Abstract
Histidine protein kinases have been explored as potential antibacterial drug targets. The recent identification of two-component histidine kinases in fungi has led us to investigate the antifungal properties of three bacterial histidine kinase inhibitors (RWJ-49815, RWJ-49968, and RWJ-61907). All three compounds were found to inhibit growth of the Saccharomyces cerevisiae and Candida albicans strains, with MICs ranging from 1 to 20 μg/ml. However, deletion of SLN1, the only histidine kinase in S. cerevisiae, did not alter drug efficacy. In vitro kinase assays were performed by using the Sln1 histidine kinase purified from bacteria as a fusion protein to glutathione S-transferase. RWJ-49815 and RWJ-49968 inhibited kinase a 50% inhibitory concentration of 10 μM, whereas RWJ-61907 failed to inhibit at concentrations up to 100 μM. Based on these results, we conclude that these compounds have antifungal properties; however, their mode of action appears to be independent of histidine kinase inhibition.
Fungal infections are an increasingly important health threat as the number of immunocompromised individuals continues to rise. A limited repertoire of effective antifungal drugs with acceptably low host toxicity has motivated the search for novel antifungal drug targets, but the identification of targets unique to fungi has been a challenge, given the remarkable similarity between fungal and mammalian metabolic and signal transduction pathways. There are, however, several promising antifungal target evaluations under active investigation. Enzymes involved in the biosynthesis of the fungal cell wall, lipid composition of the plasma membrane, and DNA and protein synthesis have been targeted, with various degrees of success (8, 9). Recently, the list of potential antifungal targets has expanded to include histidine protein kinases (HPKs), a class of signal transduction molecules once thought to be restricted to the procaryote kingdom but now known to exist in fungi and other lower eucaryotes and plants (24).
HPKs and two-component regulation are used extensively in procaryotic signal transduction processes that allow bacteria to adapt and respond to the environment. Two-component signal transduction systems are so named because at their core are two modules, the histidine kinase and a response regulator. In response to an environmental signal, the kinase autophosphorylates a conserved His residue. The His-phosphate is then transferred to an aspartyl residue on the response regulator, thereby altering its activity. Recently, it has been shown that two-component systems are essential for the growth and viability of Bacillus subtilis, establishing HPKs as important new targets for antibacterial drug design (6). Natural product and synthetic inhibitors of bacterial HPKs have been described that include hydrophobic tyramines and substituted salicylanilides (11, 15, 22, 23). These compounds exhibit MICs in the range of 1 to 10 μg/ml and inhibit bacterial HPKs 50% inhibitory concentrations (IC50s) in the low micromolar range (4). However, despite the correlation between bactericidal activity and inhibition of HPKs, it has not been established that HPK inhibition accounts for the bactericidal activity.
Although two-component signal transduction pathways have been described in bacteria, fungi, slime molds, and plants (reviewed in reference 24), HPKs and two-component pathways have not been found in vertebrates, despite considerable effort. Saccharomyces cerevisiae encodes one membrane-associated HPK (Sln1) and two response regulators, Ssk1 and Skn7, that regulate osmotic and oxidative stress response (12–14, 19, 20, 25). A second family of eucaryotic histidine kinase represented by NIK1 has been isolated from Neurospora crassa (1). Pathogenic fungi, Candida albicans and Aspergillus nidulans, appear to encode both NIK1 and SLN1 types of histidine kinases (2, 17, 21). Although they differ in the overall domain organization, there is a very high degree of sequence conservation between individual HPK domains, presumably indicating a conserved catalytic mechanism. Deletion of S. cerevisiae SLN1 causes derepression of the HOG1 osmosensing pathway, resulting in either cell death or a severe growth defect, depending on the strain background (16). Deletion of NIK1 in C. albicans results in a defect in hyphal formation which may affect virulence (2, 17, 21). Based on these observations, inhibitors of two-component signaling pathways might be effective antifungal agents. However, this idea has not been tested.
In this report we show that a set of antibacterial HPK inhibitors also have antifungal properties. A subset of these compounds also inhibits Sln1 histidine kinase activity in vitro; however, the inhibition of HPK activity does not correlate with the antifungal MICs. Finally, the antifungal properties of this group of compounds are unaffected by deletion of SLN1, the only known HPK in S. cerevisiae. Thus, the antifungal properties of these compounds do not appear to be due to inhibition of two-component HPKs.
