Abstract
Objective:
The aim of this study was to examine modifiable mediators for socioeconomic disparities in childhood obesity in the United States.
Methods:
This study used the data of 1,211 mother-child dyads from a US national birth cohort from pregnancy to 6 years post partum. Socioeconomic status was indicated by maternal education (college graduate vs. less) and family income (>185% vs. ≤185% of the poverty line). Single- and multiple-factor mediation analyses were conducted for socioeconomic disparities in childhood obesity at 6 years, adjusting for demographics.
Results:
The confounder-adjusted relative risk of childhood obesity was 1.79 for low maternal education and 1.42 for low family income. Low-maternal-education-related obesity was individually mediated by maternal preconception BMI (percentage of indirect effect, 8.8%), smoking during pregnancy (7.0%), infant weight gain (14.4%), child sleep duration (11.4%), and TV viewing during weekdays at 6 years (4.9%). Low-family-income-related obesity was mediated by maternal preconception BMI (18.5%), smoking during pregnancy (6.3%), child sleep duration (12.8%), and the home learning environment at 6 years (26.2%). In multiple-mediator analysis, significant mediators together mediated 54.0% of maternal-education-related or 39.4% of family-income-related disparities.
Conclusions:
Maternal preconception BMI, smoking during pregnancy, infant weight gain, child sleep, TV viewing, and the home learning environment substantially mediated socioeconomic disparities in childhood obesity in the United States.
INTRODUCTION
Childhood obesity is a major public health issue in the United States (US) and worldwide (1–3). The 2017–2018 National Health and Nutrition Examination Survey (NHANES) showed that 19.3% of US children had obesity and another 16.1% had overweight (4). Among other social determinants (e.g., race and ethnicity, neighborhood, transportation, media use, food access, schools) (5), there are large socioeconomic disparities in the prevalence of childhood obesity in the US (6) and many other developed countries (7). Children from socioeconomically disadvantaged families (e.g., low parental education [25% vs. 7%] (6) and/or low family income [26% vs. 16%] (6)) are particularly vulnerable to obesity.
There is an increasing interest in using mediation analyses to identify the potential determinants for socioeconomic disparities in childhood obesity. Existing research has yielded fairly consistent evidence on insufficient breastfeeding, parental body mass index (BMI), and TV viewing time; mixed evidence on maternal age, smoking during pregnancy, and child birth weight; and limited evidence on day care attendance, parenting practices, maternal depression, and alcohol consumption (8,9). However, only two published studies in this field were conducted among US children. A US study found maternal depression and permissive parenting style partially mediated the association between low socioeconomic status (SES) and BMI gain from age 2 to 11 years (10). The contribution of this study to the literature is limited by missed infancy growth outcomes and a narrow scope of mediators. Another US study found that the associations between low SES and childhood obesity at age 2 years were mainly mediated by nonrecommended infant feeding practices, including predominant formula feeding, early introduction of solid foods, and putting the child to bed with a bottle (11). A major limitation of this study was the relatively young age (2 years) for childhood obesity outcome, which did not allow for examining other important mediators in later life, especially the child’s own lifestyles such as eating, physical activities, and sleep. In addition, neither US study built a causal network (i.e., a conceptual framework to clarify the network of interrelations among multiple causal factors or mediators [e.g., smokers are less likely to breastfeed]) with time series and bidirectional relations being considered (12). Causal network research with a comprehensive list of potential mediators is much needed to advance our poor knowledge of the underlying determinants of socioeconomic disparities in childhood obesity in the US.
This study aimed to examine the potentially modifiable root mediators for socioeconomic disparities in obesity among US children. We built a causal network of multiple mediators through structural equation modeling (SEM). Our list of potential mediators covers the whole early-life period: preconception (e.g., maternal obesity); pregnancy (e.g., maternal smoking); delivery (e.g., cesarean delivery); infancy (e.g., feeding); and early childhood (e.g., sleep). We chose to focus on potentially modifiable factors because they had straight-forward implications for intervention.
METHODS
Study design and participants
We analyzed existing data from the Infant Feeding Practices Study II (IFPS II, n = 3,033 newborns) from May 2005 through June 2007 (13) and its Year 6 Follow-Up (Y6FU, n = 1,542 children) at 6 years in 2012 (14). The IFPS II was conducted by the US Food and Drug Administration (FDA) in collaboration with the Centers for Disease Control and Prevention (CDC). This longitudinal study followed mother-child dyads from late pregnancy (∼7 months of pregnancy) through 6 years post partum via mailed survey questionnaires or, sometimes, phone interviews, if needed. The sampling frame for the IFPS II was a nationally distributed consumer opinion panel of >500,000 households (13). Within the panel, all pregnant women in the third trimester were included in the initial sample. Questionnaires were then mailed to them over 8 months to achieve the desired sample size. Among 14,017 potentially eligible pregnant women, a total of 4,902 (35.0%) completed questionnaires and others either refused (46) or did not respond (9,069). The total study sample consisted of 4,902 adult pregnant women aged 18 years or older and 3,033 full-term or nearly full-term newborns (gestational age ≥ 35 complete weeks). Additional criteria for eligible newborns included the following: 1) lack of medical conditions in the mother-infant dyad that would affect feeding; 2) birth weight ≥ 5 lb; 3) singleton; and 4) not staying in neonatal intensive care for >3 days. After birth, a total of 2,095 mother-child dyads were followed to 6 months, 1,807 were followed to 12 months, and 1,542 were followed to 6 years. Mothers received a small gift (<$3 value) for completing each regular IFPS II questionnaire, $10 cash incentive for the dietary history questionnaire, and $10 for the Y6FU questionnaire. The study protocols were approved by the Research Involving Human Subjects Committee of the FDA. Mothers provided signed or oral consent for their own and their child’s participation. Details on the study design have been published elsewhere (13,14).
For the purpose of this analysis, we restricted the total eligible sample to those 1,211 mother-child dyads with complete data on the following: 1) at least one of the two SES measures of our interest, including maternal education and family income (exposures); and 2) child weight and height to calculate BMI at age 6 years (outcomes). As shown in Supporting Information Table S1, mothers in the analytic sample had higher education, family income, and mean age at pregnancy and they were more likely to be of non-Hispanic White race and married compared with their counterparts in the excluded sample. This secondary data analysis used deidentified public-use data from the CDC and was approved by the State University of New York at Buffalo Institutional Review Board.
Child growth measures (outcomes)
In the Y6FU survey, mothers reported the child’s weight and height as measured by trained health professionals at the child’s most recent doctor’s visit (Table 1). The mean child age for these clinical anthropometric measures was 6.08 (SD 0.43) years. Child BMI was calculated as weight in kilograms divided by height in meters squared. Based on the CDC 2000 Growth Charts, we calculated child BMI percentile by sex (boys vs. girls) and age (monthly intervals) (15). Accordingly, we defined childhood obesity as BMI ≥95th percentile (16).
TABLE 1.
