Abstract
Many pathogenic bacteria utilize two-component systems consisting of a histidine protein kinase (HPK) and a response regulator (RR) for signal transduction. During the search for novel inhibitors, several chemical series, including benzoxazines, benzimidazoles, bis-phenols, cyclohexenes, trityls, and salicylanilides, were identified that inhibited the purified HPK-RR pairs KinA-Spo0F and NRII-NRI, with 50% inhibitory concentrations (IC50s) ranging from 1.9 to >500 μM and MICs ranging from 0.5 to >16 μg/ml for gram-positive bacteria. However, additional observations suggested that mechanisms other than HPK inhibition might contribute to antibacterial activity. In the present work, representative compounds from the six different series of inhibitors were analyzed for their effects on membrane integrity and macromolecular synthesis. At 4× MIC, 17 of 24 compounds compromised the integrity of the bacterial cell membrane within 10 min, as measured by uptake of propidium iodide. In this set, compounds with lower IC50s tended to cause greater membrane disruption. Eleven of 12 compounds inhibited cellular incorporation of radiolabeled thymidine and uridine >97% in 5 min and amino acids >80% in 15 min. The HPK inhibitor that allowed >25% precursor incorporation had no measurable MIC (>16 μg/ml). Fifteen of 24 compounds also caused hemolysis of equine erythrocytes. Thus, the antibacterial HPK inhibitors caused a rapid decrease in cellular incorporation of RNA, DNA, and protein precursors, possibly as a result of the concomitant disruption of the cytoplasmic membrane. Bacterial killing by these HPK inhibitors may therefore be due to multiple mechanisms, independent of HPK inhibition.
Two-component signal transduction systems (TCS) are regulatory mechanisms ubiquitous among bacteria (36, 44) and often control the expression of virulence traits (10, 18, 19). In addition, TCS are associated with regulation of resistance mechanisms for β-lactams (3, 14), polymyxin B (13), tetracycline (42), and vancomycin (4). In their simplest form they consist of a histidine protein kinase (HPK) and a response regulator (RR) (36).
At least five features have made TCS attractive targets for the development of novel antimicrobial agents: (i) they are present in most bacterial species (11, 36); (ii) most bacteria contain multiple TCS, each generally controlling different functions (32, 33, 48); (iii) HPKs and also RRs have a high degree of homology around the active sites (36, 48); (iv) they have not been found in either invertebrates or vertebrates (2, 7, 24); and (v) X-ray crystallographic structures exist for several RRs, including CheY (43) and Spo0F (27), and for the HPKs ArcB (20) and CheA (51). These features suggested that an inhibitor of multiple TCS of a bacterial pathogen could be identified that did not affect cellular functions of its eukaryotic host. An agent with such properties would be expected to interfere with the adaptive responses of the pathogen, attenuate its virulence, and possibly inhibit its growth.
Our group (16, 17, 22, 23, 26, 46, 49) and others (9, 40) have recently described several chemical series of compounds displaying inhibitory activity against TCS. In our laboratory, soluble KinA-Spo0F was chosen as the prototype TCS and used in the primary screening assay. A second soluble TCS, NRII-NRI, was used in secondary assays. Several series of compounds, including benzoxazines (23), benzimidazoles (16), bis-phenols (49), cyclohexenes (22, 46), trityls (5), and salicylanilides (17, 26), inhibited the purified HPK-RR pair KinA-Spo0F with 50% inhibitory concentrations (IC50s) ranging from 1.9 to >500 μM and MICs ranging from 0.5 to >16 μg/ml for gram-positive bacteria. Compounds such as RWJ-49815 and selected salicylanilides and cyclohexenes were furthermore shown to inhibit TCS in bacterial cells at concentrations insufficient to inhibit growth (5, 26, 46). Though this suggested that inhibition of the TCS preceded growth inhibition, it did not necessarily imply a causal relationship. Many of these compounds were hydrophobic, displayed acute in vivo toxicity in mice (30), and did not exhibit a strong correlation between HPK IC50s and MICs, thus suggesting that mechanisms other than HPK inhibition might also be operative for growth inhibition.
In the present work we have examined the ability of selected TCS inhibitors to interfere with the integrity of cell membranes from Staphylococcus aureus and mammalian blood cells as well as with the biosynthesis of various macromolecules. Our results suggest that the characterized compounds exhibit several modes of action and that their effects on bacterial growth may occur through mechanisms other than TCS inhibition.
(This work was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 1998.)
MATERIALS AND METHODS
Reagents.
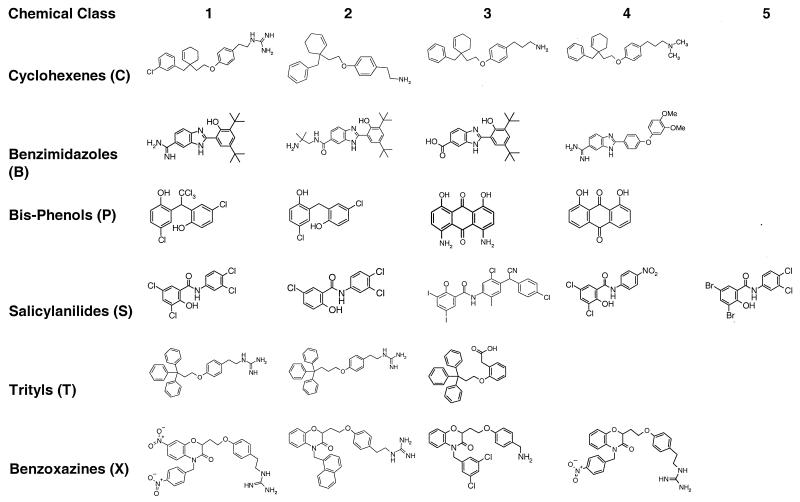
Levofloxacin was provided by Daiichi Seiyaku Co., Ltd., Kyoto, Japan. The bis-phenol P-3 (CAS 128-94-9) was purchased from Aldrich (Milwaukee, Wis.). Polymyxin B, gramicidin S, rifampin, and tetracycline were purchased from Sigma Chemical Company (St. Louis, Mo.). Cation-adjusted Mueller-Hinton broth (CAMHB) and Trypticase soy agar were purchased from BBL (Cockeysville, Md.). Bacto Peptone was purchased from Difco (Detroit, Mich.). All other exploratory compounds (Fig. 1) were synthesized in the laboratories at The R. W. Johnson Pharmaceutical Research Institute (Raritan, N.J.).