MATERIALS AND METHODS
Yeast strains and growth conditions.
S. cerevisiae strains used in this study were derived from laboratory stocks (J. S. Fassler) using standard yeast crossing and transformation techniques (3). JF1735 (MATa sln1::TRP1 ssk1::LEU2 his4-917 leu2-1 lys2-128δ trp1Δ1 ura3-52) was derived from JF1331 (MATa his4-917 leu2-1 lys2-128δ trp1Δ1 ura3-52) by carrying out the disruption of the SLN1 and SSK1 as described previously (14, 25). JF1331 is a descendant of S288c. Yeast cultures were grown at 30°C in rich medium (yeast extract-peptone-dextrose [YEPD]: 1% Bacto yeast extract, 2% Bacto Peptone) with glucose (2% [wt/vol]) as a primary carbon source. C. albicans CAF2-1 (Δura3::imm434/URA3) is derived from SC5314 (7).
Inhibitors.
RWJ compounds 49815, 49968, and 61907 were provided by the R. W. Johnson Pharmaceutical Research Institute (4, 22).
Yeast growth inhibition assays.
Compounds were added at the indicated concentrations to exponentially-growing yeast cultures (5 × 106 cells/ml), and the growth was monitored by measuring the optical density at 660 nm (OD660). Cultures were incubated at 30°C with shaking.
Microdilution MICs (80% inhibition) were determined by using the National Committee for Clinical Laboratory Standards procedure (18) with the following modifications. Compounds were dissolved in dimethyl sulfoxide (DMSO) (100%) to create 10 mM stock solutions. Molecular weights of the compounds tested were as follows: for RWJ-49815, 480.61; for RWJ-49968, 460.96; and for RWJ-61907, 442.35. All compounds were diluted into YEPD to attain the specified concentration and a final DMSO concentration of 2%. The MIC was determined in 96-well microtiter plates. Each well received 200 μl of cells (106 cells/ml) and the indicated concentration of the test compound. Each compound was tested in duplicate on two independent occasions. Plates were incubated at 30°C (S. cerevisiae) or 37°C (C. albicans) for 24 h, each well was mixed, and the OD660 was read by using a Titertek Multiskan MCC-340 microtiter plate reader.
Sln1 histidine kinase assay.
Expression and affinity purification of glutathione S-transferase (GST)-Sln1 kinase has been described previously (14). Briefly, glutathione beads containing approximately 10 μg of GST-Sln1 (50% slurry) were added to buffer C (50 mM Tris [pH 7.5], 50 mM KCl, 5 mM MgCl2, 0.1% β-mercaptoethanol, 50% glycerol). Inhibition experiments were performed by adding the compound to be tested in buffer C containing 5% DMSO. The kinase reaction (final volume, 50 μl) was initiated by the addition of 3.5 μl of [γ-32P]ATP (3,000 Ci/mmol; Amersham) followed by incubation at room temperature for 60 min. Unincorporated [γ-32P]ATP was removed by washing four times with 1 ml of buffer C. The beads were resuspended to obtain a volume of 80 μl by using buffer C, and then 20 μl of 5× protein loading buffer (30 mM Tris [pH 6.7], 10% sodium dodecyl sulfate [SDS], 60% glycerol, 0.8 M β-mercaptoethanol, 0.001% bromophenol blue) was added. Samples were incubated at room temperature for 15 min and then resolved by SDS-polyacrylamide gel electrophoresis (PAGE) (10% polyacrylamide). Phosphorylation of the kinase (32P) was determined by measuring the emission of radioactivity by using a Packard instant imager or the gel was transferred to nitrocellulose for autoradiography.
RESULTS AND DISCUSSION
Bacterial HPK inhibitors also inhibit yeast cell growth.
Inhibition of two-component signaling pathways has been identified as a promising target for antibacterial drug design (reviewed in reference 5). For example, a family of hydrophobic tyramines have been described that inhibit two-component histidine kinases and growth of gram-positive bacteria in the micromolar concentration range (4). However, despite excellent correlation between growth inhibition and HPK inhibition, it has not been possible to conclude that the bactericidal activity resulted from kinase inhibition, because of the large number of two-component kinases in bacteria. Two-component systems have recently been described in S. cerevisiae (16, 19), Neurospora crassa (1), and Candida albicans (17, 21). In S. cerevisiae, loss of Sln1 function is lethal, due to a hyperstimulation of the HOG1 mitogen-activated protein (MAP) kinase pathway (Fig. 1). Based on these observations, we reasoned that antibacterial histidine kinase inhibitors might also possess antifungal properties.
