Abstract
In the present study, we investigated the antifungal activity and cytotoxicity of a novel membrane-active peptide, KKVVFKVKFKK (MP). MP inhibited the growth of various pathogenic fungi isolated from patients and of fluconazole-resistant fungi at concentrations of 2 to 32 μg/ml. MP had potent fungicidal activity; the minimal fungicidal concentrations of the peptide were no more than fourfold greater than the MICs. Time course experiments of MP-induced killing of Candida albicans ATCC 36232 showed that the rate of killing was rapid and depended on the concentration of MP. MP had a strong synergism with other antifungal drugs; the fractional inhibitory concentration index values of MP with amphotericin B and fluconazole for C. albicans were 0.16 and 0.02, respectively. The 50% inhibitory concentrations of MP for NIH 3T3 and Jurkat cells were approximately 100 times higher than the MIC for C. albicans ATCC 36232, indicating that MP had high selectivity between the fungal and mammalian cells. These results suggest that MP has great advantages in the development of antifungal agents.
The incidence of fungal infections has increased dramatically in the past 20 years because of the increase in the number of people whose immune systems are compromised by AIDS, aging, organ transplantation, or cancer therapy (2, 6). Most of the current antifungal drugs, such as fluconazole, simply reduce the growth of fungi. Amphotericin B is a potent fungicidal agent, but it is very toxic to the kidney and to the hematopoietic and central nervous systems (1, 16). The development of resistant fungal strains in response to the widespread use of current antifungal drugs will cause serious problems in the future (11). The recent emergence of fungal infections and resistant strains has stimulated the development of antifungal drugs with different mechanisms (4, 12, 22).
In the past few years, host defense molecules have been isolated from a variety of natural sources (3, 5, 7, 18). Interestingly, these molecules are small peptides or proteins, some of which have antibacterial and antifungal activities (3, 13, 20, 23). The mode of action of these peptides is related to an increase in membrane permeability and to the disruption of the structure of cell membrane. These peptides have received attention because of their mechanism of perturbing the membrane of the pathogen; however, native defense peptides themselves cannot be used as therapeutic agents because of their low level of activity and poor bioavailabilities.
In a previous study, we designed novel combinatorial libraries consisting of simplified amino acid sequences to screen for a peptide active against Candida albicans membrane (14). A novel decapeptide named KSL, KKVVFKVKFK, which was active against C. albicans and bacteria, was identified in that study (14). Recently, we developed a depsipeptide named MP, KKVVFKVKFKK, that had more potent activity against C. albicans than KSL; we accomplished this by the addition of lysine residue at the C-terminal end of KSL. (The analogs of a membrane-active decapeptide named KSL, KKVVFKVKFK, were synthesized and their characteristics were studied. The depsipeptide, named MP, KKVVFKVKFKK, which was developed by the addition of lysine residue at the C-terminal end of KSL, also acted on the lipid membrane of microorganisms and had more potent activity against C. albicans than did KSL [15]). In the present study the antifungal activity and cytotoxicity of MP were intensively studied. The antifungal activity of the peptide against various pathogenic fungi isolated from patients and fluconazole-resistant fungi, its synergism with other antifungal drugs, the rate of fungicidal activity, and its cytotoxicity against mammalian cell were all measured.
MP irreversibly inhibited the growth of various pathogenic and fluconazole-resistant fungi, but it had low cytotoxicity against mammalian cells. The rate of fungicidal activity of MP was rapid and concentration dependent. MP had a strong synergism with other antifungal drugs; the addition of MP increased the activity of fluconazole and amphotericin B against C. albicans by more than 312- and 62.5-fold, respectively, whereas the addition of MP did not increase the hemolytic activity of amphotericin B. These results suggest that MP has great potential for the development of a novel antifungal drug.
MATERIALS AND METHODS
Synthesis of peptides.
