Keywords: podocytes, proteinuria, transcriptional coactivator with PDZ-binding motif
Abstract
The podocyte is an important component of the glomerular filtration barrier, and maintenance of the integrity of its highly specified structure and function is critical for normal kidney function. Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) are two crucial effectors of the Hippo signaling pathway, and recent studies have shown that podocyte-specific YAP deletion causes podocyte apoptosis and the development of focal segmental glomerulosclerosis followed by progressive renal failure. In the present study, we investigated a potential role of the YAP paralog TAZ in podocytes. TAZ was found to be constitutively active in podocytes, and mice with podocyte-specific deletion of TAZ (TazpodKO) developed proteinuria starting at 4 wk of age and had increased podocyte apoptosis. Using primary cultured podocytes or immortalized mouse podocytes from Tazflox/flox mice, we found that TAZ is a transcriptional activator for TEAD-dependent expression of synaptopodin, zonula occludens-1, and zonula occludens-2. This is the first study to determine that TAZ plays an important role in the maintenance of the structure and function of podocytes.
NEW & NOTEWORTHY Podocytes play an important role in maintaining the integrity of the structure and function of the kidney. We observed that mice with selective deletion of transcriptional coactivator with PDZ-binding motif (TAZ) in podocytes developed proteinuria. TAZ is constitutively active and critical for expression of synaptopodin, zonula occludens-1, and zonula occludens-2 in podocytes. The findings of this study implicate TAZ as an important mediator of podocyte structural integrity and provide further insights into the role of Hippo-Yes-associated protein/TAZ in podocyte biology.
INTRODUCTION
The integrity of the glomerular filtration barrier is crucial for normal kidney function. The glomerular filtration barrier is composed of three distinct components: endothelial cells, the glomerular basement membrane, and podocytes. A network of primary and smaller secondary and tertiary cellular processes of highly specialized podocytes covers the outside of the glomerular capillary. Therefore, podocytes are constantly exposed to mechanical forces of tensile stress and fluid flow shear stress and are sensitive to changes in these mechanical forces (1–3). Ion channels, adhesion proteins, and proteins associated with the cytoskeleton are all critical elements to transduce the mechanical forces into biochemical signals in podocytes (4). The well-organized actin cytoskeletons of foot processes coordinating with the delicate slit diaphragms (SDs) play important roles in maintaining normal structure and function of podocytes (4–6). Linker proteins such as CD2-associated protein and the noncatalytic region of tyrosine kinase proteins are important to link the fine-tuned structure of SDs to the actin cytoskeleton podocyte (7, 8). Mutations of many genes encoding the intrinsic SD components or their interaction partners have been reported to mediate the development of proteinuria and progressive glomerular injury (9).
The SD structure is different from the typical tight junctions between epithelial cells due to an absence of E-cadherin and a porous structure (10). Zonula occludens (ZO)-1 and ZO-2 proteins belong to the membrane-associated guanylate kinase homolog family of proteins and are critical scaffolding proteins for the assembly of tight junctional complexes by multiple protein-binding domains, including three PDZ domains (11). Either ZO-1 or ZO-2 deficiency in mice causes embryonic death at embryonic days 10.5 or 7.5, respectively (12), suggesting that they do not play redundant roles during embryonic development (13, 14). ZO-1 is exclusively associated with tight junctions (15). ZO-2 also connects many tight junctional integral proteins, including ZO-1 in epithelia cells (16). In addition, ZO-2 was also detected in the nucleus of epithelial cells, where it associates with nuclear matrix proteins and some transcription factors (17, 18). Most tight junction components are downregulated or lost in differentiated podocytes except for ZO-1 and ZO-2 (9, 19). In podocytes, ZO-1 is concentrated at SD junction membranes and is an important binding partner of nephrin (20). Mice with podocyte-specific deletion of the Tjp1 gene, which encodes ZO-1, showed significant growth retardation and severe proteinuria starting at 2 wk of age (19, 21). ZO-2 protein is also expressed in podocytes, and overexpression of ZO-2 in podocytes protects from adriamycin-induced injury (22). Mice with podocyte-specific deletion of ZO-2 did not display any overt defect, but ZO-2 deletion exacerbated podocyte injury in mice with ZO-1 deficiency (19).
Transcriptional coactivator with PDZ-binding motif (TAZ), also referred as WW domain containing transcription regulator 1 (WWTR1), and its paralog Yes-associated protein (YAP) are effectors of the Hippo signaling pathway. YAP and TAZ share 45% amino acid identity and play redundant roles in the morula stage of mouse development and in controlling adult cardiac growth (23, 24). However, global deletion of the Yap gene in mice causes embryonic lethality (at embryonic day 8.5) (25), whereas Taz gene deficiency causes death at the perinatal stage in only a subset of mice, but the surviving adult mice develop bilateral kidney cysts and a pulmonary emphysema-like phenotype (26, 27). The Hippo signaling pathway negatively regulates the activity of YAP/TAZ to control cell growth, proliferation, and apoptosis (28). Recent studies have indicated that YAP preferentially regulates gene sets associated with cell division and cell cycle progression, whereas TAZ primarily regulates genes that associate with cell migration and extracellular matrix remodeling (29). In addition, YAP/TAZ have been recognized as critical sensors to perceive the tissue environment and communicate with it. YAP/TAZ activity in a cell is under direct control of its shape and polarity (30), which is in turn dictated by the cytoskeletal structure of cells. TAZ was first identified as a novel transcriptional coactivator by screening proteins that interact with 14-3-3 binding protein, and it is highly expressed in the mammalian kidney, especially in renal proximal tubule epithelial cells (31, 32). YAP is primarily confined to the nuclei in podocytes and plays an important role in maintaining cell survival and the highly specialized structure of podocytes (33–35). At the age of 5–6 wk, mice with podocyte-specific YAP deficiency present with proteinuria followed by the development of progressive focal segmental glomerulosclerosis and renal failure (33). However, the potential pathophysiological role of TAZ in podocytes has not been previously studied.
In the present study, we determined that TAZ is also a constitutively active protein predominantly localizing to the nucleus of podocytes, and mice with podocyte-specific Taz gene deficiency (TazpodKO) also developed overt proteinuria starting at the age of 4 wk, with the development of podocyte apoptosis, podocyte loss, and glomerulosclerosis. Further experiments revealed that TAZ plays an important role in regulating expression of ZO-1, ZO-2, and synaptopodin to maintain the normal structure and function of podocytes.
