Abstract
To continuously process neural activity underlying sensation, movement, and cognition, the central nervous system requires a homeostatic microenvironment that is not only enriched in nutrients to meet its high metabolic demands but also devoid of toxins that might harm the sensitive neural tissues. This highly regulated microenvironment is made possible by two unique features of CNS vasculature absent in the peripheral organs. First, the blood–blood barrier, which partitions the circulating blood from the CNS, acts as a gatekeeper to facilitate the selective trafficking of substances between the blood and parenchyma. And second, neurovascular coupling ensures that, following local neural activation, regional blood flow is increased to quickly supply more nutrients and remove metabolic waste. Here, we review how neural and vascular activity act on one another with regards to these two properties.
ToC blurb
The homeostatic CNS environment is maintained by the function of the blood–blood barrier (BBB) and neurovascular coupling (NVC). Kaplan, Chow and Gu describe how neural and vascular activity act on one another with regard to BBB and NVC.
Introduction
The brain is a highly vascularized organ, with every neuron positioned within 15μm of a blood vessel1. This proximity allows for ready exchange of nutrients and waste products, enabling the high metabolic activity of the brain despite its limited intrinsic energy storage. Furthermore, the flux of blood is finely targeted to active areas through selective dilation and contraction of blood vessels, even allowing for the use of blood flow as a proxy for brain activity in functional MRI (fMRI; through the blood oxygen level-dependent (BOLD) response). For more than a century, it has also been observed that exchange of molecules across this dense vasculature in both directions is highly restricted2,3. These emergent properties — the blood–brain barrier (BBB) and neurovascular coupling (NVC) — are owed to the concerted action of the several cell types that together comprise the neurovascular unit (NVU).
The CNS vasculature itself is made up of different segments, each with a molecularly distinct composition of cell types4,5. Arteries, and the arterioles that branch off them, are the most upstream with respect to blood flow. The arterial endothelial cells (aECs) are enwrapped by arteriolar smooth muscle cells (SMCs), which can acutely constrict or dilate these vessels to control blood flow into the downstream capillary bed. Capillaries make up 85% of the vasculature of the brain6 and are the principle contributors to BBB function. Capillary endothelial cells (cECs) are very tightly associated with pericytes, which are a type of mural cell related to, but molecularly and functionally distinct from, SMCs (Table 1). In the adult vasculature, capillaries are also surrounded by astrocyte end-feet. Finally, blood from the capillaries is drained into venules and veins. Venules and veins represent the smallest fraction of CNS blood vessels. Although venous endothelial cells, similar to aECs, are surrounded by SMCs, these SMCs are molecularly distinct from arteriolar SMCs4, and the vasomotion of veins is thought to be a passive result from changes in upstream blood flow7. These cell types, along with the surrounding neurons, are the principal constituents of the NVU (Fig. 1). For a comprehensive overview of NVU cell types, readers may refer to several excellent reviews8–10.
Table 1 |.
Molecular markers of segments of the neurovascular unit
| Marker or dye | Gene name | Vascular segment | Genetic construct |
|---|---|---|---|
| Smooth muscle cells | |||
| Smooth muscle actin | Acta2 | Arteries and venules | Acta2–CreER214 |
| Acta2–mCherry215 | |||
| Myosin heavy chain 11 | Myh11 (also known as Smmhc) | Arteries and venules | Myh11–CreER216 |
| Calponin | Cnn1 | Arteries | CNN1 gene trap LacZ reporter217 |
| Transgelin (also known as smooth muscle protein 22) | Tagln | Arteries | Tagln–Cre218 |
| Pericytes | |||
| Aminopeptidase N (also known as CD13) | Anpep | Capillaries and venules24 | |
| ATP-binding cassette, sub-family C | Abcc9 | Capillaries and venules | abcc9–GAL4 (in zebrafish)219 |
| Genetic intersection with platelet-derived growth factor-β and chondroitin sulfate proteoglycan 4 | Pdgfrb; Cspg4 (also known as Ng2) | Capillaries | Pdgfrb–Flp; Cspg2–Frt-STOP-Frt-Cre–ER76 |
| NeuroTrace 525 | – | Capillaries162 | – |
| Arterial endothelial cells | |||
| Bone marrow tyrosine kinase | Bmx | Arteries | Bmx–Cre–ER220 |
| Bmx–LacZ221 | |||
| Connexin 40 (also known as gap junction protein 5) | Gja5 | Arteries206 | Gja5–Cre–ER222 |
| Capillary endothelial cells | |||
| Major facilitator superfamily domain containing 2A | Mfsd2a | Capillary and venule | Mfsd2a–Cre–ER223 |
| Artery | |||
| Hydrazide 633 | – | Arteries160 | – |
ER, oestrogen receptor.
Fig. 1 |. The neurovascular unit and cerebrovascular anatomy.
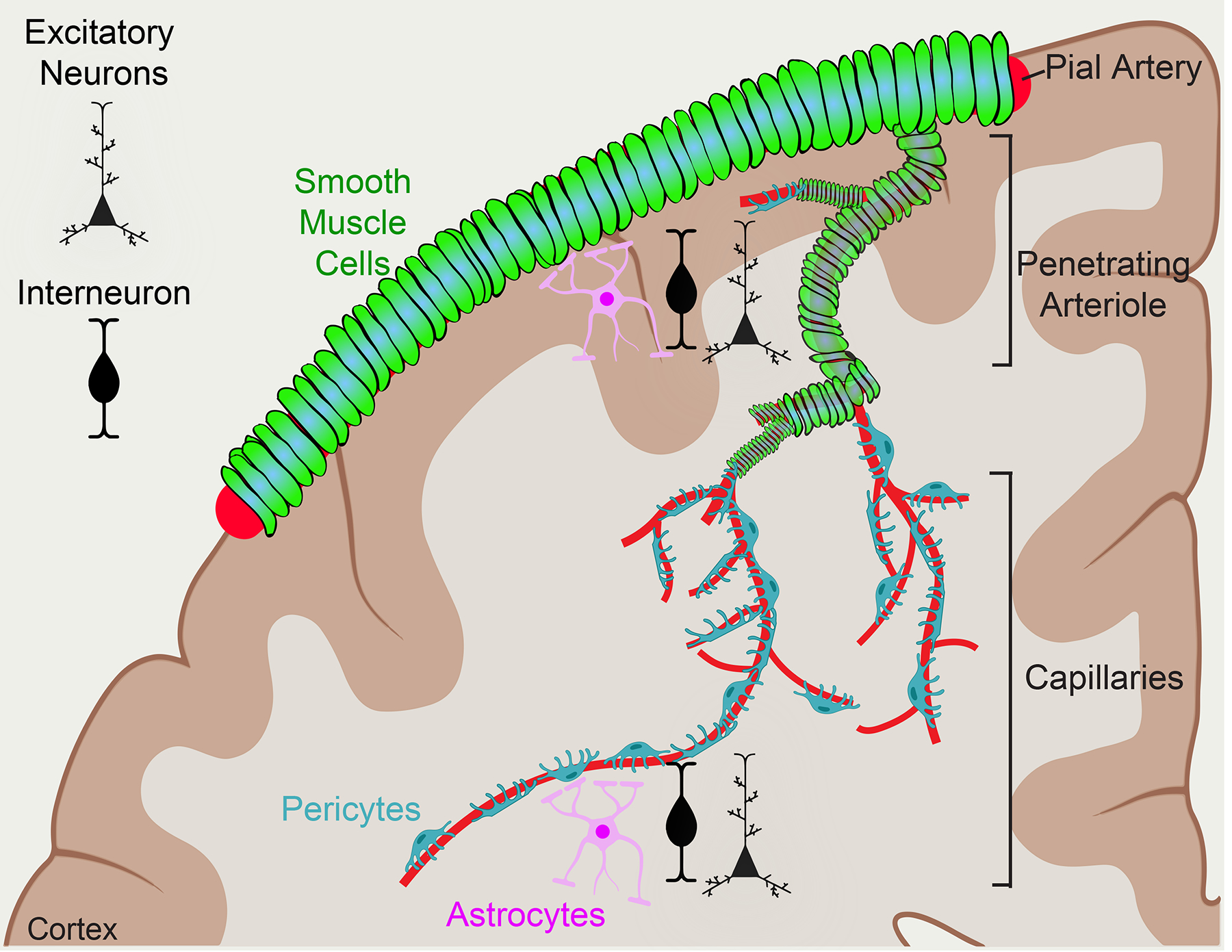
Pial arteries line the surface of the brain and are ensheathed by contractile smooth muscle cells (SMCs). Pial arteries then descend into the brain parenchyma, narrowing and branching to become penetrating arterioles, which then branch even more to become the dense network of capillaries. Pericytes surround capillaries and are near end-feet of astrocytes and dendrites of neurons. Together, these cells form the collective neurovascular unit.
Serving as the interface between the periphery and the CNS, signalingsignaling within and across the NVU is critical in health and disease. Recent work has shed greater light on the cell types and molecular pathways that regulate BBB function and NVC in the CNS. In particular, many groups have published single-cell and bulk transcriptomic data illustrating the molecular composition of different cell types of the NVU4,11–19. Notably, many of these factors are shared between both the BBB and NVC, and dysfunction in one is often correlated with dysfunction of the other.
In this Review, we discuss recent advances in our understanding of how the NVU mediates communication between the CNS parenchyma and the vasculature in the context of the BBB and NVC. We focus on how activity in the parenchyma can influence the vasculature, and vice versa. First, we cover mechanisms important to BBB function and how those mechanisms can be regulated by the activity of cells in the NVU. Second, we discuss the available evidence for how neural activity can acutely communicate to the vasculature to lead to spatially restricted changes in blood flow. For specific discussion of the CNS vasculature in disease, readers may consult other recent reviews6,20.
The blood–brain barrier
The ultrastructural basis for the BBB was first described by Reese and Karnovsky, who showed that an intravenously injected tracer cannot pass through specialized tight junctions (TJs) between CNS ECs21. The same tracer can, however, readily pass through the cell–cell junctions in the peripheral endothelium (paracellular leakage)22. Thus, historically, the restricted permeability of brain vasculature has been attributed to TJs, specialized contacts between adjacent ECs that prohibit paracellular passage of water-soluble molecules. However, substances can also cross ECs by transcytosis, a process by which material enters endocytic vesicles that traffic across the cell and release their contents on the other side (Fig. 2). Indeed, peripheral ECs have been found to have numerous tracer-filled vesicles whereas CNS ECs contain very few21. Recent evidence shows that the inhibition of transcytosis in CNS ECs is an active process to ensure BBB integrity and that full barrier integrity requires restriction of both paracellular and transcellular leakage10,23–27. However, the passage of molecules across the BBB is not completely absent, as BBB ECs abundantly express nutrient transporters, efflux transporters, and have some level of receptor-mediated transcytosis, all of which allow for molecule-specific exchange between the blood and the CNS28 (Fig. 2).
Fig. 2 |. Properties of the blood–brain barrier.
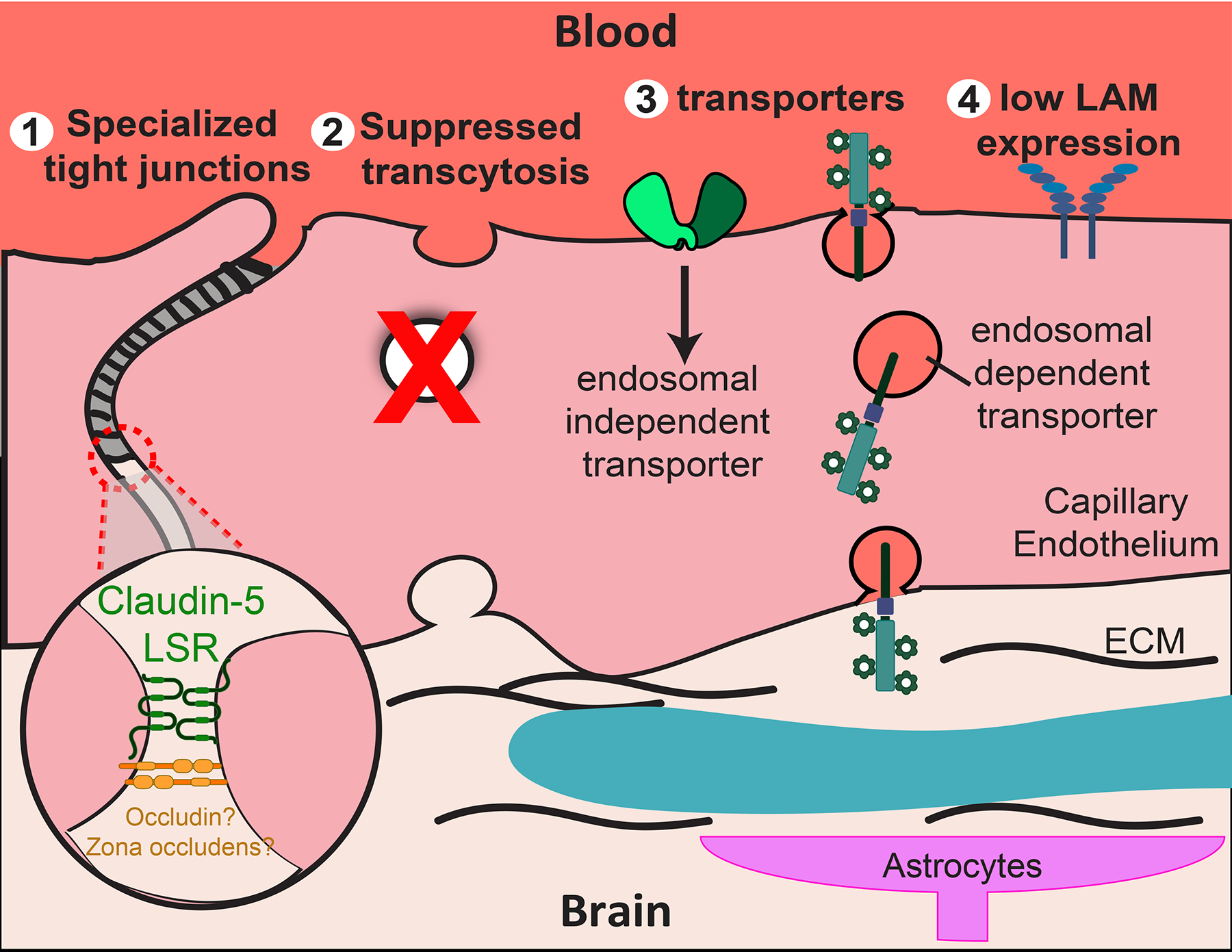
The CNS capillary endothelium has four cellular properties that contribute to the function of the blood–brain barrier (BBB) by strictly regulating the molecular trafficking between the blood and the brain. Specialized tight junctions limit paracellular flux between endothelial cells, including claudin 5 and/or lipolysis-stimulated lipoprotein receptor (LSR) (1). The specific contribution of occludin and zona occludens (ZO) remains elusive, as knockout mice lack phenotypes of BBB dysfunction. Suppression of transcytosis limits transcellular flux through CNS capillary endothelium (2). Molecule-specific transport allows the strict passage of desirable molecules such as nutrients (3). This can be categorized into endocytosis-independent transport and endocytosis-dependent transport. Low LAM expression on the luminal wall of the blood vessel maintains low levels of leukocyte adhesion and thus low levels of immune surveillance in the CNS (4). ‘?’ indicates some confounding evidence, as described in the text.
