Dear Editor,
Cyclins and cyclin-dependent kinases regulate both mitotic and meiotic cell cycles [1]. Most cyclins are ubiquitously expressed; however, a few are unique to the germline. Cyclin B3 (CCNB3)—an evolutionarily conserved type B cyclin—is meiosis-specific in mammals. In Drosophila and Caenorhabditis elegans, CCNB3 is required for early embryonic divisions. In mice, Ccnb3—an X-linked gene—is required for female fertility but dispensable for male fertility. Specifically, RNAi and knockout studies have shown that mouse CCNB3 is required for metaphase I to anaphase I transition in oocytes [2–4]. Ccnb3-deficient oocytes fail to extrude the first polar body and are arrested at metaphase I, due to a failure in degradation of securin (inhibitor of the protease separase) and CCNB1 (a component of the metaphase promoting factor). Upon fertilization, Ccnb3-deficient oocyte skips the first meiotic cell division (Meiosis I) but extrudes one polar body through segregation of sister chromatids (Meiosis II), resulting in non-viable triploid embryos [3]. By generation and study of an independent Ccnb3 knockout mouse line, we have confirmed these previous findings and further explored the embryonic requirement for Ccnb3.
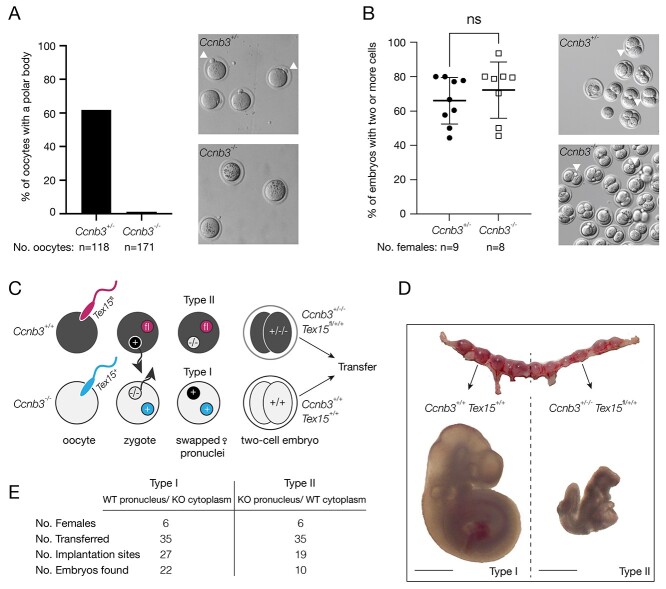
We disrupted Ccnb3 in mice using the CRISPR/Cas9 approach. Two guide RNAs targeted the intronic regions flanking exons 6 and 7 of Ccnb3 (Supplementary Figure S1A). Ccnb3-/Y males exhibited normal histology of testis, testis weight, sperm count, and were fertile (Supplementary Figure S1B–D). Ccnb3−/− females displayed normal ovarian histology but were infertile (Supplementary Figure S1E). Although 61.9% oocytes from Ccnb3+/− females extruded a polar body, only 1.2% oocytes from Ccnb3−/− females did so (Figure 1A). We next tested the competency of Ccnb3-deficient oocytes for fertilization by quantifying progression to the two-cell stage of embryogenesis 36 h after mating with wild-type males. Ccnb3-deficient oocytes were fertilized and extruded a single polar body. The resulting zygotes progressed to the two-cell stage in the same percentage as wild-type zygotes, showing that CCNB3 is dispensable for fertilization (Figure 1B). Although Ccnb3−/− females readily became pregnant, their pregnancies were never carried to term. To further examine the development of the embryos derived from Ccnb3-deficient oocytes, we mated Ccnb3+/− and Ccnb3−/− females to wild-type males and collected embryos at 9.5 days after fertilization (E9.5). Examination with light microscopy revealed malformations of embryos conceived to Ccnb3−/− females, indicating a disruption in embryogenesis consistent with other mouse models of triploidy [5]. This result suggests that the embryos derived from Ccnb3−/− females were able to develop to the blastocyst stage and implant.
Figure 1.
