Abstract
In utero exposure to glucocorticoids in late gestation programs changes in cardiovascular function. The objective of this study was to determine the degree to which angiotensin II mediates sex-biased changes in autonomic function as well as basal and stress-responsive cardiovascular function following in utero glucocorticoid exposure. Pregnant rats were administered the synthetic glucocorticoid dexamethasone (Dex; 0.4 mg/kg/day sc) or vehicle on gestation days 18–21. Mean arterial pressure, heart rate, and heart rate variability (HRV) were measured via radiotelemetry in freely moving, conscious adult rats. To evaluate the impact of stress, rats were placed in a restraint tube for 20 min. In a separate cohort of rats, restraint stress was performed before and after chronic treatment with the angiotensin type 1 receptor antagonist, losartan (30 mg/kg/day ip). Frequency domain analysis of HRV was evaluated, and data were integrated into low-frequency (LF, 0.20–0.75 Hz) and high-frequency (HF, 0.75–2.00 Hz) bands. Prenatal Dex resulted in an exaggerated pressor and heart rate response to restraint in female offspring that was attenuated by prior losartan treatment. HF power was higher in vehicle-exposed female rats compared with Dex females. Following losartan, HF power was equivalent between female vehicle and Dex-exposed rats. In utero exposure to Dex produced female-biased alterations in stress-responsive cardiovascular function, which may be indicative of a reduction in parasympathetic activity. Moreover, these findings suggest this autonomic dysregulation may be mediated, in part, by long-term changes in renin-angiotensin signaling.
NEW & NOTEWORTHY Our findings reveal the involvement of angiotensin II on sex-selective cardiovascular function and autonomic changes in adult offspring exposed to dexamethasone during the last 4 days of gestation. We show that angiotensin II receptor blockade reverses the exaggerated pressor and heart rate response to acute restraint stress and the autonomic dysregulation observed in female, but not male, offspring exposed to dexamethasone in utero.
Keywords: autonomic nervous system, blood pressure, dexamethasone, prenatal programming, sex differences
INTRODUCTION
Adverse environmental experiences in utero have been shown to predispose individuals to the development of cardiovascular disease as adults. Connections between the in utero environment and long-term health were first described by David Barker in 1989 following observations showing a relationship between low birth weight and subsequent death rates from coronary heart disease (1). His studies showed that infants who were small at birth were at high risk for developing heart disease as adults. The results of these studies gave rise to the current concept of developmental origins of health and disease.
It is now well established that prenatal insults can impact cardiovascular function of the adult (2). The glucocorticoid dexamethasone (Dex) is used clinically during the prenatal period for women at risk for preterm labor to promote lung development and prevent respiratory distress syndrome (3). According to the CDC, among pregnancy complications, preterm labor is the most prevalent during the latter part of gestation and is implicated in 10% of all pregnancies (4). Current World Health Organization guidelines recommend administering Dex over four divided doses to women at risk of delivering preterm (3). Thus, there is a need to identify the long-term consequences of this treatment on adult offspring.
We previously showed that exposure to Dex over the last 4 days of gestation results in increased depressive- and anxiety-like behaviors (5), as well as decreases in core body temperature (6) in female, but not male offspring. O’Regan et al. (7) showed that prenatal Dex exposure throughout the last 7 days of gestation resulted in a greater pressor response to restraint and amphetamine injection in adult offspring (7). The effects of glucocorticoid excess are thought to be mediated by central mechanisms since glucocorticoids are readily found in neonatal rat brains following administration of synthetic corticosteroids due to the immature blood brain barrier (8). Prenatal studies of small-for-gestational-age fetuses also show altered heart rate variability (HRV) that was characterized by higher sympathetic control, suggesting a dysregulation of autonomic nervous system (ANS) control (9) of the cardiovascular system. Studies have further demonstrated the persistence of fetal effects on cardiac function and HRV into childhood (10–13) and adulthood (14–16). Such data implicate the ANS in the developing fetus as a target for glucocorticoids.
Prenatal (17, 18) Dex has been shown to increase the hypertensive response to angiotensin II (ANG II) infusion. Prenatal Dex exposure was also shown to increase liver angiotensinogen mRNA expression and increase plasma renin activity in adult female offspring (19). The role of enhanced renin angiotensin system (RAS) activity in cardiovascular control in offspring prenatally exposed to glucocorticoids is further supported by studies showing that acute ANG II type 1 receptor (AT1R) blockade lowered blood pressure and partially normalized parasympathetic dysfunction in adult male sheep (females not tested) (20). The degree to which the RAS may sex-selectively impact autonomic dysregulation and stress-responsive cardiovascular function induced by prenatal Dex remains unknown. The present study examined the sex-selective impact of 1) in utero glucocorticoid exposure on adult cardiovascular responses to restraint stress, 2) HRV as an index of ANS regulation, and 3) the impact of chronic AT1R antagonism on these outcomes. We hypothesized that prenatal glucocorticoid exposure would induce autonomic dysregulation that would be reversed by AT1R antagonism.
METHODS
Animals
Timed-pregnant (cohort 1, n = 12 and cohort 2, n = 12), Sprague–Dawley, naive breeders (Charles River Laboratories, Wilmington, MA) arrived at the University of Arizona, College of Medicine, Phoenix Animal Facility at gestation days (GD) 7–9. They were single housed with ad libitum food and water in a temperature and humidity-controlled environment on a standard 12-h:12-h light/dark cycle (lights on at 6:00 AM and off at 6:00 PM) and allowed to acclimate for 4 days before experimental manipulations. Dams were handled daily for 5 min on GD 14–17 to minimize stress associated with experimental manipulations. Dams were randomly assigned to receive either Dex (cohort 1, n = 6 and cohort 2, n = 7) or vehicle (cohort 1, n = 6 and cohort 2, n = 5). Dex (0.4 mg/kg/day sc) or vehicle (hydroxypropyl β cyclodextrin; Veh) was administered daily on GD 18–21, as previously described (21). The timing of prenatal Dex (GD 18–21) was based on our prior studies (5, 6) and optimized for consistency with clinical administration of Dex for women at risk of preterm labor at the end of the second and beginning of the third trimester. From the standpoint of neurodevelopment, GD 18–21 in a rat would correspond to late second/early third trimester in a human (22).
Litters were reduced to 10 (∼5 males and 5 females per litter) to minimize postnatal nutritional effects. Pups were weaned at 21 days and group housed by sex and based on weight (23). Rats were housed at a minimum of two per cage until they reached a weight that precluded pair housing; single housed rats were provided Institutional Animal Care and use Committee (IACUC)-approved enrichment. All assessments were performed in male and female adult offspring (2- to 3-mo old; with a maximum of 1 male and 1 female randomly chosen from each litter). Starting at 9 wk of age, stage of estrous cycle was determined daily in female rats via vaginal lavage and cytological analysis. All procedures were approved by the University of Arizona and Arizona State University IACUCs and cared for in accordance with recommendations in the National Institutes for Health Guide for the Care and Use of Laboratory Animals (8th ed.; Pub. No. 85-23, Revised 2011).
