Abstract
Background
Tiotropium is an anticholinergic agent which has gained widespread acceptance as a once daily maintenance therapy for symptoms and exacerbations of stable chronic obstructive pulmonary disease (COPD). In the past few years there have been several systematic reviews of the efficacy of tiotropium, however, several new trials have compared tiotropium treatment with placebo, including those of a soft mist inhaler, making an update necessary.
Objectives
To evaluate data from randomised controlled trials (RCTs) comparing the efficacy of tiotropium and placebo in patients with COPD, upon clinically important endpoints.
Search methods
We searched the Cochrane Airways Group's Specialised Register of Trials (CAGR) and ClinicalTrials.gov up to February 2012.
Selection criteria
We included parallel group RCTs of three months or longer comparing treatment with tiotropium against placebo for patients with COPD.
Data collection and analysis
Two review authors independently assessed studies for inclusion and then extracted data on study quality and the outcome results. We contacted study authors and trial sponsors for additional information, and collected information on adverse effects from all trials. We analysed the data using Cochrane Review Manager 5, RevMan 5.2.
Main results
This review included 22 studies of good methodological quality that had enrolled 23,309 participants with COPD. The studies used similar designs, however, the duration varied from three months to four years. In 19 of the studies, 18 mcg tiotropium once daily via the Handihaler dry powder inhaler was evaluated, and in three studies, 5 or 10 mcg tiotropium once daily via the Respimat soft mist inhaler was evaluated. Compared to placebo, tiotropium treatment significantly improved the mean quality of life (mean difference (MD) ‐2.89; 95% confidence interval (CI) ‐3.35 to ‐2.44), increased the number of participants with a clinically significant improvement (odds ratio (OR) 1.52; 95% CI 1.38 to 1.68), and reduced the number of participants with a clinically significant deterioration (OR 0.65; 95% CI 0.59 to 0.72) in quality of life (measured by the St George's Respiratory Questionnaire (SGRQ)). Tiotropium treatment significantly reduced the number of participants suffering from exacerbations (OR 0.78; 95% CI 0.70 to 0.87). This corresponds to a need to treat 16 patients (95% CI 10 to 36) with tiotropium for a year in order to avoid one additional patient suffering exacerbations, based on the average placebo event rate of 44% from one‐year studies. Tiotropium treatment led to fewer hospitalisations due to exacerbations (OR 0.85; 95% CI 0.72 to 1.00), but there was no statistically significant difference in all‐cause hospitalisations (OR 1.00; 95% CI 0.88 to 1.13) or non‐fatal serious adverse events (OR 1.03; 95% CI 0.97 to 1.10). Additionally, there was no statistically significant difference in all‐cause mortality between the tiotropium and placebo groups (Peto OR 0.98; 95% CI 0.86 to 1.11). However, subgroup analysis found a significant difference between the studies using a dry powder inhaler and those with a soft mist inhaler (test for subgroup differences: P = 0.01). With the dry powder inhaler there were fewer deaths in the tiotropium group (Peto OR 0.92; 95% CI 0.80 to 1.05) than in the placebo group (yearly rate 2.8%), but with the soft mist inhaler there were significantly more deaths in the tiotropium group (Peto OR 1.47; 95% CI 1.04 to 2.08) than in the placebo group (yearly rate 1.8%). It is noted that the rates of patients discontinuing study treatment were uneven, with significantly fewer participants withdrawing from tiotropium treatment than from placebo treatment (OR 0.66; 95% CI 0.59 to 0.73). Participants on tiotropium had improved lung function at the end of the study compared with those on placebo (trough forced expiratory volume in one second (FEV1) MD 118.92 mL; 95% CI 113.07 to 124.77).
Authors' conclusions
This review shows that tiotropium treatment was associated with a significant improvement in patients' quality of life and it reduced the risk of exacerbations, with a number needed to treat to benefit (NNTB) of 16 to prevent one exacerbation. Tiotropium also reduced exacerbations leading to hospitalisation but no significant difference was found for hospitalisation of any cause or mortality. Thus, tiotropium appears to be a reasonable choice for the management of patients with stable COPD, as proposed in guidelines. The trials included in this review showed a difference in the risk of mortality when compared with placebo depending on the type of tiotropium delivery device used. However, these results have not been confirmed in a recent trial when 2.5 mcg or 5 mcg of tiotropium via Respimat was used in a direct comparison to the 18 mcg Handihaler.
Plain language summary
Tiotropium for managing COPD
Chronic obstructive pulmonary disease (COPD) is a lung disease which includes the conditions, chronic bronchitis and emphysema. It is caused by smoking or inhaled dust, which leads to blockage or narrowing of the airways. The symptoms include breathlessness and a chronic cough. Tiotropium is an inhaled medication that helps widen the airways (bronchodilator) for up to 24 hours, and is used to manage persistent symptoms of COPD.
We found 22 studies including 23,309 participants, comparing the long‐term effectiveness and side effects of tiotropium and placebo. Compared with placebo, tiotropium treatment led to an improvement in quality of life, fewer people had an exacerbation (worsening of COPD symptoms), or exacerbations leading to hospital admissions. The number of people that needed to be treated for a year, for one person to avoid one additional exacerbation was 16 (95% confidence interval (CI) 10 to 36). We found no statistically significant difference between the tiotropium and placebo groups in terms of the number of hospital admissions for any cause, serious adverse events or deaths during the studies. However, when we divided the data depending on whether a dry powder inhaler or a soft mist inhaler was used in the studies, these two subgroups were significantly different. With the dry powder inhaler there were fewer deaths in the tiotropium group than in the placebo group, whereas with the soft mist inhaler there were significantly more deaths in the tiotropium group than in the placebo group. Also, there was a larger number of participants that stopped study medication early in the placebo group than in the tiotropium group.
This review shows that treatment with tiotropium improves patients' quality of life, and reduces the risk of exacerbations, including exacerbations leading to hospitalisation. But tiotropium does not reduce hospitalisations for all causes or the number of deaths. Based on the evidence in this review, tiotropium appears to be a reasonable treatment choice for patients with stable COPD.
Summary of findings
Summary of findings for the main comparison. Tiotropium versus placebo for chronic obstructive pulmonary disease.
| Tiotropium versus placebo for chronic obstructive pulmonary disease | ||||||
|
Patient or population: people with COPD who have smoked for ≥ 10 pack‐years
Settings: community
Intervention: tiotropium Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Tiotropium | |||||
|
Quality of life (SGRQ) Scale 0 to 100, where 100 represents worst possible health status and 0 indicates best possible health status Follow‐up: 3 to 48 months |
See comment | See comment | MD ‐2.89 (‐3.35 to ‐2.44) | 13,034 (9 studies) | ⊕⊕⊕⊕ high | Several studies did not report results for individual treatment groups and reported MD between the groups only. The accepted threshold for a clinically significant difference is ‐4 units |
| Number of patients with a clinically significant improvement (≥ 4 units) in quality of life (SGRQ) Follow‐up: 3 to 48 months | 389 per 1000 | 492 per 1000 (468 to 517) |
OR 1.52 (1.38 to 1.68) |
11,672 (9 studies) | ⊕⊕⊕⊕ high | |
| Number of patients with a clinically significant worsening (≥ 4 units) in quality of life (SGRQ) Follow‐up: 3 to 48 months | 348 per 1000 | 257 per 1000 (239 to 277) |
OR 0.65 (0.59 to 0.72) |
11,672 (9 studies) | ⊕⊕⊕⊕ high | |
| Number of patients with one or more exacerbations Follow‐up: 3 to 48 months | 442 per 1000 | 382 per 1000 (357 to 408) | OR 0.78 (0.70 to 0.87) | 23,309 (22 studies) | ⊕⊕⊕⊕ high1 | |
| Number of patients with one or more exacerbations requiring hospitalisation Follow‐up: 3 to 48 months | 131 per 1000 | 113 per 1000 (98 to 131) | OR 0.85 (0.72 to 1.00) | 22,852 (21 studies) | ⊕⊕⊕⊖ moderate2 | |
| Number of patients with one or more hospitalisations for any cause Follow‐up: 3 to 48 months | 234 per 1000 | 234 per 1000 (212 to 257) | OR 1.00 (0.88 to 1.13) | 20,963 (19 studies) | ⊕⊕⊕⊖ moderate2 | |
| Mortality Follow‐up: 3 to 48 months | 49 per 1000 | 48 per 1000 (43 to 54) | OR 0.98 (0.86 to 1.11) | 23,309 (22 studies) | ⊕⊕⊕⊖ moderate2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; RD: Risk difference; OR: Odds ratio; SGRQ: St George's Respiratory Questionnaire | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Although there was moderate unexplained heterogeneity between the study results (I2 = 51%), this was deemed not to affect the direction of the effect or have a large effect on the size of the effect.
2 The number of participants and/or events were low, leading to wide CIs and imprecision in the result.
Background
Description of the condition
Chronic Obstructive Pulmonary Disease (COPD) is a respiratory disease characterised by chronic and progressive breathlessness, cough, sputum production, and airflow obstruction, which leads to restricted activity and poor quality of life (GOLD 2010). The World Health Organization (WHO) (WHO) has estimated that COPD is the fourth or fifth most common single cause of death worldwide, and the treatment and management costs present a significant burden to public health. In the UK the annual cost of COPD to the National Health Service (NHS) is estimated to be GBP 1.3 million per 100,000 people (NICE 2011). Furthermore, because of the slow onset and the under‐recognition of the disease, it is heavily under‐diagnosed (GOLD 2010). COPD comprises a combination of bronchitis and emphysema and involves chronic inflammation and structural changes in the lung. Cigarette smoking is the most important risk factor, however air pollution and occupational dust and chemicals are also recognised risk factors.
COPD is a progressive disease leading to reduced lung function over time, even with the best available care. There is currently no cure for COPD, although it is both a preventable and treatable disease. As yet, apart from smoking cessation and non‐pharmacological treatments such as long‐term oxygen therapy in hypoxic patients, no intervention has been shown to reduce mortality (GOLD 2010). Management of the disease is multi‐facetted and includes interventions for smoking cessation (Van der Meer 2001), pharmacological treatments (GOLD 2010), education (Effing 2007), and pulmonary rehabilitation (Lacasse 2006). Pharmacological therapy is aimed at relieving symptoms; improving exercise tolerance and quality of life; slowing decline and even improving lung function; or preventing or treating exacerbations. COPD exacerbations impair patients' quality of life (GOLD 2010). Furthermore, a large part of the economic burden of COPD is attributed to the cost of managing exacerbations, particularly those resulting in use of acute care services or hospitalisations (Hutchinson 2010). In the UK, one in eight emergency admissions to hospital is for COPD, which makes it the second largest cause of emergency admissions, and one of the most costly conditions treated by the NHS (NICE 2011). Therefore, pharmacological management aimed at reducing or preventing exacerbations is important.
Description of the intervention
COPD pharmacological management tends to begin with one treatment, with additional therapies introduced as necessary to control symptoms (GOLD 2010). The first step is often a short‐acting bronchodilator for control of breathlessness when needed: either a short‐acting beta2‐agonist (SABA) or the short‐acting anticholinergic ipratropium. For persistent or worsening breathlessness associated with lung function decline, long‐acting bronchodilators may be introduced (GOLD 2010). Long‐acting bronchodilators include long‐acting beta2‐agonists (LABAs), such as salmeterol or formoterol; and the long‐acting anticholinergic agent, tiotropium. Regular treatment with long‐acting bronchodilators may be more efficient and convenient than treatment with regular short‐acting bronchodilators (Beeh 2010). For symptomatic patients with severe or very severe COPD (forced expiratory volume in one second (FEV1) < 50% predicted), and with repeated exacerbations, GOLD 2010 recommends the addition of inhaled corticosteroids (ICS) to bronchodilator treatment.
How the intervention might work
Tiotropium is an anticholinergic agent, which blocks the action of the neurotransmitter acetylcholine. It has an antagonistic effect on muscarinic acetylcholine receptors. Tiotropium has similar affinity for the five different subtypes of muscarinic receptors (M1‐M5), however airway smooth muscle expresses only the M2 and M3 subtypes (Proskocil 2005). Activation of the M3 receptor stimulates a number of intracellular signalling cascades leading to changes in intracellular Ca2+ homeostasis and contraction. Tiotropium dissociates slowly from M3 receptors giving a bronchodilator effect lasting over 24 hours, but dissociates rapidly from M2 receptors, which appear to be feedback inhibitory receptors (Barr 2005).
Tiotropium has gained widespread acceptance as a once daily maintenance therapy in stable COPD (Barr 2005; GOLD 2010) for its effects on symptoms and exacerbations. In a previous Cochrane review (Barr 2005), tiotropium was shown to reduce the primary endpoint of COPD exacerbations compared to placebo. Within the same review, tiotropium was also associated with a significant benefit over placebo in terms of breathlessness, quality of life, and exacerbations requiring hospitalisation. Similar effects on symptoms and exacerbations were confirmed in a more recent, large RCT of almost 6000 participants followed for over four years (Tashkin 2008). There was, however, no significant effect of tiotropium on lung function decline in this longer study.
Currently, tiotropium may be delivered via two different inhalers: the HandiHaler which is a single dose dry powder inhaler; and the Respimat soft mist inhaler which is a novel, propellant‐free, multidose inhaler. Boehringer Ingleheim, the manufacturer of both formulations, has reported a higher all‐cause mortality rate associated with use of the soft mist inhaler, but not with the dry powder inhaler (Boehringer Ingelheim 2010). Anticholinergic side effects that may occur with tiotropium include dry mouth, constipation and tachycardia (Tashkin 2008), as well as major cardiovascular events (Singh 2009).
Although tiotropium is one of the more expensive drugs on the market, a systematic review suggested that tiotropium monotherapy may be associated with lower hospital and other non‐drug costs; being either cost‐saving or cost‐effective compared with other maintenance monotherapies (Mauskopf 2010). A cost‐utility analysis has presented conflicting results, suggesting that tiotropium may have an unfavourable cost‐effectiveness ratio linked to the relatively high cost of tiotropium and a relatively low number of hospitalisations in patients who are not on tiotropium treatment (Neyt 2010).
Why it is important to do this review
The potential clinical risks or benefits of treatment with tiotropium were studied in a previous systematic review (Barr 2005). However, several new trials, including those with a novel soft mist inhaler, have compared tiotropium treatment with placebo, making an update necessary. This will give a clearer picture of the true effects associated with tiotropium treatment. The review forms part of a suite of reviews on tiotropium treatment: either on its own or in various combinations with LABAs and ICS for the treatment of COPD.
Objectives
To evaluate data from randomised controlled trials (RCTs) comparing the efficacy of tiotropium and placebo in patients with chronic obstructive pulmonary disease (COPD), upon clinically important endpoints.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs with a parallel group design and of at least 12 weeks duration. We did not include cross‐over trials, as one of the primary outcomes was mortality.
Types of participants
We included studies of participants with a diagnosis of COPD, where an external set of criteria was used to screen participants for this condition (e.g. Global Initiative for Chronic Obstructive Lung Disease (GOLD), American Thoracic Society (ATS), British Thoracic Society (BTS), and Thoracic Society of Australia and New Zealand (TSANZ)).
Types of interventions
In each study, participants were randomised to receive either inhaled tiotropium bromide or placebo. Tiotropium bromide was allowed in any formulation. Participants were allowed inhaled steroids and other concomitant COPD medication, provided they were not part of the randomised treatment.
Types of outcome measures
Primary outcomes
Quality of life; measured with a scale validated for COPD, such as St George's Respiratory Questionnaire (SGRQ), Chronic Respiratory Questionnaire (CRQ).
Exacerbations; requiring oral corticosteroids and/or antibiotics.
Mortality; all‐cause.
Hospital admissions; all‐cause and due to exacerbations.
Secondary outcomes
Forced expiratory volume in one second (FEV1).
Non‐fatal serious adverse events; all‐cause and cardiovascular.
Withdrawals from study treatment.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group's Specialised Register of Trials (CAGR), which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED, and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see Appendix 1 for further details). We searched all records in the CAGR coded 'COPD' using the following terms: tiotropium OR Spiriva OR HandiHaler OR Respimat We also conducted a search of ClinicalTrials.gov in July 2011. The search terms are in Appendix 2. We searched all databases from their inception to the present and imposed no restriction on language of publication.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We searched the manufacturer's website (Boehringer Ingleheim Global trials database) for additional study information for studies identified through the electronic searches.
Data collection and analysis
Selection of studies
Two review authors (CK, JC) independently screened the titles and abstracts of citations retrieved through literature searches and obtained those deemed to be potentially relevant. We assigned each reference to a study identifier and assessed them against the inclusion criteria of this protocol.
Data extraction and management
Two review authors (CK, JC) independently extracted information from each study for the following characteristics.
Design (design, total study duration and run‐in, number of study centres and location, withdrawals, and date of study).
Participants (N, mean age, age range, gender, COPD severity, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria).
Interventions (run‐in, intervention treatment and inhaler type, control treatment and inhaler type).
Outcomes (primary and secondary outcomes specified and collected, and time points reported).
We discussed and resolved any discrepancies in the data, or consulted a third‐party where necessary.
Assessment of risk of bias in included studies
We assessed the risk of bias according to recommendations outlined in The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) for:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting; and
other bias.
We graded each potential source of bias as high, low or unclear.
Measures of treatment effect
Dichotomous data
We analysed dichotomous data variables using Mantel‐Haenzsel odds ratios (ORs) with a fixed‐effect model and 95% confidence intervals (CIs). Where events were rare we employed the Peto OR. Where count data were not available as the number of participants experiencing an event, we analysed it as continuous, time‐to‐event or rate ratios, depending on how it was reported. We transformed reported rate ratios into log rate ratios and analysed them using a fixed‐effect model and Generic Inverse Variance (GIV) in Review Manager 5 (RevMan 2011).
Continuous data
We analysed continuous outcome data as fixed‐effect mean differences (MDs) with 95% CIs. Where treatment effects were reported as a MD between treatment groups, we entered it using a fixed‐effect model and GIV in Review Manager 5 (RevMan 2011). We used end of study as time of analysis for all studies.
We used intention‐to‐treat (ITT) analysis on outcomes from all randomised participants, where possible, for primary analyses.
We calculated numbers needed to treat for primary outcomes from the pooled OR and its CI, and applied these to appropriate levels of baseline risk.
Unit of analysis issues
We analysed dichotomous data using participants as the unit of analysis (rather than events). For continuous data we preferred MD based on change from baseline over MD based on absolute values.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible.
Assessment of heterogeneity
We assessed the amount of statistical variation among the study results with the I2 measurement.
Assessment of reporting biases
We minimised reporting bias from non‐publication of studies or selective outcome reporting by using a broad search strategy, contacting study authors directly and checking references of included studies. We visually inspected funnel plots.
Data synthesis
We have presented the findings of our primary outcomes in a 'Summary of findings' table using GradePro software.
Subgroup analysis and investigation of heterogeneity
We subgrouped studies where possible, according to:
severity of disease at baseline (mild (GOLD 2010 I), moderate (GOLD 2010 II), severe (GOLD 2010 III), and very severe (GOLD 2010 IV));
tiotropium formulation (dry powder inhaler, soft mist inhaler);
concomitant medication (with or without long‐acting beta2‐agonists (LABAs), with or without inhaled corticosteroids (ICS), and with or without both LABAs and ICS); and
study duration (< 1 year, ≥ 1 year).
Sensitivity analysis
We assessed the sensitivity of our primary outcomes to degree of bias by comparing the overall results with those exclusively from trials assessed as being at low risk of bias.
Results
Description of studies
Results of the search
The search of the Cochrane Airways Group's Specialised Register of Trials (CAGR) returned 451 references (February 2012), ClinicalTrials.gov generated 119 (July 2011), and we identified four references from other sources. From these, we identified 210 as potentially relevant. After further assessment we found that 153 references belonging to 22 studies were eligible for inclusion (see Characteristics of included studies); we excluded 53 references with reasons given in the Characteristics of excluded studies tables, and five studies are awaiting classification pending retrieval and translation. Searching the manufacturer's website (Boehringer Ingleheim Global trials database) we found 22 study reports for 19 of the included studies. For the study flow diagram see Figure 1.
1.
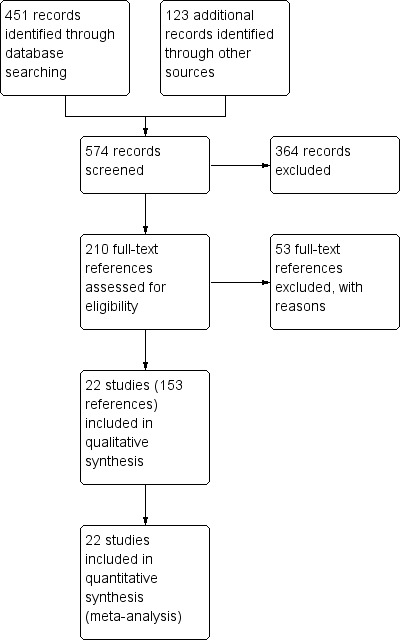
Study flow diagram.
Included studies
For details of each trial see Characteristics of included studies.
Study design
All of the included studies were randomised, double‐blind, placebo‐controlled, and of parallel group design. The study duration varied from three months to four years (see Table 2). Thirteen studies were performed in a single country and six studies were carried out in study centres in several countries. The majority of study centres were in European or North American countries. One study was performed in China (Sun 2007).
1. Study duration.
| Duration | Studies | n participants |
| 3 months |
Beeh 2006 Covelli 2005 Freeman 2007 Johansson 2008 Magnussen 2008 Moita 2008 NCT00144326 Sun 2007 Verkinde 2006 Voshaar 2008 |
4188 |
| 6 months |
Brusasco 2003 Niewoehner 2005 Trooster 2011 |
3493 |
| 9 months | Tonnel 2008 | 554 |
| 1 year |
Bateman 2010a Bateman 2010b Casaburi 2002 Chan 2007 Dusser 2006 Powrie 2007 |
8967 |
| 2 years | Cooper 2010 | 519 |
| 4 years | Tashkin 2008 | 5993 |
Sample size
The studies included 23,309 participants, of whom 12,697 were randomised to tiotropium treatment and 10,612 to placebo. The size of the studies varied greatly: Tashkin 2008 was the largest study with 5993 participants, and the smallest study only had 60 participants (Sun 2007).
Participants
The mean age of participants in the different studies was relatively similar, ranging from 60 to 68 years. Most studies had more male than female participants and a similar gender distribution in both treatment groups. In these trials the percentage of men in the studies was roughly 75%, but varied from 60% to 98%. The exception was a few studies with relatively more women, or with more uneven gender distribution between the treatment groups (Covelli 2005; Freeman 2007; Johansson 2008; Powrie 2007). Disease severity in the included studies ranged from mild to severe COPD. In a majority of the studies the patients had a mean baseline lung function of less than 50% FEV1 predicted indicating that a large proportion of participants had severe COPD. Two studies had higher mean FEV1 % predicted, Johansson 2008 at 73% and Trooster 2011 at 66% of predicted. The baseline lung function was generally well balanced between the treatment groups.
The included studies had similar inclusion and exclusion criteria. Patients of either sex, with a clinical diagnosis of stable COPD, were eligible for study entry if they were aged over 40 years and had a smoking history of at least 10 pack‐years. Participants were excluded if they had a significant disease other than COPD usually including other significant respiratory conditions such as asthma or a respiratory infection in the weeks before enrolment. The exception was Magnussen 2008 in which participants were required to have a diagnosis of asthma as well as COPD.
Interventions
All studies used tiotropium and placebo once daily. Three studies used the soft mist inhaler Respimat; Bateman 2010a (5 mcg tiotropium), Bateman 2010b and Voshaar 2008 (both 5 mcg and 10 mcg tiotropium in each study). All of the other studies used 18 mcg tiotropium via the Handihaler dry powder inhaler as the intervention.
Permitted co‐treatment
Most of the included studies allowed participants to continue previously prescribed medication and short‐acting beta2‐agonist as needed. In 13 of the included studies it was specified that anticholinergics, other than the study medication, were not allowed during the study (Bateman 2010a; Chan 2007; Covelli 2005; Dusser 2006; Johansson 2008; Magnussen 2008; Moita 2008; Niewoehner 2005; Powrie 2007; Tashkin 2008; Tonnel 2008; Verkinde 2006; Voshaar 2008). Eight studies specified that they did not allow LABAs (Bateman 2010b; Chan 2007; Dusser 2006; Johansson 2008; Moita 2008; Tonnel 2008; Verkinde 2006; Voshaar 2008), three did not allow antileukotrienes (Covelli 2005; Moita 2008; Tonnel 2008), and two did not allow ICS or ICS/LABA combination inhalers (Johansson 2008; Voshaar 2008).
Outcomes
All of the studies measured lung function using various measures including FEV1. Almost all studies reported results on exacerbations. The included studies primarily also looked at health‐related quality of life, dyspnoea, use of rescue medication, general health status and safety.
Funding
All studies except Sun 2007 were sponsored by Boehringer Ingelheim.
Excluded studies
We excluded 53 references from 35 studies as they failed to meet the eligibility criteria for the review (see Characteristics of excluded studies): 16 had a study duration shorter than 12 weeks; nine were of cross‐over study design; one was not a RCT; one was a systematic review of data; and five lacked one or both of the treatment groups ‐ tiotropium and placebo. The last three compared tiotropium to placebo, but this was part of a more complex intervention including pulmonary rehabilitation exercise programmes.
Risk of bias in included studies
An assessment of the risk of bias is presented in the Characteristics of included studies table, with an overview of the findings in Figure 2.
2.
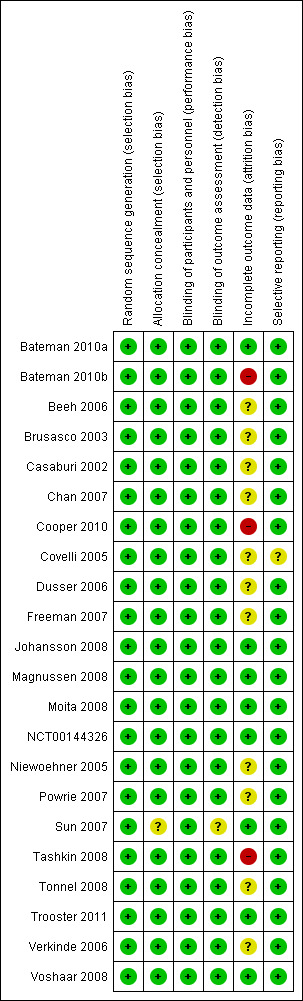
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Boehringer Ingelheim‐sponsored studies (all but Sun 2007) used randomisation lists generated using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable. Participants were then allocated study drug treatment either using a third‐party “Interactive Voice Response System” or by assigning the treatment with the lowest number available to the investigator at the time of randomisation. Sun 2007 did not describe their allocation concealment procedures.
Blinding
All of the included studies were of a double‐blind design. In all of the Boehringer Ingelheim studies, Boehringer Ingelheim was responsible for preparing and coding study treatment in a blinded fashion so that study drug and control were indistinguishable. Outcome assessors remained blinded with regard to the treatment assignments up to database lock. In Sun 2007 the placebo and the study drug had the same appearance. Brusasco 2003 and Voshaar 2008 used a double dummy design.
Incomplete outcome data
Several of the studies had relatively low rates of patients withdrawing from the study treatment in both treatment groups. These were assessed as having a low risk of attrition bias (Bateman 2010a; Johansson 2008; Magnussen 2008; Moita 2008; NCT00144326; Sun 2007; Trooster 2011; Voshaar 2008). The two longest studies Cooper 2010 (two years) and Tashkin 2008 (four years) had high withdrawal rates, especially in the placebo groups which were 39% and 45% respectively. All the other trials had a mix of relatively high and/or uneven withdrawal rates with an unclear risk of bias. However, three of the larger and longer studies followed up the vital status of participants even if they withdrew from the study treatment or prematurely discontinued study participation (Bateman 2010a; Bateman 2010b; Tashkin 2008).
Selective reporting
All of the studies reported results for outcomes included in this review that had been specified in the methods of published articles or in study reports.
Effects of interventions
See: Table 1
Primary outcome: quality of life
Many of the included studies measured health‐related quality of life using either the SGRQ, the CRQ or the Euro Quality of Life ‐ 5 dimensions questionnaire (EQ‐5D).