There is an emerging and ever-more precise picture of the molecular players capable of modulating barrier function in the CNS vasculature. But specific barrier function also exhibits spatial and temporal heterogeneity throughout the CNS. How is neural activity influenced by these variations in BBB function? Conversely, how does neural function influence BBB activity? Below, we provide a brief, inexhaustive overview of the state of our understanding of the key pathways and cell types for BBB function (for a more complete discussion, readers are referred to several recent reviews8,10,29–32). We then discuss several recent studies that highlight the interplay between barrier function and neural activity. These pose open questions as to how and which mechanisms that regulate barrier function may be at play.
Structural determinants
Specialized tight junctions.
CNS ECs form specialized TJs (Fig. 2), which are regarded as the key structural feature of the BBB, sealing the blood vessel lumen from the CNS parenchyma33–36. TJs are characterized by dense arrays of transmembrane proteins, notably claudin family members, occludin, and junctional adhesion molecules, all of which form intercellular contacts. Classically, these transmembrane factors are scaffolded by numerous other proteins, including the zona occludens proteins (ZO-1, ZO-2 and ZO-3). Many of these TJ proteins are not unique to the CNS vasculature, however, raising the question of what mechanisms distinguish the impermeability of the BBB relative to segments of peripheral continuous ECs.
A straightforward possibility is that CNS ECs have higher levels of TJ proteins, leading to denser, less-permeable TJs. Although EC TJ proteins are present in the peripheral vasculature, transcriptomics show that the levels of transcripts encoding several TJ proteins, notably occludin, are especially high in CNS ECs4,16,17. It is also possible that post-translational modifications have a role in tuning TJ protein function in CNS36–40. However, most of the evidence for such regulation stems from in vitro studies, which may fail to wholly replicate bone fide CNS ECs (Box 1). Finally, it is possible that there is an as-yet uncharacterized factor present only at BBB TJs that confers unique impermeability.
Box 1 |. Challenges of making in vitro blood–brain barrier models.
Given the complexity of studying the blood–brain barrier (BBB) in vivo, many have attempted to develop in vitro BBB models as a more tractable platform. The major challenge of in vitro models has been to recapitulate the ‘tightness’ of the BBB observed in vivo, especially as BBB properties are not intrinsic to CNS ECs but are mediated by the in situ neural environment. Notably, primary CNS ECs quickly lose their BBB properties in cell culture14,35,224,225.
Nevertheless, in the past decade, many laboratories have developed various in vitro BBB models, through the use of co-cultures with pericytes and astrocytes226, induced pluripotent stem cell (iPSC) differentiation227–229, brain organoids230 and ‘organ-on-a-chip’ approaches231in an effort to circumvent this issue. Validation of these in vitro models is typically done by measuring transendothelial electrical resistance (TEER) — a measure of TJ integrity — and the expression of BBB markers. However, non-BBB tissues, including epithelial cells232, can exhibit high TEER values and TEER is only a proxy for paracellular, not transcellular permeability. Additionally, measuring a few BBB markers is susceptible to false positives owing to antibody cross-reactivity with related non-BBB proteins46,47.
There is an urgent need to validate whether the ECs from these in vitro BBB models share similar transcriptomic profiles with CNS ECs in vivo. This is now possible given the increasing number of data sets describing the brain vasculature transcriptome4,11,12,16–18,233. Thus, basic requirements of in vitro BBB models should include transcriptomic validation and functional readouts that reflect in vivo barrier permeability.
Several groups have tested these possibilities using genetic ablation of specific TJ components. Surprisingly, knockouts of occludin41,42, ZO proteins43–45, claudin 346 (whose presence at the BBB is disputed46) and claudin 1247 do not result in gross TJ permeability. Animals lacking claudin 548 or lipolysis-stimulated lipoprotein receptor (LSR)49 (Fig. 2) do result in increased permeability to tracers smaller than about 800 Da. Curiously, CNS TJs in both of these knockout animals seem normal in electron microscopy with the evident kissing points between cell membranes, in contrast to many cell-cell junctions of peripheral vasculature ECs. Consequently, TJ dysfunction in these mice would not be detected by high-molecular-weight tracers such as horseradish peroxidase that are commonly used in electron microscopy (Box 2).
Box 2 |. Experimental methodology for assessing blood–brain barrier function.
Experimental assessment of BBB function requires measuring the degree of leakage of a molecule (that is, a tracer) from the blood to the CNS parenchyma. The choice of which molecule to use and which modality to use to measure leakage are important to consider when evaluating blood–brain barrier (BBB) integrity.
Detection modality
Most commonly used assays of BBB integrity involve sacrificing the experimental animal and measuring the amount of tracer that has leaked into the parenchyma. These approaches are versatile and allow for high spatial resolution, as with electron microscopy. By their nature, however, they do not allow for sampling the same subject over time and are not possible in humans. One of the most powerful non-invasive detection modalities is MRI with contrast agents (for example, longitudinal dynamic contrast with Gd-DPTA234,235), which allows for measuring permeability of the BBB through the whole CNS138,166. However, MRI-based imaging has relatively low spatial resolution and limited molecular capabilities, which can make it difficult to draw mechanistic conclusions.
Less common in animal models but useful in human subjects236, sampling cerebrospinal fluid (CSF) for presence of molecules from the blood can be used to approximate BBB function since normally the BBB would ensure separation of these fluids. But this method can be ambiguous as it is a function of leakage as well as CSF production and clearance. Nor does it resolve precisely where potential leakage may be occurring from the blood into the CSF. Finally, some groups have used functional readouts of BBB permeability by injecting neuroactive, but BBB-impermeable compounds. Therefore, their impact on neural activity would only manifest if there is BBB breakdown. An example is penicillin, which can act as a GABA receptor antagonist. With a leaky BBB, it can access the parenchyma, block inhibitory neuron activity, and result in net increases in neural activity.
Tracer size
Even when compromised, tight junctions (TJs) at the BBB typically only allow leakage of relatively low-molecular-weight molecules (up to ~1 kDa). Many common larger tracers, such as Evans blue, high-molecular-weight dextrans and horseradish peroxidase (HRP), may therefore show no leakage across the BBB even if TJ integrity is compromised. Common low-molecular-weight tracers include microperoxidase for electron microscopy and cadaverine for fluorescence-based detection. Differential leakage of high- and low-molecular weight tracers can discriminate between paracellular and transcellular BBB leakage.
Endogenous versus exogenous tracers
Molecules such as immunoglobulin G (IgG) and fibrinogen are naturally in the blood and are BBB-impermeable under normal conditions. Monitoring the CNS content of these endogenous molecules can be a useful alternative to delivering exogenous tracer, but does not provide kinetic information on leakage. Furthermore, most readily detectable endogenous molecules are too large to rigorously report on TJ functionality.
The at-most mild leakage levels in these knockout studies raise several questions. Is there compensation by other TJ proteins when a single TJ component is knocked out? Is there some other factor that is strictly necessary for BBB TJ function? Additionally, blood-borne macromolecules can influence CNS activity if they leak out of the vasculature. Can a reduction in TJ protein levels in disease explain paracellular leakage of these large molecules given that knockouts of individual TJ proteins only lead to small molecule leakage? Perhaps coordinated downregulation of several TJ components causes greater TJ permeability than does complete knockout of a single component. One further possibility is that TJ proteins at the BBB have additional roles besides providing strictly structural blockade of cell–cell junctions. These may include modulating signaling or transcriptional regulation, as has been demonstrated in vitro and in epithelial tissues50–54, but the role of such alternative pathways in CNS ECs has to date been poorly studied.
Suppressed transcytosis.
Unlike peripheral ECs, which readily transcytose material across the endothelium, CNS ECs suppress nonspecific transcytosis. The cell-biological mechanisms regulating transcytosis have been extensively studied in epithelial cells, but less is known in the context of ECs. Generally, transcytosis can proceed either through receptor-mediated transcytosis or fluid-phase transcytosis. The former conveys molecule-specific transport, whereas the latter can be nonspecific. Although various vesicular transcytosis pathways have been implicated at the BBB 10,27,55–57, the clathrin- and caveolae-mediated pathways are the most well-studied and have been extensively reviewed58,59. Very little is known, however, about the intracellular trafficking in the ECs that is responsible for sorting material to be transcytosed (as opposed to degraded or recycled back to the vessel lumen)60,61. A better grasp on the molecular mechanisms that regulate the intracellular trafficking of endosomes in CNS ECs could have important clinical applications for drug delivery across the BBB and for mitigating pathological BBB transcytosis62.
NVU regulation of the BBB
Although barrier functionality is ultimately localized to the ECs, BBB properties are not intrinsic for CNS ECs. Instead, BBB induction and maintenance rely on the local environment — that is, signaling from other cells in the NVU onto ECs. Such interactions include: secretion of WNT ligands by neurons and astroglia63,64; processing of transforming growth factor-β (TGFβ) by astrocytes65,66; signaling through extracellular matrix (ECM) factors secreted by pericytes and astrocytes67,68; and direct cell–cell contacts between ECs and mural cells69. The influence of the neural niche on BBB induction has been classically demonstrated in studies showing that transplanting neural tissue into the periphery can drive ectopic BBB formation70,71. Here, we highlight the contributions of just astrocytes and pericytes, owing to recent investigations into their roles in BBB maintenance and NVC. Readers may consult recent reviews for the contributions of other NVU cell types, including microglia and oligodendrocyte precursor cells (OPCs), among others9,10,72.
Pericytes.
Pericytes are recruited to the CNS vasculature early in development as ECs invade the neural tube. Early studies demonstrated that reducing pericyte recruitment by antagonizing platelet-derived growth factor receptor-β (PDGFRβ) signaling during development prevents functional BBB formation. These animals show substantial increases in transcytosis, as well as tight junction abnormalities23,24,73.
When pericyte recruitment is constitutively inhibited throughout development, adult animals continue to have leaky CNS vasculature24,73,74. Although these studies demonstrate that pericytes are required for BBB formation, they do not address whether pericytes are required for BBB maintenance after BBB formation. Correlative evidence suggested they are. First, reductions in the pericyte coverage of CNS capillaries with age coincide with increased BBB permeability as well as decreased cerebral blood flow73. Second, across different regions even in the CNS, vascular impermeability correlates well with pericyte coverage75.
Two recent studies directly test the role of pericytes in adult animals using pericyte ablation. First, Park et al. conditionally expressed diphtheria toxin subunit A (DTA) in mural cells, severely depleting both SMCs and pericytes throughout the entire mouse69. Remarkably, the authors did not observe BBB leakage even after 2 weeks, suggesting pericytes are important for initial BBB formation but not maintenance. However, in another study, Nikolakopoulou et al. used an intersectional genetic approach to specifically ablate roughly 60% of pericytes, sparing SMCs76. Using this approach, the authors observed BBB disruption, although it was much less severe than the leakage observed in models of pericyte deficiency through development. Interestingly, Nikolakopoulou et al. also did not observe an increase in transcytosis upon pericyte depletion, which is apparent in animals with constitutive pericyte depletion23,24.
A possible reason for this discrepancy may be methodological differences between the two studies. First, different molecules were used as proxies for BBB permeability (see Box 2): Park et al. examined leakage of an intravenously injected 70 kDa dextran by staining postmortem. By contrast, Nikolakopoulou et al. monitored leakage of intravenously injected gadolinium-diethylenetriamine pentaacetic acid (Gd-DPTA, molecular weight ~500Da) with MRI, and stained postmortem for infiltration of endogenous plasma proteins into the parenchyma. It may be possible that pericyte ablation makes the BBB susceptible to passage of specific molecules and not others. Second, the pericyte ablation techniques differed: whereas Park et al. directly expressed the cytotoxic subunit of the diphtheria toxin in all mural cells69, Nikolakopoulou et al. targeted the expression of the diphtheria toxin receptor and then administered diphtheria toxin (DT) systemically76. Although both of these approaches to cell ablation should be equivalent, subtle differences may exist between them77. Finally, it is notable that Nikolakopoulou et al. found more barrier disruption despite ablating a lower percentage of total pericytes. In light of this, other recent studies manipulating specific pericyte genes, including Foxf2 and Rbpj, have observed pronounced effects on BBB permeability without reduction in pericyte coverage of CNS blood vessels78,79. This illustrates the potential for dysfunctional pericytes to drive signaling pathways in ECs that reduce BBB integrity.
Astrocytes.
Astrocytes, like pericytes, are critical NVU constituents implicated in BBB and NVC regulation. Astrocyte end-feet tile CNS capillaries, and secrete trophic factors and ECM proteins. Indeed, just the steric coverage of the vasculature by astrocyte end-feet may provide a degree of barrier function80. Furthermore, the role of astrocytes in regulating neural function is now quite well appreciated81. These simultaneous direct interactions with neurons and the rest of the NVU make astrocytes attractive candidates for relaying signals between the parenchyma and the vasculature. Unlike pericytes, however, they mature postnatally after the barrier has formed82. Determining their precise role in barrier maintenance and regulation has remained elusive, partly because the diversity of astrocyte populations can make them experimentally difficult to address12.
Astrocytes are one of the principle sources of ECM critical to maintaining BBB function. Thus, animals in which the gene encoding laminin (Lamc1) is conditionally knocked out of astrocytes exhibit changes in NVU characteristics. These include reduced coverage by aquaporin4-positive end-feet, a change in pericyte differentiation, and considerable BBB permeability83,84. Secretion of soluble factors such as apolipoprotein E by astrocytes has also been shown to prevent BBB dysfunction68.
Analogously to pericytes, several groups have attempted to investigate astrocyte influence on NVU function via DT-mediated ablation. DT-mediated reduction of the numbers of either Gfap+85 or Aldh1l1+86 astrocytes manifested in profound effects on neuron function and survival, but curiously had limited observable effects on BBB function, as assayed by measuring the leakage of the endogenous plasma proteins. Specific elimination of astrocyte–vascular contacts is possible in a low-throughput manner87, but a systemic method to ablate such contacts without affecting the ability of astrocytes to provide trophic support to neurons is needed to clarify their role in BBB maintenance.
EC molecular pathways
Many signaling pathways in CNS ECs are crucial for barrier formation and maintenance. These including the WNT–β-catenin, TGFβ66,88, Hedgehog89, Notch90, angiopoietin69 and retinoic acid91 pathways. These pathways tend to also be important for CNS angiogenesis and vascular patterning, so it can be challenging to disentangle their barrier-specific effects. Systematic reviews of BBB-relevant pathways can be found in other reviews9,92,93; here we focus on two of the pathways with clear implications in barrier function in development and adult animals, WNT and TGFβ.
WNT signaling.