MATERIALS AND METHODS
Materials
Antibodies against TAZ (No. 4883), YAP (No. 14074), lamin B2 (No. 12255), ZO-2 (No. 2847), Bcl2 (No. 3498), cleaved caspase-3 (No. 9664), pan-TEAD (No. 13295), and β-actin (No. 4970) were from Cell Signaling Technology (Beverly, MA). Antibodies against ZO-1 (No. ab221547 for immunofluorescence), synaptopodin (ab259976 for immunoblot analysis), TAZ (ab110239), and Wilms’ tumor-1 (WT1; ab89901) were from Abcam (Cambridge, MA). Antibodies against ZO-1 were purchased from EMD Millipore (AB2272 for immunoblot analysis). Antibody against nephrin (AF3159) was purchased from R&D Systems (Minneapolis, MN). Antibody against synaptopodin (BM5086 for immunofluorescence) was from Origen Technologies (Rockville, MD). IRDye680RD or IRDye800CW secondary antibodies were from LI-COR (Lincoln, NE). Alexa Fluor 488- or Alexa Fluor 594-conjugated secondary antibodies for immunofluorescence staining were from Life Technologies (Grand Island, NY). Antibody against TAZ (HPA007415) for immunofluorescence staining and all other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Animal Experiments
The Vanderbilt University Institutional Animal Care and Use Committee approved all experiments, and all experiments were conducted according to National Institutes of Health guidelines. TazPodKO mice and wild-type control (TazPodWT) mice were generated by crossing Tazflox/flox mice (36) with podocin.Cre mice (37). Spot urine was collected from TazPodWT and TazPodKO mice at the indicated age for analysis. Mice were euthanized at the indicated time points, and kidney tissues were collected for analysis. Immortalized Tazflox/flox mice (immortalizedTaz) were generated by crossing Tazflox/flox mice with mice containing the Immortomouse transgene (H-2Kb-tsA58) (38).
Isolation of Glomeruli and Primary Culture of Podocytes From Wild-Type Mice or Immortalized Tazflox/flox Mice
Glomeruli were isolated as previously described (39). Briefly, anesthetized 2-mo-old wild-type mice or immortalized Tazflox/flox mice were perfused with 8 × 107 Dynabeads M-450 (Invitrogen, Carlsbad, CA). The kidneys were removed and minced on ice followed by digestion at 37°C with 1 mg/mL collagenase and 100 U/mL DNase I for 15 min. The digested tissues were filtered through a stainless testing sieve (212-μm opening, Fisher Scientific) followed by 100-μm Fisherbrand cell strainers (Thermo Fisher Scientific, Cincinnati, OH). The passthrough was pelleted by gentle centrifugation (150 g, 3 min), and glomeruli containing Dynabeads were collected using a DynaMag-2 Magnet (Invitrogen). Finally, glomeruli were harvested in RPMI-1640 medium containing 10% FBS after the free Dynabeads were removed by passage through 40-μm Fisherbrand cell strainers (Thermo Fisher Scientific). For podocyte culturing, the isolated glomeruli were cultured from wild-type or immortalized Tazflox/flox mice on dishes coated with collagen type IV (0.01%) and laminin (10 μg/mL) in RPMI-1640 medium containing 10% FBS. Four days later, the glomerular cores were removed by gentle trypsinization. The immortal podocytes (immortalizedTaz podocytes) were maintained at 33°C in RPMI-1640 medium containing 100 U/mL of interferon-γ and 10% FBS for expansion. Before experiments, podocytes were induced to differentiate by shifting them to 37°C and culturing in RPMI-1640 medium containing 10% FBS without interferon-γ for 10 days.
Quantitative Real-Time PCR Analysis
Relative mRNA expression levels of actin cytoskeleton regulators in primary cultured podocytes or differentiated immortalizedTaz podocytes were validated by quantitative real-time PCR. Relative gene expression was quantified using iQ SYBR Green Supermix (Bio-Rad) in the QuantStudio 3 real-time PCR system. All raw threshold cycle (Ct) values were normalized to β-actin, and the 2−ΔΔCT method was applied to compare fold changes of genes between the experimental group and the control group. All PCR primers (Supplemental Table S1; all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.17069663) were from Integrated DNA Technologies.
Mouse Kidney Histology Analysis
Mouse kidneys were harvested from TazPodWT and TazPodKO mice at the indicated time points and embedded in paraffin. Five-micrometer tissue sections were stained with a periodic acid-Schiff kit (Sigma, St. Louis, MO) according to the manufacturer’s instruction, and images were captured using an Axiocam MRc (Carl Zeiss).
TUNEL Staining
Deparaffinized and rehydrated 5-μm sections from TazpodKO and TazpodWT mice were subjected to TUNEL staining using the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI) according to the manufacturer’s instructions.
Immunofluorescence Analysis
Immunofluorescence staining was performed on paraffin-embedded tissues or cultured podocytes fixed by 4% paraformaldehyde using standard techniques as previously described (40, 41). Images were captured using a Nikon TE300 fluorescence microscope (Diagnostic Instruments) or an Olympus FV1000 Invert Confocal Microscope (Vanderbilt Medical Center Cell Image Shared Resource Core).
Transfection of siRNA or Infection of Adenovirus Cre in Differentiated Podocytes
Control and ON-TARGETplus mouse siRNA SMARTpool: Nontargeting Pool (D-001810-10-05), Wwtr1 (L-041057-01-0020), Yap1 (L-046247-01-0020), large tumor suppressor 1 (Lats1) (L-063467-00-0005), and large tumor suppressor 2 (Lats2) (L-044602-00-0005) were purchased from Dharmacon (Thermo Fisher Scientific). Seventy-two hours after transfection of the indicated siRNAs using Lipofectamine RNAiMAX Reagent (Thermo Fisher Scientific) in primary podocytes or infection with adenovirus expressing Cre under a cytomegalovirus (CMV) promoter (Ad-cre) or adenovirus containing an empty CMV promoter (Ad-null) in differentiated immortalizedTaz podocytes, the cells were lysed in RIPA buffer for immunoblot analysis or fixed in 4% paraformaldehyde for immunofluorescence staining.
Chromatin Immunoprecipitation Assay
The chromatin immunoprecipitation assay was performed using the MAGnify Chromatin Immunoprecipitation System (Thermo Fisher Scientific) following the manufacturer’s instructions. Specifically, differentiated immortalizedTaz podocytes were cross-linked by 1% formaldehyde. Cell nuclei were lysed and sonicated to generate DNA fragments with an average size of 0.5 kb. Antibodies against TAZ (ab110239) or pan-TEAD were used for immunoprecipitation with the sonicated chromatin fragments. The primers for PCR analysis were as follows: forward primer 5′- AAGGCCATGGAGCAATCTCAA-3′ and reverse primer 5′- GGGCTCAGTCCTCATACACA-3′ for the Tjp1 gene; forward primer 5′- TCAACCCGTTCCACTCCCCT-3′ and reverse primer 5′- GCTTTCAGTCTTTCCGCAGG-3′ for the Tjp2 gene; and forward primer 5′- CTTGGCACCCCCAAATCTCA-3′ and reverse primer 5′- GCCACCACCAGCAGAATCTA-3′ for the synaptopodin (Synop) gene.
Immunoblot Analysis
The procedures for immunoblot analysis were performed as we have previously described (40, 42).
Statistics
Data are presented as means ± SE for at least three separate experiments (each in triplicate). An unpaired Student’s t test was used for statistical analysis; for multiple group comparisons, ANOVA with Bonferroni corrections using GraphPad Prism version 7 were used. P values of <0.05 compared with control were considered statistically significant.