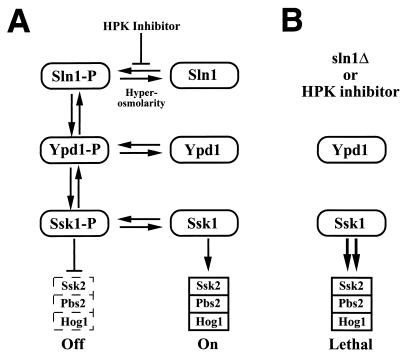
FIG. 1.
Model for the SLN1 osmosensing pathway illustrating how deletion or inhibition of Sln1 kinase may lead to cell death or growth inhibition. (A) Under physiological conditions, the histidine kinase activity of Sln1 is controlled by the osmotic balance. Hyperosmotic conditions shift the equilibrium to the unphosphorylated form of Sln1, and the Hog1 MAP kinase is activated. (B) Deletion of the SLN1 gene or inhibition of Sln1 kinase activity leads to an accumulation of unphosphorylated Ssk1 which, in turn, causes hyperstimulation of the HOG MAP kinase pathway and cell death.
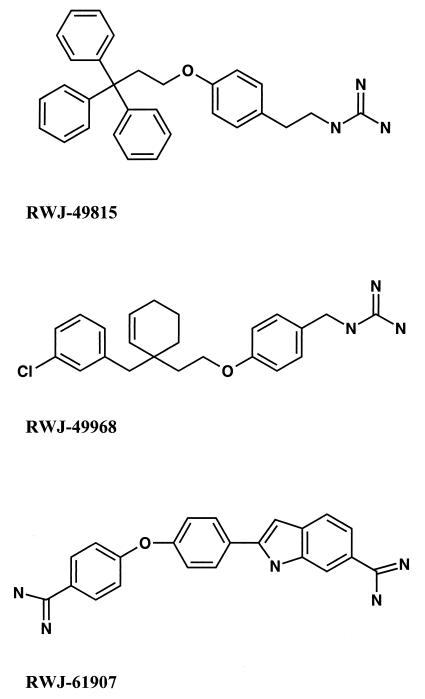
Three inhibitors of bacterial HPKs (RWJ-49815, RWJ-49968, and RWJ-61907) were examined in this study. These compounds are members of the tyramine, cyclohexene, and indole series, respectively, of compounds evaluated as antibacterial agents (Fig. 2). Antifungal activity was determined by a microdilution MIC method with S. cerevisiae and C. albicans strains. All three compounds inhibit yeast cell growth, but only RWJ-49968 and RWJ-61907 MICs are less than 20 μg/ml (Table 1). RWJ-61907 was the most active inhibitor, followed by RWJ-49968 and RWJ-49815. In all cases, the antifungal MICs are approximately 5- to 10-fold higher than the corresponding antibacterial MICs, which range from 0.12 μg/ml (RWJ-61907) to 1 to 2 μg/ml (RWJ-49815 and RWJ-49968) (4, 8a).
FIG. 2.
Chemical structures of compounds reported to inhibit bacterial two-component HPKs (4, 19).
TABLE 1.
MICs of three bacterial HPK inhibitors for yeast
| Strain | MICa (μg/ml) of:
|
||
|---|---|---|---|
| RWJ-49815 | RWJ-49968 | RWJ-61907 | |
| S. cerevisiae JF1331b | >20 | 5–10 | 2.5–5 |
| C. albicans CAF2-1c | 5–10 | 2.5–5 | 1.25–2.5 |
MICs were determined as described in Materials and Methods.
JF1331 is an S. cerevisiae strain from our lab collection (J. S. Fassler).
C. albicans CAF2-1 is described in reference 7.
Sln1 histidine kinase inhibition.