Individual peptides were synthesized on Rink amide methylbenzhydrylamine resin (PerSeptive Biosystem GmbH, Hamburg, Germany) by using 9-fluorenylmethoxycarbonyl chemistry (9, 10, 17) with a 431A automatic peptide synthesizer (Applied Biosystems, Foster City, Calif.). Cleavage of the peptide from the resin was achieved by treatment with a mixture of trifluoroacetic acid (TFA)-thioanisole-ethanedithiol-H2O in a ratio of 80:5:2.5:5 (vol/vol) at room temperature for 12 h. After filtration of the resin and a washing with TFA, a gentle stream of nitrogen was used to remove the excess TFA. The crude peptide was triturated with diethyl ether chilled at −20°C and then centrifuged at 3,000 × g for 10 min. The peptide was purified by high-performance liquid chromatography with a Waters Delta Pak C18 column (25 by 100 mm; Waters, Milford, Mass.). Amino acid analysis and electrospray mass spectrometry on a Platform II spectrometer (Fisons Instruments, Manchester, United Kingdom) were used to characterize the purified peptide.
Antifungal assay.
In vitro antifungal assays were performed by the broth microdilution method according to the recommendation of the National Committee for Clinical Laboratory Standards (21). RPMI 1640 (Gibco BRL, Gaithersburg, Md.) was used as the assay medium. Candida cells freshly grown on slopes of Sabouraud dextrose agar (logarithmic phase) were suspended in physiological saline, and the cell concentration was adjusted to 104 cells per 1 ml of 2×-concentrated medium for use as the inoculum. Peptide solution was added to the wells of a 96-well plate (100 μl per well) and serially diluted twofold. The final concentrations of peptide mixtures ranged from 0.2 to 500 μg/ml. After inoculation (100 μl per well, 5 × 103 cells per ml), the 96-well plate was incubated at 30°C for 48 h, and the absorbance was measured at 620 nm by using an enzyme-linked immunosorbent assay reader (SLT, Salzburg, Austria) to assess cell growth. The MIC was defined as the lowest concentration exhibiting no visible growth compared with the control cell. To measure the minimal fungicidal concentration (MFC), 100 μl of cell suspension was taken from each well, centrifuged, and washed three times with fresh Sabouraud broth. Then, each cell suspension was vortexed vigorously for 10 s, plated on a Sabouraud dextrose agar plate, and incubated at 30°C for 48 h. Fungal cells were then enumerated. The MFC was defined as the lowest concentration of the peptide in which no growth occurred.
Killing time assay.
C. albicans ATCC 36232 grown in RPMI 1640 medium was added to each flask to yield suspensions containing 2 × 104 CFU/ml, and then the peptide was added to the flask. The final concentrations of the peptide were 5 and 10 μg/ml. The flask contents were mixed and incubated in a water bath at 37°C. Next, 100 μl of cell suspension was taken from each flask at known time intervals, centrifuged, and washed three times with fresh RPMI 1640. Each cell suspension was then vortexed vigorously for 10 s, plated onto a Sabouraud dextrose agar plate, and incubated at 30°C for 48 h. The number of viable cells was determined by counting colonies on a Sabouraud dextrose agar plate. Each number of viable cells was determined from three independent experiments.
Cytotoxicity assay.
NIH 3T3 cells and Jurkat cells were plated in 96-well plates containing DMEM (Gibco BRL) supplemented with 10% calf serum and RPMI 1640 supplemented with 10% fetal bovine serum, respectively. After 16 h, the peptide solution was added into the wells of the 96-well plates (the final concentrations of peptide mixture ranged from 0.2 to 500 μg/ml). The plates were then incubated at 37°C in a CO2 incubator for 24 h. Next, the cells were stained with trypan blue, and the viable cells were counted. In the case of NIH 3T3 cells, the cells were trypsinized before the staining and counting. Each number of viable cells was determined from three independent experiments performed in duplicate.
Hemolytic assay.