List of outcomes, exposures, covariates, and mediators
| Time point at data collection | Method of data collection | |
|---|---|---|
| Child growth (outcomes) | ||
| Child weight | Age 6 | Doctor's visit (reported by mother) |
| Child height | Age 6 | Doctor's visit (reported by mother) |
| Child BMI | Age 6 | Calculated; BMI = weight in kg/height in m2 |
| Child obesity | Age 6 | Calculated; BMI ≥ 95th percentile |
| SES measures (exposures) | ||
| Maternal education | Late pregnancy (∼7 months; enrollment) | Paper survey via mailed questionnaires |
| Family income (US dollars) | Late pregnancy | Paper survey via mailed questionnaires |
| Family income (percentage of poverty line) | Late pregnancy | Calculated; percentage = family income/federal poverty line |
| Covariates | ||
| Maternal age at pregnancy (y) | Late pregnancy | Paper survey via mailed questionnaires |
| Maternal race and ethnicity | Late pregnancy | Paper survey via mailed questionnaires |
| Parity | Late pregnancy | Paper survey via mailed questionnaires |
| Marital status | Late pregnancy | Paper survey via mailed questionnaires |
| Child sex | 1 month post natal (neonatal) | Paper survey via mailed questionnaires |
| Child age at 6-year follow-up (y) | Age 6 | Calculated; child age = survey date - date of birth |
| Mediators | ||
| Maternal height (m) | Late pregnancy | Paper survey via mailed questionnaires |
| Maternal preconception weight (kg) | Late pregnancy | Paper survey via mailed questionnaires |
| Maternal preconception BMI (kg/m2) | Late pregnancy | Paper survey via mailed questionnaires |
| Paternal preconception weight status | Late pregnancy | Paper survey via mailed questionnaires |
| GWG (kg) | 1 month post natal | Paper survey via mailed questionnaires |
| Prenatal Healthy Eating Index | Late pregnancy | Paper survey via mailed questionnaires (Food Frequency Questionnaire) |
| Gestational age at first prenatal visit (wk) | Late pregnancy | Paper survey via mailed questionnaires |
| Maternal smoking during pregnancy | Late pregnancy | Paper survey via mailed questionnaires |
| Maternal number of cigarettes smoked per day during pregnancy | Late pregnancy | Paper survey via mailed questionnaires |
| Gestational diabetes | Late pregnancy | Paper survey via mailed questionnaires |
| Method of delivery | 1 month post natal | Paper survey via mailed questionnaires |
| Date of birth | Birth | Phone interview |
| Gestational age at birth (wk) | Birth | Calculated; gestational age = date of birth - last menstrual period |
| Birth weight (kg) | Birth | Phone interview |
| Large-for-gestational-age birth | Birth | Calculated; birth weight > 90th percentile by sex and gestational age |
| Birth length (cm) | 1 month post natal | Paper survey via mailed questionnaires |
| Edinburgh postnatal depression score | 2 months post natal | Paper survey via mailed questionnaires |
| Postnatal Healthy Eating Index | 3 months post natal | Paper survey via mailed questionnaires (Food Frequency Questionnaire) |
| Breastfeeding duration (wk) | 1–12 months post natal | Paper survey via mailed questionnaires |
| Exclusive breastfeeding duration (wk) | 1–12 months post natal | Paper survey via mailed questionnaires |
| Timing of formula initiation (wk) | 1–12 months post natal | Paper survey via mailed questionnaires |
| Timing of introduction of solid foods (wk) | 1–12 months post natal | Paper survey via mailed questionnaires |
| Infant dietary pattern adherence score | 12 months post natal | Paper survey via mailed questionnaires |
| Child sleep duration at 1 year (h) | 12 months post natal | Paper survey via mailed questionnaires |
| Nonmaternal infant care at 1 year | 12 months post natal | Paper survey via mailed questionnaires |
| Infant weight gain (kg) | 3, 7, and 12 months post natal | Calculated; infant weight gain = postnatal weight at doctor's visit - birth weight |
| Child sleep duration at 6 years (h) | Age 6 | Paper survey via mailed questionnaires or phone interview if needed |
| Child dietary pattern adherence score | Age 6 | Paper survey via mailed questionnaires or phone interview if needed |
| Score of child food and eating environment | Age 6 | Paper survey via mailed questionnaires or phone interview if needed |
| Score of child home learning environment | Age 6 | Paper survey via mailed questionnaires or phone interview if needed |
| Number of days per week with ≥60 minutes of child MVPA | Age 6 | Paper survey via mailed questionnaires or phone interview if needed |
| Child TV viewing per day on weekdays (h) | Age 6 | Paper survey via mailed questionnaires or phone interview if needed |
| Child TV viewing per day on weekends (h) | Age 6 | Paper survey via mailed questionnaires or phone interview if needed |
Abbreviations: GWG, gestational weight gain; MVPA, moderate or vigorous physical activity; SES, socioeconomic status.
SES measures (exposures)
We focused on maternal education and family income as the key SES exposure measures because existing evidence has supported their strong influences on childhood obesity (17), although we recognized that other SES factors such as paternal education, parental occupation, and neighborhood quality also play roles (18).
In the IFPS II, mothers reported their highest educational attainment at enrollment (late pregnancy). The response options for maternal education included “1–7 years grade school,” “8 years grade school,” “1–3 years high school,” “high school graduate,” “1–3 years college,” “college graduate,” and “postgraduate.” There was a limited number of children stratified by the original categories of maternal education. Therefore, we grouped maternal education into two categories (3 years of college or less vs. college graduate or higher) to ensure a sufficient sample size for each final category and thus statistical power for mediation analysis. This binary classification of maternal education has been used in previous research on childhood obesity (19). Our decision on the cutoff point of maternal education was also supported by the striking gap in the prevalence of childhood obesity between “1–3 years college” (19.4%) and “college graduate” (11.3%) compared with the moderate gaps between “high school or lower” (23.1%) and “1–3 years college” (19.4%) or between “college graduate” (11.3%) and “postgraduate” (8.3%).
Mothers also reported family annual income at enrollment. Based on household size and income, a household-level poverty variable was created as the ratio of household income to the federal poverty line. We classified family income into two categories: ≤185% and >185% of the federal poverty line. This cutoff point was based on the income criterion for the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), a US social safety net program for low-income pregnant or postpartum women and their young children (20).
Mediator measures
We considered potentially modifiable early-life determinants for obesity reported in the literature that coexist with low SES as mediators for socioeconomic disparities in childhood obesity.
Preconception and prenatal mediators
Mothers reported their height, preconception weight, and total gestational weight gain (GWG). Maternal obesity was defined as preconception BMI ≥ 30.0 (21). We defined excessive GWG as total GWG greater than 18.0, 16.0, 11.5, and 9.0 kg for women with underweight (preconception BMI < 18.5), normal weight (18.5–24.9), overweight (25.0–29.9), and obesity (≥30.0), respectively (21). Mothers also reported whether the baby’s father had overweight or obesity before conception. In late pregnancy, mothers self-reported the timing of their first prenatal visit, smoking status during pregnancy (yes vs. no; number of cigarettes smoked per day), and whether they had type 1, type 2, or gestational diabetes. Mothers’ dietary intake (i.e., frequency and amount of foods, beverages, and supplements) in the past month was measured using Food Frequency Questionnaires repeatedly during late pregnancy and 3 months post partum, and we calculated the maternal Healthy Eating Index for each period (22).
Mediators at delivery and during infancy
Mothers reported their method of delivery, child’s birth weight and length, and gestational age in the birth screener (phone interview) and neonatal survey. Large-for-gestational-age birth was defined as birth weight >90th percentile within a US reference population (23). Mothers reported weight, length, and age at their infant’s recent doctor’s visit in the 3-, 5-, 7-, and 12-month surveys (13,24). Mothers reported the infant’s longest night’s sleep duration and use of nonmaternal infant care (e.g., initial age and hours). Mothers reported their ever and current breastfeeding, breastfeeding duration, initial and current use of formula, and the timing of introduction of solid foods. In each IFPS II survey, mothers reported their infant’s intake of 18 types of foods in the past 7 days, from which we derived infant dietary patterns using principal component analysis (24). Supporting Information Table S2 shows infant dietary patterns at 12 months of age. Maternal postnatal depression was measured with the Edinburgh Postnatal Depression Scale (25).
Early-childhood mediators
In the Y6FU, mothers reported the child’s frequency of consuming 22 food and beverage groups (26), which was used to derive six dietary patterns (Supporting Information Table S3). Child food and eating environment was measured with four home-related (e.g., availability of fruits or vegetables) and two school-related items (e.g., salads and fast food from school cafeteria) (27). Mothers reported the child’s number of days per week with at least 60 minutes of moderate or vigorous physical activity (28), number of hours per day the child watched TV or played video games (28), and nighttime sleep duration during weekdays and weekends (29). The home learning environment was measured with three items selected from the Home Observation for Measurement of the Environment-Short Form (HOME-SF) (30): the frequency of parents’ reading aloud to the child, child participation in special lessons or organizations related to extracurricular activities, and attendance of musical or theatrical performances. Previous research has shown that a low HOME-SF score was associated with childhood obesity (31).
Confounders
We considered demographic and pregnancy characteristics as potential confounders, including maternal age in years, race and ethnicity, parity, marital status, child’s sex, and child actual age at the Y6FU.
Statistical analysis
Based on the causal step method (32), we examined the mediating roles of potentially modifiable early-life factors in associations between SES measures and childhood obesity at 6 years in five steps. Step 1 was to examine the overall associations between SES measures (exposure) and childhood obesity (outcome) using a multivariable log-binomial regression model (Table 2) (33). Step 2 was to examine the associations between SES measures and early-life factors (mediators; Table 3). Step 3 was to examine the associations between early-life factors and childhood obesity (Table 3).