FIG. 1.
Structures of various chemical classes of TCS inhibitors. Previous designations for selected compounds are RWJ-49815 for T-1 (5), 9 for S-1, 14 for S-2, 1 for S-3, and 23 for S-4 (26).
Bacterial strains.
S. aureus ATCC 29213 was used for all assays.
Enzyme purification.
KinA was purified by ion exchange and affinity column chromatography from lysates of Escherichia coli carrying recombinant plasmid pJM8118, in which the kinA gene is under the control of the inducible tac promoter, as previously described (37). His6-Spo0F was purified by affinity chromatography from a lysate of E. coli BL21(DE3) containing an NdeB-BamHI insert of a PCR amplicon of the spo0F gene of Bacillus subtilis cloned into the pET16b vector (Novagen, Madison, Wis.), which was obtained from J. Hoch. His6-NRI was expressed from the pPRO-EX-1 vector in E. coli DH5α (45). Both histidine-tagged proteins were purified by using nickel resin affinity chromatography. The NRII protein was purified from a lysate of E. coli RB9132 provided by J. Hoch containing the glnL gene expressed by the pJLA501 vector as previously described by Ninfa et al. (35). NRII was purified by affinity and gel filtration chromatography.
Enzyme inhibition assays.
KinA-Spo0F and NRII-NRI autophosphorylation and phosphotransferase activities were analyzed by a gel-based assay (5). IC50s were determined graphically after scanning the gel with the Bio-Rad GS-250 phosphoimager (Hercules, Calif.). The IC50 was defined as the concentration of compound that inhibited KinA or NRII phosphorylation by 50%.
MIC.
Broth microdilution MIC determinations were performed according to National Committee for Clinical Laboratory Standards methods (34). The MIC was defined as the lowest concentration of compound or drug that inhibited visible growth.
Membrane damage.
The BacLight kit from Molecular Probes, Inc. (Eugene, Oreg.) was used to assess membrane damage. In this assay, the SYTO-9 and propidium iodide stains compete for binding to the bacterial nucleic acid. SYTO-9 labels cells with both damaged and intact membranes, whereas propidium iodide penetrates only cells with damaged membranes. S. aureus was grown overnight in CAMHB at 37°C with aeration (200 rpm). The culture was diluted 1:40 in fresh CAMHB and grown to an optical density at 600 nm (OD600) of 0.5 to 0.6. The bacterial suspension was centrifuged at 10,000 × g for 15 min, and the cell pellet was washed once in filter-sterilized distilled water. The cell pellet was resuspended to 1/10 of the original volume and then diluted 1:20 into either water or water containing test compounds at 4× MIC. Bacteria and compounds were incubated at room temperature (∼23°C) on a tube rocker for 10 min. At the end of the incubation period, a sample was removed for CFU determination, and the remaining suspension was centrifuged at 10,000 × g for 10 min, washed once in water, and resuspended to an OD670 of 0.325. A volume of 100 μl of the bacterial suspension was removed and added to a 96-well Cytofluor plate (PerSeptive Biosystems). An equal volume of the BacLight reagent was then added to each well, and the plates were incubated in the dark for 15 min at room temperature. At the end of the incubation period, green fluorescence (SYTO-9) was read at 530 nm, and red fluorescence (propidium iodide) was read at 645 nm with a PerSeptive Biosystems Cytofluor 2350 (excitation wavelength, 485 nm). The ratio of green to red fluorescence was normalized to the untreated control and expressed as a percentage of the control.
Bacterial viability.
Following the 10-min exposure to the compound, a sample was removed and inoculated into chilled 0.1% peptone. The culture was serially diluted and plated in Trypticase soy agar for CFU determination. Agar plates were incubated at 37°C for 18 to 20 h. Bacterial colonies were counted, and the log decrease in CFU/milliliter compared to the untreated control was calculated.
Macromolecular synthesis.
Macromolecular synthesis in S. aureus was evaluated by measuring the incorporation of the appropriate radiolabeled precursors into bacteria prior to treatment with trichloroacetic acid (TCA) (41). The inoculum was prepared by incubating S. aureus bacteria overnight at 35°C in CAMHB with shaking at 200 rpm. The culture was then diluted 1:50 in fresh prewarmed CAMHB and incubated for 1 h. Broth was removed from the bacteria by centrifugation for 15 min at 3,500 × g. The S. aureus pellet was suspended in M9 minimal medium and adjusted to an OD450 of 0.2. The bacterial cells were then exposed to the test compounds and control antibacterials at 4× MIC for 10 min. A nonspecific binding control, containing medium alone, was also prepared. DNA synthesis was measured by labeling 1 ml of culture with 1 μl of [3H]thymidine (76 Ci/mmol at 0.1 μCi/ml, TRK686; Amersham) for 5 min. RNA synthesis was determined by adding 5 μl of [3H]uridine (40 Ci/mmol at 0.5 μCi/ml, TRK410; Amersham) to 1 ml of S. aureus bacteria for 5 min. Protein synthesis was measured by labeling 1 ml of S. aureus bacteria with 100 μl of 3H-amino acid mixture (40 Ci/mmol at 10 μCi/ml, TRK410; Amersham) for 15 min. Following the radiolabeling, 1 ml of cold 10% TCA was added to all samples; for amino acid studies, unlabeled amino acids (0.5 mg/ml) were added to each amino acid-labeled sample simultaneously with the TCA. All samples were filtered through a glass microfiber filter (GF/A, 25-mm; Whatman) by using a Millipore 12 sample manifold. Each filter was first washed with 5 ml of ice-cold 10% TCA, followed by 5 ml of cold distilled water. The dried filters were counted in a liquid scintillation counter (LS 6000TA; Beckman).
