Abstract
Since the eruption of the worldwide SARS-CoV-2 pandemic in late 2019/early 2020, multiple elective surgical interventions were postponed. Through pandemic measures, elective operation capacities were reduced in favour of intensive care treatment for critically ill SARS-CoV-2 patients. Although intermittent low-incidence infection rates allowed an increase in elective surgery, surgeons have to include long-term pulmonary and extrapulmonary complications of SARS-CoV-2 infections (especially “Long Covid”) in their perioperative management considerations and risk assessment procedures. This review summarizes recent consensus statements and recommendations regarding the timepoint for surgical intervention after SARS-CoV-2 infection released by respective German societies and professional representatives including DGC/BDC (Germany Society of Surgery/Professional Association of German Surgeons e.V.) and DGAI/BDA (Germany Society of Anesthesiology and Intensive Care Medicine/Professional Association of German Anesthesiologists e.V.) within the scope of the recent literature. The current literature reveals that patients with pre- and perioperative SARS-CoV-2 infection have a dramatically deteriorated postoperative outcome. Thereby, perioperative mortality is mainly caused by pulmonary and thromboembolic complications. Notably, perioperative mortality decreases to normal values over time depending on the duration of SARS-CoV-2 infection.
Keywords: Postponement, Operation, Surgery, COVID-19, SARS-CoV-2, Pandemic
Pathophysiology of COVID-19 (coronavirus disease 2019)
During SARS-CoV-2 (severe acute respiratory syndrome coronavirus type 2) pandemic a large amount of elective surgical operations had to be postponed or even cancelled [1, 2]. Literature reveals that several subgroups of patients (e.g. emergency and oncologic surgery) were inadequately treated caused by increased time-to-diagnosis and time-to-intervention [3, 4]. Furthermore, an enormous economic disaster for surgical departments was caused by a lack of intensive care and surgical capacities [1]. Surgeons have to learn about and to work with the SARS-CoV-2 infection and with patients with COVID-19 as an accompanying part of patients’ history and perioperative management. Only a few percent of patients could become infected with SARS-CoV-2 in the perioperative setting [5, 6]; however, the rate of unreported cases could be higher due to failure in patient testing and clinically silent infections [7, 8]. It is a known fact that SARS-CoV-2 and its variants will be part of everyday work in the next decade [9]. Since the first cases in December 2019 the SARS-CoV-2 pandemic [10], the virus spread quickly [11] and became a global health crisis [12]. On March 11th, 2020, the World Health Organization (WHO) declared the SARS-CoV-2 eruption to a worldwide pandemic [13]. As typical for coronaviruses, SARS-CoV-2 infection initiates by binding of the spike protein (S protein) to the cellular ACE2 (angiotensin-converting enzyme 2) receptor [14–16] after priming by the transmembrane serine protease (TMPRSS2) [14]. The ACE2 receptor is expressed ubiquitously, but mainly in the lung, kidney, gastrointestinal (GI) tract [17] and the heart [18]. Extrapulmonary manifestations [19] of SARS-CoV-2 infection could be explained by the enzyme Furin, which promotes the SARS-CoV-2 attachment and which is expressed in a variety of organs [20]. Depending on the immune status of the infected host, the manifestation and the course of the disease can be heterogenous in the patient population: The spectrum reaches from asymptomatic patients, mild cough and fever up to critical illness with intensive care treatment and total disruption of the lung parenchyma and other organ manifestations and consecutive organ failure [21–23]. Different mortality rates are reported in the literature [21–24], but sometimes the case-fatality rate is also reported [25]. The data vary, for example in different countries, in the timing of the pandemic (different waves), depending on the age of the patient [26] and between virus variants [27]. According to the Robert-Koch Institute (RKI), a total of 1.8% of all persons for whom confirmed SARS-CoV-2 infections have been transmitted in Germany have died in association with COVID-19 disease (23 November 2021) [23]. Severe courses of SARS-CoV-2 infection are particularly dangerous, with a case-fatality rate of approximately 49% [25]. In the first wave of COVID-19 in Germany, the course of the disease was mainly mild (80% of cases). The mortality was reported to be 5.6% of all laboratory-confirmed cases, varying between 0 and 30% depending on age [28]. In the second wave, a higher mortality rate was detected worldwide due to the emergence of different virus variants [29]. Severely infected COVID-19 patients suffer from severe respiratory failure in most cases and die frequently due to acute respiratory distress syndrome (ARDS) [30]; thus, ventilated patients have a particularly high mortality risk [31]. One possible explanation for the increased incidence of severe pulmonary complications such as ARDS is the degradation of ACE2 during SARS-CoV-2 infection [32], because of the loss of the lung-protective effect attributed to ACE2 [33]. Severe courses are furthermore accompanied by extrapulmonary manifestations like thromboembolic events [34–37]. In addition to an increased incidence of acute ischemic strokes [38], the thrombus burden of ST-elevation myocardial infarction (STEMI) is also increased in cases with concomitant SARS-CoV-2 infection [39]. Intensive research on SARS-CoV-2 revealed that the virus frequently leads to neurologic disorders like encephalopathy, stroke or cerebral seizure [40], musculoskeletal weakness and impaired concentration, especially in elderly patients [41].
Not only short-term but also long-term damage have been reported, especially in patients following severe COVID-19 infection with critical illness [42], but also in younger patients with a mild course of infection [43]. There is currently an undifferentiated terminology to describe persistence or reemergence of symptoms after SARS-CoV-2 infection, summarized as “Long Covid” or “post-COVID-19 syndrome” [44]. Therefore, National Institute for Health and Care Excellence (NICE) guidelines define “acute COVID-19” as COVID-19-associated symptoms lasting up to 4 weeks after infection and “ongoing symptomatic COVID-19” as COVID-19-associated symptoms lasting 4–12 weeks after infection. “Post-COVID-19 syndrome” is characterized by COVID-19-associated symptoms lasting longer than 12 weeks after infection. The term “Long Covid” is used for both “ongoing symptomatic COVID-19” and “post-COVID syndrome” [45]. These terms are used in the same way in the German guideline on Long Covid [46]. Risk factors for the development of Long Covid include older age and higher body mass index and female sex. The symptoms of fatigue, headache, dyspnea, hoarse voice and myalgia that occur during the first week of infection crystallized as good predictive factors for the development of Long Covid [47]. So far, the literature does not provide a reliable estimation for the incidence of Long Covid [23, 46]. In Long Covid, the most common manifestation is chronic fatigue syndrome [42, 48–50]. The causes for the development of a Long Covid are still unclear. However, pathologic and persistent systemic inflammation in response to viral and antigenic remnants, as well as the ongoing persistence of SARS-CoV-2 infection, are discussed in the pathophysiology of the disease [51, 52]. Other possible causes such as immune cell dysfunction with the development of autoimmune processes and alteration of the microbiome of the gastrointestinal tract are also still a matter of debate [53, 54]. In the following, we will present the evidence for possible existing additive negative effects of passed SARS-CoV-2 infection on the outcome of surgical patients. The optimal timing for elective surgery will be highlighted. In addition, the applications of risk scores and useful primary prophylactic treatments to prevent postoperative complications are discussed thoroughly.