Recurrent pregnancy loss in mice lacking CCNB3. (A) Percentage of ovulated oocytes from 8-week-old Ccnb3+/− and Ccnb3−/− females with a polar body. Arrowheads indicate polar bodies. The oocytes analyzed were pooled from 6 Ccnb3+/− females and 7 Ccnb3−/− females. (B) Percentage of embryos at the two-cell stage 36 h after mating. Representative mages of embryos are shown. Arrowheads indicate polar bodies. The total number of embryos analyzed: 227 from 9 Ccnb3+/− females and 181 from 8 Ccnb3−/− females. NS, statistically non-significant, by Student’s t-test. (C) Schematic of maternal pronuclei (black and white) swap experiment. Paternal pronuclei (colored) are either Tex15fl or Tex15+ (wild type). Note that paternal pronuclei were not swapped. Detailed procedure is described in Supplementary Material. (D) Images of uterine horns collected from a recipient female at E9.5. On the left are implantation sites of type I embryos. On the right are implantation sites of type II embryos. The resultant E9.5 embryos are shown below their respective sides of the uterine horn. Genotypes are shown. Scale bar, 1 mm. (E) Development of pronucleus swap embryos.
Mature oocytes are transcriptionally silent. The presence of the cytoplasmic polyadenylation element (UUUUAU; [6]) upstream of the polyadenylation signal sequence (AAUAAA) in the Ccnb3 transcripts from mouse, human, and other species suggest that Ccnb3 might be translationally dormant in immature oocytes (Supplementary Figure S1F). The initial stage of embryogenesis is driven by stored maternal transcripts and proteins in the oocyte, some of which are required for zygotic genome activation (ZGA). ZGA is a hallmark of the maternal to zygotic transition, during which maternal RNAs driving cellular processes in the oocyte and zygote are degraded, whereas the zygotic genome becomes transcriptionally active to take over the production of essential proteins. Maternal transcripts involved in ZGA are characterized by high abundance in the oocyte and pre-ZGA embryo but a sharp decrease in abundance after ZGA. Mouse and human Ccnb3 genes exhibit such a characteristic expression pattern [7], and furthermore, depletion of Ccnb3 in Ciona intestinalis causes precocious ZGA [7].
We reasoned that if stored Ccnb3 transcripts were important for ZGA in mice, their depletion would disrupt embryogenesis. To distinguish between cytoplasmic and nuclear contributions of Ccnb3, we performed pronucleus swapping experiments (Figure 1C; [8]). In these experiments, wild-type females were mated with Ccnb3+/Y Tex15fl/fl males, and Ccnb3−/− females were mated to Ccnb3+/Y Tex15+/+ males. The Tex15fl and Tex15+ alleles (autosomal) were used to discern between paternal pronuclei. Upon formation of pronuclei in one-cell embryos, the maternal pronuclei were swapped between the embryos derived from Ccnb3-deficient oocytes and those from wild-type oocytes. The swap resulted in two types of embryos: (I) embryos with a wild-type Ccnb3 pronucleus and Ccnb3-deficient cytoplasm; and (II) embryos with a maternal Ccnb3−/− pronucleus and wild-type Ccnb3 cytoplasm (Figure 1C). Types I and II embryos were transferred to the right and left sides, respectively, of the uterine horns of pseudopregnant females and allowed to develop to E9.5 (Figure 1D). An equal number (35 each) of both types of embryos were transferred to six females. Embryos were then harvested for microscopy and genotyping (Supplementary Figure S1G and Supplementary Materials). Since Ccnb3-deficient oocytes only extruded one polar body, the Ccnb3-deficient pronucleus was diploid (Ccnb3−/−) and the resulting embryos were triploid. Therefore, we anticipated that embryos with Ccnb3−/− pronuclei would degenerate regardless of the presence of Ccnb3 transcripts in the cytoplasm, because of triploidy. A total of 22 type I embryos were recovered; these embryos were morphologically normal. In contrast, only 10 type II embryos were recovered after 9.5 days and all of them were abnormal in size and shape (Figure 1E). These results demonstrate that the triploidy of embryos derived from Ccnb3-deficient oocytes is the primary cause of embryo death, i.e., such embryos can be rescued with euploid nuclei, whereas cytoplasmic Ccnb3 transcript is dispensable for ZGA and embryo development.
