalation study only). Standard and control solutions were added to normal, blank, pooled human plasma as calibration standards or controls and were used to validate the methods and perform the studies. Interassay accuracy was assessed in duplicate from three quality control samples processed over five analytical runs. The subsequent data were analyzed by analysis of variance, which partitioned the error term into a within-day component and a between-day component. The interassay accuracy in the dose-escalation study was <5%, and the range of amprenavir concentrations detected was 5 to 750 ng/ml. In the relative bioavailability study, the interassay accuracy was <9%, and the range of amprenavir concentrations detected was from 10 to 1,000 ng/ml. The calibration range in urine was 25 to 10,000 ng/ml.
Pharmacokinetics analysis.
In both the dose-escalation study and the relative bioavailability study, pharmacokinetic parameters for each participant were calculated with the concentrations in plasma obtained during each dosing period by model-independent methods (8). The peak or maximum plasma amprenavir concentration (Cmax), the time to reach Cmax (Tmax), and the plasma amprenavir concentration at 12 h (C12) were observed from the individual data sets. The apparent terminal elimination rate constant (λz) was obtained by log-linear regression of the terminal portions of the plasma concentration-versus-time curves, and the terminal-phase half-life (t1/2) was then calculated as ln(2)/λz. The area under the plasma concentration-versus-time curve (AUC) from time zero to the time of the last quantifiable sample (tlast) (AUC0–t) was calculated by the linear trapezoidal rule. AUC0–t was extrapolated from tlast to infinity (AUC0–∞) by adding Clast/λz, where tlast is the time to the last quantifiable concentration in plasma (Clast). The total apparent clearance (CL/F) was calculated as dose/AUC0–∞. The primary pharmacokinetic parameters used to evaluate bioequivalence and the effect of food were AUC0–∞ and Cmax. The apparent volume of distribution during the elimination phase (Vz/F) was calculated as dose/(λz · AUC0–∞). In the dose-escalation study, renal clearance (CLR) was calculated for each urine collection interval by estimating the rate of excretion (dAe/dt), where Ae is the cumulative amount of drug excreted in the urine, and dividing by the concentration in plasma at the midpoint of that interval. The overall CLR was calculated as the average of the estimates for the four urine collection intervals. The steady-state plasma amprenavir concentrations expected with 1,200-mg, twice-daily dosing were predicted by superposition.
Statistical analysis.
All pharmacokinetic parameters (except Tmax) were log transformed (base e) prior to analysis. A power model was used to assess the extent of dose proportionality for Cmax and AUC0–∞ across treatments. Dose proportionality for all pharmacokinetic parameters was assessed by using the reduced model, described by the following equation: log (Yij) = αi + βi log (Dj) + eij, where Dj is the dose j, and Yij is the value of the pharmacokinetic parameter for subject i at dose j. αi and βi are the intercept and slope for subject i, respectively, and eij is the residual error. The power model was fitted by restricted-maximum-likelihood methods with unrestricted variance structure by using SAS (version 6.09, SAS Institute, Inc., Cary, N.C.). A population average slope (β) and its 90% confidence interval (CI) were calculated from the individual βi values of both parameters for doses. The inclusion of 1 in the 90% CI estimated for the population average estimate of β of AUC0–∞ and Cmax indicated dose proportionality.
For the relative bioavailability study, a sample size of 18 subjects was estimated to provide more than 80% of the power (5% significance level) necessary to demonstrate the bioequivalence of the treatments (soft and hard gelatin capsules). Analyses of variance with treatment, period, and sequence effects (fixed effects) and the subject-within-sequence effect (random effect) were performed by the Mixed Linear Models procedure (SAS). Geometric-least-squares (GLS) means and the associated 95% CIs were calculated for each treatment. The ratio of the GLS means and the associated 90% CIs were calculated for Cmax and AUC0–∞ to determine the bioequivalence of the soft gelatin capsule formulation to the hard gelatin capsule formulation. The treatments were considered bioequivalent if the 90% CI for the ratios fell within the range of 0.8 to 1.25 (80 to 125%). Tmax was analyzed on a pairwise basis by a Wilcoxon signed rank test. Estimates of the median difference between formulations and between the fasting versus nonfasting conditions and the associated 90% CIs were calculated on the basis of standard nonparametric methods (5). The statistical significance of treatment means ratios was determined by two one-sided tests.