Radiotelemeter Implantation
Radiotelemetric devices [Model, cohort 1, PA-C40; or cohort 2, HD-S10; Data Sciences International (DSI), St. Paul, MN] were implanted into the abdominal aorta of adult male and female animals at ∼10 wk of age, as previously described (24, 25). Briefly, animals were anesthetized with isoflurane (VetOne, Boise, ID) and prepared for surgery using aseptic technique. A ventral midline abdominal incision was made, organs retracted, gauze moistened with sterile saline placed in the abdominal cavity, and the catheter inserted into the abdominal aorta and secured with tissue adhesive (Vetbond, 3 M Animal Care Products, St. Paul, MN). A cellulose fiber patch (DSI, St. Paul, MN) was secured with tissue adhesive. The abdominal cavity was flooded with warm lactated ringer’s solution, and the transmitter was placed into the abdomen. The abdominal wall was closed with nonabsorbable suture, while incorporating the rib of the transmitter. Buprenorphine SR (1 mg/kg sc, ZooPharm, Windsor, CO) was administered 30–45 min before the start of surgery for general analgesia, and Marcaine (Med-Vet International, Mettawa, IL) was applied to the skin suture line postsurgery for topical analgesia. Clavamox (Zoetis, Parsippany, NJ) was administered daily for 4 days to prevent infection. Animals were single housed and allowed to recover for 1 wk before experimental manipulations.
Telemetry, Measurement of Cardiovascular Parameters, and Acute Restraint Stress
Cardiovascular parameters, including mean arterial pressure (MAP) and heart rate (HR), were collected throughout the experiment. Data were collected using Dataquest ART (version 4.2). HRV was calculated with Kubios HRV analysis software (version 3.2.0), using the interbeat interval acquired from blood pressure waveforms. Frequency domain analysis of HRV was evaluated, and data were integrated into low-frequency (LF, 0.20–0.75 Hz) and high-frequency (HF, 0.75–2.00 Hz) bands, as well as the ratio of LF to HF. HF has been described as corresponding to parasympathetic control, whereas LF has been described to be comprised of both parasympathetic and sympathetic inputs (26, 27). The ratio of LF to HF has been considered an index of sympathetic control. Baseline parameters were recorded over 2 days in male animals and 5 days in female animals to capture each day of the estrous cycle, with sampling for 2 min every 2 h.
Circadian rhythm.
To establish whether basal pressure is altered by prenatal exposure to Dex by sex, we analyzed blood pressure during the dark and light cycles. Data were analyzed using the means of measurements taken at 12:00, 2:00, and 4:00 in the AM and PM from a single day to capture pressures during dark and light cycles, respectively. Times were chosen to avoid periods where other rats in the room underwent stress testing, and to capture each day of the estrous cycle, which we indicate to begin at midnight on a given day. When assessing sex differences, female data on diestrus were used.
Acute Stress—Cohort 1.
On the day of the stress test, pressure and heart rate sampling was changed to a 1-min duration every 5 min. To evaluate the effect of prenatal Dex on adult cardiovascular function in response to an acute stressor, animals were placed in a round, plexiglass restraint tube in their home cage for 20 min. Rats were then removed from the tube and pressure and heart rate recording continued through a 2-h recovery period. To minimize the impact of gonadal steroids on stress responsiveness, females were tested on diestrus, when estradiol levels are low (Fig. 1). The delta change from the average of the five data points (i.e., over a 20-min period) preceding the restraint for MAP and HR was assessed throughout the stress and recovery (SR) periods. Following the recovery period, rats were euthanized by CO2 inhalation followed by decapitation.
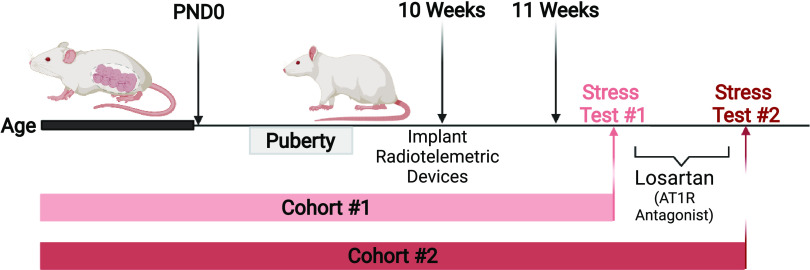
Figure 1.
Timeline for experimental protocols for cohorts 1 and 2. Created with BioRender.com and published with permission.
Impact of ANG II Receptor Type 1 Blocker on Acute Stress Responses—Cohort 2
In a separate cohort of animals, adult cardiovascular function at baseline and in response to an acute stressor was recorded as described in Acute Stress–Cohort 1 (stress test 1). To determine the degree to which stress responses are mediated by ANG II, rats were administered the AT1R antagonist losartan (30 mg/kg per day, ip) beginning the day after the first restraint test (Fig. 1). This dose of losartan was chosen based on prior studies (28) showing it induces a maximal blood pressure lowering response in Sprague-Dawley rats. After 5 days, baseline assessments of mean arterial pressure, heart rate, and HRV were performed. Baseline values were obtained over a 24-h period in males, and over 4–5 days in females to obtain measures at each stage of the estrous cycle. Subsequently, rats were placed in the restraint tube for 20 min followed by a 2-h recovery (stress test 2), as described in Acute Stress–Cohort 1 (females stressed on diestrus). Following the recovery period, rats were euthanized by CO2 inhalation followed by decapitation.
Statistical Analysis
For all line graphs, linear mixed models were implemented to ascertain mean differences in the change in MAP and HR from baseline over the stress/recovery time period. Covariates within these models include the prenatal treatment (Veh, Dex), losartan treatment (PRE, POST), time period (stress, recovery), sex (male, female), and the interaction between sex and losartan treatment. The subject ID is the random effect. Stratification analyses by sex, prenatal treatment, and time period assessed whether interactions with the remaining covariates were present. In addition, stratification analyses of Dex treatment and time period were further stratified by sex to assess interactions within each specific stratum. All P values were two sided and P < 0.05 was considered statistically significant.
Two-way ANOVA was used to measure area under the curve for changes in MAP and HR using Prism version 8 (GraphPad Software; San Diego, CA). Mixed effects analysis was used to evaluate the contributions of Dex, sex, and time of day for baseline assessments of MAP. When evaluating basal changes across the estrous cycle at baseline, mixed effects analysis was used with Dex, estrous cycle, and time of day for the independent variables. Two-way ANOVA was used to determine changes in HRV parameters by sex and Dex (GraphPad Prism). In response to the AT1R antagonist, two-way ANOVA was used to evaluate changes in HRV parameters by Dex and losartan for each sex. A P < 0.05 was considered statistically significant, and data are presented as means ± SE.
