Keywords: autonomic function, endothelial function, mechanoreflex, metaboreflex, microvascular function
Abstract
Prolonged sitting in a mild hypercapnic environment impairs peripheral vascular function. The effects of sitting interruptions using passive or active skeletal muscle contractions are still unclear. Therefore, we sought to examine the vascular effects of brief periods (2 min every half hour) of passive and active lower limb movement to interrupt prolonged sitting with mild hypercapnia in adults. Fourteen healthy adults (24 ± 2 yr) participated in three experimental visits sitting for 2.5 h in a mild hypercapnic environment (CO2 = 1,500 ppm): control (CON, no limb movement), passive lower limb movement (PASS), and active lower limb movement (ACT) during sitting. At all visits, brachial and popliteal artery flow-mediated dilation (FMD), microvascular function, plasmatic levels of nitrate/nitrite and endothelin-1, and heart rate variability were assessed before and after sitting. Brachial and popliteal artery FMDs were reduced in CON and PASS (P < 0.05) but were preserved (P > 0.05) in ACT. Microvascular function was blunted in CON (P < 0.05) but was preserved in PASS and ACT (P > 0.05). In addition, total plasma nitrate/nitrite was preserved in ACT (P > 0.05) but was reduced in CON and PASS (P < 0.05), and endothelin-1 levels were decreased in ACT (P < 0.05). Both passive and active movement induced a greater ratio between the low-frequency and high-frequency bands for heart rate variability (P < 0.05). For the first time, to our knowledge, we found that brief periods of passive leg movement can preserve microvascular function, but that an intervention that elicits larger increases in shear rate, such as low-intensity exercise, is required to fully protect both macrovascular and microvascular function and circulating vasoactive substance balance.
NEW & NOTEWORTHY Passive leg movement could not preserve macrovascular endothelial function, whereas active leg movement could protect endothelial function. Attenuated microvascular function can be salvaged by passive movement and active movement. Preservation of macrovascular hemodynamics and plasma total nitrate/nitrite and endothelin-1 during prolonged sitting requires active movement. These findings dissociate the impacts induced by mechanical stress (passive movement) from the change in metabolism (active movement) on the vasculature during prolonged sitting in a mild hypercapnic environment.
INTRODUCTION
Many adults spend more than half of their waking hours sitting, with this pattern further amplified by the recent pandemic, which can adversely affect cardiovascular health. Prolonged sitting, i.e., uninterrupted sitting for at least 1 h or longer, is indeed an independent predictor for cardiovascular diseases (CVD) (1–3) and has been associated with an increased risk for metabolic diseases (4) and all-cause mortality (5). Specifically, sitting can impair endothelial function in the legs (6–8), increase peripheral blood pressure (9, 10), and contribute to the increased susceptibility to atherosclerotic development in the lower extremity vessels (11–13). In addition, periods of prolonged sitting typically occur in mild hypercapnic environments [elevated levels of atmospheric carbon dioxide (CO2)] such as classrooms, offices, and auditoriums (14–16), which exacerbate prolonged sitting’s adverse effects on cardiovascular function (17).
Despite the known health benefits of moderate-to-vigorous physical activity, the percentage of adults in the United States who meet the guidelines for physical activity is only ∼23%, and this has been remarkably unchanged during the period 2008–2016, regardless of public health policies (18). In this context, there is a crucial need to explore evidence-based alternative behavioral strategies to mitigate the deleterious effects of sedentarism on vascular health. One strategy to improve cardiovascular health is to interrupt sitting with activity breaks such as short periods of walking or fidgeting (6, 19). These periods of activities have been documented to prevent the decline in lower limb flow-mediated dilation (FMD), a measure of vascular endothelial function, which occurs in response to prolonged sitting (6, 19). However, the optimal dose and frequency of interruptions using physical activities and the question of whether such interventions would provide sufficient protection from the compounding effects of a mild hypercapnic environment during prolonged sitting on autonomic and vascular function remain unknown.
Passive limb movement is also a potent stimulus to induce both central hemodynamic responses (20) and hyperemia in the lower limb (21) with therapeutic benefits on vascular function in chronic conditions characterized by low flow and poor vascular function (22, 23). However, whether passive movement can abolish the negative effects of prolonged sitting in a mild hypercapnic environment on vascular function has not been investigated. Besides the clinical potential of this intervention in the context of prolonged sitting, this approach would also enable us to mechanistically dissociate the effects induced by mechanical stress on the vasculature during passive stretching from the change in metabolism due to muscle contraction.
Therefore, this study comprehensively examined the effects of short-duration passive and low-intensity active lower limb movement during prolonged sitting in a mild hypercapnic environment on peripheral macrovascular endothelial function, hemodynamics, arterial stiffness, microvascular circulatory function, and autonomic nervous system activity in healthy adults. We hypothesized that both passive limb movement and active lower limb movement would preserve peripheral macrovascular and microvascular function.
MATERIALS AND METHODS
Participants
A total of 14 healthy young adults were recruited. All participants were between the ages of 19–35 yr old and had no current medical diagnoses that required treatment. All participants were free of dyslipidemia, CVD, neuromuscular disease, diabetes mellitus, and renal disease. Additional exclusion criteria included pregnant or nursing women and the use of hormonal contraceptives. Written, informed consent was obtained from all participants before the beginning of the study, and all experiments were examined in accordance with the protocol approved by the University of Nebraska Medical Center Institutional Review Board and carried out according to the Declaration of Helsinki.
Study Design
Each participant completed three experimental visits that were conducted within a randomized crossover study design with ≥ 1 wk between each visit (Fig. 1A). Each experimental visit was completed at 06:00 AM (± 1 h) after an overnight fast, and participants were asked to avoid alcohol, caffeine, and excessive physical activity for ≥ 24 h before each visit. Participants filled out an activity questionnaire that requested their average weekly activity, daily step counts obtained from a fitness tracker (Fitbits, Apple Watches, etc.), and their estimated V̇o2max from a previously completed submaximal aerobic fitness testing (YMCA cycle ergometer test) (17).
Figure 1.
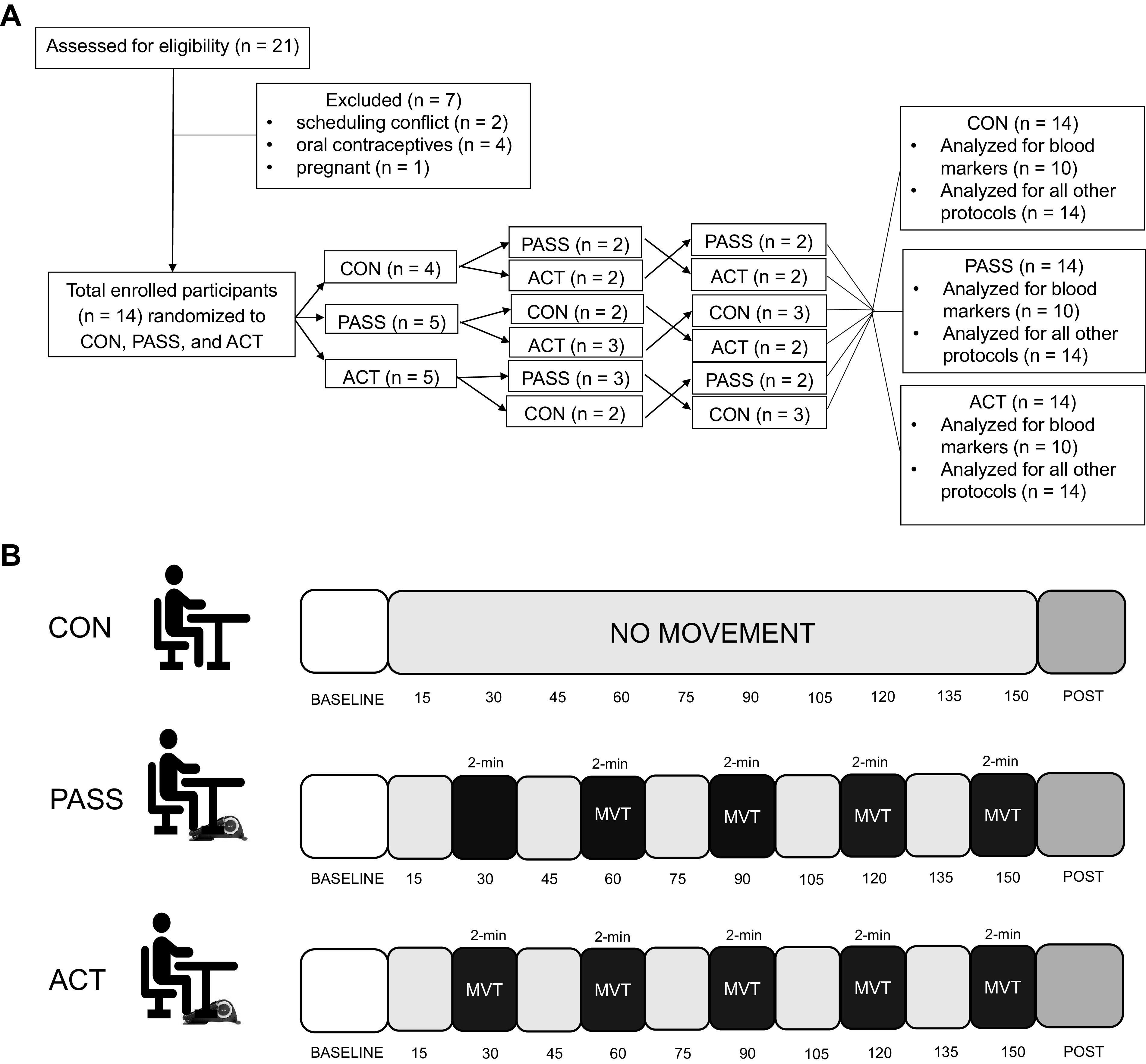
Participant allocation and study flow. A: randomized crossover study design and participant allocation to the control (CON, n = 14), passive (PASS, n = 14), and active (ACT, n = 14) groups. B: representation of the study flow for the CON, PASS, and ACT groups. Measurements were taken before sitting (baseline) and after sitting (post), which included resting heart rate, blood pressure, venous blood sampling, mean femoral arterial shear rate and blood flow, autonomic nervous system activity by heart rate variability, brachial and popliteal artery endothelial function (flow-mediated dilation, FMD), arterial stiffness (pulse-wave velocity, PWV), calf girth, and microvascular circulatory function. During sitting, measurements were taken at rest [2 min after the movement (MVT) bout in PASS and ACT if applicable] and during movement every 15 min and consisted of heart rate, blood pressure, and atmospheric CO2 monitoring.
Measurements of resting BP, resting HR, blood sampling, venous pooling, autonomic nervous system activity, endothelial function, femoral artery mean shear rate, pulse-wave velocity, and microcirculatory vascular function were obtained before and after 2.5 h of prolonged sitting in a mild hypercapnic environment (Fig. 1B). The 2.5-h sitting period was selected based on previous literature that indicated the negative effects of prolonged sitting occur within 1 h (6) and that no additional negative effects occur after 2 h (17, 24). The three experimental visits included 1) prolonged sitting with 2-min bouts of passive leg movement every 30 min, 2) prolonged sitting with 2-min bouts of active leg movement every 30 min (ACT), and 3) control prolonged sitting with no movement (CON). Movement bouts were performed using a foot elliptical (Cubii by Fitness Cubed Inc., Chicago, IL) at the rate of 1 Hz (60 cycles/min). The passive movement was induced by an investigator pedaling the participant’s feet in the elliptical, and a metronome was used to ensure a 60 cycles/min cadence. Investigators informed the participants before each passive movement bout to remain relaxed before and during the passive movement. To avoid confounding muscular contractions, participants were informed that the passive movement would begin in ∼1 min but were not informed of the exact starting point before each bout (25). The active movement was performed by the participant pedaling at a preset workload (13 W) to a metronome to ensure the same cadence. Participant cadence adherence was constantly monitored, and investigators informed them to increase or decrease speed as applicable. Our pilot data (n = 7 subjects, unpublished data) examined femoral artery mean shear rate in response to three workloads of 7 W, 8 W, and 13 W. The 13 W workload increased femoral artery mean shear rate (571.6 ± 33.4 s−1) to a level comparable with a previous study using active movement bouts during prolonged sitting (26). In addition, the change in knee joint angle was similar between the PASS and ACT groups (PASS: 32.4 ± 6.2°, ACT: 31.0 ± 5.7°). All female participants (n = 7) were tested during the early to midfollicular (days 1–10) and late luteal phases (> day 19) of the menstrual cycle to reduce the potential confounding impacts of endogenous estrogen on autonomic function (17, 27).