TABLE 2.
Sample characteristics and their associations with childhood obesity at 6 years (n = 1,211)
| n (%)/mean ± SDa | Risk of obesity, n (%) | RR of obesity (95% CI) | p value | |
|---|---|---|---|---|
| SES measures | ||||
| Maternal education | ||||
| Low (3 years of college or less) | 596 (52.0) | 122 (20.5) | 1.95 (1.46–2.60) | <0.001 |
| High (college graduate or higher) | 551 (48.0) | 58 (10.5) | Reference | |
| Family income (percentage of the poverty line) | 287.81 ± 205.97 | |||
| Low (≤185%) | 427 (35.3) | 96 (22.5) | 1.78 (1.38–2.30) | <0.001 |
| High (>185%) | 784 (64.7) | 99 (12.6) | Reference | |
| Covariates | ||||
| Maternal age at pregnancy (y) | 30.18 ± 5.36 | 0.99 (0.96–1.01) | 0.269 | |
| 18–24 | 184 (15.2) | 40 (21.7) | Reference | |
| 25–29 | 374 (30.9) | 51 (13.6) | 0.63 (0.43–0.91) | 0.015 |
| 30–34 | 388 (32.1) | 59 (15.2) | 0.70 (0.49–1.00) | 0.052 |
| ≥35 | 264 (21.8) | 45 (17.1) | 0.78 (0.54–1.15) | 0.212 |
| Maternal race and ethnicity | ||||
| Asian/Pacific Islander and others | 50 (4.2) | 3 (6.0) | 0.39 (0.13–1.19) | 0.099 |
| Hispanic | 62 (5.2) | 13 (21.0) | 1.38 (0.83–2.28) | 0.213 |
| Non-Hispanic Black | 48 (4.1) | 17 (35.4) | 2.33 (1.55–3.50) | <0.001 |
| Non-Hispanic White | 1025 (86.5) | 156 (15.2) | Reference | |
| Parity | ||||
| Nulliparous | 358 (30.3) | 52 (14.5) | Reference | |
| 1 previous birth | 487 (41.2) | 85 (17.5) | 1.20 (0.88–1.65) | 0.256 |
| ≥2 previous births | 336 (28.5) | 55 (16.4) | 1.13 (0.80–1.60) | 0.502 |
| Marital status | ||||
| Married | 956 (83.3) | 127 (13.3) | Reference | |
| Unmarried | 192 (16.7) | 52 (27.1) | 2.04 (1.54–2.71) | <0.001 |
| Child sex | ||||
| Male | 622 (51.4) | 106 (17.0) | Reference | |
| Female | 589 (48.6) | 89 (15.1) | 0.89 (0.69–1.15) | 0.361 |
| Child age at the 6-year growth measurement (y) | 6.08 ± 0.43 | 1.10 (0.57–2.12) | 0.770 | |
| Child age at the Y6FU survey (y) | 6.45 ± 0.19 |
For some variables, the sum of categories was not equal to the total because of missing data.
Abbreviations: RR, relative risk; SES, socioeconomic status; Y6FU, Year Six Follow-Up.
TABLE 3.
Associations of potential mediators with SES measures and risk of obesity
| Potential mediator | Total sample (n = 1,211) |
RR of obesity at 6 years (95% Cl) | Maternal educationb |
Family incomeb |
||||
|---|---|---|---|---|---|---|---|---|
| n (%)/mean ± SDa | Low (n = 596) |
High (n = 551) |
p value | Low (n = 427) |
High (n = 784) |
p value | ||
| n (%)/mean + SD | n (%)/mean + SD | n (%)/mean + SD | n (%)/mean + SD | |||||
| Maternal height (m) | 1.65 ± 0.07 | 2.60 (0.40–16.90) | 1.65 ± 0.07 | 1.66 ± 0.07 | 0.701 | 1.65 ± 0.07 | 1.65 ± 0.07 | 0.975 |
| Maternal preconception weight (kg) | 73.45 ± 20.46 | 1.02 (1.01–1.02) | 75.40 ± 21.75 | 71.58 ± 18.66 | 0.002 | 76.10 ± 22.44 | 72.02 ± 19.18 | 0.001 |
| Maternal preconception BMI (kg/m2) | 26.76 ± 7.03 | 1.04(1.03–1.06) | 27.49 ± 7.54 | 26.05 ±6.29 | <0.001 | 27.71 ± 7.86 | 26.25 ± 6.49 | <0.001 |
| Underweight/normal weight (<25.0) | 588 (49.2) | Reference | 263 (44.8) | 294 (53.9) | 0.002 | 180 (43.0) | 408 (52.5) | 0.004 |
| Overweight (25.0–29.9) | 312 (26.1) | 2.18 (1.57–3.03) | 156(26.6) | 139(25.5) | 116 (27.7) | 196(25.2) | ||
| Obesity (>30.0) | 296 (24.7) | 2.44 (1.77–3.36) | 168 (28.6) | 112 (20.6) | 123 (29.4) | 173 (22.3) | ||
| Paternal preconception weight status | ||||||||
| Normal weight | 952 (86.0) | Reference | 463 (85.4) | 437(86.0) | 0.782 | 324 (84.8) | 628 (86.6) | 0.411 |
| Overweight/obesity | 155 (14.0) | 1.73 (1.26–2.38) | 79 (14.6) | 71 (14.0) | 58 (15.2) | 97 (13.4) | ||
| GWG (kg) | 13.70 ± 6.24 | 0.99 (0.97–1.02) | 13.68 ± 6.89 | 13.63 ± 5.41 | 0.88 | 13.58 ± 7.02 | 13.76 ± 5.78 | 0.638 |
| Inadequate | 240 (20.8) | Reference | 127 (22.4) | 102 (19.3) | 0.112 | 92 (22.8) | 148 (19.8) | 0.084 |
| Adequate | 370 (32.1) | 0.70 (0.47–1.04) | 168 (29.7) | 187 (35.3) | 113 (28.0) | 257(34.3) | ||
| Excessive | 542 (47.1) | 1.07 (0.77–1.50) | 271 (47.9) | 240 (45.4) | 198 (49.1) | 344 (45.9) | ||
| Prenatal Healthy Eating Index | 65.99 ±8.61 | 0.97 (0.94–1.00) | 64.84 ± 9.17 | 67.36 ± 7.91 | 0.003 | 63.97 ± 9.25 | 66.78 ± 8.23 | 0.003 |
| Gestational age at first prenatal visit (wk) | ||||||||
| ≤4 | 137 (11.4) | Reference | 74 (12.5) | 51 (9.3) | 0.007 | 50(11.7) | 87(11.2) | <0.001 |
| 5–8 | 635 (52.7) | 0.82 (0.56–1.19) | 303 (51.1) | 301 (54.9) | 195 (45.8) | 440 (56.6) | ||
| 9–12 | 320 (26.6) | 0.70 (0.45–1.08) | 148 (25.0) | 159 (29.0) | 117 (27.5) | 203 (26.1) | ||
| ≥13 | 112 (9.3) | 0.91(0.54–1.53) | 68 (11.5) | 37(6.8) | 64 (15.0) | 48 (6.2) | ||
| Maternal smoking during pregnancy | ||||||||
| No | 1,118 (92.5) | Reference | 526 (88.4) | 538 (98.0) | <0.001 | 370 (86.7) | 748 (95.8) | <0.001 |
| Yes | 90 (7.5) | 1.74 (1.21–2.52) | 69 (11.6) | 11 (2.0) | 57(13.3) | 33 (4.2) | ||
| Maternal number of cigarettes smoked per day during pregnancy | 0.68 ± 2.92 | 1.05 (1.02–1.08) | 1.09 ±3.58 | 0.15 ± 1.50 | <0.001 | 1.32 ±4.03 | 0.34 ± 2.00 | <0.001 |
| Gestational diabetes | ||||||||
| No | 1,117 (92.2) | Reference | 551 (92.4) | 508 (92.2) | 0.872 | 394 (92.3) | 723 (92.2) | 0.974 |
| Yes | 94 (7.8) | 1.51 (1.02–2.23) | 45 (7.6) | 43 (7.8) | 33 (7.7) | 61 (7.8) | ||
| Method of delivery | ||||||||