RESULTS
Baseline Responses: Light versus Dark Cycle (Cohort 1)
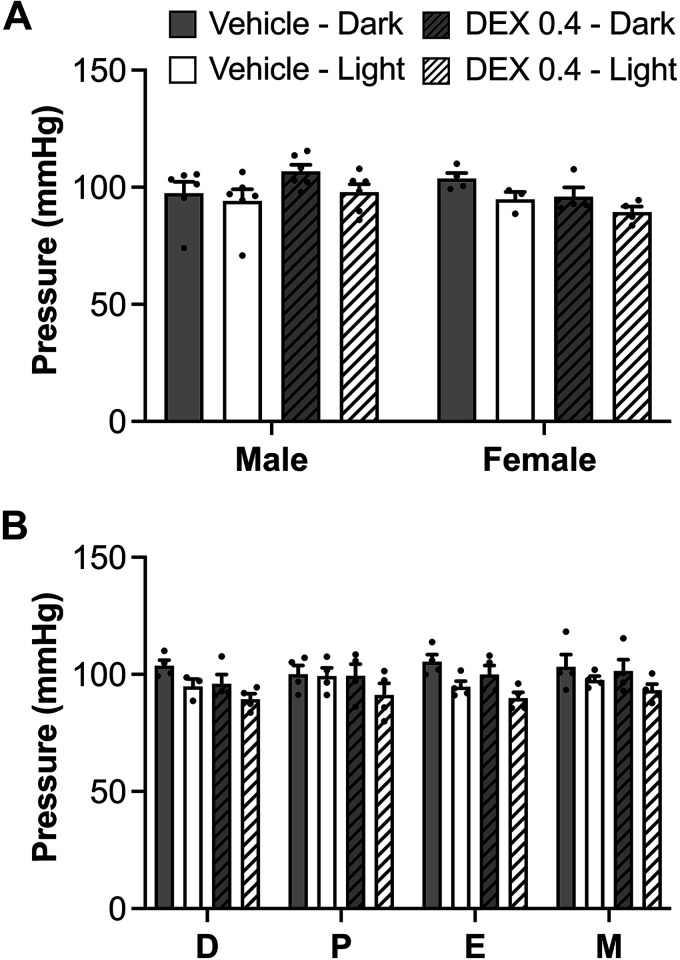
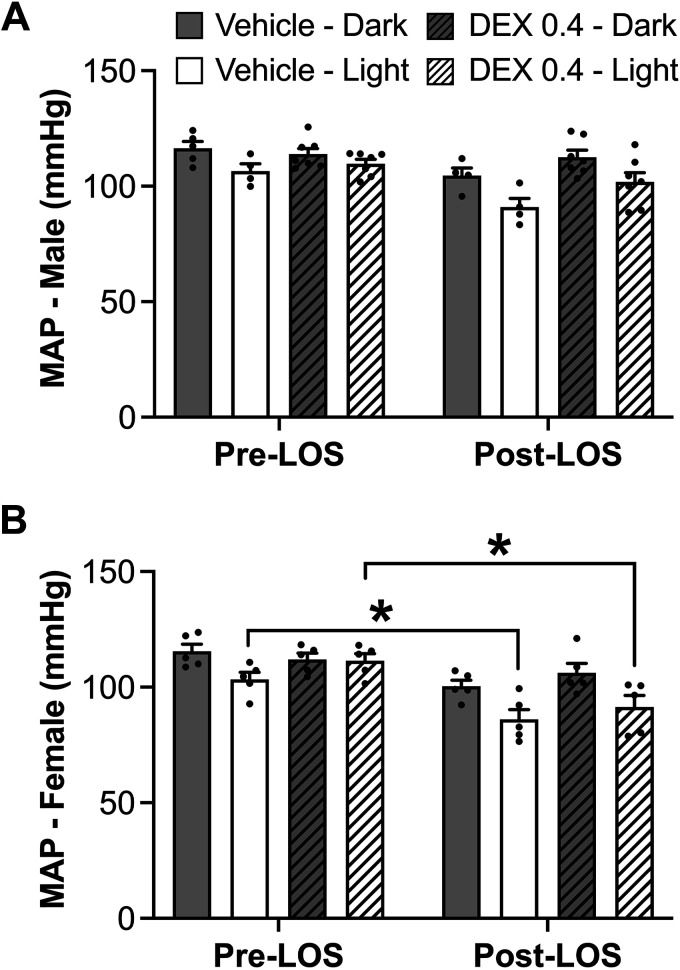
MAP.
Comparison of males with females on diestrus did not reveal sex differences in MAP or changes resulting from prenatal Dex exposure (Fig. 2A). Based on mixed-effects analysis, there was an overall main effect of time of day (P = 0.013), such that rats exhibited the expected diurnal variation with MAP reduced during the light cycle compared with the dark cycle (Fig. 2A). When assessed across the estrous cycle, there was a significant reduction in MAP (by mixed-effects analysis, P < 0.0001) during the day and a tendency toward an overall reduction in MAP in Dex-exposed rats, an effect that was most evident during the day when animal activity is low (P = 0.051). Overall, MAP was not changed across the estrous cycle (Fig. 2B).
Figure 2.
A: mean arterial pressure (MAP) in male and diestrus female rats during light and dark cycles. B: MAP in female rats across the estrous cycle during the light and dark cycles. In both cases, there was a significant main effect of time of day showing reduced MAP during the light cycle (P < 0.0001). n = 4–6 per group, means ± SE. D, diestrus; E, estrus; M, metestrus; P, proestrus.