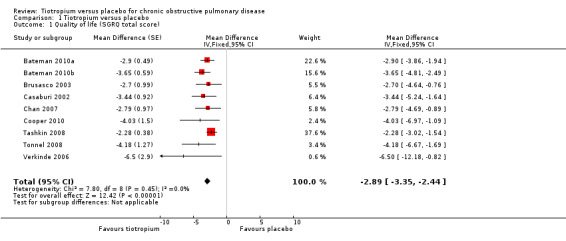
Nine studies involving 13,034 participants used the SGRQ (Bateman 2010a; Bateman 2010b; Brusasco 2003; Casaburi 2002; Chan 2007; Cooper 2010; Tashkin 2008; Tonnel 2008; Verkinde 2006). A decrease in SGRQ score denotes an improvement in quality of life and a difference of at least four units is regarded as clinically significant (SGRQ‐C manual 2008). In Bateman 2010b the 5 mcg and 10 mcg tiotropium groups were similar in size and had similar quality of life data. The two groups were therefore combined using the mean of the groups for both the MD and the standard error (SE). The SE was adjusted by (1/√1.5) to take into account the 50% increase of n. Tiotropium treatment led to a statistically significant improvement in health‐related quality of life compared to placebo (MD ‐2.89; 95% CI ‐3.35 to ‐2.44, Figure 3). These studies also reported data on the number of participants who had a clinically significant improvement (≥ ‐4 units) or worsening (≥ +4 units) in quality of life. There were significantly more participants with a clinically significant improvement in quality of life (OR 1.52; 95% CI 1.38 to 1.68, Analysis 1.2), and significantly fewer participants with a clinically significant deterioration (OR 0.65; 95% CI 0.59 to 0.72, Analysis 1.3) treated with tiotropium compared to placebo. The heterogeneity between the studies was 26% and 18% for improvement and deterioration, respectively.
3.
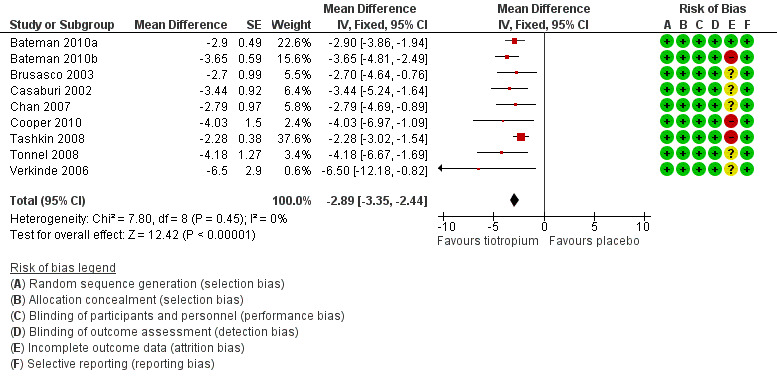
Forest plot of comparison: 1 Tiotropium versus placebo, outcome: 1.1 Quality of life (SGRQ total score).
1.2. Analysis.
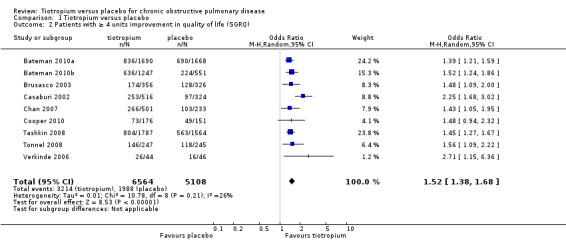
Comparison 1 Tiotropium versus placebo, Outcome 2 Patients with ≥ 4 units improvement in quality of life (SGRQ).
1.3. Analysis.
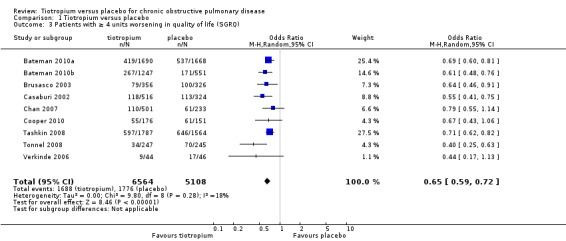
Comparison 1 Tiotropium versus placebo, Outcome 3 Patients with ≥ 4 units worsening in quality of life (SGRQ).
Subgroup analysis of participants on concomitant medication during the trials showed no statistically significant difference between participants with (518 participants) or without (270 participants) ICS use (test for subgroup differences: P = 0.56, Analysis 1.4), or with (1824 participants) or without (4114 patients) LABA use (test for subgroup differences: P = 0.38, Analysis 1.5). There was no statistically significant difference between participants with a lung function (FEV1) of more than 50% predicted (GOLD 2010 I/II, 1945 participants) and participants with FEV1 predicted of less than 50% (GOLD 2010 III/IV, 290 participants) (test for subgroup differences: P = 0.07, Analysis 1.6).
1.4. Analysis.
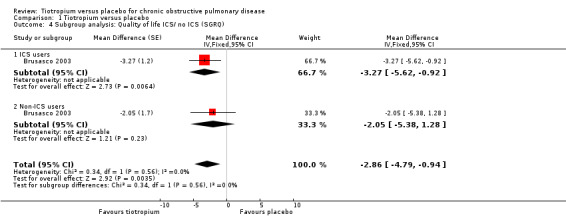
Comparison 1 Tiotropium versus placebo, Outcome 4 Subgroup analysis: Quality of life ICS/ no ICS (SGRQ).
1.5. Analysis.
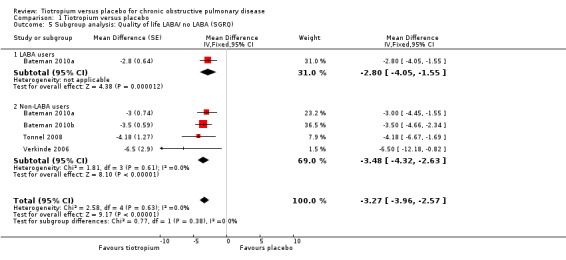
Comparison 1 Tiotropium versus placebo, Outcome 5 Subgroup analysis: Quality of life LABA/ no LABA (SGRQ).
1.6. Analysis.
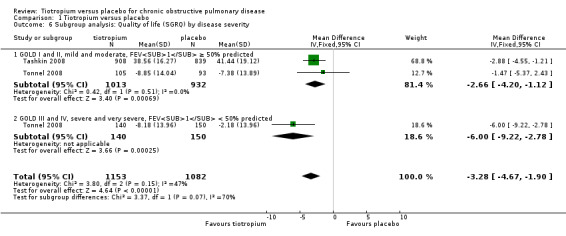
Comparison 1 Tiotropium versus placebo, Outcome 6 Subgroup analysis: Quality of life (SGRQ) by disease severity.
Three studies used the EQ‐5D questionnaire (Covelli 2005; Johansson 2008; Moita 2008), and one study used the Chronic Respiratory Questionnaire (CRQ) (NCT00144326). EQ‐5D and CRQ are different scales, but in both a higher value signifies better health. Covelli 2005 showed the tiotropium group to have a small but statistically significant improvement in quality of life compared to the placebo group (MD 0.06; 95% CI 0.02 to 0.10; Analysis 1.7). Johansson 2008, Moita 2008 and NCT00144326 showed no statistically significant difference between the groups (Johansson 2008 (MD ‐0.02; 95% CI ‐0.05 to 0.01; Analysis 1.8); Moita 2008 (P = 0.86, data analysed in a non‐parametric way, mean data therefore not available), NCT00144326 (MD ‐2.50; 95% CI ‐56.35 to 51.35)).
1.7. Analysis.
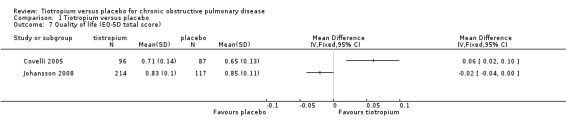
Comparison 1 Tiotropium versus placebo, Outcome 7 Quality of life (EQ‐5D total score).
1.8. Analysis.
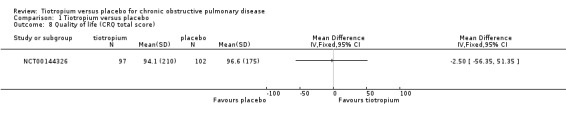
Comparison 1 Tiotropium versus placebo, Outcome 8 Quality of life (CRQ total score).
Primary outcome: exacerbations and hospital admissions
All of the studies reported COPD exacerbations as a specific outcome or as part of the safety data (22 studies, 23,309 participants). The definition of COPD exacerbation was similar among the studies; exacerbations were defined as a complex of respiratory events or symptoms (new onset or an increase in at least one of cough, sputum, dyspnoea or wheeze) that lasted at least three days and required treatment with antibiotics and/or systemic corticosteroids. All of the studies reported the number of participants with one or more exacerbation in each treatment group. There were fewer participants suffering one or more exacerbations in the tiotropium group (38%) than in the placebo group (44%). The difference between the groups was statistically significant when analysed using a fixed‐effect model (OR 0.81; 95% CI 0.76 to 0.86). There was moderate heterogeneity in the result among the studies (I2 = 51%), although a random‐effects model resulted in a similar, statistically significant result (OR 0.78; 95% CI 0.70 to 0.87, Figure 4) with a number needed to treat to benefit (NNTB) of 16 (95% CI 10 to 36) over one year (Figure 5). A funnel plot of the data did not show any obvious asymmetry indicating publication bias (Figure 6). This was confirmed by Egger 1997 test of asymmetry (P = 0.22).
4.
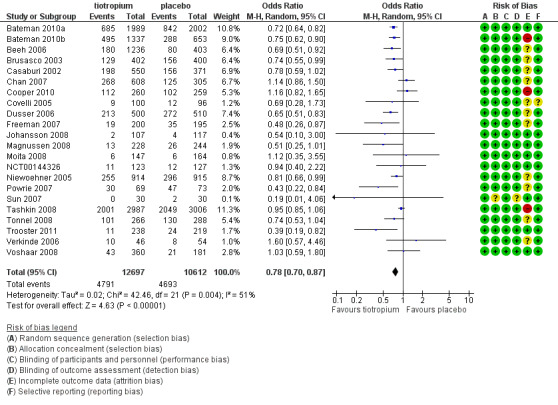
Forest plot of comparison: 1 Tiotropium versus placebo, outcome: 1.9 Patients with ≥ 1 exacerbation.
5.
In the placebo group 44 people out of 100 had one or more exacerbations over one year, compared to 38 (95% CI 34 to 41) out of 100 for the tiotropium group.
6.
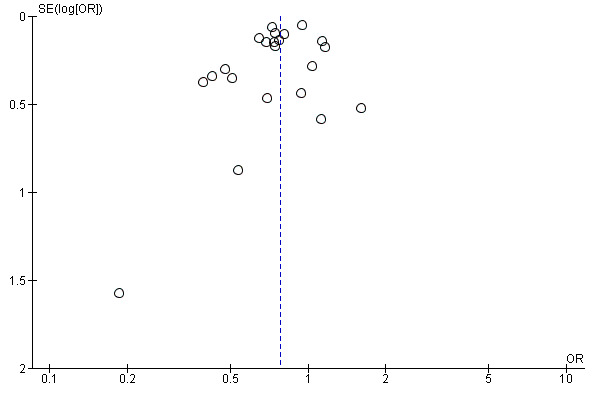
Funnel plot of comparison: 1 Tiotropium versus placebo, outcome: 1.9 Patients with ≥ 1 exacerbation.
When analysing the data according to study length and inhaler device, there was substantial heterogeneity within the subgroups. We therefore analysed the data using a random‐effects model which showed no statistically significant difference between studies of up to one year (7830 patients) and studies longer than one year (15,479 patients) (test for subgroup differences: P = 0.33, Analysis 1.10), or between the different inhalers: dry powder inhaler (16,787 participants) and soft mist inhaler (6522 participants) (test for subgroup differences: P = 0.52, Analysis 1.12). Several studies also reported exacerbation data for the different stages of disease severity (GOLD 2010 II, 3379 participants; GOLD 2010 III, 2835 participants; GOLD 2010 IV, 533 participants). Because of high heterogeneity within the subgroups, we analysed the data using a random‐effects model. This did not show any statistically significant difference among the groups (test for subgroup differences: P = 0.31, Analysis 1.13). A comparison of patients taking ICS (615 participants) or not (388 participants) showed no statistically significant difference between the groups when analysed using a random‐effects model (test for subgroup differences: P = 0.64, Analysis 1.11).
1.10. Analysis.

Comparison 1 Tiotropium versus placebo, Outcome 10 Subgroup analysis: patients with ≥ 1 exacerbation by study duration.
1.12. Analysis.
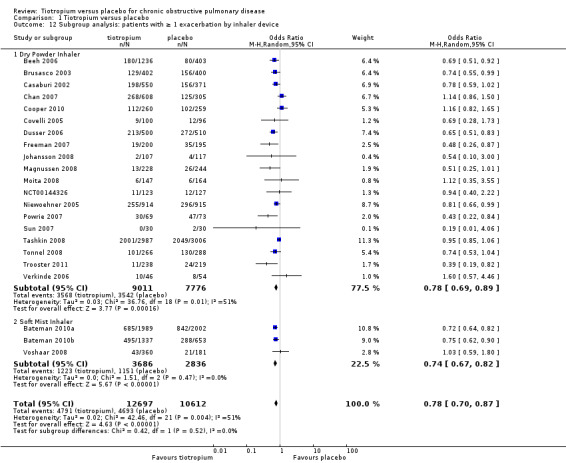
Comparison 1 Tiotropium versus placebo, Outcome 12 Subgroup analysis: patients with ≥ 1 exacerbation by inhaler device.
1.13. Analysis.
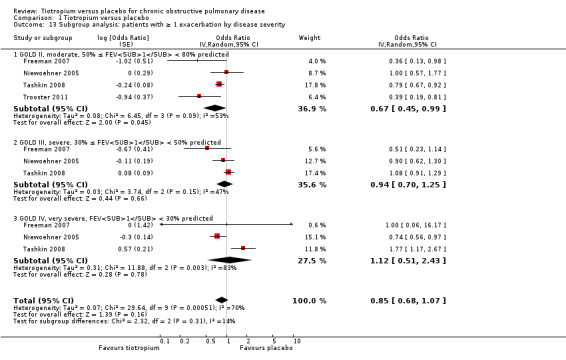
Comparison 1 Tiotropium versus placebo, Outcome 13 Subgroup analysis: patients with ≥ 1 exacerbation by disease severity.
1.11. Analysis.
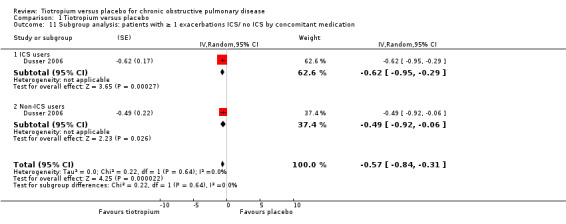
Comparison 1 Tiotropium versus placebo, Outcome 11 Subgroup analysis: patients with ≥ 1 exacerbations ICS/ no ICS by concomitant medication.
All but one study (Trooster 2011) reported exacerbations leading to hospitalisation (21 studies, 22,852 participants). There were fewer patients on tiotropium suffering one or more exacerbation(s) leading to hospitalisation (10.4%) than those on placebo (13.1%) (OR 0.85; 95% CI 0.72 to 1.00; Analysis 1.14). There was again moderate heterogeneity in this result (I2 = 37%). The heterogeneity does not seem to be explained by the use of different inhalers (dry powder inhaler, 16,330 participants; soft mist inhaler, 6522 participants; test for subgroup differences, P = 0.70; Analysis 1.17) or by study duration (< 1 year, 7373 participants; ≥ 1 year, 15,479 participants; test for subgroup differences: P = 1.00; Analysis 1.16).
1.14. Analysis.
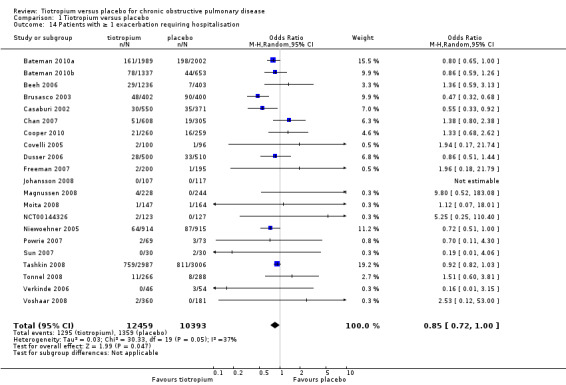
Comparison 1 Tiotropium versus placebo, Outcome 14 Patients with ≥ 1 exacerbation requiring hospitalisation.
1.17. Analysis.
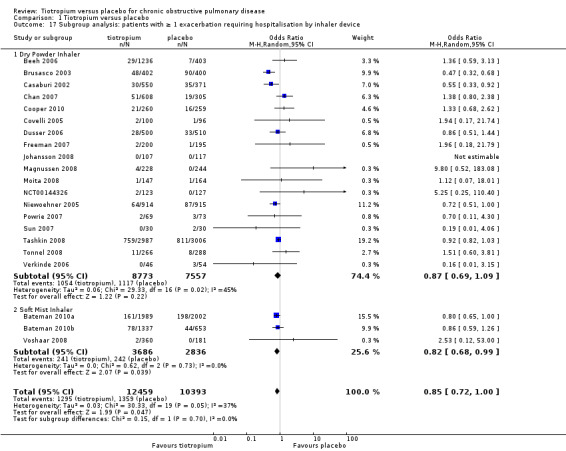
Comparison 1 Tiotropium versus placebo, Outcome 17 Subgroup analysis: patients with ≥ 1 exacerbation requiring hospitalisation by inhaler device.
1.16. Analysis.
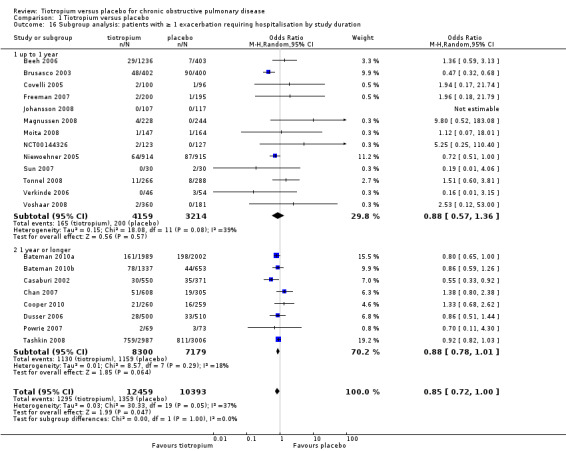
Comparison 1 Tiotropium versus placebo, Outcome 16 Subgroup analysis: patients with ≥ 1 exacerbation requiring hospitalisation by study duration.
All but three studies reported the number of participants who were hospitalised for any cause (19 studies, 20,963 participants). The exceptions were Niewoehner 2005, Sun 2007, and Trooster 2011. There was no statistically significant difference between the tiotropium and the control groups in all‐cause hospitalisations (OR 1.00; 95% CI 0.88 to 1.13; I2 = 37%; Analysis 1.15).
1.15. Analysis.

Comparison 1 Tiotropium versus placebo, Outcome 15 Patients with ≥ 1 hospitalisation (all‐cause).
Primary outcome: mortality
All studies reported the number of deaths during the treatment period in each treatment group (22 studies, 23,309 participants). Tashkin 2008 (5993 participants) was the only study to have mortality as a specified outcome, with a mortality adjudication committee evaluating the primary cause of death from blinded data. A couple of other studies also followed up all participants, including those who prematurely discontinued study treatment (Bateman 2010a; Bateman 2010b). In the pooled data there were fewer deaths in the tiotropium group (4.2%) than in the placebo group (4.9%), although the difference was not statistically significant (Peto OR 0.98; 95% CI 0.86 to 1.11; Analysis 1.19). However, there was some heterogeneity among the studies (I2 = 27%). Subgroup analysis showed no correlation with study duration (< 1 year, 7830 participants; ≥ 1 year, 15,479 participants; test for subgroup differences, P = 0.36; Analysis 1.20), but there was a statistically significant difference between the studies using the dry powder inhaler (16,787 patients) and those using a soft mist inhaler (6522 participants) (test for subgroup differences: P = 0.01; Figure 7). With the soft mist inhaler there were more deaths in the tiotropium group (2.4%) than the placebo group (1.7%) (Peto OR 1.47; 95% CI 1.04 to 2.08). With the dry powder inhaler there were fewer deaths in the tiotropium group (4.9%) than in the control group (6.1%) (Peto OR 0.92; 95% CI 0.80 to 1.05). The large difference in baseline risk is primarily caused by the large four‐year trial (Tashkin 2008), which drove up the baseline risk in the dry powder inhaler group.
1.19. Analysis.
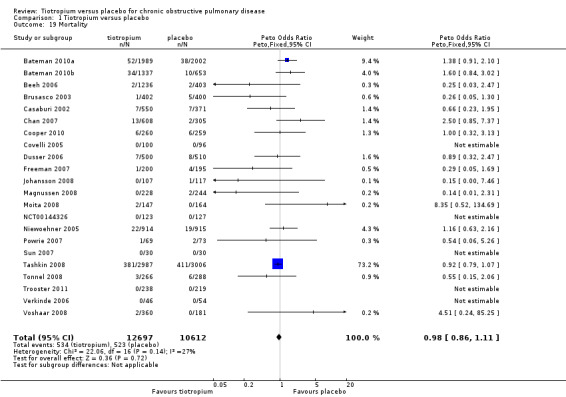
Comparison 1 Tiotropium versus placebo, Outcome 19 Mortality.
1.20. Analysis.
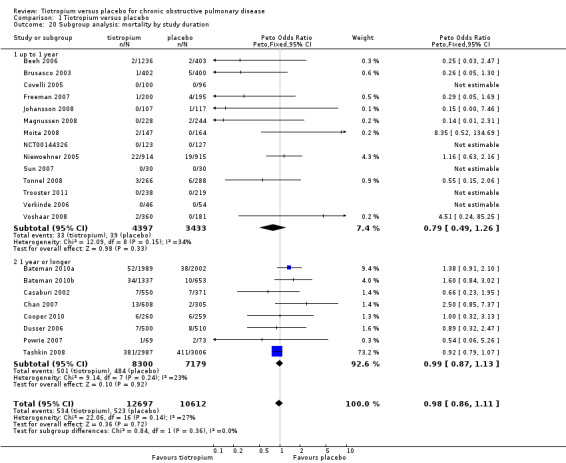
Comparison 1 Tiotropium versus placebo, Outcome 20 Subgroup analysis: mortality by study duration.
7.
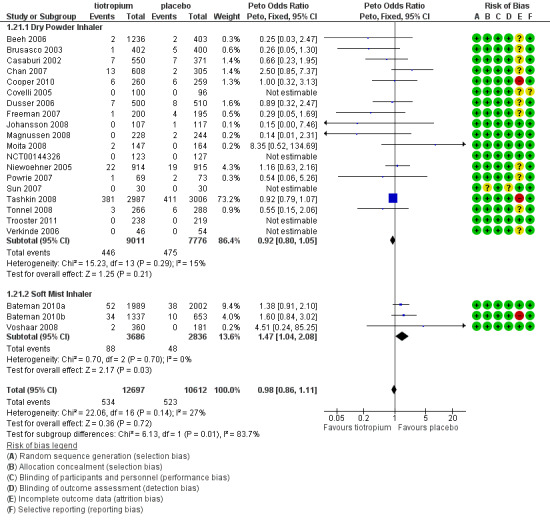
Forest plot of comparison: 1 Tiotropium versus placebo, outcome: 1.21 Subgroup analysis: mortality by inhaler device.
Furthermore, Tashkin 2008 reported a breakdown of results according to the concomitant medication taken by participants during the trial. There was no statistically significant difference in mortality among participants taking and those not taking LABAs (test for subgroup differences, P = 0.89; Analysis 1.22), ICS (test for subgroup differences, P = 0.38; Analysis 1.24), or both (test for subgroup differences, P = 1.00; Analysis 1.23). Tashkin 2008 also performed a subgroup analysis based on participants' disease severity. There was no statistically significant difference among those with mild/moderate (GOLD 2010 I/II), severe (GOLD 2010 III), and very severe (GOLD 2010 IV) COPD (test for subgroup differences, P = 0.76; Analysis 1.25).
1.22. Analysis.
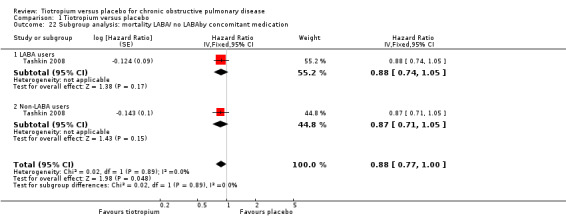
Comparison 1 Tiotropium versus placebo, Outcome 22 Subgroup analysis: mortality LABA/ no LABAby concomitant medication.
1.24. Analysis.
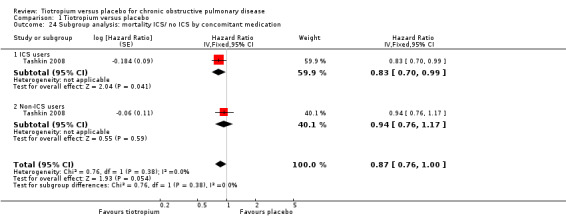
Comparison 1 Tiotropium versus placebo, Outcome 24 Subgroup analysis: mortality ICS/ no ICS by concomitant medication.
1.23. Analysis.
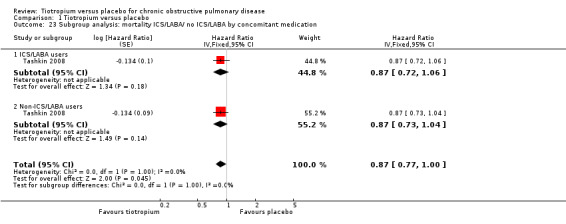
Comparison 1 Tiotropium versus placebo, Outcome 23 Subgroup analysis: mortality ICS/LABA/ no ICS/LABA by concomitant medication.
1.25. Analysis.
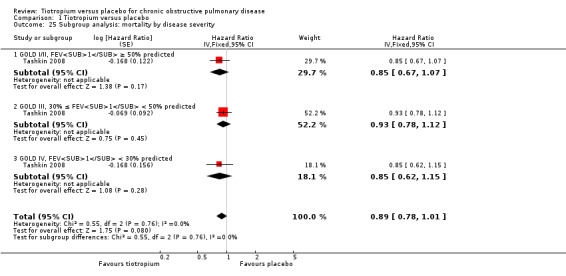
Comparison 1 Tiotropium versus placebo, Outcome 25 Subgroup analysis: mortality by disease severity.
Secondary outcome: Forced expiratory volume in one second (FEV1)
All of the included studies looked at lung function in terms of FEV1, but this was reported variously as: trough FEV1; post‐bronchodilator response at various time points; area under the curve (AUC); or as rate of decline of FEV1. Of all the different measures of FEV1' all 22 studies reported trough FEV1. In Voshaar 2008 the 5 mcg and 10 mcg tiotropium groups were similar in size and had similar FEV1 data. The two intervention groups were therefore combined using the mean of the groups for both the MD and the SE. The SE was adjusted by (1/√1.5) to take into account the 50% increase of n. The pooled analysis (22,764 participants) showed a statistically significant improvement in trough FEV1 with tiotropium compared to placebo (MD 118.92 mL; 95% CI 113.07 to 124.77; Analysis 1.26). Although there was moderate heterogeneity among the studies (I2 = 61%), the result was similar when analysed using a random‐effects model (MD 114.76 mL; 95% CI 102.91 to 126.61).
1.26. Analysis.
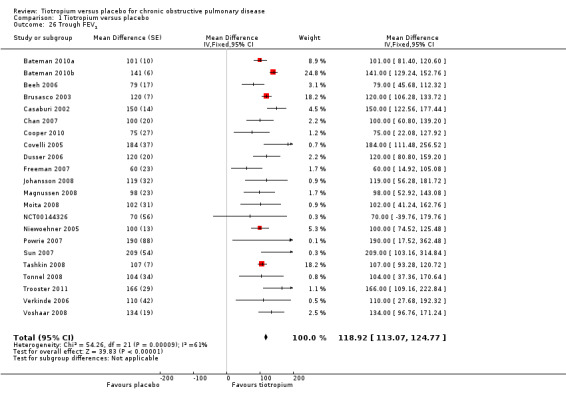
Comparison 1 Tiotropium versus placebo, Outcome 26 Trough FEV1.
Secondary outcome: all‐cause non‐fatal serious adverse events
All included studies (22 studies, 23,309 participants) reported serious adverse events. There was no statistically significant difference in the number of participants suffering one or more serious adverse event between the tiotropium and placebo groups (OR 1.03; 95% CI 0.97 to 1.10; Analysis 1.27).
1.27. Analysis.
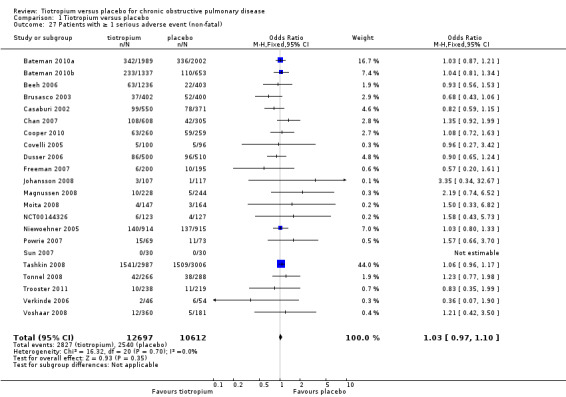
Comparison 1 Tiotropium versus placebo, Outcome 27 Patients with ≥ 1 serious adverse event (non‐fatal).