Erythrocyte hemolysis.
Hemolytic activity of the compounds was determined by using equine erythrocytes (BBL). The erythrocytes were washed three times in 10 mM Tris-HCl (pH 7.4) buffer containing 0.9% NaCl and resuspended to 1% immediately prior to assay (8, 31). A volume of 200 μl of the cell suspension was added to 1,300 μl of buffer containing the compound. Cells were added to buffer alone or to 0.5% NH4OH for the zero and 100% hemolysis controls, respectively. The cell suspension was incubated 10 min at room temperature on a tube rocker and centrifuged at 1,300 × g for 5 min. Hemoglobin release from the cells was determined by measuring A540.
RESULTS
Compound selection.
Benzimidazoles, benzoxazines, bis-phenols, cyclohexenes, trityls, and salicylanilides included in the TCS screening program were synthesized specifically for this purpose. Biochemical screening against the KinA-Spo0F enzymes yielded compounds displaying a broad range of IC50s (2 to >500 μM). MICs were then determined for the most-active inhibitors, as well as for selected, less-active compounds, in order to establish structure-activity relationships.
The compounds used in the studies described below were representative members of the different chemical series that were synthesized (Fig. 1). Compounds were chosen to provide structural variety and a broad range of TCS inhibitory activity and antibacterial activity. Two compounds were selected which did not inhibit either enzyme (compounds P-4 and S-2) but were structurally similar to compounds which inhibited one or both enzymes. These noninhibitory compounds were used to analyze the specificity of the activities of the genuine TCS inhibitors.
Enzyme inhibition assays.
Kinase inhibitory activity as measured by IC50s is shown in Table 1 (22, 23, 26, 49). Inhibition by compounds in the benzimidazole (B-), trityl (T-), and benzoxazine (X-) classes was comparable for both enzymes. For most of the compounds in the bis-phenol and salicylanilide classes (P- and S-), there was little correlation between the KinA-Spo0F and NRII-NRI results; an IC50 of <100 μM against KinA-Spo0F was not predictive of equivalent potency against NRII-NRI.
TABLE 1.
Biological effects of HPK inhibitors and closely related analogs compared to those of reference antibacterial agents with known mechanisms of action
| Chemical class and agent | IC50 (μM)a
|
MICb (μg/ml) | Membrane effects (10 min)
|
|||
|---|---|---|---|---|---|---|
| KinA | NRII | % Control (BacLight)b | Δ log (CFU/ml)b | % Hemolysisc | ||
| Cyclohexenes | ||||||
| C-1 | 18 | 17 | 2 | 2 | 3.2 | 97 |
| C-2 | 220 | 88 | 8 | 80 | 0.1 | 97 |
| C-3 | 120 | >500 | 16 | 98 | 0.1 | 100 |
| C-4 | 260 | 70 | 8 | 86 | 0.0 | 95 |
| Benzimidazoles | ||||||
| B-1 | 10 | 17 | 2 | 4 | 0.9 | 16 |
| B-2 | 10 | 13 | 2 | 4 | 2.2 | 95 |
| B-3 | 86 | 140 | 2 | 4 | 2.6 | 94 |
| B-4 | >500 | 230 | 8 | 39 | 0.0 | 12 |
| Bis-phenols | ||||||
| P-1 | 4 | 23 | 2 | 4 | 6.3 | 5 |
| P-2 | 44 | 230 | 4 | 8 | 0.3 | 1 |
| P-3 | 93 | 68 | >16 | 35 | 0.0 | 4 |
| P-4 | >500 | >500 | 8 | 76 | 0.0 | 5 |
| Salicylanilides | ||||||
| S-1 | 37 | 370 | 0.5 | 49 | −0.1 | 1 |
| S-2 | >500 | >500 | 2 | 35 | 0.3 | 4 |
| S-3 | 5 | 5 | 2 | 47 | 0.0 | 1 |
| S-4 | >500 | NDd | 4 | 83 | 0.0 | 22 |
| S-5 | 26 | 180 | 0.5 | 38 | 0.0 | 2 |
| Trityls | ||||||
| T-1 | 2 | 12 | 2 | 8 | 0.2 | 100 |
| T-2 | 9 | 16 | 2 | 10 | 2.3 | 100 |
| T-3 | 49 | 88 | 16 | 37 | 0.1 | 100 |
| Benzoxazines | ||||||
| X-1 | 53 | 180 | 2 | 22 | 0.6 | 6 |
| X-2 | 13 | 37 | 2 | 10 | 0.6 | 12 |
| X-3 | 46 | 81 | 4 | 33 | 0.3 | 87 |
| X-4 | 24 | 54 | 4 | 15 | 0.5 | 12 |
| Reference compounds | ||||||
| Tetracycline | >0.4e | ND | 0.5 | 102 | 0.0 | 4 |
| Levofloxacin | >0.1e | ND | 0.12 | 86 | 0.3 | 2 |
| Gramicidin S | >100f | ND | 4 | 2 | 3.4 | 18 |
| Rifampin | >0.007e | ND | >0.015 | 80 | 0.3 | 2 |
| Polymyxin B | >34e | ND | 64 | 18 | 0.3 | 4 |
| Untreated control | ND | ND | ND | 100 | 0.0 | 2 |
IC50s for KinA and NRII were determined in the presence of their RRs. IC50s include original data as well as data from previous studies (17, 22, 23, 26, 46, 49, 50).