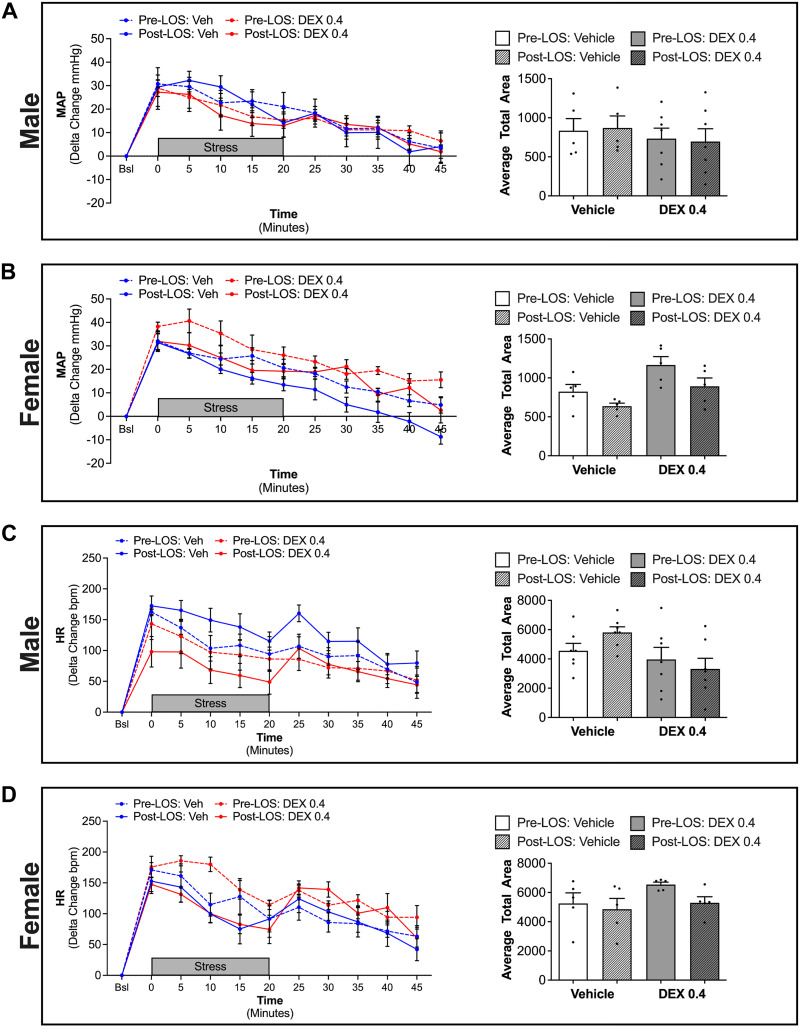
Pressor and HR Responses to Acute Restraint Stress (Cohort 1)
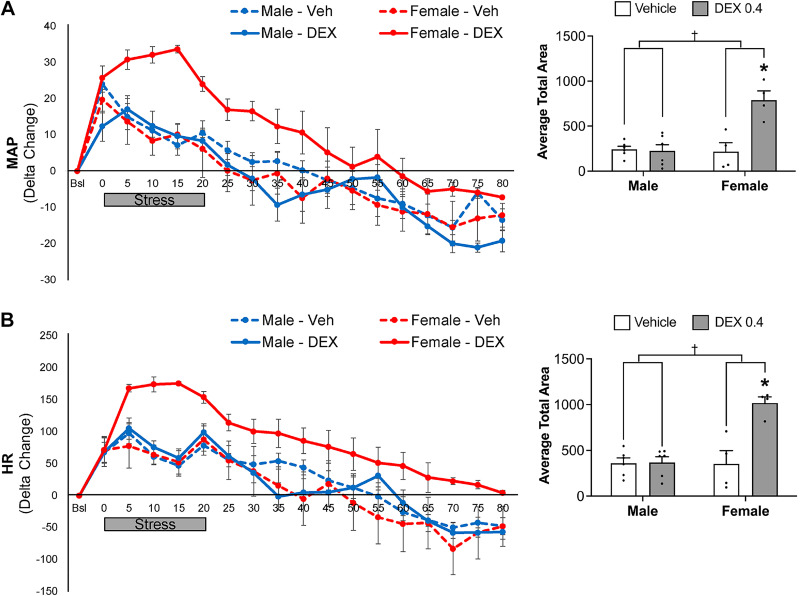
Cardiovascular responses were evaluated in response to acute restraint stress. Female rats exposed to Dex in utero displayed an exaggerated pressor (P = 0.004) and HR (P = 0.004) response to restraint, when compared with vehicle-exposed females, which persisted throughout the recovery period (Fig. 3). In contrast, in utero Dex exposure did not significantly impact pressor or HR responses to restraint in males (Fig. 3). Notably, the relative pressor and HR response was not different between vehicle-exposed male and female rats. Area under the curve analyses comparing the impact of in utero Dex exposure on stress-mediated cardiovascular responses revealed a significant prenatal exposure × sex interaction for both MAP (P = 0.001) and HR (P = 0.001).
Figure 3.
Mean arterial pressure (MAP; A) and heart rate (HR; B) during restraint and recovery periods. Line graphs depict delta change in MAP and HR from the average of 5 successive time points immediately before restraint. Gray bar depicts the 20-min stress period. Bar graphs depict the average area under the curve for MAP and HR. *P < 0.05 vs. vehicle female, †P < 0.05 sex × prenatal exposure interaction. n = 4–6 per group, means ± SE.
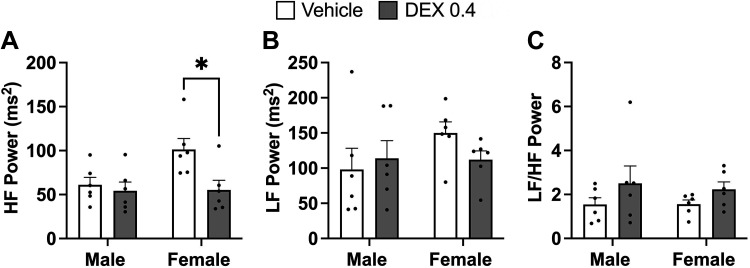
HRV.
HRV was assessed to determine the impact of in utero exposure to Dex on cardiac autonomic function (Fig. 4). Dex female HF power is significantly lower (P = 0.034) at baseline compared with vehicle-exposed females (Fig. 4A). Neither LF power nor the ratio of LF to HF was significantly influenced by prenatal Dex or by sex (Fig. 4, B and C).
Figure 4.
Assessments of high-frequency (HF) power (A), low-frequency (LF) power (B), and the ratio of LF to HF (C) in vehicle and dexamethasone (Dex)-exposed male and female rats was evaluated by two-way ANOVA. *P < 0.05 vs. vehicle female. n = 6 per group, means ± SE.
Baseline Responses—AT1R Antagonist: Light versus Dark Cycle (Cohort 2)
MAP.
The expected diurnal variation (P < 0.0001) was observed in males, based on three-way ANOVA, and there was an overall main effect of losartan to decrease MAP (P = 0.013; Fig. 5A). Although there was no significant main effect of Dex (P = 0.069), there was a significant interaction between prenatal exposure and losartan (P = 0.037), whereby vehicle-exposed males were more responsive to losartan than Dex-exposed males. Based on three-way ANOVA, female MAP (Fig. 5B) was significantly reduced by losartan (P = 0.003) and was significantly lower during the light cycle (P < 0.0001). Post hoc analyses revealed that losartan significantly reduced MAP only during the light cycle, when activity is lowest for both vehicle- (P = 0.026) and Dex-exposed (P = 0.007) rats. There was a two-way interaction for light cycle × losartan that did not reach statistical significance in both sexes (male, P = 0.064 and female, P = 0.052), whereby losartan treatment resulted in a greater change in MAP between light and dark cycles (Fig. 5).
Figure 5.