Owing to large differences among studies in whether or not serious adverse cardiovascular events were reported, or the type of events, we have not presented data on this outcome in this version of the review.
Secondary outcome: withdrawals from study treatment
All included studies (22 studies, 23,309 participants) reported the number of participants withdrawing from the study treatment before study completion. Even though there was moderate heterogeneity among the studies (I2= 44%), there were significantly fewer withdrawals in the tiotropium group compared to the placebo group when analysed using a random‐effects model (OR 0.66; 95% CI 0.59 to 0.73; Analysis 1.28).
1.28. Analysis.
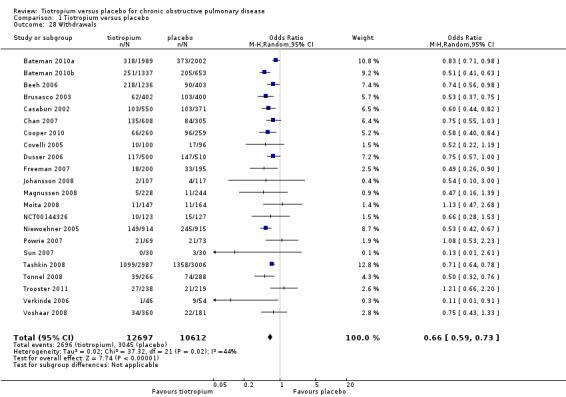
Comparison 1 Tiotropium versus placebo, Outcome 28 Withdrawals.
Discussion
Summary of main results
This systematic review set out to investigate the long‐term (three months or longer) effects of tiotropium for the treatment of COPD. We identified 22 eligible randomised, parallel group, placebo‐controlled trials with a total of 23,309 participants. All but one of the studies were sponsored by Boehringer Ingelheim (the manufacturer of tiotropium); the studies were generally of high methodological quality although there were moderate to high dropout rates and in some trials these were uneven. In this review we found that compared to placebo, treatment with tiotropium led to a significant mean improvement in quality of life (SGRQ; MD ‐2.89; 95% CI ‐3.35 to ‐2.44), however this mean improvement did not reach the accepted threshold of four units for a clinically significant difference. Yet, there were significantly more patients with a clinically important improvement (OR 1.52; 95% CI 1.38 to 1.68) and fewer patients with a clinically important worsening (OR 0.65; 95% CI 0.59 to 0.72) on tiotropium compared to placebo. Furthermore, tiotropium treatment significantly lowered the risk of exacerbations (OR 0.78; 95% CI 0.70 to 0.87), which would correspond to a total of approximately 16 COPD patients having to be treated with tiotropium for a year to prevent one additional exacerbation. We could not show a difference in the risk of exacerbation related to either disease severity or the type of tiotropium inhaler used. There were fewer patients with severe exacerbations leading to hospitalisation, and deaths among patients on tiotropium than on placebo, but the differences were not statistically significant. We found a statistically significant difference in the number of deaths in participants using the two different types of tiotropium inhaler, but event numbers were low and may have been affected by withdrawal rates which were generally higher than the mortality rates. Patients taking tiotropium using the soft mist inhaler had a significantly increased mortality risk compared to placebo, whereas fewer patients on tiotropium using the dry powder inhaler died then patients on no treatment. Of the secondary outcomes, the trough FEV1 was significantly improved with tiotropium compared with placebo. A significantly lower number of participants withdrew from the study treatment prematurely in the tiotropium group compared to placebo. There was no statistically significant difference between the number of participants suffering serious adverse events in the intervention and the control groups.
Overall completeness and applicability of evidence
Tiotropium has been on the market for several years. It was approved in Europe in 2002 and in the United States in 2004. To date, numerous trials on tiotropium have been completed. This review includes 22 long‐term studies of high methodological standard, giving robust evidence regarding the relative risks and benefits of tiotropium treatment.
When analysing the quality of life data in this review as a MD with a 95% CI, tiotropium treatment led to a statistically significant improvement in health‐related quality of life compared to placebo, although the point estimate and the whole CI were below the threshold of clinical significance (‐4 units). However, analysing the quality of life data as the number of patients with a clinically significant improvement or worsening in quality of life (more than ±4 units) showed a statistically significant difference favouring tiotropium for both outcomes. This shows that even though the mean improvement in SGRQ and the 95% CI were both less than the clinically significant change of 4 units, this is still compatible with significant differences in the proportion of individual patients who experienced a change of four units or more in either direction on their SGRQ.
Although a minimal clinically significant difference value for FEV1 is not well‐established, the MD in trough FEV1 with tiotropium treatment compared to placebo reached 119 mL in this review, which is within the range of values of 100 to 140 mL that has been suggested (Cazzola 2008).
One of the included studies (Magnussen 2008) enrolled COPD patients with concomitant asthma. They must have had an acute bronchodilator response ≥ 200 mL and ≥ 12% of pre‐bronchodilator FEV1 either at the screening visit or during the past five years, yet still had a post‐bronchodilator FEV1 < 80% predicted and a post‐bronchodilator ratio of FEV1/forced vital capacity (FVC) < 70%. It is estimated that 10% to 20% of COPD patients have features of asthma (Magnussen 2008). Although this subgroup may represent a substantial part of the COPD population, research regarding the efficacy of different treatments for this group has been limited, as most COPD trials exclude people with any features of asthma. This study did not carry much weight in any of the analyses and its results were similar to other trials. Removing it did not affect the overall results.
This review included data from the use of both the dry powder inhaler and soft mist tiotropium inhaler. Although the majority of studies used the dry powder inhaler, almost a third of the participants were enrolled in studies of the soft mist inhaler (6522 patients). The two inhalers were associated with similar improvements in quality of life and reduction of exacerbations, but subgroup comparisons between the results of different trials in the original version of this review highlighted a statistically significant difference in all‐cause mortality between the inhalers. However, as this finding is the result of subgroup analysis it should be interpreted carefully (Cates 2011; Oxman 1992). Notwithstanding this reservation, the difference in mortality was noted by the manufacturer of the two inhalers.
Recently, a large, industry‐supported prospective randomised, double blind study has found no significant increase in the risk of death or major cardiovascular adverse events with soft mist (Respimat 2.5 mcg or 5 mcg) compared with dry powder (Handihaler 18 mcg) delivery devices for tiotropium (Wise 2013). Over 17,000 participants were followed for a mean period of 2.3 years. The study included patients with a history of cardiac disease and stable heart failure. The trial did not include a placebo group, so we cannot assess whether tiotropium provided a benefit on overall mortality. All three treatment arms had a similar effect on reducing exacerbations. The trial followed up vital status for over 99% of the people who were randomised, but was subject to withdrawal rates of 21‐23% in all arms. Nevertheless the Wise 2013 trial provides the least biased evidence currently available, and has allayed some of the concerns of differences in mortality between the delivery devices (at the doses used and in the population studied). .
Information about the efficacy and risks of tiotropium in different ethnic groups is limited. The studies included in this review were conducted in a range of different countries though, when these were specified, they were countries with a predominantly white population. Specific ethnic subgroup analyses have been published for both the Niewoehner 2005 study, looking at the response to tiotropium in African‐American participants (Rice 2008), and Tashkin 2008 which examined the subgroup of Asian participants (Fukuchi 2011). Both concluded that tiotropium reduced COPD exacerbations and associated health‐care use to a similar extent in the subgroup as in the whole study population, although the number of participants in the subgroups was low (150 African‐American (Rice 2008), and 362 Asian (Fukuchi 2011) participants). The only study with a non‐white population was one small study undertaken in China (Sun 2007, 30 participants). An additional four placebo‐controlled trials of tiotropium, which may be eligible for inclusion in this review, have been undertaken in China (Gu 2007; Min 2006; Xia 2007; Yin 2010). However, these were identified too late to be included in this version of the review and so we will assess them in the next update (see Characteristics of studies awaiting classification).
Concerns have been expressed about the cardiovascular safety of tiotropium (Singh 2009). We had planned in the protocol to look at the effect of tiotropium on serious adverse cardiovascular events. However, a more recent systematic review, including 19,545 randomised patients in studies of four weeks or longer, showed that tiotropium was associated with a reduction in the risk of serious cardiovascular events (Celli 2010). In this review we did not try to obtain cardiovascular event data for the included studies from the manufacturer, nor additional studies published since Celli 2010, so as not to delay publication of this review.
Quality of the evidence
The studies included in this review were of high methodological quality. All but one of the included studies were sponsored by Boehringer Ingelheim, and conducted with similar protocols and definitions. The funnel plot of the exacerbation data, which included all 22 studies, showed no obvious signs of publication bias (Figure 6). However, for several of the outcomes studied in this review, events are relatively rare, leading to wide CIs and imprecision in the result (Table 1). Also, as these were long‐term COPD trials, many of them suffered from high and also uneven rates of patients withdrawing from study treatment before the end of the study, or withdrawing from the study altogether. The unknown status of many participants who withdraw from the study, whether they are lost to follow‐up or decline further study, and the large proportion of patients who may have stopped study medication early, has to be taken into account when looking at the evidence. This is especially important for outcomes such as mortality, which have few events compared to withdrawals. In many cases it is the healthier patients or the patients on active treatment that stay in the study, which might lead to a more conservative estimate of the treatment effect (Decramer 2011; Kesten 2007). However, in this review the studies contributing the greater part of the evidence for mortality followed up the vital status of participants who withdrew from the study treatment and from the study.
For many of the outcomes there was a certain amount of heterogeneity among the studies. This was addressed with a priori subgroup analyses and random‐effects analyses which take into account heterogeneity in study design. In most cases the causes of the heterogeneity were not able to be identified.
Potential biases in the review process
We performed comprehensive searches to identify relevant studies. We contacted authors of studies with missing data. The manufacturer (Boehringer Ingelheim), which sponsored all but one of the included studies, was accommodating in supplying information about study designs and missing data for several of the studies.
Agreements and disagreements with other studies or reviews
Our results are generally consistent with the findings of others.
A systematic review from 2010 looked at RCTs comparing the effect of tiotropium to placebo or active‐control arms on quality of life and dyspnoea (Kaplan 2010). This review described the quality of life result from nine trials comparing tiotropium to placebo. Similarly to the present review, Kaplan 2010 showed a statistically significant improvement with tiotropium in all trials except the two using concurrent pulmonary rehabilitation, which were excluded from our review (Ambrosino 2008; Casaburi 2005). Yohannes 2011 is another systematic review which looked at the effectiveness of tiotropium versus placebo, ipratropium or LABA. The review had similar selection criteria to our review, but at the time only identified 11 studies comparing tiotropium with placebo. The review reported comparable results for patients with a clinically significant improvement in quality of life (six studies, OR 1.61; 95% CI 1.38 to 1.88) comparable to this review (nine studies, OR 1.54; 95% CI 1.40 to 1.70). They also examined the proportion of patients with a clinically significant change in the Transition Dyspnoea Index (TDI) which was in favour of tiotropium (two studies, OR 1.96; 95% CI 1.58 to 2.44). Similarly to our review, Yohannes 2011 showed a significant reduction in the OR of patients having exacerbations (11 studies, OR 0.83; 95% CI 0.72 to 0.94) and exacerbation‐related hospitalisations (seven studies, OR 0.89; 95% CI 0.80 to 0.98). They also showed no statistically significant difference in the number of patients experiencing a serious adverse event (OR 1.06; 95% CI 0.97 to 1.17).
Celli 2010 is a safety review of Boehringer Ingelheim‐sponsored tiotropium trials (19,545 patients). It included data from 18 of the studies included in our review, plus an additional eight trials, which did not fit the inclusion criteria for this review. The pooled result showed a significant decrease in both fatal (RR 0.88; 95% CI 0.77 to 1.00) and serious adverse events (RR 0.94; 95% CI 0.89 to 1.00, including fatal events) with tiotropium. Meta‐analysis of cardiovascular data from these trials showed tiotropium to be associated with a reduction in major cardiovascular events (RR 0.83; 95% CI 0.71 to 0.98) and fatal cardiovascular events (RR 0.77; 95% CI 0.60 to 0.98). The cardiovascular composite endpoint included fatal events in the system organ classes cardiac and vascular disorders combined with myocardial infarction (fatal and non‐fatal), stroke (fatal and non‐fatal), and the preferred terms sudden death, sudden cardiac death, and cardiac death.
Kesten 2009 is another safety review of Boehringer Ingelheim‐sponsored tiotropium trials but only covering trials using the dry powder inhaler. It included 24 trials with a minimum of four weeks duration. Of these 17 were included in our review. Presenting the data as a risk difference per 100 patient‐years at risk showed a significantly lowered risk for mortality with tiotropium compared to placebo (RD ‐0.63; 95% CI ‐1.14 to ‐0.12) (Kesten 2009). The lower number of deaths in the tiotropium group compared to placebo corresponds well with the results for mortality in the dry powder inhaler group in our review. Kesten 2009 found a statistically significant decrease in serious adverse events (RD ‐1.41; 95% CI ‐2.81 to ‐0.00) using tiotropium dry powder inhaler. Our meta‐analysis of this outcome did not show a statistically significant difference in the numbers of patients with non‐fatal serious adverse events between tiotropium and placebo, whether looking at all studies or just studies using the dry powder inhaler. The discrepancy in the results may be due to Kesten 2009 including fatal serious adverse events in their data.
Singh 2011 presented mortality data from a systematic search of soft mist inhaler trials. The review included the same trials as this review, showing an increased risk of mortality with the soft mist inhaler. Another systematic review has looked at direct comparisons between the soft mist inhaler and other inhaler devices (Ram 2011). It found three studies looking at the difference between tiotropium via soft mist inhaler and tiotropium via dry powder inhaler. These were short‐term (three to four weeks) cross‐over or parallel group trials, hence not eligible for our review. Similarly to our trial, they showed no statistically significant difference between soft mist and dry powder inhaler in the risk of exacerbation (715 patients, RR 0.94; 95% CI 0.58 to 1.54). The results of a large head‐to‐head study comparing the dry powder and the soft mist inhaler for tiotropium have now been reported (Wise 2013) and have not confirmed any important difference in mortality between the soft mist and dry powder inhalers.
Authors' conclusions
Implications for practice.
Compared with placebo, tiotropium treatment was associated with an improvement in COPD patients' quality of life and a reduction in the risk of exacerbations, including exacerbations leading to hospitalisation. Furthermore, tiotropium did not appear to significantly reduce serious adverse events or mortality, but it did lead to an improvement in lung function.
This review confirms guideline recommendations for the use of tiotropium in the management of patients with stable COPD, particularly if reduction in exacerbations is the goal. The trials included in the review showed that tiotropium delivered via the Respimat soft mist inhaler was associated with a statistically significant increased risk of mortality. However, it should be emphasised that these were subgroup analyses and not head‐to‐head comparisons. A recent large double‐blind trial of the two delivery devices found no substantial difference in mortality using 2.5 mcg or 5 mcg of tiotropium via Respimat in comparison to 18 mcg via Handihaler.
The quality of life data suggest some patients will notice a clear symptomatic benefit with tiotropium treatment and some will not. Given the cost of the medication, it is debatable whether or not to continue treatment indefinitely in those COPD patients who do not have frequent exacerbations, and have no difference in quality of life with tiotropium.
Implications for research.
Because of the high and often uneven withdrawal rates in COPD trials, new trials should follow up the vital status of all participants, even if they have withdrawn from the study.
Other areas for study include whether the lack of difference seen in serious adverse event rates is an artefact of how events have been counted, or a result of an effect of the medication that outweighs the benefits in terms of reduced exacerbations. The results of this review need to be considered in the light of other Cochrane reviews looking at tiotropium versus LABA and ICS, as well as reviews where it is used as add‐on therapy. This should be considered alongside new and existing evidence on safety concerns for the difference inhaler devices.
We suggest there is a need for studies in other ethnic groups and settings, and that cost‐effectiveness data is sought to assist in the clinical decision of whether or not it is prescribed.
Feedback
Feeback regarding missing SGRQ data and PRISMA flow diagram
Summary
In reading your review, we had a few concerns, listed below:
Figure 1: Inconsistency with numbers in flow diagram (Figure 1); 4 references unaccounted for.
Potential attrition bias in St. George’s Respiratory Questionnaire (SGRQ) outcomes: We examined the three largest trials that accounted for about 63% of the weight in your SGRQ questionnaire responder forest plot (Analysis 1.2):
For the Bateman 2010a, 633/3991 SGRQ scores are missing
For the Bateman 2010b the trial authors state that 192/1990 SGRQ scores are missing. However, in your review, you had 1247 patients listed in the tiotropium group, and 551 in the placebo group. We were unable to determine where these numbers were obtained from.
For the Tashkin 2008 1125/5992 SGRQ scores are missing. There is also some confusion as the number of SGRQ scores listed in the forest plot of your review (Analysis 1.2) and in addition, the mean difference reported in Analysis 1.1 for the Tashkin 2008 trial was ‐2.28, whereas the mean difference reported in the trial itself was ‐2.7. We were curious as to how you determined the mean difference, as well as the denominators for each group that you included in Analysis 1.2 for the Tashkin 2008 trial.
1.1. Analysis.
Comparison 1 Tiotropium versus placebo, Outcome 1 Quality of life (SGRQ total score).
In light of the above, were any sensitivity analyses done to account for the missing data, and were the authors contacted to determine why there was such a large amount of SGRQ data missing?
To obtain a crude estimate of the potential impact the missing data, we constructed forest plots (RevMan 2011) based on two possible scenarios with assumptions about the missing data using the data presented in (Analysis 1.2).
Assumption 1: Imputation of negative outcomes (non‐response) for missing data in the tiotropium group, and positive outcomes (response: >4 point decrease in SGRQ) for missing data in the placebo group gives an odds ratio 1.12 [0.58, 2.18].
Assumption 2: Imputation of positive outcomes (response: >4 point decrease in SGRQ) for missing data in the tiotropium group, and negative outcomes (non‐response) for missing data in the placebo group gives an odds ratio of 2.26 [1.33, 3.84].
However, there is significant heterogeneity (I2= 98‐99%) when applying the listed assumptions and so results should be interpreted with caution.
As illustrated above, the missing data can skew the pooled effect towards either response or non‐response to tiotropium. As a result, we feel that readers should be cautioned on the limitations of the data presented and the grade of the quality of evidence should be reassessed. We believe that the true effect of tiotropium on quality of life is difficult to ascertain, and until adequate information is provided, we believe that it is impossible to conclude with confidence that tiotropium significantly improves quality of life as measured by St George’s Respiratory Questionnaire scores.
Reply
We thank the feedback authors for their interest in our review and for raising the issue of attrition bias in the responder analysis for the SGRQ outcome. We obtained additional information from the trial sponsors relating the number of participants in each group who suffered a deterioration of 4 units or more in their total SGRQ score at the end of each trial. We were interested in this information to see if the improvement in SGRQ reflected in the responder analysis reported in the papers (for the proportion of people who improved by 4 units or more in their total SGRQ score) was reflected in a similar reduction in those who deteriorated. This accounts for the difference between the number of participants in each trial arm reported in the published papers and those entered in the review.
In terms of the Mean Difference in SGRQ in the Tashkin 2008 trial, we entered the data from the end of the trial, obtained from Figure 2D in the paper. The paper reported a mean difference of ‐2.28 units over the total duration of the trial; this was not an outcome that was reported in the other studies, which is why we did not enter this data.
Whilst we agree that sensitivity analysis of current data is limited for providing further information on missing participants, we believe that the estimates presented by the feedback authors demonstrate the extreme upper and lower limits which would not be typical of the distribution of results seen within any treatment or control group for SGRQ response. Similarly, the high levels of heterogeneity associated with the assumptions highlight that it becomes increasingly unlikely to see a trend towards all the participants who withdrawal from one arm of the trial being responders, and all those from the other arm as not.
There is evidence that those who withdraw from COPD trials tend to have worse outcomes than those who remain (Kesten 2007). In view of this we have focused on the three largest studies (to match the analysis carried out by the authors of the feedback) and have also used the responder data at the end of the first year for Tashkin 2008 (as provided by the sponsors). We have carried out our own sensitivity analysis based on two assumptions: firstly that none of those who are missing were responders (Analysis 1.29) and secondly that 20% of those who are missing were responders (Analysis 1.30).
1.29. Analysis.
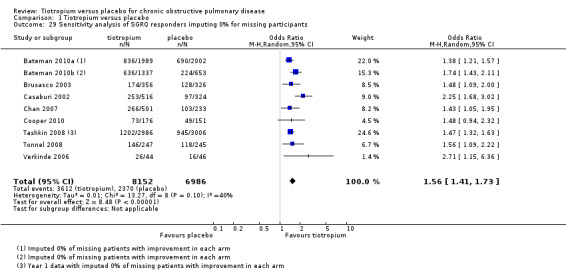
Comparison 1 Tiotropium versus placebo, Outcome 29 Sensitivity analysis of SGRQ responders imputing 0% for missing participants.
1.30. Analysis.
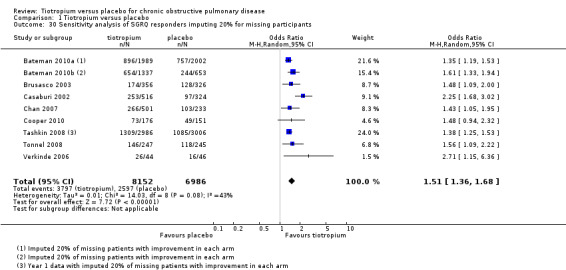
Comparison 1 Tiotropium versus placebo, Outcome 30 Sensitivity analysis of SGRQ responders imputing 20% for missing participants.
We regard this as a more plausible range of outcomes for the missing participants. We can see no reason (beyond the play of chance) for imbalance in the likelihood of improvement between the tiotropium and placebo arms. This sensitivity analysis changes the point estimates slightly for these three studies, but makes little difference to the pooled results. On the basis of these assumptions, which we regard as more plausible than those proposed in the feedback, we still conclude that it is likely quality of life improves significantly for more people on tiotropium than on placebo.
We also thank the feedback authors for highlighting the inconsistency in the flow diagram (Figure 1) which will be addressed when the review is next updated.
Contributors
Catharina Yih
Alfie Chung
Aaron M Tejani
Lower Mainland Pharmacy Services, BC, Canada
Feedback regarding presentation of uncertainty and missing data, 26 October 2016
Summary
Thank you for your insightful review on tiotropium.
In your conclusions, you state that tiotropium reduces the risk of exacerbations with a number needed to benefit (NNTB) of 16 to prevent one exacerbation. We believe that perhaps this statement is too definitive and there is a degree of uncertainty around the effects of tiotropium on exacerbations that is not conveyed.
Firstly, for exacerbations you report a number needed to benefit (NNTB) of 16 over one year based on the results of your analysis. However, this may not be an appropriate estimate of the true NNTB over a single year given the studies included in your analysis ranged from 3‐48 months (2).
Secondly, it is difficult to be certain of effect size on exacerbations given the high level of attrition in the included studies. To highlight the fact that exacerbations could be missed in the studies with high attrition we looked at the largest study in your analysis, Tashkin 2008. In this trial patients who discontinued study drug were asked to return for a voluntary follow‐up visit 30 days after cessation, but after this visit no exacerbation data was collected. This means exacerbation data was not collected after patients left the trial and likely numerous exacerbations were not accounted for.
In the Tashkin 2008 trial, there was a difference of 48 exacerbations between the tiotropium and placebo group and 2457 patients who did not complete the trial. Assuming a similar rate of exacerbations in those who did not complete the study, as many as 1600 exacerbations could be unaccounted for. Depending on the rates in each arm, this could strengthen or weaken the benefit of tiotropium greatly.
Similarly in the 2nd largest trial included in the review, Bateman 2010a, there was a difference of 157 exacerbations between the tiotropium and placebo group and 691 patients who did not complete the trial. Assuming a similar rate of exacerbations as seen in the trial there is the potential for up to 270 exacerbations that were not recorded.
We believe the number of missing patients, especially from large studies that were heavily weighted in the analysis, should be taken into account when making your conclusions. Based on the attrition and the uncertainty about what happened to patients who left, the direction of the effect on exacerbations is still unknown.
References
Higgins JPT, Green S (editors). Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration, 2011. Available from: www.cochrane‐handbook.org
Suissa S. Number needed to treat: enigmatic results for exacerbations in COPD. Eur Respir J. 2015;45:875–8.
Reply
Thank you for your interest in our review and for your feedback. Responses to your points are made below. While we have not made any changes to the review, your comments will be helpful at the time of the next update.
The reported NNTB is presented with an associated measure of statistical uncertainty in the review. In this instance, the 95% confidence interval for the NNTB of 16 is 10 to 36. This estimate should be taken into consideration when interpreting the findings from the review.
This feedback highlights one of the difficulties when generating a NNTB for studies of different durations. 'Extrapolating' the NNTB to fit the period of the longest trial duration is the more conservative approach, rather than using the average or the shortest trial duration. The authors would like to highlight that in this case the NNTB of 16 is based on the rate of exacerbations in patients treated with tiotropium from all of the included trials, however the baseline risk of exacerbation (i.e. the exacerbation rate for patients on placebo) is based on the trials with a one year follow‐up as the risk differences are very unlikely to be consistent across baseline event rates from trials with different follow‐up. However, the authors agree that there is limited evidence surrounding whether the treatment benefits of tiotropium over placebo remain stable or vary over time. This will be useful to highlight in future updates of the review.
The risk from attrition bias highlighted in this feedback is an issue commonly faced in systematic reviews. These specific examples raise the possibility that a number of exacerbation events were not recorded in participants who did not complete the study. As suggested, it is unclear whether this could either strengthen or weaken the measured benefit of tiotropium when compared to placebo. However, this systematic review shows that the rate of withdrawals are higher for patients in the placebo arms than for patients on tiotropium, and in general patients who withdraw from studies tend to have more severe disease than people who stay in the study and may be more likely to have an exacerbation (1). Hence, the calculated NNT is more likely to be an underestimate of the effectiveness of tiotropium over placebo rather than the other way around. As mentioned above, this will also be useful to highlight in future updates of the review.
References
Rennard S.I. ATS 2012, http://www.atsjournals.org/doi/pdf/10.1164/ajrccm‐conference.2012.185.1_MeetingAbstracts.A2943 accessed 09/11/16.
Contributors
Jessica Beach, Aaron Tejani
Faculty of Pharmaceutical Sciences, University of British Columbia, Vancouver, Canada.
What's new
| Date | Event | Description |
|---|---|---|
| 15 November 2016 | Feedback has been incorporated | Feedback received and responded to. No changes made to review text. |
History
Protocol first published: Issue 8, 2011 Review first published: Issue 7, 2012
| Date | Event | Description |
|---|---|---|
| 26 January 2015 | Feedback has been incorporated | Feedback and response added. Added two sensitivity analyses with imputations for responders and non‐responders to illustrate the feedback response. No changes made to review. |
| 26 January 2015 | Amended | Feedback added |
| 12 May 2014 | New citation required and conclusions have changed | No new literature search has been run. Information from a large randomised trial comparing the safety of Respimat with Handihaler delivery devices has been added to the review. This trial was ongoing at the time of publication of the last version of this review and we felt it had to be included in the review, although the review is not being updated with a new search at this time. |
| 6 June 2013 | Amended | Typo in QoL treatment effect in summary of findings table corrected. JC affiliation updated. Author affiliations updated. |
| 12 April 2013 | Amended | Funder acknowledgement added |
Acknowledgements
We are grateful to Elizabeth Stovold for help designing the search strategy.