S. aureus 29213 was used for this assay.
Erythrocytes treated with 0.5% ammonium hydroxide were completely hemolyzed.
ND, not determined.
The highest concentration tested (micromolar) corresponded to 4× MIC (reported in micrograms/milliliter).
The highest concentration tested corresponded to 6× MIC.
Antibacterial activity.
Susceptibility of S. aureus to the test compounds and selected reference agents is shown in Table 1. With the exception of the benzimidazoles and trityls, there was no strong correlation between antibacterial activity and enzyme inhibition. For the benzimidazoles, compounds with IC50s of <100 μM against KinA had MICs of 2 μg/ml, whereas B-4 with IC50s ≥230 μM had a MIC of 8 μg/ml. In the trityl series, T-1 and T-2 with IC50s ≤20 μM also demonstrated comparable antimicrobial activity against S. aureus, with MICs of 2 μg/ml. For the cyclohexenes, the NRII IC50s had a positive correlation with antibacterial activity; however, there was no correlation with KinA inhibition. The MICs of compounds B-4, S-2, S-4, and P-4, with structural similarities to other members of their class, ranged from 2 to 8 μg/ml despite the lack of measurable TCS inhibitory activity.
Membrane damage.
Bacterial membrane damage was examined by using the BacLight assay. As the BacLight data in Table 1 indicate, 71% (17 of 24) of the compounds—primarily in the benzimidazole, bis-phenol, trityl, and benzoxazine classes—altered the permeability of the membrane, resulting in <40% of control value. C-1 was the only cyclohexene that damaged the bacterial membrane within 10 min. As a class, the salicylanilides displayed moderate membrane effects, though these compounds had good antibacterial activity. Gramicidin S is a compound that depolarizes the membrane by forming channels (25), and polymyxin B is a cationic detergent that binds lipoteichoic acid and may induce autolysis (12, 39). As expected among the controls, gramicidin S and polymyxin B severely compromised the bacterial cell membrane. Levofloxacin, tetracycline, and rifampin had little effect on membrane integrity.
Bacterial viability.
Because bacterial membrane damage is not necessarily a lethal event, the ability of compound-exposed cells to form colonies on solid agar medium was determined. In contrast to the BacLight data that indicated membrane damage, only 6 of 24 compounds appreciably reduced the number of CFU compared to the control (≥0.9-log reduction in CFU/ml) during the 10-min exposure. The cyclohexenes showed a positive correlation between the BacLight data and the reduction in CFU. The benzimidazole B-1, the trityl T-1, and the benzoxazine X-2 significantly damaged the membrane at 10 min (≥90% permeability compared to the no-drug control) yet showed less than a 1-log drop in CFU/ml. By 60 min, however, there was a 2- to 6-log drop in CFU/ml for these compounds without further membrane damage (data not shown). Thus, the observable change in membrane damage as detected by the BacLight assay occurs rapidly and precedes the decrease in viability. Note that these are results of a short-term exposure (10 min) to compound in water, rather than the 16 to 20 h of exposure in medium routinely used in MIC determinations.
Macromolecular synthesis.
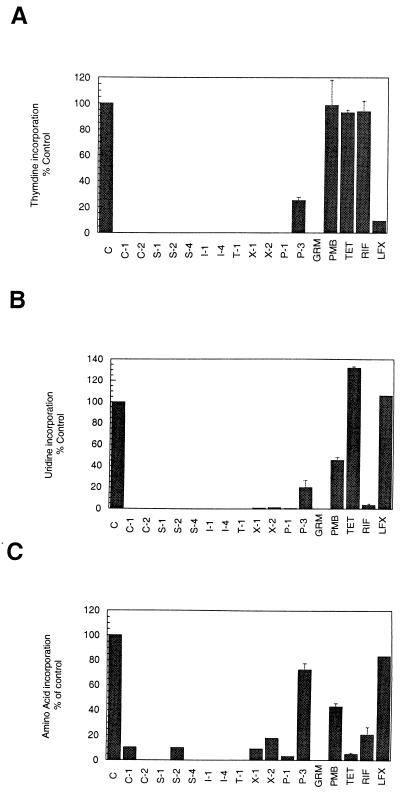
Twelve synthetic compounds were tested in the three macromolecular synthesis assays to evaluate [3H]thymidine, [3H]uridine, and 3H-amino acid mixture incorporation into DNA, RNA, and protein, respectively, after short exposure to the TCS inhibitors. The results of these assays are summarized in Fig. 2. Five antibacterial agents with various mechanisms of action were tested as controls. All of the compounds tested, except P-3, inhibited incorporation of all three macromolecular precursors. The bis-phenol P-3 inhibited thymidine and uridine incorporation but had minor effects on amino acid incorporation.
FIG. 2.
Effect of selected TCS inhibitors on incorporation of [3H]thymidine into DNA (A), [3H]uridine into RNA (B), and a mixture of 3H-amino acids into protein (C). GRM, gramicidin S; PMB, polymyxin B; TET, tetracycline; RIF, rifampin; LFX, levofloxacin.
The control antibacterial agents behaved as expected. Levofloxacin, a DNA topoisomerase inhibitor, inhibited [3H]thymidine incorporation (DNA synthesis) but did not inhibit [3H]uridine and 3H-amino acid incorporation. Tetracycline, a protein synthesis inhibitor, inhibited amino acid incorporation only. Rifampin, an RNA synthesis inhibitor, inhibited both [3H]uridine and 3H-amino acid incorporation. Two membrane-damaging agents were also tested. Polymyxin B did not affect incorporation of [3H]thymidine but decreased both [3H]uridine and 3H-amino acid incorporation, whereas gramicidin S inhibited the incorporation of all three precursors.