WNT signaling is crucial for CNS-specific angiogenesis and barriergenesis94. WNT pathway activation in ECs is detectable at early stages of CNS vascularization, starting around embryonic day 9.5 (E9.5) in mice95, before formation of a functional barrier23,25. Although WNT activity in ECs drops substantially at postnatal stages, after completion of vascular patterning, it remains necessary for barrier function throughout life95–97. In this pathway, one of several WNT ligands is secreted in the local environment, binds to Frizzled receptors on ECs, and signals to prevent β-catenin degradation. This enables β-catenin translocation into the nucleus, where it induces gene expression. Notably, astrocytes and neurons are important sources of WNT ligands64.
Several proteins important for cell-autonomous barrier function are downstream of WNT, including claudin 5 and the glucose uniporter GLUT1, which are upregulated by canonical WNT signaling15. WNT signaling also downregulates plasmalemma vesicle-associated protein (PLVAP), a marker associated with fenestrated vessels that do not possess a barrier. Furthermore, recent studies suggest that canonical WNT signaling influences suppression of transcytosis: antagonizing canonical WNT signaling by deleting the gene encoding the WNT co-receptor low-density lipoprotein receptor-related protein 5 (LRP5) decreases the expression of the lipid transporter, MFSD2A (a suppressor of transcytosis98), whereas β-catenin gain-of-function mice display upregulation of MFSD2A15. Although WNT signaling seems critical to barrier function throughout the CNS, different CNS regions have principal dependence on different WNT ligands (for example, norrin in the cerebellum and retina, but WNT7A and WNT7B in the cortex)99.
As WNT signaling regulates many BBB genes at once, could inducing WNT signaling in non-barrier ECs be sufficient for barrier formation? Recently, two studies have explored this question in the circumventricular organs (CVOs). CVOs are regions in the brain which have vasculature that naturally has a leaky BBB100 to allow for neurons to sense systemic signals, such as blood osmolarity101. β-catenin gain-of-function in the CVOs results in upregulation of BBB-related genes and decreased permeability to intravenously injected tracers15,102. Intriguingly, β-catenin stabilization alone is not as effective at inducing barrier properties in tissues that are not developmentally part of the CNS, such as the anterior pituitary gland, liver or lung15,17. Furthermore, fine regulation of β-catenin activity with other secreted factors such as WNT inhibitory factor 1 (WIF1)15,103 may be an additional means for the neural environment to tune barrier function. It will be interesting to further explore the extent to which WNT signaling regulates endothelial barrier function generally.
TGFβ signaling.
TGFβ signaling is important for cell-fate determination for every cell type of the NVU79,90. The ligand is subject to multiple post-translational regulatory steps, and the pathway is pleiotropic, particularly in ECs. Binding of TGFβ to the receptor, ALK1, leads to phosphorylation of SMAD1 and SMAD5 and a leaky, proliferative state in ECs, whereas binding to ALK5 (also known as TGFβ receptor 1) leads to phosphorylation of SMAD2 and SMAD3 and a stable quiescent state104,105. These multiple points of regulation make TGFβ signaling an important factor in BBB activity modulation.
Knocking out different components in the TGFβ pathway result in gross defects in vasculogenesis and angiogenesis, and often embryonic lethality106,107. Knocking out critical factors in TGFβ–ALK5 signaling in endothelial cells90, neurons108, or OPCs109 results in gross BBB dysfunction and hyperproliferative ECs. In adult mice, acutely blocking TGFβ signaling in the retina has also resulted in barrier disruption, but these animals exhibit gross vascular defects including EC cell death, making barrier-specific conclusions difficult110. At the same time, leaky BBB states are also often associated with upregulation in extracellular positive regulators of TGFβ signaling, such as thrombospondin17,65,66,79. More work needs to be done using acute manipulations of specific TGFβ signaling components to disentangle its role in adult barrier regulation from its importance in development.
Multiple pathways are involved in BBB regulation, and they can share similar molecules and feed back onto one another. Some confusion may be lifted by measuring pathway activity directly, for instance with reporter mice (which are available for the WNT111 and TGFβ112 pathways), or by staining for known downstream targets, such as lymphoid enhancer-binding factor 1 (LEF1) or phosphorylated SMAD proteins for the WNT or TGFβ pathways, respectively.
Does neural activity modulate the BBB?
Given the intimate and reciprocal relationship between the nervous system and the vascular system in the brain113,114, an exciting, but incompletely explored question is whether changes in neural activity can modulate BBB function through the mechanisms mentioned in the above section. Neural activity has been demonstrated to modulate developmental CNS angiogenesis115,116, and the neural control of blood flow has been studied for decades (see subsequent section). But, in a similar way to how NVC serves to efficiently match moment-to-moment energy demand, might neural activity also tune BBB transporter composition to match other demands? Does neural activity modulate general barrier permeability? Below, we highlight recent work examining these questions.
One possible mechanism for BBB modulation in response to neural activity is direct action of neurotransmitters on cells of the NVU. Indeed, glial cells have abundant neurotransmitter receptors. In the developing retina, Müller glia are activated by spillover of neurotransmitters released during spontaneous waves of neural activity117, which are necessary for refinement of the neural circuit underlying vision118,119. Müller glia are also critical to secretion of Norrin, which in turn activates the WNT pathway in retinal ECs, promoting barrier formation64. Inhibiting the spontaneous neural activity mediated by retinal cholinergic neurons impairs both angiogenesis and barriergenesis in the deep retinal vascular plexus120, further demonstrating the influence of neural activity on barrier function.
Acute increases in neural activity in adult animals have also been implicated in changes in BBB function. After being housed in darkness, mice exposed to light show substantial changes in BBB-related gene transcription in the vasculature of the visual cortex121, including increases in angiopoietin 2 (ANG2), which can then antagonize ANG1–TIE2 signaling and lead to BBB disruption122. Additionally, direct activation of neural activity with transcranial magnetic stimulation led to measurable increases in BBB permeability in mice and human participants, although in this case leakage may have been principally restricted to perivascular spaces123. It will be interesting to examine relative contributions of paracellular and transcellular permeability in these models as well as the functional consequence of BBB opening therein.
Aberrantly high neural activity has also been correlated with BBB opening. In epilepsy, which features pathologically high amounts of glutamatergic activity, robust BBB opening is observed, consistent with BBB opening in response to direct application of 1 mM glutamate123. There is in vivo evidence for active ionotropic glutamate receptors in ECs124, and in vitro evidence suggests that glutamate can act on ECs directly, inducing paracellular leakage125. Transcriptomic data, however, fails to find expression of ionotropic receptors in CNS ECs, and evidence for BBB modulation by physiological levels126 of glutamate is lacking. To what degree does normal neural activity cause general BBB opening? And how would this opening compare with neural activity ultimately leading to barriergenesis in development? More work is needed to explore whether specific subsets of neurons can mediate barrier opening or barrier sealing, or if different levels of general neural activity are responsible for differential effects on the BBB.
The BBB may also open to specific molecules in response to neural activity. An intriguing example of this is insulin-like growth factor 1 (IGF1), a peptide growth hormone that is essential for brain development and neurogenesis127–129. IGF1 is mainly secreted by the liver into the blood and typically bound to carrier proteins such as IGF binding protein 3 (IGFBP3). IGF1 acts through the IGF1 receptor (IGF1R), which is abundant both in brain ECs and in the parenchyma4,11. Interestingly, increasing neural activity — through exercise130, exploration of novel environments131 or whisker stimulation131 — all result in increased brain IGF1 levels. Conversely, inhibiting neural activity using tetrodotoxin prevents brain IGF1 accumulation131.
How does IGF1 transit from the blood to the brain, then? One possible mechanism is that activity-induced hyperaemia may result in increased blood-borne IGF1 bound to IGFPBP3 delivered to active brain areas132. Neural activity-induced enzymatic processing of IGFBP3 by matrix metalloproteinase 9 (MMP9)133,134 allows IGF1 to bind LRP1131,135 and/or IGF1R136, (which are expressed at the BBB), and to transcytose into the parenchyma specifically in areas where neural activity is elevated. Studying IGF1 trafficking in the context of endothelial-specific deletions of these proteins may help to resolve the mechanism of transport into active brain areas.
Collectively, these studies suggest that neural activity can affect barrier permeability. In normal physiology, these effects are likely to be finely tuned and spatiotemporally restricted in order to avoid pathology. It will be exciting to unravel the contexts in which neural activity can influence the BBB and whether specific classes of activity have differential effects on BBB function. To get at these mechanisms, future studies will benefit from cutting-edge pharmacological and optogenetic tools to acutely and specifically manipulate neural activity and study the impact on barrier function.
Does BBB permeability modulate neuronal functions?
The blood contains many molecules, including many cytokines, that can act as ligands for neural and glial receptors. Their passage into the parenchyma is regulated by the BBB, making it unsurprising that breakdown of BBB integrity has often been found to correlate with neural dysfunction and behavioural phenotypes137,138. Direct experimental evidence is available, for example, in recent work demonstrating that infusion of albumin into the parenchyma results in neural hyperexcitability88,139. However, barrier function also controls nutrient and waste exchange with the parenchyma, so decreases in permeability may also have considerable effects on neural activity.
For instance, recent work in mice has shown that EC-specific genetic ablation of Slc7a5 (which encodes a transporter of neutral amino acids) results in an altered metabolic profile in the brain140. These animals display autism spectrum disorder (ASD)-like behaviour (including reduced social interactions), which was rescued by intraventricular injection of leucine and isoleucine, thus bypassing the BBB140. Importantly, there are known mutations in the human SLC7A5 gene that are associated with ASD140. Similarly, reductions in GLUT1, an important glucose uniporter, can have pathological effects on neural function and are associated with Alzheimer disease. Interestingly, haploinsufficiency of the gene that encodes GLUT1, Slc2a1, at the BBB also results in abnormal TJs and BBB leakage141, which may exacerbate possible reductions in energy availability with additional leakage of molecules such as fibrinogen.
Similarly, MFSD2A functions to transport phospholipids from the outer to the inner leaflet of the plasma membrane in brain ECs, including omega-3 fatty acids such as docosahexaenoic acid (DHA), which is critical for brain development. Enrichment of these unsaturated phospholipids changes the plasma membrane lipid composition, which in turn inhibits caveolae formation to suppress nonspecific fluid transcytosis across the BBB25,142. Known human mutations in Mfsd2a are associated with microcephaly, and in mice and zebrafish, loss of MFSD2A results in microcephaly, cognitive impairment and BBB breakdown25,142–145. Furthermore, EC-specific knockouts of Mfsd2a also result in microcephaly, highlighting the importance of MFSD2A at the BBB in regulating the specific transport of nutrients crucial for brain function during development145. Interestingly, epistasis experiments show that double knockouts of Cav1 and Mfsd2a rescue the BBB leakage but not the microcephaly26, suggesting the gross neural pathology observed in Mfsd2a knockout animals is due to impaired nutrient transport that is critical for neuronal survival during early development, and that BBB leakage is not due to neuronal defects. It will be interesting to acutely knock out MFSD2A in adult animals when neuronal survival is no longer critically dependent on transport of these essential fatty acids.
Aside from nutrient exchange, changes in BBB function can result in permeability to signaling molecules that influence neural function. Emerging evidence has demonstrated that variation in BBB permeability between individuals can affect their susceptibility to neurological and psychiatric diseases. For example, the resilience to social stress among wild-type laboratory mice correlates positively with levels of various BBB-related proteins, including claudin 5146. In In stress-susceptible mice, social stress led to the BBB becoming more permeable in the nucleus accumbens and hippocampus. Notably, transient reduction of claudin 5 via short-hairpin RNA (shRNA) in stressed mice exacerbated depressive-like behaviour, suggesting that the BBB dysfunction is directly causative for the neuronal phenotype146.
Stress-susceptible mice also showed substantially higher levels of the neuropoietic family member, interleukin-6 (IL-6), than did resilient mice147, raising the possibility that BBB disruption allows for leakage of this chemokine into the parenchyma, where it alters neuronal function and thus behaviour. Indeed, direct infusion of IL-6 into the nucleus accumbens resulted in increased stress-susceptibility146, whereas IL-6 knockout mice are more resilient to stress-related depression148. As mice globally lacking claudin 5 still show restricted paracellular leakage of molecules the size of IL-648, it is not clear if TJ disruption is what causes the observed leakage in these animals.
It has also recently been demonstrated that maternal inflammation triggered by injection of the immune stimulant polyinosinic:polycytidylic acid poly(I:C)) results in increased neural activity specifically in the primary somatosensory cortex of the pups, and ASD-like behaviour in these animals via increased IL-17 signaling149. Notably, this phenotype was only observed if the injection was performed at E12.5, before the BBB has fully matured, but not at E15.5, after functional BBB formation23,25. This suggests that the timing of maternal inflammation relative to functional BBB formation in the fetus might contribute to the offspring’s susceptibility of neurological and psychiatric disorders.
The blood carries many neuroactive molecules, including growth factors and cytokines, that are normally BBB-impermeable. At the same time, the BBB specifically facilitates passage of nutrients in, and waste products out, of the parenchyma. Changes in BBB permeability, therefore, stand to have considerable effects on neural function, as described above. Moreover, the nature of this effect will be critically dependent on the spatiotemporal dynamics of BBB permeability.
Future directions
It has long been appreciated that maintenance of CNS homeostasis is highly dependent on the tight restriction of the entry of molecules from the circulation. However, although generally increased barrier permeability correlates with various pathologies, the healthy BBB is not a monolithic passive barrier. Rather, transit of various molecules through the BBB occurs in specific regions of the brain and during certain periods of development. It will be interesting to learn the functional importance of these modulations of barrier function. For instance, EC TJs are functional throughout CNS angiogenesis, but transcellular leakage is repressed only later; what role, then, if any, does the transcellular leakage have during this period? And more generally, are vascular proliferation and BBB function necessarily antagonistic processes?
Besides the examples discussed here, there are several other emerging lines of evidence suggesting modulation of BBB permeability throughout life. For example, sleep and circadian mechanisms seem to regulate barrier permeability, at least in invertebrate models150,151, and it will be very interesting to see if and how these findings apply to mammals. However, when BBB permeability correlates with neural activity, it can be unclear whether BBB opening causally changes neural activity or vice versa, or whether there is just a correlation. Furthermore, BBB permeability and blood flow can feed back onto one another, particularly in ageing. The development of sensitive real-time assays of BBB permeability 152 to accompany real-time imaging and stimulation of neural activity may help shed light on this relationship.
Neurovascular coupling
The ability of neural activity to increase local cerebral blood flow — that is, NVC — has been recognized for more than a century153. In vivo studies have demonstrated that NVC is rapid, with changes in blood flow occurring less than a second following neural activity second154–156, and that the vessel responses can occur hundreds of microns to millimeters away from the centre of neural activation154,155. Despite decades of investigation, the mechanisms underlying NVC and the extent of spatiotemporal correlation between dynamics of neural activity and vascular responses have remained poorly understood. Recent advances have provided insights into the complexity of NVC, and its involvement of coordinated crosstalk among neurons, astrocytes, mural cells, and ECs154,157,158. Here, we highlight the studies that have informed our understanding of the cellular and molecular mechanisms underlying NVC as well as some experimental caveats (Box 3).