Boehringer Ingelheim have been helpful clarifying and supplying additional information about studies sponsored by them.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Airways Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| CENTRAL (the Cochrane Library) | Quarterly (4 issues per year) |
| PscyINFO (Ovid) | Monthly |
| CINAHL (Ebsco) | Monthly |
| AMED (Ebsco) | Monthly |
Hand‐searches: Core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE Search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Appendix 2. Search terms ClinicalTrials.gov
intervention: tiotropium
condition: COPD
study type: interventional studies
Data and analyses
Comparison 1. Tiotropium versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life (SGRQ total score) | 9 | Mean Difference (Fixed, 95% CI) | ‐2.89 [‐3.35, ‐2.44] | |
| 2 Patients with ≥ 4 units improvement in quality of life (SGRQ) | 9 | 11672 | Odds Ratio (M‐H, Random, 95% CI) | 1.52 [1.38, 1.68] |
| 3 Patients with ≥ 4 units worsening in quality of life (SGRQ) | 9 | 11672 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.59, 0.72] |
| 4 Subgroup analysis: Quality of life ICS/ no ICS (SGRQ) | 1 | Mean Difference (Fixed, 95% CI) | ‐2.86 [‐4.79, ‐0.94] | |
| 4.1 ICS users | 1 | Mean Difference (Fixed, 95% CI) | ‐3.27 [‐5.62, ‐0.92] | |
| 4.2 Non‐ICS users | 1 | Mean Difference (Fixed, 95% CI) | ‐2.05 [‐5.38, 1.28] | |
| 5 Subgroup analysis: Quality of life LABA/ no LABA (SGRQ) | 4 | Mean Difference (Fixed, 95% CI) | ‐3.27 [‐3.96, ‐2.57] | |
| 5.1 LABA users | 1 | Mean Difference (Fixed, 95% CI) | ‐2.8 [‐4.05, ‐1.55] | |
| 5.2 Non‐LABA users | 4 | Mean Difference (Fixed, 95% CI) | ‐3.48 [‐4.32, ‐2.63] | |
| 6 Subgroup analysis: Quality of life (SGRQ) by disease severity | 2 | 2235 | Mean Difference (IV, Fixed, 95% CI) | ‐3.28 [‐4.67, ‐1.90] |
| 6.1 GOLD I and II, mild and moderate, FEV1 ≥ 50% predicted | 2 | 1945 | Mean Difference (IV, Fixed, 95% CI) | ‐2.66 [‐4.20, ‐1.12] |
| 6.2 GOLD III and IV, severe and very severe, FEV1 < 50% predicted | 1 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐6.00 [‐9.22, ‐2.78] |
| 7 Quality of life (EQ‐5D total score) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Quality of life (CRQ total score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Patients with ≥ 1 exacerbation | 22 | 23309 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.70, 0.87] |
| 10 Subgroup analysis: patients with ≥ 1 exacerbation by study duration | 22 | 23309 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.70, 0.87] |
| 10.1 up to 1 year | 14 | 7830 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.66, 0.84] |
| 10.2 1 year or longer | 8 | 15479 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.95] |
| 11 Subgroup analysis: patients with ≥ 1 exacerbations ICS/ no ICS by concomitant medication | 1 | (Random, 95% CI) | ‐0.57 [‐0.84, ‐0.31] | |
| 11.1 ICS users | 1 | (Random, 95% CI) | ‐0.62 [‐0.95, ‐0.29] | |
| 11.2 Non‐ICS users | 1 | (Random, 95% CI) | ‐0.49 [‐0.92, ‐0.06] | |
| 12 Subgroup analysis: patients with ≥ 1 exacerbation by inhaler device | 22 | 23309 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.70, 0.87] |
| 12.1 Dry Powder Inhaler | 19 | 16787 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.69, 0.89] |
| 12.2 Soft Mist Inhaler | 3 | 6522 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.67, 0.82] |
| 13 Subgroup analysis: patients with ≥ 1 exacerbation by disease severity | 4 | Odds Ratio (Random, 95% CI) | 0.85 [0.68, 1.07] | |
| 13.1 GOLD II, moderate, 50% ≤ FEV1 < 80% predicted | 4 | Odds Ratio (Random, 95% CI) | 0.67 [0.45, 0.99] | |
| 13.2 GOLD III, severe, 30% ≤ FEV1 < 50% predicted | 3 | Odds Ratio (Random, 95% CI) | 0.94 [0.70, 1.25] | |
| 13.3 GOLD IV, very severe, FEV1 < 30% predicted | 3 | Odds Ratio (Random, 95% CI) | 1.12 [0.51, 2.43] | |
| 14 Patients with ≥ 1 exacerbation requiring hospitalisation | 21 | 22852 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.00] |
| 15 Patients with ≥ 1 hospitalisation (all‐cause) | 19 | 20963 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.88, 1.13] |
| 16 Subgroup analysis: patients with ≥ 1 exacerbation requiring hospitalisation by study duration | 21 | 22852 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.00] |
| 16.1 up to 1 year | 13 | 7373 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.57, 1.36] |
| 16.2 1 year or longer | 8 | 15479 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.78, 1.01] |
| 17 Subgroup analysis: patients with ≥ 1 exacerbation requiring hospitalisation by inhaler device | 21 | 22852 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.00] |
| 17.1 Dry Powder Inhaler | 18 | 16330 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.69, 1.09] |
| 17.2 Soft Mist Inhaler | 3 | 6522 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.68, 0.99] |
| 18 Subgroup analysis: patients with ≥ 1 exacerbation requiring hospitalisation by disease severity | 1 | 5895 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.05] |
| 18.1 GOLD II, moderate, 50% ≤ FEV1 < 80% predicted | 1 | 2739 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.61, 0.91] |
| 18.2 GOLD III, severe, 30% ≤ FEV1 < 50% predicted | 1 | 2635 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.90, 1.25] |
| 18.3 GOLD IV, very severe, FEV1 < 30% predicted | 1 | 521 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.77, 1.53] |
| 19 Mortality | 22 | 23309 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.86, 1.11] |
| 20 Subgroup analysis: mortality by study duration | 22 | 23309 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.86, 1.11] |
| 20.1 up to 1 year | 14 | 7830 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.49, 1.26] |
| 20.2 1 year or longer | 8 | 15479 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.87, 1.13] |
| 21 Subgroup analysis: mortality by inhaler device | 22 | 23309 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.86, 1.11] |
| 21.1 Dry Powder Inhaler | 19 | 16787 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.80, 1.05] |
| 21.2 Soft Mist Inhaler | 3 | 6522 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.47 [1.04, 2.08] |
| 22 Subgroup analysis: mortality LABA/ no LABAby concomitant medication | 1 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.77, 1.00] | |
| 22.1 LABA users | 1 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.74, 1.05] | |
| 22.2 Non‐LABA users | 1 | Hazard Ratio (Fixed, 95% CI) | 0.87 [0.71, 1.05] | |
| 23 Subgroup analysis: mortality ICS/LABA/ no ICS/LABA by concomitant medication | 1 | Hazard Ratio (Fixed, 95% CI) | 0.87 [0.77, 1.00] | |
| 23.1 ICS/LABA users | 1 | Hazard Ratio (Fixed, 95% CI) | 0.87 [0.72, 1.06] | |
| 23.2 Non‐ICS/LABA users | 1 | Hazard Ratio (Fixed, 95% CI) | 0.87 [0.73, 1.04] | |
| 24 Subgroup analysis: mortality ICS/ no ICS by concomitant medication | 1 | Hazard Ratio (Fixed, 95% CI) | 0.87 [0.76, 1.00] | |
| 24.1 ICS users | 1 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.70, 0.99] | |
| 24.2 Non‐ICS users | 1 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.76, 1.17] | |
| 25 Subgroup analysis: mortality by disease severity | 1 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.78, 1.01] | |
| 25.1 GOLD I/II, FEV1 ≥ 50% predicted | 1 | Hazard Ratio (Fixed, 95% CI) | 0.85 [0.67, 1.07] | |
| 25.2 GOLD III, 30% ≤ FEV1 < 50% predicted | 1 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.78, 1.12] | |
| 25.3 GOLD IV, FEV1 < 30% predicted | 1 | Hazard Ratio (Fixed, 95% CI) | 0.85 [0.62, 1.15] | |
| 26 Trough FEV1 | 22 | Mean Difference (Fixed, 95% CI) | 118.92 [113.07, 124.77] | |
| 27 Patients with ≥ 1 serious adverse event (non‐fatal) | 22 | 23309 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.97, 1.10] |
| 28 Withdrawals | 22 | 23309 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.59, 0.73] |
| 29 Sensitivity analysis of SGRQ responders imputing 0% for missing participants | 9 | 15138 | Odds Ratio (M‐H, Random, 95% CI) | 1.56 [1.41, 1.73] |
| 30 Sensitivity analysis of SGRQ responders imputing 20% for missing participants | 9 | 15138 | Odds Ratio (M‐H, Random, 95% CI) | 1.51 [1.36, 1.68] |
1.9. Analysis.
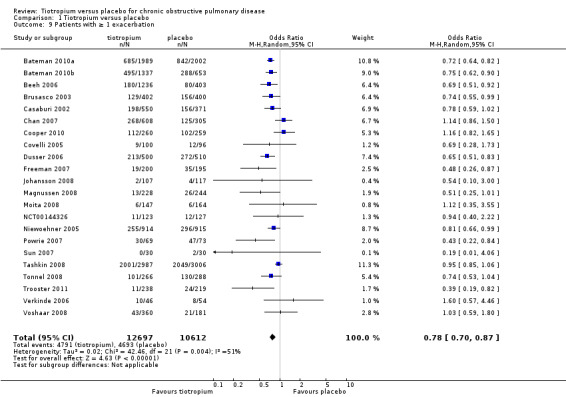
Comparison 1 Tiotropium versus placebo, Outcome 9 Patients with ≥ 1 exacerbation.
1.18. Analysis.
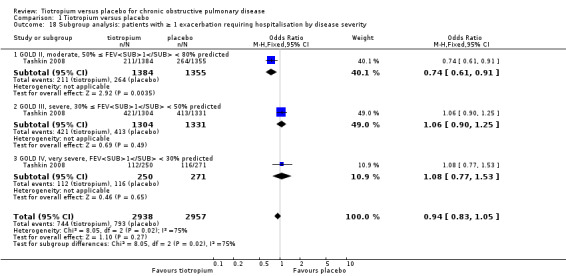
Comparison 1 Tiotropium versus placebo, Outcome 18 Subgroup analysis: patients with ≥ 1 exacerbation requiring hospitalisation by disease severity.
1.21. Analysis.
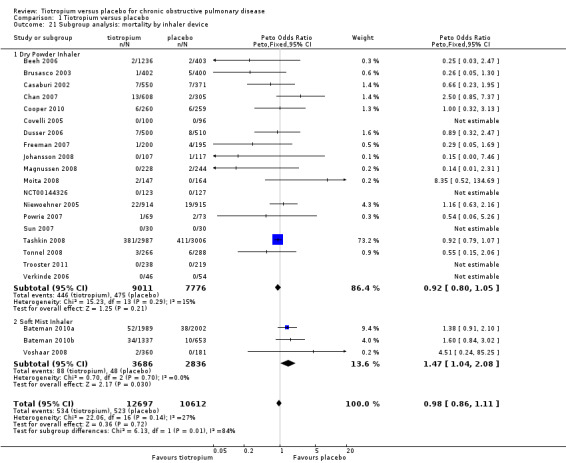
Comparison 1 Tiotropium versus placebo, Outcome 21 Subgroup analysis: mortality by inhaler device.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bateman 2010a.
| Methods | Design: a randomised, double‐blind, placebo‐controlled, parallel group trial with recruitment from October 2006 to December 2007. The trial included 336 outpatient centres spanning five continents and involving 31 countries. Duration of treatment was 48 weeks | |
| Participants |
Population: 3991 patients with COPD, as defined by GOLD guidelines, were randomised to tiotropium (1989) and placebo (2002) Baseline Characteristics: mean age 65 years, 78% male, mean FEV1 1.1 L, mean FEV1 predicted 40%, 45 pack‐years smoking history Inclusion Criteria: COPD patients of either sex were eligible for study entry if they were aged > 40 years, had pre‐bronchodilator FEV1 of ≤ 60% of predicted normal and a FEV1 / FVC ≤ 70%, and were current or ex‐smokers (smoking history of ≥ 10 pack‐years) Exclusion Criteria: patients were excluded if they had a significant disease other than COPD that, in the investigator’s judgment, could affect the patient’s ability to complete the trial, or if they had clinically significant abnormal results of haematology, urinalysis, or blood chemistry tests, a history of asthma or allergic rhinitis, or a blood eosinophil count of ≥ 600/mm3. Other exclusion criteria included previous lung resection surgery, participation in a pulmonary rehabilitation programme in the previous six weeks, and regular daytime oxygen use (>1 h/day). Less stringent exclusion criteria relating to cardiovascular disorders were employed, with the aim of making the patient sample more representative of the range of COPD patients typically encountered in clinical practice, but patients with a history of unstable arrhythmias, myocardial infarction in the previous 6 months or heart failure requiring in‐hospital treatment in the previous 12 months were excluded. Patients who had previously used tiotropium delivered via Respimat were also excluded, but those who had previously used tiotropium delivered via HandiHaler could enter the trial if they stopped taking it at least 28 days before the randomisation visit |
|
| Interventions |
1. tiotropium 5 mcg (two puffs of 2.5 mcg each) once daily in the morning 2. placebo (two puffs) once daily in the morning Inhaler device: Respimat inhaler Co‐medication: Salbutamol pMDI was provided to all patients for use as rescue medication at any time during the study. All respiratory medications were permitted during the trial other than inhaled anticholinergics. |
|
| Outcomes |
Primary: trough FEV1 response, i.e. the difference between predose FEV1 on Day 1 of the treatment period and the corresponding value after 48 weeks of treatment, and time to first COPD exacerbation Secondary: mean number of COPD exacerbations per patient‐year; the total number of exacerbations that resulted in urgent visits to a health care provider or emergency department; the number of hospitalisations for COPD; the total number of hospitalisations for all‐causes; changes in health‐related quality of life, dyspnoea, lung function |
|
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.372, European Clinical Trials Database 2006‐001009‐27, ClinicalTrials.gov NCT00387088 Definitions: exacerbations were defined as a complex of respiratory events or symptoms that lasted ≥ 3 days and required treatment with antibiotics and/or systemic corticosteroids, or prompted the investigator to change the patient’s regular respiratory medication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment allocation was determined by a computer‐generated randomisation code provided by Boehringer Ingelheim. Randomisation was stratified by study centre and within centres, and performed in blocks to ensure balanced distribution of the treatment groups at any time |
| Allocation concealment (selection bias) | Low risk | Individuals directly involved in the conduct and analysis of the trial had no access to the allocation sequence until after the trial was completed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identity of treatments was blinded to investigators, assessors and patients. Tiotropium and placebo were both inhaled via the Respimat inhaler once daily in the morning |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Identity of treatments was blinded to investigators, assessors and patients |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal rates were 16.0% in the tiotropium group and 18.6% in the placebo group. Mortality was assessed for the planned duration of the trial for all patients, including those who prematurely discontinued study medication |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
Bateman 2010b.
| Methods | Design: two identical, multicentre, multinational, randomised, double‐blind, parallel‐group studies. The run‐in phase was two weeks and the duration of treatment was 48 weeks, recruitment for the studies took place from January 2003 to December 2005 | |
| Participants |
Population: 1990 patients with COPD, as defined by ATS guidelines, were randomised to tiotropium 5 mcg (670), tiotropium 10 mcg (667), and placebo (653) Baseline Characteristics: mean age 65 years, 74% male, mean FEV1 1.06 L, mean FEV1 predicted 38%, 48 pack‐years smoking history Inclusion Criteria: males and females aged ≥ 40 years with a diagnosis of COPD and stable, moderate‐to‐severe airway obstruction (pre‐bronchodilator FEV1 ≤ 60% predicted and FEV1 ≤ 70% of FVC), and with a smoking history of ≥ 10 pack‐years were included Exclusion Criteria: patients with a confounding disease, including other significant respiratory conditions, were excluded, as were those who had a disease that might put them at risk because of study participation. Other exclusion criteria included known hypersensitivity to anticholinergics or any component of the Respimat inhalation solution; drugs contraindicated with anticholinergics; prior use of Spiriva HandiHaler; regular use of daytime oxygen therapy, oral beta‐adrenergics, or LABAs; or significant alcohol or drug abuse |
|
| Interventions |
1. Orally inhaled tiotropium 5 mcg, 2 actuations of 2.5 mcg tiotropium once daily in the morning 2. Orally inhaled tiotropium 10 mcg, 2 actuations of 5 mcg tiotropium once daily in the morning 3. 2 actuations of placebo inhalation solution once daily in the morning Inhaler device: soft mist inhaler Co‐medication: oral (up to 10 mg daily of prednisone) and ICS, theophylline preparations, mucolytic agents and antileukotrienes were allowed if stabilised for at least six weeks prior to and during the study. Patients on LABAs and ICS were switched to a monoproduct ICS prior to run‐in. Salbutamol metered‐dose inhaler (MDI) was used as rescue medication |
|
| Outcomes |
Primary: there were 4 co‐primary endpoints for both studies:
Secondary: secondary endpoints included FVC, peak expiratory flow rate (PEFR) and weekly mean number of occasions (per day as needed) that rescue medication was used. COPD symptom scores (wheezing, shortness of breath, coughing, and tightness of chest) were based on the investigator’s assessment of the patient’s condition during the week just prior to the clinic visit. The Physician's Global Evaluation, was based on the physician’s opinion of the patient’s overall clinical condition, and the Patient's Global Rating, was performed by the patients. Detailed information on exacerbations and COPD exacerbation‐related hospitalisations were recorded. Clinical efficacy measures, including spirometry and health‐related quality of life (HRQoL), patient diary cards information (predose and evening PEFR, occasions of rescue medication use, and drug compliance, i.e. whether treatment was taken or not) were measured throughout the 48‐week treatment period |
|
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.254 / 205.255, ClinicalTrials.gov NCT00168844 / NCT00168831 Definitions: exacerbation defined as respiratory adverse events lasting ≥3 days and requiring treatment with antibiotics and/or oral corticosteroids and/or a significant change in prescribed respiratory medication including inhaled bronchodilators. Note: all‐cause mortality included patients who had discontinued the study. Cardiovascular safety was monitored in a subset of patients using 12‐lead electrocardiogram (ECG) and Holter monitoring |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boehringer Ingelheim was responsible for preparing and coding study medication in a blinded fashion (Boehringer Ingelheim study drug and control were indistinguishable). Patients, investigators and study personnel remained blinded with regard to the treatment assignments up to database lock |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc., were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The withdrawal rates were relatively large and uneven (tiotropium 5 mcg 17.2%, tiotropium 10 mcg 20.4%, placebo 31.4%). However, information on vital status was collected for all patients, including patients who discontinued prematurely |
| Selective reporting (reporting bias) | Low risk | Data for cardiovascular safety not reported. All other specified outcomes were reported |
Beeh 2006.
| Methods | Design: a randomised, double blind, parallel group, placebo controlled study. The study took place in 294 respiratory trial centres in Germany. They were outpatient clinics predominantly run by chest specialists with a few run by general internal physicians (N < 10). The duration of treatment was 12 weeks | |
| Participants |
Population: 1639 patients with COPD were randomised to tiotropium (1236) and placebo (403) Baseline Characteristics: mean age 62 years, 76% male, mean FEV1 1.3 L, mean FEV1 predicted 45%, 36 pack‐years smoking history Inclusion Criteria: stable COPD, FEV1 ≤ 70% predicted, FEV1/FVC ratio of < 0.7, smoking history of at least 10 pack‐years, at least 40 years of age Exclusion Criteria: history of asthma, atopic disease which was suggestive of asthma, requirement for long‐term oxygen therapy, respiratory infection in the six weeks prior to screening, significant co‐morbidities |
|
| Interventions |
1. 18 mcg tiotropium bromide once daily 2. Placebo once daily Inhaler device: dry powder inhaler Co‐medication: LABAs were not permitted during the course of the study. Short‐acting relief medications were substituted for fenoterol as needed |
|
| Outcomes | Lung function and exacerbations were evaluated by respective pulmonary function tests (spirometry), before (trough value), and 2 hours after inhalation of study medication, FVC, Inspiratory Vital Capacity, and tolerability | |
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.257, ClinicalTrials.gov NCT00274573 Definitions: exacerbations were defined as a respiratory event which lasted for more than 3 days which required treatment or significant increase in the dose of COPD medication (bronchodilator and/or systemic corticosteroids or treatment with antibiotics) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boehringer Ingelheim was responsible for preparing and coding study medication in a blinded fashion (Boehringer Ingelheim study drug and control were indistinguishable). Patients, investigators and study personnel remained blinded with regard to the treatment assignments up to database lock |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc., were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The withdrawal rates were relatively even (tiotropium 17.6%, placebo 22.3%) |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
Brusasco 2003.
| Methods | Design: two randomised, double‐blind, double dummy, parallel group design studies. The run‐in phase was two weeks and the duration of treatment was 24 weeks. The studies were performed in 18 countries. The only difference in the two studies was the duration of serial spirometry in the clinic (12 hours in one study, 3 hours in the second) | |
| Participants |
Population: 802 patients with COPD were randomised to tiotropium 18 mcg (402) and placebo (400) Baseline Characteristics: mean age 64 years, 77% male, mean FEV1 1.1 L, mean FEV1 predicted 39%, 43 pack‐years smoking history Inclusion Criteria: patients were required to have relatively stable airway obstruction with FEV1 < 65% of predicted normal and < 70% of FVC, > 40 years of age, with a smoking history of > 10 pack‐years Exclusion Criteria: patients with a history of asthma, allergic rhinitis, atopy, or with an increased total eosinophil count were excluded. Other exclusion criteria included use of supplemental oxygen or an upper respiratory tract infection in the six weeks before screening. Those patients with a significant disease other than COPD were not enrolled. A significant disease was defined as a disease that, in the opinion of the investigator, would put the patient at risk because of participation in the study or a disease which would influence the results of the study |
|
| Interventions |
1. Tiotropium 18 mcg once daily plus MDI (salmeterol) placebo 2. A combination of salmeterol and tiotropium placebos Inhaler device: dry powder inhaler Co‐medication: patients were allowed to continue previously prescribed regular inhaled steroids or regular oral steroids, not exceeding a dose equivalent to approximately 10 mg prednisone daily |
|
| Outcomes | FEV1, FVC, dyspnoea (evaluated using the Baseline Dyspnoea Index (BDI) and the TDI), HRQoL (determined using the SGRQ), exacerbations of COPD (number of exacerbations, number of exacerbation days, percentage of patients with at least one COPD exacerbation, time to first COPD exacerbation), hospital admissions (hospital admissions for any reason, number of hospital admissions for an exacerbation, days hospitalised, percentage of patients with at least one hospital admission for a COPD exacerbation, time to first hospital admission due to a COPD exacerbation), concomitant medications, non‐scheduled contacts with physicians and other health care providers (use of the intensive care unit), disability days (days unable to perform daily activities), and employment status | |
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.130 / 205.137 Definitions: exacerbations were defined as a complex of respiratory symptoms (new onset or an increase in at least one of cough, sputum, dyspnoea, wheeze, chest discomfort) lasting at least 3 days and usually associated with a therapeutic intervention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boehringer Ingelheim was responsible for preparing and coding study medication in a blinded fashion (Boehringer Ingelheim study drug and control were indistinguishable). Patients, investigators and study personnel remained blinded with regard to the treatment assignments up to database lock. Double dummy technique was used to blind different application devices |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc., were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The withdrawal rates were relatively uneven (tiotropium (15.4%), placebo (25.8%)) |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
Casaburi 2002.
| Methods | Design: two double‐blind, placebo‐controlled trials with 49 weeks treatment duration in 50 clinical centres in United States | |
| Participants |
Population: 921 patients with COPD, as defined by ATS guidelines, were randomised to tiotropium (550) and placebo (371) Baseline Characteristics: mean age 65 years, 64% male, mean FEV1 1.0 L, mean FEV1 predicted 39%, 59 to 63 pack‐years smoking history Inclusion Criteria: the study groups consisted of outpatients of either sex who were ≥ 40 years old and had a clinical diagnosis of COPD. Participants were required to have at least a 10 pack‐year smoking history, clinically stable airway obstruction, and a FEV1 of ≤ 65% of predicted normal values and ≤ 70% of FVC Exclusion Criteria: patients were excluded if they had a history of asthma, allergic rhinitis or atopy or a total blood eosinophil count of ≥ 600 cells mm3. Bronchodilator responsiveness was not an entry criterion. Patients were also excluded if they required regular daytime supplemental oxygen or were on doses exceeding the equivalent of 10 mg prednisone daily during the month prior to entering the study. In addition, patients were excluded if they had a recent history of myocardial infarction (≤ 1 yr), heart failure (≤ 3 yrs) or cardiac arrhythmia requiring drug therapy |
|
| Interventions |
1. Tiotropium 18 mcg once each morning 2. Placebo once each morning Inhaler device: dry powder inhaler Co‐medication: patients were permitted an albuterol MDI, as needed, stable doses of theophylline (i.e. unchanging doses that had been used for ≥ 6 weeks prior to entry), inhaled glucocorticosteroids and the equivalent of ≤ 10 mg*day‐1 oral prednisone throughout the study period. Finally, to treat acute COPD exacerbations during the trial, investigators were permitted to administer any additional medication deemed necessary (excluding anticholinergic or LABAs). After 13 weeks, the investigators were permitted to prescribe glucocorticosteroids or theophylline preparations as necessary |
|
| Outcomes | FEV1 and FVC (1 hour prior to dosing, just prior to dosing, and 30, 60, 120 and 180 min after study drug administration), PEFR measurements in their home twice daily (upon arising and at bedtime), exacerbation, BDI, TDI, Generic health status; Short Form 36 (SF‐36), disease specific health status; SGRQ, the investigator recorded COPD symptom scores after reviewing the patient’s daily diary (for wheezing, shortness of breath, coughing and chest tightness) and recorded a global evaluation of the patient’s overall condition (1: poor; 8: excellent) | |
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.117 / 205.128 Definitions: an exacerbation was defined as a complex of respiratory events (i.e. cough, wheezing, dyspnoea or sputum production) lasting > 3 days. These were generally treated with antibiotics and/or oral steroids |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Subjects took active medication (tiotropium in lactose) or placebo (lactose) from identically appearing capsules via a dry powder inhaler device |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The withdrawal rates were uneven (tiotropium 18.7%, placebo 27.8%) |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
Chan 2007.
| Methods | Design: a multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group study with 48 weeks treatment duration, conducted in 101 centres in Canada involving 72 specialists and 29 general practitioners from 24 January 2002 to 07 May 2004 | |
| Participants |
Population: 913 patients with COPD were randomised to tiotropium (608) and placebo (305) Baseline Characteristics: mean age 67 years, 60% male, mean FEV1 0.97 L, mean FEV1 predicted 39%, 51 pack‐years smoking history Inclusion Criteria: male and female outpatients aged 40 years or older, with a clinical diagnosis of COPD (FEV1 65% predicted or less and FEV1/FVC 70% or less) were considered for inclusion in the present study. Participants were required to have a smoking history of 10 pack‐years or greater. The inclusion criteria relating to ‘exacerbation history’ initially required that patients had experienced one or more exacerbation(s) within the past year (requiring treatment with antibiotics and/or oral steroids), but not within the six weeks before entering the study. However, due to slower than expected enrolment, this criterion was amended to include patients with fewer exacerbations (one exacerbation in the past two years) Exclusion Criteria: history of asthma, allergic rhinitis or atopy; a recent lower respiratory tract infection or any exacerbation (within the previous six weeks); a recent history of myocardial infarction (within the previous six months) or cardiac arrhythmia requiring drug therapy; and oral corticosteroid use at unstable doses during the six weeks before entering the study or at a stable dose exceeding the equivalent of 10 mg prednisone daily. In addition, those patients with a significant disease other than COPD that would put the patient at risk because of participation in the study, or patients with a disease that may have influenced the results of the study, were not enrolled |
|
| Interventions |
1. Tiotropium 18 mcg once daily 2. Placebo once daily Inhaler device: dry powder inhaler Co‐medication: during the treatment period, patients were permitted oral corticosteroids (at a stable dose of 10 mcg or less of prednisone daily or equivalent), stable doses of ICS, theophylline preparations, mucolytic preparations (not containing bronchodilators), LABAs and, for acute symptom relief, as‐needed salbutamol MDI. Patients were not allowed to use inhaled anticholinergics (other than the study drug) or oral beta2‐agonists during the treatment period. To treat COPD exacerbations during the trial, the investigators were permitted to administer any additional medication deemed necessary |
|
| Outcomes |
Primary: morning predose (trough) FEV1 at study end Secondary: predose FVC and forced expiratory volume in six seconds (FEV6), HRQoL (SGRQ), exacerbations and associated hospitalisations, and the number of courses of both oral steroids and antibiotics administered for the treatment of exacerbations |
|
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.259, ClinicalTrials.gov NCT00277264 Definitions: an exacerbation was defined as a complex of respiratory symptoms (new onset or an increase in at least one of cough, sputum, sputum purulence, dyspnoea, wheeze, chest discomfort) lasting at least three days and requiring treatment with antibiotics and/or systemic steroids |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boehringer Ingelheim was responsible for preparing and coding study medication in a blinded fashion (Boehringer Ingelheim study drug and control were indistinguishable). Patients, investigators and study personnel remained blinded with regard to the treatment assignments up to database lock |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal rates were 22.2% in the tiotropium group and 27.5% in the placebo group |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
Cooper 2010.
