This editorial refers to ‘Role of plakophilin-2 expression on exercise-related progression of arrhythmogenic right ventricular cardiomyopathy: a translational study’, by M. Cerrone et al., https://doi.org/10.1093/eurheartj/ehab772.
We used to think that exercise is beneficial for all hearts. Nevertheless, long before we knew about desmosomal disease, we observed that some athletes were prone to right ventricular dysfunction, sometimes accompanied by malignant ventricular arrhythmias. In 2006, it was shown that exercise induced right-sided cardiomyopathy in plakophilin-2- (PKP2) deficient mice.1 This result led to clinical studies on exercise in arrhythmogenic right ventricular cardiomyopathy (ARVC) patients, but it was not until 2013 that the association between exercise and severe ARVC in humans was established, showing that exercise accelerated and aggravated ARVC.2,3 Ventricular arrhythmias were more frequent and occurred at a younger age in ARVC patients defined as athletes (>1 h of exercise/week)2 and both right and left ventricular function were decreased in ARVC patients with >4 h of exercise/week.3 Furthermore, exercise was also associated with subtle structural changes in so far healthy genotype-positive family members. Further studies showed that high-intensity exercise was more harmful than exercise of long duration in ARVC,4 also affecting left ventricular function.5 Therefore, the past 15 years have been important in understanding that patients with desmosomal disease are vulnerable to exercise, and exercise restrictions are now an established recommendation.6
Cardiac desmosomes provide cell–cell adhesion between cardiomyocytes. Exercise increases cardiac wall stress, particularly of the right ventricle, with increased demand on desmosome integrity,7 which leads to desmosomal disruption, cardiomyocyte necrosis, and fibrofatty replacement of the myocardium. In recent years, it has become evident not only that the desmosomes regulate cell–cell mechanical coupling, but that these structures also have important roles in cell signalling pathways.8 This includes pathways involving cell proliferation and apoptosis, electrolyte signalling, mitochondrial function, metabolic components, and more. Hence, the pathogenicity associated with dysfunctional cardiac desmosomes may not only be due to the resulting mechanical cardiomyocyte disruption but may also be mediated by several complex cell signalling mechanisms.
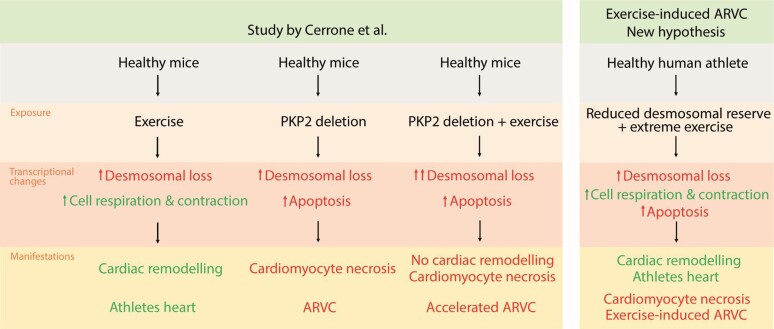
In this issue of the European Heart Journal, Cerrone et al.9 present their study describing the role of PKP2 expression in exercise-related progression of ARVC. The study was performed in a well-renowned lab and they collaborated with well-experienced ARVC centres. They investigated the effect of endurance training in PKP2-deficient mice and compared this with controls. As expected, PKP2-deficient mice developed ARVC, with decreased cardiac muscle mass, ventricular dysfunction, and right-sided heart failure compared with controls. Not surprisingly, exercise exposure aggravated the ARVC phenotype. In the control group (normal PKP2), however, exercise resulted in increased left ventricular wall thickness and improved cardiac function, in line with previously described cardiac remodelling secondary to endurance training.10 In the healthy exercise-exposed mice, the authors observed up-regulation of several transcripts, including transcripts coding for cytoskeletal proteins, cell respiration, and mitochondrial metabolism. Surprisingly, among the down-regulated transcripts in healthy, exercised mice were several desmosomal components and other intercalated disc-related genes, as well as scaffolding and ion channel proteins. Similarly, induction of PKP2 deficiency in healthy mice resulted in down-regulation of desmosomal components, but in addition increased apoptosis and development of the ARVC phenotype. When PKP2-deficient mice were exposed to exercise, they showed accelerated progression of ARVC and a more severe phenotype than sedentary mice. These findings led the authors to hypothesize that vigorous exercise diminishes desmosomal function, but that a cardiomyocyte ‘desmosomal reserve’ enables healthy subjects to tolerate exercise. In contrast, subjects with genetically impaired desmosomes and no ‘desmosomal reserve’ will not tolerate the additional impairment of desmosomal function, explaining the acceleration of disease in ARVC patients with high exercise exposure.