RESULTS
Subject demographics. (i) Dose-escalation study.
All 18 HIV-infected subjects (15 males and 3 females) enrolled in the study completed treatment with all doses. No significant demographic differences were apparent between the 12 subjects who received amprenavir and the 6 subjects who received placebo (Table 1). Only 1 study participant (in the amprenavir treatment group) had a prior diagnosis of AIDS (Centers for Disease Control and Prevention [CDC] classification C); the other 17 subjects were asymptomatic (CDC classification A).
TABLE 1.
Subject demographic and HIV-related data for dose-escalation and relative bioavailability studies
| Characteristic | Dose-escalation study
|
Relative bio-availability of amprenavir (n = 18) | |
|---|---|---|---|
| Amprenavir (n = 12) | Placebo (n = 6) | ||
| No. of subjects | |||
| Male | 9 | 6 | 15 |
| Female | 3 | 0 | 3 |
| Race | |||
| White | 12 | 5 | 10 |
| Black | 0 | 0 | 7 |
| Other | 0 | 1 | 1 |
| Age (yr)a | 33 ± 6.7 | 29 ± 5.0 | 31.94 ± 5.21 |
| Wt (kg)a | 64 ± 9.6 | 73 ± 3.3 | 71.58 ± 8.55 |
| Ht (cm)a | 171 ± 9.5 | 179 ± 8.2 | 174.5 ± 6.69 |
| HIV infection status (no. of subjects) | |||
| Asymptomatic (CDC classification A) | 11 | 6 | 15 |
| Symptomatic (CDC classification B) | 0 | 0 | 3 |
| AIDS (CDC classi-fication C) | 1 | 0 | 0 |
| CD4+ count (cells/mm3)b | 392 ± 127.2 | 484 ± 202.5 | 387 ± 250 |
| CD8+ count (cells/mm3)b | 1,194 ± 683 | 699 ± 250.5 | 755.5 ± 269 |
Values are given as means ± standard deviations.
Values are given as medians ± standard deviations.
(ii) Relative bioavailability study.
All 18 HIV-infected subjects (15 males and 3 females) enrolled in the study completed all treatments according to the study protocol. There were no apparent significant demographic differences among the subjects (Table 1). Fifteen subjects were asymptomatic (CDC classification A) and 3 were symptomatic but did not have AIDS (CDC classification B).
Safety and tolerability. (i) Dose-escalation study.
No deaths or withdrawals due to clinical adverse events occurred. Of the 12 amprenavir-treated subjects and the 6 placebo-treated subjects, 15 reported a total of 40 clinical adverse experiences judged as possibly related to study drug (amprenavir or placebo). With one exception, all of the drug-related adverse experiences were mild to moderate in intensity. The most common clinical adverse experiences reported as possibly attributable to amprenavir were headache, nausea, and hypesthesia. The frequency of occurrence of headache ranged from 8 to 25% and decreased as the dose increased: 25% for periods two (300 mg) and three (600 mg), 17% for period four (900 mg), and 8% for period five (1,200 mg). Headache occurred in placebo-treated subjects at comparable rates, with a frequency of occurrence of 17% (during periods one, three, and five) and 33% (during periods two and four). One instance of a severe headache occurred during period three; all others were of mild to moderate intensity. Nausea was reported in 8% of amprenavir-treated subjects during period one (150 mg) and in 17% of subjects during period five (1,200 mg). Nausea was not reported by any subjects who received placebo. Hypesthesia was reported in 17% of amprenavir-treated subjects during period five and in 17% of placebo-treated subjects (during period four).
No clinically significant changes were observed in any laboratory value. Subjects with hematology and/or clinical chemistry values that were either high or low relative to a reference range during one or more amprenavir dosing periods also had the same high or low values prior to the start of the study.