Mean arterial pressure (MAP) assessed during dark and light cycles in male (A) and female (B) rats exposed to vehicle or dexamethasone (Dex) in utero before and after losartan (Los) treatment. Data were evaluated by three-way ANOVA. A: main effect of Los (P = 0.0128), main effect of light cycle (P < 0.0001), and prenatal exposure × Los (P = 0.0374). B: main effect of Los, P = 0.0033; and main effect of light cycle, P = 0.0004. *P < 0.05 vs. pre-Los. n = 5–7 per group, means ± SE.
Pressor and HR Responses to Acute Restraint Stress—AT1R Antagonist (Cohort 2)
In male rats, treatment with losartan had no impact on the MAP response to restraint stress, regardless of prenatal exposure (Fig. 6A). Using linear mixed model analyses, female rats showed an overall significant effect of both losartan (P < 0.001) and Dex (P = 0.007) on MAP (Fig. 6A). During the period of restraint, losartan reversed the enhanced pressor response observed in Dex-exposed females. However, during the recovery period, although the MAP of both vehicle- and Dex-exposed female rats was significantly reduced by losartan, the MAP of Dex-exposed rats was significantly higher than in the vehicle rats. Area-under-the-curve analysis revealed main effects of Dex (P = 0.023) and losartan treatment (P = 0.005) on MAP in female rats (Fig. 6A).
Figure 6.
Mean arterial pressure (MAP; A and B) and heart rate (HR; C and D) during restraint and recovery periods before and after losartan (Los) treatment. Line graphs depict delta change in MAP and HR from the average of five successive time points immediately before restraint. Gray bar depicts the 20-min stress period. Bar graphs depict the average area under the curve for MAP and HR. Linear mixed model analysis of MAP in females (line graphs in B) revealed effects of Los (P < 0.001) and Dex (P = 0.007). Two-way ANOVA of area under the curve analysis of female MAP identified main effects of Los (P = 0.005) and Dex (P = 0.023). n = 5–7 per group, means ± SE.
Based on linear mixed model analyses, when stratified by prenatal exposure, there was a significant effect of losartan treatment to increase HR in vehicle-exposed males (P < 0.001). No significant effect was observed in Dex-exposed rats (P = 0.117; Fig. 6B). In female rats, linear mixed model analyses revealed an overall significant effect of Dex (P = 0.001) and losartan (P < 0.001). During the restraint period, losartan treatment of Dex-exposed female rats completely reversed the enhanced HR response (Fig. 6B). Immediately following the period of restraint there was a marked increase in HR that corresponds with a higher degree of activity of the rats as they exit the restraint tube.
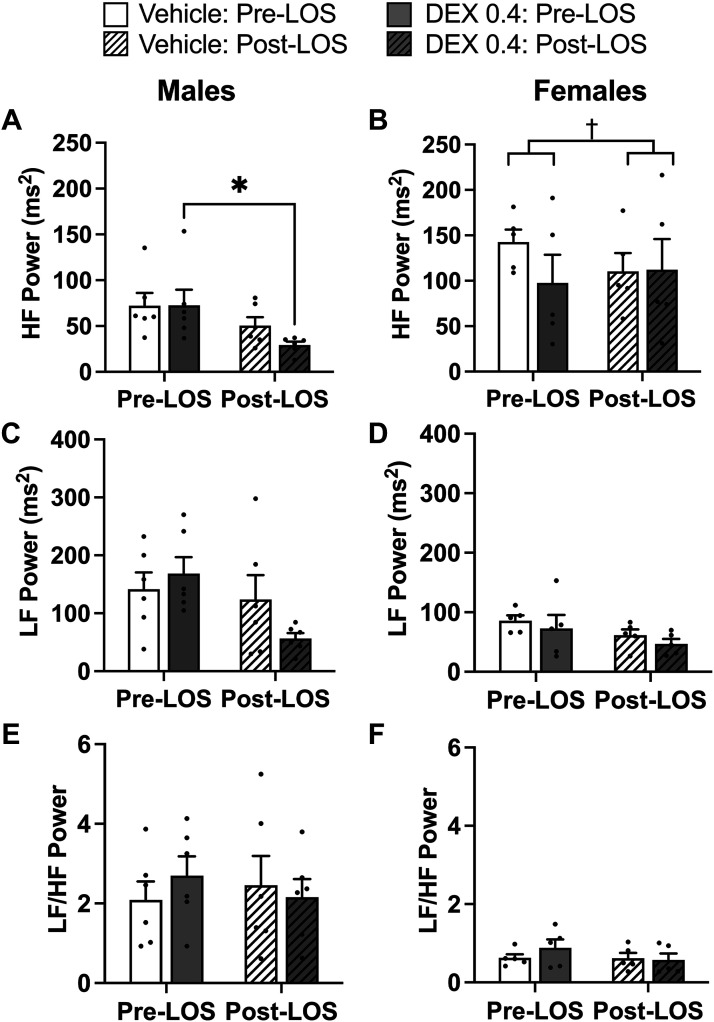
HRV—AT1R antagonist.
In males, losartan significantly reduced HF power only in Dex-exposed rats (P = 0.021; Fig. 7). In females, there was a differential impact of losartan on Dex- and vehicle-exposed rats (two-way interaction; prenatal exposure × losartan treatment, P = 0.004), such that at the end of the study HF power was similar between the prenatal exposure groups (Fig. 7). LF power and the ratio of LF to HF were not significantly impacted by prenatal exposure or losartan in either males or females (Fig. 7).
Figure 7.
Assessments of high-frequency (HF) power (A and B), low-frequency (LF) power (C and D), and the ratio of LF to HF (E and F) in vehicle and dexamethasone (Dex)-exposed male and female rats was evaluated by two-way ANOVA. *P < 0.05 vs. Male Dex, †prenatal exposure × losartan interaction (P = 0.0042). n = 5–6 per group, means ± SE.
DISCUSSION
The major findings of the present study are 1) prenatal glucocorticoid exposure produces exaggerated pressor and HR response to acute restraint stress in female, but not male, offspring; 2) prenatal glucocorticoid exposure reduces the high-frequency power component of female heart rate variability, suggesting a withdrawal of parasympathetic drive; and 3) AT1R antagonism attenuates the enhanced pressor and heart rate response to restraint stress and normalizes heart rate variability in female rats exposed to Dex in utero.