As a supplement to the experimental animal study design, the authors performed a substudy including 14 PKP2 mutation-positive athletes and 14 case-matched controls. ARVC athletes had not developed the typical athlete’s heart remodelling with an increase in left ventricular wall thickness or the same degree of left ventricular dilatation as the controls, indicating loss of the positive effects of exercise. In addition, ARVC athletes showed a trend towards maladaptive remodelling, with an increased right-sided vs. left-sided dilatation ratio, supporting the lack of positive effects, and aggravated negative effects, of exercise in ARVC.
The hypothesis of a cardiomyocyte desmosomal reserve is an interesting concept. It provides a supplemental causal explanation to the ‘wall stress theory’7 as a possible mechanism behind the exercise-induced worsened prognosis in ARVC patients. The wall stress theory focuses on the mechanical properties of the desmosomes—to provide cell–cell adhesion. The myocardium is exposed to mechanical stress during a normal cardiac cycle. Dysfunctional cardiac desmosomes are not able to provide sufficient cell–cell adhesion during such strain, resulting in desmosomal disruption and progressive cardiomyocyte necrosis. Exercise further increases the cardiac wall stress, particularly in the thin-walled right ventricle, providing an environmental stressor in addition to the genetic predisposition.7 The wall stress theory is a reasonable and acknowledged cause of exercise intolerance in ARVC, but there is room for several contributing factors. It is well described that exercise triggers initiation of a set of complex adaption mechanisms in cardiomyocytes.10,11 Several causal factors may co-exist and possibly have additive effects. Hence, the negative effect of exercise in ARVC patients is most likely to be multifactorial, including mechanical, hormonal, and transcriptional changes. The ‘desmosomal reserve theory’ is a welcomed contribution to the understanding of how exercise affects cardiac desmosomes and cardiac function.
The complexity of transcriptomic changes induced by external stressors needs to be recognized, as one stressor may lead to both up- and down-regulation of transcripts involved in similar signalling pathways. The study by Cerrone et al. showed that exercise down-regulated transcription of desmosomal and many other intercalated disc components, but also that the cardiac intermediate filament desmin was up-regulated by exposure to exercise. Furthermore, previous studies have shown that exercise induces up-regulation of several intercalated disc components.8 Hence, exercise may initiate signals to both enhance and reduce cell–cell adhesion. Moreover, a translational study has limitations that need to be considered. A murine model cannot be directly extrapolated to a human model,11 and the findings need to be interpreted with care. Another important limitation of the study is the use of one specific exercise protocol. Different forms of exercise result in different adaptations in healthy hearts. The type of exercise performed is of great importance to the outcome in ARVC patients, with high-intensity exercise as the major culprit.4 It would have been interesting to see a comparison of the effect of different exercise protocols on the phenotypic ARVC expression in PKP2-deficient mice.
One of the most interesting questions following this study is if the concept of reduced desmosomal reserve can help explain the previously proposed concept of exercise-induced ARVC in gene-elusive patients.12,13 These patients develop ARVC-mimicking disease after a great amount of exercise, often with severe ventricular arrhythmias, but without a clear family history and without a known genetic predisposition.14 Importantly, exercise-induced gene-elusive ARVC athletes may develop ‘athletes’ heart’ similar to healthy athletes,15 in contrast to desmosomally deficient ARVC patients. One may speculate that these individuals have an initial normal, positive response to exercise with cardiac remodelling and development of athlete's heart, but then eventually fail to compensate desmosomal loss due to a reduced desmosomal reserve, thereby inducing ARVC-mimicking features (Graphical Abstract). This explanation model needs further exploration.
Graphical Abstract.
The study by Cerrone et al. showed that exercise reduced the cardiac desmosomal function. This was well tolerated in healthy murine hearts, which had a ‘desmosomal reserve’, but was catastrophic for mice with PKP2 deficiency. One can hypothesize that the desmosomal reserve may be part of the explanation of why some athletes without a known genetic predisposition develop ARVC. ARVC, arrhythmogenic right ventricular cardiomyopathy; PKP2, plakophillin-2.