(ii) Relative bioavailability study.
Of the 31 adverse events reported by 14 subjects (one reported prior to starting the first treatment), 14 were determined to have a possible relationship to study drug. No serious adverse events were reported. The most frequently reported adverse experience attributable to study drug was headache (of mild intensity), which occurred in 10 of the 14 subjects who had an adverse experience. Headache was reported by four subjects who received the hard gelatin capsule formulation in the fasting state, three subjects who received the soft gelatin capsule formulation in the fasting state, and three subjects who received the soft gelatin capsules in the nonfasting state. In contrast to the dose-escalation study, none of the subjects experienced nausea.
No clinically significant changes in either hematology or clinical chemistry values were noted, although some values did change from the baseline values sporadically.
Pharmacokinetics. (i) Dose-escalation study.
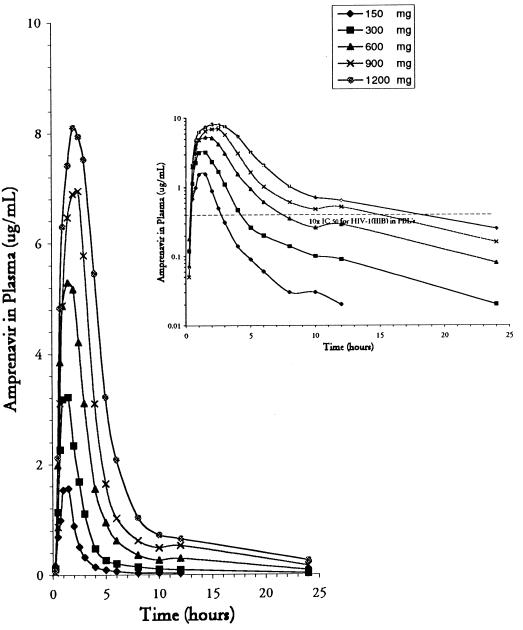
The mean plasma amprenavir concentration after the administration of five different single, oral doses from 15 min to 24 h postdosing is shown in Fig. 1. Highlighted in the inset of Fig. 1 is the average plasma concentration versus time with respect to 10 times the mean in vitro IC50 (0.04 μg/ml) for HIV-1IIIB in peripheral blood lymphocytes. At 8 and 12 h after dosing, the concentrations of the two highest doses were greater than 10 times the in vitro IC50: 0.61 μg/ml at 8 h and 0.52 μg/ml at 12 h for the 900-mg dose and 1.03 μg/ml at 8 h and 0.64 μg/ml at 12 h for the 1,200-mg dose.
FIG. 1.
Average concentration of amprenavir in plasma versus time after the administration of single oral doses of 150, 300, 600, 900, and 1,200 mg. The graph in the inset shows a logarithmic plot of average concentration versus time with a horizontal reference line for 10 times the IC50 for HIV-1IIIB in peripheral blood lymphocytes.
Mean pharmacokinetic parameter estimates for all doses are presented in Table 2. The increase in the mean AUC0–∞ was greater than dose proportional over the five ascending doses tested. On the basis of the power model, the mean slope for the linear regression line of ln(AUC0–∞) versus ln(dose) was 1.25 (associated 90% CI, 1.16 to 1.35). In contrast, the increase in the mean Cmax was less than dose proportional. The mean slope for ln(Cmax) versus ln(dose) determined from the power model was 0.78 (associated 90% CI, 0.78 to 0.86). In more than half of the individual profiles, a small second peak (shoulder) occurred 6 to 12 h after administration.
TABLE 2.