Box 3 |. Four experimental caveats of studying neurovascular coupling.
Although mechanisms underlying neurovascular coupling (NVC) has been intensely investigated, there have been conflicting results, which may be attributed to four experimental caveats. Here, we contextualize these caveats by highlighting studies investigating the role of astrocytic calcium in NVC.
Ex vivo versus in vivo
Many past studies used ex vivo preparations, including acute brain slices and isolated vessels, to study NVC237. However, such preparations do not account for many of the physiological dimensions of NVC, as they lack the vascular tone associated with the blood flow and pressure in an intact brain, neural and vascular connections are severed, and tissue is immersed in an artificial composition of nutrients for viability. Cumulatively, these conditions can deviate haemodynamic responses from those observed in vivo. For example, calcium signaling via Itpr2 was shown to be required for arteriolar dilation upon glutamate stimulation in brain slices238, but in vivo imaging in Itpr2-null mice found that, following sensory stimulation, arteriolar dilation persisted, even though increases in astrocytic calcium were abolished191–193. Thus, Itpr2 is dispensable for NVC in vivo.
Anaesthesia versus awake
As some experimental manipulations in studies of NVC in vivo are invasive, anaesthetics are often used. The mechanisms of many anaesthetics are poorly understood but can affect neural239, astrocytic240 and vascular functions241,242, and different anaesthetics have different effects on NVC243–245. Furthermore, anaesthetized and awake subjects display different NVC responses245–247. Sensory-evoked NVC responses in awake mice are larger and faster than those in anaesthetized mice248. Anaesthesia may explain the confounding kinetics of astrocytic calcium and vasodilation, as increases in astrocytic calcium preceded vasodilation in anaesthetized mice whereas the reverse was true in awake mice156,186. Thus, anaesthesia could substantially confound interpretations of NVC studies, and future studies should confirm findings in awake subjects.
Artificial versus natural stimuli
Many past studies used artificial stimuli to evoke NVC, including calcium uncaging, implanted electrode stimulation and pharmacological agents186,237. However, it is imperative to assess whether these artificial stimuli are reflective of normal physiology, especially when their use produces conflicting conclusions. For example, in anaesthetized mice, light-mediated calcium uncaging in astrocytes induces robust vasodilation186, whereas increasing astrocytic calcium through the use of Gq-DREADDs (Gq-associated designer receptors exclusively activated by designer drugs)249 did not evoke vasodilation191. Moreover, vasodilation evoked by a physiological stimulus preceded increases in astrocytic calcium in anaesthetized191 and awake mice156. Although artificial stimuli are invaluable and necessary to obtain a mechanistic understanding of NVC, cross-examinations with natural stimuli are needed too.
Pharmacology versus genetics
Past studies have used pharmacology to examine the molecular mechanisms underlying NVC. Although researchers can use pharmacology for acute perturbations, it often lacks molecular and cellular specificity. Furthermore, owing to the BBB, delivery and bioavailability of drugs are challenging in vivo. These caveats could explain why conclusions using pharmacology conflict with some results using genetics. For example, astrocytes were suggested to synthesize prostaglandins via cyclooxygenase 1 (COX1) to trigger vasodilation following increases in intracellular calcium, because antagonizing COX1 using SC-560 impaired vasodilation following a stimulus186. However, sensory-evoked NVC was reported to be unaffected in anaesthetized COX1-null mice190. Instead, genetically ablating or pharmacologically antagonizing COX2 results in impaired sensory-evoked NVC173,179. Although SC-560 inhibits COX1 less potently than it does COX2250, the studies that used this compound may have used a sufficiently high concentration to also block COX2. Brain RNA-sequencing studies identified that the gene encoding COX1 is expressed at low levels in astrocytes but robustly expressed in interneurons and microglia4,168, which could confound the alleged contribution of astrocytes in NVC186. Although pharmacology is invaluable for understanding NVC mechanisms, future studies should verify findings using genetics.
What executes neurovascular coupling?
In the brain’s vascular network, pial arteries descend into the brain parenchyma, narrow and branch into penetrating arterioles and eventually branch into a dense network of capillaries159. Interestingly, arteries and arterioles constitute only a small fraction of the entire brain vasculature and can, themselves, be far away from active neurons. Nevertheless, they are the only vascular segments ensheathed by contractile SMCs, which canonically have ability to regulate cerebral blood flow155,160–162. There is also evidence that pericytes can be contractile and control blood flow by regulating capillary diameter163–165. Notably, compared with aged wild-type controls, aged, moderately pericyte-deficient mice exhibit reduced capillary vasomotion in response to stimulus without reductions in neural activity. These mice also display BBB leakage and increased cerebral hypoxia164. Similar blood-flow deficits are seen in moderately pericyte-deficient mice before these animals display other, more overt pathologies166. However, the contribution to NVC of pericyte-mediated regulation of capillary diameter has been controversial, and recent studies have challenged this model, reporting that capillaries fail to dilate upon neural activity161,162.
Part of this controversy may stem from inconsistent criteria used to distinguish SMCs from pericytes, and arterioles from capillaries. Classically these criteria have been morphological, including vessel size, branch order in the vascular tree, or shape of the mural cells covering the vessel161,167. Unfortunately all these distinctions become imprecise at precapillary arterioles161. A molecular definition of capillaries and arterioles (and therefore pericytes and aSMCs) may stand to alleviate some confusion.
In fact, studies using unbiased single-cell RNA sequencing have already adopted the presence and absence of SMA (Acta2) expression to cluster brain SMCs and pericytes, respectively4,168. Notably, prior work shows cells that with canonical SMC morphology show positive staining for SMA while canonical pericytes do not167. Furthermore, transcriptomic studies have also identified other potentially unique molecular markers to further define the various mural cells, such as Tagln and Myh11 for SMCs and Abcc9 and Kcnj8 for pericytes (Table 1). Going forward, these may be preferable to NG2 or PDGFRβ, which have been used to identify pericytes but are also expressed by SMCs163,164,169. Table 1 also highlights two reagents, Hydrazide155,160 and NeuroTrace 525162, which have been serendipitously shown to label large arteries and pericytes, respectively. Importantly, they can be used in live animals, presenting an attractive in vivo avenue to accurate identification of arteries and capillaries.
Using molecular criteria to define mural cells, Hill et al. examined the precapillary arteriolar vessels and showed that, although some morphologically hybrid mural cells express SMA, the adjacent downstream mural cells can lack SMA despite being on the same vascular branch161,162. Furthermore, SMA+ mural cells are also found on vessels with diameters as small as 3 μm and on fourth-order vascular branches downstream of pial arteries161,162 — morphological criteria that Hall et al. used to classify pericytes and capillaries163. Using in vivo two-photon imaging of sensory-evoked neural activity, Hill et al. found that, regardless of morphological criteria such as cell shape, vessel diameter or vessel branch order, only SMA+ mural cells dilate, whereas SMA− mural cells failed to display vasomotion161.
As described in the BBB section, intersectional genetic strategies can be used to specifically target pericytes76. Using such an approach, Nikolakopoulou et al. showed that specific ablation of ~60% of pericytes results in decreased baseline cerebral blood flow. In follow-up work, Kisler et al. found that these mice with acute pericyte reduction have reduced capillary vasomotion but not reduced arteriole vasomotion165. The extent of the role of pericytes in acute vasomotion, the sensing of neural activity, signal propagation and the fine-tuning of vascular tone should be further investigated with consistent, molecularly defined studies.
Finally, although the field has generally used mural cells to categorize the various vascular segments, it has comparatively ignored ECs despite the fact that ECs undergo distinct genetic programmes for arterial–venous and capillary differentiation, which influences the differential mural-cell recruitment to arteries, capillaries and veins170. Consequently, ECs from different vascular segments also display different functions and transcriptomes.
Molecular and cellular mechanisms
NVC begins with increased neural activation that eventually induces vasodilation and increases blood flow. Although the signals that induce NVC have been investigated, broad questions remain unresolved. Can any activated neuron induce vasodilation, or are there specific subtypes of neurons that mediate NVC? Is NVC regulated by general or specific neural signals? Do neurons directly signal to SMCs, or do they signal to other cell types that then communicate to SMCs? Here, we review and discuss the evidence that helps to address these questions.
Which neurons induce neurovascular coupling?
Given that activation of excitatory neurons in the cortex elicits an increase in net neural activity, many studies have investigated the contribution of excitatory neurons to NVC. Optogenetics and chemogenetics have enabled specific activation of excitatory neurons, confirming that their activation causes increased local blood flow. For example, activating channelrhodopsin expressed in excitatory neurons specifically (for example, in CaMK2+ or Emx1+ neurons) increases local blood flow in anaesthetized rodents171–173.
Interestingly, inhibitory neurons tend to closely associate with blood vessels174. Specific activation of inhibitory neurons in the cortex (by targeting Vgat+ neurons) using optogenetics also increased blood flow in anaesthetized and awake mice, despite causing a net decrease in neural activity175,176. Similarly, chemogenetic stimulation of parvalbumin-expressing neurons in the dentate gyrus leads to hyperaemia132.
These findings highlight two interesting observations: that activation of both excitatory and inhibitory neurons can induce NVC and that NVC can occur despite a net decrease in overall neural activity. However, it remains undetermined if all or specific subtypes of excitatory and inhibitory neurons can induce NVC177. Single-cell RNA sequencing has revealed that there are at least 56 glutamatergic and 61 GABAergic subtypes of neuron in the mouse cortex168. Thus, future studies should determine if specific subtypes of neurons and circuits differentially contribute to NVC.
What are the neural signals that induce neurovascular coupling?
By understanding the specific cellular players that mediate NVC, we can pinpoint the molecules released by neurons that can directly or indirectly communicate to arterioles to induce NVC. This will address whether NVC is induced by common signals released by all neurons or by specific neurotransmitters, neuropeptides, and neuromodulators that are unique to specific subtypes of neurons. In support of the former hypothesis, studies have reported that signals released by all activated neurons, such as K+, can induce vasodilation both ex vivo and in vivo178. Given that all neurons release K+ during action potentials, these findings suggest that any neuron firing action potentials can elicit vasodilation. Although it is possible that increased extracellular K+ elevates the excitability of specific neurons to then release other, vasoactive factors, increased extracellular K+ was found to be sufficient to directly dilate isolated brain arterioles ex vivo178.
By contrast, many studies have reported that specific neural signals induce NVC. Excitatory neurons express cyclooxygenase 2 (COX2) to generate prostaglandin E2, which putatively binds to EP2 receptors (encoded by Ptger2) and EP4 receptors (encoded by Ptger4) on SMCs, causing them to relax173. Consistent with this, single-cell RNA-sequencing studies demonstrate that COX2 is expressed in excitatory neurons4,168, and COX2-knockout mice have impaired NVC in response to sensory-evoked neural activity179.
In addition, specific neurotransmitters, neuropeptides and neuromodulators, including vasoactive intestinal peptide (VIP), neuropeptide Y (NPY), somatostatin (SST) and nitric oxide synthase 1 (NOS1), each of which define various subtypes of inhibitory interneurons, have been implicated in modulating NVC ex vivo. Notably, however, in vivo evidence is still largely lacking. Bath application of VIP increases vasodilation in ex vivo brain slices, whereas NPY causes vasoconstriction180–182. However, Vip-knockout mice have not been studied to assess for defects in NVC. Similarly, although NPY has been implicated in vasoconstriction176, Npy-knockout mice have yet to be assessed for NVC deficits. Finally, there are three NOS isoforms: NOS1 (also known as neuronal NOS (nNOS)), NOS2 (also known as inducible NOS (iNOS)), and NOS3 (also known as endothelial NOS (eNOS)). Although all three enzymes use l-arginine to synthesize nitric oxide (NO), a potent vasodilator, the expression of these isoforms varies across tissues and organs.
Nos1 is expressed by multiple populations of neurons throughout the cortex168,183. Surprisingly, early work analyzing global Nos1-knockout mice found they have normal sensory-evoked NVC in the barrel cortex as assessed under anaesthesia by laser-Doppler flowmetry in vivo184. By contrast, subsequent work in the same genetic system found impaired sensory-evoked NVC in the cerebellum185. Additionally, acute short intefering RNA (siRNA)-mediated knockdown of Nos1 in the dentate gyrus results in substantially reduced NVC in awake mice132. Inducible, conditional deletion of Nos1, or additional genetic ablation of other NOS isoforms, could resolve potential compensation issues and demonstrate the role of nNOS in NVC.
Do neurons signal directly to SMCs or indirectly via other cell types?
Do excitatory and inhibitory neurons release vasodilatory signals directly onto SMCs (Fig. 3), or do these neurons first release signals to other cell types, which then release vasoactive cues to SMCs? Supporting the former scenario, excitatory pyramidal neurons express COX2 to metabolize arachidonic acid to PGE2, which is released onto SMCs to target EP2 and EP4 receptors to promote relaxation173. However, both EP2 and EP4 receptors are undetectable in SMCs by single-cell RNA sequencing4,168 but are expressed in GABAergic interneurons4,168. Thus, excitatory neurons might release PGE2 that binds to EP2 receptors or EP4 receptors on interneurons, which then release vasoactive cues onto SMCs.
Fig. 3 |. The direct pathway to elicit neurovascular coupling.