Hemolysis.
Eukaryotic membrane damage by these compounds was assessed by examining hemolysis of equine erythrocytes. Fifteen of 24 (63%) compounds tested lysed erythrocytes (greater than 10% of the control). Background hemolysis of the untreated control erythrocytes was 2%. The cyclohexene and trityl series showed nearly complete hemolysis of the cells, as shown in Table 1. The bis-phenols were not hemolytic, whereas both the benzimidazoles and benzoxazines caused varying degrees of hemolytic activity. Only one salicylanilide, S-4, caused hemolysis (22%). Gramicidin S was the only reference compound to cause appreciable hemolysis (18% of the control). Hemolytic activity did not correlate with bacterial membrane damage.
DISCUSSION
New analogs of existing antibiotics generally result in therapeutic agents whose utility to treat infectious disease is compromised by emergent drug resistance. Agents with new mechanisms of action are needed to provide a longer-term solution to this problem. Inhibition of the bacterial two-component signal transduction systems has been an appealing solution to the problem of antimicrobial drug resistance (5, 40). Two-component systems are ubiquitous among bacteria and are multiply present in each species where they regulate adaptive responses, including the regulation of virulence traits (10, 18, 19) and resistance mechanisms (3, 4, 13, 14, 42). Furthermore, they have in common structural motifs not found among higher eukaryotes, thus suggesting that agents capable of inhibiting them could have the specificity and selectivity inherent to safe and effective antimicrobial agents. However, as shown above, these expectations were not met by the six structural classes of inhibitors examined in our studies.
Twenty-three of the 24 compounds evaluated as putative HPK inhibitors either appreciably affected the membrane integrity of S. aureus or caused hemolysis of equine erythrocytes. The IC50s of the remaining compound, the bis-phenol P-4 which caused appreciably less damage to the S. aureus membrane, were >500 μM in both enzyme assays. Furthermore, for a subset of the 24 compounds, bacterial viability was affected within a 10-min exposure to compound. In addition, 11 of 12 compounds reduced the incorporation of thymidine, uridine, and amino acids into macromolecules by more than 80%. This result strongly suggests that the observed reductions resulted from a general effect upon either uptake or intracellular retention of the precursors and not on each of the individual macromolecular biosynthetic processes per se. Indeed, the membrane effects are very similar to those seen with some peptide antimicrobial agents. For example, as seen in our studies as well as in previous work (21), the cyclic peptide gramicidin S disrupts cell membranes in gram-positive bacteria, and the cyclic peptide daptomycin simultaneously inhibits the biosynthesis of RNA, DNA, peptidoglycan, and lipid (6). Daptomycin also inhibits amino acid transport by disrupting the membrane potential (1). The 11 inhibitors of macromolecular synthesis in this study may be acting in a similar manner.
The data suggest that certain structural features may be affecting membrane integrity. All of the examined compounds appeared to alter uptake of small molecules, such as propidium iodide, and macromolecular synthesis precursors. In the trityl series, replacement of the guanidinium by a carboxyl reduced the effect on the bacterial membrane, whereas extension of the alkanyl of T-1 and T-2 resulted in compounds causing increased membrane alteration. For the bis-phenols, addition of the hydrophobic trichloromethyl group (P-1) drastically increased bactericidal activity. In contrast, the addition of amino groups on P-3 reduced both the effects on membrane integrity and bactericidal activity. Similarly, for the cyclohexene C-1, the substitution of a Cl for H and guanidine for amine reduced bacterial membrane integrity and increased bactericidal activity. However, all compounds in this series were hemolytic. For the benzimidazoles, compounds containing the di-t-butylphenol moiety (B-1 to B-3) appeared to be associated with serious membrane damage. Replacement of the carboxyl (B-3) or aminoalkylamide (B-2) moiety with the amidine functionality (B-1) reduced bactericidal activity and hemolysis. In the benzoxazine series, the levels of bactericidal activity and bacterial membrane damage were fairly consistent, despite significant variation in structure (X-1 through X-4).
Clearly, the salicylanilide class of compounds behaved differently from the other inhibitors. This class contained the most-potent antibacterial agents; yet the enzymatic inhibitory activity was inconsistent throughout the series, with S-3 (closantel) being the most active. There was no correlation between enzymatic activity and membrane damage; all compounds caused moderate membrane damage (35 to 80% of the control). Prolonged exposure of S-1 to the cells (60 min) did not increase membrane damage as measured by the BacLight reagents, and it did not reduce the CFU/milliliter compared to the control (data not shown), consistent with the report of salicylanilide antibacterial agents as static (15). Closantel (S-3) is a hydrogen ionophore and is known to uncouple oxidative phosphorylation in mitochondria (28, 47). Both salicylanilides and halogenated benzimidazoles are known to act as uncouplers of oxidative phosphorylation, though they differ in their specific mechanisms of action (52). Furthermore, whereas salicylanilides act as bacteriostatic agents, benzimidazoles are often bactericidal (see especially B-2 and B-3). Therefore, it is likely that salicylanilides and benzimidazoles have multiple mechanisms of action and that they differ in their primary targets.
It is clear from the results presented here that compounds differed greatly among themselves in their patterns of effects on S. aureus membranes and horse erythrocyte membranes and in their short-term bactericidal effects. These differences were noticeable even when compounds of the same series were being compared. Since these effects are operative on both prokaryotic and eukaryotic membranes, they cannot be the consequence of the selective activity of these compounds on bacterial TCS. Hence, these compounds have multiple mechanisms of action, since they also must be acting upon targets other than bacterial TCS.