The detailed method for hemolytic assay was described elsewhere (8). Packed mouse erythrocytes were washed three times with buffer (150 mM KCl, 5 mM Tris-HCl, pH 7.4), and then packed erythrocytes were suspended in 10 volumes of the same buffer (stock cell suspension). For antibiotic treatment, the cell stock suspension was diluted 25-fold with the same buffer and preincubated in the water bath at 37°C for 15 min, and then the test sample was added. After incubation for 1 h, the sample was centrifuged at 4,000 × g for 5 min, and the absorbance of the supernatant was determined at 540 nm. Hemolysis effected by 0.1% Triton X-100 was considered to be 100%.
RESULTS
Antifungal activity of MP and its d-enantiomer, d-MP.
The MICs and MFCs of MP and its d-enantiomer (d-MP) for various pathogenic fungi isolated from patients were determined by the fungal testing laboratory at the University of Texas Health Science Center at San Antonio. As revealed in Table 1, MP inhibited the growth of Candida spp., Cryptococcus spp., and Histoplasma spp. at concentrations ranging from 2 to 32 μg/ml but did not inhibit Aspergillus spp. at up to 32 μg/ml. This peptide had fungicidal activity against Candida spp., Cryptococcus spp., and Histoplasma spp. at concentrations ranging from 2 to 32 μg/ml. d-MP had an activity similar to that of MP against pathogenic fungi except for Coccidioides immitis. d-MP was much more active than MP against C. immitis; the MIC range of d-MP for C. immitis was 4 to 8 μg/ml, while that of MP was more than 32 μg/ml. We also measured the activity of MP and d-MP against current-drug-resistant C. albicans and Candida krusei. As shown in Table 2, the activity of fluconazole against C. krusei ATCC 200917 was decreased by more than 30-fold, while the MIC of peptides for the fluconazole-resistant Candida strains was not changed.
TABLE 1.
MICs and MFCs of antifungal compounds against fungi isolated from patientsa
| Lab no. | Isolate | MIC and MFC (μg/ml) of:
|
||||||
|---|---|---|---|---|---|---|---|---|
| MP
|
d-MP
|
Amphotericin B
|
Fluconazole
|
|||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC80 | ||
| 96-820 | Aspergillus fumigatus | >32 | >32 | >32 | >32 | 0.5 | 4 | >64 |
| 96-808 | Aspergillus flavus | >32 | >32 | >32 | >32 | 1 | 2 | >64 |
| 96-807 | Aspergillus niger | >32 | >32 | 16 | 32 | 0.5 | 0.5 | >64 |
| 96-825 | Aspergillus terreus | >32 | >32 | >32 | >32 | 2 | 16 | >64 |
| 96-818 | Fusarium solani | 16 | >32 | 16 | 32 | 2 | 4 | >64 |
| 96-708 | Coccidioides immitis | >32 | >32 | 8 | 32 | 0.5 | 2 | 16 |
| 94-2259 | Histoplasma capsulatum | 8 | 16 | 4 | 16 | 0.12 | 0.25 | 4 |
| 96-801 | Candida albicans | 8 | 8 | 16 | 16 | 0.25 | 0.5 | 64 |
| 96-802 | Candida albicans | 8 | 8 | 16 | 16 | 1 | 2 | >64 |
| 96-803 | Candida albicans | 8 | 8 | 16 | >32 | 0.25 | 0.5 | 1 |
| 96-814 | Candida albicans | 8 | 32 | 16 | >32 | 0.25 | 1 | 2 |
| 96-817 | Candida albicans | 8 | 32 | 32 | 32 | 0.12 | 1 | 1 |
| 96-821 | Candida glabrata | 8 | 16 | 8 | >32 | 0.5 | 2 | 16 |
| 96-827 | Candida glabrata | 32 | >32 | 16 | 32 | 0.5 | 2 | >64 |
| 96-804 | Candida tropicalis | 4 | 4 | 8 | 16 | 0.25 | 1 | 2 |
| 96-805 | Candida tropicalis | 4 | 4 | 4 | 8 | 0.25 | 2 | 1 |
| 96-812 | Candida parapsilosis | 8 | >32 | 16 | 32 | 0.12 | 0.5 | 2 |
| 96-819 | Candida parapsilosis | 8 | 8 | 8 | 8 | 0.5 | 2 | 4 |
| 96-806 | Cryptococcus neoformans | 4 | 8 | 2 | 2 | 0.25 | 1 | 16 |
| 96-823 | Cryptococcus neoformans | 4 | 4 | 4 | 4 | 0.25 | 2 | 4 |
| 96-824 | Cryptococcus neoformans | 2 | 2 | 2 | 2 | 0.25 | 0.5 | 16 |
| 96-830 | Cryptococcus neoformans | 4 | 4 | 2 | 4 | 0.25 | 1 | 1 |
| 96-839 | Cryptococcus neoformans | 2 | 2 | 2 | 2 | 0.5 | 1 | 4 |
MICs and MFCs were measured by the fungal testing lab at the University of Texas Health Science Center at San Antonio in accordance with the recommendations of the National Committee for Clinical Laboratory Standards.