| Methods | Design: A randomised, parallel group, double‐blind, placebo‐controlled study with 96 weeks (two years) treatment duration, conducted in 60 centres | |
| Participants |
Population: 519 patients with COPD were randomised to tiotropium (260) and placebo (259) Baseline Characteristics: mean age 65 years, 77% male, mean FEV1 1.1 L, mean FEV1 predicted 38%, 52 pack‐years smoking history Inclusion Criteria: male or female, ≥ 40 years old, with a diagnosis of COPD (pre‐bronchodilator FEV1 ≤ 60% predicted, post‐bronchodilator FEV1 ≤ 65% predicted, FEV1/FVC < 70%), smoking history ≥ 10 pack‐years Exclusion Criteria: history of asthma |
|
| Interventions |
1. Tiotropium 18 mcg oral inhalation 2. Placebo oral inhalation Inhaler device: dry powder inhaler Co‐medication: concomitant use of theophylline preparations, mucolytics, ICS, LABAs and oral steroids was allowed. During the treatment period, patients were not allowed to use antileukotrienes, cromolyns, antibiotics, antileukotrienes, long‐acting anticholinergics, or any other investigational drug. |
|
| Outcomes |
Primary: Endurance Time (ET) including secondary endpoint ET at visits 4 to 9 and post‐hoc analysis (90% of maximum work rate treadmill ET Secondary: ET at 100 weeks, pulmonary function tests, Lung function (FEV1, FVC), Quality of life SGRQ, Modified Borg scale, exacerbations of COPD, Physician’s and Patient’s Global Evaluation |
|
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.368, ClinicalTrials.gov NCT00525512 Definitions: COPD exacerbations defined as a complex of respiratory symptoms (increase or new onset) of more than 1 of the following: cough, sputum, wheezing, dyspnoea, or chest tightness with a duration of at least three days requiring treatment with antibiotics and/or systemic steroids and/or hospital admission. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | A third‐party Interactive Voice Response System was used to randomise patients via a unique randomisation number to study drug medication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boehringer Ingelheim was responsible for preparing and coding study medication in a blinded fashion (Boehringer Ingelheim study drug and control were indistinguishable). Patients, investigators and study personnel remained blinded with regard to the treatment assignments up to database lock |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal rates were relatively high and uneven (tiotropium 27.3%, placebo 39.0%) |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
Covelli 2005.
| Methods | Design: a randomised, double‐blind, placebo‐controlled, parallel‐group trial with 12 weeks treatment duration, conducted at 12 sites in the USA between July 2003 and March 2004 | |
| Participants |
Population: 196 patients with COPD were randomised to tiotropium (100) and placebo (96) Baseline Characteristics: mean age 65 years, 47% to 66% male, mean FEV1 1.0 L, mean FEV1 predicted 39%, 66 pack‐years smoking history Inclusion Criteria: patients included in the study had a clinical diagnosis of COPD, were at least 40 years of age, and had a smoking history of at least 10 pack‐years. Patients were required to have a FEV1 of 60% or less of predicted normal and a FVC of 70% or less Exclusion Criteria: patients with significant disease other than COPD were excluded. Significant disease was defined as a disease or a condition that, in the opinion of the investigator, may put the patient at risk because of participation in the study, or may influence either the results of the study or the patient’s ability to participate in the study. Patients also were excluded if they had a history of asthma or atopy, had abnormal liver enzyme levels or evidence of chronic renal dysfunction, or had experienced a respiratory tract infection or COPD exacerbation within six weeks of randomisation. In addition, patients were excluded if they were taking systematic corticosteroids at unstable dosages or prednisone 10 mg/day or greater (or its equivalent), or were using oxygen for more than 12 hours/day. Patients with pre‐existing cardiovascular disease were permitted to participate in the trial unless they had experienced myocardial infarction within the preceding six months, hospitalisation for heart failure within the preceding year, or life threatening arrhythmias requiring intervention or change in drug therapy within the last year |
|
| Interventions |
1. 18 mcg tiotropium 2. Matching placebo Inhaler device: dry powder inhaler Co‐medication: during the study, treatment with respiratory drugs such as ICS, both SABAs and LABAs, and theophyllines was permitted; however, treatment with cromones, leukotriene antagonists, and inhaled anticholinergics was not permitted |
|
| Outcomes | ECG, Holter Monitoring Primary: morning trough FEV1 after 12 weeks of treatment (day 84) Secondary: predose FEV1 on day 56 and FVC on days 56 and 84, postdose FEV1 (90 min) and FVC on all test days, patient and physician global COPD ratings, scores on the EuroQol Health Questionnaire (EQ‐5D), and treatment with rescue medication |
|
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.284, ClinicalTrials.gov NCT00239460 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | A third‐party Interactive Voice Response System was used to randomise patients via a unique randomisation number to study drug medication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boehringer Ingelheim was responsible for preparing and coding study medication in a blinded fashion (Boehringer Ingelheim study drug and control were indistinguishable). Patients, investigators and study personnel remained blinded with regard to the treatment assignments up to database lock |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal rates were relatively low, but uneven (tiotropium 10%, placebo 17%) |
| Selective reporting (reporting bias) | Unclear risk | For quality of life the results were only described but no data presented |
Dusser 2006.
| Methods | Design: a double‐blind, parallel‐group trial with 48 weeks (one year) treatment duration, conducted at 177 centres in France from October 2000 to October 2003 | |
| Participants |
Population: 1010 patients with COPD, as defined by BTS guidelines, were randomised to tiotropium (500) and placebo (510) Baseline Characteristics: mean age 65 years, 88% male, mean FEV1 1.4 L, mean FEV1 predicted 48%, 43 pack‐years smoking history Inclusion Criteria: male and female patients aged ≥ 40 yrs old with a clinical diagnosis of COPD (pre‐bronchodilator FEV1 30% to 65% predicted and FEV1/slow vital capacity (SVC) ≤ 70% predicted) were eligible for inclusion in the study. Participants were also required to have a smoking history of ≥ 10 pack‐years and one or more exacerbations in the last year (as reported in the patient’s medical file), but not within the six weeks prior to entering the study. Exclusion Criteria: history of asthma, allergic rhinitis or atopy; a recent lower respiratory tract infection or any exacerbation (within the previous six weeks); regular use of daytime oxygen therapy; oral corticosteroid use at unstable doses six weeks prior to entering the study or at a dose exceeding the equivalent of 10 mg prednisone daily. In addition, those patients with a significant disease other than COPD that would put the patient at risk because of participation in the study, or a disease that would influence the results of the study, were not enrolled. |
|
| Interventions |
1. Tiotropium 18 mcg once daily 2. Placebo once daily Inhaler device: dry powder inhaler Co‐medication: patients were permitted SABAs, as needed, for acute symptom relief. Concomitant use of ICS and oral steroids (at a dose of 10 mg prednisone daily or equivalent) was allowed if the dosage was stable for ≥ 6 weeks before study entry. To treat COPD exacerbations during the trial, the investigators were permitted to administer any additional medication deemed necessary (excluding anticholinergics and LABAs). During the treatment period, patients were not allowed to use oral or inhaled LABAs, inhaled anticholinergics (other than the study drug) or theophylline. |
|
| Outcomes | Exacerbations of COPD, hospital admissions due to a COPD exacerbation, concomitant medications and non‐scheduled contacts with physicians, peak expiratory flow (PEF) measurements, number of puffs of ‘‘as‐needed’’ rescue medication, respiratory condition using a graduated numerical scale (0: poor; 10: excellent), FEV1, FVC, SVC and IC | |
| Notes |
Funding: Boehringer Ingelheim Definitions: an exacerbation was defined as the onset of at least one clinical descriptor (worsening of dyspnoea, cough or sputum production; appearance of purulent sputum; fever (> 38°C); appearance of new chest radiograph abnormality) lasting ≥ 2 days and requiring a new prescription or an increase in the dose of beta2‐agonists, antibiotics, corticosteroids or bronchodilators. The severity of an exacerbation was defined as severe, moderate or mild. A severe exacerbation was classified as an exacerbation requiring hospitalisation or an exacerbation plus one or more of the following criteria: FEV1 and/or PEF drop > 30% from baseline on ≥ 2 consecutive days; partial pressure of oxygen (Pa,O2) drops ≥ 10 mmHg (≥ 1.33 kPa) from baseline or if Pa,O2 drops to ≤ 60 mmHg (≤ 7.98 kPa); partial pressure of carbon dioxide (Pa,CO2) increases ≥ 5 mmHg (≥ 0.66 kPa) from baseline or if Pa,CO2 increases to ≥ 45 mmHg (5.98 kPa). (FEV1, PEF and arterial blood gases were monitored in patients who were hospitalised with a severe exacerbation or if deemed necessary by the investigator.) A moderate exacerbation was classified as at least three clinical descriptors excluding severe exacerbations. A mild exacerbation was classified as one or two clinical descriptors. In order to compare the results of this study more directly with those from previous exacerbation trials, a post‐hoc analysis was conducted, which used a more generalised classification of exacerbation severity based on health resource utilisation and treatment use. A severe exacerbation was classified as one requiring hospitalisation. A moderate exacerbation was defined as one requiring treatment with systemic steroids and/or antibiotics. All remaining events were classified as mild exacerbations. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boehringer Ingelheim was responsible for preparing and coding study medication in a blinded fashion (Boehringer Ingelheim study drug and control were indistinguishable). Patients, investigators and study personnel remained blinded with regard to the treatment assignments up to database lock |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal rates were relatively large (tiotropium 23.4%, placebo 28.8%) |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
Freeman 2007.
| Methods | Design: randomised, double‐blind, placebo‐controlled, parallel‐group study with 12 weeks treatment duration, conducted at 44 primary care centres throughout England, Scotland and Wales from October 2002 to October 2003 | |
| Participants |
Population: 395 patients with COPD were randomised to tiotropium (200) and placebo (195) Baseline Characteristics: mean age 65 years, 50% to 59% male, mean FEV1 1.3 L, mean FEV1 predicted 49%, 37 pack‐years smoking history Inclusion Criteria: patients were required to have a COPD diagnosis according to BTS criteria and recent stable disease (no exacerbation or respiratory infection within four weeks), with airway obstruction FEV1 between 30% and 65% of predicted normal value and FEV1/FVC ≤ 70% pre‐bronchodilators. Patients had to be at least 40 years old, have at least a 10 pack‐year smoking history and had to be receiving SABAs as rescue medication (salbutamol or terbutaline MDI or dry powder inhaler) with no anticholinergic drug prescribed in the preceding year. Patients had to be able to undergo spirometry and be able to use the HandiHaler device Exclusion Criteria: patients with a history of allergy or asthma were excluded. Patients were excluded if they had any other significant medical condition that might interfere with the study or preclude their use of study medication, such as known hypersensitivity to anticholinergic drugs, known symptomatic prostatic hypertrophy, narrow angle glaucoma, severe cardiovascular disease, or recent myocardial infarction (≤ 1 year). Patients who were on long‐term oxygen therapy were also excluded |
|
| Interventions |
1. Tiotropium 18 mcg 2. Placebo Inhaler device: dry powder inhaler Co‐medication: usual treatment |
|
| Outcomes |
Primary: trough FEV1 response end of study Secondary: trough FEV1 response after 2 and 6 weeks, trough FVC response after 2, 6 and 12 weeks, mean daily SABA use, COPD exacerbations, dyspnoea measured by the Oxygen Cost Diagram |
|
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.276, ClinicalTrials.gov NCT00274079 Definitions: an exacerbation of COPD was defined as a complex of respiratory events/symptoms with duration of three or more days (from patient's diary card) requiring a change in treatment (including patient‐initiated increases). A complex of respiratory events/symptoms meant ≥ two of the following (increase of symptom or new onset): shortness of breath, sputum production (volume), cough, wheezing and chest tightness. The change in (or requirement of) treatment included prescription of antibiotics and/or systemic steroids and/or a significant change (including increase) of the prescribed respiratory medication (bronchodilators including theophylline) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medication and placebo were delivered by identical‐appearing lactose‐based inhalers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The withdrawal rates were relatively low, but uneven (tiotropium 9.5%, placebo 17.9%) |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
Johansson 2008.
| Methods | Design: randomised, double‐blind, parallel‐group study with 12 weeks treatment duration, conducted at 27 centres in Sweden from March 2004 to July 2005 | |
| Participants |
Population: 224 patients with COPD, as defined by GOLD guidelines, were randomised to tiotropium (107) and placebo (117) Baseline Characteristics: mean age 62 years, 43% to 53% male, mean FEV1 2.1 L, mean FEV1 predicted 73%, 31 pack‐years smoking history Inclusion Criteria: outpatients aged > 40 years old with a diagnosis of mild COPD by 2003 Swedish guidelines (post‐bronchodilator FEV1/FVC < 70% and FEV1 > 60% predicted); smoking history of > 10 pack‐years; and a Medical Research Council dyspnoea score of > 2 Exclusion Criteria: history of asthma, allergic rhinitis or atopy; blood eosinophil count > 600/mm3; recent lower respiratory tract infection or any exacerbation (within the previous six weeks); recent history of myocardial infarction (within the previous six months); unstable cardiac arrhythmia; regular use of oxygen therapy; use of oral or inhaled steroids (within the previous three months); and significant diseases other than COPD |
|
| Interventions |
1. Tiotropium 18 mcg once daily in the morning 2. Placebo once daily in the morning Inhaler device: dry powder inhaler Co‐medication: patients were permitted salbutamol MDI as rescue medication, as‐needed, for acute symptom relief, with an 8‐hour washout period before spirometry. Use of short‐acting anticholinergics, beta2‐agonists (other than rescue medication), oral or ICS, or theophylline, was not permitted. However, to treat COPD exacerbations, investigators could prescribe antibiotics and oral corticosteroids (for < 2 weeks) or theophylline (for ≤ 7 days) |
|
| Outcomes |
Primary: change in FEV1 area under the curve from predose (zero time) to 2 hours postdose (AUC0‐2 h), from baseline to 12 weeks Secondary: FEV1 and FVC trough responses, use of rescue medication, adverse events, dyspnoea BDI, Medical Research Council dyspnoea scale, HRQL (generic European Quality of Life Questionnaire, EuroQol (EQ‐5D and VAS), use of rescue medication, adverse events, COPD exacerbations |
|
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.281, ClinicalTrials.gov NCT00144196 Definitions: an exacerbation of COPD is a complex of COPD‐related respiratory symptoms (increase or new onset) of a duration of at least three days, usually requiring treatment with systemic corticosteroids or antibiotics. COPD‐related respiratory symptoms consist of more than one of the following: cough, wheeze, dyspnoea, chest congestion, shortness of breath, chest tightness, or sputum production |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boehringer Ingelheim was responsible for preparing and coding study medication in a blinded fashion (Boehringer Ingelheim study drug and control were indistinguishable). Patients, investigators and study personnel remained blinded with regard to the treatment assignments up to database lock |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The withdrawal rates were low (tiotropium 1.9%, placebo 3.4%) |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
Magnussen 2008.
| Methods | Design: randomised, double‐blind, placebo‐controlled study with 12 weeks treatment duration, conducted at 67 centres distributed within Belgium, Canada, Germany, Denmark, France, Italy, the Netherlands, and South Africa | |
| Participants |
Population: 472 patients with COPD, as defined by GOLD guidelines, were randomised to tiotropium (228) and placebo (244) Baseline Characteristics: mean age 60 years, 61% male, mean FEV1 1.5 L, mean FEV1 predicted 53%, 34 pack‐years smoking history Inclusion Criteria: patients were required to have a physician‐diagnosis of asthma (before the age of 30 years), a diagnosis of COPD, post‐bronchodilator FEV1 < 80% predicted normal and a post‐bronchodilator ratio of FEV1/FVC < 70%. Other inclusion criteria were: smoking history > 10 pack‐years, age ≥ 40 years, treatment with ICS for ≥ 1 year prior to study entry, and an acute bronchodilator response ≥ 200 ml and ≥ 12% of pre‐bronchodilator FEV1 at the screening visit or documented during the past five years in the patient clinic records |
|
| Interventions |
1. Tiotropium 18 mcg once daily 2. Placebo once daily Inhaler device: dry powder inhaler Co‐medication: patients were allowed to continue treatment with inhaled LABAs, ICS, oral steroids (≤ 10 mg/day prednisone or equivalent), theophyllines, leukotriene antagonists, and cromones as concomitant medication. Salbutamol was provided for as‐needed acute symptom relief. Patients were not allowed to take anticholinergic therapy other than study drug during the randomisation period |
|
| Outcomes | Spirometry: FEV1 and FVC area under the curve (AUC) 0‐6h, PEFR, symptom relief: rescue medication use | |
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.301, ClinicalTrials.gov NCT00152984 Definitions: an exacerbation of COPD and asthma was defined as an adverse event which was a worsening of disease meeting the criteria for an serious adverse event, or led to treatment discontinuation, or required changed concomitant medication, or was an unexpected deterioration from baseline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boehringer Ingelheim was responsible for preparing and coding study medication in a blinded fashion (Boehringer Ingelheim study drug and control were indistinguishable). Patients, investigators and study personnel remained blinded with regard to the treatment assignments up to database lock |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal rates were low (tiotropium 2.2%, placebo 4.5%) |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
Moita 2008.
| Methods | Design: a randomised, double‐blind, parallel‐group, placebo‐controlled study with 12 weeks treatment duration, conducted at 31 centres in Portugal | |
| Participants |
Population: 311 patients with COPD, as defined by ATS guidelines were randomised to tiotropium (147) and placebo (164) Baseline Characteristics: mean age 64 years, 95% male, mean FEV1 1.2 L, mean FEV1 predicted 38% to 44%, 54 to 60 pack‐years smoking history Inclusion Criteria: males or females aged ≥ 40 years with a diagnosis of COPD (FEV1 ≤ 70% of predicted and FEV1/FVC ≤ 70%) and a smoking history of ≥ 10 pack‐years were eligible for inclusion Exclusion Criteria: patients were not included if they had a history of asthma, allergic rhinitis, atopy, myocardial infarction, unstable arrhythmia, or if they had any clinically significant disease that might put the patient at risk because of study participation. Patients with > 3 exacerbations of COPD in the preceding year or an exacerbation or lower respiratory tract infection within the six weeks prior to randomisation were also excluded |
|
| Interventions |
1. Tiotropium 18 mcg once daily 2. Placebo once daily Inhaler device: dry powder inhaler Co‐medication: concomitant use of prn salbutamol MDI (100 mg/puff; withheld for at least 6 hours prior to each clinic visit), LABAs and continued use of theophylline preparations (excluding 24 hour preparations) (both withheld for at least 24 hours prior to each clinic visit) were allowed during the study period. Concomitant use of mucolytics, orally ICS, minimal doses of oral corticosteroids (equivalent to prednisone ≤ 10 mg/day or ≤ 20 mg/alternate days) were allowed if the dosage was stabilised for at least six weeks before the study. Temporary increases in the dose of theophylline preparation of ≤ 7 days or addition/increased dose of oral steroids for ≤ 2 weeks were allowed for the treatment of an exacerbation during the study period. If appropriate, scheduled visits were postponed for at least one week, but not more than two weeks. Use of antibiotics was not restricted. Short‐acting anticholinergics, oral beta2‐agonists, antileukotrienes, and other investigational drugs were not allowed during the study |
|
| Outcomes |
Primary: change in trough FEV1 after 12 weeks of treatment Secondary: trough FEV1 after six weeks of treatment, trough FVC after 6 and 12 weeks of treatment, assessment of COPD symptoms, Physician's Global Evaluation, Quality of Life Questionnaire (EQ‐5D) and use of daytime and night‐time rescue medication (salbutamol MDI 100 mcg/puff). Rescue medication use, cigarette consumption and drug compliance were recorded in patient diary cards. Adverse events were collected throughout the study |
|
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.282, ClinicalTrials.gov NCT00239408 Definitions: an exacerbation was defined as an increase or new onset of more than one of the following respiratory symptoms (cough, sputum, sputum purulence, wheezing, dyspnoea) with a duration of three or more days requiring treatment with antibiotics and/or systemic (oral, intramuscular or intravenous) steroids |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boehringer Ingelheim was responsible for preparing and coding study medication in a blinded fashion (Boehringer Ingelheim study drug and control were indistinguishable). Patients, investigators and study personnel remained blinded with regard to the treatment assignments up to database lock |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The withdrawal rates were low and even (tiotropium 7.5%, placebo 6.7%) |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
NCT00144326.
| Methods | Design: randomised, double‐blind, placebo‐controlled, parallel‐group, multicentre study with 12 weeks treatment duration | |
| Participants |
Population: 250 patients with COPD were randomised to tiotropium (123) and placebo (127) Baseline Characteristics: mean age 63 years, 78% male, mean FEV1 1.3 L, mean FEV1 predicted 46% Inclusion Criteria: ambulatory patients of either sex; > 40 years old, diagnosed with COPD (FEV1 < 60% of the predicted value and FEV1/FVC < 70%); smokers or ex‐smokers with a history of having smoked at least 10 pack‐years |
|
| Interventions |
1. Tiotropium 18 mcg once daily by oral inhalation 2. Placebo once daily by oral inhalation Inhaler device: dry powder inhaler Co‐medication: patients were permitted to use SABAs, as needed, for acute symptom relief. Concomitant use of theophylline preparations, mucolytics, ICS, antibiotics, antihistamines, and oral steroids was allowed. During the treatment period, patients were not allowed to use beta‐blockers, cromolyns, antileukotrienes, inhaled LABAs, long‐acting anticholinergics, or any other investigational drug |
|
| Outcomes |
Primary: difference in daily physical activity measured in vector magnitude units (VMUs) by the triaxial Stayhealthy RT3 accelerometer at the end of the treatment period Secondary: the difference in physical activity measured in VMUs by the triaxial Stayhealthy RT3 accelerometer at 1 month and 2 month treatment period, trough (10±3 minutes predose) FEV1, peak FEV1 as measured by the maximum post‐bronchodilator value obtained within 2 hours of testing on study days (30±5, 60±10, and 120±10 minutes), trough and peak SVC measured at the same time as the FEV1 on each study day, trough and peak inspiratory capacity (IC) measured at the same time as the FEV1 on each study day, trough and peak forced inspiratory volume in one second (FIV1) measured at the same time as the FEV1 on each study day, distance covered in the six‐minute walk distance (6MWD), modified Borg Dyspnoea Scale, quality of life as measured by the Chronic Respiratory Questionnaire (CRQ), use of salbutamol (rescue medication) during the treatment period, time point at which a 20% improvement from baseline in physical activity was achieved, physician’s Global Assessment |
|
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.269, ClinicalTrials.gov NCT00144326 Definitions: an exacerbation of COPD was defined as a complex of respiratory symptoms (two or more) COPD‐related (increased or new onset) with a duration of at least three days. COPD‐related respiratory symptoms consisted of cough, wheeze, dyspnoea, shortness of breath, chest tightness, or increase in production and/or purulence in sputum. These symptoms must have been accompanied with antibiotic treatment and/or systemic corticoids (oral, intramuscular or endovenous) or with a significant change in respiratory medication prescribed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boehringer Ingelheim was responsible for preparing and coding study medication in a blinded fashion (Boehringer Ingelheim study drug and control were indistinguishable). Patients, investigators and study personnel remained blinded with regard to the treatment assignments up to database lock |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The withdrawal rates were relatively low and even (tiotropium 8.1%, placebo 11.8%) |
| Selective reporting (reporting bias) | Low risk | No published report available, but results for all specified outcomes were supplied on request |
Niewoehner 2005.
| Methods | Design: a randomised, double‐blind, parallel‐group, placebo‐controlled study with six months treatment duration, conducted at 26 Veterans Affairs medical centres in the United States | |
| Participants |
Population: 1829 patients with COPD were randomised to tiotropium (914) and placebo (915) Baseline Characteristics: mean age 68 years, 98% male, mean FEV1 1.0 L, mean FEV1 predicted 36%, 68 pack‐years smoking history Inclusion Criteria: men and women, 40 years or older, a cigarette smoking history of 10 pack‐years or more, a clinical diagnosis of COPD, and an FEV1 of 60% predicted or less and 70% or less of the FVC Exclusion Criteria: a clinical diagnosis of asthma, a myocardial infarction within the previous six months, a serious cardiac arrhythmia or hospitalisation for heart failure within the previous year, known moderate to severe renal impairment, moderate to severe symptomatic prostatic hypertrophy or bladder‐neck obstruction, narrow‐angle glaucoma, current radiation or chemotherapy for a malignant condition, or inability to give informed consent. We also excluded patients who took systemic corticosteroids at unstable doses, or in regular daily doses of 20 mg or more of prednisone (or equivalent), or who had not fully recovered from an exacerbation for at least 30 days before the first study visit |
|
| Interventions |
1. Tiotropium 18 mcg by inhalation once daily 2. Placebo by inhalation once daily Inhaler device: dry powder inhaler Co‐medication: participants otherwise received usual medical care, except that they could not take any open‐label anticholinergic bronchodilator. They continued taking all other respiratory medications (including ICS and LABAs), and primary providers were allowed to prescribe additional medications according to medical need. Primary providers also prescribed antibiotics and systemic steroid prescriptions for exacerbations without restrictions |
|
| Outcomes |
Primary: percentage of patients experiencing at least 1 exacerbation and the percentage of patients with at least one hospitalisation due to a COPD exacerbation Secondary: time to first COPD exacerbation and time to first hospitalisation due to COPD exacerbation, the frequencies of exacerbations and of exacerbation‐related health care utilisation (hospitalisations, hospitalisation days, unscheduled clinic visits, antibiotic treatment days, and systemic corticosteroid treatment days), the frequencies of all‐cause hospitalisations and hospitalisation days, and results of spirometry |
|
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.266, ClinicalTrials.gov NCT00274547 Definitions: an exacerbation was defined as a complex of respiratory symptoms (increase or new‐onset) of more than one of the following: cough, sputum, wheezing, dyspnoea, or chest tightness with a duration of at least three days requiring treatment with antibiotics or systemic steroids, hospitalisation, or both. An event was considered to be a hospitalisation if a patient was held and treated for an acute respiratory condition in an urgent care department or in an observation unit for longer than 24 hours. Admissions to nursing homes or other extended care facilities were not considered hospitalisations |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients were allocated in equal numbers to receive tiotropium or placebo according to a centrally generated blocked randomisation list. We generated a single randomisation and assigned blocks to centres |
| Allocation concealment (selection bias) | Low risk | Blinding of supplies was performed at Boehringer Ingelheim before distribution to investigational sites |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The double‐blinding remained in place until all patients were clinically complete or until a serious adverse event required unbinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The withdrawal rates were relatively large and uneven (tiotropium 16.3%, placebo 26.8%) |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
Powrie 2007.
| Methods | Design: a randomised, double‐blind, parallel‐group, placebo‐controlled study with one year treatment duration, conducted at a single‐centre, the London Chest Hospital (UK) | |
| Participants |
Population: 142 patients with COPD were randomised to tiotropium (69) and placebo (73) Baseline Characteristics: mean age 66 years, 41%‐ to 48% male, mean FEV1 1.3 L, mean FEV1 predicted 50%, 55 pack‐years smoking history Inclusion Criteria: patients aged ≥ 40 years with a diagnosis of COPD (FEV1 , 80% of the predicted value and FEV1/FVC , 70%) and a minimum 10‐ pack‐year smoking history Exclusion Criteria: patients with a history of asthma or atopy were excluded, as were those on long‐term oxygen therapy or with another clinically significant disease. |
|
| Interventions |
1. 18 mcg tiotropium once daily 2. Placebo once daily Inhaler device: dry powder inhaler Co‐medication: anticholinergics other than the study drug were not permitted during the course of the study. |
|
| Outcomes |
Primary: the concentration of interleukin (IL)‐6 in sputum Secondary: sputum IL‐8 and myeloperoxidase (MPO) levels, serum IL‐6 and C‐reactive protein (CRP) levels, sputum bacterial colonisation, FEV1 and exacerbation frequency |
|
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.270, ClinicalTrials.gov NCT00405236 Definitions: an exacerbation was defined as the presence, of > 2 days consecutively, of an increase in any two major symptoms (dyspnoea, sputum purulence and sputum volume) or in one major and one minor symptom (wheeze, sore throat, cough and symptoms of a common cold) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boehringer Ingelheim was responsible for preparing and coding study medication in a blinded fashion (Boehringer Ingelheim study drug and control were indistinguishable). Patients, investigators and study personnel remained blinded with regard to the treatment assignments up to database lock |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The withdrawal rates were high but relatively even (tiotropium 30.4%, placebo 28.8%) |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
Sun 2007.