RESULTS
Generation of Podocyte-Specific Taz Knockout Mice
To investigate the potential role of TAZ in podocytes, we crossed Tazflox/flox mice36 with podocin-Cre recombinase transgenic mice (37) to generate TazpodKO mice (Tazflox/flox;podocin-cre+) and wild-type TazpodWT mice (Tazflox/flox;podocin-cre−; Fig. 1A). Immunoblot analysis of isolated glomerular lysates from TazpodKO and TazpodWT mice confirmed the success of TAZ deletion (Fig. 1B). Interestingly, upregulation of YAP expression was detected in glomeruli from TazpodKO mice. Immunofluorescence staining of kidney samples further confirmed the efficacy of TAZ deletion and upregulation of YAP in TazpodKO mice (Fig. 1C). Of note, immunofluorescence data indicated that both TAZ and YAP are primarily localized in the nucleus of podocytes in TazpodWT mice, which was further confirmed by immunoblot analysis of nuclear and cytosol fractions of isolated glomeruli from TazpodWT mice (Fig. 1D). In addition, upregulation of both cytoplasmic and nuclear YAP was confirmed by immunoblot analysis (Fig. 1D). Evidence of nuclear localization of TAZ in podocytes suggested a constitutive role of TAZ in podocyte structure and function (43).
Figure 1.
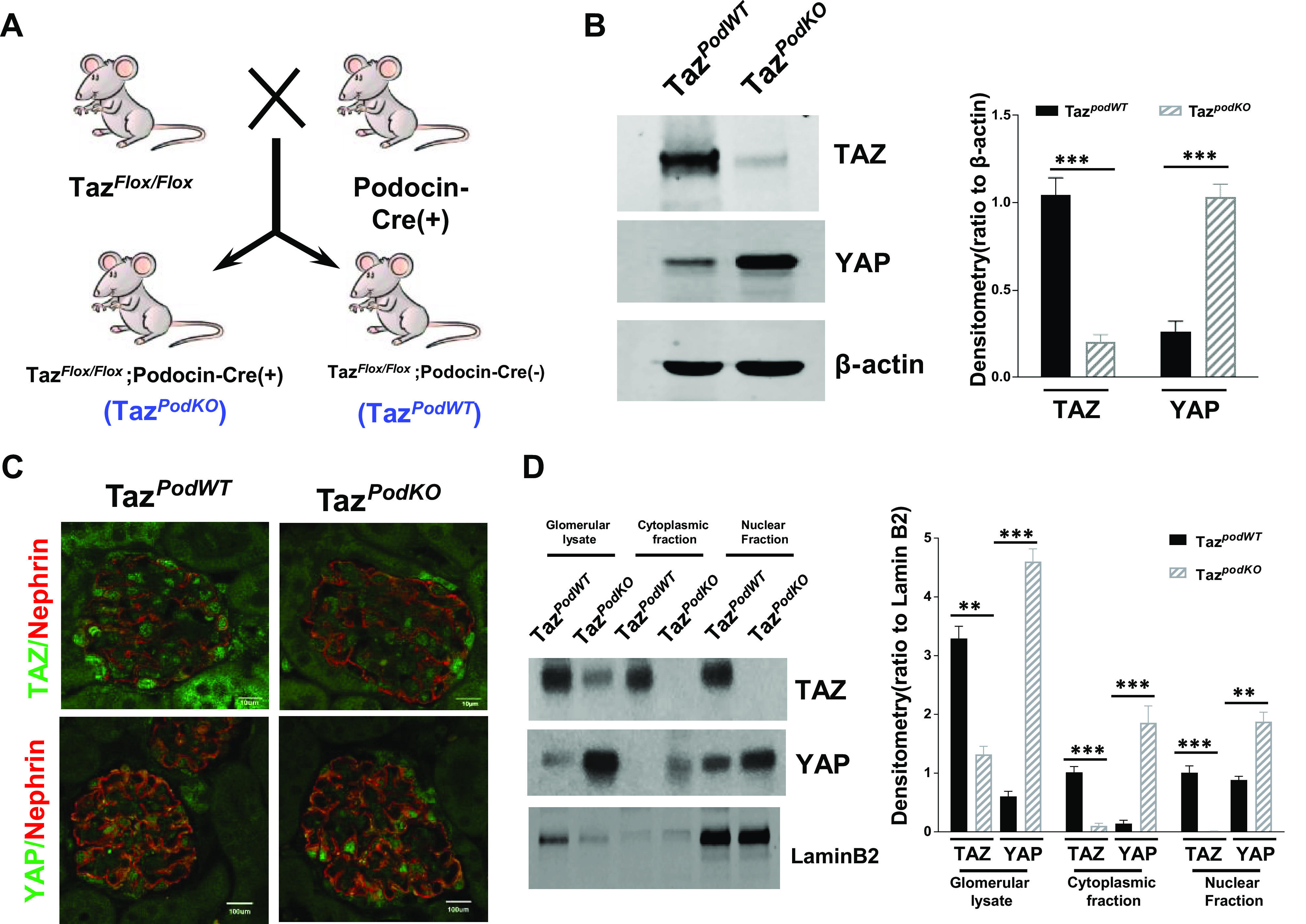
Generation of podocyte-specific transcriptional coactivator with PDZ-binding motif (Taz) knockout mice. A: scheme of generation of TazpodKO and TazpodWT mice. B: immunoblot analysis of isolated glomerular lysates indicated reduced TAZ but upregulated Yes-associated protein (YAP) expression. C: immunofluorescence staining showed that TAZ was effectively deleted but YAP was upregulated in TazpodKO mouse podocytes (red: nephrin, a podocyte marker; green: TAZ or YAP). Original magnification: ×600. D: immunoblot analysis of nuclear and cytosolic fractions of isolated glomeruli confirmed that both TAZ and YAP are primarily confined to the nuclei of glomerular cells, and both cytoplasmic and nuclear YAP were upregulated in TazpodKO mice. ***P < 0.001, **P < 0.01. WT, wild type.
Development of Proteinuria and Segmental Glomerular Sclerosis in TazpodKO Mice
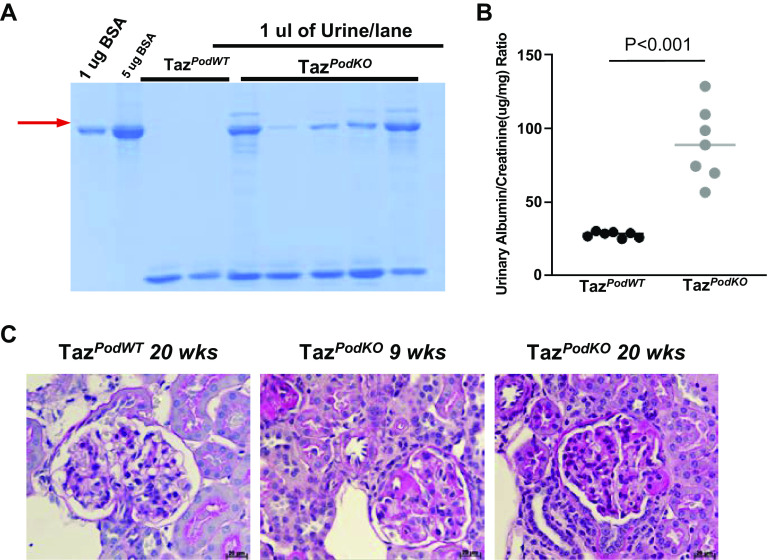
TazpodKO mice were born in the same Mendelian ratio as TazpodWT mice, and no obvious development difference was observed between TazpodKO and TazpodWT mice. We collected spot urine from TazpodKO and TazpodWT mice weekly beginning at an age of 3 wk to screen for proteinuria by Coomassie blue-stained SDS-PAGE gels. Surprisingly, 41.7% of TazpodKO mice (n = 36) developed mild proteinuria at an age of 4 wk (Fig. 2A). Albumin-to-creatinine ratio analysis of mouse spot urine at 5 wk of age confirmed increased urinary protein (89.57 ± 12.67 µg/mg in TazpodKO mice vs. 27.86 ± 1.55 µg/mg in TazpodWT mice, n = 7; Fig. 2B). Segmental glomerular sclerosis was detected in TazpodKO mice by 8–9 wk of age (Fig. 2C). These findings suggested that deletion of TAZ caused defects in podocytes, which was not completely compensated by upregulation of YAP in podocytes (Fig. 1, B–D).