In the present study, prenatal exposure to Dex did not significantly impact baseline MAP in adult offspring, compared with vehicle-exposed rats. In some instances, prenatal glucocorticoids during late gestation in rats has been shown to promote hypertension (19, 29–32) or hypotension in adult offspring (7). Reported differences in blood pressure may be explained by factors such as timing, duration, and dose of glucocorticoid administered prenatally, as well as age of offspring and method for blood pressure assessment. Studies investigating both male and female offspring following in utero glucocorticoid exposure reported elevated basal MAP in Dex-exposed animals in both sexes (29, 31, 33). O’Regan et al. (19) initially showed that prenatal Dex resulted in elevated SBP in female offspring but later determined that the hypertensive response observed in females was likely a consequence of the stress induced by restraint for tail cuff plethysmography, suggesting a susceptibility to stress-induced hypertension in females. They subsequently demonstrated, using radiotelemetry, a mild reduction in arterial pressure in Dex-exposed rats of both sexes compared with vehicle, with a greater hypertensive response to restraint in Dex-exposed rats (7). Similarly, Rogers et al. (32) demonstrated a greater elevation in blood pressure using tail cuff plethysmography than when measured by radiotelemetry (telemetry results reported in males only). Thus, the lack of basal hypertension observed in our study may be due, at least in part, to the use of radiotelemetry, as it minimizes stress and provides a direct measurement of arterial pressure in a conscious and freely moving animal in its home cage.
We demonstrate that females, but not males, exposed to Dex in utero displayed an exaggerated pressor and heart rate response to restraint stress. The relative impact of late gestation Dex on stress-responsive cardiovascular function of adult male and female offspring is not consistently described in the literature. Igosheva et al. (34) demonstrated that male and female offspring of rats stressed with restraint, light and heat over GD 15–21 displayed a greater blood pressure response to restraint stress compared with controls. O’Regan et al. (7) showed that prenatal Dex administered over GD 15–21 resulted in an exaggerated elevation in arterial pressure in response to restraint and to amphetamine stimulation in both male and female offspring at 8–10 mo of age. In addition, they demonstrated that mesenteric arteries isolated from male and female rats exposed to Dex in utero were more sensitive to norepinephrine stimulation (7). Differences in timing and duration of Dex exposure (7, 19, 29–32, 34) may explain the lack of an exaggerated stress response in male animals in the present study. Specifically, Dex administered between GD 15 and 21 has been shown to reduce the prenatal testosterone surge that typically begins between GD 16 and 17 and peaks around GD 17–18 (35). Testosterone has been shown to play an important organizational role in the development of the hypothalamic pituitary adrenal axis as neonatal gonadectomy results in exaggerated corticosterone release in response to restraint stress in adulthood (36, 37). Thus, it may be that administration of Dex after the initiation of this critical window, as in the present study, preserves, at least in part, the protective effect of testosterone in mitigating the impact of prenatal Dex on stress-responsive cardiovascular effects. Furthermore, the timing of blood pressure measurement may account for sex-specific variations in pressor responses. O’Regan et al. (7) observed elevated stress-responsive MAP in 8–10-mo-old offspring. Rogers et al. (32) showed basal hypertensive responses in females beginning at 8–9 mo of age, but not at 2 mo.
The exaggerated stress-induced pressor and HR responses in female rats exposed to Dex in utero may be due to a dysregulation of the autonomic nervous system. We showed that vehicle-exposed females exhibit a tendency toward higher HF power when compared with males (P = 0.0811). The HF component of HRV is a well-established marker of parasympathetic activity (26, 27, 38, 39). Our findings are thus consistent with the literature showing that females have a greater reliance on parasympathetic drive than males (40, 41). Prenatal Dex produced female-specific changes in heart rate variability resulting in lower HF power when compared with vehicle-exposed female rats. This decrease in parasympathetic activity may underlie the exaggerated cardiovascular response we reported in females. Identification of the mechanisms underlying the altered stress response is important as vagal dysregulation has been shown to be a significant risk factor for heart disease (42, 43).
The present study investigated the degree to which ANG II signaling mediates the altered cardiovascular responses to stress and autonomic function. ANG II modulates central cardiovascular responses to stress by stimulating sympathetic nervous system outflow centrally and norepinephrine release peripherally (44). Thus, alterations in stress-responsive MAP and heart rate in adulthood may be due to changes in the renin angiotensin system (RAS). Prenatal Dex exposure has been shown to elevate the hypertensive response to ANG II in female spontaneously hypertensive rats (17). Furthermore, prenatal glucocorticoid exposure has been shown to increase renal AT1R expression (45–47), renal ANG II production (43), and plasma renin activity (19). However, whether RAS activity increased or decreased in male versus female offspring varied with the nature or timing of the exposure. In the present study, we show that RAS blockade with the AT1R antagonist losartan ameliorates the exaggerated pressor and heart rate response to restraint in females exposed to Dex over the last 4 days of gestation. Moreover, losartan normalizes heart rate variability in Dex-exposed female rats such that HF power is no longer significantly different from vehicle-exposed rats. This suggests that altered ANG II signaling in Dex-exposed females may be driving lower HF power leading to an exaggerated pressor and HR response to restraint.
It remains to be determined whether the ANG II-mediated hyperresponsiveness to stress is due to direct actions of ANG II acting locally to stimulate norepinephrine release from nerve terminals or centrally, by binding AT1 receptors in the subfornical organ, an important activator of central autonomic signaling, which lies outside of the blood brain barrier and thus accessible to losartan. Activation of ANG II receptors within the subfornical has been shown to stimulate the sympathetic and inhibit the parasympathetic neural pathways (48). In addition, changes not just in expression but also in sensitivity to AT1 and/or adrenergic receptor signaling may result in enhanced ANG II-mediated stress responsiveness.
Taken together, our findings demonstrate that exposure to Dex over the last 4 days of gestation produces sex-specific changes in stress-responsive cardiovascular function that may be due, in part, to altered ANG II signaling and a dysregulation of autonomic function. Specifically, prenatal Dex resulted in exaggerated cardiovascular responses to restraint in female offspring that was reversed by treatment with the AT1R antagonist losartan. Moreover, reductions in high-frequency power in Dex-exposed females suggest impaired parasympathetic signaling that was restored by losartan treatment.
The sex difference revealed in the present study suggests a potential role for gonadal steroid hormones in mediating these effects. Whether these are due to organizational or activational effects is yet to be determined. Prenatal Dex is administered to women at risk of preterm birth, which affects 10% of all pregnancies in the United States (4). The use of Dex has resulted in a significant improvement in neonatal survival (49, 50). However, the long-term consequences of this exposure may result in increased risk of cardiovascular disease later in life. Importantly, Dex is also administered to pregnant women who are COVID positive and require supplemental oxygen (51, 52). Understanding the impact of prenatal Dex exposure on cardiovascular programing is critical for evaluating the future risk of disease and developing appropriate preventative and therapeutic strategies.
GRANTS
This work was supported by the National Institute of Mental Health Grant U54MH118919 (to T.M.H. and R.J.H.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.J.H. and T.M.H. conceived and designed research; L.M., B.H., and D.L.C. performed experiments; L.M., B.H., D.L.C., P.K., and T.M.H. analyzed data; L.M., R.J.H., and T.M.H. interpreted results of experiments; L.M. prepared figures; L.M. drafted manuscript; B.H., D.L.C., and T.M.H. edited and revised manuscript; L.M., B.H., D.L.C., P.K., and T.M.H. approved final version of manuscript.
