Abstract
This paper describes a microscale fibroplasia and contraction model that is based on fibrin-embedded lung fibroblasts and provides a convenient visual readout of fibrosis. Cell-laden fibrin microgel drops are formed by aqueous two-phase microprinting. The cells deposit extracellular matrix (ECM) molecules such as collagen while fibrin is gradually degraded. Ultimately, the cells contract the collagen-rich matrix to form a compact cell-ECM spheroid. The size of the spheroid provides the visual readout of the extent of fibroplasia. Stimulation of this wound-healing model with the profibrotic cytokine TGF-β1 leads to an excessive scar formation response that manifests as increased collagen production and larger cell-ECM spheroids. Addition of drugs also shifted the scarring profile: the FDA-approved fibrosis drugs (nintedanib and pirfenidone) and a PAI-1 inhibitor (TM5275) significantly reduced cell-ECM spheroid size. Not only is the assay useful for evaluation of antifibrotic drug effects, it is relatively sensitive; one of the few in vitro fibroplasia assays that can detect pirfenidone effects at submillimolar concentrations. Although this paper focuses on lung fibrosis, the approach opens opportunities for studying a broad range of fibrotic diseases and for evaluating antifibrotic therapeutics.
Keywords: fibrosis, fibroplasia, wound healing, phenotypic assay, fibrin, fibrinolysis, collagen
Insight box statement
Fibrin is widely implemented in tissue engineering applications for its advantages as a natural biopolymer that is formed by quick enzymatic cross-linking and can undergo cell-mediated degradation. These unique qualities of fibrin scaffolds arise from fibrin’s role as a temporary extracellular matrix framework in wound-healing and tissue remodeling. We utilized fibroblast-laden fibrin microdroplets to mimic the fibrin clots formed following tissue injury, which enables a unique microscale fibrosis assay that induces fibroplasia followed by dramatic ECM contraction. Results are evaluated using the label-free, direct visual readout of contracted ECM cross-sectional area. This readout was highly sensitive to multiple antifibrotic drugs, making this assay promising for drug testing with fibrotic diseases, cardiovascular diseases and cancer.
INTRODUCTION
Fibroplasia, the process by which wounds become filled with layers of fibroblasts, myofibroblasts and extracellular matrix (ECM) proteins, is prevalent in a wide range of fibrotic diseases [1–6]. Despite significant clinical need, it is not well understood how fibroplasia becomes pathological and although therapeutics have been shown to slow the progression, there is a lack of therapeutics that can halt or reverse this process. This difficulty arises, at least in part, due to the lack of convenient yet physiologically relevant in vitro models of fibroplasia. The existing fibroplasia models require large amounts of cells (generally ≥1 M fibroblasts), typically require time-consuming procedures to obtain readouts, and are not always sensitive to efficacy of clinically implemented therapeutics. Here, we describe a microscale, contracting scar-in-a-drop where 10 000 lung fibroblasts in 4 μl fibrin droplets are monitored over the course of 9–12 days and provide a direct visual readout of fibroplasia. Importantly, the assay is relatively sensitive to clinically approved idiopathic pulmonary fibrosis (IPF) drugs at concentrations that are lower than many other in vitro fibroplasia assays.
The method starts with the deposition of fibroblast-laden fibrin gel droplets (typically 4 μl and 10 000 cells) using a modification of an aqueous two-phase bioprinting method we reported recently [7]. To promote fibroplasia, the fibrin cultures were stimulated with 2% fetal bovine serum (FBS) and TGF-β1, conditions similar to classic macromolecular crowding-based fibroplasia assays [8] and fibrin gel-based fibroplasia assays [9, 10] although our assay uses lower FBS concentrations. Prior surface-attached fibrin gel assays, sometimes referred to as fibrin contraction assays, have monitored thinning of a thick fibrin gel structure [9]. Because our surface-bound fibrin droplets are already thin and because our observations are of the projected 2D area, our assay does not monitor contraction of the fibrin gel structure itself. Rather, the fibrin scaffold acts as a support for cellular deposition of ECM molecules [11] such as collagen [10]. Even as the cells deposit ECM molecules, cellular contraction of the collagen-containing gel is inhibited while there is sufficient fibrin that is also present [12]. Eventually, as most of the fibrin degrades, the relatively opaque gel changes to a more translucent appearance and the collagen-rich fibrin-poor cell-laden gel contracts into a compact spheroid. The extent of fibroplasia is then conveniently quantified by imaging the 2D projected area of the spheroid. A larger final contracted area in this phenotypic fibroplasia assay can be indicative of increased ECM deposition or cellular proliferation.
