Abstract
Graft-versus-host disease (GVHD) is an important cause of morbidity and mortality after allogeneic hematopoietic cell transplantation (HCT). Many studies have suggested that human herpesvirus-6B (HHV-6B) plays a role in acute GVHD (aGVHD) after HCT. Our objective was to systematically summarize and analyze evidence regarding HHV-6B reactivation and development of aGVHD. PubMed and EMBASE databases were searched using terms for HHV-6, HCT, and aGVHD, yielding 865 unique results. Case reports, reviews, articles focusing on inherited chromosomally integrated HHV-6, poster presentations, and articles not published in English were excluded. The remaining 467 articles were reviewed for the following requirements: a statistical analysis of HHV-6B reactivation and a GVHD was described, HHV-6B reactivation was defined by PCR, and blood (plasma, serum, or peripheral blood mononuclear cells) was used for HHV-6B PCR. Data were abstracted from publications that met these criteria (n = 33). Publications were assigned to 1 of 3 groups: (1) HHV-6B reactivation was analyzed as a time-dependent risk factor for subsequent aGVHD (n = 14), (2) aGVHD was analyzed as a time-dependent risk factor for subsequent HHV-6B reactivation (n = 1), and (3) analysis without temporal specification (n = 18). A statistically significant association (P < .05) between HHV-6B reactivation and aGVHD was observed in 10 of 14 studies (71%) in group 1, 0 of 1 study (0%) in Group 2, and 8 of 18 studies (44.4%) in Group 3. Of the 14 studies that analyzed HHV-6B as a risk factor for subsequent aGVHD, 11 performed a multivariate analysis and reported a hazard ratio, which reached statistical significance in 9 of these s tudies. Meta-analysis of these 11 studies demonstrated a statistically significant association between HHV-6B and subsequent grades II to IV aGVHD (hazard ratio, 2.65; 95% confidence interval, 1.89 to 3.72; P < .001).HHV-6B reactivation is associated with aGVHD, and when studies have a temporal component to their design, HHV-6B reactivation is associated with subsequent aGVHD. Further research is needed to investigate whether antiviral prophylaxis reduces incidence or severity of aGVHD.
Keywords: Humanherpesvirus-6, Graft-versus-hostdisease, Riskfactor
INTRODUCTION
Graft-versus-host disease (GVHD) is an important cause of morbidity and mortality after allogeneic hematopoietic cell transplantation (HCT) [1]. Approximately 40% of allogeneic HCT recipients from HLA-identical sibling donors will develop acute GVHD (aGVHD) despite immunosuppressive prophylaxis; the incidence is even higher for recipients of allogeneic HCT from unrelated donors (~60%) [2]. aGVHD presents within the first 100 days post-transplantation, typically around the time of engraftment, but late-onset aGVHD can occur beyond 100 days, often when immunosuppression is reduced [1,3]. Over the last few decades there has been a decrease in incidence and severity of aGVHD because of more effective prevention strategies [4,5]. However, GVHD remains a significant burden on HCT recipients through end-organ damage and immunosuppressive treatment regimens, which also increase risk of opportunistic infections [6]. aGVHD is believed to occur after damage to host tissues from pretransplant chemotherapy and irradiation. A complex cytokine storm follows and leads to activation of donor lymphocytes, mainly T cells, which then cause direct cytotoxicity of targeted organs, primarily the skin, gastrointestinal tract, and liver [7,8]. Recent studies have suggested that changes in the gut microbiota can also contribute to the pathogenesis and severity of GVHD [9].
Human herpesvirus 6B (HHV-6B) is a ubiquitous beta-herpesvirus that infects over 90% of people within the first 2 years of life [10]. Typically, HHV-6B reactivation occurs in approximately 40% of allogeneic HCT recipients (range, 13.9%−93.6%; Table 1). However, the reactivation rate of HHV-6 can vary greatly depending on the level of immunosuppression, the sensitivity of the diagnostic assay, and the stem cell source (the reactivation rate may surpass 90% in T cell–depleted umbilical cord blood transplant [CBT] recipients [14]). HHV-6B is the most frequent cause of encephalitis in HCT recipients [15]. Other signs and syndromes associated with HHV-6 reactivation include fever, rash, diarrhea, thrombocytopenia, and pneumonitis [16]. Several studies have suggested that HHV-6B plays a role in aGVHD after HCT [11,12,17–30]. Our objective was to systematically summarize and analyze evidence regarding HHV-6B reactivation and development of aGVHD in HCT recipients.
Table 1.
Characteristics of Enrolled Studies and Meta-Analyzed Studies
| Characteristics | Group 1: HHV-6 Reactivation Analyzed as a Risk Factor for aGVHD | Group 2: aGVHD Analyzed as a Risk Factor for HHV-6 Reactivation | Group 3: No Temporal Specification for HHV-6 vs. aGVHD Analysis | |||
|---|---|---|---|---|---|---|
|
|
||||||
| Whole* | Meta-analysis* | Whole* | Meta-analysis*,† | Whole* | Meta-analysis* | |
| Total studies | 14 | 11 | 1 | N/A | 18 | 12 |
| Median patients in cohort (range) | 103 (49–315) | 108 (68–315) | 44 | N/A | 48.5(15–366) | 64.5 (23–366) |
| Prospective cohort | 10(71.4) | 7 (63.6) | 1 (100) | N/A | 12(66.7) | 9 (75) |
| Retrospective cohort | 4 (28.6) | 4 (36.4) | 0 | N/A | 4(22.2) | 2(16.7) |
| Case control | 0 | 0 | 0 | N/A | 2(11.1) | 1 (8.3) |
| Studies that administered HHV-6 antiviral prophylaxis (ganciclovir, foscarnet, or cidofovir) | 3*(21.4) | 3‡ (27.3) | 1§(100) | N/A | 0 | 0 |
| Studies that found a significant association between HHV-6 and aGVHD (P < .05) | 10(71.4) | 8 (72.7) | 0 | N/A | 8 (44.4) | 6 (50) |
| Median year of publication (range) | 2013(1997–2017) | 2014(2005–2017) | 2015 | N/A | 2006(1995–2016) | 2008(1999–2016) |
| Total patients | 1773 | 1605 | 44 | N/A | 1553 | 1303 |
| Patients who reactivated HHV-6 | 784 (44.2) | 702 (43.7) | 29 (65.9) | N/A | 668(43.1) | 555 (42.6) |
| Median reactivation, % (range) | 47.7 (16.7–93.6) | 47(16.7–93.6) | 65.9 | N/A | 56(13.9–87) | 58(13.9–87) |
| Total CBT patients | 521 (29.4) | 491 (30.6) | 44(100) | N/A | 307(19.8) | 306 (23.5) |
| Median CBT, % (range) | 21 (0–100) | 20.1 (.9–100) | 100 | N/A | 1.1 (0–100) | 23.4(0–100) |
| Type of PCR used to diagnose HHV-6 reactivation | ||||||
| qPCR | 13 (92.9) | 11 (100) | 1 (100) | N/A | 11 (61.1) | 8 (66.7) |
| Qualitative PCR | 0 | 0 | 0 | N/A | 2(11.1) | 2 (16.7) |
| Nested PCR | 1 (7.1) | 0 | 0 | N/A | 5 (27.8) | 2 (16.7) |
| Sample used for PCR | ||||||
| Plasma | 12(85.7) | 10(90.9) | 1 (100) | N/A | 7 (38.9) | 5(41.7) |
| Whole blood | 0 | 0 | 0 | N/A | 5 (27.8) | 4(33.3) |
| PBMCs/PBLs | 1 (7.1) | 1 (9.1) | 0 | N/A | 5 (27.8) | 2 (16.7) |
| Plasma + PBMC/PBLs | 1 (7.1) | 0 | 0 | N/A | 1 (5.6) | 1 (8.3) |
Values are n (%) unless otherwise defined. N/A indicates not applicable as meta-analysis was not performed for this group; PBLS, peripheral blood leukocytes.
“Whole” and “meta-analysis” indicate all enrolled studies and studies providing sufficient data to perform meta-analysis, respectively.
Meta-analysis for group 2 was not performed because of an insufficient number of studies.
Two studies [11,12] administered HHV-6 prophylaxis before HCT, and 1 study [13] administered HHV-6 prophylaxis until day+100 after HCT.
Ganciclovir was administered during conditioning followed by valacyclovir for first 100 days after HCT.