Experimental Conditions
After descriptive and baseline measurements, each participant sat for 2.5 h in an environmental chamber (Darwin Chambers Company, St. Louis, MO) during each experimental condition. Natural respiration produced by the participant and investigators was used to generate the mild hypercapnic environment. Atmospheric CO2 was set to ∼1,500 ppm, which is a typical atmospheric CO2 level in classrooms and offices (14–16) and exacerbates the negative effects of prolonged sitting (17). Atmospheric conditions (temperature, humidity, and CO2 concentration) were monitored every 15 min during sitting (Fig. 1B). Temperature and relative humidity were monitored using a handheld weather meter (Kestrel Meters, Brooklyn, PA) and were maintained at 22°C and 50% relative humidity, respectively. Atmospheric CO2 concentrations were monitored with a CO2 meter (Extech CO260 CO/CO2 Meter+; Psychrometer, Nashua, NH). In addition, measurements of both resting HR and BP and during-movement (PASS and ACT movement bouts) HR and BP were taken during sitting. Participants were asked to bring nonstimulating reading material or to do work on a laptop to reduce any extraneous muscle contractions during the sitting period (17).
Body Composition, Anthropometrics, and Handgrip Strength
Height and handgrip strength were assessed upon arrival at the first visit. Body mass, body mass index (BMI), body composition, and volume of the thigh and shank were assessed at all three visits. Height was measured to the nearest 0.5 cm. Body composition was measured using bioelectrical impedance analysis (InBody H20N, InBody Europe B.V., Amsterdam, The Netherlands). Leg volume was quantified by measuring thigh and lower leg skinfolds and circumferences of three sites (distal, middle, and proximal) using standard calipers and measuring tape as previously described (28–30). Thigh and lower leg volumes were calculated as previously described and reported as leg muscle mass (28–30). Venous pooling was estimated by measuring calf circumference at the largest circumference of the calf (24). Handgrip strength was measured for both hands using a handgrip dynamometer (JAMAR, Bolingbrook, IL) as previously described (31).
Indirect Calorimetry
Indirect calorimetry was used for the first 20 min and at the 150 min time point of sitting to assess fractional content of expired CO2 (FECO2) and tidal volume (Vt) in normocapnic and hypercapnic conditions using a gas- and flow-calibrated metabolic cart (ParvoMedics TrueOne Metabolic System, Sandy, UT).
Blood Pressure and Heart Rate at Rest and during Movement
Resting systolic blood pressure (SBP), diastolic blood pressure (DBP), and HR were measured using an automatic sphygmomanometer (Omron BP786N; Omron Corp., Kyoto, Japan) before and after sitting as previously described (17), and mean arterial pressure (MAP) was calculated as MAP = DBP + 1/3 (SBP – DBP). Resting measurements were repeated every 15 min during sitting using an automated sphygmomanometer and a Suunto watch and chest band (Suunto Spartan Sport Watch and Smart Sensor, Suunto, Vantaa, Finland). To ensure a rested state after movement bouts, a 2-min recovery period was used before resting measurements for the PASS and ACT groups. During movement, HR and BP were measured during the movement bouts in the PASS and ACT groups. The HR was recorded continuously during each 2-min movement bout, and BP was measured at the 1-min point during each movement bout.
Blood Sampling
Blood samples were drawn from an antecubital vein using EDTA tubes (∼10 mL) before and after prolonged sitting. Samples were centrifuged for 10 min at 3,500 rpm at 4°C, and plasma samples were stored in microcentrifuge tubes at −80°C. Later analyses for NO bioavailability (total nitrate/nitrite) and endothelin-1 (ET-1) were measured with a nitrate/nitrite colorimetric assay kit (Cat. No. 780001, Cayman Chemical, Ann Arbor, MI) and an ET-1 ELISA kit (No. EIAET, Thermo Fisher Scientific, Waltham, MA), respectively, according to the manufacturer’s instructions.
Autonomic Nervous System Activity Assessments
Autonomic nervous system function was measured using HRV and a head-up tilt test. Each participant was fastened to the tilt table (9520 Manual Economy Tilt Table, Bailey Manufacturing, Lodi, OH) in a supine position (180°) for 20 min. HR was recorded for 2 min using the Suunto watch and band. Following the recording, the participant was tilted to 70° and was informed to stay as rested as possible. After 20 min at 70°, HR was recorded for 2 min. The raw RR interval data were extracted from these HR recordings and analyzed using Kubios HRV Standard (Kubios Oy, Boston, MA) software (32), and using participant total power, low frequency (LF) power, high frequency (HF) power, and LF/HF ratio were recorded and normalized (17, 33).
Endothelial Function and Femoral Artery Blood Flow and Mean Arterial Shear Rate
Flow-mediated dilation (FMD) was used to measure endothelial function in the brachial and popliteal arteries with a Doppler ultrasound system (Terason uSmart 3300, Terason Division Teratech Corporation, Burlington, MA), a 3-lead electrocardiogram system (7700 Series Trigger Monitor, Ivy Biomedical Systems Inc., Branford, CT), and a rapid-inflation cuff system (E20 Rapid Cuff and cuff model SC5, D.E. Hokanson, Bellevue, WA) as previously described (17). Popliteal artery FMD was assessed first with the rapid-inflation cuff secured just distal to the popliteal fossa with the participant prone, and brachial artery FMD was assessed second with the cuff placed just distal to the antecubital fossa with the participant supine. For both procedures, resting artery diameter was recorded 1–2 cm proximal to the cuff for 5 min with the ultrasound probe. The cuff then was inflated (250 mmHg) for 5 min. After cuff release, arterial diameter was recorded continuously for 5 min on R-wave trigger. Arterial images were analyzed with Vascular Research Tools 6 (Medical Imaging Applications, LLC, Coralville, IA). The most stable 30–60 s of baseline (including ≥ 10 cardiac cycles) were averaged as the resting baseline arterial diameter (34) and percent changes were calculated as previously described (35).
Femoral artery blood flow and mean shear rate were assessed with the ultrasound system (36). The common femoral artery was located with the probe ∼1–2 cm distal to the inguinal ligament but proximal to the deep and superficial bifurcations at 10 MHz. The femoral artery diameter was measured at a perpendicular angle along the central axis of the artery. The blood velocity was collected at a frequency of 5 MHz with an insonation angle of ≤ 60°. Second-by-second blood velocity was used to calculate mean velocity (Vm). Blood flow was calculated as Vm × π (vessel diameter/2)2 × 60, with blood flow in mL·min−1 (36). Mean shear rate was calculated as 4 × Vm/artery diameter (36).
Pulse-Wave Velocity
Measurements of arterial stiffness were assessed using pulse-wave velocity (PWV). Peripheral arterial stiffness was assessed with carotid-to-radial PWV, carotid-to-distal PWV, and lower limb PWV as previously described (17, 37), and central arterial stiffness was assessed using carotid-to-femoral PWV as previously described with participants in the supine position (17). Briefly, carotid-to-radial and carotid-to-distal PWV were measured using applanation tonometry (Complior Analyze, Alam Medical, Saint-Quentin-Fallavier, France). The pulse waveforms were recorded on the system for ∼30–60 s. Lower extremity PWV was measured using applanation tonometry and an oscillometric cuff (SphygmoCor XCEL, AtCor Medical, Sydney, Australia) and calculated as previously described (37). Carotid-to-femoral PWV was assessed using the same XCEL system as previously described (17).
Microvascular Circulatory Function
Microvascular oxygenation was quantified using noninvasive near-infrared spectroscopy (NIRS) as previously described (17). This device provides continuous measurements of oxygenated ([HbO2]), deoxygenated ([HHb]), and total ([Hbtot]) hemoglobin levels as well as a tissue oxygenation index (TOI; i.e., [HbO2]/[Hbtot]). Briefly, the NIRS device (PortaLite, Artinis, Eisteinweg, The Netherlands) was secured to the skin over the medial gastrocnemius of the dominant leg and wrapped with a commercially available black bandage with the participant in an upright seated position. A rapid-inflation cuff (model SC12D, D. E. Hokanson, Bellevue, WA) was placed just proximal to the knee. NIRS measurements were taken continuously throughout the entire protocol at a sampling rate of 10 Hz. Baseline measurements of tissue oxygen saturation were taken for 5 min. The cuff was inflated to 275 mmHg for 5 min. Following deflation, tissue oxygen recovery was continuously recorded until the values became stable (38).
Hemoglobin and myoglobin possess indistinguishable spectral characteristics in the NIRS signal; therefore, the signal is considered to be primarily derived from Hb (39). The signals were analyzed according to a modified Beer–Lambert’s law, and a constant differential pathlength factor was not used due to the assumption that constant optical scattering of the photons has been demonstrated to affect alterations in NIRS signals (40). Data were expressed as relative changes with respect to baseline as a percentage (TOI). Tissue reoxygenation was estimated by calculating the initial slope of TOI recovery, which has been used as an index of microvascular function (17, 41, 42).
Statistical Analysis
The Shapiro–Wilk test was used to test normality of the data. A one-way ANOVA was used for group comparisons at baseline (CON, PASS, and ACT). A two-way analysis of variance (ANOVA) with repeated measures [group (CON, PASS, and ACT) × time (before and after 2.5 h of sitting)] was used to compare the difference of changes at pre- and post-sitting within and between groups. When a significant interaction was noted, Tukey’s test was used for post hoc comparisons. GraphPad Prism v. 9.0.2 (GraphPad Software, San Diego, CA) was used for statistical analyses. Statistical significance was set at P < 0.05. Data are presented as means ± SD unless otherwise noted.
RESULTS
Participant Characteristics and Calf Girth
Participants (age: 23.6 ± 2.3 yr, height: 170.5 ± 7.6 cm, right-hand grip strength: 38.5 ± 6.4 kg, left handgrip strength: 35.5 ± 6.4 kg) averaged 12,666 ± 2,743 steps per day and a V̇o2max of 46.4 ± 6.5 mL·kg−1·min−1, and participant characteristics were not different between study visits (Table 1). No adverse events were reported, and all participants were able to complete all experimental visits. Unlike ACT (Δ 0.5 ± 0.5 cm, P > 0.05), calf girth was significantly increased after sitting in CON (Δ 1.6 ± 0.2 cm, P < 0.05) and PASS (Δ 1.4 ± 0.3 cm, P < 0.05).
Table 1.
Participant characteristics at control (CON, n = 14), passive (PASS, n = 14), and active (ACT, n = 14) visits
| CON, n = 14 | PASS, n = 14 | ACT, n = 14 | |
|---|---|---|---|
| Body mass, kg | 73.6 ± 13.3 | 72.6 ± 13.4 | 72.8 ± 13.1 |
| Body fat, % | 17.5 ± 5.5 | 17.8 ± 3.5 | 16.9 ± 2.8 |
| BMI, kg/m2 | 24.5 ± 3.7 | 24.4 ± 3.8 | 24.3 ± 3.7 |
| Heart rate, beats/min | 63.7 ± 8.0 | 67.5 ± 11.2 | 65.4 ± 5.8 |
| Systolic BP, mmHg | 109.4 ± 10.6 | 109.8 ± 10.5 | 108.3 ± 10.9 |
| Diastolic BP, mmHg | 67.9 ± 3.7 | 67.1 ± 7.2 | 69.3 ± 9.3 |
| MAP, mmHg | 81.8 ± 6.0 | 81.3 ± 8.3 | 82.3 ± 9.8 |
| Leg muscle mass, kg | 14.2 ± 3.1 | 14.1 ± 3.4 | 14.4 ± 3.2 |
BMI, body mass index; BP, blood pressure; MAP, mean arterial pressure.
Values are represented as means ± SD. One-way ANOVA. n, Number of subjects.