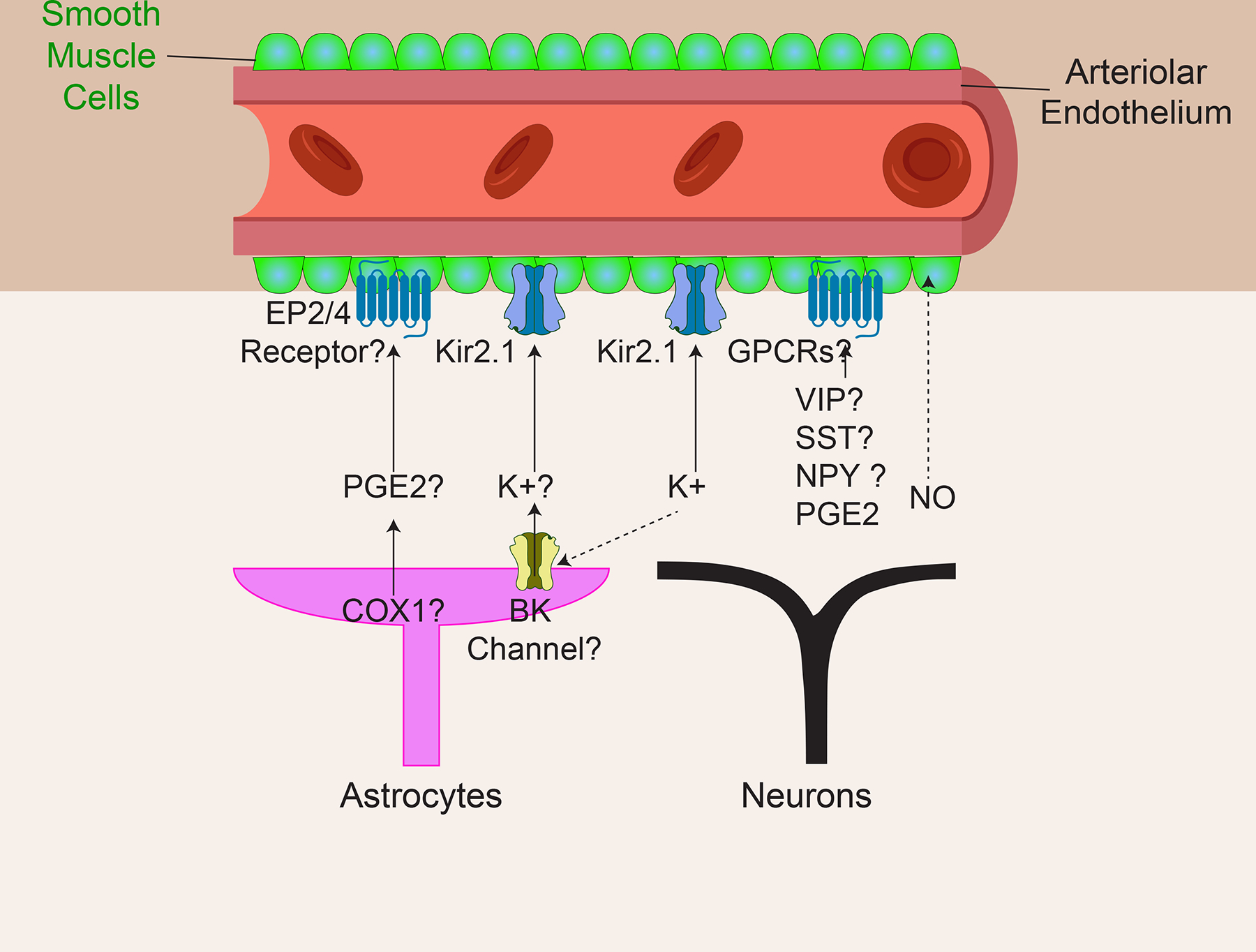
Brain cells, including excitatory neurons, interneurons and astrocytes, can release vasoactive compounds directly onto the smooth muscle cells (SMCs) located near the surface of the brain. Potential neural signals, which may include prostaglandin E₂ (PGE2), K+ ions, vasoactive intestinal peptide (VIP), somatostatin (SST) and nitric oxide (NO), may act on or travel through putative receptors or channels on SMCs, including the prostaglandin EP2 and EP4 receptors, the inward-rectifier potassium ion channel 2.1 (Kir2.1) and G protein-coupled receptors (GPCRs). ‘?’ indicates some confounding evidence, as described in the text. Dotted arrows indicate hypothesized modes of action.
Analogously, certain subtypes of interneurons have been reported to secrete vasoactive compounds directly onto SMCs158,182. In particular, the role of VIP in NVC in vivo remains unclear. Specifically, the receptors for VIP — VPAC1 and VPAC2 — are expressed only at low levels in SMCs and are instead mainly found in excitatory neurons4,168. Cell-type-specific deletion of these receptors in SMCs will help elucidate the role of VIP signaling in NVC in vivo.
A recent study found that interneurons release NPY to enhance the vasocontraction phase of NVC in vivo176. Pharmacological antagonism of NPY type 1 receptor (NPY1R; encoded by Npy1r) attenuated the magnitude of the contraction176. Although single-cell RNA sequencing demonstrates that Npy1r is expressed by SMCs, it is also expressed by excitatory and inhibitory neurons4,168. Thus, pharmacological approaches may antagonize NPY1Rs on neurons and indirectly impair NVC. A cell-type-specific deletion of Npy1r would determine the role of NPY signaling in SMCs.
Many studies have reported that neurons first signal to astrocytes, which then signal to SMCs to mediate NVC. Excitatory pyramidal neurons release glutamate, which binds to metabotropic glutamate receptors 1 and 5 (mGluR1 and mGluR5) on astrocytes. This leads to the opening of inositol triphosphate receptors (IP3R) on the endoplasmic reticulum to elevate intracellular calcium in astrocytes, activating COX1 to metabolize arachidonic acid to PGE2186 and opening BK channels (encoded by Kcnma1) to release K+ — which hyperpolarizes SMCs187, triggering NVC. However, the role of these astrocytic molecular players in triggering NVC has been controversial. First, mGluR1 and mGluR5 were claimed to be essential for NVC, since blocking them impairs NVC as assessed by laser-Doppler flowmetry in vivo188. But a later study demonstrated that astrocytes in adult rodents lack these receptors189. Next, the requirement for COX-1 in NVC has been questioned, as COX1-null mice have normal NVC as assessed by laser-Doppler flowmetry190. Furthermore, the role of astrocytic calcium in NVC has also been controversial. Itpr2 encodes the predominant IP3R in astrocytes, and three studies showed that although Itpr2-null mice have abolished calcium release in astrocytes, their arteriolar dilation upon sensory-evoked neural activity remains intact in vivo191–193. Last, although astrocytes express the BK channel, its role in NVC is unclear, as knocking out Kcnma1 does not impair NVC upon sensory-evoked neural activity194.
Moreover, there are also temporal discrepancies between the increase in astrocytic calcium and arteriolar dilation. Although many studies found that increased astrocytic calcium precedes arteriolar dilation186,188, a recent study showed that the onset of astrocytic calcium release occurs after, and is in fact triggered by, arteriolar dilation156. Collectively, these studies do not provide clear conclusions about the role of astrocytes in NVC. However, it is still possible that astrocytes may signal to SMCs via other mechanisms independent of calcium and the molecular players discussed here to mediate NVC.
How is neurovascular coupling sensed?
Previous NVC models postulated that neurons and astrocytes release vasodilatory factors onto the SMCs of penetrating arterioles, causing them to relax and dilate195. However, changes in neural activity occur deep in the brain parenchyma within capillary beds, whereas SMCs only surround upstream arterioles, up to 200 microns away. It is unlikely that locally generated factors, such as NO, can diffuse over this long distance in a few hundred milliseconds to elicit a vasodilatory response in such a short timeframe154. Moreover, such a diffusive mechanism would stand to lose the spatial specificity of vasodilation. Thus, the previous models did not account for the spatiotemporal realities of NVC dictated by the anatomy of the vascular network.
Recent evidence suggests ECs play a pivotal role in NVC196, with capillaries in deeper cortical layers sensing neural activity (Fig. 4). Capillary ECs (cECs) are ideal for sensing neural activity because they are deep in the brain and close to all neurons1. One study found that brain cECs express the potassium channel Kir2.1 and therefore can sense increases in extracellular potassium generated during neural activity; indeed, mice lacking Kir2.1 in ECs showed attenuated NVC178. Other studies have also reported that brain ECs express neurotransmitter receptors, including those for glutamate124 and GABA197. Conditional genetic ablation of the Grin1-encoded subunit of the NMDA receptor (NMDAR) in ECs attenuates sensory-evoked NVC198. Although conditional genetic ablation of GABA type A receptors (GABAARs) in ECs impairs brain vascularization and interneuron migration and leads to behavioural defects, NVC effects have not been examined199. Nevertheless, collectively, these studies support the notion that ECs are the ideal sensors of neural activity.
Fig. 4 |. The endothelium-dependent model of neurovascular coupling.
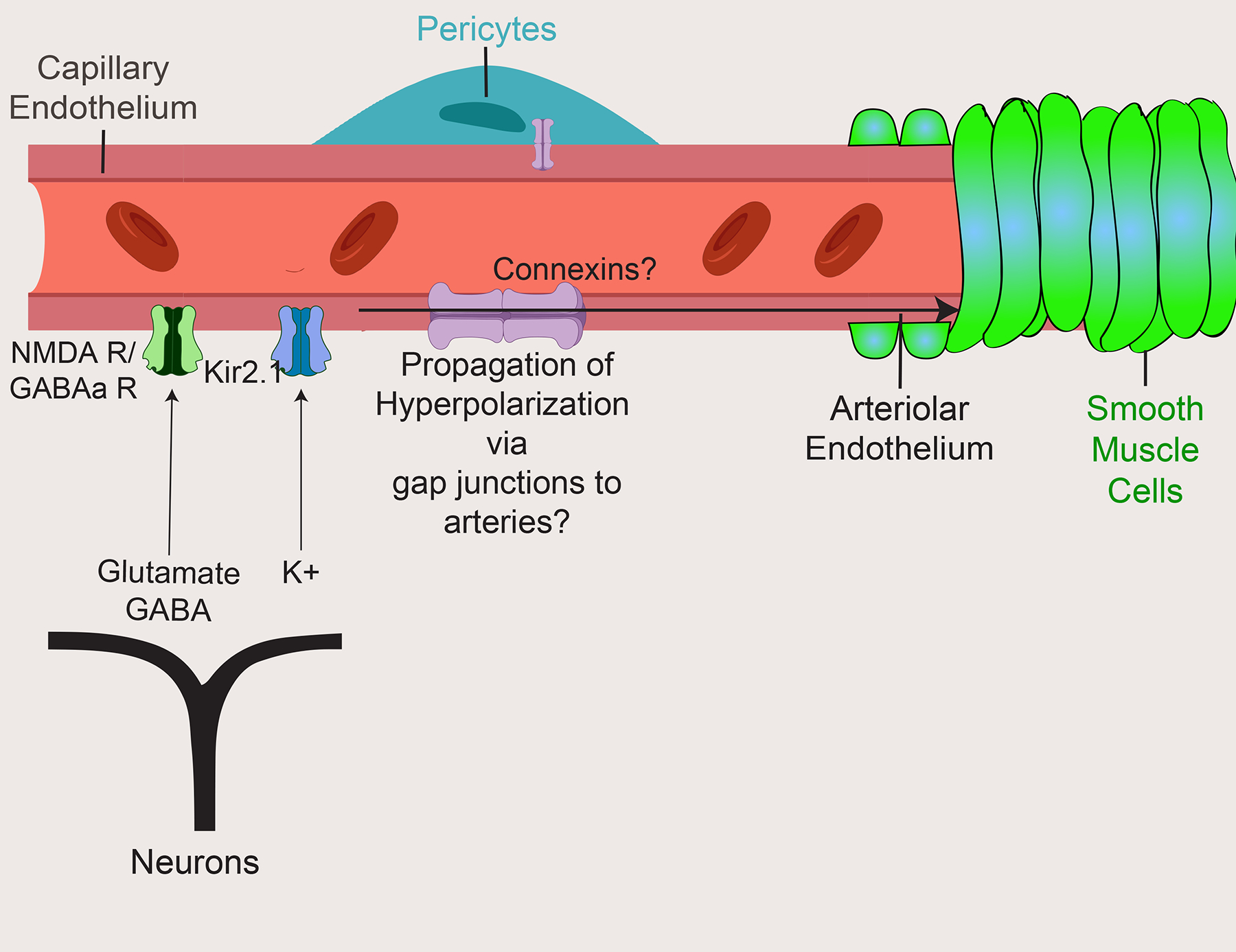
Most brain cells are juxtaposed to capillaries and can release neural signals, including glutamate, GABA and K+, to capillary endothelial cells, which express the corresponding neurotransmitter receptor and ion channels such as NMDA receptors (NMDARs) and GABA type A receptors (GABAARs) as well as the inward-rectifier potassium ion channel 2.1 (Kir2.1). The occurrence of neural activity may be signaled via a retrograde propagation of hyperpolarization through putative gap junctions between capillary endothelial cells to upstream arteries, to dilate and increase blood flow. ‘?’ indicates some confounding evidence as described in the text.
How is neurovascular coupling propagated?
In addition to acting as sensors of neural activity, ECs have been proposed to ‘retrogradely’ propagate this information up the vascular tree to dilate upstream arterioles, via electrical coupling155,178,196. As the spread of electrical signals across ECs is fast and can traverse long distances, this model fits the spatiotemporal constraints inherent to NVC. Indeed, peripheral ECs are electrically coupled via gap junctions and can rapidly conduct signals between ECs. These waves of ions, including potassium and calcium, trigger EC release of vasodilatory factors onto SMCs200. A similar mechanism may exist in the brain vasculature, as micropipette application of 6–10 mM potassium to capillaries generated robust hyperpolarization in ECs that was transmitted retrogradely to penetrating arterioles at the estimated speed of 2 millimetres per second178. Many have speculated that, like peripheral ECs, the brain ECs are also coupled by gap junctions, allowing for the spread of electrical hyperpolarization up the vascular tree from capillaries to arteries. However, the evidence for gap-junction coupling in brain ECs has been scarce. Most vascular gap junction studies have been performed in the peripheral vasculature201–205 but very few studies have examined the role of gap junctions in the brain vasculature206, especially in the context of NVC in vivo. Furthermore, studies examining the role of gap junctions in the cerebral vasomotion mostly used putative gap junction blockers207,208, which have been repeatedly demonstrated to have non-gap-junction-related effects209,210. These effects include blocking ion channels such as GABAARs211 and, more pertinently, endothelial small- and intermediate-conductance calcium-activated K+ channels (IK and SK channels), which are implicated in NVC212. Thus, the role of gap junctions, and the identities of the connexins that form them, in NVC remain unknown. Given the advent of new genetic and imaging tools, it is finally possible to study the effect of gap junctions on NVC using cell-type-specific deletion of their various connexin components.
How does the endothelium communicate to SMCs to mediate NVU?
Once the electrical conductance travels from the capillary endothelium to the arterial endothelium, how do arterial endothelial cells then communicate to the underlying SMCs to mediate NVC? Interestingly, a recent study demonstrate that the arterial endothelial cells actively mediate NVC through cell-type-specific mechanisms213. Unlike capillary endothelium, the arterial endothelium lacks MFSD2A, and aECs have abundant caveolae, which relay signals to SMCs to mediate arterial dilation213. Indeed, arterial endothelium-specific ablation of caveolae impairs vasodilation during NVC. Furthermore, the caveolae-mediated pathway is independent of the eNOS-mediated pathway, as ablation of both caveolae and eNOS completely abolished NVC, whereas single genetic ablation of Cav1 or Nos3 resulted in partial impairment, demonstrating that a caveolae-mediated pathway in the arterial endothelium is a major contributor to NVC213. Future work should investigate the mechanism by which caveolae facilitate signaling to SMCs to promote dilation.
Future directions
NVC is a complex process involving the coordination of multiple cells and feedback cycles. The cellular players and molecular determinants involved in NVC have been intensely investigated. As highlighted in this Review, many NVC studies have resulted in incomplete conclusions. We believe that with the development of novel technologies to investigate molecular and cellular mechanisms in vivo, the field should revisit the old dogmas of NVC. These will lead to substantial breakthroughs in our understanding of the sequence of events underlying the mechanisms of NVC. Insights gleaned from future mechanistic NVC studies could also facilitate the development of novel therapeutics to enhance cerebral blood flow in disease, as well as better interpretation of the BOLD signal that is crucial for human brain-imaging studies.