METHODS
Literature Search Strategy
A systematic inquiry of publications indexed in the PubMed database was performed using the search phrase “(HHV-6 OR HHV6 OR herpesvirus-6 OR HHV-6A OR HHV6A OR herpesvirus-6A OR HHV-6B OR HHV6B OR herpesvirus-6B) AND (graft versus host OR GVHD OR cord blood OR bone marrow OR stem cell)” published from November 1987 to April 2017, which yielded 653 results. Next, a systematic inquiry of publications indexed in the EMBASE database was performed using the combination of the 3 search phrases:
“human herpesvirus 6”/exp OR “human herpesvirus 6” OR “human herpesvirus 6a”/exp OR “human herpesvirus 6a” OR “human herpesvirus 6b”/exp OR “human herpesvirus 6b” OR “hhv6” (n = 5081)
“acute graft versus host disease”/exp OR “acute graft versus host disease” OR “acute graft versus host reaction”/exp OR “acute graft versus host reaction” OR “agvhd”/exp OR “agvhd” OR “graft versus host” OR “gvhd”/exp OR “gvhd” OR “graft versus host reaction”/exp OR “graft versus host reaction” (n = 61,767)
“hematopoietic stem cell transplantation”/exp OR “hematopoietic stem cell transplantation” OR “hematopoietic stem cell therapy”/exp OR “hematopoietic stem cell therapy” OR “umbilical cord blood”/exp OR “umbilical cord blood” OR “cord blood”/exp OR “cord blood” OR “cord blood stem cell transplantation”/exp OR “cord blood stem cell transplantation” OR “bone marrow”/exp OR “bone marrow” OR “bone marrow transplantation”/exp OR “bone marrow transplantation” OR “bone marrow transfusion”/exp OR “bone marrow transfusion” (n = 480,335)
This search algorithm produced 366 results.
A total of 1019 results from both inquiries were identified. Of these, 154 were duplicates, leaving 865 unique results. No efforts were made to identify unpublished work.
Study Selection
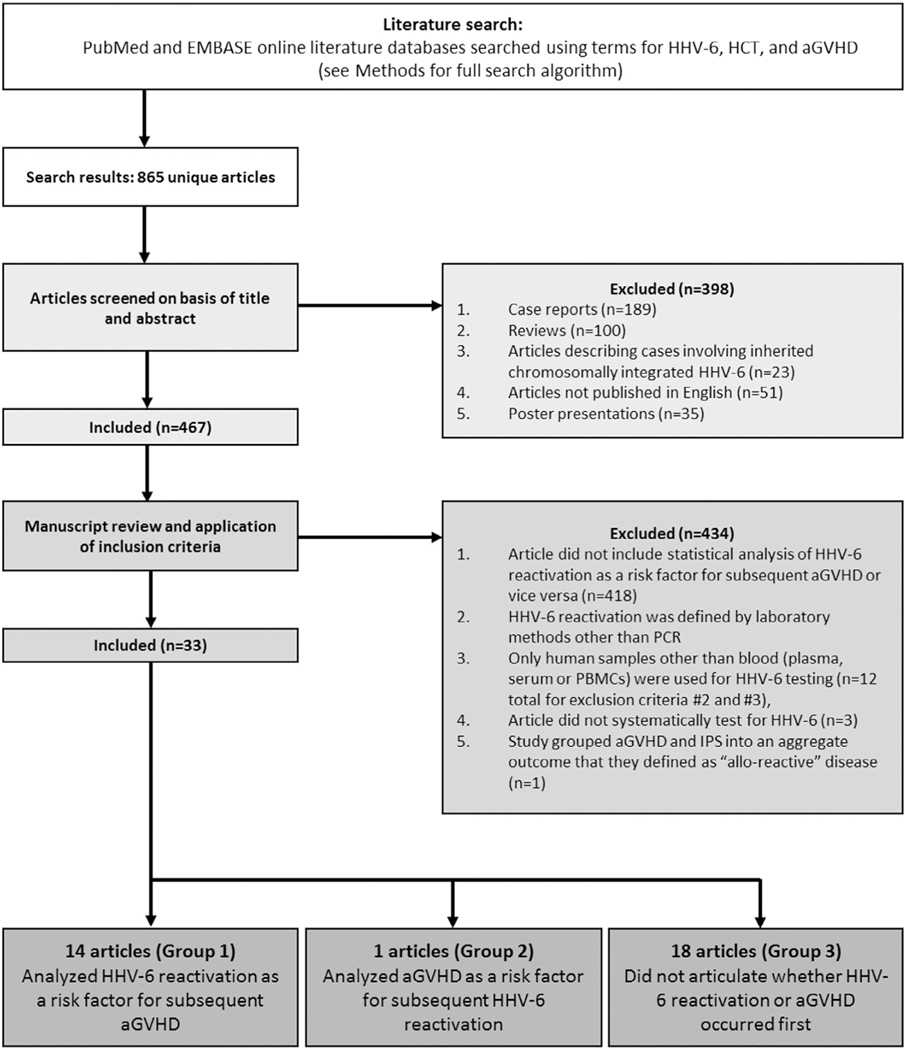
Figure 1 shows a flowchart of study selection. Case reports (n = 189), reviews (n = 100), articles describing cases involving inherited chromosomally integrated HHV-6 (ciHHV-6; n = 23), articles not published in English (n = 51), and poster presentations (n = 35) were excluded, resulting in 467 remaining articles.
Figure 1.
Flow chart.
Next, these articles were reviewed for the following requirements: (1) description of a statistical analysis assessing the relationship between HHV-6B reactivation and aGVHD (418 articles excluded for requirement 1), (2) HHV-6B reactivation was defined by HHV-6B DNA detection by PCR, (3) blood (plasma, serum, or peripheral blood mononuclear cells [PBMCs]) specimens were used for HHV-6 testing (12 articles excluded for requirements 2 and 3), and (4) patients were systematically tested for HHV-6 regardless of clinical symptoms and signs of disease (3 articles excluded for requirement 4). One article was deemed ineligible for inclusion because the authors grouped aGVHD and idiopathic pulmonary syndrome into an aggregate outcome they defined as “allo-reactive” disease.
Data were abstracted from publications that met the inclusion criteria (n = 33) by 1 investigator (T.L.P.) and confirmed by another (K.C.). An effort was made to contact investigators to confirm data. Corresponding authors were given 8 weeks to respond to inquiries.
Publications were assigned to 1 of 3 groups based on the temporal nature of the HHV-6B and aGVHD analysis by 2 investigators (T.L.P> and D.M.Z.): (1) HHV-6B reactivation was analyzed as a time-dependent variable with subsequent aGVHD as the outcome, (2) aGVHD was analyzed as a time-dependent variable with subsequent HHV-6B reactivation as the outcome, and (3) analysis was performed without temporal specification. If the corresponding authors did not respond to inquiries regarding whether they treated aGVHD and HHV-6B reactivation as time-dependent variables within the 8 weeks, their study was placed in group 3.
Data Extraction
Publications that met the inclusion criteria were subjected to data extraction by 1 investigator (T.L.P.). Study design, subject demographic and clinical information, and results were collected (Tables 1 to 3). HHV-6B and aGVHD statistical results were confirmed by a second investigator (K.C.).
Table 3.