Heart Rate Variability
No significant changes were observed (P > 0.05) within or between conditions for LF power (CON: Δ 3.7 ± 2.2 n.u., PASS: Δ 10.6 ± 2.2 n.u., ACT: Δ 13.8 ± 3.8 n.u.) and HF power (CON: Δ −3.6 ± 2.2 n.u., PASS: Δ −10.6 ± 2.2 n.u., ACT: Δ −13.9 ± 3.6 n.u.) (Table 2). However, the ΔLF/HF ratio in PASS (1.4 ± 0.1) was significantly greater (P < 0.05) than CON (Δ 0.6 ± 1.1), and the ΔLF/HF ratio was significantly greater (P < 0.05) in ACT (Δ 2.1 ± 0.6) compared with both CON and PASS (Table 2).
Table 2.
Heart rate and heart rate variability, pulse-wave velocity, blood pressure, and arterial hemodynamics before and after prolonged sitting at the control (CON, n = 14), passive (PASS, n = 14), and active (ACT, n = 14) visits
| CON, n = 14 |
PASS, n = 14 |
ACT, n = 14 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Δ | Pre | Post | Δ | Pre | Post | Δ | |
| Heart rate and HRV variables | |||||||||
| Heart rate, beats/min | 63.7 ± 8.2 | 62.9 ± 9.1 | −0.8 ± 0.9 | 67.5 ± 11.2 | 68.2 ± 10.9 | 0.6 ± 0.3 | 65.4 ± 5.8 | 61.5 ± 9.6 | −3.9 ± 3.8 |
| Total power, ms2 | 4811.3 ± 2646.2 | 4155.5 ± 2302.2 | −655.8 ± 344.1 | 4211.7 ± 1006.1 | 4163.9 ± 814.0 | −47.8 ± 192.1 | 4944.7 ± 1089.5 | 4556.1 ± 855.3 | −388.6 ± 233.7 |
| LF power, n.u. | 73.8 ± 5.4 | 77.4 ± 3.3 | 3.7 ± 2.2 | 66.6 ± 6.7 | 77.2 ± 4.5 | 10.6 ± 2.2 | 66.3 ± 7.5 | 80.2 ± 3.7 | 13.8 ± 3.8 |
| HF power, n.u. | 26.1 ± 5.5 | 22.5 ± 3.3 | −3.6 ± 2.2 | 33.4 ± 6.7 | 22.7 ± 4.5 | −10.6 ± 2.2 | 33.6 ± 7.5 | 19.7 ± 3.9 | −13.9 ± 3.6 |
| LF/HF ratio | 2.8 ± 4.1 | 3.4 ± 3.0 | 0.6 ± 1.1 | 2.0 ± 2.4 | 3.4 ± 2.3 | 1.4 ± 0.1* | 2.0 ± 2.7 | 4.1 ± 2.1 | 2.1 ± 0.6*† |
| Pulse-wave velocity | |||||||||
| Carotid-to-radial PWV, m/s | 8.7 ± 0.5 | 8.9 ± 0.3 | 0.2 ± 0.2 | 8.5 ± 0.3 | 8.6 ± 0.4 | 0.1 ± 0.1 | 8.5 ± 0.3 | 8.4 ± 0.2 | −0.1 ± 0.1 |
| Carotid-to-ankle PWV, m/s | 7.9 ± 0.1 | 7.8 ± 0.2 | −0.1 ± 0.1 | 8.2 ± 0.2 | 7.9 ± 0.2 | −0.3 ± 0.0 | 7.9 ± 0.2 | 7.6 ± 0.2 | −0.2 ± 0.0 |
| Carotid-to-femoral PWV, m/s | 7.4 ± 0.3 | 7.5 ± 0.3 | 0.1 ± 0.0 | 7.2 ± 0.3 | 7.7 ± 0.3 | 0.5 ± 0.6 | 7.2 ± 0.3 | 7.5 ± 0.3 | 0.3 ± 0.0 |
| Femoral-to-ankle PWV, m/s | 10.1 ± 0.8 | 10.1 ± 0.1 | 0.0 ± 0.7 | 10.1 ± 0.1 | 10.2 ± 0.1 | 0.1 ± 0.0 | 10.1 ± 0.2 | 10.2 ± 0.1 | 0.1 ± 0.0 |
| Blood pressure | |||||||||
| Systolic BP, mmHg | 109.6 ± 3.0 | 106.3 ± 2.3 | −3.3 ± 0.7 | 110.3 ± 1.9 | 107.3 ± 2.2 | −3.0 ± 0.2 | 109.1 ± 2.8 | 111.6 ± 3.0 | 2.5 ± 0.2 |
| Diastolic BP, mmHg | 67.2 ± 1.4 | 67.2 ± 2.3 | 0.0 ± 1.0 | 67.5 ± 2.4 | 67.3 ± 2.0 | −0.2 ± 0.4 | 65.9 ± 2.0 | 65.1 ± 1.7 | −0.8 ± 0.3 |
| MAP, mmHg | 81.0 ± 1.6 | 80.6 ± 1.2 | −0.4 ± 0.4 | 79.0 ± 1.1 | 81.3 ± 2.2 | 2.3 ± 1.1 | 78.3 ± 1.2 | 79.2 ± 1.4 | 0.9 ± 0.2 |
| Shear rate and blood flow | |||||||||
| CFA shear rate, s−1 | 355.5 ± 23.2 | 235.7 ± 17.6* | −119.8 ± 5.6 | 346.6 ± 22.7 | 250.1 ± 13.5* | −96.5 ± 9.2 | 356.1 ± 20.3 | 453.3 ± 23.3*† | 97.2 ± 3.1 |
| CFA blood flow, mL·min−1 | 362.2 ± 14.8 | 250.3 ± 17.6* | −112.0 ± 2.8 | 358.6 ± 14.6 | 281.6 ± 16.2* | −77.0 ± 1.6 | 356.2 ± 14.5 | 427.4 ± 17.4*† | 71.2 ± 2.9 |
Heart rate variability metrics are normalized to total power (n.u.). BP, blood pressure; CFA, common femoral artery; HF, high frequency; LF, low frequency; MAP, mean arterial pressure; PWV, pulse-wave velocity. Values are represented as means ± SE. Two-way ANOVA with repeated measures and Tukey’s post hoc test. *P < 0.05 vs. pre. †P < 0.05 vs. PASS and ACT; n, number of subjects.
Endothelial Function, Pulse-Wave Velocity, and Hemodynamics during Movement
Popliteal artery FMD was significantly reduced (P < 0.05) after sitting in CON and PASS but preserved in ACT (P > 0.05). Post-ACT was significantly greater than post-CON and post-PASS (P < 0.05) (Fig. 2A, Supplemental Fig. S1A; see https://doi.org/10.6084/m9.figshare.17708567). In addition, the sitting-induced reduction in popliteal artery FMD (CON: Δ −3.6 ± 0.4% and PASS: Δ −3.2 ± 0.4%) was significantly less in ACT (Δ −0.2 ± 0.2%) (Fig. 2B). Brachial artery FMD was significantly reduced (P < 0.05) after sitting in CON and PASS but was preserved (P > 0.05) in ACT. Post-ACT was significantly greater than post-CON and post-PASS (P < 0.05) (Fig. 2C, Supplemental Fig. S1B). The sitting-induced change in brachial artery FMD (CON: Δ −3.6 ± 0.5% and PASS: Δ −3.3 ± 0.4%) was significantly less in ACT (Δ −0.7 ± 0.4%) (Fig. 2D). In contrast, pulse-wave velocity was not significantly different within and between conditions (Table 2). During active movement, there was an increase in femoral mean arterial shear rate (Δ 269.7 ± 18.8 s−1, P < 0.05) but not during passive movement (Δ 30.5 ± 4.9 s−1, P > 0.05). Brachial artery mean shear rate did not increase (P > 0.05) during active movement (Δ 11.3 ± 4.3 s−1) or passive movement (Δ 6.0 ± 3.9 s−1).
Figure 2.
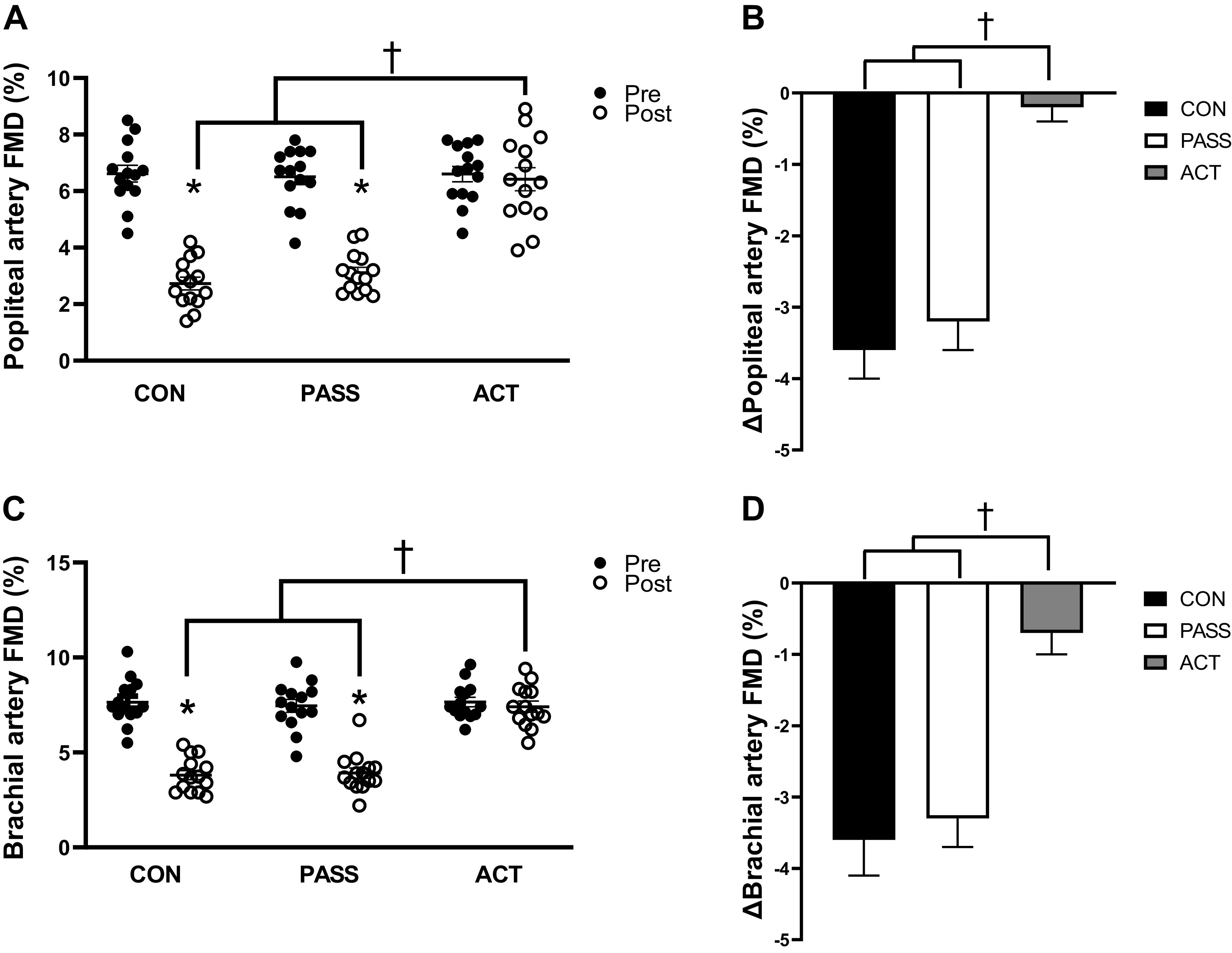
Flow-mediated dilation (FMD, %) and change (pre to post) in FMD (%) in the brachial and popliteal arteries pre- and post-prolonged sitting in control (CON, n = 14), passive movement (PASS, n = 14), and active (ACT, n = 14) groups. A: popliteal artery FMD was significantly reduced after prolonged sitting in the CON and PASS groups, whereas FMD was maintained after prolonged sitting in the ACT group. Post-CON and post-PASS FMDs were significantly lower than post-ACT. B: the reduction in popliteal artery FMD was significantly greater in both CON and PASS groups compared with the ACT group. C: brachial artery FMD was significantly reduced after prolonged sitting in the CON and PASS groups, whereas FMD was maintained in the ACT group. Post-CON and post-PASS FMDs were significantly lower than post-ACT. D: the reduction in brachial artery FMD was significantly greater in both CON and PASS groups compared with the ACT group. Values are represented as means ± SE. A and C: two-way ANOVA with repeated measures and Tukey’s post hoc test. B and D: one-way ANOVA and Tukey’s post hoc test. *P < 0.05 vs. Pre. †P < 0.05 vs. ACT.