| Vaginal | 849 (70.2) | Reference | 419 (70.4) | 390(70.8) | 0.894 | 310(72.6) | 539 (68.8) | 0.172 |
| Cesarean delivery | 361 (29.8) | 1.32 (1.01–1.72) | 176 (29.6) | 161 (29.2) | 117 (27.4) | 244 (31.2) | ||
| Large-for-gestational-age | ||||||||
| No | 1,075 (88.8) | Reference | 535 (89.8) | 478 (86.8) | 0.112 | 382 (89.5) | 693 (88.4) | 0.574 |
| Yes | 136(11.2) | 1.49 (1.06–2.10) | 61 (10.2) | 73 (13.2) | 45 (10.5) | 91(11.6) | ||
| Gestational age at birth (wk) | 39.32 ± 1.26 | 1.02 (0.91–1.14) | 39.28 ± 1.29 | 39.34 ± 1.22 | 0.41 | 39.34 ± 1.31 | 39.31 ± 1.24 | 0.687 |
| Birth weight (kg) | 3.46 ± 0.50 | 1.33 (1.05–1.69) | 3.44 ± 0.48 | 3.51 ± 0.51 | 0.018 | 3.44 ± 0.53 | 3.47 ± 0.48 | 0.26 |
| Birth length (cm) | 50.81 ±2.68 | 1.02 (0.96–1.07) | 50.63 ±2.63 | 51.03 ± 2.70 | 0.001 | 50.58 ±2.67 | 50.93 ±2.68 | 0.029 |
| Edinburgh postnatal depression score at 2 months | 6.50 ± 4.47 | 1.03 (1.00–1.06) | 6.75 ± 4.68 | 6.12 ±4.21 | 0.026 | 6.84 ±4.79 | 6.32 ± 4.28 | 0.074 |
| Postnatal Healthy Eating Index at 3 months | 64.42 ± 9.67 | 0.98 (0.96–1.00) | 62.21 ± 9.73 | 66.64 ± 9.06 | <0.001 | 62.78 ± 10.19 | 65.09 ± 9.38 | 0.007 |
| Breastfeeding duration (wk) | 30.68 ± 30.47 | 1.00(0.99–1.00) | 25.03 ± 28.32 | 37.77 ± 30.86 | <0.001 | 27.41 ± 29.86 | 32.43 ±30.67 | 0.007 |
| Exclusive breastfeeding duration (wk) | 4.84 ± 8.32 | 0.96 (0.97–1.01) | 3.55 ± 6.86 | 6.63 ± 9.68 | <0.001 | 4.31 ±8.09 | 5.13 ±8.44 | 0.141 |
| Timing of formula initiation (wk) | 10.24 ± 10.69 | 0.97(0.95–0.99) | 8.34 ±8.49 | 13.09 ± 12.91 | <0.001 | 8.87 ±9.51 | 11.01 ± 11.23 | 0.003 |
| Timing of introduction of solid foods (wk) | 26.66 ±8.15 | 0.97(0.95–0.99) | 25.61 ± 9.06 | 27.78 ± 6.58 | <0.001 | 25.47 ±8.55 | 27.29 ± 7.86 | <0.001 |
| Infant dietary pattern adherence score | ||||||||
| Infant guideline solids at 1 year | 0.06 ± 1.00 | 0.79 (0.64–0.99) | -0.08 ± 0.99 | 0.18 ± 0.96 | <0.001 | -0.11 ± 1.02 | 0.14 ± 0.98 | 0.001 |
| High sugar/fat/protein at 1 year | -0.09 ± 0.93 | 1.23 (1.08–1.40) | 0.09 ± 0.95 | -0.26 ± 0.78 | <0.001 | 0.19 ± 1.19 | -0.21 ± 0.74 | <0.001 |
| High dairy at 1 year | -0.05 ± 1.01 | 1.01 (0.85–1.20) | 0.10 ± 1.00 | -0.17 ± 1.01 | <0.001 | -0.03 ± 0.98 | -0.05 ± 1.03 | 0.796 |
| Formula/baby cereal at 1 year | 0.03 ± 1.01 | 1.08 (0.94–1.25) | 0.03 ± 1.05 | -0.02 ± 0.94 | 0.486 | 0.05 ± 1.12 | 0.02 ± 0.96 | 0.679 |
| Child sleep duration at 1 year (h) | ||||||||
| ≥4 | 49 (5.4) | Reference | 21(5.2) | 27 (5.8) | <0.001 | 26 (9.0) | 23 (3.7) | <0.001 |
| 5–6 | 129 (14.2) | 0.70 (0.37–1.30) | 66 (16.4) | 56(12.0) | 42 (14.5) | 87 (14.1) | ||
| 7–8 | 159 (17.5) | 0.85 (0.48–1.51) | 85(21.1) | 59 (12.6) | 73 (25.3) | 86(13.9) | ||
| ≥9 | 571 (62.9) | 0.48 (0.28–0.82) | 231 (57.3) | 326 (69.7) | 148 (51.2) | 423 (68.3) | ||
| Nonmaternal infant care at 1 year | ||||||||
| No | 435 (47.3) | Reference | 175 (42.9) | 238 (50.2) | 0.03 | 177 (60.6) | 307 (49.0) | 0.001 |
| Yes | 484(52.7) | 1.11 (0.81–1.52) | 233 (57.1) | 236 (49.8) | 115 (39.4) | 320(51.0) | ||
| Infant weight gain (kg) | ||||||||
| From birth to 3 months | 2.07 ± 0.77 | 1.12 (0.90–1.40) | 2.10 ± 0.78 | 2.05 ± 0.76 | 0.291 | 2.03 ± 0.81 | 2.09 ± 0.75 | 0.232 |
| From birth to 7 months | 4.42 ± 1.08 | 1.37(1.19–1.57) | 4.54 ± 1.17 | 4.29 ± 0.95 | <0.001 | 4.40 ± 1.14 | 4.43 ± 1.04 | 0.625 |
| From birth to 12 months | 6.16 ± 1.24 | 1.29 (1.13–1.48) | 6.19 ± 1.34 | 6.07 ± 1.11 | 0.147 | 6.09 ± 1.37 | 6.19 ± 1.18 | 0.291 |
| Child sleep duration at 6 years (h) | 9.71 ± 1.06 | 0.68 (0.62–0.75) | 9.53 ± 1.11 | 9.93 ± 0.94 | <0.001 | 9.49 ± 1.13 | 9.83 ± 0.99 | <0.001 |
| Child dietary pattern adherence score at 6 years | ||||||||
| Fast foods | 0.02 ± 1.07 | 1.08 (1.00–1.16) | 0.18 ± 1.31 | -0.20 ± 0.63 | <0.001 | 0.19 ± 1.49 | -0.08 ± 0.72 | <0.001 |
| Guideline foods | 0.01 ± 1.02 | 0.90 (0.78–1.02) | -0.07 ± 1.08 | 0.14 ± 0.94 | <0.001 | -0.01 ± 1.11 | 0.03 ± 0.97 | 0.525 |
| Breakfast-type foods | 0.00 ± 1.02 | 0.94 (0.79–1.12) | -0.06 ± 1.02 | 0.08 ± 0.96 | 0.014 | -0.05 ± 1.01 | 0.03 ± 1.03 | 0.236 |
| Staple foods | 0.01 ± 1.05 | 1.01 (0.87–1.16) | -0.02 ± 1.13 | 0.03 ± 0.99 | 0.42 | 0.06 ± 1.20 | -0.02 ± 0.96 | 0.193 |
| Dairy foods | 0.01 ± 1.03 | 0.94 (0.82–1.08) | 0.03 ± 1.09 | 0.00 ± 0.97 | 0.625 | 0.00 ± 1.04 | 0.02 ± 1.03 | 0.841 |
| Package foods | -0.01 ± 1.02 | 1.04 (0.92–1.19) | -0.01 ± 1.05 | -0.03 ± 0.90 | 0.803 | 0.13 ± 1.14 | -0.08 ± 0.93 | <0.001 |
| Score of child food and eating environment at 6 years | 28.03 ± 2.41 | 0.98 (0.51–1.90) | 27.92 ± 2.53 | 28.19 ±2.08 | 0.137 | 28.09 ±2.37 | 27.91 ± 2.48 | 0.378 |
| Score of child home learning environment at 6 years | 2.21 ± 0.86 | 0.77 (0.67–0.88) | 2.01 ± 0.91 | 2.43 ± 0.75 | <0.001 | 1.89 ± 0.93 | 2.38 ± 0.78 | <0.001 |
| Number of days per week with ≥60 minutes of child MVPAat 6 years | 5.49 ± 1.60 | 0.95 (0.88–1.02) | 5.54 ± 1.59 | 5.48 ± 1.55 | 0.51 | 5.46 ± 1.64 | 5.50 ± 1.58 | 0.706 |
| Child TV viewing per day on weekdays at 6 years (h) | 1.50 ± 1.15 | 1.18 (1.08–1.28) | 1.68 ± 1.25 | 1.28 ± 0.96 | <0.001 | 1.61 ± 1.26 | 1.44 ± 1.09 | 0.012 |
| Child TV viewing per day on weekends at 6 years (h) | 2.70 ± 1.64 | 1.08 (1.00–1.15) | 2.95 ± 1.70 | 2.41 ± 1.43 | <0.001 | 2.81 ± 1.72 | 2.64 ± 1.60 | 0.091 |
For some variables, the sum of categories was not equal to the total because of missing data.