Studies in Which Directionality of Analysis Was Not Articulated (Group 3)
| Reference (Study Design) | Graft Source | No. of Patients | Testing Method | Reactivation Percent (Frequency) | Médian Day of HHV-6 Réactivation (Range) | Médian Day of aGVHD Onset (Range) | HHV-6 Active Antivirals: Indication for Treatment | HR[95% Cl]; P(aGVHD Grade) |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Definition of Active Infection | ||||||||
| Quintela 2016 [33]* (case control) | BM: 42% CB:16% PBSC: 42% | 366 | qPCRWB >2 consecutive positive qPCR results (>3 logio copies/mL) | 20.5 (75/366) 13.9 (51/366)* | N/A | N/A | Foscarnet, ganciclovir, or cidofovir: CMV and/or HHV-6 infection (46/366 patients were treated) | P=.52 (all grades) |
| Inazawa2015 [26](prospective cohort)† | BM: 57% CB:37% PBSC: 6% | 105 | Qualitative PCRWB >50 copies | 60(63/105) | N/A | N/A | N/A | UP = .046† (all grades) UP = .005† (stage of skin GVHD) |
| Mutlu 2014 [28],¶ (prospective cohort) | BM:100% | 15 | qPCRWB >250 copies/mL | 53 (8/15) | Day 14 (6–23) | N/A | N/A | UP = .001 (grade 4)¶ |
| Ogata 2013 [34] (prospective cohort) | BM/PBSC: 29% BM: 44% CB:27% | 230 | qPCRp >50 copies/mL | 72.2 (166/230) | N/A | N/A | Ganciclovir treatment: CMV reactivation Foscarnet, Ganciclovir, or valganciclovir: HHV-6 reactivation at the discretion of clinicians | UP = .0003 (any reactivation) (grades II-IV) UP = .02 (> 10,000 copies/mL) (grades II-IV) |
| Jeulin 2013 [29] (retrospective cohort) | BM: 47% CB:20% PBSC: 33% BM+PBSC: .5% | 220 | Qualitative PCR & qPCRWB >2500 copies/mL | 20(44/220) | N/A | N/A | Foscarnet, ganciclovir, or cidofovir treatment: CMV or HHV-6 (persistent with clinical signs) reactivation | UP = .0028 (all grades) |
| Kullberg-Lindh 2011 [35];¶ (retrospective cohort) | BM: 66% CB: 2% PBSC: 32% | 47 | qPCRs >200–400 gEq/mL | 27.7(13/47) | N/A | N/A | Foscarnet or ganciclovir treatment: CMV reactivation | UP = .16 (all grades) MP = .38 (all grades )¶ |
| Betts 2011 [36] ‡ (prospective cohort) | BM: 34% CB:66% | 82 | qPCRWB >500 copies/mL (high level viremia >25,000 copies/mL) | 56.1 (46/82) | Day 23 (range 10–168) | N/A | Foscarnet, ganciclovir, valganciclovir or cidofovir treatment: CMV reactivation, and/or HHV-6 reactivation with clinical complications | UP = .12 (grades II-IV) UP = .55* (grades II-IV) |
| Chevallier 2010 [37] (retrospective cohort) | BM: 73% CB:27% | 55 | qPCRp >1 PCR >31og/mL or >2 consecutive PCRs between 2–31og/mL | 52.7 (29/55) | CB: Day 36 (16–74) BM: Day 58 (24–100) | N/A | Foscarnet or ganciclovir treatment: CMV reactivation or disease | P=NS (grades II-IV) |
| Yamane 2007 [38] (prospective cohort) | BM/PBSC: 24% BM: 48% CB:28% | 46 | qPCRp >200 copies/mL | 47.8 (22/46) | Week 3 (week 2-week 5) | N/A | Foscarnet or ganciclovir treatment: CMV reactivation | UP = .269 (grades II-IV) |
| Ogata 2006 [39] (prospective cohort) | BM: 84% PBSC: 16% | 50 | qPCRp >50 copies/mL | 48(24/50) | Day 18 (0–48) | N/A | Ganciclovir treatment: CMV reactivation or HHV-6 disease | UP = .08 (grades II-IV) |
| Tomonari 2005 [40] (retrospective cohort) | CB: 100% | 23 | qPCRs Any HHV-6 DNA detection | 87(20/23) | N/A | Day 32 (range 10–42) | Ganciclovir treatment: CMV reactivation | UP< .05 (grades II-IV) |
| Radonic 2005 [41],¶ (prospective cohort) | BM: 23% PBSC: 77% | 82 | qPCRp >200 copies/mL | 42.7 (35/82) | Day 41 (5–212) | N/A | N/A | UP = .047 (grades II-IV) mHR2.3 [1.1–4.7]; P =.023 (grades II-IV)1 |
| Ljungman 2000 [42], §(prospective cohort) | BM: 80% PBSC: 20% | 74 | Nested PCRPBL Any HHV-6 DNA detection | 78.4(58/74) | N/A | N/A | Foscarnet or ganciclovir treatment: CMV reactivation | UP =NS (Grades II-IV) |
| Imbert-Marcille 2000 [43] (prospective cohort) | BM: 68% PBSC: 32% | 28 | Qualitative pcrpbmc+p Any HHV-6 DNA detection | 42 (12/28) | Day 17 (6–117) | N/A | Ganciclovir treatment: CMV reactivation | UP =.04 (grades III-IV) |
| Wang 1999 [44] (prospective cohort) | BM: 33% PBSC: 67% | 24 | Nested PCRPBL any HHV-6 DNA detection in >3 consecutive samples | 83 (20/24) | N/A | N/A | N/A | UP =.009 (grades II-IV) |
| Maeda 1999 [45],¶ (prospective cohort) | BM: 58% PBSC: 42% | 38 | Nested PCRPBL Any HHV-6 DNA detection | 56 (9/16; peak week 3) | N/A | N/A | N/A | UP = .051 (grades II-IV)¶ |
| Wang 1996 [46],‡ (prospective cohort) | BM: 100% | 37 | Nested PCRPBL Any HHV-6 DNA detection | 70(26/37) | N/A | N/A | Foscarnet or ganciclovir treatment: CMV reactivation | P =NS (grades II-IV) |
| Appleton 1995 [47]∥,¶(prospective case control) | BM: 100% | 31 | Nested pcrpbl+sb Any HHV-6 DNA detection in PBL or skin biopsy | 71 (22/31) | N/A | N/A | N/A | PBL: P =.85(allgrades) Biopsy: UOdds ratio 22.00 [2.3213]; P=.008† (severe GVHD)¶ |
HHV-6 infection (20.5%) was defined as HHV-6 DNAemia ≥ 3 log 10 copies/mL whole blood, whereas active infection (13.9%) was ≥ 2 consecutive HHV-6 DNAemia > 3 log10 copies/mL whole blood.
Univariate analysis of onset of aGVHD vs. HHV-6 reactivation(P = .016). Univariate analysis of stage of aGVHD vs. HHV-6 reactivation (P = .005).
Univariate analyses: [incidence of aGVHD] in no viremia vs. HHV-6 viremia (P = .12), [incidence of aGVHD] in low level viremia (<25,000 copies/mL WB) vs. High level viremia (≥25,000 copies/mL WB)(P = .55).
All patients from Wang et al. 1996 were included in Ljungman et al. 2000, so only Ljungman et al. 2000 was used for the meta-analysis.
Odds ratio was determined using HHV-6 DNA detection in after BM transplant biopsy as a risk factor for severe aGVHD.
No odds ratio or 2 × 2 table provided (ie, study was excluded from meta-analysis).
Meta-Analysis
Study result consistency was assessed using a test of heterogeneity, which refers to the total variation in outcomes between studies. Publication bias was evaluated using a funnel plot. Day +100 and estimates for GVHD grades II to IV were chosen for analysis if a study reported multiple time points or grades, respectively. Random-effects models, which take into account the differences among participants within studies and allow for differing effect sizes across studies, were used for meta-analysis. Hazard ratios (HRs; Table 2) or calculated odds ratios (Table 3) were transformed by taking their natural logarithms (ln). Standard errors were calculated from the ln (HR) or ln (odds ratio) estimates. To view the effects of patient age (pediatric versus adult), HHV-6B prophylaxis, CBT (cut-off,>20%), and the effect of different definitions of HHV-6B reactivation (high level of >1000 copies/mL versus any or lower level), we performed stratified analyses. A forest plot, sorted by publication date, was used to visualize all study results. Statistical analyses were completed in STATA version 14 (StataCorp LP, College Station, TX).
Table 2.
HHV-6 Reactivation as a Risk Factor for Subsequent aGVHD (Group 1)
| Reference (Study Design) | Graft Source | No. of Patietns | Testing Method | Reactivation Percent (Frequency) | Median Day (Post-HCT) of HHV-6 Reactivation (Range) | Median Day of aGVHD Onset (Range) | HHV-6 Active Antivirals: Indication for Treatment | HR [95% Cl]; P (aGVHD grade) |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Definition of Active Infection | ||||||||
| Admiraal 2017 [17],∥ (retrospective cohort) | BM: 45% CB: 52% PBSC: 3% | 273 | qPCRp > 1000 copies/mL | 27 (74/273) | N/A | N/A | Foscarnet treatment: HHV-6 disease, eg, encephalitis or bone marrow suppression Ganciclovir or foscarnet: CMV reactivation Cidofovir: adenovirus reactivation | “HR3.47 [2.11–5.7]; P<.0001 (grades II-IV) “HR2.74 [1.22–6.15]; P=.018 (grades III-IV) |
| Cirrone 2016 [11] (prospective cohort) | CB: 100% | 92 | qPCRp >200 copiesjmL | 65.2 (60/92) | Day 26 (IQR, 19–33) | Day 42 (N/A) | Ganciclovir prophylaxis (administered pretransplant): CMV-seropositive donor andfor recipient | “HR3.00 [1.4–6.4]; P=.004 (grade N/A) |
| Aoki 2015 [18] (retrospective cohort) | BM: 73% CB: 27% | 236 | qPCRp > 125 copiesjmL | 58.5(138/236) | N/A | N/A | N/A | “HR 1.87 [1.13–3.09]; P=.01 (grades II-IV) |