Blood Pressure and Heart Rate at Rest and during Movement
There were no significant differences (P > 0.05) in resting HR, SBP, DBP, or MAP within or between groups at rest during prolonged sitting (Table 3). During movement, ACT demonstrated significantly greater (P < 0.05) HR and systolic BP at several time points compared with both CON and PASS (Table 3).
Table 3.
Physiological outcomes every 30 min during sitting and during movement at the control (CON, n = 14), passive (PASS, n = 14), and active (ACT, n = 14) visits
| CON, n = 14 |
PASS, n = 14 |
ACT, n = 14 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time, Min | 30 | 60 | 90 | 120 | 150 | 30 | 60 | 90 | 120 | 150 | 30 | 60 | 90 | 120 | 150 |
| Physiological outcomes during sitting | |||||||||||||||
| Heart rate, beats/min | 60.7 ± 1.7 | 61.2 ± 1.6 | 59.5 ± 1.8 | 60.5 ± 1.6 | 59.3 ± 2.2 | 63.5 ± 2.6 | 59.8 ± 3.2 | 57.7 ± 2.5 | 58.8 ± 2.7 | 56.4 ± 2.5 | 62.7 ± 2.9 | 63.1 ± 2.2 | 63.2 ± 2.9 | 63.6 ± 2.7 | 62.6 ± 2.3 |
| Systolic BP, mmHg | 100.9 ± 3.1 | 102.7 ± 3.1 | 104.3 ± 1.8 | 106.6 ± 2.2 | 110.6 ± 2.7 | 103.4 ± 1.7 | 102.9 ± 1.8 | 108.3 ± 1.3 | 104.5 ± 2.4 | 107.3 ± 2.3 | 109.2 ± 2.2 | 110.8 ± 2.0 | 112.0 ± 2.6 | 113.1 ± 2.3 | 113.0 ± 2.6 |
| Diastolic BP, mmHg | 65.6 ± 3.3 | 66.0 ± 1.5 | 65.3 ± 2.0 | 66.2 ± 1.2 | 69.5 ± 1.2 | 64.4 ± 1.7 | 64.1 ± 1.3 | 67.4 ± 2.0 | 65.4 ± 1.4 | 67.3 ± 2.0 | 66.1 ± 1.7 | 65.7 ± 1.3 | 64.6 ± 1.6 | 66.8 ± 1.8 | 65.6 ± 1.6 |
| MAP, mmHg | 77.3 ± 3.1 | 78.2 ± 3.1 | 78.3 ± 1.8 | 79.7 ± 2.2 | 83.2 ± 2.7 | 77.4 ± 1.7 | 77.0 ± 1.8 | 81.0 ± 1.3 | 78.4 ± 2.4 | 80.6 ± 2.3 | 80.5 ± 2.7 | 80.7 ± 2.4 | 80.4 ± 3.6 | 82.2 ± 3.1 | 81.4 ± 3.3 |
| Physiological outcomes during movement | |||||||||||||||
| Heart rate, beats/min | 60.7 ± 1.7 | 61.2 ± 1.6 | 59.5 ± 1.8 | 60.5 ± 1.6 | 59.3 ± 2.2 | 63.5 ± 2.6 | 60.6 ± 2.3 | 61.3 ± 2.5 | 61.9 ± 2.7 | 62.6 ± 2.9 | 81.5 ± 2.7*† | 80.2 ± 2.5*† | 82.8 ± 2.0*† | 83.8 ± 2.5*† | 83.9 ± 2.3*† |
| Systolic BP, mmHg | 100.9 ± 3.1 | 102.7 ± 3.1 | 104.2 ± 1.8 | 106.6 ± 2.2 | 110.6 ± 2.7 | 103.9 ± 2.1 | 103.7 ± 1.9 | 109.1 ± 2.3 | 105.1 ± 2.1 | 107.8 ± 1.2 | 110.9 ± 1.7 | 117.5 ± 3.6*† | 116.5 ± 3.1*† | 116.3 ± 2.2*† | 115.5 ± 1.7 |
| Diastolic BP, mmHg | 65.6 ± 1.1 | 66.6 ± 3.3 | 66.0 ± 1.5 | 65.3 ± 2.0 | 66.2 ± 1.2 | 64.9 ± 2.2 | 65.9 ± 1.7 | 65.8 ± 1.4 | 65.9 ± 2.7 | 67.5 ± 1.2 | 68.2 ± 2.8 | 67.4 ± 2.5 | 67.1 ± 2.2 | 67.6 ± 1.6 | 67.2 ± 1.4 |
| MAP, mmHg | 77.4 ± 3.0 | 77.9 ± 3.2 | 78.8 ± 1.8 | 79.1 ± 2.2 | 81.0 ± 2.5 | 78.6 ± 2.3 | 78.4 ± 2.6 | 80.3 ± 3.0 | 80.0 ± 2.4 | 80.9 ± 2.2 | 81.9 ± 2.7 | 83.9 ± 2.8 | 83.9 ± 3.0 | 83.6 ± 3.2 | 83.1 ± 1.8 |
BP, blood pressure; MAP, mean arterial pressure. Values are represented as means ± SE. Two-way ANOVA with repeated measures and Tukey’s post hoc test. *P < 0.05 vs. CON. †P < 0.05 vs. PASS.
Microvascular Circulatory Function
Following cuff deflation, TOI recovery rate was significantly blunted (P < 0.05) after sitting in CON, and post-CON was significantly lower (P < 0.05) than post-PASS and post-ACT, whereas post-ACT was significantly greater (P < 0.05) than post-PASS (Fig. 3A, Supplemental Fig. S2). In addition, sitting-induced reduction in TOI recovery rate (post − pre, Δ) in CON (Δ −12.1 ± 1.4%·min−1) was significantly lower (P < 0.05) than PASS (Δ −3.4 ± 2.0%·min−1) and ACT (Δ 4.2 ± 1.3%·min−1), and PASS was significantly lower than ACT (Fig. 3B).
Figure 3.
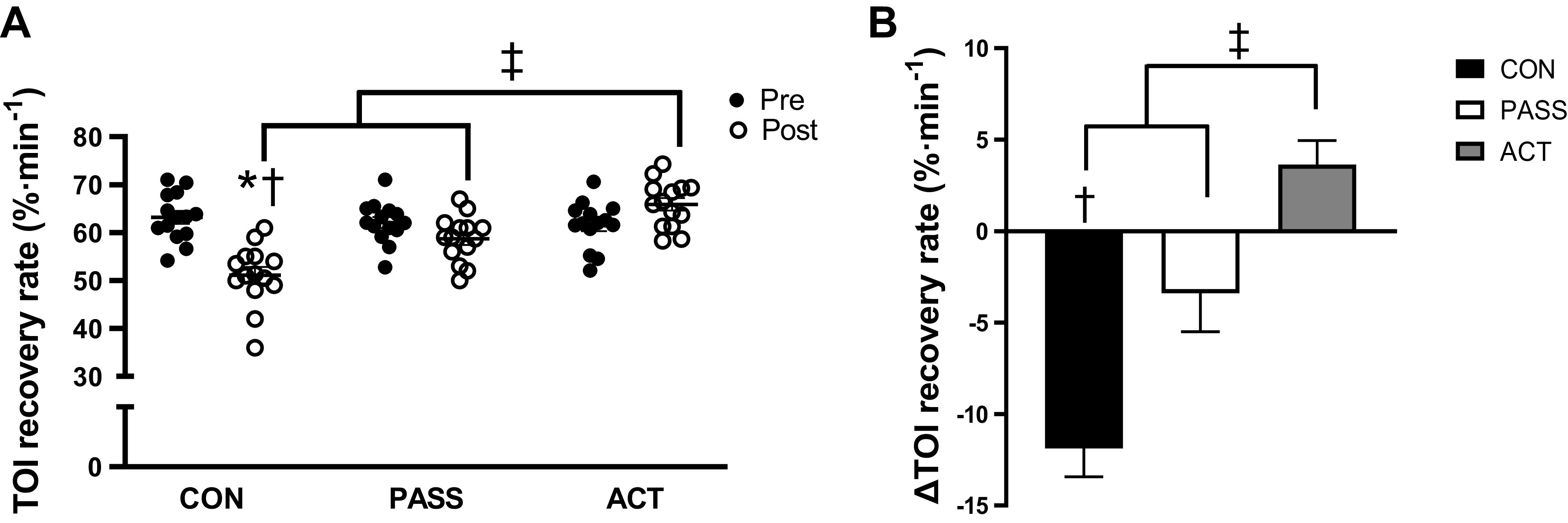
Measurements of microvascular circulatory function before and after prolonged sitting in control (CON, n = 14), passive movement (PASS, n = 14), and active movement (ACT, n = 14) groups. A: tissue oxygenation index (TOI) recovery rate (%·min−1) was significantly reduced after sitting in the CON group, and post-CON was significantly lower than both post-PASS and post-ACT. Post-ACT was significantly greater than both post-CON and post-PASS. B: reduction in TOI recovery rate (%·min−1) was significantly greater in CON compared with PASS and ACT, and the reduction in PASS was significantly greater than ACT. Values are represented as means ± SE. A: two-way ANOVA with repeated measures and Tukey’s post hoc test. B: one-way ANOVA and Tukey’s post hoc test. *P < 0.05 vs. Pre. †P < 0.05 vs. PASS. ‡P < 0.05 vs. ACT.