Possible synergies between SARS-CoV-2 infection and surgical intervention on the immune system
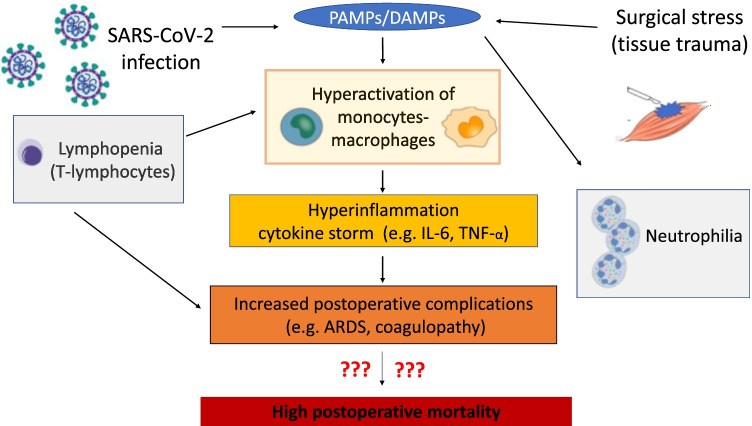
The most common postoperative complications and major causes of death after surgery are postoperative infections [55, 56] and thromboembolic events [57–59]. Surgery results in a hyperinflammation-induced procoagulant status in the perioperative phase due to impairments of the immune system [60]. Notably, not only in postoperative patients, but also in patients with severe SARS-CoV-2 infection, a frequent cause of death is caused by modulation of the immune system, leading to severe pulmonary (ARDS) [10, 61, 62] and thromboembolic complications [34, 36, 63–69]. Thromboembolic complications are not only characterized by the formation of microthrombi in the lungs [70]. Rather, COVID-19 is a systemic vascular disease with multiple manifestation sites [71, 72] and platelet activation [73]. In severe SARS-CoV-2 infection, this procoagulant state is diagnostically associated with elevated D-dimer levels [64, 74]. The common immunomodulatory effects of SARS-CoV-2 infection and surgical therapy are depicted in Fig. 1.
Fig. 1.
Common immunomodulatory effects of SARS-CoV-2 infection and surgical therapy on postoperative mortality. Both SARS-CoV-2 infection and surgical therapy lead to hyperactivation of macrophages through tissue damage of various causes, which first leads to local hyperinflammation. In the following course, a systemic cytokine storm may occur. In this line, lymphopenia and neutrophilia are induced. These SARS-CoV-2 driven effects on the immune system negatively influence on postoperative immune competence of patients and lead to severe postoperative complications such as ARDS, sepsis and thromboembolism. The question now concerns the impact of perioperative SARS-CoV-2 infection on postoperative mortality. ARDS, acute respiratory distress syndrome; PAMPS, pathogen-associated molecular patterns; DAMPS, damage-associated molecular patterns; IL-6, interleukin-6, TNF-α, tumour necrosis factor-α (modified from [75]; Icons from [76, 77])
Initially, SARS-CoV-2 is recognized by endothelial cells and monocytes/macrophages through its viral RNA, known as PAMP (pathogen-associated molecular patterns). PAMPs activate monocytes/macrophages and cause dysfunctional proinflammatory cytokine response leading to a cytokine storm with consecutive hyperinflammation [76, 78–82]. The level of cytokine release in these cases correlates positively with the severity of the disease [79, 83–85]. Tissue damage induced by hyperinflammation in the context of SARS-CoV-2 infection and iatrogenic tissue damage in the setting of surgery each result in the release of DAMPs from the damaged cells [86, 87]. Iatrogenic tissue damage may also cause hypersecretion of proinflammatory cytokines resulting in a vicious circle [85, 88]. In the initial acute stage of SARS-CoV-2 infection, there is also a characteristic lymphopenia [61, 78, 79, 89] and the extent of initial lymphopenia by itself often correlates with the severity of the SARS-CoV-2 infection [90]. For this reason, severe courses have been observed especially in elderly patients [89] with an already weakened immune system [91]. Hyperinflammation may also be aggravated by the presence of lymphopenia, as the reduced number of T lymphocytes may not adequately inhibit macrophages in their proinflammatory activity. Lymphopenia also exists postoperatively as part of the systemic stress response, but it differs from SARS-CoV-2-induced lymphopenia because of other pathomechanisms (e.g. endocrine responses involving cortisol release [92]). Another common immunomodulatory reaction that can be triggered by both SARS-CoV-2 infection and surgical therapy is neutrophilia [22, 93]. In the synopsis of the immunogenic changes just presented, an increased neutrophil-to-lymphocyte ratio (NLR) can be detected especially in severe SARS-CoV-2 infections of critically ill patients [89] and can also be used as a predictive marker for postoperative complications [94].
In summary, synergistic immunopathologic mechanisms of SARS-CoV-2 and surgery are to be expected in patients with perioperative SARS-CoV-2 infection.
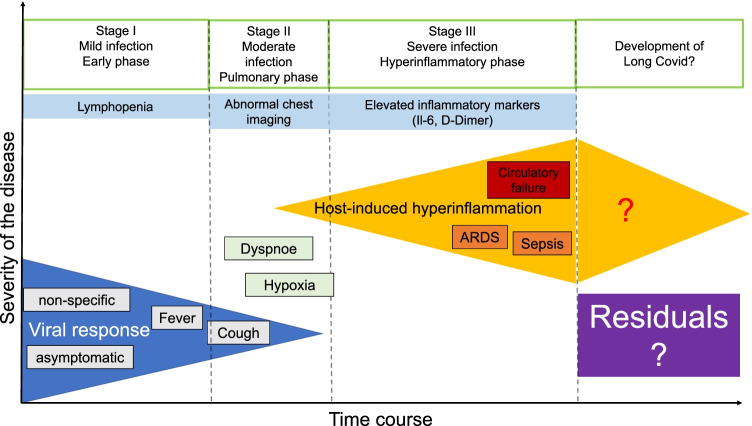
For surgeons planning elective surgical interventions, it is important to know and to learn about the time course and the possibility of prolonged dysfunction of the immune system in Long Covid: In the early phase of SARS-CoV-2 infection, there is a mild inflammation characterized by a high viral load in the frequently asymptomatic or by coughing and fever, mildly symptomatic patient. Subsequently, moderate infection with most common pulmonary manifestation and dyspnea may occur. Severe courses are characterized by an endogenous hyperinflammatory response, that frequently lead to ARDS, sepsis and even circulatory failure. A prolonged course of SARS-CoV-2 infection with a very slow regression of the immunologic activation [95] and the development of prolonged COVID-19 Long Covid should be considered for the affected patients when planning elective surgical procedures (s. Fig. 2) [96].
Fig. 2.
The different diseases phases of SARS-CoV-2 infection in relation to the severity of COVID-19. The initial phase is characterized by mild infection with cough and fever or even presents asymptomatically. Blood examinations might give evidence for lymphopenia and neutrophilia. The prognosis at this stage is very good. In case of progression of the infection, a transition to a pulmonary phase with clinical and morphological development of pneumonia can be found, which makes frequently hospitalization necessary. The prognosis depends on the severity of pulmonary function impairment or respiratory insufficiency and comorbidities of the affected patients. Transition to the 3rd phase results in the development of a systemic extrapulmonary syndrome with a systemic increase in proinflammatory markers. The prognosis is poor due to the development of sepsis with multiple organ failure and/or ARDS (modified from [96])
The different stages and varying clinical courses of SARS-CoV-2 infection underline the complexity of planning surgical interventions with additional (surgical) trauma in the affected patients. An increasing amount of literature has been published in that field, which has to be considered for the safety of future patients during the pandemic and which is reviewed in the following.