This fibrin gel-based contracting scar-in-a-drop was tested with normal and diseased primary human lung fibroblasts from multiple donors, with and without presence of TGF-β1 and nintedanib, to evaluate consistency in response to therapeutic stimuli. We further tested the response of normal and diseased lung fibroblasts to pirfenidone and a PAI-1 inhibitor and found the assay to clearly reveal antifibrotic effects of these antifibrotic agents. To our knowledge, this is one of the smallest high-throughput assay for monitoring fibroplasia that we are aware of. The assay is also convenient in allowing label-free, direct readouts of fibroplasia. Importantly, this assay is also similar to or more sensitive than other fibroplasia assays for detecting antifibrotic activity of the two FDA-approved IPF drugs, nintedanib and pirfenidone.
MATERIALS AND METHODS
Cell culture and ATPS reagents
A stock solution of DEX (20% w/w dextran T500; Sigma) was prepared in PBS on a rocker overnight. A stock solution of PEG (6% w/w, 35 k MW; Sigma) was prepared in fully supplemented culture media with 10% deionized water to balance osmolality. Both stock solutions were passed through a 0.22 μm sterilizing syringe filter before storage. PEG working solutions were stored for up to 2 weeks at 4°C. Thrombin (Human Alpha Thrombin; Enzyme Research Labs) was also added to the PEG solution at a concentration of 0.1 U/ml immediately preceding experiments. Fibrinogen-DEX solutions were prepared by diluting fibrinogen stock solution (human fibrinogen 3; Enzyme Research Labs) to a final concentration of 4 mg/ml in a sterile solution of 4% 10× DMEM, 15% DEX stock solution (to a final concentration of 3% dextran), and 50% cell suspension in growth media. For all experiments, the cell suspension was diluted for 2500 cells per microliter in the final fibrinogen-DEX solution.
Cell preparation
Human primary lung fibroblasts were used in all experiments presented in this paper. Unless otherwise noted, experiments utilized normal human lung fibroblasts (NHLF B lot#0000580583; Lonza) from a 79 year old female with a history of smoking. For experiments evaluating donor variability, the following cells were utilized: NHLF A (NHLF lot#0000608197; Lonza) from a 67-year-old male, IPF A (IPF lot#0000627840; Lonza) from a 52-year-old male, and IPF B (IPF lot#6F5002; Lonza) from an 83-year-old male. All cells were cultured in fibroblast growth media (FGM; Lonza). Cells were passaged at 80–90% confluence, and were subcultured in 1:3 ratios by trypsinization. When at the desired confluence, cells were washed with PBS and 0.05% trypsin solution was added to the flask. Cells were incubated for 2 min, and then harvested and centrifuged (200 × g, 5 min) in a conical tube. The supernatant was aspirated and the cell pellet was resuspended in FBS-free culture media. When used in fibrin degradation experiments, cells were resuspended at 2× the final desired concentration (2500 cells/μl unless otherwise indicated). All experiments were conducted with cells at or below passage 8 except for high-passage experiments conducted at passage 12. In all experiments, media was changed every 48 h and any media additives (plasminogen, TGF-β1, drugs, etc) were included.
ATPS printing of fibrin microgels
ATPS printing of fibrin microscaffolds has previously been documented by our lab in a prior publication [7]. Briefly, working solutions of PEG with 0.1 U/ml of thrombin were warmed to 37°C and pipetted into a 96-well plate. For production of droplets, fibrinogen-DEX solutions with cell suspension were maintained at 37°C and 4 μl per assay (unless otherwise noted) was pipetted directly into the PEG-thrombin media using a semiautomated repeater pipette (Repeater E3X; Eppendorf). Following dispensing of the DEX phase, the plates were placed in an ambient air incubator at 37°C for 30 min to allow the thrombin to enzymatically crosslink the fibrinogen into a fibrin matrix (Fig. 1a). The PEG-enriched media was removed using a 12-channel micropipette and replaced with 100 μl of fully supplemented media in each well. When applicable, this media addition was supplemented with stimuli as detailed in Section Phenotypic evaluation of stimuli. For the duration of each experiment, assay plates were imaged every 2 h at 4× with an automated cell culture monitoring system (Incucyte S3; Sartorius). As the assay proceeded, the fibroblasts progressively remodeled the fibrin scaffold as illustrated in Fig. 1c.