Vasoactive Metabolites
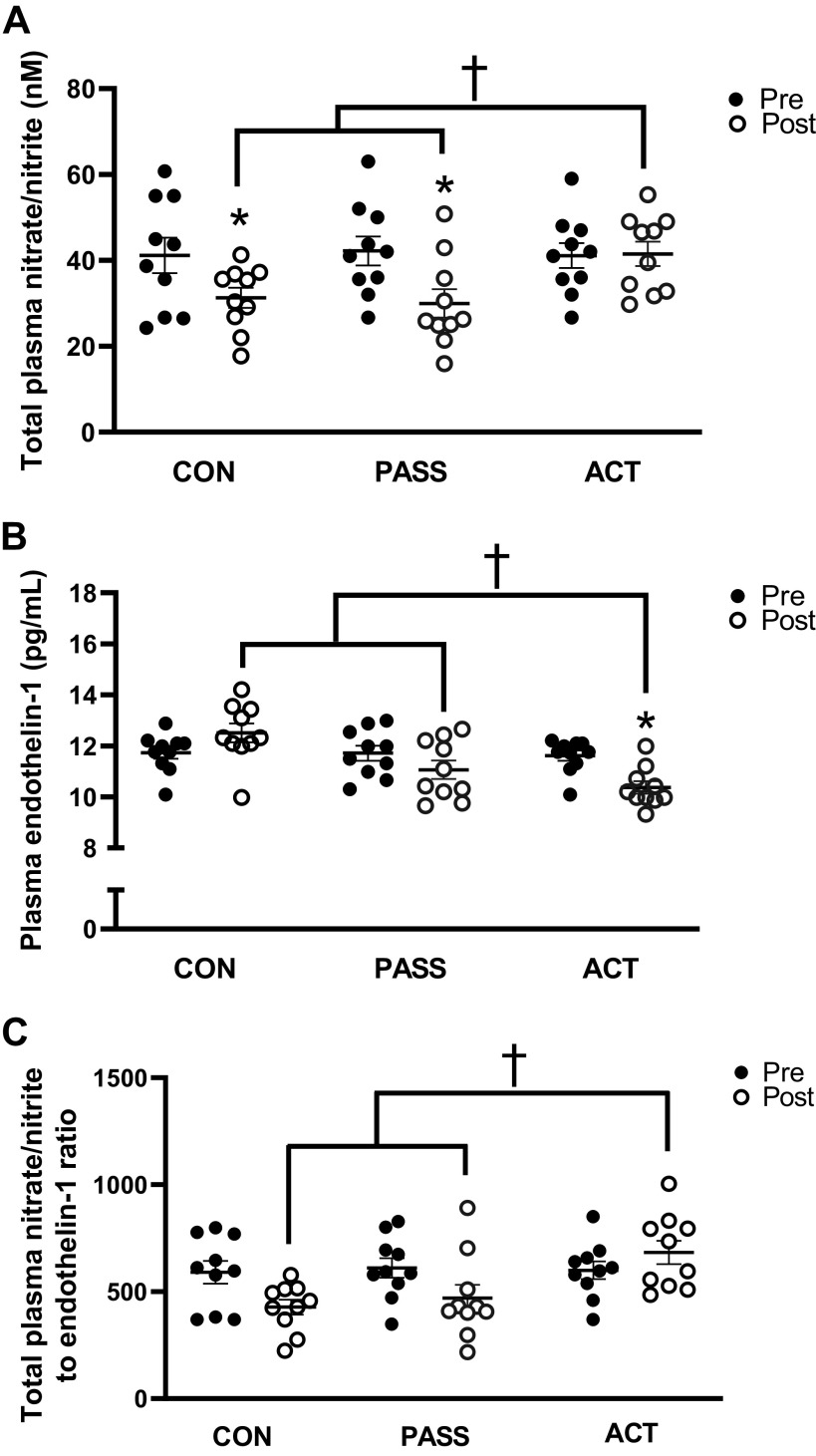
Total plasma nitrate/nitrite was significantly reduced (P < 0.05) after sitting in CON (Δ −9.9 ± 3.2 nM) and PASS (Δ −12.2 ± 3.6 nM), whereas it was not significantly changed (P > 0.05) in ACT (Δ 0.4 ± 4.4 nM) (Fig. 4A, Supplemental Fig. S3A). In addition, post-ACT was significantly greater (P < 0.05) than post-CON and post-PASS. Plasma levels of ET-1 were significantly reduced (P < 0.05) after sitting in ACT (Δ −1.2 ± 0.3 pg/mL), which were significantly lower than post-CON (Δ 0.8 ± 0.3 pg/mL) and post-PASS (Δ −0.7 ± 0.5 pg/mL) (Fig. 4B, Supplemental Fig. S3B). There was a trend (P = 0.07) for a lower total nitrate/nitrite to ET-1 ratio after sitting in CON (Δ −162.3 ± 43.0), but remained statistically similar (P > 0.05) after sitting in PASS (Δ −141.3 ± 67.5) and ACT (Δ 83.9 ± 62.3) (Fig. 4C, Supplemental Fig. S3C). However, the total nitrate/nitrite to ET-1 ratio in post-CON and post-PASS was significantly lower (P < 0.05) than post-ACT (Fig. 4C, Supplemental Fig. S3C).
Figure 4.
Total plasma nitrate/nitrite levels (nM), endothlin-1 levels (pg/mL), and total plasma nitrate/nitrite to endothelin-1 ratios pre- and post-prolonged sitting in control (CON, n = 10), passive (PASS, n = 10), and active (ACT, n = 10) groups. A: total plasma nitrate/nitrite was significantly reduced after prolonged sitting in the CON and PASS groups, whereas it was maintained in the ACT group. Post-CON and post-PASS were significantly lower than post-ACT. B: endothelin-1 was significantly reduced after prolonged sitting in the ACT condition, and post-ACT was significantly greater than post-CON and post-PASS. C: the total plasma nitrate/nitrite to endothelin-1 ratio in post-CON and post-PASS was significantly lower than post-ACT. Values are represented as means ± SE. Two-way ANOVA with repeated measures and Tukey’s post hoc test. *P < 0.05 vs. Pre. †P < 0.05 vs. ACT
Indirect Calorimetry
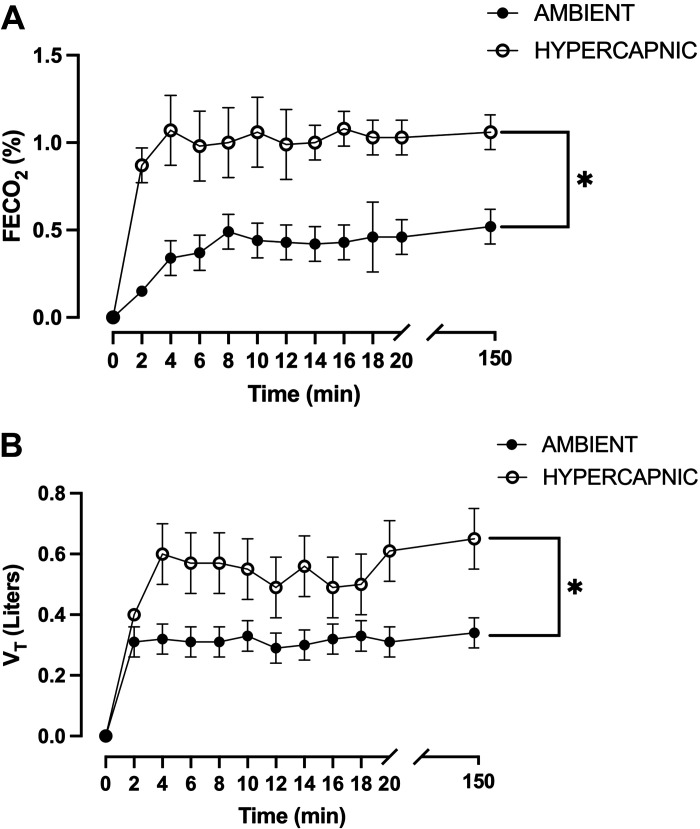
Indirect calorimetry was measured in a subset of participants (n = 8) and indicated a significant increase (P < 0.05) in FECO2 in the hypercapnic condition (CO2 ∼1,500 ppm) compared with a normocapnic condition (CO2 ∼400 ppm) (Fig. 5A) and resulted in a significant increase (P < 0.05) in tidal volume (Vt) (Fig. 5B).
Figure 5.
Fraction of end tidal carbon dioxide (FECO2) and tidal volume (Vt) during sitting time in ambient (CO2 = 400 ppm) and mild hypercapnic (CO2 = 1,500 ppm) conditions in a subset of participants. A: FECO2 is significantly higher in a mild hypercapnic condition (n = 8) at all time points compared with FECO2 in an ambient condition (n = 8). B: Vt is significantly higher in a mild hypercapnic condition (n = 8) at 4 min, 12 min, 14 min, 16 min, and 150 min compared with Vt in an ambient condition (n = 8). Values are represented as means ± SE. Two-way ANOVA with repeated measures and Tukey’s post hoc test. *P < 0.05 vs. ambient.
DISCUSSION
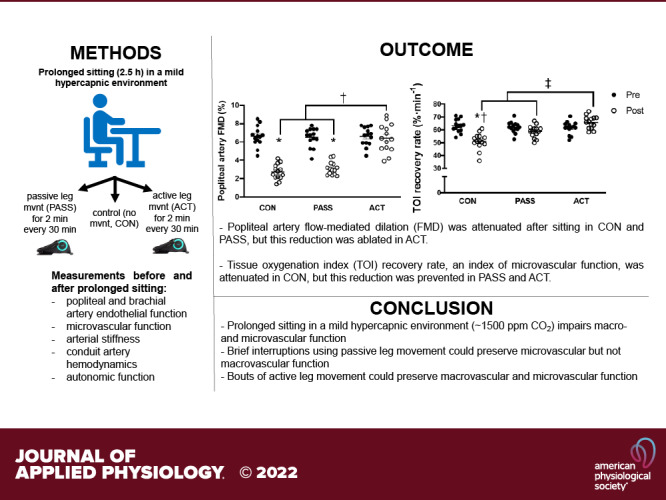
The goal of this study was to comprehensively examine the effects of short duration passive and low-intensity active lower limb movement during prolonged sitting in a mild hypercapnic environment on autonomic nervous system activity, peripheral endothelial function, arterial stiffness, and microvascular circulatory function in healthy young adults. The main findings of the study were that 1) prolonged sitting combined with a mild hypercapnic environment impaired both popliteal and brachial artery endothelial function along with microvascular reoxygenation, 2) brief periods of sitting interruption using passive lower limb movement preserved microcirculatory function measured by NIRS, whereas both brachial and popliteal artery FMD remained impaired, 3) sitting interruptions with short duration low-intensity active movement ablated the deleterious effect of prolonged sitting in a mild hypercapnic environment on both microcirculatory function and FMD (popliteal and brachial arteries), preserved total plasma nitrate/nitrite, and decreased ET-1 levels, and 4) both passive and active movement induced a shift toward greater activation of the sympathetic nervous system (SNS) relative to the parasympathetic nervous system (PNS) measured by the ratio of LF/HF. Together, these findings suggest only a partial protection from passive leg movement to interrupt prolonged sitting in a mild hypercapnic environment, whereas short-duration low-intensity active movement elicited a robust protection on both microvascular and macrovascular function from prolonged sitting, which appears to be mediated through both hemodynamic changes and blood circulating factors (adequate balance between NO bioavailability and the potent vasoconstrictor ET-1). Also, our results suggest that sympathetic outflow likely did not play a major role in the adverse effects of prolonged sitting in mild hypercapnic environment on macrovascular and microvascular function.
Effect of Prolonged Sitting in a Mild Hypercapnic Environment on Vascular Function
In line with our previous results (17), prolonged sitting combined with a mild hypercapnic environment substantially attenuated popliteal artery endothelial function (Δ −3.6 ± 0.4%, Fig. 2, A and B, Supplemental Fig. S1A). It is noteworthy that the magnitude of this change is higher than the specific effect of prolonged sitting (3–6 h) on popliteal artery FMD (−2.51%) estimated in a recent meta-analysis (43). In addition, brachial artery FMD (Δ −3.6 ± 0.5%) was also significantly blunted following sitting in a mild hypercapnic environment in this study (Fig. 2, C and D, Supplemental Fig. S1B) and in our previous work (17). These findings support the compounding effect of a mild hypercapnic environment during sitting on vascular function, as prolonged sitting alone is not associated with changes in brachial artery FMD (43). In addition, we also confirmed our previous findings (17) of impaired microcirculation in the lower extremity skeletal muscle. Specifically, we found that the rate of microvascular reoxygenation measured in the lower limb by NIRS, a commonly used index of microvascular function (41, 42), is blunted after prolonged sitting in a mild hypercapnic environment (Fig. 3, A and B, Supplemental Fig. S2).
Several mechanisms may be underlying these vascular deficits. In our previous study (17), the mechanism(s) by which a mild hypercapnic environment exacerbates lower extremity vascular dysfunction were not entirely clear. We proposed that alterations in local hemodynamics and imbalances in vasoactive mediators rather than autonomic function were likely mechanisms (17), and these conjectures are partially confirmed in this work. It appears that the negative effects of prolonged sitting on the vasculature are not due to autonomic dysregulation, as we noted these decrements in vascular function occurred without any significant change in autonomic nervous system activity as inferred from several metrics of heart rate variability (LF power, HF power, LF/HF ratio) (Table 2). Also, resting heart rate, MAP, and arterial stiffness measured by PWV in several locations of the arterial vasculature (carotid-to-radial, carotid-to-ankle, carotid-to-femoral, and femoral-to-ankle) were not affected after sitting (Table 2), which suggests that the attenuations of vascular endothelial function and microcirculation are likely due to local hemodynamic and/or blood circulating factors.
A commonly proposed pair of mechanisms underlying sitting-induced impairments in macrovascular endothelial function is a reduction in arterial shear stress and an increase in lower extremity venous pooling (7, 24, 44, 45). Together, these factors may impair the signal-transduction cascade for NO production and ET-1 release, resulting in an imbalance toward a vasoconstrictive state in the resistance vessels (46). Our results are consistent with these mechanisms, as calf girth, a proxy for blood pooling, was significantly increased (Δ 1.6 ± 0.2 cm) after sitting in CON and was concomitant to a lower femoral artery mean shear rate (Δ −119.8 ± 5.6 s−1) (Table 2). Therefore, blood pooling in the lower extremity may have reduced limb blood flow and arterial shear stress, ultimately resulting in lower endothelial-mediated dilation in the lower limb (45, 47).