TABLE 2.
MICs of MP and d-MP against fungal strains resistant to current antifungal agents
| Strain | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| MP | d-MP | Amphotericin B | Fluconazole | |
| C. albicans ATCC 36232 | 3.12 | 3.12 | 0.6 | 0.3 |
| C. albicans ATCC 200955 | 6.25 | 6.25 | 2.5 | 0.6 |
| C. krusei ATCC 90878 | 3.12 | 3.12 | 1.25 | >10 |
| C. krusei ATCC 200917 | 3.12 | 3.12 | 1.25 | >10 |
Killing time assay.
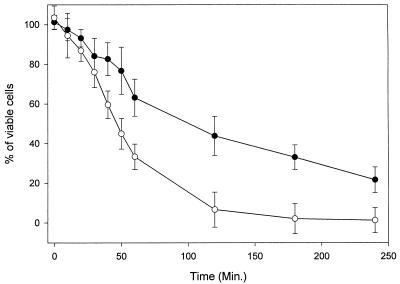
As shown in Fig. 1, 50% of C. albicans cells were killed after 100 min of incubation with 5 μg of MP per ml. As the concentration of peptide was increased, the rate of killing was increased. A total of 50% of the cells were killed after 60 min of exposure to 10 μg of MP per ml. This result indicated that the killing of C. albicans by MP was rapid and concentration dependent.
FIG. 1.
Time course of MP-induced killing of C. albicans. C. albicans cells were incubated for the indicated periods at 30°C with MP. Symbols: ●, 5 μg of MP per ml; ○, 10 μg of MP per ml.
Synergic effect of MP with other antifungal drugs.
The synergic effect of MP in combination with amphotericin B and fluconazole against C. albicans ATCC 36232 and fluconazole-resistant C. krusei ATCC 200917 was studied. As shown in Table 3, the fractional inhibitory concentration index (FIC) values of MP and d-MP for fluconazole and amphotericin B against C. albicans ATCC 36232 were calculated to be ca. 0.02 and 0.16, respectively, indicating that MP had stronger synergism with fluconazole than with amphotericin B against C. albicans. MP and d-MP also had a strong synergism with amphotericin B against C. krusei (FIC index = 0.25), but MP had no synergism with fluconazole against C. krusei.
TABLE 3.
FIC indices for the synergism of the peptides with amphotericin B and fluconazole, as measured by using C. albicans ATCC 36232 and C. krusei ATCC 200917 as the target organisms
| Peptide | FIC index
|
|||
|---|---|---|---|---|
|
C. albicans ATCC 36232
|
C. krusei ATCC 200917
|
|||
| Amphotericin B | Fluconazole | Amphotericin B | Fluconazole | |
| MP | 0.16 | 0.02 | 0.25 | No synergic effect |
| d-MP | 0.16 | 0.02 | 0.25 | No synergic effect |
Cytotoxicity assay.