Conclusions
The work discussed in this Review demonstrates the tight interplay between neural activity and its vasculature. As many neuropathologies and ageing are associated with dysfunction of both the BBB and NVC, a clearer mechanistic understanding of this interplay in health and disease will be crucial for the development of new therapies.
The complexity of the NVU has historically made studying the physiology of the BBB and NVC daunting. The NVU has considerable heterogeneity and zonation, and is composed of several highly related but functionally distinct cell types. Fortunately, recent technical advances have helped push past these hurdles and stand to clarify mechanistic questions in BBB regulation and NVC. For instance, single-cell sequencing and multiplexed fluorescent in situ hybridization (FISH) has helped to distinguish which cell types express molecules important for BBB function and NVC. This, in combination with cell-type-specific gain-of-function and loss-of-function genetics, is a powerful tool for elucidating mechanisms. Additionally, the increasingly large optogenetic, chemogenetic and biosensor toolkits provides means of acutely controlling and detecting activity in live animals. These approaches stand to answer many long-held questions in the BBB and NVC fields.
Acknowledgements
The authors thank all the Gu Lab members for reading and providing feedback on the Review. This work was supported by a Quan Fellowship (B.W.C.), a Mahoney Postdoctoral Fellowship (L.K.), a US National Institutes of Health DP1 NS092473 Pioneer Award (C.G.), and Fidelity Biosciences Research Initiative (C.G.). The research of C.G. was also supported in part by a Faculty Scholar grant from the Howard Hughes Medical Institute.
Glossary terms:
- Blood–brain barrier
A physiological barrier formed by CNS endothelial cells to regulate the trafficking of molecules between the blood and the brain
- Neurovascular coupling
The process by which local neural activation can rapidly increase local blood flow; it is the basis of functional MRI
- Mural cell
A collective term to describe the cell types that wrap around blood vessels, including the smooth muscle cells on arteries and pericytes on capillaries
- Diphtheria toxin
A toxin that inhibits protein synthesis, leading to cell death
- Angiogenesis
Growth of new blood vessels from existing blood vessels
- Circumventricular organs
(CVOs). Midline brain structures with permeable vasculature allowing for ready exchange of molecules between neurons and the blood
- Polyinosinic:polycytidylic acid
(Poly(I:C)). Synthetic mimic of double-stranded RNA mimicking the effect of viral infection on the immune system
- Caveolae
Flask-shaped vesicular structures formed by caveolins approximately 70nm in diameter
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Tsai PS et al. Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of nuclei and vessels. J. Neurosci 29, 14553–14570 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehrlich P Das Sauerstoff-Bedürfniss des Organismus: eine farbenanalytische Studie. Hirschwald, Berlin (1885). doi: 10.1038/nbt0797-647 [DOI] [Google Scholar]
- 3.York PE-R and T. WN & 1906, undefined. Ueber die beziehungen von chemischer constitution, vertheilung, und pharmakologischen wirkung. Collected Studies on Immunity [Google Scholar]
- 4.Vanlandewijck M et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475–480 (2018). [DOI] [PubMed] [Google Scholar]; This study provided a single cell transcriptomic analysis of the BBB vasculature with direct comparison to peripheral vasculature.
- 5.Simionescu M, Simionescu N & Palade GE Segmental differentiations of cell junctions in the vascular endothelium: Arteries and veins. J. Cell Biol 68, 705–723 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sweeney MD, Sagare AP & Zlokovic BV Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol 14, 133–150 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drew PJ, Shih AY & Kleinfeld D Fluctuating and sensory-induced vasodynamics in rodent cortex extend arteriole capacity. Proc. Natl. Acad. Sci 108, 8473–8478 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow BW & Gu C The Molecular Constituents of the Blood-Brain Barrier. Trends Neurosci 38, 598–608 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Z, Nelson AR, Betsholtz C & Zlokovic BV Establishment and Dysfunction of the Blood-Brain Barrier. Cell 163, 1064–1078 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langen UH, Ayloo S & Gu C Development and Cell Biology of the Blood-Brain Barrier. Annu. Rev. Cell Dev. Biol 35, annurev-cellbio-100617–062608 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y et al. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J. Neurosci (2014). doi: 10.1523/jneurosci.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cahoy JD et al. A Transcriptome Database for Astrocytes, Neurons, and Oligodendrocytes: A New Resource for Understanding Brain Development and Function. J. Neurosci 28, 264–278 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaum N et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562, 367–372 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabbagh MF & Nathans J A genome-wide view of the de-differentiation of central nervous system endothelial cells in culture. Elife 9, 1–19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y et al. Beta-catenin signaling regulates barrier-specific gene expression in circumventricular organ and ocular vasculatures. Elife 1–36 (2019). doi: 10.7554/eLife.43257 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that β-catenin gain-of-function conveys barrier properties to the normally leaky vasculature of CVOs.
- 16.Sabbagh MF et al. Transcriptional and epigenomic landscapes of CNS and non-CNS vascular endothelial cells. Elife 1–44 (2018). doi: 10.7554/eLife.36187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munji RN et al. Profiling the mouse brain endothelial transcriptome in health and disease models reveals a core blood–brain barrier dysfunction module. Nat. Neurosci (2019). doi: 10.1038/s41593-019-0497-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daneman R et al. The mouse blood-brain barrier transcriptome: A new resource for understanding the development and function of brain endothelial cells. PLoS One 5, 1–16 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yousef H et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat. Med 25, 988–1000 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Profaci CP, Munji RN, Pulido RS & Daneman R The blood–brain barrier in health and disease: Important unanswered questions. J. Exp. Med 217, 1–16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reese TS & Karnovsky MJ Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol 34, 207–217 (1967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karnovsky MJ The ultrastructural basis of capillary permeability studied with peroxidase as a tracer. J. Cell Biol 35, 213–236 (1967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daneman R, Zhou L, Kebede AA & Barres BA Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that inhibition of pericyte recruitment in development results in BBB dysfunction proportional to the pericyte reduction.
- 24.Armulik A et al. Pericytes regulate the blood-brain barrier. Nature 468, 557–561 (2010). [DOI] [PubMed] [Google Scholar]; This study showed that inhibition of pericyte recruitment during development results in aberrant transcytosis at the BBB throughout life.
- 25.Ben-zvi A et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509, 507–511 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andreone BJ et al. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 94, 1–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that Mfsd2a’s lipid transport function suppresses caveolae-mediated transcytosis at the BBB.
- 27.Ayloo S & Gu C Transcytosis at the blood–brain barrier. Curr. Opin. Neurobiol 57, 32–38 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schinkel AH et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 77, 491–502 (1994). [DOI] [PubMed] [Google Scholar]
- 29.Alvarez JI, Katayama T & Prat A Glial influence on the blood brain barrier. Glia 61, 1939–1958 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Brown NM, Pfau SJ & Gu C Bridging barriers: A comparative look at the blood-brain barrier across organisms. Genes Dev 32, 466–478 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siegenthaler JA, Sohet F & Daneman R ‘Sealing off the CNS’: Cellular and molecular regulation of blood-brain barriergenesis. Curr. Opin. Neurobiol 23, 1057–1064 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanchette M & Daneman R Formation and maintenance of the BBB. Mech. Dev 138, 8–16 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Brightman MW & Reese TS Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol 40, 648–677 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simionescu M, Simionescu N & Palade GE Characteristic endothelial junctions in different segments of the vascular system. Thromb. Res 8, 247–256 (1976). [DOI] [PubMed] [Google Scholar]
- 35.Liebner S, Kniesel U, Kalbacher H & Wolburg H Correlation of tight junction morphology with the expression of tight junction proteins in blood-brain barrier endothelial cells. Eur. J. Cell Biol 79, 707–717 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Stamatovic SM, Johnson AM, Keep RF & Andjelkovic AV Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers 4, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murakami T, Felinski EA & Antonetti DA Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J. Biol. Chem 284, 21036–21046 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishizaki T et al. Cyclic AMP induces phosphorylation of claudin-5 immunoprecipitates and expression of claudin-5 gene in blood-brain-barrier endothelial cells via protein kinase A-dependent and -independent pathways. Exp. Cell Res 290, 275–288 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Dörfel MJ & Huber O Modulation of Tight Junction Structure and Function by Kinases and Phosphatases Targeting Occludin. J. Biomed. Biotechnol 2012, 1–14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Itallie CM, Gambling TM, Carson JL & Anderson JM Palmitoylation of claudins is required for efficient tight-junction localization. J. Cell Sci 118, 1427–1436 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Saitou M et al. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J. Cell Biol 141, 397–408 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saitou M et al. Complex Phenotype of Mice Lacking Occludin, a Component of Tight Junction Strands. Mol. Biol. Cell 11, 4131–4142 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Umeda K et al. ZO-1 and ZO-2 Independently Determine Where Claudins Are Polymerized in Tight-Junction Strand Formation. Cell 126, 741–754 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Katsuno T et al. Deficiency of Zonula Occludens-1 Causes Embryonic Lethal Phenotype Associated with Defected Yolk Sac Angiogenesis and Apoptosis of Embryonic Cells. Mol. Biol. Cell 19, 2465–2475 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu J et al. Early Embryonic Lethality of Mice Lacking ZO-2, but Not ZO-3, Reveals Critical and Nonredundant Roles for Individual Zonula Occludens Proteins in Mammalian Development. Mol. Cell. Biol 28, 1669–1678 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castro Dias M et al. Claudin-3-deficient C57BL/6J mice display intact brain barriers. Sci. Rep 9, 1–16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castro Dias M et al. Claudin-12 is not required for blood–brain barrier tight junction function. Fluids Barriers CNS 16, 30 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nitta T et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol 161, 653–660 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that knockout of the principle tight junction component at the BBB results in BBB permeability to small molecular weight molecules.
- 49.Sohet F et al. LSR/angulin-1 is a tricellular tight junction protein involved in blood-brain barrier formation. J. Cell Biol 208, 703–711 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding L et al. Inflammation and disruption of the mucosal architecture in claudin-7-deficient mice. Gastroenterology 142, 305–315 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu Z et al. A non-tight junction function of claudin-7-Interaction with integrin signaling in suppressing lung cancer cell proliferation and detachment. Mol. Cancer 14, 1–15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huerta M et al. Cyclin D1 Is Transcriptionally Down-Regulated by ZO-2 via an E Box and the Transcription Factor c-Myc. Mol. Biol. Cell 18, 4826–4836 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sourisseau T et al. Regulation of PCNA and Cyclin D1 Expression and Epithelial Morphogenesis by the ZO-1-Regulated Transcription Factor ZONAB/DbpA. Mol. Cell. Biol 26, 2387–2398 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stamatovic SM, Sladojevic N, Keep RF & Andjelkovic AV Relocalization of Junctional Adhesion Molecule A during Inflammatory Stimulation of Brain Endothelial Cells. Mol. Cell. Biol 32, 3414–3427 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.TUMA PL & HUBBARD AL Transcytosis: Crossing Cellular Barriers. Physiol. Rev 83, 871–932 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Mayor S, Parton RG & Donaldson JG Clathrin-independent pathways of endocytosis. Cold Spring Harb. Perspect. Biol 6, 1–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villaseñor R, Lampe J, Schwaninger M & Collin L Intracellular transport and regulation of transcytosis across the blood–brain barrier. Cell. Mol. Life Sci (2018). doi: 10.1007/s00018-018-2982-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naslavsky N & Caplan S The enigmatic endosome – sorting the ins and outs of endocytic trafficking. J. Cell Sci 131, jcs216499 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parton RG Caveolae : Structure, Function, and Relationship to Disease. Annu. Rev. Cell Dev. Biol 34, 111–136 (2018). [DOI] [PubMed] [Google Scholar]
- 60.De Bock M et al. Into rather unexplored terrain-transcellular transport across the blood-brain barrier. Glia 64, 1097–1123 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Preston JE, Joan Abbott N & Begley DJ Transcytosis of macromolecules at the blood-brain barrier. Advances in Pharmacology 71, (Elsevier Inc., 2014). [DOI] [PubMed] [Google Scholar]
- 62.Knowland D et al. Stepwise Recruitment of Transcellular and Paracellular Pathways Underlies Blood-Brain Barrier Breakdown in Stroke. Neuron 82, 603–617 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ye X et al. Norrin, Frizzled-4, and Lrp5 Signaling in Endothelial Cells Controls a Genetic Program for Retinal Vascularization. Cell 139, 285–298 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye X, Smallwood P & Nathans J Expression of the Norrie disease gene (Ndp) in developing and adult mouse eye, ear, and brain. Gene Expr. Patterns 11, 151–155 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anbalagan S et al. Pituicyte Cues Regulate the Development of Permeable Neuro-Vascular Interfaces. Dev. Cell 47, 711–726.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Cambier S et al. Integrin αvβ8-mediated activation of transforming growth factor-β by perivascular astrocytes: An angiogenic control switch. Am. J. Pathol 166, 1883–1894 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gautam J, Miner JH & Yao Y Loss of Endothelial Laminin α5 Exacerbates Hemorrhagic Brain Injury. Transl. Stroke Res 10, 705–718 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bell RD et al. Apolipoprotein e controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park DY et al. Plastic roles of pericytes in the blood-retinal barrier. Nat. Commun 8, 1–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Janzer RC & Raff MC Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 325, 253–257 (1987). [DOI] [PubMed] [Google Scholar]
- 71.Stewart PA & Wiley MJ Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: A study using quail-chick transplantation chimeras. Dev. Biol 84, 183–192 (1981). [DOI] [PubMed] [Google Scholar]
- 72.Daneman R & Prat A The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol 7, a020412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bell RD et al. Pericytes Control Key Neurovascular Functions and Neuronal Phenotype in the Adult Brain and during Brain Aging. Neuron 68, 409–427 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that pericyte deficient mice have progressively worsening BBB function as well as degeneration of the neurovascular unit.
- 74.Villaseñor R et al. Region-specific permeability of the blood–brain barrier upon pericyte loss. J. Cereb. Blood Flow Metab 37, 3683–3694 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Winkler EA, Sengillo JD, Bell RD, Wang J & Zlokovic BV Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J. Cereb. Blood Flow Metab 32, 1841–1852 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nikolakopoulou AM et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat. Neurosci 22, 1089–1098 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a intersectional genetic approach, the authors reported to genetically target pericytes specifically and avoid manipulating SMCs.