| Methods | Design: a randomised, double‐blind, parallel‐group, placebo‐controlled study with three months treatment duration, conducted in China | |
| Participants |
Population: 60 patients with COPD were randomised to tiotropium (30) and placebo (30) Baseline Characteristics: mean age 62 years, 63% to ‐77% male, mean FEV1 1.3 L, mean FEV1 predicted 47% Inclusion Criteria: a diagnosis of stable COPD, and an age of 18 to 70 years Exclusion Criteria: severe bronchial asthma, severe COPD, bronchiectasia, congestive heart‐ failure, pulmonary tuberculosis, systematic infection, particularly respiratory tract infection, in past two weeks before enrolment, severe heart, liver, kidney, blood system, nerve system, mental diseases and glaucoma, oversensitive to the experiment drugs, attended other trials in past one month, systematic chronic diseases such as hypertension, diabetes, hyperthyroid, etc. |
|
| Interventions |
1. 18 mcg of tiotropium once daily 2. A matching placebo once daily Inhaler device: dry powder inhaler Co‐medication: salbutamol as needed |
|
| Outcomes |
Primary: symptom improvement (%) = (scores before – scores after)/scores before x 100%; controlled: > 75%; marked improvement: 50% to 75%; improvement: 25% to 50%; no improvement: < 25% Secondary: COPD worsening: grade I: self treatment; Grade II: patients need to be treated by clinic; Grade III: hospitalisation is needed. Clinical symptoms including: cough, sputum, whoop, breathing difficulty, and lung rales; lung function including: 1 h predose FEV1 and FEV1 % predicted, FVC and FEV1/FVC; safety index including: blood, urine, liver and kidney function, chest X‐ray and ECG |
|
| Notes |
Funding: not specified Definitions: exacerbations level 1: could be treated by patients themselves; level 2: needed to be treated by Dept. of outpatients or Dept. of Emergency; level 3: needed hospitalisation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | SAS software was used by stratification randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study. The placebo had the same appearance as the intervention drug |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The withdrawal rates were relatively low but uneven (tiotropium 0%, placebo 10%) |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
Tashkin 2008.
| Methods | Design: a randomised, double‐blind, parallel‐group, placebo‐controlled study with four‐year treatment duration, conducted at 490 investigational centres in 37 countries. Patients were recruited from January 2003 through March 2004; the study ended in February 2008 | |
| Participants |
Population: 5993 patients with COPD were randomised to tiotropium (2987) and placebo (3006) Baseline Characteristics: mean age 65 years, 75% male, mean FEV1 1.1 L, mean FEV1 predicted 39%, 49 pack‐years smoking history Inclusion Criteria: a diagnosis of COPD, an age of 40 years or more, a smoking history of at least 10 pack‐years, a post‐bronchodilator FEV1 of 70% or less of the predicted value, and an FEV1 of 70% or less of the FVC (after supervised administration of 80 mcg of ipratropium (four actuations), followed by 400 mcg of albuterol (four actuations) 60 minutes later) Exclusion Criteria: a history of asthma, a COPD exacerbation or respiratory infection within four weeks before screening, a history of pulmonary resection, use of supplemental oxygen for more than 12 hours per day, and the presence of a coexisting illness that could preclude participation in the study or interfere with the study results |
|
| Interventions |
1. 18 mcg of tiotropium once daily 2. A matching placebo once daily Inhaler device: dry powder inhaler Co‐medication: all respiratory medications, except other inhaled anticholinergic drugs, were permitted during the trial |
|
| Outcomes |
Primary: yearly rate of decline in the mean FEV1 before the use of a study drug and short‐acting bronchodilators in the morning (pre‐bronchodilator) and after the use of a study drug (post‐bronchodilator) from day 30 (steady state) until completion of double‐blind treatment Secondary: rate of decline in the mean FVC and SVC, health‐related quality of life, as measured by the total score on SGRQ, exacerbations of COPD and related hospitalisations; and the rate of death from any cause and from lower respiratory conditions |
|
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.235, ClinicalTrials.gov NCT00144339 Definitions: exacerbations were defined as an increase in or the new onset of more than one respiratory symptom (cough, sputum, sputum purulence, wheezing, or dyspnoea) lasting three days or more and requiring treatment with an antibiotic or a systemic corticosteroid |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned in a 1:1 ratio to receive either tiotropium or placebo with the use of centralised randomisation in blocks of four, stratified according to site. The randomisation list will be generated using a validated system, which involves a pseudo‐random number generator so that the resulting treatment sequence will be both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | An Interactive Voice Response System will be used for patient randomisation and drug supply management. Each site will be provided with a telephone number (with 24‐hour access) and password that will connect them to a series of instructions on how to assign a medication kit to a patient |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of the study drugs will be such that the treatments will be indistinguishable |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An independent data and safety monitoring committee reviewed data throughout the trial. A mortality adjudication committee evaluated the primary cause of death from blinded data |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The withdrawal rates were high (tiotropium 36.8%, placebo 45.2%). However, data regarding vital status were systematically requested for patients who prematurely discontinued study participation on a recorded date determined as four years from the first day of administration of a study drug |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
Tonnel 2008.
| Methods | Design: a randomised, double‐blind, parallel‐group, placebo‐controlled study with nine months treatment duration, conducted at 123 centres in France. Patients were recruited between May 2002 and June 2003, and follow‐up was from August 2002 through April 2004 | |
| Participants |
Population: 554 patients with COPD, as defined by ATS guidelines, were randomised to tiotropium (266) and placebo (288) Baseline Characteristics: mean age 64 years, 86% male, mean FEV1 1.4 L, mean FEV1 predicted 44%, 44 pack‐years smoking history Inclusion Criteria: male and female outpatients aged ≥ 40 years with a clinical diagnosis of COPD (pre‐ and post‐bronchodilator FEV1 20% to– 70% predicted and FEV1/SVC ≤ 70%,) corresponding to mild, moderate, or severe COPD according to 1995 ATS and a smoking history of > 10 pack‐years were eligible for inclusion in the study. Exclusion Criteria: a history of asthma, allergic rhinitis, or atopy; regular use of daytime oxygen therapy; a recent respiratory tract infection (within the previous six weeks); a recent history of myocardial infarction (within the previous six months); cardiac arrhythmia requiring drug therapy (within the previous year); or hospitalisation for either heart failure or pulmonary edema (within the previous 3 years). |
|
| Interventions |
1. Tiotropium 18 mcg once daily 2. Placebo ones daily Inhaler device: dry powder inhaler Co‐medication: patients were permitted to use salbutamol (Ventolin®; GlaxoSmithKline, UK) delivered via a MDI, as needed, for acute symptom relief. Concomitant use of theophylline preparations (excluding 24‐hour preparations), mucolytics, (ICS), and oral steroids (at a dose of < 10 mg prednisone daily or equivalent) was allowed if the dosage was stabilised for ≥ 6 weeks before study entry. During the treatment period, patients were not allowed to use beta‐blockers, antileukotrienes, oral or inhaled LABAs, short‐acting anticholinergics, or any other investigational drug. One 10‐day course of oral steroids was permitted for the treatment of a COPD exacerbation during the study period. Investigators were also permitted to administer antibiotics as deemed necessary for the treatment of exacerbations. |
|
| Outcomes |
Primary: the proportion of patients achieving a reduction of at least 4 units in the SGRQ total score at study end Secondary: Visual Simplified Respiratory Questionnaire (VSRQ) total score (improvement in health status), FEV1, FVC, IC, SVC, and FIV1; measured at selected sites only), exacerbations of COPD |
|
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.256, ClinicalTrials.gov NCT00274053 Definitions: an acute exacerbation was defined as a sustained worsening of the patient’s COPD (from the stable state and beyond normal day‐to‐day variation) that was acute in onset and necessitated a change in regular medication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were assigned using a computer‐generated randomisation schedule, with no stratification (block size of 4) |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boehringer Ingelheim was responsible for preparing and coding study medication in a blinded fashion (Boehringer Ingelheim study drug and control were indistinguishable). Patients, investigators and study personnel remained blinded with regard to the treatment assignments up to database lock |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The withdrawal rates were uneven (tiotropium 14.7%, placebo 25.7%) |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
Trooster 2011.
| Methods | Design: a randomised, double‐blind, parallel‐group, placebo‐controlled study with 24 weeks (6 months) treatment duration, conducted at 70 centres (of which, 11 did not randomise subjects); Belgium 4 centres; Canada 4 centres (of which, 2 did not randomise subjects), Czech Republic 12 centres; Germany 5 centres; Greece 4 centres; Netherlands 4 centres; Portugal 3 centres; Ukraine 7 centres; United Kingdom 4 centres; (of which, 2 did not randomise subjects); and United States 23 centres (7 did not randomise subjects). The study took place from April 2007 to July 2010 | |
| Participants |
Population: 457 patients with COPD were randomised to tiotropium (238) and placebo (219) Baseline Characteristics: mean age 62 years, 69% male, mean FEV1 2.0 L, mean FEV1 predicted 66% Inclusion Criteria: subjects were men and women current or ex‐smokers (smoking history of ≥ 10 pack‐years) with GOLD Stage 2 COPD, post‐bronchodilator FEV1 ≥ 50% and < 80% of predicted normal, and were from 40 to 80 years of age. Subjects were required to have post‐bronchodilator FEV1/FVC ratio < 70% (Week ‐4 [screening]) and a Medical Research Council dyspnoea score of ≥ 2 Exclusion Criteria: subjects could not be treated previously with maintenance medications for chronic respiratory disease (e.g. LABAs, inhaled anticholinergics, inhaled or systemic corticosteroids, theophylline, leukotriene receptor antagonists) within six months prior to screening and who had symptomatic shortness of breath). |
|
| Interventions |
1. Tiotropium 18 mcg once daily in the morning 2. Placebo once daily in the morning Inhaler device: dry powder inhaler Co‐medication: albuterol (salbutamol) was provided for use by all subjects (for use as rescue therapy) during the screening and treatment period |
|
| Outcomes | Spirometry: predose FEV1 and FVC measurements, postdose measurements were performed at 30, 60, 120, and 180 minutes (±5 minutes) postdose, Activity Monitor, Physician’s and Patient’s Global Assessment, Work Productivity and Activity Impairment (WPAI) Questionnaire, Subject Diary: the number of rescue albuterol (salbutamol) inhalations | |
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.365, ClinicalTrials.gov NCT00523991 Definitions: an exacerbation of COPD was defined as a complex of respiratory symptoms (increase or new onset) of more than one of the following: cough, sputum, sputum purulence, wheezing, and dyspnoea with duration of at least three days requiring treatment with antibiotics and/or systemic steroids. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boehringer Ingelheim was responsible for preparing and coding study medication in a blinded fashion (Boehringer Ingelheim study drug and control were indistinguishable). Patients, investigators and study personnel remained blinded with regard to the treatment assignments up to database lock |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The withdrawal rates were relatively low and even (tiotropium 11.3%, placebo 9.6%) |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
Verkinde 2006.
| Methods | Design: a randomised, double‐blind, parallel‐group, placebo‐controlled study with 12 weeks treatment duration, conducted at 10 sites in France | |
| Participants |
Population: 100 patients with COPD were randomised to tiotropium (46) and placebo (54) Baseline Characteristics: mean age 60 years, 94% male, mean FEV1 1.1 L, mean FEV1 predicted 35%, 44 pack‐years smoking history Inclusion Criteria: male and female outpatients aged ≥ 40 years with at least a 10 pack‐year smoking history and moderate‐to‐severe COPD (FEV1 ≤ 50% of predicted, and FEV1 /SVC ≤ 70%), with lung hyperinflation (residual volume (RV) measured using whole‐body plethysmography ≥ 125% of predicted) were eligible for inclusion in the study. RV is a static lung volume that reflects lung hyperinflation Exclusion Criteria: a history of asthma, allergic rhinitis or atopy; a blood eosinophil count ≥ 600 cells/mm3; a recent history of myocardial infarction (within the previous year), congestive heart failure (within the previous three years), or cardiac arrhythmia requiring drug therapy; recent lower respiratory tract infection; regular use of supplemental oxygen; oral corticosteroid use at unstable doses during the six weeks prior to entering the study or at a stable dose exceeding the equivalent of 10 mg prednisone daily |
|
| Interventions |
1. once‐daily inhaled tiotropium 18 mcg 2. once‐daily inhaled placebo Inhaler device: dry powder inhaler Co‐medication: during the treatment period, patients were permitted oral corticosteroids (at a dose of ≤ 10 mg/day prednisone or equivalent), ICS, theophylline preparations, mucolytic agents and salbutamol MDI, as needed, for acute symptom relief. Use of short‐acting anticholinergics, oral beta2‐agonists, or LABAs was not allowed |
|
| Outcomes |
Primary: the change from baseline in trough FVC Secondary: FVC, IC and SVC were measured to assess indirectly lung volumes and FEV1 to assess airflow limitation. Daily PEFR measurements. Exercise capacity was assessed using the incremental shuttle walking test (SWT). Exertional dyspnoea was assessed using the modified Borg scale. Dyspnoea during activities of daily living was evaluated using the BDI. Changes from baseline were measured using the TDI. HRQoL was determined using the SGRQ |
|
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.215 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boehringer Ingelheim was responsible for preparing and coding study medication in a blinded fashion (Boehringer Ingelheim study drug and control were indistinguishable). Patients, investigators and study personnel remained blinded with regard to the treatment assignments up to database lock |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The withdrawal rates were relatively low but uneven (tiotropium 2.2%, placebo 16.7%) |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
Voshaar 2008.
| Methods | Design: two identical, multicentre,randomised, double‐blind, parallel‐group studies. The run‐in phase was two weeks and the duration of treatment was 12 weeks. The studies were conducted in 39 centres across Germany, Italy, South Africa and Switzerland and in 25 centres across the USA and Canada from November 2002 to December 2003 | |
| Participants |
Population: 541 patients with COPD were randomised to tiotropium 5 mcg (180), tiotropium 10 mcg (180), and placebo (181) Baseline Characteristics: mean age 64 years, 70% male, mean FEV1 1.1 L, mean FEV1 predicted 52%, 52 pack‐years smoking history Inclusion Criteria: males and females aged ≥ 40 years with a diagnosis of COPD, moderate‐to‐severe airway obstruction with a pre‐bronchodilator FEV1 of ≤ 60% of predicted normal, FEV1/FVC ≤ 70%, (based on ECCS values) and a smoking history of ≥ 10 pack‐years were included. Exclusion Criteria: patients were excluded if they had a history of asthma, allergic rhinitis, any other significant respiratory illness or if they had a condition that could influence their ability to participate in the study. Other exclusion criteria included known hypersensitivity to anticholinergics, prior use of tiotropium, regular use of daytime oxygen therapy, significant alcohol or drug abuse or participation in another study. Pregnant or nursing women, or women of childbearing potential not using contraception, were also excluded. |
|
| Interventions |
1. Tiotropium 5 mcg once daily 2. Tiotropium 10 mcg once daily 3. Placebo once daily Inhaler device: soft mist inhaler Co‐medication: rescue medication (salbutamol pMDI) was permitted as needed during the study. Oral corticosteroids (equivalent of < 10 mg prednisone per day), orally ICS, theophyllines and mucolytics were allowed if stabilised for at least six weeks prior to and throughout the study. Oral beta‐adrenergics and other investigational drugs were not allowed for at least 1 month prior to run‐in. Cromolyn sodium and nedocromil sodium were not allowed for at least 3 months prior to run‐in. Anticholinergics, inhaled beta‐adrenergics other than salbutamol or fixed combination inhalers were also not allowed during the treatment period. |
|
| Outcomes |
Primary: the change in trough FEV1 after 12 weeks of treatment Secondary: FVC, PEFR and the number of patients achieving a 15% increase above baseline in FEV1, the weekly mean number of occasions per day that rescue medication (salbutamol) was used, the severity of COPD symptoms (i.e. wheezing, shortness of breath, coughing and tightness of chest), which was based on the physician’s assessment of the patient’s condition during the week prior to a clinic visit, and was rated from 0 (not present) to 3 (severe); the Physician's Global Evaluation of the patient’s condition, which was rated on an 8‐point scale from poor (1 to 2) to excellent |
|
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 205.251 / 205.252, ClinicalTrials.gov NCT00239473 / NCT00240435 Definitions: a COPD exacerbation was defined as a complex of respiratory events/symptoms with a duration of three days or more requiring a change in treatment. A complex of respiratory events/symptoms meant two or more of the following (increase of symptom or new onset): shortness of breath/dyspnoea/shallow, rapid breathing, sputum production, occurrence of purulent sputum, cough, wheezing, or chest tightness. The change in or requirement of treatment included the following: prescription of antibiotics and/or systemic corticosteroids, and/or a significant change of the prescribed respiratory medication (bronchodilators including theophylline). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by Boehringer Ingelheim using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The double‐dummy feature prevented both investigators and patients from differentiating active drug from placebo, despite the different inhaler devices, which could otherwise not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The withdrawal rates were relatively low (tiotropium 5mcg 8.9%, tiotropium 10mcg 10%, and placebo 12.2%) |
| Selective reporting (reporting bias) | Low risk | Results for all specified outcomes were reported |
ATS: American Thoracic Society BDI: Baseline Dyspnoea Index BTS: British Thoracic Society COPD: chronic obstructive pulmonary disease ECG: electrocardiogram FEV1: forced expiratory volume in one second FEV 6 : forced expiratory volume in six second s
FIV1: forced inspiratory volume in one second FVC: forced vital capacity GOLD: Global Initiative for Chronic Obstructive Lung Disease HRQoL: health‐related quality of life IC: inspiratory capacity ICS: inhaled corticosteroids ICU: intensive care unit LABA: long‐acting beta2‐agonist MDI: metered‐dose inhaler PEF: peak expiratory flow PEFR: peak expiratory flow rate SABA: short ‐acting beta2‐agonist SGRQ: St George's Respiratory Questionnaire SVC: slow vital capacity TDI: Transition Dyspnoea Index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ambrosino 2008 | Part of a more complex intervention with 8 weeks pulmonary rehabilitation before 12 weeks of tiotropium versus placebo treatment |
| Baloira 2005 | Less than 12 weeks study duration |
| Bedard 2011 | Less than 12 weeks study duration |
| Calverley 2000 | Less than 12 weeks study duration |
| Casaburi 2005 | Part of a more complex intervention with 8 weeks pulmonary rehabilitation before 12 weeks of tiotropium versus placebo treatment |
| Celli 2002 | Less than 12 weeks study duration |
| da Fonseca 2010 | Part of a more complex intervention with an exercise programme |
| de Guia 2004 | Tiotropium versus ipratropium, no placebo group, less than 12 weeks study duration |
| Diba 2009 | Less than 12 weeks study duration |
| Diba 2011 | Cross‐over study design |
| Friedman 1998 | Less than 12 weeks study duration |
| Fuhr 2010 | Cross‐over study design |
| Gelb 2011 | Cross‐over study design |
| Gurzhiy 2007 | Not an RCT as the study groups were moderate versus severe COPD, less than 12 weeks study duration |
| Halpin 2006 | Systematic review of data |
| Hasani 2001 | Less than 12 weeks study duration |
| Hasani 2001b | Less than 12 weeks study duration |
| Hirata 2003 | Tiotropium versus oxitropium, no placebo group |
| Kerstjens 2004 | Tiotropium in combination with ipratropium or fenoterol or placebo, cross‐over study design |
| Langley 2002 | Less than 12 weeks study duration |
| McNicholas 2001 | Less than 12 weeks study duration |
| Meshcheriakova 2007 | Tiotropium + ICS/LABA or tiotropium + ICS/LABA + threshold positive expiratory pressure (PEP) and inspiratory muscle trainer (IMT) devices (PID) training or tiotropium + ICS/LABA + physical training or ICS/LABA |
| O'Donnell 2002 | Less than 12 weeks study duration |
| O'Donnell 2004a | Less than 12 weeks study duration |
| O'Donnell 2005 | Less than 12 weeks study duration |
| O'Donnell 2005a | Cross‐over study design |
| Olson 2009 | Less than 12 weeks study duration |
| Reisner 2011 | Cross‐over study design |
| Rossi 2008 | Cross‐over study design, less than 12 weeks study duration |
| Schilling 2000 | Less than 12 weeks study duration |
| Schurmann 2004 | Cross‐over study design, less than 12 weeks study duration |
| Sposato 2005 | Less than 12 weeks study duration |
| ten Hacken 2007 | Cross‐over study design |
| van Noord 2006 | Cross‐over study design, less than 12 weeks study duration |
| Vincken 2001 | Tiotropium versus ipratropium, no placebo group |
COPD: chronic obstructive pulmonary disease ICS: inhaled corticosteroids IMT: inspiratory muscle training LABA: long‐acting beta2‐agonist PEP: positive expiratory pressure
Characteristics of studies awaiting assessment [ordered by study ID]
Gu 2007.
| Methods | Design: 12 weeks, double‐blind, randomised, placebo‐controlled, parallel group study |
| Participants |
Population: 57 patients Inclusion criteria: FEV1/FVC ≤ 0.70, FEV1 ≥ 30% predicted |
| Interventions | |
| Outcomes | Dyspnoea scale, 6MWD, FEV1/FVC, FEV1%, IC |
| Notes |
Min 2006.
| Methods | Design: 12 weeks, double‐blind, randomised, placebo‐controlled, parallel group study |
| Participants |
Population: 43 patients Inclusion criteria: FEV1/FVC ≤ 0.70, FEV1 ≥ 30% predicted |
| Interventions |
1. Tiotropium 18 mcg once daily 2. Placebo once daily |
| Outcomes | FEV1, FVC, FEV1/FVC, FEV1%, safety |
| Notes |
NCT00528996.
| Methods | Design: 24 weeks, multinational, randomised, double‐blind, parallel group study |
| Participants |
Population: 2080 patients with COPD Inclusion criteria: FEV1 of < 80% of predicted, FEV1/FVC ≤ 70%, > 40 years of age, smoking history of > 10 pack‐years |
| Interventions |
1. 50 mcg BEA 2180 once daily 2. 100 mcg BEA 2180 once daily 3. 200 mcg BEA 2180 once daily 4. Placebo once daily 5. Tiotropium bromide once daily Inhaler device: Respimat soft mist inhaler |
| Outcomes | Primary: trough FEV1 response after 24 weeks Secondary: trough FEV1 response after 1, 2, 4, 8, 12, and 18 weeks, safety |
| Notes |
Funding: Boehringer Ingelheim Study number: Boehringer Ingelheim 1205.14, ClinicalTrials.gov NCT00528996 |
Xia 2007.
| Methods | Design: 12 weeks, randomised, double‐blind, placebo‐controlled, parallel group study |
| Participants |
Population: 50 patients Inclusion criteria: FEV1/FVC ≤ 0.70, FEV1 ≥ 30% predicted |
| Interventions | |
| Outcomes | FEV1, FVC, FEV1/FVC, FEV1% |
| Notes |
Yin 2010.
| Methods | Design: 12 week, multicentre, randomised, double‐blind, placebo‐controlled study |
| Participants | Population: 205 patients with stable stage I or II COPD |
| Interventions |
1. Tiotropium 18 mcg once daily 2. Placebo |
| Outcomes | Clinical symptoms, adverse events |
| Notes |
COPD: chronic obstructive pulmonary disease FEV1: forced expiratory volume in one second FVC: forced vital capacity 6MWD: six‐minute walk distance
Differences between protocol and review
We had planned in the protocol to look at the effect of tiotropium on serious adverse cardiovascular events. However, a more recent systematic review, including 19,545 randomised patients in studies of four weeks or longer, showed that tiotropium was associated with a reduction in the risk of serious cardiovascular events (Celli 2010). In this review we did not try to obtain cardiovascular event data for the included studies from the manufacturer, nor additional studies published since Celli 2010, so as not to delay publication of this review.
Contributions of authors
Charlotta Karner (CK) and Jimmy Chong (JC) identified eligible trials and extracted data. CK performed the statistical analysis and wrote the review. JC and Phillippa Poole (PP) contributed to the interpretation of findings and writing of the final draft.
Sources of support
Internal sources
-
St George's University of London, UK.
CK is supported by St George's University of London
External sources
-
NIHR, UK.
Programme grant funding
Declarations of interest
None known.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Bateman 2010a {published and unpublished data}
- Bateman ED. A randomised, double‐blind, placebo‐controlled, parallel‐group study to assess long term (one‐year) efficacy and safety of tiotropium inhalation solution 5 mcg (2 puffs of 2.5 mcg) delivered by the Respimat® inhaler in patients with chronic obstructive pulmonary disease (COPD). www.trials.boehringer‐ingelheim.com. [trial number: 205.372]
- Bateman ED, Tashkin D, Siafakas N, Dahl R, Towse L, Massey D, et al. A one‐year trial of tiotropium Respimat plus usual therapy in COPD patients. Respiratory Medicine 2010;104(10):1460‐72. [CTG: NCT00387088] [DOI] [PubMed] [Google Scholar]
Bateman 2010b {published and unpublished data}
- Bateman E, Singh D, Smith D, Disse B, Towse L, Massey D, et al. Efficacy and safety of tiotropium Respimat SMI in COPD in two 1‐year randomized studies. International Journal of Chronic Obstructive Pulmonary Disease 2010;5:197‐208. [CTG: NCT00168831; CTG: NCT00168844] [PMC free article] [PubMed] [Google Scholar]
- Cooper, C. A retrospective collection of vital status and pulmonary medication usage data for patients with chronic obstructive pulmonary disease (COPD) who withdrew prematurely from either of two one‐year trials (205.254, 205.255) of tiotropium inhalation solution delivered by the Respimat® inhaler. www.trials.boehringer‐ingelheim.com (accessed 05 March 2008). [trial number: 205.392]
- Hodder R, Pavia D, Lee A, Bateman E. Lack of paradoxical bronchoconstriction after administration of tiotropium via Respimat Soft Mist Inhaler in COPD. International Journal of Chronic Obstructive Pulmonary Disease 2011;6:245‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SD. A randomised, double‐blind, placebo‐controlled, parallel group efficacy and safety comparison of one‐year treatment of two doses (5 μg [2 actuations of 2.5 μg] and 10 μg [2 actuations of 5 μg]) of tiotropium inhalation solution delivered by the Respimat® inhaler in patients with chronic obstructive pulmonary disease (COPD). www.trials.boehringer‐ingelheim.com. [trial number: 205.255]
- Smith D. A Randomised, Double‐Blind Placebo Controlled, Parallel Group Efficacy and Safety Comparison of One‐year Treatment of Two Doses (5 μg [2 actuations of 2.5 μg] and 10 μg [2 actuations of 5 μg]) of Tiotropium Inhalation Solution Delivered by the Respimat® Inhaler in Patients with Chronic Obstructive Pulmonary Disease (COPD). www.trials.boehringer‐ingelheim.com. [trial number: 205.254]
Beeh 2006 {published and unpublished data}
- Beeh KM, Beier J, Buhl R, Gerken FMN. Efficacy of tiotropium (spriva) in patients with COPD switched from previous treatment with short‐acting anticholinergics [Abstract]. Proceedings of the American Thoracic Society; 2006 May 19‐24; San Diego. 2006:A113 [Poster J27].
- Beeh KM, Beier J, Buhl R, Stark‐Lorenzen P, Gerken F, Metzdorf N, et al. Efficacy of tiotropium bromide (Spiriva) in patients with chronic‐obstructive pulmonary disease (COPD) of different severities [Wirksamkeit von Tiotropiumbromid (Spiriva)bei verschiedenen Schweregraden derchronisch−obstruktiven Lungenerkrankung (COPD)]. Pneumologie 2006;60(6):341‐6. [CTG: NCT00274573] [DOI] [PubMed] [Google Scholar]
- Beeh KM, Beier J, Buhl R, Strak‐Lorenzen P, Gerken F, Metzdorf N. Efficacy of tiotropium in patients with mild to moderate COPD [Abstract]. American Thoracic Society 2004 International Conference; 2004 May 21‐26; Florida, USA. 2004:P513.
- Boehringer Ingelheim. Acute and long‐term effects of once daily oral inhalation of tiotropium 18 mcg dry powder inhalation capsules in a placebo controlled parallel group design study in patients with chronic obstructive pulmonary disease (COPD) of different severity. www.trials.boehringer‐ingelheim.com. [trial number: 205.257]
Brusasco 2003 {published and unpublished data}
- Bateman ED, Hodder R, Miravitlles M, Lee A, Towse L, Serby C. A comparative trial of tiotropium, salmeterol and placebo: health‐related quality of life. European Respiratory Journal 2001;18(Suppl 33):26. [Google Scholar]
- Bateman ED, Jenkins C, Korducki L, Keston S. Tiotropium (TIO) improves health care resource utilization (HRU) and patient disability in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A111. [Google Scholar]
- Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 2003;58(5):399‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusasco V, Menjoge SS, Kesten S. Flow and volume responders following treatment with tiotropium and salmeterol in patients with COPD [Abstract]. American Thoracic Society 99th International Conference; 2003; May 16 ‐ 21; Seattle. 2003:B024 Poster 420.
- Brusasco V, Menjoge SS, Kesten S. Flow and volume responders over 12 hours following treatment with tiotropium or salmeterol in patients with COPD [Abstract]. American Thoracic Society Annual Meeting; 2006; May 19‐24; San Diego. 2006:A110 [Poster J10].