Values of pharmacokinetic parameters for amprenavir after administration of escalating single, oral doses to 12 subjectsa
| Dose of amprenavir (mg) | AUC0–∞ (h · μg/ml) | Cmax (μg/ml) | Cmax/C12 | Tmax (h) | t1/2 (h) | Vz/F (liters) | CL/F (ml/min) | CLR (ml/min) |
|---|---|---|---|---|---|---|---|---|
| 150 | 4.03 (55) | 2.00 (57) | 112 (77) | 1.13 (34) | 7.95 (62) | 482 (57) | 808 (57) | 3.37 (36) |
| 300 | 9.13 (56) | 3.51 (44) | 48 (43) | 1.31 (35) | 7.08 (50) | 460 (76) | 757 (62) | 3.07 (48) |
| 600 | 21.16 (60) | 6.27 (52) | 29 (50) | 1.56 (37) | 8.00 (41) | 429 (58) | 651 (59) | 3.71 (24) |
| 900 | 32.73 (49) | 7.76 (31) | 19 (43) | 1.92 (35) | 7.83 (48) | 336 (41) | 547 (42) | 3.86 (24) |
| 1,200 | 47.14 (46) | 9.11 (30) | 18 (39) | 2.09 (38) | 9.54 (71) | 388 (75) | 519 (49) | 4.70 (35) |
All values are means, given together with percent coefficient of variance in parentheses.
Tmax was reached rapidly for all doses, between 1 and 2 h (range, 1.13 to 2.09 h) after dosing. The mean t1/2 was approximately 8 h (range, 7.08 to 9.54 h) and was relatively consistent between doses. CL/F tended to decrease with increasing doses, as indicated by the results from the power model analysis: the mean slope was −0.25 (90% CI, −0.35 to −0.17). While this trend was largely the result of decreases for three subjects (all males) with the highest CL/F, analysis of CL/F without these outliers indicated that CL/F still decreased with dose: the mean slope was −0.20 (90% CI, −0.29 to −0.11). The mean CLR was similar for all doses, ranging from 3.07 to 4.70 ml/min. Vz/F decreased with increasing doses from 150 to 900 mg and then increased slightly with increasing doses from 900 to 1,200 mg (range, 482 to 388 liters).
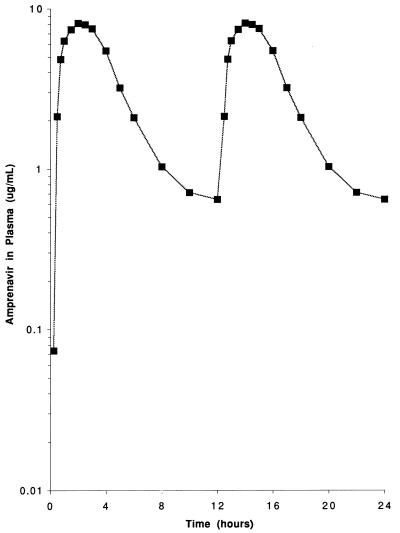
Steady-state plasma amprenavir concentrations for multiple doses (1,200 mg administered every 12 h) were predicted by superposition. The predicted steady-state plasma amprenavir concentrations over a 24-h period are shown in Fig. 2. The predicted steady-state maximum Cmax (Cmax,ss) and steady-state C12 (C12,ss) for amprenavir were estimated to be 9 and 1 μg/ml, respectively.
FIG. 2.
Simulated steady-state plasma amprenavir concentrations on the basis of twice-daily dosing with 1,200 mg.
The concentration of amprenavir in urine, which was determined for each subject during each dosing interval, showed that the average urinary recovery (percentage of the administered dose recovered as unchanged drug in urine) over the 24 h postdosing was low, ranging from 0.38 to 1.31%. The cumulative amount of amprenavir recovered in the urine increased with increasing dose. Most of the renal excretion of the drug (74 to 86%) occurred during the first 4 h after dosing.
(ii) Relative bioavailability study.