Figure 1.
ATPS fibrin printing and cell-mediated remodeling: (a) process schematic of ATPS generation of microscale fibrin droplets and subsequent remodeling. After the initial pipetting step, thrombin from the PEG phase diffuses into the dextran phase for controlled conversion of fibrinogen into fibrin over the incubation period. Subsequent remodeling includes concurrent fibrinolysis and collagen deposition, followed by contraction. (b) Characteristic brightfield microscope images (taken at 4× magnification) illustrate the assay progression when stimulated with 2 ng/ml of TGF-β1. Scale bars are 1 mm. (c) Microscale illustration shows the changes in ECM organization at stages of remodeling. Fibrosis denotes deposition and accumulation of fibrous extracellular protein.
Histologic analysis of fibrin microgels
Contracted cell-ECM spheroids were harvested after 9–12 days of culture. These structures were prepared for histology, stained and imaged as previously described for cultured spheroids [13]. Briefly, the spheroids were washed with PBS and fixed in 4% paraformaldehyde (Alfa Aesar) for 1 h at room temperature. The structures were stained with 0.5% methylene blue solution in PBS for 10 min at room temperature to aid in visualization during histology. Samples were placed in a cryomold containing optimal cutting temperature compound, and flash frozen in cooled isopentane. Ten micrometer-sections were obtained using a CryoStar NX70 cryostat (Thermo Fisher Scientific).
Upon warming to room temperature, the sections were washed with PBS, permeabilized with 0.2% Triton-X 100, and blocked for 1 h at room temperature with 4% bovine serum albumin (Millipore Sigma). Sections were stained for 30 min at room temperature with Sirius red (0.1% of Sirius red in saturated aqueous picric acid), as previously described for collagen bundle staining [14]. The samples were then washed with PBS, stained with DAPI for 15 min at room temperature, and coverslipped. Samples were imaged using a DMi8 microscope (Leica) equipped with 10× and 20× air objectives. Fluorescence was detected using Texas Red channel settings as previously described [15]. Mean fluorescence intensity (MFI) was quantified in ImageJ as the average pixel intensity within the sections.
mRNA quantification by qPCR
RNA extraction and qPCR: Cells from 12 wells at the indicated time points were pooled together per condition, and lysed with 350 μl of RLT lysis buffer. RNA was extracted using an RNeasy Mini Kit (Qiagen, #74104) and was performed according to the manufacturer’s instructions. RNA sample concentration was measured using a NanoDrop OneC Spectrophotometer (Thermo Fisher Scientific). A High-Capacity RNA-to-cDNA Kit (Applied Biosystems, #4387406) was used for reverse transcription; 400 ng of RNA for each sample was mixed with 10 μl primer, 1 μl reverse transcriptase enzyme and nuclease-free water to bring the final reaction volume to 20 μl. The reaction was performed for 60 min at 37°C, followed by 5 min at 95°C using a Veriti Thermal Cycler (Applied Biosystems). qPCR was performed using a QuantStudio 3 Real-Time PCR System (Applied Biosystems). Each reaction consisted of 1 μl cDNA, 10 μl TaqMan Fast advanced master mix (Applied Biosystems, #4444556), 1 μl primer, and 6 μl nuclease-free water. TaqMan primers (Applied Biosystems) for smooth muscle actin (ACTA2, Hs00426835_g1), plasminogen activator (PLAT, Hs00263492_m1), Plasminogen activator inhibitor-1 (SERPINE1, Hs00167155_m1), collagen type I (COL1A1, Hs00164004_m1), plasminogen activator (PLAU, Hs01547054_m1) and Ki67 (MKI67, Hs01032443_m1) were utilized. The QuantStudio 3 was programmed with a 2 min hold at 95°C, followed by 40 cycles of 95°C for 1 s and 60°C for 20 s. Each sample was run with biological triplicates. The relative gene expression was calculated using the 2-ΔΔCT method, with glyceraldehyde-3-phosphate dehydrogenase as the housekeeping gene (GAPDH, Hs02786624_g1). Fold changes were normalized with respect to the time zero timepoint with no TGF-β1 stimulation, and are reported as the mean with the error bars representing the minimum and maximum values.
Brightfield determination of final area
After each experiment, the final projected areas of the cell-ECM spheroids were determined from brightfield images taken at the final time point using a benchtop imaging system (2× objective; EVOS M7000; ThermoFisher). These images were then segmented through a process of pixel classification, thresholding, and morphological filtering in order to isolate the cell-ECM construct area from the background.