Increased postoperative mortality in cases of perioperative SARS-CoV-2 infection
At the beginning of the SARS-CoV-2 pandemic, most guidelines focused on perioperative management and hygiene precautions of SARS-CoV2-positive patients. The intention was to control the spread of infection and protect other patients and healthcare workers from infection [97–99]. However at this time, due to missing studies, no recommendations could be made for the optimal time period between SARS-CoV-2 infection and elective surgery. Up to date, there are many publications, analyzing the perioperative mortality of patients with perioperative SARS-CoV-2 infection [100] and giving an evidence-based statement on postponing elective surgical interventions [101]. Table 1 summarizes some selected publications from the onset of the pandemic to the appearance of studies that examine the time interval in more detail [8, 102–110].
Table 1.
Summary of selective publications on perioperative outcome after SARS-CoV-2 infection
| Authors | Title | Journal | Publication date | Country | Study design | Period of surgery | Sample size | Diagnosis/start of COVID-19 infection | Examined perioperative period (detection of SARS-CoV-2 infection) | Mortality | Most common complications | Recommendation distance from operation to SARS-CoV-2 infection | Some limitations |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lei et al. [8] | Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection | EClinicalMedicine (The Lancet) | April/2020 | China (Hubei province, Wuhan) | Retrospective cohort study | 01/2020–02/2020 | 34 | Onset of clinical symptoms | During the incubation period of COVID-19 infection | 20.5% (patients with perioperative COVID-19 infection), no comparison group | Pneumonia, ARDS, secondary infection | Preoperative quarantine period, exclusion of new COVID-19 infection | Small sample size, PCR tests preoperative not performed as standard |
| CovidSurg Collaborative [102] | Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study | The Lancet | May/2020 | international (24 countries, predominantly Europe and North America) | Retrospective cohort study | 01/2020–03/2020 | 1128 | PCR test or clinical suspicion or radiological signs | 7 days preoperative to 30 days postoperative | 30-day mortality rate: 23.8% (perioperative COVID-19 infection), 43.1% (emergency surgery, postoperative COVID-19 diagnosis, pulmonary complications), no comparison group | Pulmonary complications | Generous postponement of operations, balancing the consequences of postponed surgery and expected postoperative mortality with perioperative COVD-19 infection (risk factors: male and advanced age) | Not always PCR test used for diagnosis |
| Kahlberg et al. [103] | Vascular surgery during COVID-19 emergency in Hub Hospitals of Lombardy: experience on 305 patients | European Journal of Vascular & Endovascular Surgery | November/2020 | Italy (Lombardy) | Prospective study | 03/2020–04/2020 | 305 | PCR test and clinical suspicion with radiological signs | Pre- and postoperative | COVID vs non-COVID patient: In-hospital mortality: 25% vs 6%, Elective: 20.0% vs 2.8%, Emergent: 27.9% vs 13.2% | Multiorgan failure, respiratory failure | In surgical planning: consider COVID-19 infection as a negative prognostic factor (pulmonary and vascular complications) | Not always PCR test used for diagnosis |
| Mi et al. [104] | Characteristics and Early Prognosis of COVID-19 Infection in Fracture Patients | The Journal of Bone And Joint Surgery | May/2020 | China (Hubei province, Wuhan) | Retrospective cohort study | 01/2020–02/2020 | 10 | PCR test and/or radiological signs | COVID-19 infection before admission, postoperative | Of 2 patients with COVID-19 infection detected by PCR test and surgical treatment 1 died | Pulmonary complications | Surgical treatment should be carried out cautiously or non-operative care should be chosen | Very small sample size, not always PCR test used for diagnosis |
| COVIDSurg Collaborative [105] | Delaying surgery for patients with previous SARS-CoV-2 infection | British Journal of Surgery | November/2020 | International (16 countries, predominantly Italy, UK, Spain) | Prospective cohort study | 01/2020–03/2020 | 122 | PCR test | preoperative | 30-day mortality 3.4% (all patients with positive PCR test), 7.7% (1–2 weeks after positive PCR test), 3–4% (2–4 weeks after positive PCR test), 0% (> 4 weeks after positive PCR test), no comparison group | Pulmonary complications (10.7% COVID-19 infection vs 3.6% no COVID-19 infection) | Postponement of surgery > 4 weeks after positive PCR result | Small sample size |
| Doglietto et al. [106] | Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID-19) in Italy | JAMA Surgery | June/2020 | Italy (Brescia) | Retrospective cohort study | 02/2020–04/2020 | 123 | PCR test and/or radiological signs (chest radiography and/or computed tomography) | Preoperative or within 1 week after surgery | COVID vs non-COVID patient: 30-day mortality: 19.51% vs 2.44% | Pulmonary and thrombotic complications | Postpone surgery if possible, because of increased mortality has been demonstrated | Not always PCR test used for diagnosis, single-center study |
| Catton et al. [107] | Planned surgery in the COVID-19 pandemic: a prospective cohort study from Nottingham | Langenbeck’ s Archives of Surgery | May/2021 | UK (Nottingham) | Prospective cohort study | 03/2020–04/2020 | 597 | PCR test confirmed suspected cases (temperature measurement and questionnaire or imaging) | 2 days preoperative to 30 days postoperative | 30-day mortality: 0.7% (all postoperative patients)vs 25% (postoperative patients with COVID-19 infection) | No information | Patient should be informed about increased mortality rate in COVID-19 infection after surgery. Urgent and cancer operations can take place with a low incidence of COVID-19 infection | Not always PCR test used for diagnosis, mortality not clearly attributable to COVID19 infection (e.g. palliative situation) small number of COVID-19 diagnosis or suspected COVID-19 infections (18 patients) |
| Jonker et al. [108] | Perioperative SARS-CoV-2 infections increase mortality, pulmonary complications and thromboembolic events: a Dutch, multicenter, matched-cohort clinical study | Surgery | September/2020 | Netherlands | Retro- and prospective cohort study | 02/2020–06/2020 | 558 screened for the study, 503 included in data analysis | PCR test or clinical suspicion plus radiological signs (computed tomography of the chest) | 30 days before surgery or within 30 days postoperatively | COVID vs non-COVID patient: 30-day mortality: 12% vs 4% | Pulmonary and thromboembolic complications | Postponing elective surgeries and, if possible, emergency surgeries, altered protocols of thromboembolic prophylaxis | Not always PCR test used for diagnosis |
| COVIDSurg Collaborative & GlobalSurg Collaborative [109] | Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study | Anaesthesia | March/2021 | International (116 countries) | prospective cohort study | 10/2020 | 140 231 | PCR test or rapid antigen test or computed tomography of the chest or antibody test or clinical suspicion | Preoperative | 30-day mortality (weeks after COVID-19 diagnosis): 9.1% (0–2 weeks), 6.9% (3–4 weeks), 5.5% (5–6 weeks), 2% (> 7 weeks), 1.4% (no preoperative COVID-19 infection) | Pulmonary complications | Postpone surgery > 7 weeks after COVID-19 infection, longer for patients with persistent symptoms | Not always PCR test used for diagnosis |
| National emergency laparotomy audit [110] | The impact of COVID-19 on emergency laparotomy – an interim report of the national emergency laparotomy audit | Royal College of Anaesthetists | March/2021 | England and Wales | Retrospective cohort study | 03/2020–09/2020 | 10,546 | PCR test or clinical suspicion | Pre- and postoperative | COVID vs non-COVID patient: 30-day mortality: 12.5% vs 7.2% | No data | Due to increased postoperative mortality with COVID-19 infection, high-risk patients should be offered alternative/conservative therapies | Not always PCR test used for diagnosis |
The studies were published from 2020 to mid-2021 and examine only a small time interval in 2020, which only represents the early global onset of the pandemic [8, 102–110]. Unfortunately, there are some limitations of the 10 studies, shown in Table 1. The majority of studies (7 out of 10 studies) is not international, but focus only on certain countries [8, 103, 104, 106–108, 110]. Only half of the studies were prospectively conducted [103, 105, 107–109]. The studies show a high degree of heterogeneity in terms of:
The contingent of surgeries investigated (investigation of heterogeneous surgeries [8, 102, 106, 108, 109] versus investigation of a specific type of surgery [103–105, 107, 110],
The high range in case-loads of the studies (ranging from 10 to 140,231 patients),
The modality of SARS-CoV-2 diagnosis (e.g. clinical symptoms [8], clinical suspicion and/or chest imaging with or without confirmation by either rapid antigen or PCR-testing
The perioperative period was studied (many studies without precise data [8, 103–105, 109, 110], with the largest period 30 days before to 30 days after surgery [108].