Our findings underscore the requirement for CCNB3 in female meiosis and suggest that ZGA-specific functions of CCNB3 observed in invertebrate species are not likely conserved in mammals. The Ccnb3-deficient mouse model is similar to a human infertility condition—recurrent pregnancy loss (RPL). RPL is characterized by two or more consecutive pregnancy losses in the first trimester. Recent case studies have identified CCNB3 mutations as genetic causes of RPL in women [9, 10]. Because CCNB3 is X-linked and CCNB3 is dispensable for male fertility, deleterious mutations may be propagated through males and heterozygous females. Clinically, mutations in CCNB3 are likely to be frequent underlying genetic causes of RPL in humans.
Supplementary Material
Footnotes
† Grant Support: This work was supported by NIH/NICHD T32HD083185, R01HD069592, and P50HD068157.
Contributor Information
Jessica Y Chotiner, Department of Biomedical Sciences, University of Pennsylvania School of Veterinary Medicine, Philadelphia, PA, USA; Cell and Molecular Biology Graduate Program, University of Pennsylvania, Philadelphia, PA, USA.
N Adrian Leu, Department of Biomedical Sciences, University of Pennsylvania School of Veterinary Medicine, Philadelphia, PA, USA.
Yang Xu, Department of Biomedical Sciences, University of Pennsylvania School of Veterinary Medicine, Philadelphia, PA, USA.
P Jeremy Wang, Department of Biomedical Sciences, University of Pennsylvania School of Veterinary Medicine, Philadelphia, PA, USA; Cell and Molecular Biology Graduate Program, University of Pennsylvania, Philadelphia, PA, USA.
Conflict of interest
The authors declare they have no conflicts of interest.
References
- 1. Chotiner JY, Wolgemuth DJ, Wang PJ. Functions of cyclins and CDKs in mammalian gametogenesisdagger. Biol Reprod 2019; 101:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang T, Qi ST, Huang L, Ma XS, Ouyang YC, Hou Y, Shen W, Schatten H, Sun QY. Cyclin B3 controls anaphase onset independent of spindle assembly checkpoint in meiotic oocytes. Cell Cycle 2015; 14:2648–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Y, Wang L, Zhang L, He Z, Feng G, Sun H, Wang J, Li Z, Liu C, Han J, Mao J, Li P, et al. Cyclin B3 is required for metaphase to anaphase transition in oocyte meiosis I. J Cell Biol 2019; 218:1553–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karasu ME, Bouftas N, Keeney S, Wassmann K. Cyclin B3 promotes anaphase I onset in oocyte meiosis. J Cell Biol 2019; 218:1265–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Niemierko A. Postimplantation development of CB-induced triploid mouse embryos. J Embryol Exp Morphol 1981; 66:81–89. [PubMed] [Google Scholar]
- 6. Mendez R, Richter JD. Translational control by CPEB: a means to the end. Nat Rev Mol Cell Biol 2001; 2:521–529. [DOI] [PubMed] [Google Scholar]
- 7. Treen N, Heist T, Wang W, Levine M. Depletion of maternal cyclin B3 contributes to zygotic genome activation in the Ciona embryo. Curr Biol 2018; 28:1150–1156.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McGrath J, Solter D. Nuclear transplantation in the mouse embryo by microsurgery and cell fusion. Science 1983; 220:1300–1302. [DOI] [PubMed] [Google Scholar]
- 9. Fatemi N, Salehi N, Pignata L, Palumbo P, Cubellis MV, Ramazanali F, Ray P, Varkiani M, Reyhani-Sabet F, Biglari A, Sparago A, Acurzio B, et al. Biallelic variant in cyclin B3 is associated with failure of maternal meiosis II and recurrent digynic triploidy. J Med Genet 2021; 58:783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rezaei M, Buckett W, Bareke E, Surti U, Majewski J, Slim R. A protein-truncating mutation in CCNB3 in a patient with recurrent miscarriages and failure of meiosis I. J Med Genet 2021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.