Low parental education was defined as 3 years of college or lower, and high education was defined as college graduate or higher; low family income was defined as ≤185% of the poverty line, and high family income was defined as >185% of the poverty line.
Abbreviations: GWG, gestational weight gain; MVPA, moderate or vigorous physical activity; RR, relative risk.
Step 4 was the single-mediator analysis to examine the mediation of each of the significant early-life factors in both Step 2 and Step 3 in the associations between SES measures and childhood obesity (Table 4). Specifically, the SAS procedure of CAUSALMED (SAS Institute Inc.) (34) was used to estimate the direct effects of low SES on risk of childhood obesity at 6 years and its indirect effect through each potential mediator. We chose the SAS CAUSALMED procedure over other existing approaches because it uniquely incorporates convenient estimations of the indirect effect as well as its 95% CI and percentage (i.e., indirect effect/[direct effect + indirect effect]).
TABLE 4.
Single-mediator analysis for socioeconomic disparities in childhood obesity at 6 years
| Sample size (n) | Excess RR (95% CI)a |
Percentage of indirect effectb | |||
|---|---|---|---|---|---|
| Total effect | Direct effect | Indirect effect | |||
| Maternal-education-related disparity in childhood obesity (low vs. high education)c | |||||
| Maternal preconception BMI (kg/m2) | 1,085 | 0.85 (0.28 to 1.41) | 0.77 (0.21 to 1.34) | 0.07 (0.03 to 0.12) | 8.8 |
| Prenatal Healthy Eating Index | 394 | 1.21 (−0.05 to 2.48) | 1.17 (−0.10 to 2.45) | 0.04 (−0.04 to 0.12) | 3.3 |
| Postnatal Healthy Eating Index at 3 months | 559 | 0.89 (0.06 to 1.73) | 0.85 (0.00 to 1.69) | 0.05 (−0.05 to 0.14) | 5.3 |
| Maternal smoking during pregnancy (binary, yes vs. no) | 1,096 | 0.75 (0.19 to 1.30) | 0.69 (0.13 to 1.25) | 0.05 (0.01 to 0.10) | 7.0 |
| Maternal number of cigarettes smoked per day during pregnancy | 1,096 | 0.88 (0.34 to 1.42) | 0.84 (0.30 to 1.38) | 0.04 (0.02 to 0.07) | 4.8 |
| Birth weight (kg) | 1,099 | 0.80 (0.23 to 1.37) | 0.83 (0.26 to 1.40) | −0.03 (−0.05 to 0.00) | N/A |
| Timing of formula initiation (wk) | 871 | 1.08 (0.39 to 1.77) | 1.01 (0.33 to 1.69) | 0.07 (−0.01 to 0.16) | 6.6 |
| Timing of introduction of solid foods (wk) | 977 | 0.67 (0.10 to 1.24) | 0.63 (0.06 to 1.20) | 0.04 (0.00 to 0.08) | 5.8 |
| Adherence score to infant guideline solids at 1 year | 693 | 0.24 (−0.25 to 0.73) | 0.19 (−0.30 to 0.67) | 0.05 (−0.01 to 0.12) | 22.6 |
| Adherence score to high sugar/fat/protein at 1 year | 693 | 0.26 (−0.28 to 0.80) | 0.20 (−0.34 to 0.74) | 0.06 (0.00 to 0.13) | 23.5 |
| Infant weight gain from birth to 7 months (kg) | 854 | 0.82 (0.28 to 1.37) | 0.71 (0.15 to 1.26) | 0.12 (0.05 to 0.19) | 14.4 |
| Child sleep duration at 1 year (h) | 831 | 0.41 (−0.08 to 0.89) | 0.38 (−0.10 to 0.87) | 0.03 (−0.01 to 0.06) | 6.3 |
| Child sleep duration at 6 years (h) | 1,090 | 0.78 (0.21 to 1.34) | 0.69 (0.13 to 1.25) | 0.09 (0.03 to 0.14) | 11.4 |
| Score of child home learning environment at 6 years | 1,096 | 0.75 (0.19 to 1.30) | 0.69 (0.13 to 1.25) | 0.06 (0.00 to 0.12) | 7.7 |
| Child TV viewing per day on weekdays at 6 years (h) | 1,093 | 1.20 (0.60 to 1.80) | 1.14 (0.54 to 1.74) | 0.06 (0.02 to 0.10) | 4.9 |
| Child TV viewing per day on weekends at 6 years (h) | 1,085 | 0.78 (0.21 to 1.35) | 0.77 (0.20 to 1.34) | 0.01 (−0.03 to 0.05) | 1.2 |
| Family-income-related disparity in childhood obesity (low vs. high income)c | |||||
| Maternal preconception BMI (kg/m2) | 1,087 | 0.51 (0.14 to 0.88) | 0.42 (0.06 to 0.77) | 0.10 (0.04 to 0.15) | 18.5 |
| Prenatal Healthy Eating Index | 395 | 0.10 (−0.56 to 0.77) | 0.05 (−0.62 to 0.71) | 0.06 (−0.03 to 0.15) | 55.9 |
| Postnatal Healthy Eating Index at 3 months | 560 | 0.37 (−0.25 to 0.99) | 0.35 (−0.27 to 0.96) | 0.03 (−0.02 to 0.07) | 6.8 |
| Maternal smoking during pregnancy (binary, yes vs. no) | 1,098 | 0.39 (−0.05 to 0.83) | 0.36 (−0.08 to 0.80) | 0.03 (−0.01 to 0.06) | 7.1 |
| Maternal number of cigarettes smoked per day during pregnancy | 1,098 | 0.73 (0.31 to 1.14) | 0.68 (0.26 to 1.10) | 0.05 (0.02 to 0.08) | 6.3 |
| Timing of formula initiation (wk) | 873 | 0.85 (0.36 to 1.34) | 0.84 (0.35 to 1.32) | 0.01 (−0.02 to 0.04) | 1.4 |
| Timing of introduction of solid foods (wk) | 979 | 0.55 (0.01 to 1.09) | 0.53 (−0.01 to 1.07) | 0.02 (−0.01 to 0.05) | 3.6 |
| Adherence score to infant guideline solids at 1 year | 695 | 0.34 (−0.27 to 0.94) | 0.29 (−0.31 to 0.89) | 0.05 (−0.01 to 0.12) | 15.3 |
| Adherence score to high sugar/fat/protein at 1 year | 695 | 0.28 (−0.31 to 0.87) | 0.22 (−0.38 to 0.82) | 0.06 (−0.01 to 0.12) | 20.5 |
| Child sleep duration at 1 year (h) | 833 | 0.35 (−0.18 to 0.88) | 0.33 (−0.21 to 0.86) | 0.03 (−0.01 to 0.07) | 7.4 |
| Child sleep duration at 6 years (h) | 1,092 | 0.70 (0.32 to 1.08) | 0.61 (0.23 to 0.99) | 0.09 (0.02 to 0.16) | 12.8 |
| Score of child home learning environment at 6 years | 1,098 | 0.41 (−0.03 to 0.84) | 0.30 (−0.13 to 0.73) | 0.11 (0.04 to 0.18) | 26.2 |
| Child TV viewing per day on weekdays at 6 years (h) | 1,095 | 0.43 (−0.01 to 0.87) | 0.42 (−0.02 to 0.85) | 0.02 (−0.01 to 0.04) | 3.4 |
Excess RR = RR − 1. Significant indirect effects are in bold (p < 0.05).