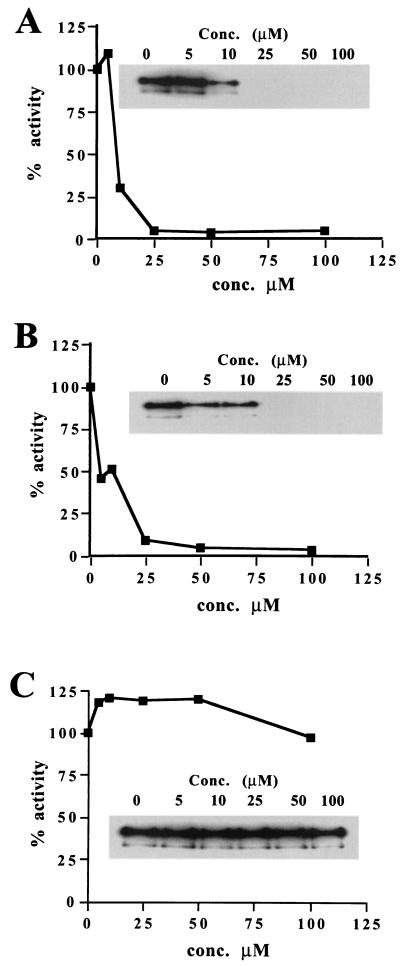
Next, an in vitro kinase reaction was used to determine directly if RWJ-49815, RWJ-49968, and RWJ-61907 inhibit the HPK activity of yeast Sln1. The autokinase activity of purified GST-Sln1 was tested with and without incubation with the inhibitors (Fig. 3). Two of the compounds, RWJ-49815 and RWJ-49968, inhibit Sln1 kinase, IC50s of approximately 10 μM. In contrast, RWJ-61907 failed to inhibit Sln1 kinase at concentrations up to 100 μM despite an MIC of 2.5 to 5.0 μg/ml (Table 1). This was somewhat surprising, given the presumption that the antibacterial and antifungal properties of these compounds were due to HPK inhibition. This raises the possibility that the kinase is not the target of inhibition. However, we could not rule out that RWJ-61907 is metabolically converted in yeast to a form that inhibits HPK in vivo. One way to address this possibility is to compare the abilities of these compounds to inhibit growth of wild type and yeast cells in which the SLN1 gene has been deleted (sln1Δ).
FIG. 3.
Inhibition of GST-Sln1 kinase by RWJ-49815 (A), RWJ-49968 (B), or RWJ-61907 (C). Results are expressed as a function of GST-Sln1 phosphorylation (Sln1-PO4) in the absence of drug after a 60-min incubation. The inset shows the autoradiogram of GST-Sln1-PO4 used to quantitate autokinase activity. This is a representative experiment; essentially the same results were obtained on three separate occasions.
Growth inhibition of SLN1 and sln1Δ yeast strains.
Our current understanding of the Sln1 osmosensing pathway provides us with a unique opportunity to address the target specificity of putative HPK inhibitors (Fig. 1). Sln1 is the only two-component histidine kinase in S. cerevisiae. Deletion of the SLN1 gene results in a severe growth defect or cell death, depending on the strain (15). Lethality can be suppressed by deletion of the response regulator SSK1 or of the genes encoding components of the HOG1 MAP kinase pathway, SSK2 (MEK kinase), PBS2 (MEK), or HOG1 itself (16). For example, an sln1Δ strain is inviable, whereas an sln1Δ ssk1Δ strain grows normally. It follows that a true anti-HPK drug will inhibit a wild-type (SLN1) strain but will be ineffective against an sln1Δ ssk1Δ strain.
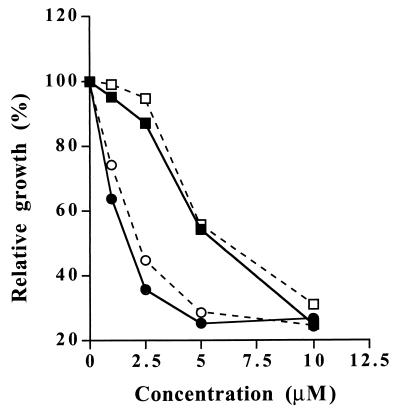
We compared the antifungal properties of RWJ-49815, RWJ-49968, and RWJ-61907 in SLN1 (JF1331) and sln1Δ ssk1Δ (JF1735) strains. As seen in Fig. 4, no difference in drug sensitivity is observed. Both strains are inhibited equally by the drug, regardless of whether a histidine kinase is present (SLN1 [JF1331]) or absent (sln1Δ ssk1Δ [JF1735]). This indicates that the antifungal properties of these antibacterial HPK inhibitors are attributable to a mechanism distinct from histidine kinase inhibition. Based on these results, a reevaluation of the proposed antibacterial target of these compounds is warranted. A similar conclusion was reached in a recent study in which it was found that antibacterial HPK inhibitors cause general membrane damage (10). The isolation and characterization of drug-resistant yeast revertants might lead to a better understanding of the true mechanism of action of these compounds.