- 77.Ruedl C & Jung S DTR-mediated conditional cell ablation—Progress and challenges. Eur. J. Immunol 48, 1114–1119 (2018). [DOI] [PubMed] [Google Scholar]
- 78.Reyahi A et al. Foxf2 Is Required for Brain Pericyte Differentiation and Development and Maintenance of the Blood-Brain Barrier. Dev. Cell 34, 19–32 (2015). [DOI] [PubMed] [Google Scholar]
- 79.Diéguez-Hurtado R et al. Loss of the transcription factor RBPJ induces disease-promoting properties in brain pericytes. Nat. Commun 10, 2817 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kutuzov N, Flyvbjerg H & Lauritzen M Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood–brain barrier. Proc. Natl. Acad. Sci 201802155 (2018). doi: 10.1073/pnas.1802155115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sofroniew MV & Vinters HV Astrocytes: Biology and pathology. Acta Neuropathologica 119, 7–35 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Freeman MR Specification and morphogenesis of astrocytes. Science (80-. ). 330, 774–778 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yao Y, Chen ZL, Norris EH & Strickland S Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat. Commun 5, 1–12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen Z-L et al. Ablation of astrocytic laminin impairs vascular smooth muscle cell function and leads to hemorrhagic stroke. J. Cell Biol 202, 381–395 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schreiner B et al. Astrocyte Depletion Impairs Redox Homeostasis and Triggers Neuronal Loss in the Adult CNS. Cell Rep 12, 1377–1384 (2015). [DOI] [PubMed] [Google Scholar]
- 86.Tsai HH et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science (80-. ). 337, 358–362 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kubotera H et al. Astrocytic endfeet re-cover blood vessels after removal by laser ablation. Sci. Rep 9, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Senatorov VV et al. Blood-brain barrier dysfunction in aging induces hyperactivation of TGFβ signaling and chronic yet reversible neural dysfunction. Sci. Transl. Med 11, eaaw8283 (2019). [DOI] [PubMed] [Google Scholar]
- 89.Alvarez JI et al. The Hedgehog Pathway Promotes Blood-Brain Barrier Integrity and CNS Immune Quiescence. Science (80-. ) 334, 1727–1732 (2011). [DOI] [PubMed] [Google Scholar]
- 90.Li F et al. Endothelial Smad4 Maintains Cerebrovascular Integrity by Activating N-Cadherin through Cooperation with Notch. Dev. Cell 20, 291–302 (2011). [DOI] [PubMed] [Google Scholar]
- 91.Mizee MR et al. Retinoic Acid Induces Blood-Brain Barrier Development. J. Neurosci 33, 1660–1671 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sweeney MD, Ayyadurai S & Zlokovic BV Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat. Neurosci 19, 771–783 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Obermeier B, Daneman R & Ransohoff RM Development, maintenance and disruption of the blood-brain barrier. Nat. Med 19, 1584–1596 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Daneman R et al. Wnt/ -catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci 106, 641–646 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liebner S et al. Wnt/β-catenin signaling controls development of the blood–brain barrier. J. Cell Biol 183, 409–417 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y et al. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell 151, 1332–1344 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou Y & Nathans J Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical Wnt signaling. Dev. Cell 31, 248–256 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen J et al. Retinal expression of Wnt-pathway mediated genes in low-density lipoprotein receptor-related protein 5 (Lrp5) knockout mice. PLoS One 7, 1–10 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Y et al. Interplay of the Norrin and Wnt7a/Wnt7b signaling systems in blood–brain barrier and blood–retina barrier development and maintenance. Proc. Natl. Acad. Sci 201813217 (2018). doi: 10.1073/pnas.1813217115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weindl A & Joynt RJ Barrier properties of the subcommissural organ. Arch. Neurol 29, 16–22 (1973). [DOI] [PubMed] [Google Scholar]
- 101.Ufnal M & Skrzypecki J Blood borne hormones in a cross-talk between peripheral and brain mechanisms regulating blood pressure, the role of circumventricular organs. Neuropeptides (2014). doi: 10.1016/j.npep.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 102.Benz F et al. Low Wnt/β-catenin signaling determines leaky vessels in the subfornical organ and affects water homeostasis in mice. Elife (2019). doi: 10.7554/eLife.43818 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that β-catenin gain-of-function conveys barrier properties to the normally leaky subfornical organ, affecting neural activity in water-restricted mice.
- 103.Yu L et al. Wnt signaling is altered by spinal cord neuronal dysfunction in amyotrophic lateral sclerosis transgenic mice. Neurochem. Res (2013). doi: 10.1007/s11064-013-1096-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Meeteren LA & ten Dijke P Regulation of endothelial cell plasticity by TGF-β. Cell Tissue Res 347, 177–186 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ten Dijke P & Arthur HM Extracellular control of TGFβ signaling in vascular development and disease. Nat. Rev. Mol. Cell Biol 8, 857–869 (2007). [DOI] [PubMed] [Google Scholar]
- 106.Lan Y et al. Essential Role of Endothelial Smad4 in Vascular Remodeling and Integrity. Mol. Cell. Biol 27, 7683–7692 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martin JS et al. Analysis of Homozygous TGFβ1 Null Mouse Embryos Demonstrates Defects in Yolk Sac. Vasculogenesis and Hematopoiesis. Ann. N. Y. Acad. Sci 752, 300–308 (1995). [DOI] [PubMed] [Google Scholar]
- 108.Hellbach N et al. Neural deletion of Tgfbr2 impairs angiogenesis through an altered secretome. Hum. Mol. Genet 23, 6177–6190 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seo JH et al. Oligodendrocyte Precursor Cells Support Blood-Brain Barrier Integrity via TGF-β Signaling. PLoS One 9, e103174 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Walshe TE et al. TGF-β is required for vascular barrier function, endothelial survival and homeostasis of the adult microvasculature. PLoS One 4, 1–16 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ferrer-Vaquer A et al. A sensitive and bright single-cell resolution live imaging reporter of Wnt/-catenin signaling in the mouse. BMC Dev. Biol 10, 121 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin AH et al. Global Analysis of Smad2/3-Dependent TGF-β Signaling in Living Mice Reveals Prominent Tissue-Specific Responses to Injury. J. Immunol 175, 547–554 (2005). [DOI] [PubMed] [Google Scholar]
- 113.Andreone BJ, Lacoste B & Gu C Neuronal and vascular interactions. Neurosci Res 38, 25–46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lacoste B & Gu C Control of cerebrovascular patterning by neural activity during postnatal development. Mech. Dev 138, 43–49 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Whiteus C, Freitas C & Grutzendler J Perturbed neural activity disrupts cerebral angiogenesis during a postnatal critical period. Nature 505, 407–411 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lacoste B et al. Sensory-Related Neural Activity Regulates the Structure of Vascular Networks in the Cerebral Cortex. Neuron 83, 1117–1130 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rosa JM et al. Neuron-glia signaling in developing retina mediated by neurotransmitter spillover. Elife 4, 1–20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Feller MB, Wellis DP, Stellwagen D, Werblin FS & Shatz CJ Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science (80-. ) 272, 1182–1187 (1996). [DOI] [PubMed] [Google Scholar]
- 119.Huberman AD, Feller MB & Chapman B Mechanisms Underlying Development of Visual Maps and Receptive Fields. Annu. Rev. Neurosci 31, 479–509 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weiner GA et al. Cholinergic neural activity directs retinal layer-specific angiogenesis and blood retinal barrier formation. Nat. Commun 10, 2477 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hrvatin S et al. Single-cell analysis of experience-dependent transcriptomic states in the mouse visual cortex. Nat. Neurosci 21, 120–129 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Augustin HG, Young Koh G, Thurston G & Alitalo K Control of vascular morphogenesis and homeostasis through the angiopoietin - Tie system. Nat. Rev. Mol. Cell Biol 10, 165–177 (2009). [DOI] [PubMed] [Google Scholar]
- 123.Vazana U et al. Glutamate-Mediated Blood-Brain Barrier Opening: Implications for Neuroprotection and Drug Delivery. J. Neurosci 36, 7727–7739 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lu L et al. Astrocytes drive cortical vasodilatory signaling by activating endothelial NMDA receptors. J. Cereb. Blood Flow Metab 39, 481–496 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.András IE et al. The NMDA and AMPA/KA Receptors are Involved in Glutamate-Induced Alterations of Occludin Expression and Phosphorylation in Brain Endothelial Cells. J. Cereb. Blood Flow Metab 27, 1431–1443 (2007). [DOI] [PubMed] [Google Scholar]
- 126.Moussawi K, Riegel A, Nair S & Kalivas PW Extracellular Glutamate: Functional Compartments Operate in Different Concentration Ranges. Front. Syst. Neurosci 5, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu JP, Baker J, Perkins AS, Robertson EJ & Efstratiadis A Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75, 59–72 (1993). [PubMed] [Google Scholar]
- 128.Beck KD, Powell-Braxtont L, Widmer HR, Valverde J & Hefti F Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron 14, 717–730 (1995). [DOI] [PubMed] [Google Scholar]
- 129.Aberg MA, Aberg ND, Hedbäcker H, Oscarsson J & Eriksson PS Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci 20, 2896–903 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Trejo JL, Carro E & Torres-Aleman I Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci 21, 1628–1634 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nishijima T et al. Neuronal Activity Drives Localized Blood-Brain-Barrier Transport of Serum Insulin-like Growth Factor-I into the CNS. Neuron 67, 834–846 (2010). [DOI] [PubMed] [Google Scholar]; This study showed that driving neural activity by either electrical or sensory stimulation leads to accumulation of IGF-1 from the circulation in the stimulated brain regions.
- 132.Shen J et al. Neurovascular Coupling in the Dentate Gyrus Regulates Adult Hippocampal Neurogenesis. Neuron 103, 878–890.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mañes S et al. The matrix metalloproteinase-9 regulates the insulin-like growth factor-triggered autocrine response in DU-145 carcinoma cells. J. Biol. Chem 274, 6935–6945 (1999). [DOI] [PubMed] [Google Scholar]
- 134.Reinhard SM, Razak K & Ethell IM A delicate balance: Role of MMP-9 in brain development and pathophysiology of neurodevelopmental disorders. Front. Cell. Neurosci 9, 1–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang SS et al. Cellular growth inhibition by IGFBP-3 and TGF-β1 requires LRP-1. FASEB J 17, 2068–2081 (2003). [DOI] [PubMed] [Google Scholar]
- 136.Zapf A, Hsu D & Olefsky JM Comparison of the intracellular itineraries of insulin-like growth factor-i and insulin and their receptors in rat-1 fibroblasts. Endocrinology (1994). doi: 10.1210/endo.134.6.8194471 [DOI] [PubMed] [Google Scholar]
- 137.Nation D et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med 25, 270–276 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tomkins O et al. Frequent blood-brain barrier disruption in the human cerebral cortex. Cell. Mol. Neurobiol 21, 675–691 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Milikovsky DZ et al. Paroxysmal slow cortical activity in Alzheimer’s disease and epilepsy is associated with blood-brain barrier dysfunction. Sci. Transl. Med 11, eaaw8954 (2019). [DOI] [PubMed] [Google Scholar]
- 140.Tărlungeanu DC et al. Impaired Amino Acid Transport at the Blood Brain Barrier Is a Cause of Autism Spectrum Disorder. Cell 167, 1481–1494.e18 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Winkler EA et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci 18, 521–530 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nguyen LN et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509, 503–506 (2014). [DOI] [PubMed] [Google Scholar]
- 143.Guemez-Gamboa A et al. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat. Genet 47, 809–813 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Alakbarzade V et al. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat. Genet 47, 814–817 (2015). [DOI] [PubMed] [Google Scholar]
- 145.Chan JP et al. The lysolipid transporter Mfsd2a regulates lipogenesis in the developing brain. PLoS Biol 1–30 (2018). doi: 10.1371/journal.pbio.2006443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Menard C et al. Social stress induces neurovascular pathology promoting depression. Nat Neurosci 20, 1752–1760 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that Claudin-5 modulates BBB permeability, leading to susceptibility to social stress.
- 147.Hodes GE et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci 111, 16136–16141 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chourbaji S et al. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol. Dis 23, 587–594 (2006). [DOI] [PubMed] [Google Scholar]
- 149.Shin Yim Y et al. Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature 549, 482–487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pan W & Kastin AJ The blood-brain barrier: Regulatory roles in wakefulness and sleep. Neuroscientist 23, 124–136 (2017). [DOI] [PubMed] [Google Scholar]
- 151.Zhang SL, Yue Z, Arnold DM, Artiushin G & Sehgal A A Circadian Clock in the Blood-Brain Barrier Regulates Xenobiotic Efflux. Cell 173, 130–139.e10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Berthiaume AA et al. Dynamic Remodeling of Pericytes In Vivo Maintains Capillary Coverage in the Adult Mouse Brain. Cell Rep 22, 8–16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sherington CS & Roy CS On the Regulation of the Blood-Supply of the Brain. J. Physiol 11, (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hillman EMC Coupling Mechanism and Significance of the BOLD Signal: A Status Report. Annu. Rev. Neurosci 37, 161–181 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.O’Herron P et al. Neural correlates of single-vessel haemodynamic responses in vivo. Nature 534, 378–382 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tran CHT, Peringod G & Gordon GR Astrocytes Integrate Behavioral State and Vascular Signals during Functional Hyperemia. Neuron 1–16 (2018). doi: 10.1016/j.neuron.2018.09.045 [DOI] [PubMed] [Google Scholar]
- 157.Kisler K, Nelson AR, Montagne A & Zlokovic BV Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nature Reviews Neuroscience 18, 419–434 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Iadecola C The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 96, 17–42 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Blinder P et al. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat. Neurosci 16, 889–897 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shen Z, Lu Z, Chhatbar PY, O’Herron P & Kara P An artery-specific fluorescent dye for studying neurovascular coupling. Nat. Methods 9, 273–276 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study serendipitously identified a dye, Hydrazide 633, that can robustly label arteries.
- 161.Hill RA et al. Regional Blood Flow in the Normal and Ischemic Brain Is Controlled by Arteriolar Smooth Muscle Cell Contractility and Not by Capillary Pericytes. Neuron 87, 95–110 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used acta2 (SMA) as a molecular marker to distinguish SMCs and pericytes and reported that only SMA+ mural cells display vasomotion.
- 162.Damisah EC, Hill RA, Tong L, Murray KN & Grutzendler J A fluoro-Nissl dye identifies pericytes as distinct vascular mural cells during in vivo brain imaging. Nat. Neurosci 20, 1023–1032 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified a dye, Neurotrace 525, that can specifically label pericytes and not SMA+ mural cell. The authors reported that Neurotrace 525+ vessels do not display vasomotion.
- 163.Hall CN et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55–60 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported that using morphological and general mural cell markers, pericytes on capillaries can dilate upon neural activation.
- 164.Kisler K et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat. Neurosci 20, 406–416 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kisler K et al. Acute Ablation of Cortical Pericytes Leads to Rapid Neurovascular Uncoupling. Front. Cell. Neurosci 14, 1–8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Montagne A et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat. Med 24, 326–337 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 167.Armulik A, Genové G & Betsholtz C Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Dev. Cell 21, 193–215 (2011). [DOI] [PubMed] [Google Scholar]
- 168.Tasic B et al. Shared and distinct transcriptomic cell types across neocortical areas. Nature 563, 72–78 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mishra A et al. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat. Neurosci 19, 1619–1627 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang HU, Chen ZF & Anderson DJ Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93, 741–753 (1998). [DOI] [PubMed] [Google Scholar]
- 171.Lee JH et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788–792 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ji L et al. Cortical Neurovascular Coupling Driven by Stimulation of Channelrhodopsin-2. PLoS One 7, e46607 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lacroix A et al. COX-2-derived prostaglandin E2 produced by pyramidal neurons contributes to neurovascular coupling in the rodent cerebral cortex. J. Neurosci 35, 11791–11810 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vaucher E, Tong XK, Cholet N, Lantin S & Hamel E GABA neurons provide a rich input to microvessels but not nitric oxide neurons in the rat cerebral cortex: A means for direct regulation of local cerebral blood flow. J. Comp. Neurol 421, 161–171 (2000). [PubMed] [Google Scholar]
- 175.Anenberg E, Chan AW, Xie Y, LeDue JM & Murphy TH Optogenetic stimulation of GABA neurons can decrease local neuronal activity while increasing cortical blood flow. J. Cereb. Blood Flow Metab 35, 1579–1586 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Uhlirova H et al. Cell type specificity of neurovascular coupling in cerebral cortex. Elife 5, 1–23 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified that selective activation of GABAergic interneurons can also induce neurovascular coupling despite an overall net decreased in neural activity. Furthermore, the authors identified that NPY+ interneurons may modulate the vasoconstriction phase.