Percentage of indirect effect = indirect effect/total effect.
Low education was defined as 3 years of college or lower, and high education was defined as college graduate or higher; low family income was defined as ≤185% of the poverty line, and high family income was defined as >185% of the poverty line.
Abbreviation: RR, relative risk.
Step 5 was the multiple-mediator analysis to examine the causal network of all significant mediators (p < 0.05) individually screened in Step 4. We considered all plausible interrelations among these mediators, based on prior literature and theoretical hypotheses. To allow the causal network to be inclusive, a higher cutoff point of p value (<0.10) was used for the final multiple-mediator model. Specifically, we built the causal network through the SEM using the “gsem” command in Stata (StataCorp LLC) with maximum likelihood estimation, log-transformation of non-normal variables, and Bayesian information criterion for the goodness of fit of models (35). SEM could handle multiple and time-varying mediators, reveal their complex relation, provide in-depth understanding on the causal chains with mediators at different levels (e.g., distant vs. proximal), and incorporate the measurement errors.
Note that adjustment for multiple comparisons might not be necessary in this study, as our hypothesis-driven statistical tests were independent from each other (36). Sample weights were not available from the CDC owing to the unrepresentative sampling frame of the IFPS II (13).
RESULTS
Sample characteristics
Among the 1,211 eligible mother-child dyads, 48.0% of mothers had a college degree or higher educational attainment, and 64.7% had a family income >185% of the poverty line (Table 2). Mean (SD) maternal age was 30.18 (5.36) years; 4.2% of mothers self-identified as Asian/Pacific Islander and others, 5.2% as Hispanic, 4.1% as non-Hispanic Black, and 86.5% as non-Hispanic White; 30.3% were nulliparous; and 83.3% were married. Among the children, 51.4% were boys, and the mean age at the Y6FU survey was 6.45 (0.19) years. The main reason for excluding other participants was missing data on child growth outcomes at 6 years due to nonresponse, loss of contact, or refusal in the Y6FU.
Socioeconomic disparities in childhood obesity
There was an inverse dose-response association between maternal education and childhood obesity (Supporting Information Table S4). When classified as a binary variable, low maternal education (i.e., not a college graduate) was associated with a high risk of childhood obesity (20.5% vs. 10.5%; relative risk [RR]: 1.95 [95% CI: 1.46–2.60]; p < 0.001; Table 2). Low family income (i.e., ≤185% of poverty line) was also associated with a high risk of childhood obesity (22.5% vs. 12.6%; RR: 1.78 [95% CI: 1.38–2.30]; p < 0.001). Adjustment for demographic and pregnancy confounders attenuated the RR of childhood obesity for low family income to 1.42 (95% CI: 1.03–1.97), but it did not substantially change the RR for low maternal education (1.79 [95% CI: 1.31–2.46]).
Single-mediator analysis of socioeconomic disparities in childhood obesity
As shown in Table 3, a considerable number of potential mediators were associated with both SES measures (exposure) and childhood obesity at 6 years (outcome) and were therefore eligible for the single-mediator analysis. The following mediators remained significant in the single-mediator analysis (Table 4) for low-maternal-education-related childhood obesity: maternal preconception BMI (percentage of indirect effect, 8.8%); maternal smoking during pregnancy (yes vs. no, 7.0%; number of cigarettes smoked per day, 4.8%); infant weight gain from birth to 7 months (14.4%); child sleep duration at 6 years (11.4%); and TV viewing during weekdays at 6 years (4.9%). In addition, the association between low family income and childhood obesity was substantially mediated by maternal preconception BMI (18.5%), maternal number of cigarettes smoked per day during pregnancy (6.3%), child sleep duration at 6 years (12.8%), and the home learning environment at 6 years (26.2%).
Multiple-mediator analysis of socioeconomic disparities in childhood obesity
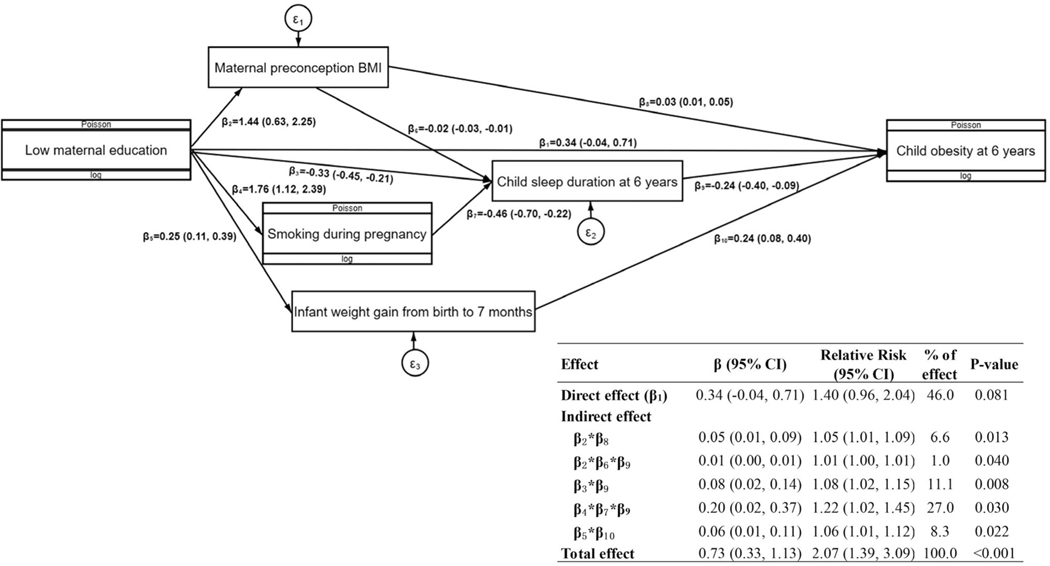
Figure 1 shows the proposed causal network for multiple mediators in the association between maternal education and risk of childhood obesity at 6 years, adjusting for confounders. The mediators that remained significant in the final SEM included maternal preconception BMI, maternal smoking during pregnancy, infant weight gain from birth to 7 months, and child sleep duration at 6 years. In addition, we identified associations between some mediators: maternal preconception BMI → child sleep duration at 6 years; and maternal smoking during pregnancy → child sleep duration at 6 years. The five pathways of indirect effects together mediated 54.0% of the total effect of maternal education on childhood obesity.
FIGURE 1.
Proposed causal network for multiple mediators in the association between maternal education and risk of childhood obesity at 6 years. The parameters were estimated from structural equation modeling, adjusting for confounders including maternal age, race and ethnicity, parity, marital status, child sex, and child’s actual age at the 6-year follow-up
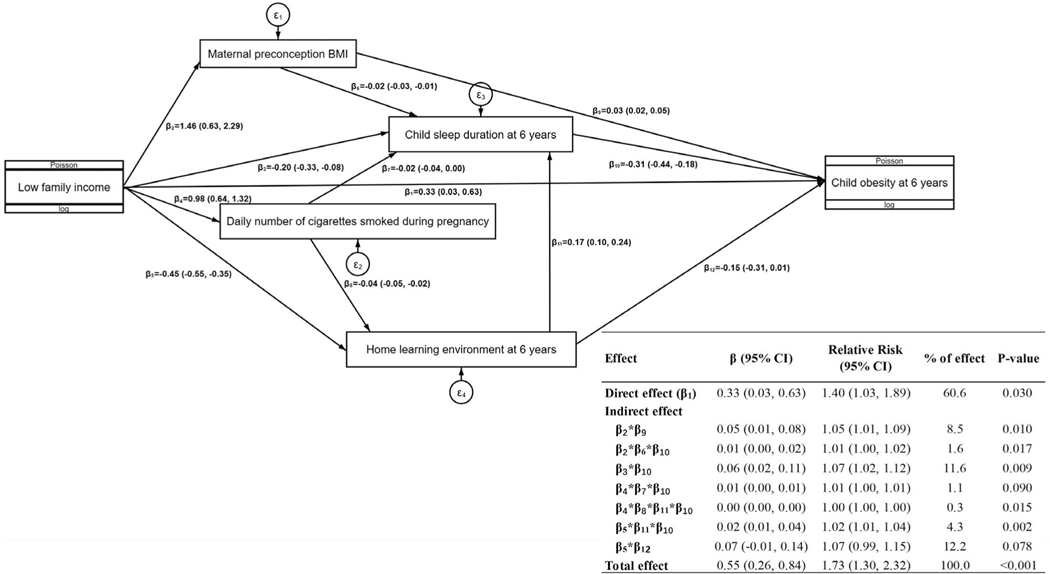
Figure 2 shows the proposed causal network for multiple mediators in the association between family income and risk of childhood obesity at 6 years. The mediators in the final SEM included maternal preconception BMI, maternal number of cigarettes smoked per day during pregnancy, and child sleep duration at 6 years, with an extra mediator of the home learning environment at 6 years. We also identified the following associations between some mediators: maternal preconception BMI → child sleep duration at 6 years; maternal number of cigarettes smoked per day during pregnancy → child sleep duration at 6 years; maternal number of cigarettes smoked per day during pregnancy → the home learning environment at 6 years; and the home learning environment at 6 years → child sleep duration at 6 years. The seven pathways of indirect effects together mediated 39.4% of the total effect of family income on childhood obesity.