We congratulate the authors on their interesting study. It launches a novel and interesting concept. The hypothesis of impaired desmosomal reserve represents a supplementary explanation to the exercise-induced increased wall stress theory as causes of accelerated desmosomal dysfunction in ARVC athletes. Importantly, it provides a new and interesting step forward in the unresolved topic of exercise-induced ARVC.
Conflict of interest: None declared.
Contributor Information
Kristina H. Haugaa, Department of Cardiology, Karolinska University Hospital, Stockholm, Sweden Department of Medicine, Huddinge, Karolinska Institutet, Stockholm, Sweden; ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, Oslo, Norway.
Christine Rootwelt-Norberg, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, Oslo, Norway; Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway.
References
- 1. Kirchhof P, Fabritz L, Zwiener M, Witt H, Schäfers M, Zellerhoff S, et al. Age- and training-dependent development of arrhythmogenic right ventricular cardiomyopathy in heterozygous plakoglobin-deficient mice. Circulation 2006;114:1799–1806. [DOI] [PubMed] [Google Scholar]
- 2. James CA, Bhonsale A, Tichnell C, Murray B, Russell SD, Tandri H, et al. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol 2013;62:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saberniak J, Hasselberg NE, Borgquist R, Platonov PG, Sarvari SI, Smith HJ, et al. Vigorous physical activity impairs myocardial function in patients with arrhythmogenic right ventricular cardiomyopathy and in mutation positive family members. Eur J Heart Fail 2014;16:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lie ØH, Dejgaard LA, Saberniak J, Rootwelt C, Stokke MK, Edvardsen T, et al. Harmful effects of exercise intensity and exercise duration in patients with arrhythmogenic cardiomyopathy. JACC Clin Electrophysiol 2018;4:744–753. [DOI] [PubMed] [Google Scholar]
- 5. Lie ØH, Chivulescu M, Rootwelt-Norberg C, Ribe M, Bogsrud MP, Lyseggen E, et al. Left ventricular dysfunction in arrhythmogenic cardiomyopathy: association with exercise exposure, genetic basis, and prognosis. J Am Heart Assoc 2021;10:e018680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace 2015;17:1601–1687. [DOI] [PubMed] [Google Scholar]
- 7. Prior D, La Gerche A. Exercise and arrhythmogenic right ventricular cardiomyopathy. Heart Lung Circ 2020;29:547–555. [DOI] [PubMed] [Google Scholar]
- 8. Gasperetti A, James CA, Cerrone M, Delmar M, Calkins H, Duru F. Arrhythmogenic right ventricular cardiomyopathy and sports activity: from molecular pathways in diseased hearts to new insights into the athletic heart mimicry. Eur Heart J 2021;42:1231–1243. [DOI] [PubMed] [Google Scholar]
- 9. Cerrone M, Marrón-Liñares G, van Opbergen C, Costa S, Bourfiss M, Perez-Hernandez M, et al. Role of plakophilin-2 expression on exercise-related progression of arrhythmogenic right ventricular cardiomyopathy: a translational study. Eur Heart J 2022;43:1251–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharma S, Merghani A, Mont L. Exercise and the heart: the good, the bad, and the ugly. Eur Heart J 2015;36:1445–1453. [DOI] [PubMed] [Google Scholar]
- 11. Vega RB, Konhilas JP, Kelly DP, Leinwand LA. Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metab 2017;25:1012–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. La Gerche A, Robberecht C, Kuiperi C, Nuyens D, Willems R, de Ravel T, et al. Lower than expected desmosomal gene mutation prevalence in endurance athletes with complex ventricular arrhythmias of right ventricular origin. Heart 2010;96:1268–1274. [DOI] [PubMed] [Google Scholar]
- 13. Benito B, Gay-Jordi G, Serrano-Mollar A, Guasch E, Shi Y, Tardif JC, et al. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation 2011;123:13–22. [DOI] [PubMed] [Google Scholar]
- 14. Heidbuchel H. The athlete’s heart is a proarrhythmic heart, and what that means for clinical decision making. Europace 2018;20:1401–1411. [DOI] [PubMed] [Google Scholar]
- 15. Lie ØH, Klaboe LG, Dejgaard LA, Skjølsvik ET, Grimsmo J, Bosse G, et al. Cardiac phenotypes and markers of adverse outcome in elite athletes with ventricular arrhythmias. JACC Cardiovasc Imaging 2021;14:148–158. [DOI] [PubMed] [Google Scholar]