Interestingly, it is unlikely that attenuated arterial shear stress contributed to the reductions in brachial artery FMD, given that we allowed for small arm movements, similar to previous studies (17, 24, 48). Both Restaino et al. (24) and Thosar et al. (48) reported local hemodynamic alterations in the brachial artery following prolonged sitting, but brachial artery FMD was not changed. Therefore, it may be more likely that alterations in circulating factors, rather than local hemodynamic changes, are the mechanism underlying the reduction in brachial FMD after prolonged sitting in a mild hypercapnic environment. We found that total plasma nitrate/nitrite from the systemic circulation was significantly lower after sitting, thus suggesting a lower NO bioavailability (Fig. 4A, Supplemental Fig. S3A). In addition, ET-1, a potent vasoconstrictor, was not decreased in this condition, thus ruling out a potential contribution from this vasoactive molecule to sitting-induced endothelial dysfunction (Fig. 4B, Supplemental Fig. S3B). These results are consistent with our previous findings in a similar population of young healthy adults (17) and suggest that attenuated NO and/or a potential NO bioavailability/ET-1 ratio imbalance (Fig. 4C, Supplemental Fig. S3C), rather than excessive ET-1 release, may be a key contributing mechanism underlying sitting-induced endothelial dysfunction in a mild hypercapnic environment.
Although most previous studies have been centered around macrovascular dysfunction as a consequence of prolonged sitting, microvascular reactivity (Fig. 3, A and B, Supplemental Fig. S2) also appears to be blunted after prolonged sitting (49–52). We and others have proposed vasoactive substance imbalance as a potential mechanism (17, 50) as confirmed by the attenuated NO bioavailability (Fig. 4A, Supplemental Fig. S3A). Combined with reduced mean arterial shear rate (Table 2), these findings support the notion that local hemodynamic and circulating factors, such as reduced peripheral arterial shear stress and NO bioavailability, may be responsible for the reduction in microvascular function.
Sitting Interruptions with Passive Leg Movement Elicit Different Effects on Macro- and Microcirculatory Function
Passive leg movement has been reported to elicit beneficial central and peripheral hemodynamic responses through mechanical compression of the vasculature stimulating NO release and through the likely activation of group III muscle afferents feedback in healthy and clinical populations (20–23). A novel aspect of the present work was to investigate the potential protective effects of brief periods of sitting interruption using passive leg movement. As expected, passive leg movement triggered SNS activity and elicited a detectable peripheral hemodynamic response, as indicated by both an increase in LF power (Δ 51.0 ± 2.5 n.u.) and mean femoral artery shear rate (Δ 30.5 ± 4.9 s−1) during movement. However, somewhat contrary to our hypothesis, passive limb movement did not protect macrovascular endothelial function from the detrimental effects of sitting, as indicated by significantly lower popliteal artery FMD (Δ −3.2 ± 0.4%) and brachial artery FMD (Δ −3.3 ± 0.4%) (Fig. 2, Supplemental Fig. S1), whereas postischemia microvascular reoxygenation of the lower limb, an index of microcirculatory reactivity, was preserved (Fig. 3, A and B, Supplemental Fig. S2). Interestingly, these functional effects occurred in conjunction with decreased systemic levels in total plasma nitrate/nitrite (Δ −12.2 ± 3.6 nM, P < 0.05, Fig. 4A, Supplemental Fig. S3A) and blood pooling (calf girth: Δ 1.4 ± 0.3 cm, P < 0.05) as a consequence of sitting.
The apparent dissociation between macrocirculatory and microcirculatory function in response to sitting interruption by passive leg movement provides some interesting insights into sitting-induced vascular dysfunction. It has indeed been documented that the passive leg movement hyperemic response is largely (∼80%) NO-mediated and is mainly driven by downstream microvascular dilation (20, 53–55). In this study, it may be speculated that the intermittent increases in shear stress induced by passive leg movement upregulated NO production in the microcirculation, and in turn, protected microcirculatory function from the deleterious effects of prolonged sitting. However, the magnitude of this stimulus was not sufficient to provide a robust and sustainable increase in blood flow (and therefore shear stress) and prevent blood pooling, ultimately resulting in lower systemic NO bioavailability (Fig. 4A, Supplemental Fig. S3A). These results collectively suggest that passive movement may offer microvascular but not macrovascular protection during prolonged sitting in a mild hypercapnic environment.
Sitting Interruptions with Short-Duration Low-Intensity Active Leg Movement (Group III and Group IV Activation) Fully Protect Vascular Function
Despite growing evidence that prolonged sitting compromises vascular function, the development of effective strategies to protect the vasculature from the effects of sitting is still in its infancy. Interestingly, sitting interruption with short bouts (2 min) of low-intensity active leg movement (13 W) was performed every 30 min, which elicited a modest increase in femoral artery mean shear rate (Δ 97.2 ± 3.1 s−1) and prevented significant venous pooling (calf girth: Δ 0.5 ± 0.5 cm, P > 0.05), was sufficient to fully protect vascular endothelial function in both the popliteal (Δ −0.2 ± 0.2%) and brachial arteries (Δ −0.7 ± 0.4%) (Table 2, Fig. 2, Supplemental Fig. S1) in this study. In addition, microvascular reoxygenation postischemia was fully preserved after sitting in the ACT condition (Δ 4.2 ± 1.3%·min−1) (Fig. 3, Supplemental Fig. S2).
The protective effect on vascular function of the exercise modality used in this study has several implications in terms of strategies to interrupt sitting. First, this finding indicates that even modest (∼ 570 s−1) increases in shear rate are sufficient to preserve vascular function from prolonged sitting. For context, the transient increase in peak shear rate induced in this study by cycling at 13 W is quantitatively similar to the changes resulting from 10 calf raises (26). Second, the total activity duration was only 10 min (5 times, 2 min) over the 2.5 h of sitting thus suggesting that even a brief period of sitting interruptions can elicit a protection to sitting-induced vascular dysfunction. Our results thus confirm the results of previous studies that used more frequent and longer period of muscle activity (15–45 min) to protect vascular function (6, 19), and extend this finding to even shorter (10 min/3 h) low-intensity muscle contraction. In contrast, Peddie et al. (56) did not report a beneficial effect of standing or walking for the same total activity durations (2 min every 30 min). However, both interventions induced a marginal change in shear rate (< 60 s−1), i.e., 10 times lower than this study.
The bent-artery system, unique to sitting, has been shown to alter shear rate profiles and enable turbulent flow (7). In ACT, it is probable that increased shear rate due to the periodic movement bouts (Δ 269.7 ± 18.8 s−1) intermittently produced more favorable shear and flow profiles (i.e., greater laminar flow as opposed to turbulent flow), thus resulting in NO production and ET-1 attenuation (46, 57). This increased shear rate also may be explained by increased local skeletal muscle metabolite accumulation (e.g., potassium, adenosine, hydrogen ions, phosphates, etc.), which may also alter local vasomotor responses. Herein, we measured both total plasma nitrate/nitrite and ET-1 concentrations. Unlike CON during which plasma nitrate/nitrite levels were decreased, brief bouts of repeated muscle contraction were sufficient to maintain plasma nitrate/nitrite levels, which in turn were significantly greater than post-CON and post-PASS (Fig. 4A, Supplemental Fig. S3A). In addition, ET-1 levels were significantly decreased after ACT (Fig. 4B, Supplemental Fig. S3B). As a result, the NO bioavailability/ET-1 ratio was significantly higher (P < 0.05) after sitting in the ACT condition compared with both post-CON and post-PASS (Fig. 4C, Supplemental Fig. S3C). Interestingly, heterogeneous findings regarding vasoactive substances have been noted in previous work. This may be due to the intensity of the stimuli used during prolonged sitting (8, 26). Evans et al. (58) found that calf raises performed while seated (10 repetitions every 10 min at 0.3 Hz) over 3 h of prolonged sitting did not alter circulating ET-1 concentrations. However, Climie et al. (8) had participants perform a series of body weight resistance exercises (3 min every 30 min at 0.5 Hz) over 5 h of prolonged sitting, and they found an ablated rise in ET-1 levels and no changes in total plasma nitrate/nitrite. In this study, we controlled for frequency (2 min every 30 min at 1 Hz) and used a constant workload (13 W). Based on the number of repetitions performed in our study (120 repetitions every 30 min), our stimulus may be considered more comparable with that of Climie et al. (90 repetitions every 30 min) (8), which may be why our circulating vasoactive substance findings are more similar to their work as opposed to Evans et al. (30 repetitions over 30 min) (26).
In addition, the preservation of circulating factors, rather than increases in local arterial shear stress, may be the mechanism underlying the protection of brachial FMD in the ACT group. Brachial artery mean shear rate does not significantly increase during an active movement bout (Δ 11.3 ± 4.3 s−1), whereas femoral artery mean shear rate increases (Δ 269.7 ± 18.8 s −1). These findings are aligned with that of Restaino et al. (24), as they found that a 10-min walking bout after prolonged sitting restored lower extremity but not upper extremity hemodynamics. The preservation of circulating factors, as mediated by lower extremity arterial shear stress (59–62), may be a primary factor underlying brachial artery FMD preservation in ACT. However, future investigation of upper extremity hemodynamics in conjunction with circulating biomarkers during prolonged sitting in a mild hypercapnic environment is warranted to further understand these potential mechanisms.
Together, these findings indicate that short-duration low-intensity muscular contraction is sufficient to preserve vasomotor balance and prevent both macrovascular (Fig. 2, Supplemental Fig. S1) and microvascular endothelial dysfunction (Fig. 3, Supplemental Fig. S2) during prolonged sitting in a mild hypercapnic environment. The combination of the likely group III/IV muscle afferent activation in ACT also induced a rise in sympathetic output (indicated by LF power) compared with baseline, which elicited increases in HR and likely contributed to reduce blood pooling through venoconstriction. Combined with local change in vasomotor tone, these reflex mechanisms likely contributed to increased local lower extremity shear stress and venous return as well as circulating vasoactive substances, thus bolstering the protective effects of low-intensity exercise on vascular function against prolonged sitting in a mild hypercapnic environment.
Experimental Considerations
As expected, we found that the fraction of expired CO2 (FECO2) was significantly increased (P < 0.05) resulting in increased tidal volume (Vt) and ventilation rate (P < 0.05) during sitting in hypercapnia when compared with normocapnia condition (Fig. 5, A and B). The increase in FECO2 and Vt confirms that our experimental condition (CO2 ∼1,500 ppm) was sufficient to trigger peripheral chemoreceptor activation (63–66). Despite this stimulation of ventilation, the mild hypercapnic environment did not appear to significantly increase sympathetic nerve activity measured from heart rate variability metrics and did not trigger changes in MAP, suggestive of changes in total peripheral resistance (Tables 2 and 3). To examine the roles of chemoreceptor activation during prolonged sitting in mild hypercapnic environment, blood pH, CO2, and O2 level assessments are warranted in future work.
Previous research has used both passive and active movement to activate group III and group III/IV afferents, respectively, in human research (20, 55, 67, 68). Although we did not directly assess muscle afferent activation, we have evidence that may indirectly support the activation of these afferents. Although our resting post-sitting HRV data show that both LF (represents sympathetic activity) and HF (represents parasympathetic activity) were not significantly changed after prolonged sitting in CON, PASS, or ACT within a mild hypercapnic environment, the change in sympathetic dominance (ΔLF/HF) was significantly higher (P < 0.05) in PASS and ACT compared with CON (Table 2). These results could potentially be due to the muscular contraction-induced increase in LF during passive movement [group III stimulation (Δ 51.0 ± 2.5 n.u.)] and during active movement [the combination of group III/IV stimulation (Δ 64.1 ± 2.5 n.u.)]. Of note, previous studies suggested that passive leg movement alone has been shown to increase sympathetic output, which then leads to increases in cardiac output and heart rate, whereas limb blood flow concomitantly increased (69, 70). Although we used a similar passive leg movement strategy (69, 70), our results indicated no significant changes in heart rate, shear rate, and local leg blood flow (Tables 2 and 3), which might be attributed to the effect of prolonged sitting on the hemodynamic response to passive leg movement (52, 71). We speculate that these results supply indirect evidence of group III and group III/IV afferent activation in response to passive and active leg movement; however, a more direct assessment of afferent activation is warranted in future work, such as MSNA.