| Verhoeven 2015 [19] (prospective cohort) | BM: 71% CB: 7% PBSC: 22% | 106 | qPCRp+s >25 copiesjmL | 48(51/106) | Day 20 (1–44) | N/A | Foscarnet, Ganciclovir, or cidofovir: Indication not specified, but reported that 6/51 patients received these antivirals during HHV-6 reactivation | “HR 14.2 [2.1–94.4]; P=.006 (grades II-IV) |
| Violago 2015 [13],* (prospective cohort) | BM: 49% CB: 21% PBSC: 30% | 100 | qPCRp > 1000 copiesjmL | 19% (19/100) | Day 42 (N/A) | N/A | Foscarnet or ganciclovir prophylaxis (administered post-transplant until day +100): patients at risk for CMV reactivation (received by 67% of cohort) Foscarnet, ganciclovir, or cidofovir treatment: CMV or adenovirus reactivation; HHV-6 reactivation at the discretion of clinicians | UP=.028* (grades II-IV) UHR2.323 [.897–6.015]; P=.083 (grades II-IV) “HR 1.951 [.737– 5.1262]; P= .178 (grades II-IV) |
| Gotoh 2014 [27],† (prospective cohort) | BM: 43% CB: 31% PBSC: 26% | 49 | qPCRp >20 copiesjmL | 53.1 (26/49) | All patients were tested for HHV-6 on day 30 | Day 31 (13–81) | Foscarnet or ganciclovir treatment: CMV reactivation | “HR 9.957 [2.68–37.06]; P<.001f (grades II-IV) |
| Olson 2014 [14] (retrospective cohort) | CB: 100% | 125 | qPCRp >100 copies/mL (high reactivation >10,000 copies/mL) | 94(117/125) | Day 20 (10–59) | N/A | Foscarnet treatment: CMV reactivation; HHV-6 reactivation at the discretion of clinicians (311125 treated); HHV-6 disease | “HR 1.41 [.86–2.31]; P=.17 (grades II-IV)# |
| de Pagter 2013 [20],§ (retrospective cohort) | BM/PBSC: 95% CB: 5% | 108 | qPCRp > 1,000 copiesjmL | 17(18/108) | Day 18 (0–35) | N/A | Foscarnet or valganciclovir treatment: CMV reactivation | UHR 7.4 [3.84–14.3]; P<.001 (grades II-IV) “HR6.07 [2.9–12.7]; P<.001 (grades II-IV) |
| Zerr2012 [22] (prospective cohort) | BM: 19% CB: 7% PBSC: 74% | 315 | qPCRp >25 copies/mL (high reactivation >1,000 copies/mL) | 35(111/315) | Day 20 (IQR, 15–28) | Day 27 (IQR, 19–41) | Foscarnet or ganciclovir CMV reactivation | “aHR2.39 [1.60–3.56]; P<.001 (grades II-IV)# |
| Tormo 2010[31],‡ (prospective cohort) | BM: 3% CB: 19% PBSC: 78% | 68 | qPCRp > 10 copiesjmL | 39.7 (27/68) | Day 20 (7–44) | N/A | Foscarnet, ganciclovir, or valganciclovir treatment: CMV reactivation | HR.9[.3–0.9];P = .6* (grades II-IV) |
| Wang 2008 [12] (prospective cohort) | BM: 8% | 72 | qpCRPBMC | 48.6 (35/72) | Day 21 (7–84) | Day 26 (9–73) | Ganciclovir prophylaxis (administered pretransplant): indication not specified | “HR8.9 [2.9–31.0]; P=.0006 (grades II-IV by day +30) “HR 6.1 [2.1–17.8]; P= .001 (grades II-IV by day +50) (.continued on next page) |
| BM+PBSC/BM +PBSC+CB: 81% | >10 copies/10 [6] cells | Foscarnet or ganciclovir treatment: CMV reactivation | MHR 4.8 [1.7–13.6]; P =.0028 (grades II-IV by day +100) | |||||
| de Pagter 2008 [24]∥¶ (prospective cohort) | BM: 64% CB: 26% PBSC: 10% | 58 | qPCRP >250 copies/mL (high reactivation >1000 copies/mL) | 67(39/58) | Day 16(0–120) | N/A | Foscarnet or ganciclovir treatment: CMV reactivation Foscarnet treatment: HHV-6 disease (3 of 58 treated) | UOR5.3 [1.1–28.0]; P =.049 (grades II-IV)# mOR 7.8 [1.2–50.1]; P =.032 (grades II-IV)# |
| Zerr 2005 [25] (prospective cohort) | BM: 81% CB: 1% PBSC: 18% | 110 | qPCRP >25 copies/mL | 47(52/110) | Day 23 (IQR, 19–28) | N/A | Foscarnet or ganciclovir treatment: CMV reactivation | UHR5.6 [1.6–19]; P = .01 (grades III-IV) MaHR4.9 [1.5–16]; P =.02 (grades III-IV) |
| Chan 1997 [32],¶(prospective cohort) | BM:100% | 61 | Nested PCRpbl+ p AnyHHV-6DNA detection | 28(17/61) | N/A | N/A | Ganciclovir prophylaxis (administered until day +120 post-transplant or engraftment): matched unrelated transplant recipients (7/61 patients) Ganciclovir treatment: CMV reactivation | UP=.61 (grade N/A)¶ |
U indicates univariate analysis; M, multivariate analysis; P, plasma; S, serum; PBL, peripheral blood leukocyte; BM, bone marrow; CB, cord blood; PBSC, peripheral blood stem cells.
Incidence of aGVHD in HHV-6–postitve patients vs. HHV-6–negative (P = .028).
HHV-6 testing was performed only on day +30 post-HCT. This study did provide an HR but tested for HHV-6B only on day +30 post-HCT. We judged this to be systematic HHV-6 testing so we included it in our systematic review. However, this method is not homogenous with the rest of the studies included in our meta-analysis (weekly or biweekly HHV-6 testing). Thus, we did not include Gotoh et al. [27] in our meta-analysis.
Cryopreserved specimens were used for analysis and availability of sufficient volume of plasma for PCR was a limiting factor that precluded analysis of a larger number of specimens (median 6 samples per patient [range, 3 to 11] by day +75).
There is some overlap between de Pagter 2013 (patients from UMC Utrecht and Erasmus MC from January 2004 to May 2008: median age at transplant 40 years [range 17–65]) and Admiraal 2017 (patients from UMC Utretcht from January 2004 to September 2014: median age at transplant 8.4 years [range, .1–22.7]). Based on this information, the overlap was judged to be small. Thus, both studies were included in the meta-analysis.
All patients from de Pagter et al. 2008 were included in Admiraal et al. 2017, so only Admiraal et al. 2017 was used for the meta-analysis.
Study was excluded from meta-analysis (ie, no HR was reported).
High reactivation(>1000 copies/mL for Zerr 2012, de Pagter 2008 or >10,000 copies/mL in Olson 2014) was used as the predictor for aGVHD.
Study Quality Assessment
This systematic review and meta-analysis was performed according to PRISMA guidelines [48].
RESULTS
In total, 33 publications met eligibility criteria for inclusion. Fourteen studies analyzed HHV-6B reactivation as a time-dependent variable with subsequent aGVHD as the outcome (group 1), 1 study analyzed aGVHD as a time-dependent variable with subsequent HHV-6B reactivation as the outcome (group 2), and 18 publications performed an analysis of HHV-6B and aGVHD without temporal specification (group 3). Table 1 summarizes the characteristics of the 33 studies that met our inclusion criteria.
Studies Examining HHV-6B Reactivation as a Risk Factor for Subsequent aGVHD
Of the 14 studies that analyzed HHV-6B as a predictor for subsequent aGVHD, 10 studies reported a statistically significant (P < .05) association (Table 2). Ten of 14 studies were prospective cohort studies (71.4%), whereas the remaining 4 were retrospective cohort studies (28.6%). Bone marrow and peripheral blood stem cell transplants were included in most studies, whereas CBTs became more prevalent within this past decade. The size of the cohorts varied (median, 103; range 49 to 315); the 3 largest studies (n > 200) all reported a statistically significant association between HHV-6B reactivation and aGVHD. Twelve of 14 studies performed a multivariate analysis, 10 of which reported a statistically significant association.
Plasma was the most common sample used for HHV-6B testing (12/14, 85.7%), and quantitative PCR (qPCR) was the most common method of diagnosing HHV-6B reactivation (13/14, 92.9%). Overall, a total of 1773 patients were examined for HHV-6B in these 14 studies, and 784 of these patients (44.2%) reactivated HHV-6 (range, 17% to 94%). Studies that used a threshold for reactivation set at >1000 HHV-6 DNA copies/mL reported an HHV-6B reactivation rate of 23% (range, 17% to 27%) compared with 52% (range, 28% to 94%) when any level of detection or a threshold <1000 copies/mL was accepted as reactivation.
Violago et al. [13] administered HHV-6B active antiviral prophylaxis (valganciclovir/ganciclovir or foscarnet) after HCT to patients at risk for cytomegalovirus (CMV) infection (n = 67, 67% of cohort); no other study administered HHV-6B active antiviral prophylaxis after HCT. Of the 14 studies, only Zerr et al. [22] systematically examined their cohort for ciHHV-6. Nine of 14 (64%) studies [12,13,17,19,20,24,27,31,32] examined other pathogens besides HHV-6B (including CMV, Epstein Barr virus [EBV], varicella zoster virus, BK virus, herpes simplex virus, adenovirus, and HHV-7) as risk factors for aGVHD, and only Admiraal et al. [17] reported a statistically significant association between a non-HHV-6 pathogen, EBV, and aGVHD. Although 4 other studies [13,19,24,27] examined EBV as a risk factor for aGVHD, none observed a statistically significant association.