FIGURE 2.
Proposed causal network for multiple mediators in the association between family income and risk of childhood obesity at 6 years. The parameters were estimated from structural equation modeling, adjusting for confounders including maternal age, race and ethnicity, parity, marital status, child sex, and child’s actual age at the 6-year follow-up
DISCUSSION
Within a US prebirth cohort, we confirmed that both low maternal education and low family income were associated with a high risk of childhood obesity. We also made unique contributions to the science by identifying several modifiable mediators in early life that might explain these disparities among young US children. They included maternal preconception BMI, smoking during pregnancy, infant weight gain, child sleep, the home learning environment, and child TV viewing. In single-mediator analysis, each of them could explain a considerable proportion (range: 4.8%−26.2%) of SES-related disparities in childhood obesity. In multiple-mediator analysis, all significant mediators together mediated a substantial proportion of the maternal-education-related (54.0%) and the family-income-related (39.4%) disparities in childhood obesity. Our novel findings can inform effective intervention to narrow socioeconomic disparities in childhood obesity in the US and possibly other developed countries.
Maternal preconception BMI
In our sample, preconception BMI consistently mediated the associations between both SES measures (i.e., maternal education and family income) and childhood obesity. Similarly, two previous studies have found that maternal preconception BMI largely mediated the association between low SES and early-childhood weight gain (37,38). Although it is challenging to intervene preconception weight because of its multifactorial determinants, there is some pioneering research on preconception or interpregnancy weight-loss intervention through behavioral changes (39).
Maternal smoking during pregnancy
Maternal smoking during pregnancy also consistently mediated the effects of both SES measures on childhood obesity. Our result was supported by two previous studies (37,38) in which maternal smoking during pregnancy largely mediated the association between low SES and rapid infant weight gain from birth to age 1 year. It is effective and safe to use behavioral, pharmaceutical, and economic interventions to assist pregnant women to stop smoking (40). For example, our own smoking cessation intervention achieved a 63.3% smoking abstinence rate through a multicomponent intervention (41). In addition, we found that intervention-assisted maternal smoking cessation, especially if quitting occurred before 27 weeks, could substantially reduce the risk of rapid infant BMI gain (42). However, maternal smoking cessation during pregnancy remains an opportunity neglected in existing childhood obesity interventions.
Infant weight gain
In our analysis, infant weight gain only mediated the effects of maternal education on childhood obesity, but not the effect of family income. One possible reason might be that infant weight gain is more subject to maternal influences such as infant feeding practices, infant daily activities, monitoring infant growth trajectories, and adhering to well-child visits. There are some intervention programs that have been tested to prevent rapid infant weight gain, but the results have been mixed (43,44).
Child sleep
Our research might be the first study, to our knowledge, to observe that short sleep duration could largely (>10% of total effect) mediate the effects of both SES measures on risk of childhood obesity. In the multiple-mediator analysis, child sleep also mediated some effects of maternal preconception BMI, smoking during pregnancy, and the home learning environment on childhood obesity. Altogether, child sleep seemed to play a crucial role in the causal network between low SES and childhood obesity, which warrants further intervention research. There have been some existing intervention programs that could effectively improve child sleep duration and quality (45).
The home learning environment
An unexpected finding in this analysis was that the home learning environment mediated the largest percentage (26.2%) of the effect of low family income on childhood obesity. It should be noted that two of the three HOME-SF items used to measure the home learning environment in our analysis (i.e., special lessons or organizations related to extracurricular activities and attendance of musical or theatrical performances) might be intertwined with family income, as these activities often require financial and time resources. Other less resource-dependent mediators related to the home learning environment, including alternative cognitive reinforcers to food (e.g., library books, chess, board games), warrant further investigation and may have broader implications. Compared with conventional early-life factors for obesity, the home learning environment, including cognitive stimulation, is a relatively new direction for childhood obesity (31). We were not aware of any existing childhood obesity intervention programs that have specifically focused on the home learning environment.
Other potential mediators
TV viewing was a significant and moderate mediator in our single-mediator analysis for the maternal-education-related disparity in childhood obesity, but it became nonsignificant in the multiple-mediator analysis. This suggested that the effect of TV viewing might overlap with other stronger mediators such as child sleep duration. We could not replicate the finding from a previous US study, in which some nonrecommended infant feeding practices (e.g., predominant formula feeding, early introduction of solid foods) mediated the association between low SES and childhood obesity at age 2 years (11). This inconsistency suggested that the potential mediation effects of these infant feeding practices on socioeconomic disparities in childhood obesity might diminish with age. Similarly, we did not find any significant mediation by child diet or moderate or vigorous physical activity at 6 years. A possible reason was low accuracy of maternal retrospective recalls of child diet and physical activity, especially with simplified items in the Y6FU.
Study limitations
The study limitations included the following: 1) although the IFPS II sample was geographically national (44 states and the District of Columbia), it did not represent the total US population because the sample was not randomly obtained from the general population and overrepresented well-educated, middle-income, employed, non-Hispanic White, nonsmoking, low-parity, and breastfeeding mothers (13), which could limit the generalizability of our results; 2) survey-based data were subject to recall bias, including the mother-reported child’s weight and height as measured at the child’s most recent doctor’s visit; 3) lack of information on other potential mediators such as food security, parenting, and neighborhood social and built environment, which might explain why there was still a large direct effect of SES on childhood obesity unexplained by our multiple-mediator models, especially for the family-income-related disparities (∼60%); 4) a potential violation of positivity, especially in the multiple-mediator analysis; 5) inclusion of 6-year mediators (e.g., child sleep, TV viewing) was subject to risk of reverse causality; 6) our findings should be interpreted as associational rather than causal; 7) we did not examine the potential effect modification by child sex or race and ethnicity because of insufficient statistical power; 8) we did not include postpartum maternal smoking in the mediation analysis because of the concern of its high correlation (multicollinearity) with maternal smoking during pregnancy; 9) we did not examine disparities by other important SES indicators, including paternal education and parental employment/occupation; (18) and 10) the relatively old data (the last follow-up in 2012) might impact the implications to current practice.
CONCLUSION
In the US, socioeconomic disparities in childhood obesity could be substantially mediated by several potentially modifiable early-life factors, including maternal preconception BMI, smoking during pregnancy, infant weight gain, child sleep, the home learning environment, and child TV viewing. Further research in external cohorts is needed to confirm our novel findings and to further test potential differences in other age groups and in other countries. Effective intervention targeting these significant modifiable mediators holds promise to improve obesity-related health equity among socioeconomically disadvantaged children.
Supplementary Material
Study Importance.
What is already known?
There are large socioeconomic disparities in the prevalence of childhood obesity in the United States and many other developed countries.
What does this study add?
Maternal preconception BMI, smoking during pregnancy, infant weight gain, child sleep, the home learning environment, and child TV viewing substantially mediated socioeconomic disparities in childhood obesity in the United States.
How might these results change the direction of research or the focus of clinical practice?
Effective intervention targeting these significant modifiable mediators holds promise to improve obesity-related health equity among socioeconomically disadvantaged children.