- Brusasco V, Thompson P, Vincken W, Lee A, Towse L, Witek TJ. Improvement of dyspnea following of six months treatment with tiotropium but not with salmeterol in patients with COPD. European Respiratory Journal 2001;18(Suppl 33):26. [Google Scholar]
- Donohue JF. Alterations in bronchodilator effectiveness over six months with tiotropium and salmeterol. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A227. [Google Scholar]
- Donohue JF, Menjoge S, Kesten S. Tolerance to bronchodilating effects of salmeterol in COPD. Respiratory Medicine 2003;97(9):1014‐20. [DOI] [PubMed] [Google Scholar]
- Donohue JF, Noord JA, Bateman ED, Langley SJ, Lee A, Witek TJ, et al. A 6‐month, placebo‐controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 2002;122(1):47‐55. [DOI] [PubMed] [Google Scholar]
- Donohue JF, Noord JA, Langley SJ, Lee A, Kesten S, Towse LJ. Superior bronchodilaton of once daily tiotropium compared to twice daily salmeterol in patients with COPD. European Respiratory Journal 2001;18(Suppl 33):26. [Google Scholar]
- Friedman M, Morera G, Menjoge S, Kesten S. Reduced COPD exacerbations with tiotropium. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A270. [Google Scholar]
- Gunther K, Lee A, Kesten S, Towse L. Superior bronchodilation of once daily tiotropium compared with twice daily salmeterol in patients with COPD [Abstract]. International Primary Care Respiratory Group Congress; 2002; June 7‐9; Amsterdam. 2002:112.
- Hodder R, Kesten S, Menjoge S, Viel K. Outcomes in COPD patients receiving tiotropium or salmeterol plus treatment with inhaled corticosteroids. International Journal of COPD 2007;2(2):157‐67. [PMC free article] [PubMed] [Google Scholar]
- Langley J, Briggs Jr D, Bhattacharya S, Kesten S, Cassino C. Spirometric outcomes of COPD patients with tiotropium compared with salmeterol according to previous maintenance long acting beta‐agonist use [Abstract]. European Respiratory Journal 2004;24(Suppl 48):289. [Google Scholar]
Casaburi 2002 {published and unpublished data}
- Adams SG, Anzueto A, Briggs DD, Menjoge SS, Kesten S. Tiotropium in COPD patients not previously receiving maintenance respiratory medications. Respiratory Medicine 2006;100(9):1495‐503. [DOI] [PubMed] [Google Scholar]
- Anzueto A, Kesten S. Effects of tiotropium in COPD patients only treated with PRN albuterol [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26; Orlando. 2004:C47 Poster F81.
- Anzueto A, Tashkin D, Menjoge S, Kesten S. One‐year analysis of longitudinal changes in spirometry in patients with COPD receiving tiotropium. Pulmonary Pharmacology and Therapeutics 2005;18(2):75‐81. [DOI] [PubMed] [Google Scholar]
- Casaburi R, Briggs DD Jr, Donohue JF, Serby CW, Menjoge SS, The US Tiotropium Study Group. The spirometric efficacy of once‐daily dosing with tiotropium in stable COPD: a 13‐week multicenter trial. Chest 2000;118(5):1294‐302. [DOI] [PubMed] [Google Scholar]
- Casaburi R, Mahler DA, Jones PW, Wanner A, San Pedro G, Zuwallack RL, et al. A long‐term evaluation of once‐daily inhaled tiotropium in chronic obstructive pulmonary disease. European Respiratory Journal 2002;19(2):217‐24. [DOI] [PubMed] [Google Scholar]
- Friedman M, Bell T, Menjoge SS, Anton S, Koch P. Cost consequences of tiotropium plus existing therapy versus existing therapy alone following one year of treatment in patients with COPD. European Respiratory Journal 2001;18(Suppl 33):5. [Google Scholar]
- Friedman M, Menjoge SS, Anton SF, Kesten S. Healthcare costs with tiotropium plus usual care versus usual care alone following 1 year of treatment in patients with chronic obstructive pulmonary disorder (COPD). Pharmacoeconomics 2004;22(11):741‐9. [DOI] [PubMed] [Google Scholar]
- Kesten S, Flanders J, Menjoge SS, Serby C. Maintenance of bronchodilation following tiotropium in patients with mild, moderate and severe COPD in one‐year placebo‐controlled clinical trials [Abstract]. International Primary Care Respiratory Group Congress; 2002; June 7‐9; Amsterdam. 2002:24.
- Kesten S, Menjoge S. Can patient demographics be used to predict future choices for antibiotics, steroids, or both for exacerbations of COPD? [Abstract]. National COPD Conference; 2003; November 14‐15, 2003; Arlington, Virginia. 2003:Abstract no: 1189.
- Kesten S, Menjoge SS, Witek T. The impact of COPD exacerbations (EXAC) on health related quality of life (HRQL) is attenuated by tiotropium [Abstract]. International Primary Care Respiratory Group Congress; 2002; June 7‐9; Amsterdam. 2002:23.
- Mahler DA, Montner P, Brazinsky SA, Goodwin B, Menjoge SS, Witek TJ. Tiotropium (spiriva™), a new long‐acting anticholinergic bronchodilator, improves dyspnea in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A892. [Google Scholar]
- San Pedro G, Elias DJ, Serby CW, Witek TJ. Tiotropium (spiriva™): one year bronchodilator efficacy established with once daily dosing in COPD patients. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A749. [DOI] [PubMed] [Google Scholar]
- Serby CW. Impact of tiotropium on COPD exacerbations: 1‐Yr studies versus placebo or ipratropium. European Respiratory Review 2002;12(82):16‐7. [Google Scholar]
- Serby CW, Schwartzstein, Jones, Ries, Killian. Tiotropium: 1‐Yr studies versus placebo/ipratropium. European Respiratory Review 2002;12(82):40‐2. [Google Scholar]
- Spencer S. Decline in health status over one year is eliminated by tiotropium. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A228. [Google Scholar]
- Spencer S, Jones PW. Short‐term changes in health status with tiotropium predict long‐term changes in health status [Abstract]. European Respiratory Society Annual Congress; 2002; September 14‐18; Stockholm. 2002:P1611.
- Tashkin D, Kesten S. Long‐term treatment benefits with tiotropium in COPD patients with and without short‐term bronchodilator responses. Chest 2003;123(5):1441‐9. [DOI] [PubMed] [Google Scholar]
- Witek TJ Jr, Mahler DA. Meaningful effect size and patterns of response of the transition dyspnea index. Journal of Clinical Epidemiology 2003;56(3):248‐55. [DOI] [PubMed] [Google Scholar]
- Zuwallack R, Jones PW, Kotch A, Goodwin B, Menjoge SS, Serby CW. Tiotropium (spiriva™) improves health status in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A892. [Google Scholar]
- Zuwallack R, Morganroth J, Lanes S, Kesten S. Electrocardiographic evaluations in COPD patients treated with tiotropium in one‐year clinical trials [Abstract]. American Thoracic Society 99th International Conference; 2003 May 16‐21; Seattle. 2003:A035 Poster D78.
Chan 2007 {published and unpublished data}
- Chan C. Spiriva assessment of FEV1 (SAFE). The effect of inhaled tiotropium bromide (18 mcg once daily) on the change in FEV1 during long‐term treatment in patients with COPD. A one‐year parallel group, double‐blind, randomised, placebo‐controlled study. Boehringer Ingelheim Clinical Trial Register 2005. [trial number : 205.259]
- Chan CK, Maltais F, Sigouin C, Haddon JM, Ford GT, Group SS. A randomized controlled trial to assess the efficacy of tiotropium in Canadian patients with chronic obstructive pulmonary disease. Canadian Respiratory Journal 2007;14(8):465‐72. [CTG: NCT00277264] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2010 {published and unpublished data}
- Cooper C. A randomized, double‐blind, placebo‐controlled two‐year trial to examine the changes in exercise endurance and COPD patients treated with tiotropium (Spiriva® HandiHaler®) 18 μg once daily (EXACTT trial). www.trials.boehringer‐ingelheim.com. [trial number: 205.368]
- Cooper CB, Abrazado M, Legg D, Kesten S. Development and implementation of treadmill exercise testing protocols in COPD. International Journal of COPD 2010;5:375‐85. [CTG: NCT00525512] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CB, Celli B, Wise RA, Jardim JR, Legg D, Guo J, et al. "Treadmill endurance during 2 years" treatment with tiotropium in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2011;183:A4586. [Google Scholar]
Covelli 2005 {published and unpublished data}
- Boehringer Ingelheim. Efficacy and safety (including 24‐hour holter monitoring) of tiotropium inhalation capsules in patients with chronic obstructive pulmonary disease (a 12‐week, parallel group, randomized, placebo‐controlled, double‐blind study). www.trials.boehringer‐ingelheim.com. [trial number: 205.284]
- Covelli H, Bhattacharya S, Cassino C, Conoscenti C, Kesten S. Absence of electrocardiographic findings and improved function with once‐daily tiotropium in patients with chronic obstructive pulmonary disease. Pharmacotherapy 2005; Vol. 25, issue 12:1708‐18. [CTG: NCT00239460] [DOI] [PubMed]
- Kesten S, Cassino C, Conoscenti C. Absence of electrocardiographic findings with daily tiotropium in patients with chronic obstructive pulmonary disease [Abstract]. Chest 2004;126(4 Suppl):837S. [DOI] [PubMed] [Google Scholar]
Dusser 2006 {published and unpublished data}
- Dusser D. Effect of inhaled tiotropium bromide (18 mcg once daily) on the severity of airflow obstruction during long‐term treatment in patients with moderately severe COPD. Impact on severity and incidence of exacerbations. A one‐year parallel‐group, double‐blind, randomized, placebo‐controlled study. www.trials.boehringer‐ingelheim.com. [trial number: 205.214]
- Dusser D, Bravo ML, Lacono P. The effect of tiotropium on exacerbations and airflow in patients with COPD. European Respiratory Journal 2006;27(3):547‐55. [CTG: NCT00274014] [DOI] [PubMed] [Google Scholar]
- Dusser D, Bravo ML, Lacono P. Tiotropium reduces health resource utilization associated with COPD exacerbations [Abstract]. European Respiratory Journal 2004;24(Suppl 48):513. [Google Scholar]
- Dusser D, Bravo ML, Lanono P. Tiotropium COPD exacerbations the MISTRAL study [Abstract]. European Respiratory Journal 2004;24(Suppl 48):513. [Google Scholar]
- Dusser D, Viel K, Bravo ML, Lacono P. Tiotropium reduces moderate‐to‐severe exacerbations in COPD patients irrespective of concomitant use of inhaled corticosteroids (ICS) [Abstract]. Proceedings of the American Thoracic Society; 2006 May 19‐24; San Diego. 2006:A603 [Poster H6].
- Dusser D, Viel K, Rouyrre N, Lancono P. Tiotropium reduces COPD exacerbations requiring treatment with systemic corticosteroids, antibiotics, or hospitalizations [Abstract]. Proceedings of the American Thoracic Society; 2006 May 19‐24; San Diego. 2006:A603 [Poster H5].
Freeman 2007 {published and unpublished data}
- Freeman D. A randomised, double‐blind, parallel group, 12 week study, comparing the effect of once daily tiotropium lactose capsule with placebo in patients with chronic obstructive pulmonary disease (COPD), naive to anticholinergic agents in addition to receiving their usual COPD care. www.trials.boehringer‐ingelheim.com. [trial number: 205.276]
- Freeman D, Lee A, Price D. Efficacy and safety of tiotropium in COPD patients in primary care‐the SPiRiva Usual CarE (SPRUCE) study. Respiratory Research 2007;8:45. [CTG: NCT00274079] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D, Sarno M, White L, Lee A, Price D. Spruce: tiotropium in UK primary care [Abstract]. Thorax 2004;59(Suppl II):ii100. [Google Scholar]
- Price D, Sarno M, Lee A, Freeman D. SPiRiva usual carE‐ SPRUCE ‐ tiotropium in a UK primary care COPD population other regular inhaled treatment [Abstract]. American Thoracic Society 2005 International Conference; 2005 May 20‐25; San Diego. 2005:[A43] [Poster: F26].
Johansson 2008 {published and unpublished data}
- Johansson G. A 12‐week, double‐blind, randomised, parallel‐group, multi‐centre study evaluating the efficacy of tiotropium versus placebo in patients with mild COPD according to Swedish guidelines (SPIRIMILD). www.trials.boehringer‐ingelheim.com. [trial number: 205.281]
- Johansson G, Lindberg A, Romberg K, Nordstrom L, Gerken F, Roquet A. Bronchodilator efficacy of tiotropium in patients with mild COPD [Abstract]. European Respiratory Journal 2006;28(Suppl 50):616s [E3614]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson G, Lindberg A, Romberg K, Nordstrom L, Gerken F, Roquet A. Bronchodilator efficacy of tiotropium in patients with mild to moderate COPD. Primary Care Respiratory Journal 2008; Vol. 17, issue 3:169‐75. [CTG: NCT00144196] [DOI] [PMC free article] [PubMed]
Magnussen 2008 {published and unpublished data}
- Boehringer Ingelheim. A trial evaluating the efficacy and safety of inhaled tiotropium 18 µg qd in patients with COPD and a concomitant diagnosis of asthma. www.clinicaltrials.gov 2005.
- Magnussen H. A 12‐week randomised, double blind, placebo‐controlled, parallel group trial evaluating the efficacy and safety of inhaled tiotropium 18 μg q.d. in patients with COPD and a concomitant diagnosis of asthma. www.trials.boehringer‐ingelheim.com. [trial number: 205.301]
- Magnussen H, Bugnas B, Noord J, Schmidt P, Gerken F, Kesten S. Improvements with tiotropium in COPD patients with concomitant asthma. Respiratory Medicine 2008;102(1):50‐6. [CTG: NCT00152984] [DOI] [PubMed] [Google Scholar]
- Magnussen H, Bugnas B, Noord J, Schmidt P, Gerken F, Kesten S, et al. Spirometric improvements with tiotropium in COPD patients with concomitant asthma [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco. 2007:Poster #A5.
Moita 2008 {published and unpublished data}
- Boehringer Ingelheim. Spiriva® assessment of FEV1 ‐ (SAFE‐Portugal). The effect of inhaled tiotropium bromide (18 mcg once daily) on the change in FEV1 during treatment in patients with COPD. A three‐month parallel group, double‐blind, randomised, placebo‐controlled study. www.trials.boehringer‐ingelheim.com. [trial number: 205.282]
- Moita J, Barbara C, Cardoso J, Costa R, Sousa M, Ruiz J, et al. Tiotropium improves FEV1 in patients with COPD irrespective of smoking status. Pulmonary Pharmacology and Therapeutics 2008;21(1):146‐51. [CTG: NCT00239408] [DOI] [PubMed] [Google Scholar]
- Moita J, Costa R, Barbara C, Cardosa J, Rulz J, Sousa M. The effect of tiotropium bromide in a stable cohort of COPD patients in Portugal [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1955. [Google Scholar]
NCT00144326 {published and unpublished data}
- García Río, F. A randomised, double‐blind, placebo‐controlled, 12 week trial to evaluate the effect of tiotropium inhalation capsules on the magnitude of exercise, measured using an accelerometer, in patients with chronic obstructive pulmonary disease (COPD). www.trials.boehringer‐ingelheim.com (accessed 15 Oct 2007). [CTG: NCT00144326; trial number: 205.269]
Niewoehner 2005 {published and unpublished data}
- Anonymous. Tiotropium reduces exacerbations in COPD. Pharmaceutical Journal 2004;272(7302):696. [Google Scholar]
- Kesten S, Plautz M, Piquette CA, Habib MP, Niewoehner DE. Premature discontinuation of patients: a potential bias in COPD clinical trials. European Respiratory Journal 2007;30(5):898‐906. [DOI] [PubMed] [Google Scholar]
- Niewoehner D. A randomized, double‐blind, placebo‐controlled, parallel group trial assessing the proportion of patients experiencing an exacerbation and proportion of patients hospitalized for an exacerbation over 6 months during treatment with tiotropium 18 mcg capsule once daily in patients with COPD in a Veterans Affairs setting. www.trials.boehringer‐ingelheim.com. [trial number: 205.266]
- Niewoehner D, Gonzalez‐Rothi R, Shigeoka J, Korducki L, Cassino C, Kesten S, et al. Reduced COPD exacerbations in patients without first‐dose peak FEV increases 15% with tiotropium [Abstract] American Thoracic Society 2005 International Conference; 2005 May 20‐25; San Diego, California. San Diego, 2005:[B93] [Poster: 319].
- Niewoehner D, Rice K, Cote C, Korducki L, Cassino C, Kesten S. Characteristics of COPD and response to once‐daily tiotropium in African‐Americans in the VA medical system [Abstract]. American Thoracic Society 2005 International Conference; 2005 May 20‐25; San Diego. San Diego, 2005:[A43] [Poster: F41].
- Niewoehner D, Rice K, Cote C, Paulson D, Cooper JA, Korducki L, et al. Reduced COPD exacerbations and associated health care utilization with once‐daily tiotropium (TIO) in the VA medical system [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26; Orlando. 2004:A84.
- Niewoehner DE, Rice K, Cassino C, Leimer I, Kesten S. Reductions in COPD exacerbations with tiotropium in African‐Americans in the VA medical system [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco. 2007:Poster #A51.
- Niewoehner DE, Rice K, Cote C, Paulson D, Cooper Jr JAD, Korducki L, et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once‐daily inhaled anticholinergic bronchodilator: A randomized trial. Annals of Internal Medicine 2005;143(5):317‐326. [CTG: NCT00274547] [DOI] [PubMed] [Google Scholar]
- Rice KL, Leimer I, Kesten S, Niewoehner DE. Responses to tiotropium in African‐American and Caucasian patients with chronic obstructive pulmonary disease. Translational Research 2008;152(2):88‐94. [DOI] [PubMed] [Google Scholar]
Powrie 2007 {published and unpublished data}
- Powrie D, Wilkinson T, Donaldson G, Viel K, Jones P, Scrine K, et al. The effect of tiotropium on diary based exacerbation frequency in COPD [Abstract]. American Thoracic Society Annual Meeting; 2006 May 19‐24 2006; San Diego. 2006:A604 [Poster H11].
- Powrie DJ, Donaldson GC, Wilkinson TM, Jones P, Viel K, Kesten S, et al. Tiotropium reduces exacerbations and common colds in patients with COPD [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco, California, USA. 2007:Poster #A50.
- Powrie DJ, Wilkinson TMA, Donaldson GC, Jones P, Scrine K, Viel K, et al. Effect of tiotropium on sputum and serum inflammatory markers and exacerbations in COPD. European Respiratory Journal 2007;30(3):472‐8. [CTG: NCT00405236] [DOI] [PubMed] [Google Scholar]
- Powrie J, Wilkinson M, Donaldson C, Wedzicha A. Tiotropium reduces subjectively reported sputum production in COPD. European Respiratory Journal 2006;28(Suppl 50):661. [Google Scholar]
- Wedzicha JA. A randomised, double‐blind, parallel‐group, placebo‐controlled study to evaluate the changes in inflammatory markers and in induced sputum following treatment with tiotropium inhalation capsules 18 μg once daily in patients with chronic obstructive pulmonary disease (COPD). www.trials.boehringer‐ingelheim.com. [trial number: 205.270]
Sun 2007 {published data only}
- Sun LH, Tan Y, Qiao Y, Fang SR, Xie H. Evaluation of clinical effect and safety of tiotropium bromide in treating stable chronic obstructive pulmonary disease. Zhongguo Xinyao yu Linchuang Zazhi 2007;26(5):328‐31. [Google Scholar]
- Yan L, Lu YK. Effect of domestic tiotropium bromide inhalation in patients with COPD at stable stage. Chinese Journal of New Drugs 2010;19(2):127‐129, 138. [Google Scholar]
Tashkin 2008 {published and unpublished data}
- Antoniu SA. UPLIFT Study: The effects of long‐term therapy with inhaled tiotropium in chronic obstructive pulmonary disease. Expert Opinion on Pharmacotherapy 2009;10(4):719‐22. [DOI] [PubMed] [Google Scholar]
- Braido F. UPLIFT study: The results and their implications. Multidisciplinary Respiratory Medicine. 2009; Vol. 4, issue Suppl 1:6s‐12s.
- Celli B, Decramer M, Kesten S, Liu D, Mehra S, Tashkin DP, et al. Mortality in the 4‐year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2009;180(10):948‐55. [DOI] [PubMed] [Google Scholar]
- Celli B, Kesten S, Lystig T, Tashkin DP, Decramer M. Long‐term changes in inspiratory capacity: an analysis from the UPLIFT(R) trial [Abstract]American Thoracic Society (ATS) International Conference; 2010 May 14‐19; New Orleans. 2010, issue Meeting Abstracts:A4482.
- Corhay JL, Louis R. The UPLIFT study. Revue Medicale de Liege 2009;64(1):50‐5. [PubMed] [Google Scholar]
- Decramer M, Celli B, Burkhart D, Kesten S, Mehra S, Liu D, et al. The effect of tiotropium on COPD GOLD stage II during the four‐year UPLIFT trial [Abstract]. American Thoracic Society International Conference; 2008 May 16‐21; Toronto. 2009:A2466.
- Decramer M, Celli B, Kesten S, Liu D, Menjoge S, Senn S, et al. Statistical approaches to analysis of exacerbations in the UPLIFT(R) trial [Abstract]. American Thoracic Society (ATS) International Conference; 2010 May 14‐19; New Orleans. 2010, issue Meeting Abstracts:A1527.
- Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP, et al. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet 2009;374(9696):1171‐8. [DOI] [PubMed] [Google Scholar]
- Decramer M, Celli B, Tashkin DP, Pauwels RA, Burkhart D, Cassino C, et al. Clinical trial design considerations in assessing long‐term functional impacts of tiotropium in COPD: the UPLIFT trial. Journal of Chronic Obstructive Pulmonary Disease 2004;1(2):303‐12. [DOI] [PubMed] [Google Scholar]
- Decramer M, Kesten S, Celli B, Tashkin DP. How much lift to the UPLIFT study? ‐ Authors' reply. The Lancet 2010;375(9710):198. [DOI] [PubMed] [Google Scholar]
- Decramer M, Molenberghs G, Liu D, Celli B, Kesten S, Lystig T, et al. Premature discontinuation during the UPLIFT study. Respiratory Medicine 2011;105:1523‐30. [DOI] [PubMed] [Google Scholar]
- Fernandez L, Kesten S, Liu D, Decramer M, Tashkin D, Celli B, et al. Efficacy of tiotropium in COPD patients from Asia: A subgroup analysis from the uplift trial [Abstract]. 15th Congress of the Asian Pacific Society of Respirology; 2010 November 22‐25; Manila. 2010. [DOI] [PubMed]
- Fukuchi Y, Fernandez L, Kuo HP, Mahayiddin A, Celli B, Decramer M, et al. Efficacy of tiotropium in COPD patients from Asia: A subgroup analysis from the UPLIFT trial. Respirology 2011;16:825‐35. [DOI] [PubMed] [Google Scholar]
- Halpin DM, Decramer M, Celli B, Kesten S, Leimer I, Tashkin DP. Risk of nonlower respiratory serious adverse events following COPD exacerbations in the 4‐year UPLIFT trial. Lung 2011;189(4):261‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanania N, Kesten S, Celli B, Decramer M, Sharafkhaneh A, Lystig T, et al. Acute bronchodilator response does not predict health outcomes in patients with COPD treated with tiotropium [Abstract]. European Respiratory Society Annual Congress; 2009 September 12‐16; Vienna. 2009:[E4353].
- Hanania NA, Sharafkhaneh A, Celli B, Decramer M, Lystig T, Kesten S, et al. Acute bronchodilator responsiveness and health outcomes in COPD patients in the UPLIFT trial. Respiratory Research 2011;12(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Asai Y. UPLIFT (understanding potential long‐term impacts on function with tiotropium) study. Respiration and Circulation 2009;57(8):813‐8. [Google Scholar]
- Kesten S, Celli B, Decramer M, Liu D, Tashkin D. Adverse health consequences in COPD patients with rapid decline in FEV1 ‐ evidence from the UPLIFT trial. Respiratory Research 2011;12:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarvey L, Magder SA, Liu D, Kesten S, Manuel R, Niewoehner DE. A comparison of the classification of cause of death between site investigators and a mortality adjudication committee in the UPLIFT(R) trial [Abstract]. American Thoracic Society (ATS) International Conference; 2010 May 14‐19; New Orleans. 2010.
- Morice AH, Celli B, Kesten S, Lystig T, Tashkin D, Decramer M. COPD in young patients: A pre‐specified analysis of the four‐year trial of tiotropium (UPLIFT). Respiratory Medicine 2010;104(11):1659‐67. [DOI] [PubMed] [Google Scholar]
- Osztovits J, Feher J. Safety of tiotropium therapy in chronic obstructive lung diseases (COPD) [A tiotropiumterápia cardiovascularis biztonságossága krónikus obstruktív tüdőbetegségben (COPD)]. Orvosi Hetilap 2010;151(18):749‐50. [DOI] [PubMed] [Google Scholar]
- Paggiaro P, Rossi A. "Prince Charming"? Some considerations about the decline of FEV1 and on data from UPLIFT study. Rassegna di Patologia dell'Apparato Respiratorio 2009;24(5):278‐86. [Google Scholar]
- Rodriguez Gonzalez‐Moro JM, Lucero S, Lucas Ramos P. The UPLIFT study: future perspectives [Estudio UPLIFT: perspectivas futuras]. Archivos de Bronconeumologia 2008;44(Suppl 2):39‐48. [PubMed] [Google Scholar]
- Tashkin D. A randomized, double‐blind, placebo‐controlled, parallel group trial assessing the rate of decline of lung function with tiotropium 18 mcg inhalation capsule once daily in patients with chronic obstructive pulmonary disease (COPD). www.trials.boehringer‐ingelheim.com. [trial number: 205.235]
- Tashkin D, Celli B, Decramer M, Burkhart D, Kesten S, Cassino C. Comorbid conditions reported in patients with COPD recruited into a global clinical trial (UPLIFT) [Abstract]. European Respiratory Society 15th Annual Congress; 2005 September 17 ‐ 21; Copenhagen. 2005; Vol. 26, issue Suppl 49:Abstract No. 4203.
- Tashkin D, Celli B, Kesten S, Lystig T, Decramer M. Effect of tiotropium in men and women with COPD: Results of the 4‐year UPLIFT trial. Respiratory Medicine 2010;104(10):1495‐504. [DOI] [PubMed] [Google Scholar]
- Tashkin D, Celli B, Lystig T, Kesten S, Decramer M. Efficacy of tiotropium in COPD patients with FEV1? 60% participating in the UPLIFT trial [Abstract]. European Respiratory Society Annual Congress; 2010 September 18‐22; Barcelona, Spain. 2010:[P1186].
- Tashkin D, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4‐year trial of tiotropium in chronic obstructive pulmonary disease (UPLIFT trial). Revista Portuguesa de Pneumologia 2009;15(1):137‐40. [DOI] [PubMed] [Google Scholar]
- Tashkin DP. Impact of tiotropium on the course of moderate‐to‐very severe chronic obstructive pulmonary disease: The UPLIFT trial. Expert Review of Respiratory Medicine 2010;4(3):279‐89. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Celli B, Burkhart D, Kesten S, Liu D, Mehra S, et al. Long‐term efficacy of tiotropium in continuing smokers vs sustained ex‐smokers in the UPLIFT trial [Abstract]. American Thoracic Society International Conference; 2009 May 15‐20; San Diego. 2009:A6175 [Poster #201].
- Tashkin DP, Celli B, Decramer M, Liu D, Burkhart D, Cassino C, et al. Bronchodilator responsiveness in patients with COPD. European Respiratory Journal 2008;31(4):742‐50. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Celli B, Kesten S, Liu D, Decramer M. Reduced reporting of respiratory failure in the UPLIFT(R) trial [Abstract]. American Thoracic Society International Conference; 2010 May 14‐19; New Orleans. 2010.
- Tashkin DP, Celli B, Kesten S, Lystig T, Mehra S, Decramer M. Long‐term efficacy of tiotropium in relation to smoking status in the UPLIFT trial. European Respiratory Journal 2010;35(2):287‐94. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4‐year trial of tiotropium in chronic obstructive pulmonary disease. New England Journal of Medicine 2008; Vol. 359, issue 15:1543‐54. [CTG: NCT00144339] [DOI] [PubMed]
- Tashkin DP, Celli BR, Kesten S, Liu D, Decramer M. Cardiovascular adverse events according to GOLD stage in the UPLIFT trial. Chest 2009;136(4):52. [Google Scholar]
- Tashkin DP, Celli BR, Kesten S, Lystig T, Mehra S, Decramer M. Efficacy of tiotropium in men and women: 4‐year follow‐up in the UPLIFT trial. Chest 2009;136(4):24‐25. [Google Scholar]
- Tashkin DP, Decramer M, Celli B, Burkhart D, Cassino C, Kesten S. Baseline characteristics according to gender in a global respiratory trial [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco. 2007:Poster #924.