Studies Examining aGVHD as a Risk Factor for Subsequent HHV-6B Reactivation
Only 1 study examined aGVHD as a risk factor for subsequent HHV-6B reactivation. Hill et al. [49] prospectively studied a cohort of 44 CBT recipients and did not find a statistically significant association in multivariate analysis (HR, 2.41; 95% CI, .94 to 6.19; P = .07). Hill et al. [49] used plasma samples for qPCR, defined HHV-6B reactivation as DNAemia >25 copies/mL and reported that high-dose valacyclovir mitigated HHV-6B reactivation (adjusted HR, .39; 95% CI, .14 to 1.08). Patients with suspected ciHHV-6 were excluded from their analysis. Evaluation of aGVHD as a risk factor for reactivation of other viruses was not performed in this study.
Studies Examining HHV-6B Reactivation and aGVHD without Temporal Specification
Of the 18 studies that examined HHV-6B reactivation and aGVHD without temporal specification (group 3), 8 (44%) reported a statistically significant association (P < .05), and 1 study [45] found a marginal association (P = .051). Twelve of 18 studies were prospective cohort studies, 4 were retrospective cohort studies, and 2 were case control studies. Nine studies included CBT recipients, and 2 of these [36,40] had a majority of CBT recipients in their cohort. The overall size of the cohorts varied (median, 48 to 49 patients; range, 15 to 366), 4 of which included more than 100 patients. Only 2 of 18 studies performed a multivariate analysis for HHV-6B reactivation versus aGVHD [35,40], with a statistically significant association reported in 1 of the 2 (HR, 2.3; 95% CI, 1.1 to 4.7; P = .023) [40].
Overall, a total of 1553 patients were examined for HHV-6B reactivation in these 18 studies, of which 692 (44.6%) reactivated HHV-6B (median, 54.7%; range, 20% to 87%). The samples used for HHV-6B testing and the type of PCR testing used to identify HHV-6B reactivation varied greatly (Tables 1 and 3). Of the 1216 patients examined for HHV-6B reactivation using qPCR (11 studies), 458 (37.7%) reactivated HHV-6B (median, 53%; range, 13.9% to 87%). Of the 133 patients who had samples available for qualitative PCR testing in 2 studies, HHV-6B was detected in 75 patients (56.4%) (median, 51.4%; range, 42.9% to 60%). Of the 204 patients who had samples available for nested PCR testing in 5 studies, 135 patients (66.2%) reactivated HHV-6B (median, 78.4%; range, 56% to 85%).
HHV-6B–active antiviral prophylaxis (ganciclovir, foscarnet, or cidofovir) was not administered in any of the studies after HCT. Three studies [29,33,37] examined patients in their respective cohorts for ciHHV-6. Five of 18 studies examined pathogens other than HHV-6B versus aGVHD, and Kullberg-Lindh et al. [35] found that aGVHD was a prognostic factor for adenovirus infection, although this association was not significant in multivariate analysis.
Meta-Analysis
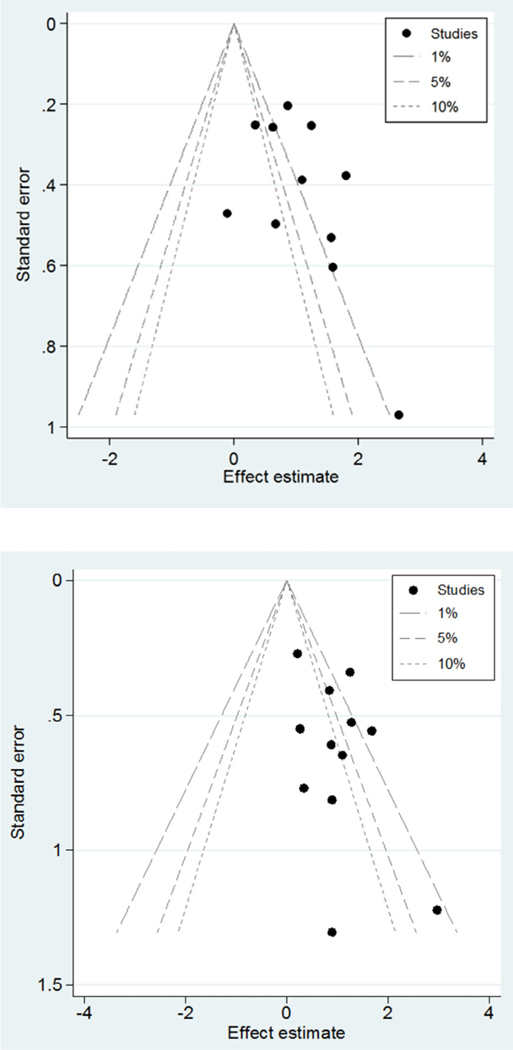
Study result consistency was assessed using a test of heterogeneity for our meta-analyses of both groups 1 and 3 (group 1, I2 = 59.7%, P = .006; group 3: I2 = 23.8%, P = .210), indicating low to moderate heterogeneity between the risk of aGVHD associated with HHV-6B reactivation (Figures 2 and 3). Additionally, review of funnel plots suggested some publication bias present in group 1.
Figure 2.
Funnel plots for group 1 (top) and group 3 (bottom).
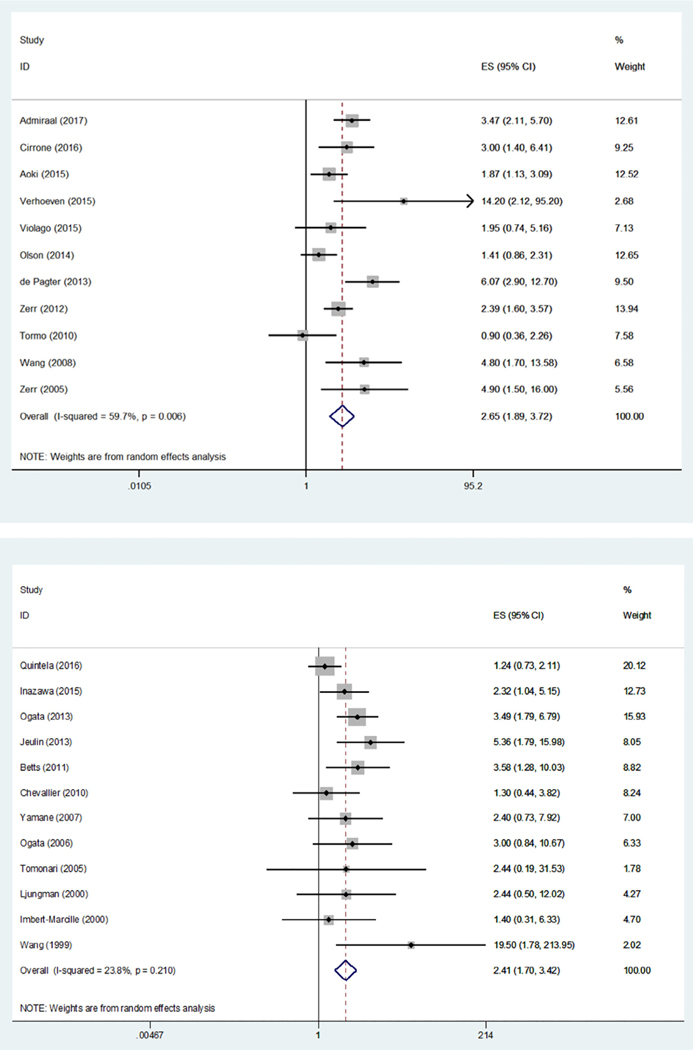
Figure 3.
Forest plots for group 1 (left) and group 3 (right).
Eleven of 14 studies in group 1 reported an HR from Cox proportional hazards regression, which reached statistical significance in 9 studies (Table 2). Meta-analysis of these 11 studies (Figure 3) demonstrated a significant association between HHV-6B and subsequent grades II to IV aGVHD (HR, 2.65; 95% CI, 1.89 to 3.72; P < .001). Every group 1 study included in our meta-analysis provided an HR for grades II to IV aGVHD except Cirrone et al. [11]; thus, we performed a sensitivity analysis excluding Cirrone et al. and found that this did not affect our results (HR, 2.64; 95% CI, 1.82 to 3.82; P < .001; data not shown). In addition, analyses stratified by patient age, HHV-6B prophylaxis, GVHD prophylaxis, CBT (cut-off, >70%), and definition of HHV-6B reactivation did not affect the overall results (data not shown).
Twelve of 18 studies in group 3 provided sufficient data to perform a meta-analysis. Meta-analysis of these 12 studies also demonstrated a significant association between HHV-6B and grades II to IV aGVHD (odds ratio, 2.41; 95% CI, 1.70 to 3.43; P < .001). An analysis stratified by CBT (cut-off, >70%) was performed and did not affect the results (data not shown). Other stratified analyses were not performed because of a lack of variability or lack of a clear cut-off in the targeted variables across the included studies.
DISCUSSION
We performed a systematic review of studies evaluating an association between HHV-6B reactivation and aGVHD. Meta-analysis of the studies that specifically analyzed HHV-6B as a risk factor for subsequent aGVHD demonstrated a significant association (HR, 2.65; 95% CI, 1.89 to 3.72; P < .001). Similarly, meta-analysis of the studies that did not perform time-dependent analyses of HHV-6B and aGVHD also demonstrated a significant association (odds ratio, 2.41; 95% CI, 1.70 to 3.43; P < .001).