The chemical series used for our program originated either from similar structures in the literature (9, 40) or from computer modelling with the X-ray crystallographic structures for CheY (43) and Spo0F (27). Given that KinA and Spo0F were both soluble enzymes, it was unexpected that our selection yielded primarily compounds that had poor specificity and that behaved as membrane disrupters. However, knowing that some of the active compounds displayed detergent-like features, we investigated the possibility that lipophilic membrane disrupters may act as KinA-Spo0F inhibitors. Among 45 commercially available detergents representing a variety of families of structures, only 12 showed KinA-Spo0F inhibitory activity at concentrations below 500 μM. The more-inhibitory detergents, whose IC50s were between 6 and 18 μM, contained C14 to C16 aliphatic chains with quaternary ammonium groups. Though aliphatic chain length was important, the charged groups were also important. Thus, whereas the IC50 of sodium dodecyl sulfate was 78 μM, dodecyl quaternary ammonium salts, as well as detergents such as polyethylene glycol ethers, taurocholic acid, Pluronic F-127, okadaic acid, N-N-bis[3-(d-gluconamido)propyl]cholamide, laudanosine methiodide, and benzyldimethylphenylammonium chloride, did not inhibit TCS at concentrations of 500 μM (23a). Therefore, inhibition of KinA-Spo0F, even by detergents, showed specificity.
Our results do not necessarily invalidate the value of bacterial TCS as novel targets for antimicrobial agents. However, they emphasize potential problems that may be inherent to the inhibitors identified through the use of these biochemical assays. Thus, notwithstanding our initial expectations, searching for agents that simultaneously inhibit multiple TCS and show good antibacterial activity may yield compounds with broad specificity and poor selectivity. This problem may now be circumvented by focusing on inhibitors for only those particular TCS that may be essential for pathogenic bacteria. Inhibition of these individual systems would then result in growth inhibition or bacterial killing. Such systems have been recently identified (29, 38) and may represent an opportunity to exploit TCS as novel targets in a more selective approach.
ACKNOWLEDGMENTS
We thank Dennis Hlasta and Mark Macielag for their contributions to the HPK project and their critical assessment of the manuscript; Barbara Foleno and Ellyn Wira for performing susceptibility testing; and Thurman Dow, Jeffrey Fernandez, Michael Loeloff, and Glenda Webb for assistance with some of the biochemical assays.
REFERENCES
- 1.Alborn W E, Jr, Allen N E, Preston D A. Daptomycin disrupts membrane potential in growing Staphylococcus aureus. Antimicrob Agents Chemother. 1991;35:2282–2287. doi: 10.1128/aac.35.11.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alex L A, Simon M I. Protein histidine kinases and signal transduction in prokaryotes and eukaryotes. Trends Genet. 1994;10:133–138. doi: 10.1016/0168-9525(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 3.Alksne L, Rasmussen B A. Expression of the AsbA1, OXA-12, and AsbM1 β-lactamases in Aeromonas jandaei AER 14 is coordinated by a two-component regulon. J Bacteriol. 1997;179:2006–2013. doi: 10.1128/jb.179.6.2006-2013.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arthur M, Molinas C, Courvalin P. The VanS-VanR two-component regulatory system controls synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol. 1992;174:2582–2591. doi: 10.1128/jb.174.8.2582-2591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett J F, Goldschmidt R M, Lawrence L E, Foleno B, Chen R, Demers J P, Johnson S, Kanojia R, Fernandez J, Bernstein J, Licata L, Donetz A, Huang S, Hlasta D J, Macielag M J, Ohemeng K, Frechette R, Frosco M B, Klaubert D H, Whiteley J M, Wang L, Hoch J A. Antibacterial agents that inhibit two-component signal transduction systems. Proc Natl Acad Sci USA. 1998;95:5317–5322. doi: 10.1073/pnas.95.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canepari P, Boaretti M, Del Mar Lleo M, Satta G. Lipoteichoic acid as a new target for activity of antibiotics: mode of action of daptomycin ( LY146032) Antimicrob Agents Chemother. 1990;34:1220–1226. doi: 10.1128/aac.34.6.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang C, Stewart R C. The two-component system. Regulation of diverse signaling pathways in prokaryotes and eukaryotes. Plant Physiol. 1998;117:723–731. doi: 10.1104/pp.117.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dathe M, Schumann M, Wieprecht T, Winkler A, Beyerman M, Krause E, Matsuzaki O, Bienert M. Peptide helicity and membrane surface charge modulate the balance of electrostatic and hydrophobic interactions with lipid bilayers and biological membranes. Biochemistry. 1996;35:12612–12622. doi: 10.1021/bi960835f. [DOI] [PubMed] [Google Scholar]
- 9.Domagala J M, Alessi D, Gracheck S, Huang L, Huband M, Olson E, Shapiro M, Singht R, Song Y, VanBogelen R, Vot D. Program and abstracts of the 29th Central Regional Meeting of the American Chemical Society, Midland, Mich. 1997. Bacterial two-component signaling as a therapeutic target in drug design: inhibition of NRII by diphenolic methanes, abstr. 44. [Google Scholar]
- 10.Dziejman M, Mekalanos J J. Two-component signal transduction and its role in the expression of bacterial virulence factors. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: ASM Press; 1995. pp. 305–317. [Google Scholar]
- 11.Egger L A, Park H, Inouye M. Signal transduction via the histidyl-aspartyl phosphorelay. Genes Cells. 1997;2:167–184. doi: 10.1046/j.1365-2443.1997.d01-311.x. [DOI] [PubMed] [Google Scholar]