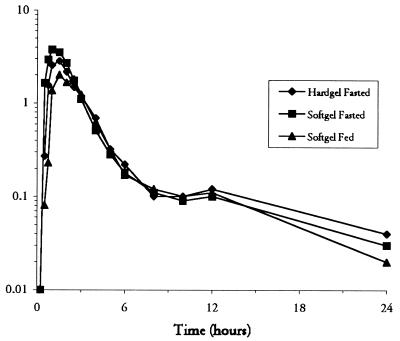
The bioavailability of the soft gelatin capsule formulation relative to that of the hard gelatin formulation, as assessed by the GLS means ratio for AUC0–∞ under fasting conditions, was 103% (Table 3). The 90% CI for the GLS means ratio for AUC0–∞ for the two formulations was within the bioequivalence acceptance range, indicating that the extent of absorption for the two formulations was similar. The drug was absorbed slightly faster from the soft gelatin capsules, as evidenced by a shorter median Tmax (1.0 versus 1.5 h) and a somewhat higher mean Cmax (4.36 versus 3.66 μg/ml; data not shown; Fig. 3). This difference was significant, and the 90% CI of the GLS means ratio for Cmax was outside of the acceptance range (1.03 to 1.53). As expected from the similarity in AUC0–∞, the GLS means for CL/F and Vz/F were similar for the two formulations (Table 3).
TABLE 3.
GLS means and means ratios for pharmacokinetic parameters after administration of single (600-mg) oral doses of amprenavir in two capsule types and under fasting versus nonfasting conditions in 18 HIV-infected subjects
| Study | AUC0–∞ (h · μg/ml) | Cmax (μg/ml) | Tmax (h)a | t1/2 (h) | CL/F (ml/min) | Vz/F (liters) |
|---|---|---|---|---|---|---|
| Amprenavir formulation | ||||||
| Hard gelatin, fastingb | 9.32 (7.20–12.08) | 3.27 (2.62–4.08) | 1.50 (0.75–2.50) | 6.30 (4.82–8.23) | 1,072 (828–1,390) | 585 (456–750) |
| Soft gelatin, fastingb | 9.58 (7.39–12.41) | 4.10 (3.28–5.11) | 1.00 (0.50–2.15) | 6.68 (5.11–8.73) | 1,044 (805–1,353) | 603 (470–774) |
| Soft gelatin, nonfastingb | 8.26 (6.37–10.70) | 2.75 (2.20–3.43) | 1.75 (0.75–4.00) | 6.15 (4.71–8.04) | 1,211 (935–1,569) | 645 (503–828) |
| Bioequivalence testing | ||||||
| Soft gelatin, fasting: hard gelatin, fastingc | 1.03 (0.92–1.14) | 1.25 (1.03–1.53) | −0.38 (−0.63–0.00) | 1.06 (0.87–1.30) | NDd | ND |
| Soft gelatin, nonfasting: soft gelatin, fasting | 0.86 (0.78–0.96) | 0.67 (0.55–0.82) | 0.75 (0.25–1.25) | 0.92 (0.75–1.13) | ND | ND |
Values are medians and ranges; ratios under bioequivalence testing are median differences.
Values for GLS means for all pharmacokinetic parameters are given together with the 95% CI range in parentheses unless indicated otherwise.
Values for GLS mean ratios are given together with the 90% CI range.
ND, not determined.
FIG. 3.
Semilogarithmic plot of median plasma amprenavir concentration versus time for three 600-mg doses given as a hard gelatin (Hardgel) capsule to fasting subjects, a soft gelatin (Softgel) capsule to fasting subjects, and a soft gelatin capsule to nonfasting subjects.
Compared with the values obtained for subjects in the fasting state, administration of the amprenavir soft gelatin formulation with food resulted in a 14% decrease in AUC0–∞ (9.58 versus 8.26 μg · h/ml), an increase in the median Tmax (1.0 versus 1.75 h), and a 33% decrease in Cmax (4.10 versus 2.75 μg/ml) (Table 3). The 90% CIs of the GLS means ratio for AUC0–∞ for the soft gelatin formulation in the nonfasting versus fasting state fell within the bioequivalence acceptance range, although the ratios for Cmax and Tmax were slightly outside (below) the bioequivalence acceptance range. The GLS means for CL/F and Vz/F were similar for the nonfasting and fasting conditions (Table 3).