Pixel classification implemented Ilastic, a freely available image classification tool developed by the European Molecular Biology Laboratory. Ilastik’s pixel classification utility implements a random forest classifier for quick and robust segmentation. In order to train the classifier, 10 characteristic images were selected to include different stages of ECM remodeling. In this step, each individual pixel is assigned a probability for belonging to layers for the background or the cell-ECM construct. Ilastik enables interactive training of the random forest classifier via user annotations of the training images. All default features (σ = 0.3 through 10 for intensity, edge and texture) were utilized for this interactive training by methodically annotating mislabeled areas of each training image. Care was taken to equally annotate background and cell-ECM areas in order to prevent the algorithm from weighting features inappropriately. Through this iterative training method, the user can evaluate interactive predictions by the algorithm and then draw additional annotations to correct mistakes. When additional training annotations no longer improved background noise and edge feature fit of the predicted mask over the cell-ECM area, the trained classifier was saved for future use. With each experiment, this trained classifier was reloaded and classification performance was evaluated on representative images (not from the training set) before use.
This pixel classification workflow performs semantic segmentation, and therefore returns a probability map for the background and cell-ECM area for each image. The probability map was transformed into background and cell-ECM area objects through thresholding. Thresholding of these probability masks then enabled generation of a single mask to isolate the cell-ECM area. A closing morphological filter with a 25 × 25 kernel was then applied to each mask in order to remove noise. The area from segmented masks was then used to quantify cell-ECM contraction.
Various concentrations of transforming growth factor type β1 (Human Recombinant TGF-β1; Peprotech) were used for validation due to its established antifibrinolytic and profibrotic qualities. TGF-β1 was added at indicated concentrations in the assay media which was used to rinse and remove ATPS polymers after incubation.
Phenotypic evaluation of stimuli
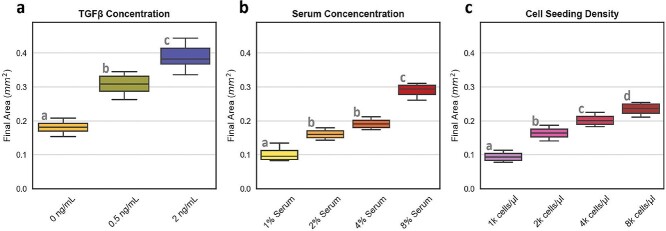
Our customized high-throughput image analysis approach was applied for phenotypic evaluation of all experiments. In order to evaluate ECM remodeling behavior with established stimuli; experiments implemented different conditions of TGF-β1, FBS and cell seeding density. TGF-β1 was introduced at concentrations of 0, 0.5, 2 and 10 ng/ml; however the highest concentration did not contract within the duration of the experiment and was therefore omitted from analysis. In order to evaluate the remodeling effects of serum, FBS (Lonza) at concentrations of 0, 1, 2, 4 and 8% by volume of the cell culture media was added during the washing step after fibrin cross-linking. For cell seeding density experiments, fibroblasts were suspended at appropriately modified concentrations in the dextran phase of the ATPS fibrin printing formulation so that assays were printed with concentrations of 1, 2, 4 and 8 thousand cells per microliter within a 4 μl assay.
In order to evaluate the capability of this assay to test the fibrinolytic and antifibrotic effects of therapeutic stimuli, a variety of drug compounds were introduced to the assays after the wash step. This included 10 μM TM5275 (MedChemExpress), 1 μM nintedanib (Selleck Chem) and 500 μM pirfenidone (Selleck Chem); all diluted and stored according to supplier data sheet recommendations. These concentrations were established in preliminary experiments that evaluated a range of concentrations used in prior literature. These stimuli were freshly mixed for each media change during experiments, and a minimum of four replicates were tested per experimental condition.
Statistical analysis
All experimental values are reported as means ± standard deviation. ANOVA tests were performed using the statsmodels library in Python 3 with the Tukey test for post-hoc pairwise comparisons.
RESULTS AND DISCUSSION
In tissue repair, fibrin formation is followed by fibroblast migration, fibrinolysis and matrix remodeling. In lung fibrosis, fibrinolytic activity is decreased and undegraded fibrin is commonly reported in human IPF patient lungs [16, 17]. We previously reported a method to print microscale cell-laden fibrin gels to quantify cell-mediated fibrinolysis [7]. Our prior study conditions, however, did not provide readouts of fibroplasia such as collagen deposition. Furthermore, the fibrinolysis assay did not show significant change in response to treatment with fibrosis drugs such as nintedanib and pirfenidone.