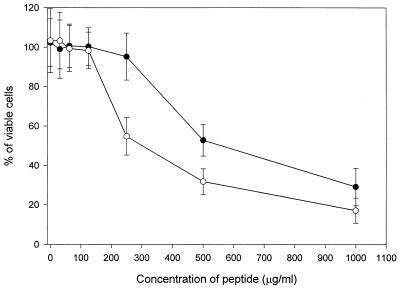
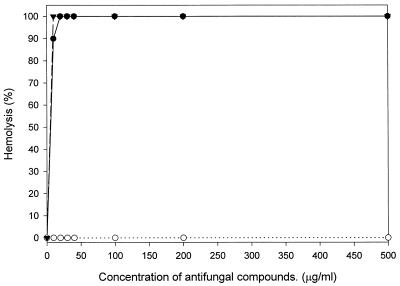
The cytotoxic activity of MP against mammalian cells was measured. As shown in Fig. 2, MP did not show cytotoxicity against NIH 3T3 cells and Jurkat cells at concentrations of up to 125 μg/ml. The 50% inhibitory concentrations (IC50s) of MP for NIH 3T3 and Jurkat cells were ca. 540 and 300 μg/ml, respectively. The IC50s were approximately 100 times higher than the MICs for C. albicans ATCC 36232. Figure 3 shows the level of lysis of the mouse erythrocytes as a function of the concentrations of MP, melittin, and amphotericin B. Amphotericin B and melittin used as a positive control caused 100% lysis at a concentration greater than 10 μg/ml, whereas MP did not show hemolytic activity at a concentration of up to 500 μg/ml. The concentration causing 50% hemolysis for MP was approximately 600 times higher than the MIC for C. albicans ATCC 36232. These results indicated that MP has a high degree of selectivity for fungal rather than mammalian cells.
FIG. 2.
Cytotoxicity of MP against mammalian cells. NIH 3T3 cells and Jurkat cells were incubated at 37°C for 24 h in a CO2 incubator with various concentrations of MP. Symbols: ●, Jurkat cells; ○, NIH 3T3 cells.
FIG. 3.
Hemolytic activity of MP. Erythrocytes were incubated in Tris buffer (150 mM KCl, 5 mM Tris-HCl, pH 7.4) with various concentrations of MP for 1 h at 37°C. Symbols: ○, MP; ●, melittin; ▾, amphotericin B.
DISCUSSION
In this work, we studied the antifungal activity and the cytotoxicity of a novel membrane-active peptide. MP and d-MP had a broad range of activities against various pathogenic fungi, including Candida spp., Cryptococcus spp., and Histoplasma spp., isolated from patients. The MICs and MFCs of d-MP were similar to those of MP, which confirmed that the major target of the peptide is the membrane of the cell and that the peptide does not form a tight interaction with chiral receptors or enzymes or with the chiral components of the lipid membrane. Interestingly, d-MP was much more active than MP against C. immitis. This difference in activity must be due to the difference in stability between MP and d-MP, because d-MP, consisting of d-amino acids, is more resistant to the protease secreted by fungi than is MP. The fact that MP also had a potent activity against the fluconazole-resistant Candida strains indicated that the peptide has a different mode of action from that of fluconazole.