DISCUSSION
The single-dose, dose-escalation trial with amprenavir described here represents the first study of this HIV-1 protease inhibitor conducted in humans. In this study, all five single, oral doses of amprenavir (150, 300, 600, 900, and 1,200 mg) were well tolerated by all HIV-infected subjects, most of whom were asymptomatic (CDC classification A or B). The most common clinical adverse experiences possibly related to the study drug were headache, nausea, and hypesthesia, although the incidences of headache and hypesthesia in drug-treated subjects were not different from those in placebo-treated subjects. In the relative bioavailability study, amprenavir was again found to be safe and well tolerated, with headache being the most frequently reported adverse event. All drug-related clinical adverse experiences were of mild to moderate intensity.
The pharmacokinetic findings from the dose-escalation study indicate that amprenavir reaches a maximum concentration rapidly (between 1 and 2 h), has adequate bioavailability (on the basis of the concentrations at 8 and 12 h after dosing), and has a relatively long t1/2 (approximately 8 h) with little renal excretion (CLR was significantly lower than the normal creatinine clearance regardless of the dose, and the cumulative urinary recovery was low, although it was dose dependent). AUC0–∞ was linear but was slightly greater than dose proportional, indicating the possibility of saturable metabolism at higher doses. This finding would most likely be attributed to first-pass metabolism since t1/2 was approximately equal at all five doses and Vz/F decreased with increasing dose. A small second concentration peak, or shoulder, in the plasma concentration-time curve was typically observed between 6 and 12 h after amprenavir administration, suggesting the possibility of secondary absorption or enterohepatic recirculation.
Renal elimination of amprenavir, as determined by the percentage of unchanged drug excreted in the urine, was low over the 24-h postdosing period but increased linearly with dose. This phenomenon may be related to the slightly greater than dose-proportional increase in AUC0–∞ over the range of doses tested. The CLR of amprenavir also tended to increase with increasing dose, although not as consistently as elimination did with AUC0–∞. The increase in CLR may be due to increases in the free drug concentration because binding to α1-acid glycoprotein can be saturable.
The median plasma amprenavir concentration at 12 h with the highest dose tested (1,200 mg) was greater than 10 times the mean in vitro IC50 for HIV-1IIIB in peripheral blood lymphocytes. Furthermore, assuming 90% plasma protein binding for amprenavir (11), the median free drug concentration of amprenavir at 12 h after the administration of single doses of 1,200 mg was 10 times the previously observed IC50 (0.006 μg/ml) of amprenavir for clinical isolates in vivo (18).
The predicted concentration of amprenavir at the end of each steady-state dosing interval following the administration of the 1,200-mg dose every 12 h was 1 μg/ml. However, the observed steady-state concentrations of amprenavir have not been as high as predicted (8a). The difference between single-dose and steady-state amprenavir concentrations may be explained by the lower concentrations of α1-acid glycoprotein in HIV-positive subjects after 3 weeks of therapy.
The overall pharmacokinetic profile of amprenavir compares favorably with those of the currently marketed protease inhibitors. Amprenavir appears to be absorbed as quickly as or more quickly than the other agents (9). Like that of most of the protease inhibitors (except indinavir), CLR is minimal (9). Amprenavir has a relatively long t1/2 (8 h), and the overall pharmacokinetic profile is conducive to twice-daily dosing.
The single-dose pharmacokinetic study of amprenavir described here was conducted with the drug in a hard gelatin capsule formulation. Because the hard gelatin formulation, a mesylate salt of amprenavir, was unstable at room temperature and had to be stored under refrigeration, a new formulation of amprenavir as a free base, which is stable at room temperature, was developed in a soft gelatin capsule. A relative bioavailability study was therefore designed and conducted to compare the single-dose pharmacokinetics of the two amprenavir formulations. A dose of 600 mg was compared since this dose yielded concentrations which were consistently above the assay limit of quantitation for the duration of the pharmacokinetic evaluation period in the initial dose-escalation study. The extent of absorption between the two formulations was found to be similar, as indicated by the AUC0–∞, Cmax, and Tmax data. The soft gelatin capsule formulation had a slightly higher AUC0–∞, a higher Cmax, and a shorter Tmax, but these differences are likely of little clinical significance. Note that the data in Table 2 (for the hard gelatin capsules) are arithmetic means, whereas the data in Table 3 are geometric means. The pharmacokinetic estimates from the two studies are statistically similar for the 600-mg dose.