Figure 2.
Development of proteinuria and segmental glomerular sclerosis in podocyte-specific transcriptional coactivator with PDZ-binding motif (Taz) knockout (TazpodKO) mice. A: representative Coomassie blue staining of 1 μL urine from TazpodKO or TazpodWT mice at 4 wk of age. B: proteinuria indicated by increases in the albumin-to-creatinine ratio in TazpodKO mice. Values are means ± SE; n = 7. C: glomerular sclerosis was detected in TazpodKO mice at 9 wk of age. Original magnification: ×400. WT, wild type.
Podocyte Apoptosis Was Detected in the Glomeruli of TazpodKO Mice
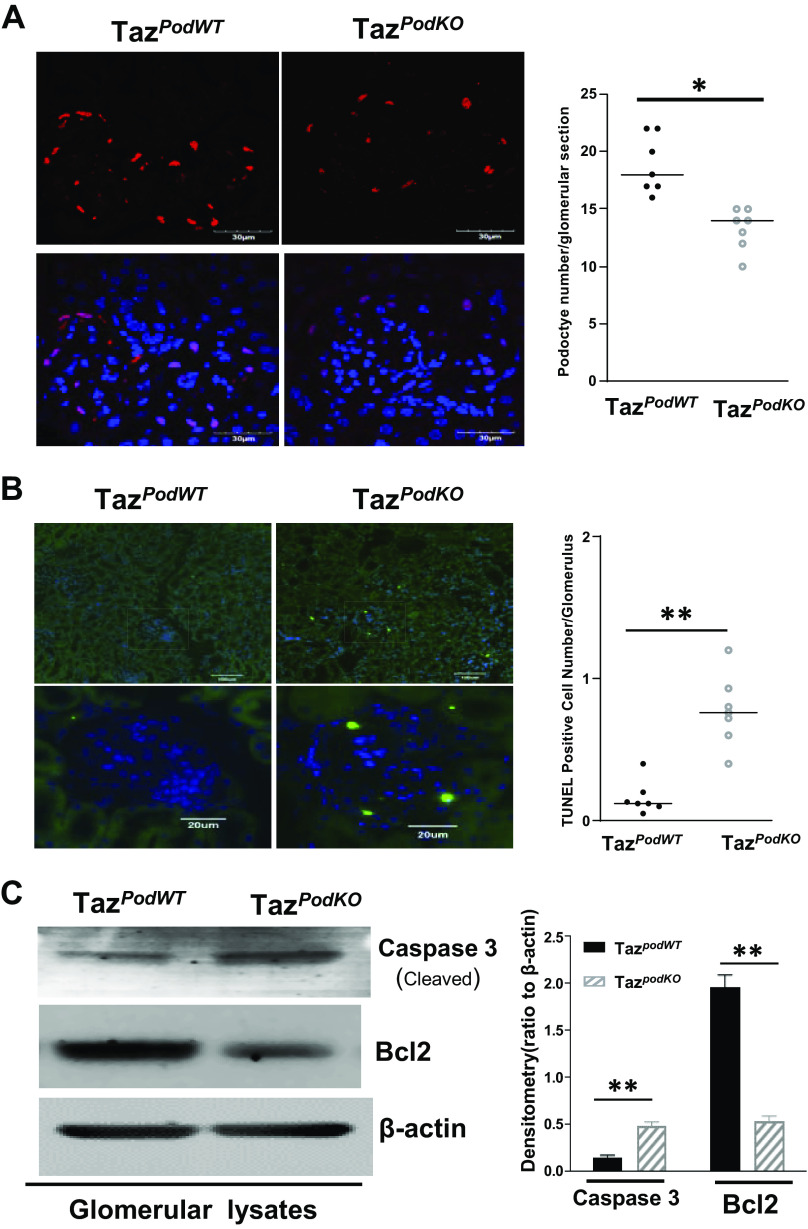
Podocytes are essential for the integrity of the glomerular filtration barrier, and proteinuria usually reflects damage and/or loss of glomerular podocytes (44, 45). We found that podocyte number/glomerulus, indicated by WT1-positive cells, was significantly lower in TazpodKO mice compared with TazpodWT mice (podocyte number/glomerulus: 17.85 ± 2.19 in TazpodWT mice vs. 13.23 ± 2.52 in TazpodKO mice, n = 7 mice/group, P < 0.01; Fig. 3A). TUNEL staining indicated increased apoptosis in TazpodKO mice (Fig. 3B). In addition, immunoblot analysis of glomerular lysates indicated a decrease in the expression of Bcl2, an antiapoptotic protein, and an increase in the expression of cleaved caspase-3, a proapoptotic protein (Fig. 3C). These data suggested that TAZ is an important protein for prevention of apoptosis in podocytes.
Figure 3.
Podocyte apoptosis was detected in the glomeruli of podocyte-specific transcriptional coactivator with PDZ-binding motif (Taz) knockout (TazpodKO) mice. A: reduced podocyte cell number indicated by Wilms’ tumor-1-positive cells was observed in TazpodKO mice. B: podocyte apoptosis indicated by increases in TUNEL-positive cells in glomeruli of TazpodKO mice. C: increased expression of cleaved capase-3 and decreased expression of Bcl2 were determined in isolated glomerular lysates from TazpodKO mice (10 fields/section, n = 7). **P < 0.01, *P < 0.05. WT, wild type.