Because the clinical implications of ciHHV-6 are still unclear, we excluded articles focusing on ciHHV-6. However, it is worth noting that in a retrospective analysis of archived pre-HCT PBMC samples from 4319 HCT donor–recipient pairs who received an allogeneic HCT from 1992 to 2013, investigators reported that grades II to IV aGVHD was more frequent when recipients or donors had ciHHV-6 [50]. Further research is needed to fully understand the clinical impact of ciHHV-6.
Other herpesviruses have been assessed as potential triggers of aGVHD. CMV and EBV, in particular, have been extensively analyzed as risk factors for GVHD; however, few studies have demonstrated a statistically significant association, and, overall, results have conflicted [12,51,52]. In a cohort study of 4394 allogeneic HCT patients (excluding CBT) spanning from 1995 to 2013, Green et al. [53] reported that CMV reactivation was not associated with subsequent development of grades II to IV aGVHD (adjusted HR, 1.09; 95% CI, .97 to 1.22) or grades III to IV aGVHD (adjusted HR, .91; 95% CI, .68 to 1.21). Furthermore, a study of 11,364 allogeneic HCT patients reported that the 100-day cumulative incidence of grades II to IV aGVHD was increased by only 1.07-fold if the donor was EBV-seropositive versus EBV-seronegative (32.2% versus 30.2%, respectively) [54]. In contrast, the presented meta-analysis demonstrates that HHV-6B has a strong statistical association with subsequent grades II to IV aGVHD (HR, 2.65; P < .001).
Use of quantitative HHV-6 results may help identify patients at particularly high risk of aGVHD. For example, Wang et al. [12] reported that the cumulative incidences of grades II to IV aGVHD in low (<100 copies/106 PBMCs), intermediate (100 to 5000 copies/106 PBMCs) and high (>5000 copies/106 PBMCs) HHV-6 viral level groups were 13.8%, 47.1%, and 54.6%, respectively. Compared with patients with low viral level of HHV-6B, patients with intermediate and high viral levels of HHV-6B had increased probability of subsequent development of grades II to IV aGVHD (P = .0005). Violago et al. [13], de Pagter et al. [20], and Admiraal et al. [17] all used a threshold of >1000 copies/mL plasma to define HHV-6 reactivation, and all reported similar reactivation rates (19% in 100 patients, 17% in 108 patients, and 27% in 273 patients, respectively). Despite this, in multivariate analysis the association between HHV-6 reactivation and subsequent aGVHD reached statistical significance in only de Pagter et al. (HR, 6.07; P < .001) and Admiraal et al. (HR, 3.47; P < .0001) but not in Violago et al. (HR, 1.95; P = .178). This inconsistency was likely due to the size of the studied cohorts, although it is possible that CMV prophylaxis, which is effective against HHV-6, could have affected Violago et al.’s results.
One possible mechanism of HHV-6 triggering or exacerbating GVHD is by interaction with and up-regulation of CD134 (also known as OX40 or TNFRSF4) [55,56], which is an HHV-6B receptor for cell entry [57]. CD134 is an immunomodulatory molecule that blocks natural regulatory T cell (Treg) activity and antagonizes generation of inducible Treg cells [58,59], and a recent study of 23 allogeneic HCT patients showed that CD134 up-regulation coincided with HHV-6B reactivation [56]. Through IL-10 and other immune mechanisms, Treg cells reduce the functional activity of autoreactive cells and are believed to prevent the development of aGVHD [60,61]. CD134 has also been suggested to play a role in GVHD pathophysiology in transplant recipients [62–65]. Results from an in vitro study [63] suggest that use of CD134-allodepleted grafts may reduce alloreactivity and GVHD without loss of pathogen-specific and leukemia-specific immunity. Studies performed in mouse models reported that use of an antiCD134 monoclonal antibody reduced the morbidity and mortality of aGVHD in mice [62,64]. Further clinical studies are needed to fully understand the implications in humans.
Additionally, some researchers have suggested that the delay or absence of immune reconstitution, especially of CD4+ T cells, facilitates viral reactivation [33,66–68], which may subsequently contribute to a number of outcomes including aGVHD, graft failure, and increased mortality [69]. After allogeneic HCT, T cell reconstitution is a long process that proceeds along 2 pathways with distinct kinetics: (1) a thymus-independent phase that begins immediately during the early post-transplant period and is mediated by adoptively transferred graft-derived T cells or recipient T cells that survived conditioning and (2) a late thymus-dependent phase that is a prolonged, multistep process that can take up to 18 months after HCT, in which lymphomyeloid progenitor cells repopulate the thymus with thymocyte precursors that can reconstitute thymopoiesis [68,70]. CD4+ lymphopenia, including CD4+/CD25+ (Treg) lymphopenia, has been associated with high levels of homeostatic cytokines like IL-7, which has been implicated in the pathogenesis of GVHD [71–73]. Given that HHV-6 reactivates early and often after HCT and can directly infect and deplete CD4+ cells [17,44,67,68,74–79], it is reasonable to hypothesize that HHV-6 could interfere with early T cell reconstitution [44,67,74–79], resulting in uncontrolled alloreactivity secondary to Treg depletion and leading to aGVHD. Coupled with our systematic review and meta-analysis, the aforementioned data provide a possible causal pathway for HHV-6B and aGVHD. However, HHV-6 reactivation could simply be an epiphenomenon; thus, more experimental data, such as results from a prospective randomized trial, are needed to confirm causality.
Although the search algorithm resulted in over 1000 articles and abstracts, there is still a possibility that some publications were not included using this strategy. Additionally, results from the funnel plot implied some evidence of publication bias in the group 1 analysis; however, there was no evidence publication bias in the group 3 analysis. Another limitation of this systematic review and meta-analysis is that the patient population, the methods (including type of HCT performed, type/dosage of immunosuppressive drugs used, HHV-6 testing procedures, GVHD prophylaxis/treatment), and the outcome definitions were not identical across all studies. In addition, the existent risk of misdiagnosis of HHV-6 skin manifestations as aGVHD [23,80], and the variability in aGVHD diagnosis and staging practices between transplant centers may further confound the comparison of findings across these studies.
Finally, the statistically significant relationship between HHV-6 reactivation and subsequent aGVHD does not prove causality. Antiviral trials are needed to define strategies to manage HHV-6B and to elucidate its role in aGVHD. Unfortunately, the available antivirals for HHV-6 can have adverse side effects (eg, bone marrow suppression, nephrotoxicity), which will make interventional studies technically challenging until less toxic HHV-6B–active antivirals are available or until there is more direct experimental evidence to support such a trial. Because HHV-6B infection can persist in the organ without a high viral load in the periphery [81–83], analysis of tissue may be the only means of identifying an active HHV-6B infection. At the National Institute of Allergy and Infectious Diseases–-sponsored workshop on roseoloviruses, leading experts in the field underscored that in-depth molecular interrogation of tissues involved in end-organ disease is required to identify pathologic and molecular signatures of HHV-6B infection [84]. Ultimately, determining whether antiviral prophylaxis or treatment reduces the incidence or severity of aGVHD while reducing HHV-6B activity will provide conclusive evidence regarding the role HHV-6B plays in aGVHD.
ACKNOWLEDGMENTS
The authors are indebted to Kristin Loomis (HHV-6 Foundation) for facilitating the network among the authors of this study and for her continuous support of HHV-6 research.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
Financial disclosure: The authors have nothing to disclose.