Some studies report only the mortality of patients with pre- or perioperative SARS-CoV-2 infection without a comparison group (mortality of patients with postoperative SARS-CoV-2 infection and ICU stay: 20.5% [8], overall 30-day mortality in patients with perioperative SARS-CoV-2 infection: 23.8% [102], overall 30-day mortality in patients with preoperative SARS-CoV-2 infection and different surgical time points after confirmed SARS-CoV-2 infection: 3.4% [105]. Mortality of patients with perioperative SARS-CoV-2 infection compared with patients without SARS-CoV-2 infection has been investigated in 4 studies and was significantly increased in cases with perioperative SARS-CoV-2 infection [103, 106, 108, 110]. Only two studies investigated the impact of the selected time interval from diagnosis of SARS-CoV-2 infection to surgery on postoperative mortality. The majority of the presented studies observed an increase in postoperative pulmonary and thromboembolic complications [8, 102–106, 108, 109] and came to the conclusion that elective surgical interventions should be postponed [102, 104–106, 108, 109]. Herein, a longer time interval between SARS-CoV-2 infection and surgery reduces postoperative mortality [105, 109]!
In the landmark trial from the COVIDSurg Collaborative and the GlobalSurg Collaborative dealing with “Timing of surgery following SARS-CoV-2 infection” the 30-day mortality rates in more than 140,000 patients (non-COVID-19 (97.8%) versus former COVID-19 (2.2%) patients) were depicted. While 30-day mortality was 1.5% (95% CI 1.4–1.5) in patients without SARS-CoV-2 infection, mortality was increased in SARS-CoV-2-positive patients depending on the time to surgery after infection. When surgery was performed 0–2 weeks after SARS-CoV-2 infection, mortality was increased (odds ratio (95% CI) 4.1 (3.3–4.8)) and decreased 5–6 weeks after SARS-CoV-2 infection (odds ratio (95% CI) 3.6 (2.0–5.2)). Finally, patients operated on 7 weeks or longer after infection had mortality similar to that of uninfected patients. These results are also evident in the different subgroups classified by age, ASA physical status, grade (on the basis of the Bupa schedule of procedures in minor and major) and urgency of surgery elective vs emergency. Indications for surgery were divided into “trauma”, “benign”, “cancer” and “obstetrics”.
Interestingly, results revealed that symptomatic patients with a prolonged COVID-19 disease without resolution of symptoms had persistently increased mortality rates and might benefit from a further postposition of the elective surgical intervention. This important trial was one of the largest global surgical studies ever conducted. The GlobalSurg Collaborative and COVIDSurg Collaborative give mandatory information on postoperative mortality after SARS-CoV-2 infection and furthermore demonstrated a time-dependent decrease in unfavourable patient outcomes after SARS-CoV-2 infection, which might be a recommendation and transferred to future patients during the global pandemic.
Planning of surgery is an individual and interdisciplinary decision
Before the SARS-CoV-2 pandemic, elective operations were postponed in children with recent severe upper respiratory tract infection [111]. The rationale was that postoperative respiratory complications were otherwise observed more frequently [112, 113]. In adults, a former respiratory infection within 1 month prior to surgery was also identified as a risk factor for postoperative pulmonary complications and increased mortality [114]. In contrast to “classical” respiratory diseases, the reasons for postponing elective surgery in the current COVID-19 situation are more complex. Operations are postponed not only because of increased postoperative complication rates, but also because of the risk and the fear of nosocomial SARS-CoV-2 infection, in-hospital spreading [5] and reduced intensive care capacities due to the pandemic as well as reserved intensive care capacities for critical ill COVID-19 patients during the phases with high incidences [115]. When deciding whether elective operations can be postponed, the indication and urgency of the operation must be considered [116, 117]. Evaluating the urgency of the operation becomes particularly difficult in patients with malignant diseases. Cancer progression and systemic spreading may lead to increased cancer-related mortality, if oncologic surgery (and medical oncologic treatment) is delayed [4]. On the other hand, cancer patients with perioperative SARS-CoV-2 infection in particular often require postoperative monitoring and ventilation at an ICU and show higher postoperative mortality [118]. Not only the type of surgery (e.g. high mortality rate of almost 40% after thoracic surgery [119]) but also the severity of COVID-19 infections (with potentially long duration until complete rehabilitation and functional recovery) and the development of a Long Covid should be taken into account for the treating surgeon. Existing comorbidities of COVID-19 patients associated with a poorer prognosis are, e.g. male gender, diabetes mellitus type II, advanced age and cardiovascular disease [30, 120–128]. Obese patients in particular are at increased risk of SARS-CoV-2 infection with a severe course [129]. This is particularly evident in ICU patients with COVID-19 [130].
Many efforts were made to analyze the impact of the time interval to a passed SARS-CoV-2 infection on postoperative mortality. On May 12th 2021, the consensus recommendation of the DGC/BDC as surgical representatives and DGAI/BDA as anesthesiologic representatives was published on the timing of planned surgical interventions after SARS-CoV-2 infection [131]. This recommendation was mainly based on the COVIDSurg Collaborative and GlobalSurg Collaborative trial mentioned above (Table 1, [109]). If possible, any planned operation should be performed seven weeks after the beginning of COVID-19 infection at the earliest convenience. This is not the case, if COVID-19 symptoms are persisting, as the COVIDSurg Collaborative and GlobalSurg Collaborative trial revealed a “normal” mortality only in patients 7 weeks after the diagnosis of COVID-19, if they are no longer symptomatic! With persistent symptoms, increased mortality was seen in patients operated 7 weeks after SARS-CoV-2 infection, thus this patient collective will benefit from a delay of at least 7 weeks. Be alerted, that the study underlying this German society recommendation refers to the suggested time interval not only to clinical symptoms, but to disease detection, where the patient does not necessarily have to be symptomatic [109]. Furthermore, it should be noted that preoperative vaccination is recommended in patients without a history of SARS-CoV-2 infection [131, 132]. An interval of at least 1 week was recommended between vaccination and elective surgery in order to distinguish possible consequences of vaccination from postoperative complications. However, to ensure that a competent immune response has occurred before surgery, the interval should be extended to 2 weeks [131, 133].