We did not normalize FMD to a shear stimulus, and since this is the first of several follow-up studies to our previous work (17), the authors deemed it appropriate to keep protocols and methods as similar as possible to make appropriate comparisons between the two studies. Although our FMD values in this study are relatively consistent with our previous work using the same methods in healthy young adults (17, 35) and show that the study population is relatively healthy according to FMD reference intervals (72), it has been recently recommended by Thijssen et al. (73) that some form of shear stimulus normalization should be considered for FMD; however, the preferred method of normalization (e.g., AUC or peak shear rate) is not entirely clear. In addition, we did not perform control experiments with participants in the supine position. Walsh et al. (7) performed a study that showed prolonged leg bending (resemblant of the sitting position) attenuates popliteal artery endothelial function when compared with an internal control (straight leg). Therefore, in a supine position, we may be able to better understand the impacts of a mild hypercapnic environment alone on macrovascular and microvascular function, as the “arterial bending” component that is unique to sitting would be eliminated. Finally, we do not know the impacts of a mild hypercapnic environment alone on total plasma nitrate/nitrite, as we did not collect blood samples in our previous study to analyze for these biomarkers (17). Climie et al. (8) demonstrated that total plasma nitrate/nitrite was unchanged after 5 h of prolonged sitting. It may be appropriate to speculate that the additive effects of a mild hypercapnic environment to prolonged sitting exacerbated the vasoactive substance imbalance, but further research is warranted.
Conclusion and Clinical Implications
This study further confirmed that prolonged sitting in a mild hypercapnic environment greatly attenuates peripheral macrovascular endothelial function and microvascular function. Our findings support the notion that local hemodynamic and/or blood circulating factors, such as reduced peripheral arterial shear stress and NO bioavailability, may be likely mechanisms underlying the reduction in vascular function after prolonged sitting. Interestingly, when sitting was interrupted by short bouts of passive leg movement, which reflexively activated the sympathetic nervous system through likely group III muscle afferent feedback and stimulated a movement-induced hyperemic response, only microvascular reactivity was preserved whereas macrovascular endothelial function was not protected. In contrast, sitting interruptions by short-duration low-intensity exercise eliciting a small increase in blood flow every 30 min (∼Δ 70 mL·min−1) were sufficient to restore vasomotor balance (higher NO bioavailability/ET-1 ratio), increase shear rate, and reduced blood pooling. These changes in local lower extremity hemodynamics and systemic circulating factors are likely mechanisms that served to protect both macrovascular and microvascular function. As the experimental conditions represent everyday environments such as offices or classrooms, the present findings provide additional insight into effective strategies to preserve vascular endothelial function to limit the deleterious effects of prolonged sitting.
SUPPLEMENTAL DATA
Supplemental Figs. S1–S3: https://doi.org/10.6084/m9.figshare.17708567.
GRANTS
This work was supported by a pilot award from the National Institutes of Health COBRE (P20GM109090), the NASA Nebraska Space Grant (NNX15AI09H and 80NSSC20M0112), the NASA Nebraska Space Grant Fellowship, The Sherwood Foundation (5444), the University of Nebraska at Omaha Graduate Research and Creative Activity (GRACA) grant, and the NIH National Heart, Lung, and Blood Institute Grant R00HL125756.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.-Y.P., T.K.W., E.J.P., and G.L. conceived and designed research; S.-Y.P., T.K.W., E.J.P., C.P.A., and S.K.Y. performed experiments; S.-Y.P., T.K.W., E.J.P., C.P.A., and S.K.Y. analyzed data; S.-Y.P., T.K.W., E.J.P., C.P.A., D.R.S., and G.L. interpreted results of experiments; T.K.W., E.J.P., and C.P.A. prepared figures; S.-Y.P., T.K.W., E.J.P., and G.L. drafted manuscript; S.-Y.P., T.K.W., E.J.P., D.R.S., and G.L. edited and revised manuscript; S.-Y.P., T.K.W., E.J.P., C.P.A., S.K.Y., D.R.S., and G.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Fitness Cubed Inc. for providing the elliptical devices.
REFERENCES
- 1.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 56: 2655–2667, 2007. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 2.Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sport Exer 41: 998–1005, 2009. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- 3.Chomistek AK, Manson JE, Stefanick ML, Lu B, Sands-Lincoln M, Going SB, Garcia L, Allison MA, Sims ST, LaMonte MJ, Johnson KC, Eaton CB. Relationship of sedentary behavior and physical activity to incident cardiovascular disease. J Am Coll Cardiol 61: 2346–2354, 2013. doi: 10.1016/j.jacc.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereira SMP, Ki M, Power C. Sedentary behaviour and biomarkers for cardiovascular disease and diabetes in mid-life: the role of television-viewing and sitting at work. PLoS One 7: e31132, 2012. doi: 10.1371/journal.pone.0031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biddle SJH, Bennie JA, Bauman AE, Chau JY, Dunstan D, Owen N, Stamatakis E, van Uffelen JGZ. Too much sitting and all-cause mortality: is there a causal link? BMC Public Health 16: 635, 2016. doi: 10.1186/s12889-016-3307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thosar SS, Bielko SL, Mather KJ, Johnston JD, Wallace JP. Effect of prolonged sitting and breaks in sitting time on endothelial function. Med Sci Sport Exer 47: 843–849, 2015. doi: 10.1249/MSS.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 7.Walsh LK, Restaino RM, Martinez-Lemus LA, Padilla J. Prolonged leg bending impairs endothelial function in the popliteal artery. Physiol Rep 5: e13478, 2017. doi: 10.14814/phy2.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Climie RE, Wheeler MJ, Grace M, Lambert EA, Cohen N, Owen N, Kingwell BA, Dunstan DW, Green DJ. Simple intermittent resistance activity mitigates the detrimental effect of prolonged unbroken sitting on arterial function in overweight and obese adults. J Appl Physiol 125: 1787–1794, 2018. doi: 10.1152/japplphysiol.00544.2018. [DOI] [PubMed] [Google Scholar]
- 9.Shvartz E, Reibold RC, White RT, Gaume JG. Hemodynamic-responses in orthostasis following 5 hours of sitting. Aviat Space Envir Md 53: 226–231, 1982. [PubMed] [Google Scholar]
- 10.Shvartz E, Gaume JG, White RT, Reibold RC. Hemodynamic-responses during prolonged sitting. J Appl Physiol Respir Environ Exerc Physiol 54: 1673–1680, 1983. doi: 10.1152/jappl.1983.54.6.1673. [DOI] [PubMed] [Google Scholar]
- 11.Ross R, Wight TN, Strandness E, Thiele B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol 114: 79–93, 1984. [PMC free article] [PubMed] [Google Scholar]
- 12.Kröger K, Kucharczik A, Hirche H, Rudofsky G. Atherosclerotic lesions are more frequent in femoral arteries than in carotid arteries independent of increasing number of risk factors. Angiology 50: 649–654, 1999. doi: 10.1177/000331979905000805. [DOI] [PubMed] [Google Scholar]
- 13.Aboyans V, McClelland RL, Allison MA, McDermott MM, Blumenthal RS, Macura K, Criqui MH. Lower extremity peripheral artery disease in the absence of traditional risk factors. The multi-ethnic study of atherosclerosis. Atherosclerosis 214: 169–173, 2011. doi: 10.1016/j.atherosclerosis.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Guan J, Yang XD, Chao-Hsin. L. Source apportionment of airborne particles in commercial aircraft cabin environment: contributions from outside and inside of cabin. Atmos Environ 89: 119–128, 2014. doi: 10.1016/j.atmosenv.2014.01.04. [DOI] [Google Scholar]
- 15.Guan J, Li Z, Yang XD. Net in-cabin emission rates of VOCs and contributions from outside and inside the aircraft cabin. Atmos Environ 111: 1–9, 2015. doi: 10.1016/j.atmosenv.2015.04.002. [DOI] [Google Scholar]
- 16.Weisel CP, Fiedler N, Weschler CJ, Ohman-Strickland PA, Mohan KR, McNeil K, Space DR. Human symptom responses to bioeffluents, short-chain carbonyls/acids, and long-chain carbonyls in a simulated aircraft cabin environment. Indoor Air 27: 1154–1167, 2017. doi: 10.1111/ina.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Headid RJ 3rd, Pekas EJ, Wooden TK, Son WM, Layec G, Shin J, Park SY. Impacts of prolonged sitting with mild hypercapnia on vascular and autonomic function in healthy recreationally active adults. Am J Physiol Heart Circ Physiol 319: H468–H480, 2020. doi: 10.1152/ajpheart.00354.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans (Online). https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf. [2021 Sep 16].