REFERENCES
- 1.Gatza E, Choi SW. Approaches for the prevention of graft-versus-host disease following hematopoietic cell transplantation. Int J Hematol Oncol. 2015;4:113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jagasia M, Arora M, Flowers ME, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vigorito AC, Campregher PV, Storer BE, et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood. 2009;114:702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norkin M, Wingard JR. Recent advances in hematopoietic stem cell transplantation. N Engl J Med. 2010;363:2091–2101.21105791 [Google Scholar]
- 5.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramadan A, Paczesny S. Various forms of tissue damage and danger signals following hematopoietic stem-cell transplantation. Front Immunol. 2015;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couriel D, Caldera H, Champlin R, Komanduri K. Acute graft-versus-host disease: pathophysiology, clinical manifestations, and management. Cancer. 2004;101:1936–1946. [DOI] [PubMed] [Google Scholar]
- 8.Shono Y, Docampo MD, Peled JU, Perobelli SM, Jenq RR. Intestinal microbiota-related effects on graft-versus-host disease. Int J Hematol. 2015;101:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathewson ND, Jenq R, Mathew AV, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol. 2016;17:505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768–776. [DOI] [PubMed] [Google Scholar]
- 11.Cirrone F, Ippoliti C, Wang H, et al. Early human herpes virus type 6 reactivation in umbilical cord blood allogeneic stem cell transplantation. Leuk Lymph. 2016;57:2555–2559. [DOI] [PubMed] [Google Scholar]
- 12.Wang LR, Dong LJ, Zhang MJ, Lu DP. Correlations of human herpesvirus 6B and CMV infection with acute GVHD in recipients of allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;42:673–677. [DOI] [PubMed] [Google Scholar]
- 13.Violago L, Jin Z, Bhatia M, et al. Human herpesvirus-6 viremia is not associated with poor clinical outcomes in children following allogeneic hematopoietic cell transplantation. Pediatr Transplant. 2015;19:737–744. [DOI] [PubMed] [Google Scholar]
- 14.Olson AL, Dahi PB, Zheng J, et al. Frequent human herpesvirus-6 viremia but low incidence of encephalitis in double-unit cord blood recipients transplanted without antithymocyte globulin. Biol Blood Marrow Transplant. 2014;20:787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill JA, Koo S, Guzman Suarez BB, et al. Cord-blood hematopoietic stem cell transplant confers an increased risk for human herpesvirus-6-associated acute limbic encephalitis: a cohort analysis. Biol Blood Marrow Transplant. 2012;18:1638–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agut H Deciphering the clinical impact of acute human herpesvirus6 (HHV-6) infections. J Clin Virol. 2011;52:164–171. [DOI] [PubMed] [Google Scholar]
- 17.Admiraal R, de Koning C, Lindemans CA, et al. Viral reactivations and associated outcomes in context of immune reconstitution after pediatric hematopoietic cell transplantation. J Allergy Clin Immunol. 2017;140:1643–1650. [DOI] [PubMed] [Google Scholar]
- 18.Aoki J , Numata A, Yamamoto E, Fujii E, Tanaka M, Kanamori H. Impact of human herpesvirus-6 reactivation on outcomes of allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:2017–2022. [DOI] [PubMed] [Google Scholar]
- 19.Verhoeven DH, Claas EC, Jol-van der Zijde CM, et al. Reactivation of human herpes virus-6 after pediatric stem cell transplantation: risk factors, onset, clinical symptoms and association with severity of acute graft-versushost disease. Pediatr Infect DisJ. 2015;34:1118–1127. [DOI] [PubMed] [Google Scholar]
- 20.de Pagter PJ, Schuurman R, Keukens L, et al. Human herpes virus 6 reactivation: important predictor for poor outcome after myeloablative, but not non-myeloablative allo-SCT. Bone Marrow Transplant. 2013;48:1460–1464. [DOI] [PubMed] [Google Scholar]
- 21.Dulery R, Salleron J, Dewilde A, et al. Early human herpesvirus type 6 reactivation after allogeneic stem cell transplantation: a large-scale clinical study. Biol Blood Marrow Transplant. 2012;18:1080–1089. [DOI] [PubMed] [Google Scholar]
- 22.Zerr DM, Boeckh M, Delaney C, et al. HHV-6 reactivation and associated sequelae after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:1700–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pichereau C, Desseaux K, Janin A, et al. The complex relationship between human herpesvirus 6 and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18:141–144. [DOI] [PubMed] [Google Scholar]
- 24.de Pagter PJ, Schuurman R, Visscher H, et al. Human herpes virus 6 plasma DNA positivity after hematopoietic stem cell transplantation in children: an important risk factor for clinical outcome. Biol Blood Marrow Transplant. 2008;14:831–839. [DOI] [PubMed] [Google Scholar]
- 25.Zerr DM, Corey L, Kim HW, Huang ML, Nguy L, Boeckh M. Clinical outcomes of human herpesvirus 6 reactivation after hematopoietic stem cell transplantation. Clin Infect Dis. 2005;40:932–940. [DOI] [PubMed] [Google Scholar]
- 26.Inazawa N, Hori T, Hatakeyama N, et al. Large-scale multiplex polymerase chain reaction assay for diagnosis of viral reactivations after allogeneic hematopoietic stem cell transplantation. J Med Virol. 2015;87:1427–1435. [DOI] [PubMed] [Google Scholar]
- 27.Gotoh M, Yoshizawa S, Katagiri S, et al. Human herpesvirus 6 reactivation on the 30th day after allogeneic hematopoietic stem cell transplantation can predict grade 2–4 acute graft-versus-host disease. Transpl Infect Dis. 2014;16:440–449. [DOI] [PubMed] [Google Scholar]
- 28.Mutlu D, Uygun V, Yazisiz H, Tezcan G, Hazar V, Colak D. Analysis of human herpes virus 6 infections with a quantitative, standardized, commercial kit in pediatric stem cell transplant recipients after transplantation. Ann Saudi Med. 2014;34:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeulin H, Agrinier N, Guery M, et al. Human herpesvirus 6 infection after allogeneic stem cell transplantation: incidence, outcome, and factors associated with HHV-6 reactivation. Transplantation. 2013;95:1292–1298. [DOI] [PubMed] [Google Scholar]
- 30.Brands-Nijenhuis AV, van Loo IH, Schouten HC, van Gelder M. Temporal relationship between HHV 6 and graft vs host disease in a patient after haplo-identical SCT and severe T-cell depletion. Bone Marrow Transplant. 2011;46:1151–1152. [DOI] [PubMed] [Google Scholar]
- 31.Tormo N, Solano C, de la Camara R, et al. An assessment of the effect of human herpesvirus-6 replication on active cytomegalovirus infection after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:653–661. [DOI] [PubMed] [Google Scholar]
- 32.Chan PK, Peiris JS, Yuen KY, et al. Human herpesvirus-6 and human herpesvirus-7 infections in bone marrow transplant recipients. J Med Virol. 1997;53:295–305. [DOI] [PubMed] [Google Scholar]
- 33.Quintela A, Escuret V, Roux S, et al. HHV-6 infection after allogeneic hematopoietic stem cell transplantation: from chromosomal integration to viral co-infections and T-cell reconstitution patterns. J Infect. 2016;72:214–222. [DOI] [PubMed] [Google Scholar]
- 34.Ogata M, Satou T, Kadota J, et al. Human herpesvirus 6 (HHV-6) reactivation and HHV-6 encephalitis after allogeneic hematopoietic cell transplantation: a multicenter, prospective study. Clin Infect Dis. 2013;53:671–681. [DOI] [PubMed] [Google Scholar]
- 35.Kullberg-Lindh C, Mellgren K, Friman V, et al. Opportunistic virus DNA levels after pediatric stem cell transplantation: serostatus matching, antithymocyte globulin, and total body irradiation are additive risk factors. Transpl Infect Dis. 2011;13:122–130. [DOI] [PubMed] [Google Scholar]
- 36.Betts BC, Young JA, Ustun C, Cao Q, Weisdorf DJ. Human herpesvirus6 infection after hematopoietic cell transplantation: is routine surveillance necessary? Biol Blood Marrow Transplant. 2011;17:1562–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chevallier P, Hebia-Fellah I, Planche L, et al. Human herpes virus 6 infection is a hallmark of cord blood transplant in adults and may participate to delayed engraftment: a comparison with matched unrelated donors as stem cell source.Bone Marrow Transplant. 2010;45:1204–1211. [DOI] [PubMed] [Google Scholar]
- 38.Yamane A, Mori T, Suzuki S, et al. Risk factors for developing human herpesvirus 6 (HHV-6) reactivation after allogeneic hematopoietic stem cell transplantation and its association with central nervous system disorders. Biol Blood Marrow Transplant. 2007;13:100–106. [DOI] [PubMed] [Google Scholar]
- 39.Ogata M, Kikuchi H, Satou T, et al. Human herpesvirus 6 DNA in plasma after allogeneic stem cell transplantation: incidence and clinical significance. J Infect Dis. 2006;193:68–79. [DOI] [PubMed] [Google Scholar]
- 40.Tomonari A, Takahashi S, Ooi J, et al. Human herpesvirus 6 variant B infection in adult patients after unrelated cord blood transplantation. Int J Hematol. 2005;81:352–355. [DOI] [PubMed] [Google Scholar]
- 41.Radonic A, Oswald O, Thulke S, et al. Infections with human herpesvirus 6 variant B delay platelet engraftment after allogeneic haematopoietic stem cell transplantation.Br J Haematol. 2005;131:480–482. [DOI] [PubMed] [Google Scholar]