- 12.Ginsburg I, Lahav M, Giesbrecht P. Effect of leukocyte hydrolases on bacteria. XVI. Activation by leukocyte factors and cationic substances of autolytic enzymes in Staphylococcus aureus: modulation by anioic polyelectrolytes in relation to survival of bacteria in inflammatory exudates. Inflammation. 1982;6:269–284. doi: 10.1007/BF00916408. [DOI] [PubMed] [Google Scholar]
- 13.Groisman E A, Kayser J, Soncini F C. Regulation of polymyxin resistance and adaptation to low-Mg2+ environments. J Bacteriol. 1997;179:7040–7045. doi: 10.1128/jb.179.22.7040-7045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guenzi E, Gasc A M, Sicard M A, Hakenbeck R. A two-component signal-transducing system is involved in competence and penicillin susceptibility in laboratory mutants of Streptococcus pneumoniae. Mol Microbiol. 1994;12:505–515. doi: 10.1111/j.1365-2958.1994.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton W A. Mechanism of the bacteriostatic action of tetrachlorosalicylanilide. Membrane-active antibacterial compound. J Gen Microbiol. 1968;50:441–458. doi: 10.1099/00221287-50-3-441. [DOI] [PubMed] [Google Scholar]
- 16.Hilliard J J, Licata L, Goldschmidt R, Baum E Z, Macielag M, Hlasta D J, Bush K. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Multiple mechanisms of action for inhibitors of histidine protein kinase from bacterial two-component systems, abstr. F-161; p. 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hlasta D J, Demers J P, Foleno B D, Fraga-Spano S A, Guan J, Hilliard J J, Macielag M J, Ohemeng K A, Sheppard C M, Sui Z, Webb G C, Weidner-Wells M A, Werblood H, Barrett J F. Novel inhibitors of bacterial two-component systems with gram positive antibacterial activity: pharmacophore identification based on the screening hit closantel. Bioorg Med Chem Lett. 1998;8:1923–1928. doi: 10.1016/s0960-894x(98)00326-6. [DOI] [PubMed] [Google Scholar]
- 18.Jones A L, DeShazer D, Woods D E. Identification and characterization of a two-component regulatory system involved in invasion of eukaryotic cells and heavy-metal resistance in Burkholderia pseudomallei. Infect Immun. 1997;65:4972–4977. doi: 10.1128/iai.65.12.4972-4977.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jungnitz H, West N P, Walker M J, Chhatwal G S, Guzmán C A. A second two-component regulatory system of Bordetella bronchiseptica required for bacterial resistance to oxidative stress, production of acid phosphatase, and in vivo persistence. Infect Immun. 1998;66:4640–4650. doi: 10.1128/iai.66.10.4640-4650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato M, Mizuno T, Shimizu T, Hakoshima T. Insights into multistep phosphorelay from the crystal structure of the C-terminal HPt domain of ArcB. Cell. 1997;88:717–723. doi: 10.1016/s0092-8674(00)81914-5. [DOI] [PubMed] [Google Scholar]
- 21.Katsu T, Kuroko M, Morikawa T, Sanchika K, Fujita Y, Yamamura H, Uda M. Mechanism of membrane damage induced by the amphipathic peptides gramicidin S and melittin. Biochim Biophys Acta. 1989;983:135–141. doi: 10.1016/0005-2736(89)90226-5. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence L, Huang S, Foleno B, Chen R, Barrett J F. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. In vitro activity and mechanism of action of a series of novel two-component system inhibitors, abstr. F-225; p. 184. [Google Scholar]
- 23.Licata L, Melton J L, Fernandez J A, Frechette R F, Beach M, Webb G, Lawrence L E, Barrett J F, Frosco M B. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. In vitro characterization of a novel class of antibacterial agents that inhibit bacterial two-component systems, abstr. F-226; p. 184. [Google Scholar]
- 23a.Loeloff, M. Unpublished results.
- 24.Loomis W F, Shaulsky G, Wang N. Histidine kinases in signal transduction pathways of eukaryotes. J Cell Sci. 1997;110:1141–1145. doi: 10.1242/jcs.110.10.1141. [DOI] [PubMed] [Google Scholar]
- 25.López-Amorós R, Comas J, Vives-Rego J. Flow cytometric assessment of Escherichia coli and Salmonella typhimurium starvation-survival in seawater using rhodamine 123, propidium iodide, and oxonol. Appl Environ Microbiol. 1995;61:2521–2526. doi: 10.1128/aem.61.7.2521-2526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macielag M J, Demers J P, Fraga-Spano S A, Hlasta D J, Johnson S G, Kanojia R M, Russell R K, Sui Z, Weidner-Wells M A, Werblood H, Foleno B D, Goldschmidt R M, Loeloff M J, Webb G C, Barrett J F. Substituted salicylanilides as inhibitors of two-component regulatory systems in bacteria. J Med Chem. 1998;41:2939–2945. doi: 10.1021/jm9803572. [DOI] [PubMed] [Google Scholar]
- 27.Madhusudan J, Zapf, Whiteley J M, Hoch J A, Xuong N H, Varughese K I. Crystal structure of a phosphatase-resistant mutant of sporulation response regulator Spo0F from Bacillus subtilis. Structure. 1996;4:679–690. doi: 10.1016/s0969-2126(96)00074-3. [DOI] [PubMed] [Google Scholar]
- 28.Maes L. Flukicidal action of closantel against immature and mature Fasciola hepatica in experimentally infected rats and sheep. Res Vet Sci. 1988;44:229–232. [PubMed] [Google Scholar]
- 29.Martin P K, Li T, Sun D, Biek D P, Schmid M B. Abstracts of the 98th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1998. Genetic evidence for the essential nature of an HK/RR signal transduction circuit in Staphylococcus aureus, abstr. B-46; p. 63. [Google Scholar]