Expression of TAZ Is Important for Maintaining Normal F-Actin Distribution in Podocytes
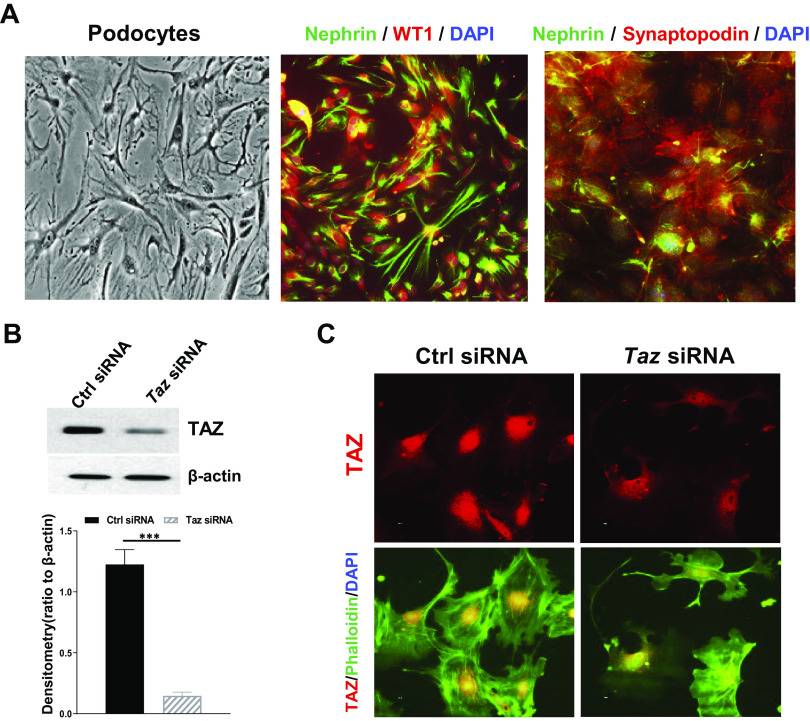
Defects of any component of the focal adhesion complex or SD in podocytes may cause proteinuria (46). To determine the role of TAZ in the maintenance of the highly specified morphology of podocytes, we isolated glomeruli and cultured primary podocytes from wild-type mice as described in materials and methods. Cultured podocytes still maintained characteristics of podocytes, including long foot processes and expression of nephrin, WT1, and synaptopodin, as shown in Fig. 4A. With knockdown of TAZ in these differentiated mouse podocytes (Fig. 4B), cell morphology changed, and normal F-actin distribution was disrupted (Fig. 4C). Of note, TAZ was in large part localized in the nuclei of cultured podocytes, which is similar to what we observed in mouse glomeruli (Fig. 1C).
Figure 4.
Transcriptional coactivator with PDZ-binding motif (TAZ) is important for maintaining normal F-actin distribution in podocytes. A: representative images of primary culture of podocytes and immunofluorescence staining of podocyte markers for the primary culture of podocytes [green: nephrin; red: Wilms’ tumor-1 (WT1) or synaptopodin; blue: DAPI]. Original magnification: ×400. B: immunoblot analysis confirmed effective silencing of TAZ expression by siRNA in cultured mouse podocytes. C: disrupted stress fiber distribution in cultured mouse podocytes transfected with Taz siRNA. ***P < 0.001. Ctrl, control.
TAZ Regulates Expression of Synaptopodin, ZO-1, and ZO-2 in Podocytes
The function and highly specialized structure of podocytes are mainly dependent on actin cytoskeleton regulation within the foot processes. The SD and focal adhesions are two key signals within foot processes that regulate the actin cytoskeletons, and dysregulation of the actin cytoskeleton is a common mediator of podocyte damage (42). To determine whether TAZ is involved in regulating expression of actin cytoskeletal proteins in podocytes, we screened gene expression of 13 focal adhesion proteins or SD components in primary podocytes with or without Taz siRNA transfection. As shown in Fig. 5A, expression of Synop (coding synaptopodin protein), Tjp1 (coding ZO-1 protein), and Tjp2 (coding ZO-2 protein) were significantly inhibited by silencing Taz expression in podocytes. Immunoblot analysis confirmed that silencing the Taz gene by siRNA markedly inhibited protein expression of synaptopodin, ZO-1, and ZO-2 (Fig. 5B and Supplemental Fig. S1). To exclude the off-target possibility of Taz siRNA, we also cultured podocytes from immortalizedTaz mice generated by crossing Tazflox/flox mice with mice containing the Immortomouse transgene (H-2Kb-tsA58). Differentiated immortalizedTaz mouse podocytes were infected with adenovirus expressing Cre under a CMV promoter (Ad-cre) or an adenovirus containing an empty CMV promoter (Ad-null) followed by quantitative PCR analysis. In these cells, we again noted that TAZ deletion inhibited synaptopodin, ZO-1, and ZO-2 mRNA and protein levels (Fig. 5, C and D). As a transcriptional activator, TAZ lacks a DNA-binding domain but contains an activation domain, whereas the transcription factor TEAD contains a DNA-binding domain but lacks an activation domain (47). TEAD has been found to bind to numerous M-CAT-Like DNA sequences (48), and our chromatin immunoprecipitation assay confirmed that both TEAD and TAZ bind to the promoter sequence of Tjp1, Tjp 2, and synop genes in differentiated immortalizedTaz mouse podocytes (Fig. 5E). Activation of the Hippo signaling pathway negatively regulates activity of both YAP/TAZ by activation of LATS1/2 to control cell proliferation, survival, and migration (49). Therefore, we transfected Lats1/2 siRNAs in differentiated podocytes and found that silencing Lats1/2 increased the TAZ protein level and modestly upregulated expression of synaptopodin, ZO-1, and ZO-2 (Fig. 5B). Transfection of constitutively active YAP (hYAP S127A) (50) did not reverse the effects of silencing Taz on the expression of synaptopodin, ZO-1, and ZO-2 (Fig. 5B), suggesting that TAZ and YAP did not play redundant regulatory roles in the protein expression of these cytoskeleton components. As an in vivo confirmation, we also detected decreases in the expression of these proteins in isolated glomeruli from TazpodKO mice compared with TazpodWT mice (Fig. 5F). Therefore, these results indicated that the expression of constitutively active TAZ in podocytes is important for the expression of synaptopodin, ZO-1, and ZO-2 and thereby maintaining normal podocyte structure and function.
Figure 5.