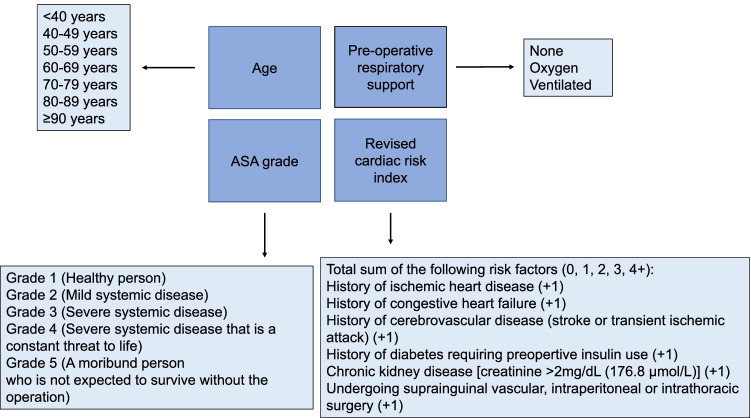
The DGCH/BDC and DGAI/BDA recommendation follows, with minor deviations, the recommendation from the Royal Australasian College of Surgeons (RACS) Victorian State Committee. On August 5th, 2020, they already recommended a symptom-free interval of 8 weeks before elective surgery [134]. Previous recommendations from the American Society of Anesthesiologists (ASA) dated December 8th, 2020, note that the time interval between symptoms and elective surgical procedures depends on the severity of the disease and should be between 4 and 12 weeks [135]. The aforementioned period of 12 weeks is supported by a study by Hsieh et al. It shows that the reconvalescence phase is 3 months after influenza-associated ARDS [136]. Prediction models already exist that estimate the mortality of COVID-19 infection [128, 137–139]. One of the first measurements for the planning of surgery and to predict mortality in patients with or after COVID-19 is COVIDSurg Mortality Score. It takes into account age, ASA grade, preoperative oxygen demand and cardiovascular comorbidities and is freely available under https://covidsurgrisk.app [140] (s. Fig. 3). For a comprehensive overview, Fig. 4 summarizes important aspects in the planning of elective surgery during the SARS-CoV-2 pandemic.
Fig. 3.
The CovidSurg Mortality Score. To estimate postoperative mortality, age, ASA and pulmonary and cardiac comorbidities are considered.
Modified from https://covidsurgrisk.app and [140]
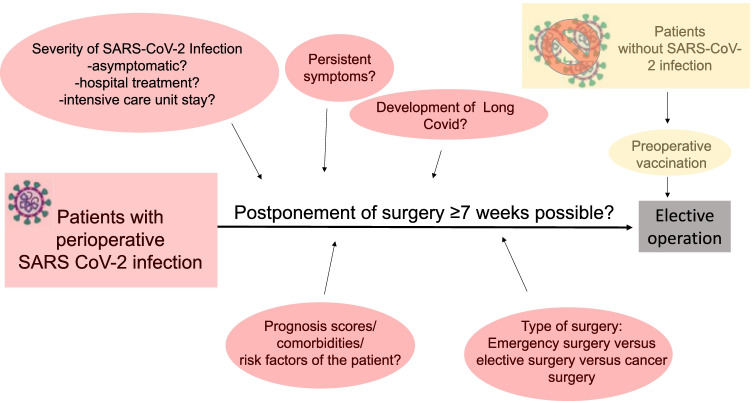
Fig. 4.
Individual and interdisciplinary factors in elective surgery planning. Summary of individual and interdisciplinary factors influencing the planning of operations in patients with and without perioperative SARS-CoV-2 infection. The SARS-CoV-2 icons by [76]
In conclusion, many factors should be considered when planning elective operations in patients with perioperative SARS-CoV-2 infection, including the severity and course of SARS-CoV-2 infection (e.g. asymptomatic versus severe course, outpatient versus inpatient/ICU treatment, rapid versus delayed recovery). Nevertheless, the type of surgery (e.g. emergency versus elective surgery, cancer versus benign surgery) has to be included in the decision-making [102]. Based on the recommendation of the DGCH/BDC and DGAI/BDA, the time interval is critical for postoperative mortality (postponing surgery by ≥ 7 weeks if possible) [109, 131]. Thus, the planning of surgery after SARS-CoV-2 infection is an individualized and interdisciplinary decision in times of the SARS-CoV-2 pandemic.
Abbreviations
- ACE2
Angiotensin-converting enzyme 2
- ARDS
Acute respiratory distress syndrome
- ASA
American Society of Anesthesiologists
- COVID-19
Coronavirus disease 2019
- DAMPs
Damage-associated molecular patterns
- GI tract
Gastrointestinal tract
- ICU
Intensive care unit
- IL-6
Interleukin-6
- NICE
The National Institute for Health and Care Excellence
- NLR
Neutrophil-to-lymphocyte index
- PAMPs
Pathogen-associated molecular patterns
- RACS
Royal Australasian College of Surgeons
- PCR
Polymerase chain reaction
- RKI
Robert-Koch Institute
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus type 2
- S protein
Spike protein
- STEMI
ST-elevation myocardial infarction
- TMPRSS2
Transmembrane serine protease
- TNF-α
Tumour necrosis factor-α
- WHO
World Health Organization
- DGC
Germany Society of Surgery
- BDC
Professional Association of German Surgeons e.V.
- DGAI
Germany Society of Anesthesiology and Intensive Care Medicine
- BDA
Professional Association of German Anesthesiologists e.V
Authors’ contributions
J. N., M. R. and A. H. wrote this manuscript together. M. D., J. G. R. and M.H. performed corrections and graphic illustration. W.P. and M.A.W. were supervisors of the scientific work.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Ethics approval and consent to participate
For this article, no patients or animals were involved.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fowler AJ, Dobbs TD, Wan YI, Laloo R, Hui S, Nepogodiev D, et al. Resource requirements for reintroducing elective surgery during the COVID-19 pandemic: modelling study. Br J Surg. 2021;108(1):97–103. doi: 10.1093/bjs/znaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVIDSurg Collaborative Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg. 2020;107(11):1440–1449. doi: 10.1002/bjs.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reichert M, Sartelli M, Weigand MA, Doppstadt C, Hecker M, Reinisch-Liese A, et al. Impact of the SARS-CoV-2 pandemic on emergency surgery services-a multi-national survey among WSES members. World J Emerg Surg. 2020;15(1):64. doi: 10.1186/s13017-020-00341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanna TP, King WD, Thibodeau S, Jalink M, Paulin GA, Harvey-Jones E, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glasbey JC, Nepogodiev D, Simoes JFF, Omar O, Li E, Venn ML, et al. Elective cancer surgery in COVID-19-free surgical pathways during the SARS-CoV-2 pandemic: an international, multicenter, comparative cohort study. J Clin Oncol. 2020;39(1):66–78. doi: 10.1200/JCO.20.01933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singhal R, Ludwig C, Rudge G, Gkoutos GV, Tahrani A, Mahawar K, et al. 30-day morbidity and mortality of bariatric surgery during the COVID-19 pandemic: a multinational cohort study of 7704 patients from 42 countries. Obes Surg. 2021;31(10):4272–4288. doi: 10.1007/s11695-021-05493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau H, Khosrawipour V, Kocbach P, Mikolajczyk A, Ichii H, Schubert J, et al. Internationally lost COVID-19 cases. J Microbiol Immunol Infect. 2020;53(3):454–458. doi: 10.1016/j.jmii.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lei S, Jiang F, Su W, Chen C, Chen J, Mei W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramesh S Govindarajulu M Parise RS Neel L Shankar T Patel S et al (2021) Emerging SARS-CoV-2 variants: a review of its mutations, its implications and vaccine efficacy. Vaccines 9(10). 10.3390/vaccines9101195 [DOI] [PMC free article] [PubMed]
- 10.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallah SI, Ghorab OK, Al-Salmi S, Abdellatif OS, Tharmaratnam T, Iskandar MA, et al. COVID-19: breaking down a global health crisis. Ann Clin Microbiol Antimicrob. 2021;20(1):35. doi: 10.1186/s12941-021-00438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO (2022) WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020 https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- 14.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280 e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du M, Cai G, Chen F, Christiani DC, Zhang Z, Wang M. Multiomics evaluation of gastrointestinal and other clinical characteristics of COVID-19. Gastroenterol. 2020;158(8):2298–2301 e7. doi: 10.1053/j.gastro.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532(2002):107–110. doi: 10.1016/S0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 19.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C, Zheng M, Yang Y, Gu X, Yang K, Li M, et al. Furin: a potential therapeutic target for COVID-19. iScience. 2020;23(10):101642. doi: 10.1016/j.isci.2020.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/s0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.RKI (2021) Epidemiologischer Steckbrief zu SARS-CoV-2 und COVID-19 https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Steckbrief.html;jsessionid=78B8A0D9803B912A1FB6271495B41588.internet101?nn=13490888#doc13776792bodyText6.