Comparison of the pharmacokinetics of the amprenavir soft gelatin formulation administered with and without food showed that food has a minimal effect on amprenavir absorption. Consumption of a standardized high-calorie, high-fat breakfast produced a small decrease in the rate and extent of absorption of amprenavir in the soft gelatin formulation. However, these differences are likely of little clinical significance.
Drug-food interaction studies with the currently available HIV protease inhibitors have revealed that the absorptions and bioavailabilities of three of the four compounds are significantly affected by the presence of food. The absorption of indinavir is appreciably reduced with a standard high-calorie meal that is also high in fat, protein, and carbohydrate content: AUC is reduced by 78% and Cmax is reduced by 86% (21). The absorptions of nelfinavir (15) and saquinavir (17) are significantly increased, by two- to threefold, with the consumption of a meal or a snack. Administration of ritonavir with a meal does not result in a clinically significant change in the drug’s extent of absorption; AUC is increased by 15% when ritonavir capsules are given with a meal (12). The effect of food on the absorption of amprenavir appears to be less than its effect on the absorption of indinavir, nelfinavir, and saquinavir.
In summary, these studies indicate that amprenavir is a safe and well-tolerated HIV protease inhibitor with favorable absorption and clearance pharmacokinetics that are only minimally affected by administration with food. The overall pharmacokinetic profile of amprenavir, including its long t1/2, supports the further evaluation of a twice-daily dosing regimen for this HIV protease inhibitor. The results of this study have supported subsequent multiple-dose studies conducted to identify the optimal dose and dosing regimen of amprenavir and to evaluate safety, efficacy, and drug resistance.
ACKNOWLEDGMENT
We thank Cindy M. Rawls for performing the bioanalytical studies.
REFERENCES
- 1.Barry M, Gibbons S, Back D, Mulcahy F. Protease inhibitors in patients with HIV disease. Clinically important pharmacokinetic considerations. Clin Pharmacokinet. 1997;32:194–209. doi: 10.2165/00003088-199732030-00003. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter C C J, Fischl M A, Hammer S M, Hirsch M S, Jacobsend D M, Katzenstein D A, Montaner J S G, Richman D D, Saag M S, Schooley R T, Thompson M A, Vella S, Yeni P G, Volberding P A. Antiretroviral therapy for HIV infection in 1998: updated recommendations of the International AIDS Society–USA panel. JAMA. 1998;280:78–86. doi: 10.1001/jama.280.1.78. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. Morbid Mortal Weekly Rep. 1998;47(RR 5):43–82. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Update: trends in AIDS incidence—United States, 1996. Morbid Mortal Weekly Rep. 1997;46:861–864. [PubMed] [Google Scholar]
- 5.Conover J W. Practical nonparametric statistics. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1980. Some methods based on ranks; pp. 213–343. [Google Scholar]
- 6.Deeks S G, Smith M, Holodniy M, Kahn J O. HIV-1 protease inhibitors: a review for clinicians. JAMA. 1997;277:145–153. [PubMed] [Google Scholar]
- 7.Flexner C. HIV-protease inhibitors. N Engl J Med. 1998;338:1281–1291. doi: 10.1056/NEJM199804303381808. [DOI] [PubMed] [Google Scholar]
- 8.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker Inc.; 1982. [Google Scholar]
- 8a.Glaxo Wellcome Inc. Data on file. Glaxo Wellcome Inc., Research Triangle Park, N.C.