- Troosters T, Celli B, Kesten S, Liu D, Mehra S, Tashkin D, et al. Effectiveness of combination therapy with tiotropium in COPD. A secondary analysis of the uplift trial [Abstract]. European Respiratory Society Annual Congress; 2009 September 12‐16; Vienna. 2009:[P3808].
- Troosters T, Celli B, Kesten S, Liu D, Tashkin D, Decramer M. Effectiveness of combination therapy with tiotropium in COPD. A secondary analysis of the UPLIFT trial [Abstract]. Primary Care Respiratory Journal 2010;19(2):A13. [Google Scholar]
- Troosters T, Celli B, Lystig T, Kesten S, Mehra S, Tashkin DP, et al. Tiotropium as a first maintenance drug in COPD: Secondary analysis of the UPLIFT trial. European Respiratory Journal 2010;36(1):65‐73. [DOI] [PubMed] [Google Scholar]
- Troosters T, Kesten S, Burkhart D, Celli B, Liu D, Mehra S, et al. Effectiveness of tiotropium as first maintenance drug in patients with COPD. Secondary analysis of the UPLIFT Trial [Abstract]. American Thoracic Society International Conference; 2009 May 15‐20; San Diego. 2009.
Tonnel 2008 {published and unpublished data}
- Tonnel AB. Effect of a 9‐month treatment of SPIRIVA® on health related quality of life in patients with chronic obstructive pulmonary disease. Validation of a new HRQoL questionnaire appropriate to common daily practice. (TIPHON Study). www.trials.boehringer‐ingelheim.com. [trial number: 205.256]
- Tonnel AB, Bravo ML, Brun M. Clinically significant improvements of health status of COPD patients after 9 months treatment with tiotropium bromide: the TIPHON study [Abstract]. American Thoracic Society International Conference; May 20‐25; San Diego. 2005:[B93] [Poster: 302].
- Tonnel AB, Perez T, Grosbois JM, Bravo ML, Brun M. Improvement in HRQoL of COPD patients after 9 months treatment with tiotropium bromide: use of a new scale for daily medical practice [Abstract]. European Respiratory Society 15th Annual Congress; 2005 September 17 ‐ 21; Copenhagen. 2005.
- Tonnel AB, Perez T, Grosbois JM, Verkindre C, Bravo ML, Brun M. Effect of tiotropium on health‐related quality of life as a primary efficacy endpoint in COPD. International Journal of COPD 2008;3(2):301‐10. [CTG: NCT00274053] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trooster 2011 {published and unpublished data}
- Carron K. A 24 week, randomized, double‐blind, placebo‐controlled, multicenter study to evaluate the efficacy and safety of 18 mcg of tiotropium inhalation capsules administered by handihaler® once‐daily plus prn albuterol (salbutamol) vs. placebo plusprn albuterol (salbutamol) in chronic obstructive pulmonary disease subjects naive to maintenance therapy. www.trials.boehringer‐ingelheim.com. [trial number: 205.365]
- Sciurba FC, Siafakas N, Troosters T, Klioze SS, Sutradhar S, Weisman I, et al. The efficacy and safety of tiotropium Handihaler, 18 µg, once daily plus PRN salbutamol versus placebo plus prn salbutamol in COPD subjects naive to maintenance therapy [Abstract]. American Thoracic Society 2011 International Conference; 2011 May 13‐18; Denver. 2011; Vol. 183:A1589.
- Troosters T, Weisman I, Dobbels F, Giardino N, Valluri SR. Assessing the impact of tiotropium on lung function and physical activity in GOLD Stage II COPD patients who are naive to maintenance respiratory therapy: a study protocol. The Open Respiratory Medicine Journal 2011;5:1‐9. [CTG: NCT00523991] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verkinde 2006 {published and unpublished data}
- Huchon G. Effect of a 12‐week treatment with inhaled tiotropium (18 mcg once daily) on lung function and static lung volumes in stable, moderate to severe COPD patients. Correlation to dyspnoea scales: a double‐blind, placebo‐controlled, randomized, parallel group study. www.trials.boehringer‐ingelheim.com. [trial number: 205.215]
- Huchon G, Verkindre C, Bart F, Aguilaniu B, Guerin JC, Lemerre C, et al. Improvements with tiotropium on endurance measure by the shuttle walking test (SWT) and on health related quality of life (HRQoL) in COPD patients. European Respiratory Society Congress; 2002 September 14‐18; Stockholm. 2002.
- Verkindre C, Bart F, Aguilaniu B, Fortin F, Guerin JC, Merre C, et al. The effect of tiotropium on hyperinflation and exercise capacity in chronic obstructive pulmonary disease. Respiration 2006;73(4):420‐7. [DOI] [PubMed] [Google Scholar]
Voshaar 2008 {published and unpublished data}
- Boehringer Ingelheim. A randomised, double‐blind, double‐dummy, placebo‐ and active‐controlled, parallel group efficacy and safety comparison of 12‐week treatment of two doses [5 mcg (2 actuations of 2.5 mcg) and 10 mcg (2 actuations of 5 mcg)] of tiotropium inhalation solution delivered by the Respimat inhaler, placebo and ipratropium bromide inhalation aerosol (MDI) in patients with chronic obstructive pulmonary disease (COPD). www.trials.boehringer‐ingelheim.com. [trial number: 205.251]
- Boehringer Ingelheim. A randomized, double‐blind, double‐dummy, placebo‐ and active‐controlled, parallel group efficacy and safety comparison of 12‐week treatment of two doses (5 Mcg and 10 Mcg) of tiotropium inhalation solution delivered by the Respimat inhaler, placebo and ipratropium bromide inhalation aerosol (MDI) in patients with chronic obstructive pulmonary disease (COPD). www.clinicaltrials.gov 2002; Vol. Sept 2004 End Date. [trial number: 205.252]
- Voshaar T, Lapidus R, Maleki‐Yazdi R, Timmer W, Rubin E, Lowe L, et al. A randomised study of tiotropium Respimat soft mist inhaler versus ipratropium PMDI in chronic obstructive pulmonary disease [Abstract]. Thorax 2006;61(Suppl 2):ii40 [S112]. [Google Scholar]
- Voshaar T, Lapidus R, Maleki‐Yazdi R, Timmer W, Rubin E, Lowe L, et al. A randomized study of tiotropium Respimat Soft Mist inhaler vs. ipratropium pMDI in COPD. Respiratory Medicine 2008;102(1):32‐41. [CTG: NCT00239473; CTG: NCT00240435] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ambrosino 2008 {published data only}
- Ambrosino N, Foglio K, Balzano G, Paggiaro PL, Lessi P, Kesten S, et al. Tiotropium and exercise training in COPD patients: effects on dyspnea and exercise tolerance. International Journal of Chronic Obstructive Pulmonary Disease 2008;3(4):771‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baloira 2005 {published data only}
- Baloira A, Vilarino C. Effects of tiotropium bromide in exercise tolerance in patients with chronic obstructive pulmonary disease [Abstract]. European Respiratory Society Congress; 2005 September 17‐21; Copenhagen. 2005; Vol. 26, issue Suppl 49:Abstract No. 1929.
Bedard 2011 {published data only}
- Bedard ME, Brouillard C, Pepin V, Milot J, Lacasse Y, Leblanc P, et al. Tiotropium improves walking endurance in chronic obstructive pulmonary disease patients. American Journal of Respiratory and Critical Care Medicine. 2011; Vol. 183:A1590.
Calverley 2000 {published data only}
- Calverley PMA, Towse LJ, Lee A. The timing of dose and pattern of bronchodilatation of tiotropium (TIO) in stable COPD. European Respiratory Journal 2000;16(Suppl 31):56s. [Google Scholar]
Casaburi 2005 {published data only}
- Casaburi R, Kukafka D, Cooper CB, Kesten S. Improvement in exercise endurance with the combination of tiotropium and rehabilitative exercise training in COPD patients [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26; Orlando. 2004:D14.
- Casaburi R, Kukafka D, Cooper CB, Witek TJ Jr, Kesten S. Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest 2005;127(3):809‐17. [DOI] [PubMed] [Google Scholar]
- Cooper CB, Kukafka D, Casburi R, Kesten S. Tiotropium augments improvements in dyspnea and health‐related quality of life following pulmonary rehabilitation in COPD patients [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26; Orlando. 2004:C22 Poster 507.
- Kesten S, Casaburi R, Kukafka D, Cooper CB. Improvement in self‐reported exercise participation with the combination of tiotropium and rehabilitative exercise training in COPD patients. International Journal of COPD 2008;3(1):127‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Celli 2002 {published data only}
- Celli BR, Zuwallack RL, Wang S, Kesten S. Improvements in inspiratory capacity with tiotropium in patients with COPD [Abstract]. European Respiratory Society Annual Congress 2002. 2002:abstract nr: P3079.
da Fonseca 2010 {published data only}
- Fonseca Reis LF, Ventura TG, Jayme S, Bispo P, Cantanhede LA. A role of tiotropium in patients with severe COPD subject to a supervised program of exercises [Abstract]. European Respiratory Society Annual Congress; 2010 September 18‐22; Barcelona, Spain. 2010:[P4872].
de Guia 2004 {published data only}
- Guia T, Punzal P, Canizares L, Sy‐Naval S, Salonga R, Tan D, et al. Evaluation of the response of Filipino COPD patients to Tiotropium (EVEREST STUDY) [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26; Orlando. 2004:C47 Poster F78.
Diba 2009 {published data only}
- Diba C, Salome CM, Berend N, Harris B, Bailey D, King GG. Effect of bronchodilator on ventilation heterogeneity in COPD [Abstract]. Respirology 2009;14(Suppl 1):A19. [Google Scholar]
Diba 2011 {published data only}
Friedman 1998 {published data only}
- Friedman M, Auerbach D, Campbell S, Dunn L, Illowite J, Littner M, et al. Tiotropium in patients with COPD. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A804. [Google Scholar]
Fuhr 2010 {published data only}
- Fuhr R, Magnussen H, Ribera A, Kirsten AM, Falques M, Caracta C, et al. Efficacy and safety of twice‐daily aclidinium bromide 400 µg compared with placebo and tiotropium 18 µg qd in moderate to severe COPD patients [Abstract]. Chest 2010;138(4):465A. [DOI] [PubMed] [Google Scholar]
- Fuhr R, Magnussen H, Ribera Llovera A, Kirsten A, Falques M, Caracta C, et al. Efficacy and safety of twice‐daily aclidinium bromide compared with tiotropium and placebo in patients with moderate to severe COPD [Abstract]. European Respiratory Society Annual Congress; 2010 September 18‐22; Barcelona, Spain. 2010:[P1236].
Gelb 2011 {published data only}
- Gelb AF, Fraser C, Zamel N. Lack of protective effect of tiotropium vs induced dynamic hyperinflation in moderate COPD. Respiratory Medicine 2011;105:755‐60. [DOI] [PubMed] [Google Scholar]
Gurzhiy 2007 {published data only}
- Gurzhiy O, Pertseva T, Lykholat O. Evaluation of mucociliary clearance 's (MCC) condition in patients with COPD influence of tiotropium bromide (TB) on MCC [Abstract]. European Respiratory Journal 2007;30(Suppl 51):73s [P582]. [Google Scholar]
Halpin 2006 {published data only}
- Halpin DMG, Menjoge S, Viel K. Tiotropium reduces the risk of COPD exacerbation irrespective of its characterization by the treatment intervention [Abstract]. European Respiratory Journal 2006;28(Suppl 50):764s [4397]. [Google Scholar]
Hasani 2001 {published data only}
- Hasani A, Agnew J, Dilworth J, Begent L, Mier A, Sarno M, et al. Effect of tiotropium on distribution pattern of aerosol particles deposited in the lung [abstract]. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A277. [Google Scholar]
- Hasani A, Agnew JE, Dilworth JP, Creer D, Mier A, Sarno M, et al. Effect of tiotropium on lung ventilatory distribution in COPD patients. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A282. [Google Scholar]
Hasani 2001b {published data only}
- Hasani A, Toms N, Creer DD, Agnew JE, Dilworth JP, Sarno M, et al. Effect of inhaled tiotropium on tracheobronchial clearance in patients with COPD. European Respiratory Journal 2001;18(Suppl 33):245s. [Google Scholar]
Hirata 2003 {published data only}
- Hirata K, Nishimura M, Ichinose M, Nishimura K, Nagai A, Yoshida M, et al. Tiotropium once daily improves health status in Japanese patients with COPD [Abstract]. American Thoracic Society 99th International Conference; 2003 May 16‐21; Seattle. 2003:A035 Poster D79.
- Ichinose M, Nishimura M, Hirata K, Nagai A, Yoshida M, Fukuchi Y. Tiotropium once daily improves spirometry over 24 hours in Japanese patient with COPD [Abstract]. American Thoracic Society Meeting; 2003 May 16‐21; Seattle. 2003:A035 Poster D80.
Kerstjens 2004 {published data only}
- Kerstjens HAM, Bantje TA, Luursema PB, Damste HEJS, Jong JW, Lee A, et al. Tiotropium maintainence therapy and the additive effects of a single dose of ipratropium bromide or fenoterol in patient with COPD [Abstract]. American Thoracic Society 100th International Conference 2004 May 21‐26 Orlando. 2004:C22 Poster 514.
Langley 2002 {published data only}
- Langley S, Towse L, Kesten S, Claverley PM. Heart rate and rhythm analysis from holter monitoring in COPD patients receiving tiotropium. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A592. [Google Scholar]
McNicholas 2001 {published data only}
- McNicholas WT, Calverley PMA, Edwards C, Lee A. Effects of anticholinergic therapy (tiotropium) on REM‐related desaturation (SaO2) and sleep quality in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A280. [Google Scholar]
Meshcheriakova 2007 {published data only}
- Meshcheriakova N, Belevskiy A, Cherniak A. The role of tiotropium bromide in physical training for patients with COPD [Abstract]. European Respiratory Journal 2007;30(Suppl 51):516s [E3091]. [Google Scholar]
O'Donnell 2002 {published data only}
- Magnussen H, O'Donnell DE, Casaburi R, Keston S, Gerken F, Flüge T. Spiriva® (tiotropium) reduces lung hyper inflation in COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A227. [Google Scholar]
- O'Donnell D, He Z, Lam M, Webb K, Flüge T, Hamilton A. Reproducibility of measurements of inspiratory capacity dyspnea intensity and exercise endurance in multicentre trials in COPD [Abstract]. European Respiratory Journal 2004;24(Suppl 48):323s. [Google Scholar]
- O'Donnell DE, Magnussen H, Aguilaniu B, Gerken F, Hamilton A, Flüge T. Spiriva (tiotropium) improves exercise tolerance in COPD [Abstract]. First National COPD Conference; 2003 November 14‐15; Arlington, Virginia. 2003:Abstract no: 1192.
- O'Donnell DE, Magnussen H, Aguilaniu B, Gerken F, Hamilton A, Flüge T. Spiriva® (Tiotropium) improves exercise tolerance in COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A227. [Google Scholar]
- O'Donnell DE, Magnussen H, Aguilaniu B, Make B, Flüge T, Hamilton A. Spiriva® (Tiotropium) reduces exertional dyspnea in COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A265. [Google Scholar]
- O'Donnell DE, Magnussen H, Gerken F, Kesten S, Flüge T. Mechanisms of improved exercise tolerance in COPD in response to tiotropium [Abstract]. European Respiratory Society Annual Congress; 2002 September 14‐18; Stockholm. 2002:abstract nr: P1826.
- O'Donnell DE, Magnussen H, Gerken F, Kesten S, Flüge T. Cardiovascular evaluation of tiotropium during constant‐work rate exercise in COPD [Abstract]. European Respiratory Journal 2003;22(Suppl 45):Abstract No: [P430]. [Google Scholar]
- O'Donnell DM. Spiriva(r) (Tiotropium) improves exercise tolerance in COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A227. [Google Scholar]
- O'Donnell DM. Spiriva(r) (Tiotropium) reduces exertional dyspnea in COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A265. [Google Scholar]
O'Donnell 2004a {published data only}
- O'Donnell D, Maltais F, Sciurba F, Kesten S, Hamilton A. SPIRIVA® (Tiotropium) improves symptom‐limited exercise tolerance in COPD patients [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26; Orlando. 2004:D23 Poster 207.
- O'Donnell D, Marciniuk D, Hernandez P, Richter K, Kesten S, Hamilton A. Spiriva® (Tiotropium) reduces lung hyperinflation at rest and during exercise in COPD patients [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26 2004; Orlando. 2004:D23 Poster 210.
- O'Donnell DE, Johnson B, Richter K, Kesten S, Hamilton A. Inspiratory capacity (IC) and dyspnea during recovery from symptom‐limited exercise in COPD patients treated with tiotropium [Abstract]. European Respiratory Journal 2004;24(Suppl 48):214s. [Google Scholar]
O'Donnell 2005 {published data only}
- O'Donnell D, Hamilton A, Kesten S. Influence of the exercise‐limiting symptom on the relationship between lung hyperinflation and endurance time in COPD patients treated with tiotropium [Abstract]. American Thoracic Society 2005 International Conference; 2005 May 20‐25; San Diego, California. 2005:[B23] [Poster: 502].
O'Donnell 2005a {published data only}
- O'Donnell D, Webb KA. The effect of tiotropium on ventilatory mechanics during exercise in COPD [Abstract]. American Thoracic Society 2005 International Conference; 2005 May 20‐25; San Diego, California. 2005:[B65] [Poster: A51].
Olson 2009 {published data only}
- Olson T, Baldi J, Afessa B, Scanlon P, Hulsebus M, Miller A, et al. Cardiac consequences of obstruction during exercise: benefits of bronchodilation [Abstract]. American Thoracic Society International Conference; 2009 May 15‐20; San Diego. 2009:A4571 [Poster #J84].
Reisner 2011 {published data only}
- Orevillo C, St. Rose E, Strom S, Fischer T, Golden M, Thomas M, et al. Glycopyrrolate MDI demonstrates comparable efficacy and safety to tiotropium DPI in a randomized, double‐blind, placebo‐controlled phase 2b study in patients with COPD [Abstract]. European Respiratory Society Annual Congress;2011 September 24‐28; Amsterdam, The Netherlands 2011;38(55):724s [P3975]. [Google Scholar]
- Reisner C, Fogarty C, Spangenthal S, Dunn L, Kerwin EM, Quinn D, et al. Novel combination of glycopyrrolate and formoterol MDI (GFF‐MDI) provides superior bronchodilation compared to its components administered alone, tiotropium DPI, and formoterol DPI in a randomized, double‐blind, placebo‐controlled phase 2b study in patients with COPD [Abstract]. American Journal of Respiratory and Critical Care Medicine. 2011; Vol. 183:A6435.
- Reisner C, St. Rose E, Strom S, Fischer T, Golden M, Thomas M, et al. Fixed combination of glycopyrrolate and formoterol MDI (GFF‐MDI) demonstrates superior inspiratory capacity (IC) compared to tiotropium DPI (Tio) following 7 days dosing, in a randomized, double‐blind, placebo‐controlled phase 2b study in patients with COPD [Abstract]. European Respiratory Society Annual Congress; 2011 September 24‐28; Amsterdam, The Netherlands 2011;38(55):150s [P879]. [Google Scholar]
Rossi 2008 {published data only}
- Rossi S, Gladly C, Baril J, Perrault H, Bourbeau J. COPD patients who respond to tiotropium with dyspnea relief: A proof of efficacy? [Abstract]. American Thoracic Society International Conference 2008 May 16‐21; Toronto. 2008:A648[#F15].
Schilling 2000 {published data only}
- Schilling JC, Witek TJ, Feifel U, Souhrada JF, Disse B. Safety and tolerability of supra‐therapeutic doses of inhaled tiotropium (TIO). European Respiratory Journal 2000;16(Suppl 31):361s. [Google Scholar]
Schurmann 2004 {published data only}
- Schurmann W, Schmidtmann S, Moroni P, Massey D, Qidan M. Patients prefer Respimat® Soft Mist TM inhaler to HFA‐MDI [Abstract]. Primary Care Respiratory Journal 2004;13(2):118 ABS044. [Google Scholar]
Sposato 2005 {published data only}
- Sposato B, Salvatore M, Giovanna MM, Alberto R, Elena C, Aldo G, et al. Formoterol or tiotropium bromide do not influence nocturnal hypoxemia in stable COPD [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1959. [Google Scholar]
ten Hacken 2007 {published data only}
- Hacken NH, Haart H, Grevink R, Thompson S, Roemer W, Postma DS. Bronchodilation improves endurance but not muscular efficiency in COPD [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco, California, USA. 2007:Poster #M72.
van Noord 2006 {published data only}
- Noord JA, Cornelissen G, Aumann JL, Platz J, Mueller A, Fogarty C. Efficacy in COPD patients of tiotropium administered via the Respimat soft mist inhaler (SMI) compared to handihaler (HH) [Abstract]. European Respiratory Journal 2006;28(Suppl 50):431s [P2516]. [Google Scholar]
Vincken 2001 {published data only}
- Vincken WG, Vermiere P, Menjoge SS, Keston S, Cornelissen PJG. Maintenance of bronchodilation following tiotropium in patients with mild, moderate and severe COPD in one year clinical trials. European Respiratory Journal 2001;18(Supp 33):331s. [Google Scholar]
References to studies awaiting assessment
Gu 2007 {published data only}
- Gu W, Sun LH, Tan Y. Effects of tiotropium on the exercise endurance of patients with COPD. Journal Southeast University (Med. Sci. Edi.) 2007;26:332–5. [Google Scholar]
Min 2006 {published data only}
- Min R, Huang M, Yin KS, Fu WZ, Yu WZ. Clinical trial on tiotropium for COPD. Jiangsu Medical Journal 2006;32:1078–9. [Google Scholar]
NCT00528996 {published data only}
- Boehringer Ingelheim. Tiotropium (SPIRIVA®) RESPIMAT®: Evaluation of fatal events – February 2010. www.trials.boehringer‐ingelheim.com (accessed 12 July 2010). [CTG: NCT00528996; trial number: 1205.14]
Xia 2007 {published data only}
- Xia QP, Chen JR, Tao YJ. The curative effect of tiotropium bromide in treating stable COPD. Journal of Clinical Pulmonary Medicine 2007;12:1327–8. [Google Scholar]
Yin 2010 {published data only}
- Yin KS, Zhang DP, Shi Y, Sun LH, Min R, Xiao YL, et al. A randomized, double‐blind, placebo‐control study of once‐daily inhaled tiotropium in chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi 2010;33(7):519‐23. [PubMed] [Google Scholar]
Additional references
Barr 2005
- Barr RG, Bourbeau J, Camargo Carlos A. Tiotropium for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD002876.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beeh 2010
- Beeh KM, Beier J. The short, the long, and the "ultra‐long": why duration of bronchodilator action matters in chronic obstructive pulmonary disease. Advances in Therapy 2010;27(3):150‐59. [DOI] [PubMed] [Google Scholar]
Boehringer Ingelheim 2010
- Boehringer Ingelheim. Tiotropium (SPIRIVA®) RESPIMAT®: Evaluation of fatal events. www.trials.boehringer‐ingelheim.com/res/trial/data/pdf/Pooled_Analysis_U10‐3255‐01.pdf 2010.
Boehringer Ingleheim Global trials database
- Global Clinical Trials Website of Boehringer Ingelheim . http://trials.boehringer‐ingelheim.com/.
Cates 2011
- Cates CJ. Safety of tiotropium. BMJ 2011;342:d2970. [DOI] [PubMed] [Google Scholar]
Cazzola 2008
Celli 2010
Decramer 2011
- Decramer M, Molenberghs G, Liu D, Celli B, Kesten S, Lystig T, et al. Premature discontinuation during the UPLIFT study. Respiratory Medicine 2011;105:1523‐30. [DOI] [PubMed] [Google Scholar]
Effing 2007
- Effing T, Monninkhof EM, Valk PD, Palen J, Herwaarden CL, Partridge MR, et al. Self‐management education for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD002990.pub2] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; Vol. 315, issue 7109:629‐34. [DOI] [PMC free article] [PubMed]
Fukuchi 2011
- Fukuchi Y, Fernandez L, Kuo HP, Mahayiddin A, Celli B, Decramer M, et al. Efficacy of tiotropium in COPD patients from Asia: A subgroup analysis from the UPLIFT trial. Respirology 2011;16:825‐35. [DOI] [PubMed] [Google Scholar]
GOLD 2010
- Global Initiative for Chronic Obstructive Lung Disease. www.goldcopd.com (accessed 14th December 2009).
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hutchinson 2010
- Hutchinson A, Brand C, Irving L, Roberts C, Thompson P, Campbell D. Acute care costs of patients admitted for management of chronic obstructive pulmonary disease exacerbations: contribution of disease severity, infection and chronic heart failure. Internal Medicine Journal 2010;40(5):364‐71. [DOI] [PubMed] [Google Scholar]
Kaplan 2010
- Kaplan A. Effect of tiotropium on quality of life in COPD: a systematic review. Primary Care Respiratory Journal 2010;19(4):315‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kesten 2007
- Kesten S, Plautz M, Piquette CA, Habib MP, Niewoehner DE. Premature discontinuation of patients: a potential bias in COPD clinical trials. European Respiratory Journal 2007;30(5):898‐906. [DOI] [PubMed] [Google Scholar]
Kesten 2009
- Kesten S, Celli B, Decramer M, Leimer I, Tashkin D. Tiotropium HandiHaler in the treatment of COPD: a safety review. International Journal of Chronic Obstructive Pulmonary Disease 2009;4:397‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lacasse 2006
- Lacasse Y, Goldstein R, Lasserson Toby J, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003793.pub2] [DOI] [PubMed] [Google Scholar]
Mauskopf 2010
- Mauskopf JA, Baker CL, Monz BU, Juniper MD. Cost effectiveness of tiotropium for chronic obstructive pulmonary disease: a systematic review of the evidence. Journal of Medical Economics 2010;13(3):403‐17. [DOI] [PubMed] [Google Scholar]
Neyt 2010
- Neyt M, Devriese S, Thiry N, Bruel A. Tiotropium's cost‐effectiveness for the treatment of COPD: a cost‐utility analysis under real‐world conditions. BMC Pulmonary Medicine 2010;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease, costing report, implementing NICE guidance. www.guidance.nice.org.uk/CG101/CostingReport/pdf/English.
Oxman 1992
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116(1):78‐84. [DOI] [PubMed] [Google Scholar]
Proskocil 2005
- Proskocil BJ, Fryer AD. Beta2‐agonist and anticholinergic drugs in the treatment of lung disease. Proceedings of the American Thoracic Society 2005;2(4):305‐10; discussion 311‐2. [DOI] [PubMed] [Google Scholar]
Ram 2011
- Ram FS, Carvallho CR, White J. Clinical effectiveness of the Respimat inhaler device in managing chronic obstructive pulmonary disease: evidence when compared with other handheld inhaler devices. International Journal of Chronic Obstructive Pulmonary Disease 2011; Vol. 6:129‐39. [DOI] [PMC free article] [PubMed]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rice 2008
- Rice KL, Leimer I, Kesten S, Niewoehner DE. Responses to tiotropium in African‐American and Caucasian patients with chronic obstructive pulmonary disease. Translational Research 2008;152(2):88‐94. [DOI] [PubMed] [Google Scholar]
SGRQ‐C manual 2008
- Jones P. St George's Respiratory Questionnaire for COPD Patients (SGRQ‐C). www.healthstatus.sgul.ac.uk/SGRQ_download/SGRQ‐C%20Manual%202008.pdf 2008.
Singh 2009
- Singh S. Review: inhaled anticholinergics increase risk of major cardiovascular events in COPD. Evidence Based Medicine 2009;14(2):42‐3. [DOI] [PubMed] [Google Scholar]
Singh 2011
- Singh S, Loke YK, Enright PL, Furberg CD. Mortality associated with tiotropium mist inhaler in patients with chronic obstructive pulmonary disease: systematic review and meta‐analysis of randomised controlled trials. BMJ 2011;342:d3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Meer 2001
- Meer Regina M, Wagena E, Ostelo RW, Jacobs JE, Schayck CP. Smoking cessation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002999] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO
- World Health Organization. Chronic respiratory diseases. www.who.int.
Wise 2013
- Wise RA, Anzueto A, Cotton D, Dahl R, Devins T, Disse B, et al. Tiotropium respimat inhaler and the risk of death in COPD. New England Jounral of Medicine 2013;369(16):1491‐501. [DOI] [PubMed] [Google Scholar]
Yohannes 2011
- Yohannes AM, Willgoss TG, Vestbo J. Tiotropium for treatment of stable COPD: a meta‐analysis of clinically relevant outcomes. Respiratory Care 2011;56(4):477‐87. [DOI] [PubMed] [Google Scholar]