Acknowledgments
Funding information
This project was supported by the Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services (HHS) under R40MC31880, titled “Socioeconomic disparities in early origins of childhood obesity and body mass index trajectories” (PI, XW), as well as R21 exploratory research support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NIH grant R21HD091515. The information, content, and/or conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by, the HRSA, HHS, or the US government. BM received support from the National Natural Science Foundation of China (grant number: 82103944), National Key R&D Program of China (grant number: 2017YFC0907200, 2017YFC0907201) and Fundamental Research Funds for the Central Universities (grant number: xzy032020033) while assisting with this project. The funder/sponsor did not participate in the work.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
REFERENCES
- 1.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315:2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fryar CD, Carrol MD, Ogden CL. Prevalence of overweight and obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2013–2014 Health E-Stats. National Center for Health Statistics; 2016. [Google Scholar]
- 3.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1:11–25. [DOI] [PubMed] [Google Scholar]
- 4.Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among Adults children and adolescents Aged 2–19 years: United States, 1963–1965 through 2017–2018. Health E-Stats. National Center for Health Statistics; 2020. [Google Scholar]
- 5.Chung KE, Romney MC. Social determinants of childhood obesity: beyond individual choices. Curr Pediatr Rev. 2012;8:237–252. [Google Scholar]
- 6.Frederick CB, Snellman K, Putnam RD. Increasing socioeconomic disparities in adolescent obesity. Proc Natl Acad Sci USA. 2014;111:1338–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Lim H. The global childhood obesity epidemic and the association between socio-economic status and childhood obesity. Int Rev Psychiatry. 2012;24:176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boone-Heinonen J, Messer L, Andrade K, Takemoto E. Connecting the dots in childhood obesity disparities: a review of growth patterns from birth to pre-adolescence. Curr Epidemiol Rep. 2016;3:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mech P, Hooley M, Skouteris H, Williams J. Parent-related mechanisms underlying the social gradient of childhood over-weight and obesity: a systematic review. Child Care Health Dev. 2016;42:603–624. [DOI] [PubMed] [Google Scholar]
- 10.Lane SP, Bluestone C, Burke CT. Trajectories of BMI from early childhood through early adolescence: SES and psychosocial predictors. Br J Health Psychol. 2013;18:66–82. [DOI] [PubMed] [Google Scholar]
- 11.Gibbs BG, Forste R. Socioeconomic status, infant feeding practices and early childhood obesity. Pediatr Obes. 2014;9:135–146. [DOI] [PubMed] [Google Scholar]
- 12.Runge J Causal network reconstruction from time series: from theoretical assumptions to practical estimation. Chaos. 2018;28:075310. doi: 10.1063/1.5025050 [DOI] [PubMed] [Google Scholar]
- 13.Fein SB, Labiner-Wolfe J, Shealy KR, Li R, Chen J, Grummer-Strawn LM. Infant Feeding Practices Study II: study methods. Pediatrics. 2008;122(suppl 2):S28–S35. [DOI] [PubMed] [Google Scholar]
- 14.Fein SB, Li R, Chen J, Scanlon KS, Grummer-Strawn LM. Methods for the year 6 follow-up study of children in the Infant Feeding Practices Study II. Pediatrics. 2014;134(suppl 1):S4–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Advance Data, no. 314. National Center for Health Statistics; 2000. [PubMed] [Google Scholar]
- 16.Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007;120(suppl 4):S193–S228. [DOI] [PubMed] [Google Scholar]
- 17.Shrewsbury V, Wardle J. Socioeconomic status and adiposity in childhood: a systematic review of cross-sectional studies 1990–2005. Obesity (Silver Spring). 2008;16:275–284. [DOI] [PubMed] [Google Scholar]
- 18.Vazquez CE, Cubbin C. Socioeconomic status and childhood obesity: a review of literature from the past decade to inform intervention research. Curr Obes Rep. 2020;9:562–570. [DOI] [PubMed] [Google Scholar]
- 19.Lumeng JC, Burke LM. Maternal prompts to eat, child compliance, and mother and child weight status. J Pediatr. 2006;149:330–335. [DOI] [PubMed] [Google Scholar]
- 20.US Department of Agriculture Food and Nutrition Service. WIC Eligibility Requirements. Updated June 19, 2020. Accessed June 6, 2020. https://www.fns.usda.gov/wic/wic-eligibility-requirements
- 21.Institute of Medicine and National Research Council. Weight Gain During Pregnancy: Reexamining the Guidelines. The National Academies Press; 2009. [PubMed] [Google Scholar]
- 22.Guenther PM, Casavale KO, Reedy J, et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. [DOI] [PubMed] [Google Scholar]
- 24.Wen X, Kong KL, Eiden RD, Sharma NN, Xie C. Sociodemographic differences and infant dietary patterns. Pediatrics. 2014;134: e1387–1398. [DOI] [PubMed] [Google Scholar]
- 25.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. [DOI] [PubMed] [Google Scholar]
- 26.Thompson FE, Midthune D, Kahle L, Dodd KW. Development and evaluation of the National Cancer Institute’s Dietary Screener Questionnaire scoring algorithms. J Nutr. 2017;147:1226–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. National Youth Physical Activity and Nutrition Study. Updated August 20, 2020. Accessed June 30, 2020. https://www.cdc.gov/healthyyouth/data/yrbs/nypans.htm
- 28.Sisson SB, Broyles ST, Baker BL, Katzmarzyk PT. Screen time, physical activity, and overweight in U.S. youth: national survey of children’s health 2003. J Adolesc Health. 2010;47:309–311. [DOI] [PubMed] [Google Scholar]
- 29.Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–1051. [PubMed] [Google Scholar]
- 30.Bradley RH, Corwyn RF, McAdoo HP, Coll CG. The home environments of children in the United States part I: variations by age, ethnicity, and poverty status. Child Dev. 2001;72:1844–1867. [DOI] [PubMed] [Google Scholar]
- 31.Strauss RS, Knight J. Influence of the home environment on the development of obesity in children. Pediatrics. 1999;103:e85. doi: 10.1542/peds.103.6.e85 [DOI] [PubMed] [Google Scholar]
- 32.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. [DOI] [PubMed] [Google Scholar]
- 33.Marschner IC, Gillett AC. Relative risk regression: reliable and flexible methods for log-binomial models. Biostatistics. 2012;13:179–192. [DOI] [PubMed] [Google Scholar]
- 34.SAS Institute Inc. The CAUSALMED Procedure. SAS/STAT 14.3 User’s Guide. SAS Institute, Inc.; 2017. [Google Scholar]
- 35.Loehlin JC. Latent Variable Models: An Introduction to Factor, Path, and Structural Analysis. 3rd ed. Lawrence Erlbaum Associates Publishers; 1998. [Google Scholar]
- 36.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 37.Van Den Berg G, Van Eijsden M, Galindo-Garre F, Vrijkotte T, Gemke R. Low maternal education is associated with increased growth velocity in the first year of life and in early childhood: the ABCD study. Eur J Pediatr. 2013;172:1451–1457. [DOI] [PubMed] [Google Scholar]
- 38.Layte R, Bennett A, McCrory C, Kearney J. Social class variation in the predictors of rapid growth in infancy and obesity at age 3 years. Int J Obes (Lond). 2014;38:82–90. [DOI] [PubMed] [Google Scholar]
- 39.Erickson ML, Mey JT, Axelrod CL, et al. Rationale and study design for lifestyle intervention in preparation for pregnancy (LIPP): a randomized controlled trial. Contemp Clin Trial. 2020;94:106024. doi: 10.1016/j.cct.2020.106024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2009:CD001055. doi: 10.1002/14651858.CD001055.pub3 [DOI] [PMC free article] [PubMed]
- 41.Wen X, Eiden RD, Justicia-Linde FE, et al. A multicomponent behavioral intervention for smoking cessation during pregnancy: a nonconcurrent multiple-baseline design. Translat Behav Med. 2019;9:308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen X, Eiden RD, Justicia-Linde FE, et al. Reducing fetal origins of childhood obesity through maternal smoking cessation during pregnancy: an intervention study. Int J Obes (Lond). 2019;43:1435–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savage JS, Birch LL, Marini M, Anzman-Frasca S, Paul IM. Effect of the INSIGHT responsive parenting intervention on rapid infant weight gain and overweight status at age 1 rear: a randomized clinical trial. JAMA Pediatr. 2016;170:742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomson JL, Goodman MH, Tussing-Humphreys LM, Landry AS. Infant growth outcomes from birth to 12 months of age: findings from the Delta Healthy Sprouts randomized comparative impact trial. Obes Sci Practice. 2018;4:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quach J, Hiscock H, Ukoumunne OC, Wake M. A brief sleep intervention improves outcomes in the school entry year: a randomized controlled trial. Pediatrics. 2011;128:692–701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.