The peptide had a strong synergism with fluconazole for C. albicans ATCC 36232; however, it had no synergism with fluconazole for fluconazole-resistant C. krusei ATCC 200917. The target of MP and fluconazole can explain this result as follows. The primary target of fluconazole is the enzyme involved in ergosterol synthesis in the cytoplasm. Since MP perturbs the membrane of the target cell, MP can increase the influx of fluconazole into the cytoplasm of the cell, resulting in the decrease in the MIC80 of fluconazole for C. albicans ATCC 36232. Studies of fluconazole resistance in C. krusei showed that the target enzyme of fluconazole in the resistant C. krusei strain was altered by point mutation and that fluconazole was not susceptible to the target enzyme any longer (19). Therefore, in the case of fluconazole-resistant C. krusei, the increased influx of fluconazole caused by MP did not increase the activity. We expected that MP had an additive effect with amphotericin B since amphotericin B, like MP, killed microorganisms through the action on the membrane as its primary target. However, interestingly, MP had a strong synergism with amphotericin B against C. albicans and C. krusei. It is possible that perturbation of the fungal membrane by MP may help the binding of amphotericin B to ergosterol and/or the formation of pores of amphotericin B. Further study is currently under way to elucidate the mechanism for the synergic effect of MP with amphotericin B. We also studied the effect of the addition of MP on the hemolytic activity of amphotericin B because amphotericin B had potent cytotoxicity against mammalian erythrocytes. Interestingly, MP did not demonstrate synergism with amphotericin B against mammalian erythrocytes. The current drug of choice for most cases of systemic mycosis is still amphotericin B, but its use is restricted by its toxicity for the kidney and for the hematopoietic and central nervous systems. The strong synergism of MP with amphotericin B suggests that the combination of amphotericin B with MP would permit a reduction in the dosage of amphotericin B needed to kill the fungi.
This study shows that MP has many potential advantages as a candidate for antifungal agents. First, MP shows fungicidal activity against pathogenic and resistant fungi and a fast killing rate but low cytotoxicity for mammalian cells. Second, MP shows strong synergism with fluconazole and amphotericin B. On the basis of these results, we suggest that this peptide can be a lead compound for the development of novel antifungal drugs.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the Korean Ministry of Science and Technology (04-02-61).
We thank M. G. Rinaldi for measuring the activities of the peptides against pathogenic fungi isolated from patients.
REFERENCES
- 1.Andreoli T E. The structure and function of amphotericin B-cholesterol pores in lipid bilayer membranes. Ann N Y Acad Sci. 1974;235:448–468. doi: 10.1111/j.1749-6632.1974.tb43283.x. [DOI] [PubMed] [Google Scholar]
- 2.Andriole V T. Infections with Aspergillus species. Clin Infect Dis. 1993;17(Suppl.):S481–S486. doi: 10.1093/clinids/17.supplement_2.s481. [DOI] [PubMed] [Google Scholar]
- 3.Barra D, Simmanco M. Amphibian skin: a promising resource for antimicrobial peptides. Trends Biotechnol. 1995;13:205–209. doi: 10.1016/S0167-7799(00)88947-7. [DOI] [PubMed] [Google Scholar]
- 4.Bergstrom J D, Dufresne C, Bills G F, Nallin-Omstead M, Byrne K. Discovery, biosynthesis, and mechanism of action of the zaragozic acids: potent inhibitors of squalene synthase. Annu Rev Microbiol. 1995;49:607–639. doi: 10.1146/annurev.mi.49.100195.003135. [DOI] [PubMed] [Google Scholar]
- 5.Berkowitz B A, Bevins C L, Zasloff M A. Magainins: a new family of membrane-active host defense peptides. Biochem Pharmacol. 1990;39:625–629. doi: 10.1016/0006-2952(90)90138-b. [DOI] [PubMed] [Google Scholar]
- 6.Bodey G, Bueltmann B, Duguid W, Gibbs D, Hanak H, Hotchi M, Mall G, Martino P, Meunier F, Milliken S. Fungal infections in cancer patients: an international autopsy survey. Eur J Clin Microbiol Infect Dis. 1992;11:99–109. doi: 10.1007/BF01967060. [DOI] [PubMed] [Google Scholar]