- 19.Morishima T, Restaino RM, Walsh LK, Kanaley JA, Fadel PJ, Padilla J. Prolonged sitting-induced leg endothelial dysfunction is prevented by fidgeting. Am J Physiol Heart Circ Physiol 311: H177–H182, 2016. doi: 10.1152/ajpheart.00297.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barrett-O'Keefe Z, Runnels S, Morgan DE, Wray DW, Richardson RS. Limb movement-induced hyperemia has a central hemodynamic component: evidence from a neural blockade study. Am J Physiol Heart Circ Physiol 299: H1693–H1700, 2010. doi: 10.1152/ajpheart.00482.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trinity JD, McDaniel J, Venturelli M, Fjeldstad AS, Ives SJ, Witman MAH, Barrett-O'Keefe Z, Amann M, Wray DW, Richardson RS. Impact of body position on central and peripheral hemodynamic contributions to movement-induced hyperemia: implications for rehabilitative medicine. Am J Physiol Heart Circ Physiol 300: H1885–H1891, 2011. doi: 10.1152/ajpheart.00038.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoier B, Walker M, Passos M, Walker PJ, Green A, Bangsbo J, Askew CD, Hellsten Y. Angiogenic response to passive movement and active exercise in individuals with peripheral arterial disease. J Appl Physiol (1985) 115: 1777–1787, 2013. doi: 10.1152/japplphysiol.00979.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phadke CP, Vierira L, Mathur S, Cipriano G Jr, Ismail F, Boulias C. Impact of passive leg cycling in persons with spinal cord injury: a systematic review. Top Spinal Cord Inj Rehabil 25: 83–96, 2019. doi: 10.1310/sci18-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Restaino RM, Holwerda SW, Credeur DP, Fadel PJ, Padilla J. Impact of prolonged sitting on lower and upper limb micro- and macrovascular dilator function. Exp Physiol 100: 829–838, 2015. doi: 10.1113/EP085238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venturelli M, Amann M, McDaniel J, Trinity JD, Fjeldstad AS, Richardson RS. Central and peripheral hemodynamic responses to passive limb movement: the role of arousal. Am J Physiol Heart Circ Physiol 302: H333–H339, 2012. doi: 10.1152/ajpheart.00851.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans WS, Stoner L, Willey Q, Kelsch E, Credeur DP, Hanson ED. Local exercise does not prevent the aortic stiffening response to acute prolonged sitting: a randomized crossover trial. J Appl Physiol (1985) 127: 781–787, 2019. doi: 10.1152/japplphysiol.00318.2019. [DOI] [PubMed] [Google Scholar]
- 27.Saeki Y, Atogami F, Takahashi K, Yoshizawa T. Reflex control of autonomic function induced by posture change during the menstrual cycle. J Auton Nerv Syst 66: 69–74, 1997. doi: 10.1016/s0165-1838(97)00067-2. [DOI] [PubMed] [Google Scholar]
- 28.Jones PR, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol 204: 63p–66p, 1969. [PubMed] [Google Scholar]
- 29.Râdegran G, Blomstrand E, Saltin B. Peak muscle perfusion and oxygen uptake in humans: importance of precise estimates of muscle mass. J Appl Physiol (1985) 87: 2375–2380, 1999. doi: 10.1152/jappl.1999.87.6.2375. [DOI] [PubMed] [Google Scholar]
- 30.Layec G, Venturelli M, Jeong EK, Richardson RS. The validity of anthropometric leg muscle volume estimation across a wide spectrum. J Appl Physiol (1985) 116: 1142–1147, 2014. doi: 10.1152/japplphysiol.01120.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park SY, Wong A, Son WM, Pekas EJ. Effects of heated water-based versus land-based exercise training on vascular function in individuals with peripheral artery disease. J Appl Physiol (1985) 128: 565–575, 2020. doi: 10.1152/japplphysiol.00744.2019. [DOI] [PubMed] [Google Scholar]
- 32.Kenny RA, O'Shea D, Parry SW. The Newcastle protocols for head-up tilt table testing in the diagnosis of vasovagal syncope, carotid sinus hypersensitivity, and related disorders. Heart 83: 564–569, 2000. doi: 10.1136/heart.83.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health 5: 258, 2017. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pekas EJ, Shin J, Headid RJ, Son WM, Layec G, Yadav SK, Scott SD, Park SY. Combined anthocyanins and bromelain supplement improves endothelial function and skeletal muscle oxygenation status in adults: a double-blind placebo-controlled randomised crossover clinical trial. Br J Nutr 125: 161–171, 2021. doi: 10.1017/S0007114520002548. [DOI] [PubMed] [Google Scholar]
- 36.Trinity JD, Barrett-O'Keefe Z, Ives SJ, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS, Wray DW. Endogenous endothelin-1 and femoral artery shear rate: impact of age and implications for atherosclerosis. J Hypertens 34: 266–273, 2016. doi: 10.1097/HJH.0000000000000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stone K, Fryer S, Kelsch E, Burnet K, Zieff G, Faulkner J, Credeur D, Lambrick D, Hanson ED, Stoner L. Validity and reliability of lower-limb pulse-wave velocity assessments using an oscillometric technique. Exp Physiol 104: 765–774, 2019. doi: 10.1113/EP087444. [DOI] [PubMed] [Google Scholar]
- 38.Ryan TE, Erickson ML, Brizendine JT, Young HJ, McCully KK. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. J Appl Physiol (1985) 113: 175–183, 2012. doi: 10.1152/japplphysiol.00319.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koga S, Kano Y, Barstow TJ, Ferreira LF, Ohmae E, Sudo M, Poole DC. Kinetics of muscle deoxygenation and microvascular PO2 during contractions in rat: comparison of optical spectroscopy and phosphorescence-quenching techniques. J Appl Physiol (1985) 112: 26–32, 2012. doi: 10.1152/japplphysiol.00925.2011. [DOI] [PubMed] [Google Scholar]
- 40.Ferreira LF, Hueber DM, Barstow TJ. Effects of assuming constant optical scattering on measurements of muscle oxygenation by near-infrared spectroscopy during exercise. J Appl Physiol (1985) 102: 358–367, 2007. doi: 10.1152/japplphysiol.00920.2005. [DOI] [PubMed] [Google Scholar]
- 41.Bopp CM, Townsend DK, Warren S, Barstow TJ. Relationship between brachial artery blood flow and total [hemoglobin+myoglobin] during post-occlusive reactive hyperemia. Microvasc Res 91: 37–43, 2014. doi: 10.1016/j.mvr.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Gayda M, Juneau M, Tardif JC, Harel F, Levesque S, Nigam A. Cardiometabolic and traditional cardiovascular risk factors and their potential impact on macrovascular and microvascular function: preliminary data. Clin Hemorheol Microcirc 59: 53–65, 2015. doi: 10.3233/CH-141816. [DOI] [PubMed] [Google Scholar]
- 43.Paterson C, Fryer S, Zieff G, Stone K, Credeur DP, Barone Gibbs B, Padilla J, Parker JK, Stoner L. The effects of acute exposure to prolonged sitting, with and without interruption, on vascular function among adults: a meta-analysis. Sports Med 50: 1929–1942, 2020. doi: 10.1007/s40279-020-01325-5. [DOI] [PubMed] [Google Scholar]
- 44.Restaino RM, Walsh LK, Morishima T, Vranish JR, Martinez-Lemus LA, Fadel PJ, Padilla J. Endothelial dysfunction following prolonged sitting is mediated by a reduction in shear stress. Am J Physiol Heart Circ Physiol 310: H648–H653, 2016. doi: 10.1152/ajpheart.00943.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delp MD, Laughlin MH. Regulation of skeletal muscle perfusion during exercise. Acta Physiol Scand 162: 411–419, 1998. doi: 10.1046/j.1365-201X.1998.0324e.x. [DOI] [PubMed] [Google Scholar]
- 46.Chistiakov DA, Orekhov AN, Bobryshev YV. Effects of shear stress on endothelial cells: go with the flow. Acta Physiol (Oxf) 219: 382–408, 2017. doi: 10.1111/apha.12725. [DOI] [PubMed] [Google Scholar]
- 47.Reynolds LJ, Credeur DP, Manrique C, Padilla J, Fadel PJ, Thyfault JP. Obesity, type 2 diabetes, and impaired insulin-stimulated blood flow: role of skeletal muscle NO synthase and endothelin-1. J Appl Physiol (1985) 122: 38–47, 2017. doi: 10.1152/japplphysiol.00286.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thosar SS, Bielko SL, Wiggins CC, Wallace JP. Differences in brachial and femoral artery responses to prolonged sitting. Cardiovasc Ultrasound 12: 50, 2014. doi: 10.1186/1476-7120-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vranish JR, Young BE, Stephens BY, Kaur J, Padilla J, Fadel PJ. Brief periods of inactivity reduce leg microvascular, but not macrovascular, function in healthy young men. Exp Physiol 103: 1425–1434, 2018. doi: 10.1113/EP086918. [DOI] [PubMed] [Google Scholar]
- 50.Vranish JR, Young BE, Kaur J, Patik JC, Padilla J, Fadel PJ. Influence of sex on microvascular and macrovascular responses to prolonged sitting. Am J Physiol Heart Circ Physiol 312: H800–H805, 2017. doi: 10.1152/ajpheart.00823.2016. [DOI] [PubMed] [Google Scholar]
- 51.Credeur DP, Miller SM, Jones R, Stoner L, Dolbow DR, Fryer SM, Stone K, McCoy SM. Impact of prolonged sitting on peripheral and central vascular health. Am J Cardiol 123: 260–266, 2019. doi: 10.1016/j.amjcard.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 52.Garten RS, Scott MC, Zúñiga TM, Hogwood AC, Fralin RC, Weggen J. A prior high-intensity exercise bout attenuates the vascular dysfunction resulting from a prolonged sedentary bout. J Phys Act Health 16: 916–924, 2019. doi: 10.1123/jpah.2018-0568. [DOI] [PubMed] [Google Scholar]
- 53.Trinity JD, McDaniel J, Venturelli M, Fjeldstad AS, Ives SJ, Witman MA, Barrett-O'Keefe Z, Amann M, Wray DW, Richardson RS. Impact of body position on central and peripheral hemodynamic contributions to movement-induced hyperemia: implications for rehabilitative medicine. Am J Physiol Heart Circ Physiol 300: H1885–H1891, 2011. doi: 10.1152/ajpheart.00038.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gifford JR, Richardson RS. CORP: ultrasound assessment of vascular function with the passive leg movement technique. J Appl Physiol (1985) 123: 1708–1720, 2017. doi: 10.1152/japplphysiol.00557.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol 590: 1413–1425, 2012. doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peddie MC, Kessell C, Bergen T, Gibbons TD, Campbell HA, Cotter JD, Rehrer NJ, Thomas KN. The effects of prolonged sitting, prolonged standing, and activity breaks on vascular function, and postprandial glucose and insulin responses: a randomised crossover trial. PLoS One 16: e0244841, 2021. doi: 10.1371/journal.pone.0244841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor FC, Dunstan DW, Homer AR, Dempsey PC, Kingwell BA, Climie RE, Owen N, Cohen ND, Larsen RN, Grace M, Eikelis N, Wheeler MJ, Townsend MK, Maniar N, Green DJ. Acute effects of interrupting prolonged sitting on vascular function in type 2 diabetes. Am J Physiol Heart Circ Physiol 320: H393–H403, 2021. doi: 10.1152/ajpheart.00422.2020. [DOI] [PubMed] [Google Scholar]
- 58.Evans WS, Hanson ED, Shill DD, Landers-Ramos RQ, Stoner L, Willey Q, Credeur DP, Prior SJ. Sitting decreases endothelial microparticles but not circulating angiogenic cells irrespective of lower leg exercises: a randomized cross-over trial. Exp Physiol 105: 1408–1419, 2020. doi: 10.1113/EP088690. [DOI] [PubMed] [Google Scholar]
- 59.Morawietz H, Talanow R, Szibor M, Rueckschloss U, Schubert A, Bartling B, Darmer D, Holtz J. Regulation of the endothelin system by shear stress in human endothelial cells. J Physiol 525: 761–770, 2000. doi: 10.1111/j.1469-7793.2000.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol 18: 677–685, 1998. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 61.Bourque SL, Davidge ST, Adams MA. The interaction between endothelin-1 and nitric oxide in the vasculature: new perspectives. Am J Physiol Regul Integr Comp Physiol 300: R1288–R1295, 2011. doi: 10.1152/ajpregu.00397.2010. [DOI] [PubMed] [Google Scholar]
- 62.Rapoport RM. Acute nitric oxide synthase inhibition and endothelin-1-dependent arterial pressure elevation. Front Pharmacol 5: 57, 2014. doi: 10.3389/fphar.2014.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nurse CA. Neurotransmission and neuromodulation in the chemosensory carotid body. Auton Neurosci 120: 1–9, 2005. doi: 10.1016/j.autneu.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 74: 829–898, 1994. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- 65.Eyzaguirre C, Zapata P. Perspectives in carotid body research. J Appl Physiol Respir Environ Exerc Physiol 57: 931–957, 1984. doi: 10.1152/jappl.1984.57.4.931. [DOI] [PubMed] [Google Scholar]
- 66.Peers C, Buckler K. Transduction of chemostimuli by the type I carotid body cell. J Membr Biol 144: 1–9, 1995. doi: 10.1007/BF00238411. [DOI] [PubMed] [Google Scholar]
- 67.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011. doi: 10.1113/jphysiol.2011.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol (1985) 109: 966–976, 2010. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Venturelli M, Layec G, Trinity J, Hart CR, Broxterman RM, Richardson RS. Single passive leg movement-induced hyperemia: a simple vascular function assessment without a chronotropic response. J Appl Physiol (1985) 122: 28–37, 2017. doi: 10.1152/japplphysiol.00806.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Broxterman RM, Trinity JD, Gifford JR, Kwon OS, Kithas AC, Hydren JR, Nelson AD, Morgan DE, Jessop JE, Bledsoe AD, Richardson RS. Single passive leg movement assessment of vascular function: contribution of nitric oxide. J Appl Physiol (1985) 123: 1468–1476, 2017. doi: 10.1152/japplphysiol.00533.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Decker KP, Feliciano PG, Kimmel MT, Hogwood AC, Weggen JB, Darling AM, Richardson JW, Garten RS. Examining sex differences in sitting-induced microvascular dysfunction: insight from acute vitamin C supplementation. Microvasc Res 135: 104147, 2021. doi: 10.1016/j.mvr.2021.104147. [DOI] [PubMed] [Google Scholar]
- 72.Holder SM, Bruno RM, Shkredova DA, Dawson EA, Jones H, Hopkins ND, Hopman MTE, Bailey TG, Coombes JS, Askew CD, Naylor L, Maiorana A, Ghiadoni L, Thompson A, Green DJ, Thijssen DHJ. Reference intervals for brachial artery flow-mediated dilation and the relation with cardiovascular risk factors. Hypertension 77: 1469–1480, 2021. doi: 10.1161/HYPERTENSIONAHA.120.15754. [DOI] [PubMed] [Google Scholar]
- 73.Thijssen DHJ, Bruno RM, van Mil A, Holder SM, Faita F, Greyling A, Zock PL, Taddei S, Deanfield JE, Luscher T, Green DJ, Ghiadoni L. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J 40: 2534–2547, 2019. doi: 10.1093/eurheartj/ehz350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1–S3: https://doi.org/10.6084/m9.figshare.17708567.