- 24.An C, Lim H, Kim D-W, Chang JH, Choi YJ, Kim SW. Machine learning prediction for mortality of patients diagnosed with COVID-19: a nationwide Korean cohort study. Sci Rep. 2020;10(1):18716. doi: 10.1038/s41598-020-75767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 26.Norgaard SK Vestergaard LS Nielsen J Richter L Schmid D Bustos N et al (2021) Real-time monitoring shows substantial excess all-cause mortality during second wave of COVID-19 in Europe, October to December 2020. Euro Surveill 26(2). 10.2807/1560-7917.ES.2021.26.1.2002023 [DOI] [PMC free article] [PubMed]
- 27.Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021;372:n579. doi: 10.1136/bmj.n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schilling J, Lehfeld AS, Schumacher D, Ullrich A, Diercke M. Krankheitsschwere der ersten COVID19-Welle in Deutschland basierend auf Meldungen gemäß Infektionsschutzgesetz. J Health Monit. 2020;5(S11):2–20. [Google Scholar]
- 29.WHO (2021) COVID-19 Weekly Epidemiological Update-23 March 2021 https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---23-march-2021.
- 30.Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lei Y, Zhang J, Schiavon CR, He M, Chen L, Shen H, et al. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2. Circ Res. 2021;128(9):1323–1326. doi: 10.1161/CIRCRESAHA.121.318902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marone EM, Bonalumi G, Curci R, Arzini A, Chierico S, Marazzi G, et al. Characteristics of venous thromboembolism in COVID-19 patients: a multicenter experience from Northern Italy. Ann Vasc Surg. 2020;68:83–87. doi: 10.1016/j.avsg.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wichmann D, Sperhake J-P, Lutgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choudry FA, Hamshere SM, Rathod KS, Akhtar MM, Archbold RA, Guttmann OP, et al. High thrombus burden in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2020;76(10):1168–1176. doi: 10.1016/j.jacc.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frontera JA, Sabadia S, Lalchan R, Fang T, Flusty B, Millar-Vernetti P, et al. A prospective study of neurologic disorders in hospitalized patients with COVID-19 in New York City. Neurology. 2021;96(4):e575–e586. doi: 10.1212/WNL.0000000000010979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/s0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tenforde MW, Kim SS, Lindsell CJ, Rose EB, Shapiro NI, Files DC, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a Multistate Health Care Systems Network — United States, March–June 2020. MMWR. 2020;69(30):993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond) 2021;53(10):737–754. doi: 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.NICE (2020) COVID-19 rapid guideline: managing the long-term effects of COVID-19. ISBN 978–1–4731–3943–5 [PubMed]
- 46.Koczulla AR, Ankermann T, Behrends U, Berlit P, Böing S, Brinkmann F, et al. S1-Leitline Post-COVID/Long-COVID. Pneumologie. 2021;75(11):869–900. doi: 10.1055/a-1551-9734. [DOI] [PubMed] [Google Scholar]
- 47.Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–627. doi: 10.1016/s2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lam MH-B, Wing Y-K, Yu MW-M, Leung C-M, Ma RCW, Kong APS, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors. Arch Intern Med. 2009;169(22):2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- 51.Ahmad MS, Shaik RA, Ahmad RK, Yusuf M, Khan M, Almutairi AB, et al. „LONG COVID”:an insight. Eur Rev Med Pharmacol Sci. 2021;25:5561–5577. doi: 10.26355/eurrev_202109_26669. [DOI] [PubMed] [Google Scholar]
- 52.Akbarialiabad H, Taghrir MH, Abdollahi A, Ghahramani N, Kumar M, Paydar S, et al. Long COVID, a comprehensive systematic scoping review. Infection. 2021;49(6):1163–1186. doi: 10.1007/s15010-021-01666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeoh YK, Zuo T, Lui GC-Y, Zhang F, Liu Q, Li AYL, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70(4):698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Proal AD, VanElzakker MB. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An overview of biological factors that may contribute to persistent symptoms. Front Microbiol. 2021;12:698169. doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect. 2017;96(1):1–15. doi: 10.1016/j.jhin.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Delgado-Rodriguez M, Gomez-Ortega A, Jlorca J, Lecuona M, Dierssen T, Sillero-Arenas M, et al. Nosocomial infection, indices of intrinsic infection risk, and in-hospital mortality in general surgery. J Hosp. 1999;41(3):203–11. doi: 10.1016/s0195-6701(99)90017-8. [DOI] [PubMed] [Google Scholar]
- 57.Encke A Haas S Kopp I Abholz H-H Bode C Bootz F et al (2015) S3-Leitlinie Prophylaxe der venösen Thromboembolie (VTE). https://www.awmf.org/uploads/tx_szleitlinien/003-001l_S3_VTE-Prophylaxe_2015-10-abgelaufen_01.pdf. Accessed 9 Mar 2022
- 58.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. Risk factors for deep vein thrombosis and pulmonary embolism. Arch Intern Med. 2000;160:809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 59.Leonardi MJ, McGory ML, Ko CY. A systematic review of deep venous thrombosis prophylaxis in cancer patients: implications for improving quality. Ann Surg Oncol. 2007;14(2):929–936. doi: 10.1245/s10434-006-9183-9. [DOI] [PubMed] [Google Scholar]
- 60.Poredos P, Poredos P, Jezovnik MK, Mavric A, Leben L, Mijovski MB, et al. Time course of inflammatory and procoagulant markers in the early period after total hip replacement. Clin Appl Thromb Hemost. 2021;27:1076029620985941. doi: 10.1177/1076029620985941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/s2213-2600(20)30076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Battaglini D, Robba C, Rocco PRM, De Abreu MG, Pelosi P, Ball L. Perioperative anaesthetic management of patients with or at risk of acute distress respiratory syndrome undergoing emergency surgery. BMC Anesthesiol. 2019;19(1):153. doi: 10.1186/s12871-019-0804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Porfidia A, Valeriani E, Pola R, Porreca E, Rutjes AWS, Di Nisio M. Venous thromboembolism in patients with COVID-19: systematic review and meta-analysis. Thromb Res. 2020;196:67–74. doi: 10.1016/j.thromres.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giannis D, Allen SL, Tsang J, Flint S, Pinhasov T, Williams S, et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood. 2021;137(20):2838–2847. doi: 10.1182/blood.2020010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, et al. Pulmonary embolism in patients with COVID-19. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 68.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roncon L, Zuin M, Barco S, Valerio L, Zuliani G, Zonzin P, et al. Incidence of acute pulmonary embolism in COVID-19 patients: systematic review and meta-analysis. Eur J Intern Med. 2020;82:29–37. doi: 10.1016/j.ejim.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/s0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gu SX, Tyagi T, Jain K, Gu VW, Lee SH, Hwa JM, et al. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol. 2021;18(3):194–209. doi: 10.1038/s41569-020-00469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goshua G, Pine AB, Meizlish ML, Chang C-H, Zhang H, Bahel P, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/s2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Besnier E, Tuech J-J, Schwarz L. We asked the experts: Covid-19 outbreak: is there still a place for scheduled surgery? “Reflection from pathophysiological data”. World J Surg. 2020;44(6):1695–1698. doi: 10.1007/s00268-020-05501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blanco-Melo D, Nilsson-Payant BE, Liu W-C, Uhl S, Hoagland D, Mølller R, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045 e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ackermann M, Anders H-J, Bilyy R, Bowlin GL, Daniel C, De Lorenzo R, et al. Patients with COVID-19: in the dark-NETs of neutrophils. Cell Death Differ. 2021;28(11):3125–3139. doi: 10.1038/s41418-021-00805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Del Valle DM, Kim-Schulze S, Huang H-H, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev. 2020;7(6):998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sims JT, Krishnan V, Chang C-Y, Engle SM, Casalini G, Rodgers GH, et al. Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19. J Allergy Clin Immunol. 2021;147(1):107–111. doi: 10.1016/j.jaci.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Longbottom ER, Torrance HDT, Owen HC, Fragkou PC, Hinds CJ, Pearse RM, et al. Features of postoperative immune suppression are reversible with interferon gamma and independent of IL-6 pathways. Ann Surg. 2016;264(2):370–377. doi: 10.1097/SLA.0000000000001484. [DOI] [PubMed] [Google Scholar]
- 86.Guo Q, Zhao Y, Li J, Liu J, Yang X, Guo X, et al. Induction of alarmin S100A8/A9 mediates activation of aberrant neutrophils in the pathogenesis of COVID-19. Cell Host Microbe. 2021;29(2):222–235 e4. doi: 10.1016/j.chom.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mokart D, Merlin M, Sannini A, Brun JP, Delpero JR, Houvenaeghel G, et al. Procalcitonin, interleukin 6 and systemic inflammatory response syndrome (SIRS): early markers of postoperative sepsis after major surgery. Br J Anaesth. 2005;94(6):767–773. doi: 10.1093/bja/aei143. [DOI] [PubMed] [Google Scholar]
- 89.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan. China Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haynes L, Eaton SM, Swain SL. Effect of age on naive CD4 responses: impact on effector generation and memory development. Springer Semin Immunopathol. 2002;24(1):53–60. doi: 10.1007/s00281-001-0095-2. [DOI] [PubMed] [Google Scholar]
- 92.Prete A, Yan Q, Al-Tarrah K, Akturk HK, Prokop LJ, Alahdab F, et al. The cortisol stress response induced by surgery: a systematic review and meta-analysis. Clin Endocrinol (Oxf) 2018;89(5):554–567. doi: 10.1111/cen.13820. [DOI] [PubMed] [Google Scholar]
- 93.Shijo H, Iwabuchi K, Hosoda S, Watanabe H, Nagaoka I, Sakakibara N. Evaluation of neutrophil functions after experimental abdominal surgical trauma. Inflamm res. 1997;47:67–74. doi: 10.1007/s000110050278. [DOI] [PubMed] [Google Scholar]
- 94.Tan TP, Arekapudi A, Metha J, Prasad A, Venkatraghavan L. Neutrophil-lymphocyte ratio as predictor of mortality and morbidity in cardiovascular surgery: a systematic review. ANZ J Surg. 2015;85(6):414–419. doi: 10.1111/ans.13036. [DOI] [PubMed] [Google Scholar]
- 95.Liu J Yang X Wang H Li Z Deng H Liu J et al (2021) Analysis of the long-term impact on cellular immunity in COVID-19-recovered individuals reveals a profound NKT cell impairment. mBio 12:e00085–21. 10.1128/mBio [DOI] [PMC free article] [PubMed]
- 96.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee JS, Yum H-K, Si HJ, Han SH, Park SY, Peck KR, et al. Guidelines for surgery of confirmed or suspected COVID-19 patients. Infect Chemother. 2020;52(3):453–459. doi: 10.3947/ic.2020.52.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coccolini F, Perrone G, Chiarugi M, Di Marzo F, Ansaloni L, Scandroglio I, et al. Surgery in COVID-19 patients: operational directives. World J Emerg Surg. 2020;15(1):25. doi: 10.1186/s13017-020-00307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coimbra R, Edwards S, Kurihara H, Bass GA, Balogh ZJ, Tilsed J, et al. European Society of Trauma and Emergency Surgery (ESTES) recommendations for trauma and emergency surgery preparation during times of COVID-19 infection. Eur J Trauma Emerg Surg. 2020;46(3):505–510. doi: 10.1007/s00068-020-01364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abbott TEF, Fowler AJ, Dobbs TD, Gibson J, Shahid T, Dias P, et al. Mortality after surgery with SARS-CoV-2 infection in England: a population-wide epidemiological study. Br J Anaesth. 2021;127(2):205–214. doi: 10.1016/j.bja.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abate SM, Mantefardo B, Basu B. Postoperative mortality among surgical patients with COVID-19: a systematic review and meta-analysis. Patient Saf Surg. 2020;14:37. doi: 10.1186/s13037-020-00262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Collaborative CovidSurg. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396(10243):27–38. doi: 10.1016/s0140-6736(20)31182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kahlberg A, Mascia D, Bellosta R, Attisani L, Pegorer M, Socrate AM, et al. Vascular surgery during COVID-19 emergency in Hub Hospitals of Lombardy: experience on 305 patients. Eur J Vasc Endovasc Surg. 2021;61(2):306–315. doi: 10.1016/j.ejvs.2020.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mi B, Chen L, Xiong Y, Xue H, Zhou W, Liu G. Characteristics and early prognosis of COVID-19 infection in fracture patients. J Bone Joint Surg Am. 2020;102(9):750–758. doi: 10.2106/JBJS.20.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.COVIDSurg Collaborative Delaying surgery for patients with a previous SARS-CoV-2 infection. Br J Surg. 2020;107(12):e601–e602. doi: 10.1002/bjs.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Doglietto F, Vezzoli M, Gheza F, Lussardi GL, Domenicucci M, Vecchiarelli L, et al. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID-19) in Italy. JAMA Surg. 2020;155(8):691–702. doi: 10.1001/jamasurg.2020.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Catton J, Banerjea A, Gregory S, Hall C, Crooks CJ, Lewis-Lloyd CA, et al. Planned surgery in the COVID-19 pandemic: a prospective cohort study from Nottingham. Langenbecks Arch Surg. 2021;406(7):2469–2477. doi: 10.1007/s00423-021-02207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jonker PKC, van der Plas WY, Steinkamp PJ, Poelstra R, Emous M, van der Meij W, et al. Perioperative SARS-CoV-2 infections increase mortality, pulmonary complications, and thromboembolic events: a Dutch, multicenter, matched-cohort clinical study. Surgery. 2021;169(2):264–274. doi: 10.1016/j.surg.2020.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.COVIDSurg Collaborative and GlobalSurg Collaborative Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia. 2021;76(6):748–758. doi: 10.1111/anae.15458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.NELA Project Team (2020) The impact of COVID-19 on emergency laparotomy-an interim report of the national emergency laparotomy audit, 23 March 2020–30 September 2020. RCoA: 1–31. https://www.nela.org.uk/downloads/COVID_analysis_08%20Mar%202021.pdf. Accessed 1 Mar 2022