- 7.Boman H G, Hultmark D. Cell-free immunity in insects. Annu Rev Microbiol. 1987;41:103–126. doi: 10.1146/annurev.mi.41.100187.000535. [DOI] [PubMed] [Google Scholar]
- 8.Cheron M, Cybulska B, Mazerski J, Grzybowska J, Czerwinski A, Borowski E. Quantitative structure-activity relationships in amphotericin B derivatives. Biochem Pharmacol. 1988;37:827–836. doi: 10.1016/0006-2952(88)90168-2. [DOI] [PubMed] [Google Scholar]
- 9.Fields G B, Tian Z, Barany G. Synthetic peptide: a user’s guide. New York, N.Y: W. H. Freeman & Co.; 1992. pp. 77–183. [Google Scholar]
- 10.Furka A, Sebestyen F, Asgedom M, Dibo G. General method for rapid synthesis of multicomponent peptide mixtures. Int J Peptide Protein Res. 1991;37:487–493. doi: 10.1111/j.1399-3011.1991.tb00765.x. [DOI] [PubMed] [Google Scholar]
- 11.Georgopapadakou N H, Walsh T J. Human mycoses: drugs and targets for emerging pathogens. Science. 1994;264:371–373. doi: 10.1126/science.8153622. [DOI] [PubMed] [Google Scholar]
- 12.Hata K, Kimura J, Miki H, Toyosawa T, Nakamura T, Katsu K. In vitro and in vivo antifungal activities of ER-30346, a novel oral triazole with a broad antifungal spectrum. Antimicrob Agents Chemother. 1996;40:2237–2242. doi: 10.1128/aac.40.10.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinz D W, Baase W A, Matthews B W. Folding and function of T4 lysozyme containing 10 consecutive alanines illustrate the redundancy of information in an amino acid sequence. Proc Natl Acad Sci USA. 1992;89:3751–3755. doi: 10.1073/pnas.89.9.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong S Y, Oh J E, Kwon M Y, Choi M J, Lee J H, Lee B L, Moon H M, Lee K H. Identification and characterization of novel antimicrobial decapeptides generated by combinatorial chemistry. Antimicrob Agents Chemother. 1998;42:2534–2541. doi: 10.1128/aac.42.10.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong, S. Y., J. E. Oh, M. Y. Kwon, J. H. Lee, B. L. Lee, and K. H. Lee. Unpublished data.
- 16.Kinsky S C. Antibiotic interaction with model membranes. Annu Rev Pharmacol. 1970;10:119–142. doi: 10.1146/annurev.pa.10.040170.001003. [DOI] [PubMed] [Google Scholar]
- 17.Lam K S, Salmon S E, Hersh E M, Hruby V J, Kazmierski W M, Knapp R J. A new type of synthetic peptide library for identifying ligand-binding activity. Nature (London) 1991;354:82–84. doi: 10.1038/354082a0. [DOI] [PubMed] [Google Scholar]
- 18.Mak P, Wojcik K, Thogersen I B, Dubin A. Isolation, antimicrobial activities, and primary structures of hamster neutrophil defensins. Infect Immun. 1996;64:4444–4449. doi: 10.1128/iai.64.11.4444-4449.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orozco A S, Higginbotham L M, Hitchcock C A, Parkinson T, Falconer D, Ibrahim A S, Ghannoum M A, Filler S G. Mechanism of fluconazole resistance in Candida krusei. Antimicrob Agents Chemother. 1998;42:2645–2649. doi: 10.1128/aac.42.10.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu X D, Harwig S S, Shafer W M, Lehrer R I. Protegrin structure and activity against Neisseria gonorrhoeae. Infect Immun. 1997;65:636–639. doi: 10.1128/iai.65.2.636-639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Tudela J L, Berenguer J, Martinez-Sugarez J V, Sanchez R. Comparison of a spectrophotometric microdilution method with RPMI–2% glucose with the National Committee for Clinical Laboratory Standards reference macrodilution method M27-P for in vitro susceptibility testing of amphotericin B, flucytosine, and fluconazole against Candida albicans. Antimicrob Agents Chemother. 1996;40:1998–2003. doi: 10.1128/aac.40.9.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugawara T, Shibazaki M, Nakahara H, Suzuki K. YM-47522, a novel antifungal antibiotic produced by Bacillus sp. II. Structure and relative stereochemistry. J Antibiot. 1996;49:345–348. doi: 10.7164/antibiotics.49.345. [DOI] [PubMed] [Google Scholar]
- 23.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci USA. 1987;84:5449. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]