Footnotes
†Deceased 27 August 2021.
REFERENCES
- 1.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 298: 564–567, 1989. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krause B, Sobrevia L, Casanello P. Epigenetics: new concepts of old phenomena in vascular physiology. Curr Vasc Pharmacol 7: 513–520, 2009. doi: 10.2174/157016109789043883. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO Recommendations on Interventions to Improve Preterm Birth Outcomes. Geneva: World Health Organization, 2015. [PubMed] [Google Scholar]
- 4.Osterman MH, Martin JA, Driscoll AK, Valenzuela CP. Births: final data for 2020. Natl Vital Stat Rep 70: 1–50, 2022. [PubMed] [Google Scholar]
- 5.Hiroi R, Carbone DL, Zuloaga DG, Bimonte-Nelson HA, Handa RJ. Sex-dependent programming effects of prenatal glucocorticoid treatment on the developing serotonin system and stress-related behaviors in adulthood. Neuroscience 320: 43–56, 2016. doi: 10.1016/j.neuroscience.2016.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone DL, Zuloaga DG, Lacagnina AF, McGivern RF, Handa RJ. Exposure to dexamethasone during late gestation causes female-specific decreases in core body temperature and prepro-thyrotropin-releasing hormone expression in the paraventricular nucleus of the hypothalamus in rats. Physiol Behav 108: 6–12, 2012. doi: 10.1016/j.physbeh.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Prenatal dexamethasone 'programmes' hypotension, but stress-induced hypertension in adult offspring. J Endocrinol 196: 343–352, 2008. doi: 10.1677/JOE-07-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arya V, Demarco VG, Issar M, Hochhaus G. Contrary to adult, neonatal rats show pronounced brain uptake of corticosteroids. Drug Metab Dispos 34: 939–942, 2006. doi: 10.1124/dmd.105.007419. [DOI] [PubMed] [Google Scholar]
- 9.Galland BC, Taylor BJ, Bolton DP, Sayers RM. Heart rate variability and cardiac reflexes in small for gestational age infants. J Appl Physiol (1985) 100: 933–939, 2006. doi: 10.1152/japplphysiol.01275.2005. [DOI] [PubMed] [Google Scholar]
- 10.Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Dev Psychobiol 31: 125–135, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 11.Thomas PW, Haslum MN, MacGillivray I, Golding MJ. Does fetal heart rate predict subsequent heart rate in childhood? Early Hum Dev 19: 147–152, 1989. doi: 10.1016/0378-3782(89)90125-4. [DOI] [PubMed] [Google Scholar]
- 12.DiPietro JA, Hodgson DM, Costigan KA, Johnson TR. Fetal antecedents of infant temperament. Child Dev 67: 2568–2583, 1996. [PubMed] [Google Scholar]
- 13.Rakow A, Katz-Salamon M, Ericson M, Edner A, Vanpée M. Decreased heart rate variability in children born with low birth weight. Pediatr Res 74: 339–343, 2013. doi: 10.1038/pr.2013.97. [DOI] [PubMed] [Google Scholar]
- 14.Ward AM, Syddall HE, Wood PJ, Chrousos GP, Phillips DI. Fetal programming of the hypothalamic-pituitary-adrenal (HPA) axis: low birth weight and central HPA regulation. J Clin Endocrinol Metab 89: 1227–1233, 2004. doi: 10.1210/jc.2003-030978. [DOI] [PubMed] [Google Scholar]
- 15.Jones A, Beda A, Ward AM, Osmond C, Phillips DI, Moore VM, Simpson DM. Size at birth and autonomic function during psychological stress. Hypertension 49: 548–555, 2007. doi: 10.1161/01.HYP.0000257196.13485.9b. [DOI] [PubMed] [Google Scholar]
- 16.Ward AM, Moore VM, Steptoe A, Cockington RA, Robinson JS, Phillips DI. Size at birth and cardiovascular responses to psychological stressors: evidence for prenatal programming in women. J Hypertens 22: 2295–2301, 2004. doi: 10.1097/00004872-200412000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Hadoke PW, Lindsay RS, Seckl JR, Walker BR, Kenyon CJ. Altered vascular contractility in adult female rats with hypertension programmed by prenatal glucocorticoid exposure. J Endocrinol 188: 435–442, 2006. doi: 10.1677/joe.1.06506. [DOI] [PubMed] [Google Scholar]
- 18.Loria AS, Pollock DM, Pollock JS. Early life stress sensitizes rats to angiotensin II-induced hypertension and vascular inflammation in adult life. Hypertension 55: 494–499, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am J Physiol Endocrinol Physiol 287: E863–E870, 2004. doi: 10.1152/ajpendo.00137.2004. [DOI] [PubMed] [Google Scholar]
- 20.Shaltout HA, Rose JC, Figueroa JP, Chappell MC, Diz DI, Averill DB. Acute AT(1)-receptor blockade reverses the hemodynamic and baroreflex impairment in adult sheep exposed to antenatal betamethasone. Am J Physiol Heart Circ Physiol 299: H541–H547, 2010. doi: 10.1152/ajpheart.00100.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carbone DL, Zuloaga DG, Hiroi R, Foradori CD, Legare ME, Handa RJ. Prenatal dexamethasone exposure potentiates diet-induced hepatosteatosis and decreases plasma IGF-I in a sex-specific fashion. Endocrinology 153: 295–306, 2012. doi: 10.1210/en.2011-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology 28: 931–937, 2007. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixon A, Maric C. 17β-estradiol attenuates diabetic kidney disease by regulating extracellular matrix and transforming growth factor-β protein expression and signaling. Am J Physiol Renal Physiol 293: F1678–F1690, 2007. doi: 10.1152/ajprenal.00079.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woolard J, Hale TM, Bushfield TL, Adams MA. Persistent lowering of arterial pressure after continuous and intermittent therapy. J Hypertens 21: 813–820, 2003. doi: 10.1097/00004872-200304000-00026. [DOI] [PubMed] [Google Scholar]
- 25.Biwer LA, Broderick TL, Xu H, Carroll C, Hale TM. Protection against L-NAME-induced reduction in cardiac output persists even after cessation of angiotensin-converting enzyme inhibitor treatment. Acta Physiol (Oxf) 207: 156–165, 2013. doi: 10.1111/j.1748-1716.2012.02474.x. [DOI] [PubMed] [Google Scholar]