- 42.Ljungman P, Wang FZ, Clark DA, et al. High levels of human herpesvirus 6 DNA in peripheral blood leucocytes are correlated to platelet engraftment and disease in allogeneic stem cell transplant patients. Br J Haematol. 2000;111:774–781. [PubMed] [Google Scholar]
- 43.Imbert-Marcille BM, Tang XW, Lepelletier D, et al. Human herpesvirus 6 infection after autologous or allogeneic stem cell transplantation: a single-center prospective longitudinal study of 92 patients. Clin Infect Dis. 2000;31:881–886. [DOI] [PubMed] [Google Scholar]
- 44.Wang FZ, Linde A, Dahl H, Ljungman P. Human herpesvirus 6 infection inhibits specific lymphocyte proliferation responses and is related to lymphocytopenia after allogeneic stem cell transplantation. Bone Marrow Transplant. 1999;24:1201–1206. [DOI] [PubMed] [Google Scholar]
- 45.Maeda Y, Teshima T, Yamada M, et al. Monitoring of human herpesviruses after allogeneic peripheral blood stem cell transplantation and bone marrow transplantation. Br J Haematol. 1999;105:295–302. [PubMed] [Google Scholar]
- 46.Wang FZ, Dahl H, Linde A, Brytting M, Ehrnst A, Ljungman P. Lymphotropic herpesviruses in allogeneic bone marrow transplantation. Blood. 1996;88:3615–3620. [PubMed] [Google Scholar]
- 47.Appleton AL, Sviland L, Peiris JS, et al. Human herpes virus-6 infection in marrow graft recipients: role in pathogenesis of graft-versus-host disease. Newcastle upon Tyne Bone Marrow Transport Group. Bone Marrow Transplant. 1995;16:777–782. [PubMed] [Google Scholar]
- 48.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill JA, Boeckh M, Leisenring WM, et al. Human herpesvirus 6B reactivation and delirium are frequent and associated events after cord blood transplantation. Bone Marrow Transplant. 2015;50:1348–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill JA, Sedlak RH, Magaret A, et al. Outcomes after allogeneic hematopoietic cell transplantation with donors or recipients harboring inherited chromosomally integrated HHV-6. Biol Blood Marrow Transplant. 2017;23: S55–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beck JC, Wagner JE, DeFor TE, et al. Impact of cytomegalovirus (CMV) reactivation after umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2010;16:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller W, Flynn P, McCullough J, et al. Cytomegalovirus infection after bone marrow transplantation: an association with acute graft-v-host disease.Blood. 1986;67:1162–1167. [PubMed] [Google Scholar]
- 53.Green MSP, Gooley T, Martin P, Boeckh M. Indirect effects of CMV infection after hematopoietic cell transplantation (HCT) in the preemptive therapy era. ID Week. San Diego, CA. 2015. [Google Scholar]
- 54.Styczynski J, Tridello G, Gil L, et al. Impact of donor Epstein-Barr virus serostatus on the incidence of graft-versus-host disease in patients with acute leukemia after hematopoietic stem-cell transplantation: a study from the Acute Leukemia and Infectious Diseases working parties of the European Society for Blood and Marrow Transplantation. J Clin Oncol. 2016;34:2212–2220. [DOI] [PubMed] [Google Scholar]
- 55.PazMorante M, Briones J, Canto E, et al. Activation-associated phenotype of CD3 T cells in acute graft-versus-host disease. Clin Exp Immunol. 2006;145:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagamata S, Nagasaka M, Kawabata A, et al. Human CD134 (OX40) expressed on T cells plays a key role for human herpesvirus 6B replication after allogeneic hematopoietic stem cell transplantation. J Clin Virol. 2018;102:50–55. [DOI] [PubMed] [Google Scholar]
- 57.Tang H, Serada S, Kawabata A, et al. CD134 is a cellular receptor specific for human herpesvirus-6B entry. Proc Natl Acad Sci USA. 2013;110:9096–9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.So T, Lee SW, Croft M. Immune regulation and control of regulatory T cells by OX40 and 4–1BB. Cytokine Growth Factor Rev. 2008;19:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vu MD, Xiao X, Gao W, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rezvani K, Mielke S, Ahmadzadeh M, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108:1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rieger K, Loddenkemper C, Maul J, et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107:1717–1723. [DOI] [PubMed] [Google Scholar]
- 62.Tsukada N, Akiba H, Kobata T, Aizawa Y, Yagita H, Okumura K. Blockade of CD134 (OX40)-CD134L interaction ameliorates lethal acute graft-versus-host disease in a murine model of allogeneic bone marrow transplantation. Blood. 2000;95:2434–2439. [PubMed] [Google Scholar]
- 63.Ge X, Brown J, Sykes M, Boussiotis VA. CD134-allodepletion allows selective elimination of alloreactive human T cells without loss of virus-specific and leukemia-specific effectors. Biol Blood Marrow Transplant. 2008;14:518–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang Y, Feng S, Tang R, Du B, Xu K, Pan X. Efficacy of pretreatment of allografts with methoxypolyethylene glycol-succinimidyl-propionic acid ester in combination with an anti-OX40L monoclonal antibody in relieving graft-versus-host disease in mice. Int J Hematol. 2010;92:609–616. [DOI] [PubMed] [Google Scholar]
- 65.Blazar BR, Sharpe AH, Chen AI, et al. Ligation of OX40 (CD134) regulates graft-versus-host disease (GVHD) and graft rejection in allogeneic bone marrow transplant recipients. Blood. 2003;101:3741–3748. [DOI] [PubMed] [Google Scholar]
- 66.Pourgheysari B, Piper KP, McLarnon A, et al. Early reconstitution of effector memory CD4+ CMV-specific T cells protects against CMV reactivation following allogeneic SCT. Bone Marrow Transplant. 2009;43:853–861. [DOI] [PubMed] [Google Scholar]
- 67.Michalek J, Horvath R, Benedik J, Hrstkova H. Human herpesvirus-6 infection in children with cancer. Pediatr Hematol Oncol. 1999;16:423–430. [DOI] [PubMed] [Google Scholar]
- 68.de Koning C, Admiraal R, Nierkens S, Boelens JJ. Human herpesvirus 6 viremia affects T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Blood Adv. 2018;2:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Broers AE, van Der Holt R, van Esser JW, et al. Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood. 2000;95:2240–2245. [PubMed] [Google Scholar]
- 70.Politikos I, Boussiotis VA. The role of the thymus in T-cell immune reconstitution after umbilical cord blood transplantation. Blood. 2014;124:3201–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Politikos I, Kim HT, Nikiforow S, et al. IL-7 and SCF levels inversely correlate with T cell reconstitution and clinical outcomes after cord blood transplantation in adults. PLoS One. 2015;10. e0132564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dean RM, Fry T, Mackall C, et al. Association of serum interleukin-7 levels with the development of acute graft-versus-host disease. J Clin Oncol. 2008;26:5735–5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thiant S, Labalette M, Trauet J, et al. Plasma levels of IL-7 and IL-15 after reduced intensity conditioned allo-SCT and relationship to acute GVHD. Bone Marrow Transplant. 2011;46:1374–1381. [DOI] [PubMed] [Google Scholar]
- 74.Yasukawa M, Inoue Y, Ohminami H, Terada K, Fujita S. Apoptosis of CD4+T lymphocytes in human herpesvirus-6 infection. J Gen Virol. 1998;79(pt1):143–147. [DOI] [PubMed] [Google Scholar]
- 75.Inoue Y, Yasukawa M, Fujita S. Induction of T-cell apoptosis by human herpesvirus 6. J Virol. 1997;71:3751–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lusso P, Markham PD, Tschachler E, et al. In vitro cellular tropism of human B-lymphotropic virus (human herpesvirus-6). J Exp Med. 1988;167:1659–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gobbi A, Stoddart CA, Malnati MS, et al. Human herpesvirus 6 (HHV-6) causes severe thymocyte depletion in SCID-hu Thy/Liv mice. J Exp Med. 1999;189:1953–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patel SJ, Zhao G, Penna VR, et al. A murine herpesvirus closely related to ubiquitous human herpesviruses causes T-cell depletion. J Virol. 2017;91. pii: e02463–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sultanova A, Chistjakovs M, Chapenko S, Donina S, Murovska M. Possible interference of human beta-herpesviruses-6 and −7 in gastrointestinal cancer development.Exp Oncol. 2013;35:93–96. [PubMed] [Google Scholar]
- 80.Yoshikawa T, Ihira M, Ohashi M, et al. Correlation between HHV-6 infection and skin rash after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2001;28:77–81. [DOI] [PubMed] [Google Scholar]
- 81.Leveque N, Boulagnon C, Brasselet C, et al. A fatal case of human herpesvirus 6 chronic myocarditis in an immunocompetent adult. J Clin Virol. 2011;52:142–145. [DOI] [PubMed] [Google Scholar]
- 82.Pischke S, Gösling J, Engelmann I, et al. High intrahepatic HHV-6 virus loads but neither CMV nor EBV are associated with decreased graft survival after diagnosis of graft hepatitis. J Hepatol. 2012;56:1063–1069. [DOI] [PubMed] [Google Scholar]
- 83.Buyse S, Roque-Afonso A-M, Vaghefi P, et al. Acute hepatitis with periportal confluent necrosis associated with human herpesvirus 6 infection in liver transplant patients. Am J Clin Pathol. 2013;140:403–409. [DOI] [PubMed] [Google Scholar]
- 84.Caserta MT, Krug LT, Pellett PE. Roseoloviruses: unmet needs and research priorities: perspective. Curr Opin Virol. 2014;9:167–169. [DOI] [PubMed] [Google Scholar]