FIG. 4.
Growth inhibition of JF1331 (SLN1) (solid lines) and JF1735 (sln1Δssk1Δ) (dashed lines) by RWJ-49815 (squares) or RWJ-61907 (circles). The indicated concentration of drug was added to exponentially-growing cultures (5 × 106 cells/ml), and the OD660 was determined after incubation at 30°C for 5.5 h. The values plotted represent the averages of triplicate samples. Individual values varied less than 10%.
ACKNOWLEDGMENTS
We thank S. Moye-Rowley (University of Iowa) for providing the CAF2-1 C. albicans strain and R. Goldschmidt, M. Macielag, and K. Bush (R. W. Johnson Pharmaceutical Research Institute, Rahway, N.J.) for providing the compounds used in this study. We also thank J. Hoch (Scripps Institute) for his interest in this project and many insightful comments along the way.
This work was supported by the National Institutes of Health (GM56719), the R. W. Johnson Pharmaceutical Research Institute (R.J.D. and J.S.F.), and grant T32AG (A.D.A.) from the NIH/National Institute on Aging.
REFERENCES
- 1.Alex L A, Borkovich K A, Simon M I. Hyphal development in Neurospora crassa: involvement of a two-component histidine kinase. Proc Natl Acad Sci USA. 1996;93:3416–3421. doi: 10.1073/pnas.93.8.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alex L A, Korch C, Selitrennikoff C P, Simon M I. COS1, a two-component histidine kinase that is involved in hyphal development in the opportunistic pathogen Candida albicans. Proc Natl Acad Sci USA. 1998;95:7069–7073. doi: 10.1073/pnas.95.12.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M. Current protocols in molecular biology. Boston, Mass: John Wiley & Sons; 1995. [Google Scholar]
- 4.Barrett J F, Goldschmidt R M, Lawrence L E, Foleno B, Chen R, Demers J P, Johnson S, Kanojia R, Fernandez J, Bernstein J, Licata L, Donetz A, Huang S, Hlasta D J, Macielag M J, Ohemeng K, Frechette R, Frosco M B, Klaubert D H, Whiteley J M, Wang L, Hoch J A. Antibacterial agents that inhibit two-component signal transduction systems. Proc Natl Acad Sci USA. 1998;95:5317–5322. doi: 10.1073/pnas.95.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett J F, Hoch J A. Two-component signal transduction as a target for microbial anti-infective therapy. Antimicrob Agents Chemother. 1998;42:1529–1536. doi: 10.1128/aac.42.7.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabret C, Hoch J A. A two-component signal transduction system essential for growth of Bacillus subtilis: implications for anti-infective therapy. J Bacteriol. 1998;180:6375–6383. doi: 10.1128/jb.180.23.6375-6383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgopapadakou N H, Walsh T J. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279–291. doi: 10.1128/aac.40.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Goldschmidt, R. Personal communication.