- 26.Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Cohen RJ. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol Heart Circ Physiol 248: H151–H153, 1985. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 27.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol 141: 122–131, 2010. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 28.Banting JD, Friberg P, Adams MA. Acute hypertension after nitric oxide synthase inhibition is mediated primarily by increased endothelin vasoconstriction. J Hypertens 14: 975–981, 1996. doi: 10.1097/00004872-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Woods LL, Weeks DA. Prenatal programming of adult blood pressure: role of maternal corticosteroids. Am J Physiol Regul Integr Comp Physiol 289: R955–R962, 2005. doi: 10.1152/ajpregu.00455.2004. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen P, Khurana S, Peltsch H, Grandbois J, Eibl J, Crispo J, Ansell D, Tai TC. Prenatal glucocorticoid exposure programs adrenal PNMT expression and adult hypertension. J Endocrinol 227: 117–127, 2015. doi: 10.1530/JOE-15-0244. [DOI] [PubMed] [Google Scholar]
- 31.Lamothe J, Khurana S, Tharmalingam S, Williamson C, Byrne CJ, Lees SJ, Khaper N, Kumar A, Tai TC. Oxidative stress mediates the fetal programming of hypertension by glucocorticoids. Antioxidants (Basel) 10: 531, 2021. doi: 10.3390/antiox10040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogers JM, Ellis-Hutchings RG, Grey BE, Zucker RM, Norwood J Jr, Grace CE, Gordon CJ, Lau C. Elevated blood pressure in offspring of rats exposed to diverse chemicals during pregnancy. Toxicol Sci 137: 436–446, 2014. doi: 10.1093/toxsci/kft248. [DOI] [PubMed] [Google Scholar]
- 33.Dodic M, Abouantoun T, O’Connor A, Wintour EM, Moritz KM. Programming effects of short prenatal exposure to dexamethasone in sheep. Hypertension 40: 729–734, 2002. doi: 10.1161/01.HYP.0000036455.62159.7E. [DOI] [PubMed] [Google Scholar]
- 34.Igosheva N, Klimova O, Anishchenko T, Glover V. Prenatal stress alters cardiovascular responses in adult rats. J Physiol 557: 273–285, 2004. doi: 10.1113/jphysiol.2003.056911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lalau JD, Aubert ML, Carmignac DF, Grégoire I, Dupouy JP. Reduction in testicular function in rats. II. Reduction by dexamethasone in fetal and neonatal rats. Neuroendocrinology 51: 289–293, 1990. doi: 10.1159/000125352. [DOI] [PubMed] [Google Scholar]
- 36.McCormick CM, Furey BF, Child M, Sawyer MJ, Donohue SM. Neonatal sex hormones have 'organizational' effects on the hypothalamic-pituitary-adrenal axis of male rats. Brain Res Dev Brain Res 105: 295–307, 1998. doi: 10.1016/s0165-3806(97)00155-7. [DOI] [PubMed] [Google Scholar]
- 37.Bingham B, Viau V. Neonatal gonadectomy and adult testosterone replacement suggest an involvement of limbic arginine vasopressin and androgen receptors in the organization of the hypothalamic-pituitary-adrenal axis. Endocrinology 149: 3581–3591, 2008. doi: 10.1210/en.2007-1796. [DOI] [PubMed] [Google Scholar]
- 38.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation 84: 482–492, 1991. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 39.Appel ML, Berger RD, Saul JP, Smith JM, Cohen RJ. Beat to beat variability in cardiovascular variables: noise or music? J Am Coll Cardiol 14: 1139–1148, 1989. doi: 10.1016/0735-1097(89)90408-7. [DOI] [PubMed] [Google Scholar]
- 40.Ryan SM, Goldberger AL, Pincus SM, Mietus J, Lipsitz LA. Gender- and age-related differences in heart rate dynamics: are women more complex than men? J Am Coll Cardiol 24: 1700–1707, 1994. doi: 10.1016/0735-1097(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 41.Evans JM, Ziegler MG, Patwardhan AR, Ott JB, Kim CS, Leonelli FM, Knapp CF. Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. J Appl Physiol (1985) 91: 2611–2618, 2001. doi: 10.1152/jappl.2001.91.6.2611. [DOI] [PubMed] [Google Scholar]
- 42.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 213: 220–222, 1981. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 43.Dagan A, Gattineni J, Habib S, Baum M. Effect of prenatal dexamethasone on postnatal serum and urinary angiotensin II levels. Am J Hypertens 23: 420–424, 2010. doi: 10.1038/ajh.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zucker IH, Xiao L, Haack KK. The central renin-angiotensin system and sympathetic nerve activity in chronic heart failure. Clin Sci (Lond) 126: 695–706, 2014. doi: 10.1042/CS20130294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh RR, Cullen-McEwen LA, Kett MM, Boon WM, Dowling J, Bertram JF, Moritz KM. Prenatal corticosterone exposure results in altered AT1/AT2, nephron deficit and hypertension in the rat offspring. J Physiol 579: 503–513, 2007. doi: 10.1113/jphysiol.2006.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moritz KM, Johnson K, Douglas-Denton R, Wintour EM, Dodic M. Maternal glucocorticoid treatment programs alterations in the renin-angiotensin system of the ovine fetal kidney. Endocrinology 143: 4455–4463, 2002. doi: 10.1210/en.2002-220534. [DOI] [PubMed] [Google Scholar]
- 47.Cuffe JS, Burgess DJ, O'Sullivan L, Singh RR, Moritz KM. Maternal corticosterone exposure in the mouse programs sex-specific renal adaptations in the renin-angiotensin-aldosterone system in 6-month offspring. Physiol Rep 4: e12754, 2016. doi: 10.14814/phy2.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferguson AV, Bains JS. Actions of angiotensin in the subfornical organ and area postrema: implications for long term control of autonomic output. Clin Exp Pharmacol Physiol 24: 96–101, 1997. doi: 10.1111/j.1440-1681.1997.tb01790.x. [DOI] [PubMed] [Google Scholar]
- 49.McGoldrick E, Stewart F, Parker R, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 12: Cd004454, 2020. doi: 10.1002/14651858.CD004454.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madarek EO, Najati N. The effect of glucocorticoid therapy in preventing early neonatal complications in preterm delivery. J Perinat Med 31: 441–443, 2003. doi: 10.1515/JPM.2003.069. [DOI] [PubMed] [Google Scholar]
- 51.Magala Ssekandi A, Sserwanja Q, Olal E, Kawuki J, Bashir Adam M. Corticosteroids use in pregnant women with COVID-19: recommendations from available evidence. J Multidiscip Healthc 14: 659–663, 2021. doi: 10.2147/JMDH.S301255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou CG, Packer CH, Hersh AR, Caughey AB. Antenatal corticosteroids for pregnant women with COVID-19 infection and preterm prelabor rupture of membranes: a decision analysis. J Matern Fetal Neonatal Med 1–9, 2020. doi: 10.1080/14767058.2020.1763951. [DOI] [PubMed] [Google Scholar]