- 111.Becke K. Anesthesia in children with a cold. Curr Opin Anaesthesiol. 2012;25(3):333–339. doi: 10.1097/ACO.0b013e3283534e80. [DOI] [PubMed] [Google Scholar]
- 112.Cohen MM, Cameron CB. Should you cancel the operation when a child has an upper respiratory tract infection? Anesth Analg. 1991;72:282–288. doi: 10.1213/00000539-199103000-00002. [DOI] [PubMed] [Google Scholar]
- 113.Bordet F, Allaouchiche B, Lansiaux S, Combet S, Pouyau A, Taylor P, et al. Risk factors for airway complications during general anaesthesia in paediatric patients. Paediatr Anaesth. 2002;12:762–769. doi: 10.1046/j.1460-9592.2002.00987.x. [DOI] [PubMed] [Google Scholar]
- 114.Canet J, Gallart L, Gomar C, Paluzie G, Vallès J, Castillo J, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anaesthesiol V. 2010;113(6):1338–1350. doi: 10.1097/ALN.0b013e3181fc6e0a. [DOI] [PubMed] [Google Scholar]
- 115.Søreide K, Hallet J, Matthews JB, Schnitzbauer AA, Line PD, Lai PBS, et al. Immediate and long-term impact of the COVID-19 pandemic on delivery of surgical services. Br J Surg. 2020;107(10):1250–1261. doi: 10.1002/bjs.11670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thoracic Surgery Outcomes Research Network Inc COVID-19 guidance for triage of operations for thoracic malignancies: a consensus statement from thoracic surgery outcomes research network. Ann Thorac Surg. 2020;110(2):692–696. doi: 10.1016/j.athoracsur.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Berger-Richardson D, Ko G, Hong NJL. Preparing for the renaissance: treating breast cancer during the COVID-19 pandemic and planning for a safe re-emergence to routine surgical care within a universal health care system. Curr Oncol. 2020;27(3):163–168. doi: 10.3747/co.27.6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/s1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li Y-k, Peng S, Li L-q, Wang Q, Ping W, Zhang N, et al. Clinical and transmission characteristics of Covid-19 – a retrospective study of 25 cases from a single thoracic surgery department. Curr Med Sci. 2020;40(2):1–6. doi: 10.1007/s11596-020-2176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan. China Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guan W-J Liang W-h Zhao Y Liang H-r Chen Z-s Li Y-m et al (2020) Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 55(5). 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed]
- 123.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020;382(26):2582. doi: 10.1056/NEJMc2021225. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 125.Guo W Li M Dong Y Zhou H Zhang Z Tian C et al (2020) Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev:e3319. 10.1002/dmrr.3319 [DOI] [PMC free article] [PubMed]
- 126.Feng Z, Yu Q, Yao S, Luo L, Zhou W, Mao X, et al. Early prediction of disease progression in COVID-19 pneumonia patients with chest CT and clinical characteristics. Nat Commun. 2020;11(1):4968. doi: 10.1038/s41467-020-18786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Meng Y, Wu P, Lu W, Liu K, Ma K, Huang L, et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. 2020;16(4):e1008520. doi: 10.1371/journal.ppat.1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jiao G Jingyi O Xueping Q Yusheng J Yaqiong C Lianxiong Y et al (2020) A tool to early predict severe corona virus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. Apr 16:ciaa443 [DOI] [PMC free article] [PubMed]
- 129.Westheim AJF, Bitorina AV, Theys J, Shiri-Sverdlov R. COVID-19 infection, progression, and vaccination: focus on obesity and related metabolic disturbances. Obes Rev. 2021;22(10):e13313. doi: 10.1111/obr.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sjögren L, Stenberg E, Thuccani M, Martikainen J, Rylander C, Wallenius V, et al. Impact of obesity on intensive care outcomes in patients with COVID-19 in Sweden-A cohort study. PLoS ONE. 2021;16(10):e0257891. doi: 10.1371/journal.pone.0257891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.DGCH/BDC und DGAI/BDA (2021) Empfehlungen von DGCH/BDC und DGAI/BDA zur Terminierung elektiver operativer Eingriffe nach Covid-19 Infektion und Impfung. https://www.dgai.de/alle-docman-dokumente/aktuelles/1696-stellungnahme-zu-operation-und-intervall-zu-covid19-impfung-oder-infektion-12-05-2021/file.html
- 132.COVIDSurg Collaborative and GlobalSurg Collaborative SARS-CoV-2 vaccination modelling for safe surgery to save lives: data from an international prospective cohort study. Br J Surg. 2021;108(9):1056–1063. doi: 10.1093/bjs/znab101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Limper U, Defosse J, Schildgen O, Wappler F. Perioperative risk evaluation in patients scheduled for elective surgery in close relation to their SARS-CoV-2 vaccination. Br J Anaesth. 2021;126(6):e225–e226. doi: 10.1016/j.bja.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sparnon T Hadfield M (2020) Guidance on delay to elective surgery post recovery from SARS-COV 2 infection (5 August 2020) https://www.surgeons.org/-/media/Project/RACS/surgeons-org/files/news/covid19-information-hub/Perioperative-Guidance-post-COVID-infection.pdf?rev=7592ce808e8c4ac19dc2c8856bde48fa%26hash=216BF43F414EA0293F1C717E4A79E43A
- 135.American Society of Anesthesiologists (ASA) and Anesthesia Patient Safety Foundation (APSF) (2021) ASA and APSFJoint Statement on Elective Surgery and Anesthesia for Patients after COVID-19 Infection (Updated March 9, 2021) https://www.asahq.org/about-asa/newsroom/news-releases/2021/03/asa-and-apsf-joint-statement-on-elective-surgery-and-anesthesia-for-patients-after-covid-19-infection-rv
- 136.Hsieh M-J, Lee W-C, Cho H-Y, Wu M-F, Hu H-C, Kao K-C, et al. Recovery of pulmonary functions, exercise capacity, and quality of life after pulmonary rehabilitation in survivors of ARDS due to severe influenza A (H1N1) pneumonitis. Influenza Other Respir Viruses. 2018;12(5):643–648. doi: 10.1111/irv.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yuan M, Yin W, Tao Z, Tan W, Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan. China PLoS One. 2020;15(3):e0230548. doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hajifathalian K, Sharaiha RZ, Kumar S, Krisko T, Skaf D, Ang B, et al. Development and external validation of a prediction risk model for short-term mortality among hospitalized U.S. COVID-19 patients: A proposal for the COVID-AID risk tool. PLoS One. 2020;15(9):e0239536. doi: 10.1371/journal.pone.0239536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tuty Kuswardhani RA, Henrina J, Pranata R, Lim AM, Lawrensia S, Suastika K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14(6):2103–2109. doi: 10.1016/j.dsx.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.COVIDSurg Collaborative Machine learning risk prediction of mortality for patients undergoing surgery with perioperative SARS-CoV-2: the COVIDSurg mortality score. Br J Surg. 2021;108(11):1274–1292. doi: 10.1093/bjs/znab183. [DOI] [PMC free article] [PubMed] [Google Scholar]