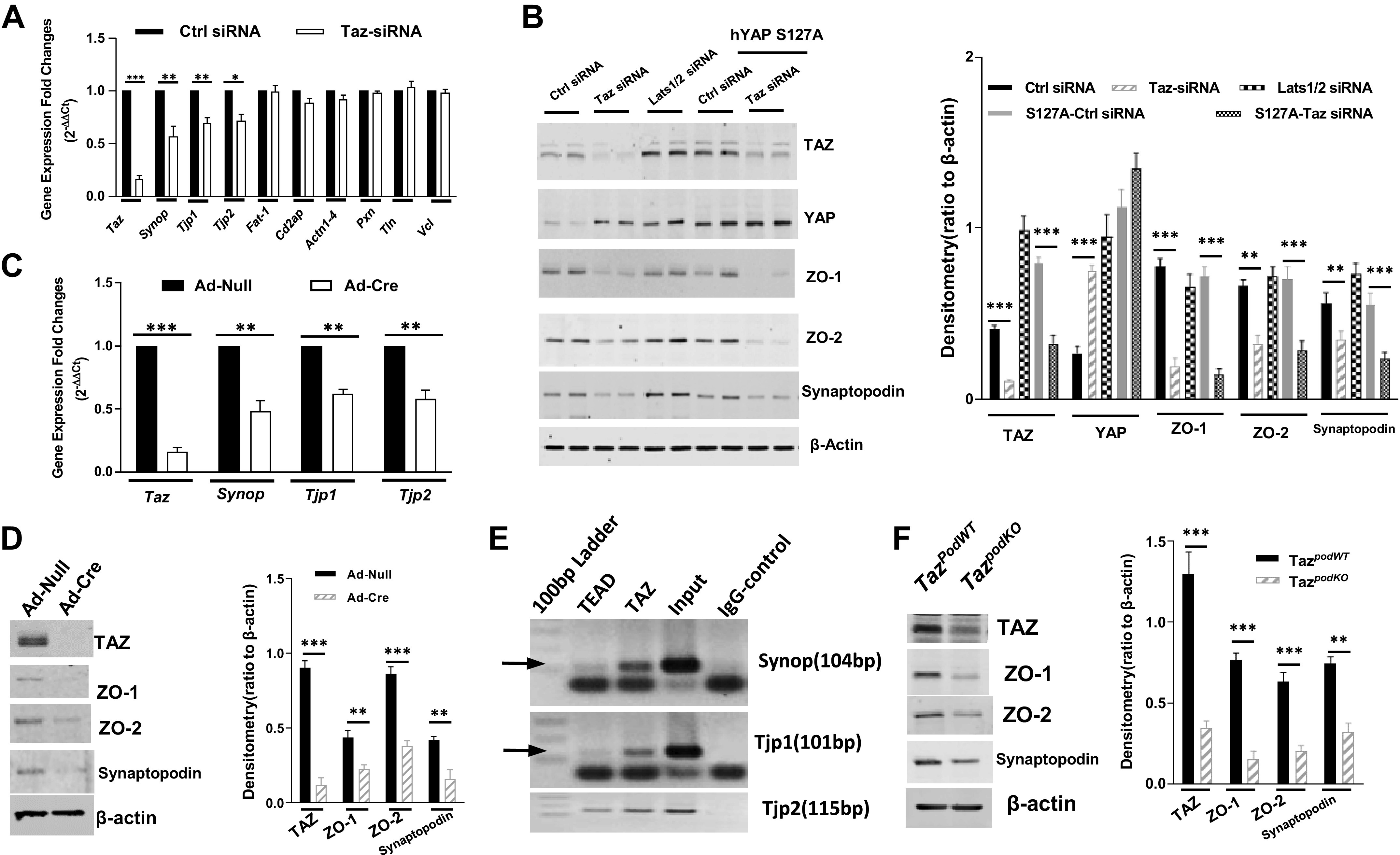
Transcriptional coactivator with PDZ-binding motif (TAZ) regulates expression of synaptopodin (Synop), zonula occludens (ZO)-1 (Tjp1), and ZO-2 (Tjp2) in podocytes. A: screening of actin cytoskeleton regulators by quantitative PCR revealed that knockdown of TAZ significantly inhibited synaptopodin, ZO-1, and ZO-2 gene expression in mouse primary podocytes. B: expression of synaptopodin, ZO-1, and ZO-2 were slightly increased by knockdown of large tumor suppressor 1/2 (Lats1/2) with siRNA transfection and was not affected by overexpression of an active Yes-associated protein (YAP) mutant. C: infection of Cre adenovirus in cultured podocytes from immortalizedTaz mice inhibited mRNA transcription of synaptopodin, ZO-1, and ZO-2. D: infection of Cre adenovirus in cultured podocytes from immortalizedTaz mice inhibited protein expression of synaptopodin, ZO-1, and ZO-2. E: chromatin immunoprecipitation assay indicated that both TEAD and TAZ bind to the promoter of Tjp1, Tjp2, and Synop genes. F: inhibition of synaptopodin, ZO-1, and ZO-2 expression was confirmed in isolated glomerular lysates from TazpodWT or TazpodKO mice. ***P < 0.001, **P< 0.01, *P < 0.05. Cd2AP, CD2-associated protein; Ctrl, control; hYAP, human YAP; WT, wild type.
DISCUSSION
In the present study, we observed a moderate increase in proteinuria in podocyte-specific Taz gene knockout (TazpodKO) mice starting at 4 wk of age and development of focal glomerulosclerosis by 9 wk of age. In addition, we also detected more podocyte apoptosis and a reduction in podocyte number/glomerulus in TazpodKO mouse kidneys. The results of the underlying molecular mechanism experiments indicated that TAZ is an important regulator of expression of actin cytoskeletal protein synaptopodin and the cell-cell interaction proteins ZO-1 and ZO-2 in podocytes.
Normal expression and distribution of the actin cytoskeleton are essential for normal function of podocytes. Synaptopodin is exclusively expressed in the foot process of completely differentiated podocytes, and it is intimately associated with actin microfilaments (51), indicating an important role to maintain the integrity of the actin cytoskeleton and to regulate podocyte migration in vitro (52). Impairment of synaptopodin expression in podocytes has been reported in some but not all forms of nephrotic syndrome (53); however, deletion of synaptopodin in mice does not cause an obvious phenotype in podocytes, suggesting that synaptopodin is dispensable for normal podocyte homeostasis. In contrast, deletion of synaptopodin exacerbates kidney injury in response to adriamycin injection in a mouse model of Alport syndrome (54, 55). Whether dysregulation of synaptopodin in some etiologies of nephrotic syndrome is related to impairment of TAZ signals in podocytes needs further studies. The tight junction proteins ZO-1 and ZO-2 are present in mature podocytes (9, 19). Interaction of ZO-1 with nephrin is important for the structural integrity of the SD (56), and podocyte-specific ZO-1-null mice develop severe proteinuria starting at 2 wk of age (19, 21). Although mice with podocyte-specific deletion of ZO-2 do not present any overt defects, ZO-2 deletion accelerates the defective podocyte function observed in podocyte-specific ZO-1 knockout mice (19). Our present study revealed that TAZ is an important transcriptional regulator for synaptopodin, ZO-1, and ZO-2 gene expression. Although we would not anticipate proteinuria in TazpodKO mice if synaptopodin were the only target of TAZ in podocytes, with concurrent downregulation of synaptopodin and the cell-cell interaction proteins ZO-1 and ZO-2 in TAZ-deficient mice, we did see both functional and structural podocyte abnormalities. However, our in vitro and in vivo data suggested that knockdown or deletion of TAZ in podocytes only partially, but not completely, inhibited expression of both ZO-1 and ZO-2, indicating that there may be other mechanisms to regulate their expression in addition to TAZ. This assumption may also explain why only moderate proteinuria was observed in TazpodKO mice and why the proteinuria did not progressively worsen with age (Supplemental Fig. S2). Further studies to indicate additional regulatory mechanisms for ZO-1 and ZO-2 in podocytes are therefore needed.
YAP and TAZ are canonical downstream effectors of the Hippo signaling pathway. They share 45% homology in protein sequences and play some redundant roles (23, 24). However, either YAP (33) or TAZ knockout in podocytes causes podocyte apoptosis and proteinuria, suggesting that they play distinct functions in maintaining podocyte structure and functions. Although podocyte deletion of YAP causes more profound and progressive glomerular injury (33), upregulation of YAP expression in podocytes and overexpression of constitutively active human YAP construct could not reverse the effects of TAZ deficiency in differentiated podocytes, providing evidence that YAP and TAZ may play distinct roles in the maintenance of normal podocyte structure and function. YAP has been documented to be predominantly confined to the nucleus of podocytes (33, 34). We also found that TAZ is partly localized in the nucleus of podocytes (Fig. 1, C and D). Nuclear localization of YAP/TAZ is an indicator of their activation, so we propose that both YAP and TAZ are constitutively active in adult mouse podocytes. Moreover, we postulate that constitutively active TAZ maintains expression of synaptopodin, ZO-1, and ZO-2, thereby endowing podocytes a highly flexible cytoskeleton to respond to the pulsatile tensile stress from the glomerular capillary loops and the fluid flow shear stress from the ultrafiltrate within Bowman’s space. However, investigation of detailed mechanisms will require further investigation.