- 9.Kakuda T N, Struble K A, Piscitelli S C. Protease inhibitors for the treatment of human immunodeficiency virus infection. Am J Health-Syst Pharm. 1998;55:233–254. doi: 10.1093/ajhp/55.3.233. [DOI] [PubMed] [Google Scholar]
- 10.Kim E E, Baker C T, Dwyer M D, Mureko M A, Rao B G, Tung R D, Navia M A. Crystal structure of HIV-1 protease in complex with VX-478, a potent and orally bioavailable inhibitor of the enzyme. J Am Chem Soc. 1995;117:1181–1182. [Google Scholar]
- 11.Livingston D J, Pazhanisamy S, Porter D J T, Partaledis J A, Tung R D, Painter G R. Weak binding of VX-478 to human plasma proteins and implications for anti-human immunodeficiency virus therapy. J Infect Dis. 1995;172:1238–1245. doi: 10.1093/infdis/172.5.1238. [DOI] [PubMed] [Google Scholar]
- 12.Medical Economics Co. Physician’s desk reference. Vol. 52. Montvale, N.J: Medical Economics Co.; 1998. pp. 459–464. [Google Scholar]
- 13.Painter G R, St Clair M H, DeMiranda P, Reynolds D, Ching S, Dornsife R, Livingston D J, Pazhanisamy S, Tung R. Abstracts of the 2nd National Conference on Human Retroviruses and Related Infections. 1995. An overview of the preclinical development of the HIV protease inhibitor VX-478 (141W94), abstr. LB5. [Google Scholar]
- 14.Partaledis J A, Yamaguchi K, Tisdale M, Blair E E, Falcione C, Maschera B, Myers R E, Pazhanisamy S, Futer O, Cullinan A B, Stuver C M, Byrn R A, Livingston D J. In vitro selection and characterization of human immunodeficiency virus type 1 (HIV-1) isolates with reduced sensitivity to hydroxyethylamino sulfonamide inhibitors of HIV-1 aspartyl protease. J Virol. 1995;69:5228–5235. doi: 10.1128/jvi.69.9.5228-5235.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quart B D, Chapman S K, Peterkin J, Webber S, Oliver S. Abstracts of the 2nd National Conference on Human Retroviruses and Related Infections. 1995. Phase I safety, tolerance, pharmacokinetics and food effect studies of AG1343, abstr. LB3. [Google Scholar]
- 16.Sadler B M, Elkins M, Hanson C, Rooney J, Millard J, Blum M R, Painter G. Program and abstracts of the 5th European Conference on Clinical Aspects and Treatment of HIV Infection. 1995. The safety and pharmacokinetics of 141W94: an HIV protease inhibitor, abstr. 564; p. 90. [Google Scholar]
- 17.Shaw T M, Williams P E O, Nuirhead G J, Harris S, Watson N, Nimmo W. Abstracts of the IXth International Conference on AIDS. 1993. Effect of timing of food and gastric pH on exposure to Ro31-8959, HIV proteinase inhibitor in healthy subjects, abstr. PO-B30-2199; p. 502. [Google Scholar]
- 18.St Clair M, Millard J, Rooney J, Tisdale M, Parry N, Sadler B, Blum M R, Painter G. In vitro activity of 141W94 (VX-478) in combination with other antiviral agents. Antivir Res. 1996;29:53–56. doi: 10.1016/0166-3542(95)00916-7. [DOI] [PubMed] [Google Scholar]
- 19.Symonds W T, Sadler B M, Chittick G E, Moss J. A clinical study of the HIV-1 protease inhibitor, 141W94 (VX-478) to evaluate a new soft gelatin capsule formulation and to determine the effects of food upon bioavailability. Antivir Res. 1996;30:A42. [Google Scholar]
- 20.Woolley J, Studenberg S, Boehlert C, Bowers G, Sinhababu A, Adams P. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Cytochrome P-450 isozyme induction, inhibition, and metabolism studies with the HIV protease inhibitor, 141W94, abstr. A-60; p. 12. [Google Scholar]
- 21.Yeh K C, Deutsch P J, Haddix H, Hesney M, Hoagland V, Ju W D, Justice S J, Osborne B, Sterrett A T, Stone J A, Woolf E, Waldman S. Single-dose pharmacokinetics of indinavir and the effect of food. Antimicrob Agents Chemother. 1998;42:332–338. doi: 10.1128/aac.42.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]