- 9.Groll A H, De Lucca A J, Walsh T J. Emerging targets for the development of novel antifungal therapeutics. Trends Microbiol. 1998;6:117–124. doi: 10.1016/s0966-842x(97)01206-7. [DOI] [PubMed] [Google Scholar]
- 10.Hilliard J J, Goldschmidt R M, Licata L, Baum E Z, Bush K. Multiple mechanisms of action for inhibitors of histidine protein kinases from bacterial two-component systems. Antimicrob Agents Chemother. 1999;43:1693–1699. doi: 10.1128/aac.43.7.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hlasta D J, Demers J P, Foleno B D, Fraga-Spano S A, Guan J, Hilliard J J, Macielag M J, Ohemeng K A, Sheppard C M, Sui Z, Webb G C, Weidner-Wells M A, Werblood H, Barrett J F. Novel inhibitors of bacterial two-component systems with gram positive antibacterial activity: pharmacophore identification based on the screening hit closantel. Bioorg Med Chem Lett. 1998;8:1923–1928. doi: 10.1016/s0960-894x(98)00326-6. [DOI] [PubMed] [Google Scholar]
- 12.Ketela T, Brown J L, Stewart R C, Bussey H. Yeast Skn7p activity is modulated by the Sln1p-Ypd1p osmosensor and contributes to regulation of the HOG pathway. Mol Gen Genet. 1998;259:372–378. doi: 10.1007/s004380050824. [DOI] [PubMed] [Google Scholar]
- 13.Krems B, Charizanis C, Entian K D. The response regulator-like protein Pos9/Skn7 of Saccharomyces cerevisiae is involved in oxidative stress resistance. Curr Genet. 1996;29:327–334. doi: 10.1007/BF02208613. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Ault A, Malone C L, Raitt D, Dean S, Johnston L H, Deschenes R J, Fassler J S. The yeast histidine protein kinase, Sln1p, mediates phosphotransfer to two response regulators, Ssk1p and Skn7p. EMBO J. 1998;17:6952–6962. doi: 10.1093/emboj/17.23.6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macielag M J, Demers J P, Fraga-Spano S A, Hlasta D J, Johnson S G, Kanojia R M, Russell R K, Sui Z, Weidner-Wells M A, Werblood H, Foleno B D, Goldschmidt R M, Loeloff M J, Webb G C, Barrett J F. Substituted salicylanilides as inhibitors of two-component regulatory systems in bacteria. J Med Chem. 1998;41:2939–2945. doi: 10.1021/jm9803572. [DOI] [PubMed] [Google Scholar]
- 16.Maeda T, Wurgler-Murphy S M, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- 17.Nagahashi S, Mio T, Ono N, Yamada-Okabe T, Arisawa M, Bussey H, Yamada-Okabe H. Isolation of CaSLN1 and CaNIK1, the genes for osmosensing histidine kinase homologues, from the pathogenic fungus Candida albicans. Microbiology. 1998;144:425–432. doi: 10.1099/00221287-144-2-425. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeast. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 19.Ota I M, Varshavsky A. A yeast protein similar to bacterial two-component regulators. Science. 1993;262:566–569. doi: 10.1126/science.8211183. [DOI] [PubMed] [Google Scholar]
- 20.Posas F, Wurgler-Murphy S M, Maeda T, Witten E A, Thai T C, Saito H. Yeast Hog1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two component” osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 21.Srikantha T, Tsai L, Daniels K, Enger L, Highley K, Soll D R. The two-component hybrid kinase regulator caNIK1 of Candida albicans. Microbiology. 1998;144:2715–2729. doi: 10.1099/00221287-144-10-2715. [DOI] [PubMed] [Google Scholar]
- 22.Urbanski M J, Xiang M A, Foleno B D, Bernstein J I, Lawrence L, Fraga-Spano S A, Goldschmidt R M, Barrett J F, Frechette R, Chen R. Abstracts of the 214th American Chemical Society National Meeting, Las Vegas, Nev. 1997. Novel cyclohexene derivatives with histidine protein kinase inhibitory activity—potential new antibacterial agents, abstr. 270. [Google Scholar]
- 23.Weidner-Wells M A, Bernstein J I, Chen R, Demers J P, Foleno B D, Fraga-Spano S A, Hlasta D J, Johnson S G, Kanojia R, Klaubert M D, Sheppard C M, Barrett J F. Abstracts of the 214th American Chemical Society National Meeting, Las Vegas, Nev. 1997. Triphenylalkyl derivatives as inhibitors of bacterial two-component regulatory systems, abstr. 148. [Google Scholar]
- 24.Wurgler-Murphy S M, Saito H. Two-component signal transducers and MAPK cascades. Trends Biochem Sci. 1997;22:172–176. doi: 10.1016/s0968-0004(97)01036-0. [DOI] [PubMed] [Google Scholar]
- 25.Yu G, Deschenes R J, Fassler J S. The essential transcription factor, Mcm1, is a downstream target of Sln1, a yeast “two-component” regulator. J Biol Chem. 1995;270:8739–8743. doi: 10.1074/jbc.270.15.8739. [DOI] [PubMed] [Google Scholar]