Perspectives and Significance
The present study has shown that TAZ is an important transcriptional activator for regulating the expression of synaptopodin, ZO-1, and ZO-2 in podocytes. Our study also suggested that although both YAP and TAZ are constitutively active in podocytes, they may regulate expression of different downstream target genes.
DATA AVAILABILITY
The data sets generated and analyzed during the present study are available from the corresponding authors on reasonable request.
SUPPLEMENTAL DATA
Supplemental Figs. S1 and S2 and Supplemental Table S1: https://doi.org/10.6084/m9.figshare.17069663.
GRANTS
This work was supported by American Diabetes Association Grant 1–18-IBS-267 (to J.C.), National Institute of Diabetes and Digestive and Kidney Diseases Grants DK51265, DK62794, and DK79341 (to R.C.H.), Department of Veterans Affairs Grant 00507969 (to R.C.H.), and support by the Vanderbilt O’Brien Kidney Center (DK114809). Immunofluorescent images were captured with the aid of the Vanderbilt Cell Imaging Shared Resource.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C. and R.C.H. conceived and designed research; J.C., X.W., and Q.H. performed experiments; J.C. and X.W. analyzed data; J.C. interpreted results of experiments; J.C. prepared figures; J.C. drafted manuscript; R.C.H. edited and revised manuscript; J.C., X.W., Q.H., and R.C.H. approved final version of manuscript.
REFERENCES
- 1.Endlich N, Kress KR, Reiser J, Uttenweiler D, Kriz W, Mundel P, Endlaich K. Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol 12: 413–422, 2001. doi: 10.1681/ASN.V123413. [DOI] [PubMed] [Google Scholar]
- 2.Friedrich C, Endlich N, Kriz W, Endlich K. Podocytes are sensitive to fluid shear stress in vitro. Am J Physiol Renal Physiol 291: F856–F865, 2006. doi: 10.1152/ajprenal.00196.2005. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava T, Dai H, Heruth DP, Alon US, Garola RE, Zhou J, Duncan RS, El-Meanawy A, McCarthy ET, Sharma R, Johnson ML, Savin VJ, Sharma M. Mechanotransduction signaling in podocytes from fluid flow shear stress. Am J Physiol Renal Physiol 314: F22–F34, 2018. doi: 10.1152/ajprenal.00325.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Endlich K, Kliewe F, Endlich N. Stressed podocytes-mechanical forces, sensors, signaling and response. Pflugers Arch 469: 937–949, 2017. doi: 10.1007/s00424-017-2025-8. [DOI] [PubMed] [Google Scholar]
- 5.Drenckhahn D, Franke RP. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest 59: 673–682, 1988. [PubMed] [Google Scholar]
- 6.Ichimura K, Kurihara H, Sakai T. Actin filament organization of foot processes in rat podocytes. J Histochem Cytochem 51: 1589–1600, 2003. doi: 10.1177/002215540305101203. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest 108: 1621–1629, 2001. doi: 10.1172/JCI200112849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440: 818–823, 2006. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- 9.Scott RP, Quaggin SE. Review series: the cell biology of renal filtration. J Cell Biol 209: 199–210, 2015. doi: 10.1083/jcb.201410017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tassin MT, Beziau A, Gubler MC, Boyer B. Spatiotemporal expression of molecules associated with junctional complexes during the in vivo maturation of renal podocytes. Int J Dev Biol 38: 45–54, 1994. [PubMed] [Google Scholar]
- 11.Fanning AS, Anderson JM. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann N Y Acad Sci 1165: 113–120, 2009. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber TB, Schmidts M, Gerke P, Schermer B, Zahn A, Hartleben B, Sellin L, Walz G, Benzing T. The carboxyl terminus of Neph family members binds to the PDZ domain protein zonula occludens-1. J Biol Chem 278: 13417–13421, 2003. doi: 10.1074/jbc.C200678200. [DOI] [PubMed] [Google Scholar]
- 13.Katsuno T, Umeda K, Matsui T, Hata M, Tamura A, Itoh M, Takeuchi K, Fujimori T, Nabeshima Y, Noda T, Tsukita S, Tsukita S. Deficiency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol Biol Cell 19: 2465–2475, 2008. doi: 10.1091/mbc.e07-12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Kausalya PJ, Phua DCY, Ali SM, Hossain Z, Hunziker W. Early embryonic lethality of mice lacking ZO-2, but Not ZO-3, reveals critical and nonredundant roles for individual zonula occludens proteins in mammalian development. Mol Cell Biol 28: 1669–1678, 2008. doi: 10.1128/MCB.00891-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol 103: 755–766, 1986. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 273: 29745–29753, 1998. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 17.Jaramillo BE, Ponce A, Moreno J, Betanzos A, Huerta M, Lopez-Bayghen E, Gonzalez-Mariscal L. Characterization of the tight junction protein ZO-2 localized at the nucleus of epithelial cells. Exp Cell Res 297: 247–258, 2004. doi: 10.1016/j.yexcr.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Betanzos A, Huerta M, Lopez-Bayghen E, Azuara E, Amerena J, González-Mariscal L. The tight junction protein ZO-2 associates with Jun, Fos and C/EBP transcription factors in epithelial cells. Exp Cell Res 292: 51–66, 2004. doi: 10.1016/j.yexcr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Itoh M, Nakadate K, Matsusaka T, Hunziker W, Sugimoto H. Effects of the differential expression of ZO-1 and ZO-2 on podocyte structure and function. Genes Cells 23: 546–556, 2018. doi: 10.1111/gtc.12598. [DOI] [PubMed] [Google Scholar]
- 20.Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, Mathur BN, Turner CA, Geske R, Montgomery CA, Starbuck M, Brandt M, Gupta A, Ramirez-Solis R, Zambrowicz BP, Powell DR. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol 21: 4829–4836, 2001. doi: 10.1128/MCB.21.14.4829-4836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itoh M, Nakadate K, Horibata Y, Matsusaka T, Xu J, Hunziker W, Sugimoto H. The structural and functional organization of the podocyte filtration slits is regulated by Tjp1/ZO-1. PLoS One 9: e106621, 2014. doi: 10.1371/journal.pone.0106621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bautista-García P, Reyes JL, Martín D, Namorado MC, Chavez-Munguía B, Soria-Castro E, Huber O, González-Mariscal L. Zona occludens-2 protects against podocyte dysfunction induced by ADR in mice. Am J Physiol Renal Physiol 304: F77–F87, 2013. doi: 10.1152/ajprenal.00089.2012. [DOI] [PubMed] [Google Scholar]