- 177.Devor A et al. Suppressed Neuronal Activity and Concurrent Arteriolar Vasoconstriction May Explain Negative Blood Oxygenation Level-Dependent Signal. J. Neurosci 27, 4452–4459 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Longden TA et al. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat. Neurosci 20, 717–726 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified that capillary endothelial cells express Kir2.1 potassium channel to sense neural activity and can relay this information via as a propagation of hyperpolarization to upstream arteries.
- 179.Niwa K, Araki E, Morham SG, Ross ME & Iadecola C Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J. Neurosci 20, 763–770 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Edvinsson L, Ekman R, Jansen I, Ottosson A & Uddman R Peptide‐containing nerve fibers in human cerebral arteries: Immunocytochemistry, radioimmunoassay and in vitro pharmacology. Ann. Neurol 21, 431–437 (1987). [DOI] [PubMed] [Google Scholar]
- 181.Huang M & Rorstad OP Effects of Vasoactive Intestinal Polypeptide, Monoamines, Prostaglandins, and 2-Chloroadenosine on Adenylate Cyclase in Rat Cerebral Microvessels. J. Neurochem 40, 719–726 (1983). [DOI] [PubMed] [Google Scholar]
- 182.Cauli B et al. Cortical GABA Interneurons in Neurovascular Coupling: Relays for Subcortical Vasoactive Pathways. J. Neurosci 24, 8940–8949 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Perrenoud Q et al. Characterization of Type I and Type II nNOS-Expressing Interneurons in the Barrel Cortex of Mouse. Front. Neural Circuits 6, 1–31 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ma J, Ayata C, Huang PL, Fishman MC & Moskowitz MA Regional cerebral blood flow response to vibrissal stimulation in mice lacking type I NOS gene expression. Am. J. Physiol. - Hear. Circ. Physiol 270, (1996). [DOI] [PubMed] [Google Scholar]
- 185.Yang G, Zhang Y, Ross ME & Iadecola C Attenuation of activity-induced increases in cerebellar blood flow in mice lacking neuronal nitric oxide synthase. Am. J. Physiol. - Hear. Circ. Physiol 285, 298–304 (2003). [DOI] [PubMed] [Google Scholar]
- 186.Takano T et al. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci 9, 260–267 (2006). [DOI] [PubMed] [Google Scholar]
- 187.Filosa JA et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat. Neurosci 9, 1397–1403 (2006). [DOI] [PubMed] [Google Scholar]
- 188.Zonta M et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat. Neurosci 6, 43–50 (2003). [DOI] [PubMed] [Google Scholar]
- 189.Sun W et al. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science (80-. ). 339, 197–200 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Niwa K, Haensel C, Ross ME & Iadecola C Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ. Res 88, 600–608 (2001). [DOI] [PubMed] [Google Scholar]
- 191.Bonder DE & McCarthy KD Astrocytic Gq-GPCR-linked IP3R-Dependent Ca2+ signaling does not mediate neurovascular coupling in mouse visual cortex in vivo. J. Neurosci 34, 13139–13150 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nizar K et al. In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J. Neurosci 33, 8411–8422 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Takata N et al. Cerebral Blood Flow Modulation by Basal Forebrain or Whisker Stimulation Can Occur Independently of Large Cytosolic Ca2+ Signaling in Astrocytes. PLoS One 8, e66525 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Girouard H et al. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc. Natl. Acad. Sci 107, 3811–3816 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Attwell D et al. Glial and neuronal control of brain blood flow. Nature 468, 232–243 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chen BR, Kozberg MG, Bouchard MB, Shaik MA & Hillman EMC A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J. Am. Heart Assoc 3, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study was the first study to highlight the importance of endothelial cells in neurovascular coupling, proposing the retrograde propagation model.
- 197.Won C et al. Autonomous vascular networks synchronize GABA neuron migration in the embryonic forebrain. Nat. Commun 4, 1–14 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hogan-Cann AD, Lu P & Anderson CM Endothelial NMDA receptors mediate activity-dependent brain hemodynamic responses in mice. Proc. Natl. Acad. Sci 201902647 (2019). doi: 10.1073/pnas.1902647116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li S et al. Endothelial cell-derived GABA signaling modulates neuronal migration and postnatal behavior. Cell Res 28, 221–248 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tallini YN et al. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: Measurements in Cx40BAC-GCaMP2 transgenic mice. Circ. Res 101, 1300–1309 (2007). [DOI] [PubMed] [Google Scholar]
- 201.Reaume A et al. Cardiac malformation in neonatal mice lacking connexin43. Science (80-. ). 267, 1831–1834 (1995). [DOI] [PubMed] [Google Scholar]
- 202.Kruger O et al. Defective vascular development in connexin 45-deficient mice. Development 127, 4179–4193 (2000). [DOI] [PubMed] [Google Scholar]
- 203.Kumai M et al. Loss of connexin45 causes a cushion defect in early cardiogenesis. Development 127, 3501–3512 (2000). [DOI] [PubMed] [Google Scholar]
- 204.Simon AM & McWhorter AR Decreased intercellular dye-transfer and downregulation of non-ablated connexins in aortic endothelium deficient in connexin37 or connexin40. Journal of Cell Science 116, 2223–2236 (2003). [DOI] [PubMed] [Google Scholar]
- 205.Saliez J et al. Role of Caveolar Compartmentation in Endothelium-Derived Hyperpolarizing Factor–Mediated Relaxation. Circulation 117, 1065–1074 (2008). [DOI] [PubMed] [Google Scholar]
- 206.Zechariah A et al. Intercellular Conduction Optimizes Arterial Network Function and Conserves Blood Flow Homeostasis During Cerebrovascular Challenges. Arterioscler. Thromb. Vasc. Biol 40, 733–750 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lagaud G, Karicheti V, Knot HJ, Christ GJ & Laher I Inhibitors of gap junctions attenuate myogenic tone in cerebral arteries. Am. J. Physiol. Circ. Physiol 283, H2177–H2186 (2002). [DOI] [PubMed] [Google Scholar]
- 208.Watanabe N, Sasaki S, Masamoto K & Hotta H Vascular gap junctions contribute to forepaw stimulation-induced vasodilation differentially in the pial and penetrating arteries in isoflurane-anesthetized rats. Front. Mol. Neurosci 11, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tare M, Coleman HA & Parkington HC Glycyrrhetinic derivatives inhibit hyperpolarization in endothelial cells of guinea pig and rat arteries. Am. J. Physiol. - Hear. Circ. Physiol 282, 335–341 (2002). [DOI] [PubMed] [Google Scholar]
- 210.Matchkov VV, Rahman A, Peng H, Nilsson H & Aalkjær C Junctional and nonjunctional effects of heptanol and glycyrrhetinic acid derivates in rat mesenteric small arteries. Br. J. Pharmacol 142, 961–972 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Connors BW Tales of a Dirty Drug: Carbenoxolone, Gap Junctions, and Seizures. Epilepsy Curr 12, 66–68 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hannah RM, Dunn KM, Bonev AD & Nelson MT Endothelial SKCa and IKCa channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J. Cereb. Blood Flow Metab 31, 1175–1186 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chow BW et al. Caveolae in CNS arterioles mediate neurovascular coupling. Nature 579, 106–110 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wendling O, Bornert JM, Chambon P & Metzger D Efficient temporally-controlled targeted mutagenesis in smooth muscle cells of the adult mouse. Genesis (2009). doi: 10.1002/dvg.20448 [DOI] [PubMed] [Google Scholar]
- 215.Armstrong JJ, Larina IV, Dickinson ME, Zimmer WE & Hirschi KK Characterization of bacterial artificial chromosome transgenic mice expressing mCherry fluorescent protein substituted for the murine smooth muscle alpha-actin gene. Genesis 48, 457–63 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wirth A et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat. Med 14, 64–68 (2008). [DOI] [PubMed] [Google Scholar]
- 217.Long X et al. Smooth muscle calponin: An unconventional CArG-dependent gene that antagonizes neointimal formation. Arterioscler. Thromb. Vasc. Biol 31, 2172–2180 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Holtwick R et al. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc. Natl. Acad. Sci. U. S. A 99, 7142–7147 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ando K et al. Peri-arterial specification of vascular mural cells from naïve mesenchyme requires notch signaling. Dev 146, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ehling M, Adams S, Benedito R & Adams RH Notch controls retinal blood vessel maturation and quiescence. Dev 140, 3051–3061 (2013). [DOI] [PubMed] [Google Scholar]
- 221.Rajantie I et al. Bmx Tyrosine Kinase Has a Redundant Function Downstream of Angiopoietin and Vascular Endothelial Growth Factor Receptors in Arterial Endothelium. Mol. Cell. Biol 21, 4647–4655 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Miquerol L et al. Endothelial plasticity drives arterial remodeling within the endocardium after myocardial infarction. Circ. Res 116, 1765–71 (2015). [DOI] [PubMed] [Google Scholar]
- 223.Pu W et al. Mfsd2a+ hepatocytes repopulate the liver during injury and regeneration. Nat. Commun 7, 13369 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lyck R et al. Culture-induced changes in blood-brain barrier transcriptome: Implications for amino-acid transporters in vivo. J. Cereb. Blood Flow Metab (2009). doi: 10.1038/jcbfm.2009.72 [DOI] [PubMed] [Google Scholar]
- 225.Urich E, Lazic SE, Molnos J, Wells I & Freskgård PO Transcriptional profiling of human brain endothelial cells reveals key properties crucial for predictive in vitro blood-brain barrier models. PLoS One (2012). doi: 10.1371/journal.pone.0038149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Miranda-Azpiazu P, Panagiotou S, Jose G & Saha S A novel dynamic multicellular co-culture system for studying individual blood-brain barrier cell types in brain diseases and cytotoxicity testing. Sci. Rep 8, 1–10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Neal EH et al. A Simplified, Fully Defined Differentiation Scheme for Producing Blood-Brain Barrier Endothelial Cells from Human iPSCs. Stem Cell Reports 12, 1380–1388 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lippmann ES et al. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol 30, 783–791 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lippmann ES, Al-Ahmad A, Azarin SM, Palecek SP & Shusta EV A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci. Rep 4, 1–10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cho CF et al. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat. Commun 8, 1–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Park TE et al. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat. Commun 10, 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Srinivasan B et al. TEER Measurement Techniques for In Vitro Barrier Model Systems. J. Lab. Autom 20, 107–126 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zeisel A et al. Molecular Architecture of the Mouse Nervous System. Cell 174, 999–1014.e22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Taheri S, Gasparovic C, Shah NJ & Rosenberg GA Quantitative measurement of blood-brain barrier permeability in human using dynamic contrast-enhanced MRI with fast T1 mapping. Magn. Reson. Med 65, 1036–1042 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Heye AK, Culling RD, Valdés Hernández MDC, Thrippleton MJ & Wardlaw JM Assessment of blood-brain barrier disruption using dynamic contrast-enhanced MRI. A systematic review. NeuroImage: Clinical 6, 262–274 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sweeney MD, Sagare AP & Zlokovic BV Cerebrospinal fluid biomarkers of neurovascular dysfunction in mild dementia and Alzheimer’s disease. J. Cereb. Blood Flow Metab 35, 1055–68 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mulligan SJ & MacVicar BA Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431, 195–199 (2004). [DOI] [PubMed] [Google Scholar]
- 238.He L, Linden DJ & Sapirstein A Astrocyte Inositol Triphosphate Receptor Type 2 and Cytosolic Phospholipase A2 Alpha Regulate Arteriole Responses in Mouse Neocortical Brain Slices. PLoS One 7, e42194 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Liang Z, King J & Zhang N Anticorrelated resting-state functional connectivity in awake rat brain. Neuroimage 59, 1190–1199 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Nimmerjahn A, Mukamel EA & Schnitzer MJ Motor Behavior Activates Bergmann Glial Networks. Neuron 62, 400–412 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Maekawa T, Tommasino C, Shapiro HM, Keifer-Goodman J & Kohlenberger RW Local cerebral blood flow and glucose utilization during isoflurane anesthesia in the rat. Anesthesiology 65, 144–151 (1986). [DOI] [PubMed] [Google Scholar]
- 242.Tsubokawa T et al. Changes in local cerebral blood flow and neuronal activity during sensory stimulation in normal and sympathectomized cats. Brain Res 190, 51–65 (1980). [DOI] [PubMed] [Google Scholar]
- 243.Miyauchi Y, Sakabe T, Maekawa T, Ishikawa T & Takeshita H Responses of EEG, cerebral oxygen consumption and blood flow to peripheral nerve stimulation during thiopentone anaesthesia in the dog. Can. Anaesth. Soc. J 32, 491–8 (1985). [DOI] [PubMed] [Google Scholar]
- 244.Lindauer U, Villringer A & Dirnagl U Characterization of CBF response to somatosensory stimulation: model and influence of anesthetics. Am. J. Physiol 264, H1223–8 (1993). [DOI] [PubMed] [Google Scholar]
- 245.Aksenov DP, Li L, Miller MJ, Iordanescu G & Wyrwicz AM Effects of anesthesia on BOLD signal and neuronal activity in the somatosensory cortex. J. Cereb. Blood Flow Metab 35, 1819–1826 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ueki M, Mies G & Hossmann K‐A Effect of alpha‐chloralose, halothane, pentobarbital and nitrous oxide anesthesia on metabolic coupling in somatosensory cortex of rat. Acta Anaesthesiol. Scand 36, 318–322 (1992). [DOI] [PubMed] [Google Scholar]
- 247.Lyons DG, Parpaleix A, Roche M & Charpak S Mapping oxygen concentration in the awake mouse brain. Elife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Andrea Pisauro M, Dhruv NT, Carandini M & Benucci A Fast hemodynamic responses in the visual cortex of the awake mouse. J. Neurosci 33, 18343–18351 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Urban DJ & Roth BL DREADDs (Designer Receptors Exclusively Activated by Designer Drugs): Chemogenetic Tools with Therapeutic Utility. Annu. Rev. Pharmacol. Toxicol 55, 399–417 (2015). [DOI] [PubMed] [Google Scholar]
- 250.Smith CJ et al. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc. Natl. Acad. Sci. U. S. A 95, 13313–13318 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]