- 30.Melton J L, Fernandez J A, Frechette R F, Beach M J, Licata L, Mehta D B, Barrett J F, Frosco M B. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. In vivo activity of a novel series of antibacterial agents that inhibit the bacterial two-component signal transduction system, abstr. F-229; p. 185. [Google Scholar]
- 31.Miyoshi S, Sashara K, Akamatsu S, Rahman H M, Katsu T, Tomochika K, Shinoda S. Purification and characterization of a hemolysin produced by Vibrio mimicus. Infect Immun. 1997;65:1830–1835. doi: 10.1128/iai.65.5.1830-1835.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizuno T. Compilation of all genes encoding two-component phosphotransfer signal transducers in the genome of Escherichia coli. DNA Res. 1997;4:161–168. doi: 10.1093/dnares/4.2.161. [DOI] [PubMed] [Google Scholar]
- 33.Mizuno T, Kaneko T, Tabata S. Compilation of all genes encoding bacterial two-component signal transducers in the genome of the cyanobacterium, Synechocystis sp. strain PCC 6803. DNA Res. 1996;3:407–414. doi: 10.1093/dnares/3.6.407. [DOI] [PubMed] [Google Scholar]
- 34.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 35.Ninfa E G, Atkinson M R, Kamberov E S, Ninfa A J. Mechanism of autophosphorylation of Escherichia coli nitrogen regulator II (NRII or NtrB): trans-phosphorylation between subunits. J Bacteriol. 1993;175:7024–7032. doi: 10.1128/jb.175.21.7024-7032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parkinson J S, Kofoid E C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 37.Perego M, Cole S P, Burbulys D, Trach K, Hoch J A. Characterization of the gene for a protein kinase which phosphorylates the sporulation-regulatory proteins Spo0A and Spo0F of Bacillus subtilis. J Bacteriol. 1989;171:6187–6196. doi: 10.1128/jb.171.11.6187-6196.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quon K C, Yang B, Domian I J, Shapiro L, Marczynski G T. Negative control of bacterial DNA replication by a cell cycle regulatory protein that binds at the chromosome origin. Proc Natl Acad Sci USA. 1998;95:120–125. doi: 10.1073/pnas.95.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renzi P M, Lee C-H. A comparative study of biological activities of lipoteichoic acid and lipopolysaccharide. J Endotoxin Res. 1995;2:431–434. [Google Scholar]
- 40.Roychoudhury S, Zielinski N A, Ninfa A J, Allen N E, Jungheim L N, Nicas T I, Chakrabarty A M. Inhibitors of two-component signal transduction systems: inhibition of alginate gene activation in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1993;90:965–969. doi: 10.1073/pnas.90.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh M P, Petersen P J, Jacobus N V, Maiese W M, Greenstein M, Steinberg D A. Mechanistic studies and biological activity of bioxalomycin α2, a novel antibiotic produced by Streptomyces viridodiastaticus subsp. “litoralis” LL-31F508. Antimicrob Agents Chemother. 1994;38:1808–1812. doi: 10.1128/aac.38.8.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevens A M, Sanders J M, Shoemaker N B, Salyers A A. Genes involved in production of plasmidlike forms by a Bacteroides conjugal chromosomal element share amino acid homology with two-component regulatory systems. J Bacteriol. 1992;174:2935–2942. doi: 10.1128/jb.174.9.2935-2942.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stock A M, Martinez-Hackert E, Rasmussen B F, West A H, Stock J B, Ringe D, Petsko G A. Structure of the magnesium-bound form of CheY and mechanism of phosphoryl transfer in bacterial chemotaxis. Biochemistry. 1993;32:13375–13380. doi: 10.1021/bi00212a001. [DOI] [PubMed] [Google Scholar]
- 44.Stock J B, Ninfa A J, Stock A M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tzeng Y-L, Hoch J A. Molecular recognition in signal transduction: the interaction surfaces of the Spo0F response regulator with its cognate phosphorelay proteins revealed by alanine scanning mutagenesis. J Mol Biol. 1997;272:200–212. doi: 10.1006/jmbi.1997.1226. [DOI] [PubMed] [Google Scholar]
- 46.Urbanski M J, Xiang M A, Foleno B D, Bernstein J I, Lawrence L, Goldschmidt R M, Barrett J F, Frechette R, Chen R. Abstracts of the 214th American Chemical Society National Meeting, Las Vegas, Nev. 1997. Novel cyclohexene derivatives with histidine protein kinase inhibitory activity—potential new antibacterial agents, abstr. MEDI-270. [Google Scholar]
- 47.Vanden Bossche H. How anthelmintics help us to understand helminths. Parasitology. 1985;90:675–685. doi: 10.1017/s0031182000052306. [DOI] [PubMed] [Google Scholar]
- 48.Volz K. Structural conservation in the CheY superfamily. Biochemistry. 1993;32:11741–11753. doi: 10.1021/bi00095a001. [DOI] [PubMed] [Google Scholar]
- 49.Webb G C, Podlogar B L, Frosco M, Foleno B D, Ohemeng K A, Barrett J F. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Simple bis-phenols inhibit bacterial two-component phospho-transfer transduction, abstr. F-230; p. 185. [Google Scholar]
- 50.Weidner-Wells M A, Bernstein J, Chen R H K, Demers J P, Foleno B, Fraga-Spano S A, Hlasta D J, Johnson S G, Kanojia R, Klaubert M D, Sheppard H C M, Barrett J F. Abstracts of the 214th American Chemical Society National Meeting, Las Vegas, Nev. 1997. Triphenylalkyl derivatives as inhibitors of bacterial two-component regulatory systems, abstr. MEDI-148. [Google Scholar]
- 51.Welch M, Chinardet N, Mourey L, Birck C, Samama J-P. Structure of the CheY-binding domain of histidine kinase CheA in complex with CheY. Nat Struct Biol. 1998;5:25–29. doi: 10.1038/nsb0198-25. [DOI] [PubMed] [Google Scholar]
- 52.Wilson D F, Ting H P, Koppelman M S. Mechanism of action of uncouplers of oxidative phosphorylation. Biochemistry. 1971;10:2897–2902. doi: 10.1021/bi00791a016. [DOI] [PubMed] [Google Scholar]