- 23.Nishioka N, Inoue K-I, Adachi K, Kiyonari H, Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson RO, Ogonuki N, Makita R, Kurihara H, Morin-Kensicki EM, Nojima H, Rossant J, Nakao K, Niwa H, Sasaki H. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell 16: 398–410, 2009. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci USA 110: 13839–13844, 2013. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG, Magnuson TR, O'Neal W, Milgram SL. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol 26: 77–87, 2006. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hossain Z, Ali SM, Ko HL, Xu J, Ng CP, Guo K, Qi Z, Ponniah S, Hong W, Hunziker W. Glomerulocystic kidney disease in mice with a targeted inactivation of Wwtr1. Proc Natl Acad Sci USA 104: 1631–1636, 2007. doi: 10.1073/pnas.0605266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makita R, Uchijima Y, Nishiyama K, Amano T, Chen Q, Takeuchi T, Mitani A, Nagase T, Yatomi Y, Aburatani H, Nakagawa O, Small EV, Cobo-Stark P, Igarashi P, Murakami M, Tominaga J, Sato T, Asano T, Kurihara Y, Kurihara H. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol Renal Physiol 294: F542–F553, 2008. doi: 10.1152/ajprenal.00201.2007. [DOI] [PubMed] [Google Scholar]
- 28.Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov 13: 63–79, 2014. doi: 10.1038/nrd4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shreberk-Shaked M, Dassa B, Sinha S, Di Agostino S, Azuri I, Mukherjee S, Aylon Y, Blandino G, Ruppin E, Oren M. A division of labor between YAP and TAZ in non-small cell lung cancer. Cancer Res 80: 4145–4157, 2020. doi: 10.1158/0008-5472.CAN-20-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol 18: 758–770, 2017. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, Hisaminato A, Fujiwara T, Ito Y, Cantley LC, Yaffe MB. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J 19: 6778–6791, 2000. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian Y, Kolb R, Hong J-H, Carroll J, Li D, You J, Bronson R, Yaffe MB, Zhou J, Benjamin T. TAZ promotes PC2 degradation through a SCFbeta-Trcp E3 ligase complex. Mol Cell Biol 27: 6383–6395, 2007. doi: 10.1128/MCB.00254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartzman M, Reginensi A, Wong JS, Basgen JM, Meliambro K, Nicholas SB, D'Agati V, McNeill H, Campbell KN. Podocyte-specific deletion of Yes-associated protein causes FSGS and progressive renal failure. J Am Soc Nephrol 27: 216–226, 2016. doi: 10.1681/ASN.2014090916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell KN, Wong JS, Gupta R, Asanuma K, Sudol M, He JC, Mundel P. Yes-associated protein (YAP) promotes cell survival by inhibiting proapoptotic dendrin signaling. J Biol Chem 288: 17057–17062, 2013. doi: 10.1074/jbc.C113.457390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meliambro K, Wong JS, Ray J, Calizo RC, Towne S, Cole B, El Salem F, Gordon RE, Kaufman L, He JC, Azeloglu EU, Campbell KN. The Hippo pathway regulator KIBRA promotes podocyte injury by inhibiting YAP signaling and disrupting actin cytoskeletal dynamics. J Biol Chem 292: 21137–21148, 2017. doi: 10.1074/jbc.M117.819029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reginensi A, Scott RP, Gregorieff A, Bagherie-Lachidan M, Chung C, Lim DS, Pawson T, Wrana J, McNeill H. Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet 9: e1003380, 2013. doi: 10.1371/journal.pgen.1003380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003. doi: 10.1002/gene.10164. [DOI] [PubMed] [Google Scholar]
- 38.Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA 88: 5096–5100, 1991. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J, Chen J-K, Nagai K, Plieth D, Tan M, Lee TC, Threadgill DW, Neilson EG, Harris RC. EGFR signaling promotes TGFβ-dependent renal fibrosis. J Am Soc Nephrol 23: 215–224, 2012. doi: 10.1681/ASN.2011070645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J, Harris RC. Interaction of the EGF receptor and the hippo pathway in the diabetic kidney. J Am Soc Nephrol 27: 1689–1700, 2016. doi: 10.1681/ASN.2015040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blaine J, Dylewski J. Regulation of the actin cytoskeleton in podocytes. Cells 9: 1700, 2020. doi: 10.3390/cells9071700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan SW, Lim CJ, Loo LS, Chong YF, Huang C, Hong W. TEADs mediate nuclear retention of TAZ to promote oncogenic transformation. J Biol Chem 284: 14347–14358, 2009. doi: 10.1074/jbc.M901568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu L, Yang H-C, Hao C-M, Lin S-T, Gu Y, Ma J. Podocyte number predicts progression of proteinuria in IgA nephropathy. Mod Pathol 23: 1241–1250, 2010. doi: 10.1038/modpathol.2010.110. [DOI] [PubMed] [Google Scholar]
- 45.Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 46.Asanuma K, Yanagida-Asanuma E, Takagi M, Kodama F, Tomino Y. The role of podocytes in proteinuria. Nephrology (Carlton) 12 Suppl 3: S15–S20, 2007. doi: 10.1111/j.1440-1797.2007.00876.x. [DOI] [PubMed] [Google Scholar]
- 47.Tian W, Yu J, Tomchick DR, Pan D, Luo X. Structural and functional analysis of the YAP-binding domain of human TEAD2. Proc Natl Acad Sci USA 107: 7293–7298, 2010. doi: 10.1073/pnas.1000293107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anbanandam A, Albarado DC, Nguyen CT, Halder G, Gao X, Veeraraghavan S. Insights into transcription enhancer factor 1 (TEF-1) activity from the solution structure of the TEA domain. Proc Natl Acad Sci USA 103: 17225–17230, 2006. doi: 10.1073/pnas.0607171103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev 30: 1–17, 2016. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J, Wang X, He Q, Bulus N, Fogo AB, Zhang MZ, Harris RC. YAP activation in renal proximal tubule cells drives diabetic renal interstitial fibrogenesis. Diabetes 69: 2446–2457, 2020. doi: 10.2337/db20-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mundel P, Heid HW, Mundel TM, Krüger M, Reiser J, Kriz W. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol 139: 193–204, 1997. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol 8: 485–491, 2006. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
- 53.Srivastava T, Garola RE, Whiting JM, Alon US. Synaptopodin expression in idiopathic nephrotic syndrome of childhood. Kidney Int 59: 118–125, 2001. doi: 10.1046/j.1523-1755.2001.00472.x. [DOI] [PubMed] [Google Scholar]
- 54.Ning L, Suleiman HY, Miner JH. Synaptopodin is dispensable for normal podocyte homeostasis but is protective in the context of acute podocyte injury. J Am Soc Nephrol 31: 2815–2832, 2020. doi: 10.1681/ASN.2020050572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ning L, Suleiman HY, Miner JH. Synaptopodin deficiency exacerbates kidney disease in a mouse model of Alport syndrome. Am J Physiol Renal Physiol 321: F12–F25, 2021. doi: 10.1152/ajprenal.00035.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagner MC, Rhodes G, Wang E, Pruthi V, Arif E, Saleem MA, Wean SE, Garg P, Verma R, Holzman LB, Gattone V, Molitoris BA, Nihalani D. Ischemic injury to kidney induces glomerular podocyte effacement and dissociation of slit diaphragm proteins Neph1 and ZO-1. J Biol Chem 283: 35579–35589, 2008. doi: 10.1074/jbc.M805507200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1 and S2 and Supplemental Table S1: https://doi.org/10.6084/m9.figshare.17069663.
Data Availability Statement
The data sets generated and analyzed during the present study are available from the corresponding authors on reasonable request.