To overcome these limitations of our previously reported miniaturized fibrinolysis assay, we learned from published larger-scale fibrin-based fibroplasia assays that were used for skin keloids [9, 10]. Specifically, we replaced the plasminogen addition step [7] with addition of FBS and TGF-β1 instead [10]. We observed that fibrin was gradually replaced by a collagen-rich ECM. Surprisingly, after 3–7 days, we also observed contraction and detachment of the remodeled cell-laden matrix into a compact cell-ECM spheroid. Although early contraction of the fibrin gel itself has been reported [9] and inhibition of collagen gel contraction by incorporation of fibrin has been reported [12], we had not found reports of detachment and contraction of the ECM produced by cells embedded in fibrin gels [9, 10]. Here, we test whether this unexpected contraction, detachment and spheroid formation process could be utilized as a convenient, image-based, direct readout of fibroplasia in fibrin droplet assays.
Fabrication of microscale fibrin scaffolds
Biological environments establish fibrin matrices through coagulation, where a cascade of clotting factors activates thrombin to enzymatically convert fibrinogen into fibrin [18]. Similarly, synthetic fibrin scaffolds are formed by exposing monomeric fibrinogen to activated thrombin [19]. Our previously established technique for generating fibrin microscaffolds utilized an ATPS with PEG and dextran to improve control over fibrin formation, which enables printing of unprecedentedly small cell-laden fibrin matrices with standard liquid handling equipment [7]. In this prior report, we evaluated fibroblast-mediated fibrinolysis by addition of exogenous plasminogen comparable to levels found in serum [20]. In the current work, we decreased availability of plasminogen to levels that may better reflect tissue levels [21], and supplemented the media with FBS. These adjustments significantly altered the trajectory of the microscale fibrin remodeling process compared to our prior approach [7] and to other published fibrin fibroplasia assays [9, 10]. Rather than strictly degrading the scaffold into fibrin degradation products and dissociated cells, these conditions induced deposition of significant amounts of collagen followed by contraction of the cell-ECM construct into a fibrotic spheroid.
In this approach, the microscale format enabled microwell plate implementations with convenient automated live imaging. In order to fit the printed microscale fibrin drops within the field of view of a 4× objective, we implemented ATPS printing as previously described [7]. The ATPS-based bioinks allowed partitioning of fibrinogen into the denser, DEX-rich, droplet phase while thrombin was allowed to diffuse in gradually from the less dense, PEG-rich, bulk phase solution. This controlled mixing of enzyme with fibrinogen delayed cross-linking of cell-laden fibrin matrices until after the fibrinogen droplets were dispensed (Fig. 1a). After a 30 min incubation period, the fibrin was sufficiently polymerized and the ATPS solutions could be removed and replaced with growth media.
During assay progression, remodeling is visually apparent in brightfield images as opaque fibrin transitioning into a translucent fibrous matrix and eventually contracting into a dense spheroid (Fig. 1b and c). This concurrent fibrinolysis and deposition of cell-secreted ECM is similar to in-fibrin fibroplasia processes reported previously [10] although the final contraction into a dense spheroid is novel. In the absence of FBS, which contains plasminogen, the initial fibrin matrix remained opaque and intact with minimal change (Supplementary Video 1). Control conditions verified that presence of both FBS and cells was necessary for degradation of the opaque fibrin scaffold, indicating that cell-mediated activation of plasminogen was necessary for fibrin degradation. Factors contributing to altered fibrinolysis, increased ECM deposition, and cell contraction are assessed in the following section.
Response to TGF-β1
Downstream signaling effects of TGF-β1 include inhibition of fibrinolysis, increased fibroblast activation, increased synthesis and deposition of ECM, inhibition of ECM breakdown, and increased contractility [22]. This section describes how TGF-β1 treatment impacts histologic staining of collagen, expression of key genes associated with fibroplasia, and size of the final contracted cell-ECM spheroids.
Final organization of deposited collagen
The most commonly used commercially available method for quantification of deposited collagen is the Sircol™ insoluble collagen assay kit, which implements the dye Sirius Red F3B due to its high specificity for collagen [23]. These kits, however, are optimized for use on fixed quantities of excised tissue. Due to the low assay volume and variability in assay final size (Fig. 2a), Sircol™ kits were not practical. Enzyme-linked immunosorbent assays (ELISAs) for soluble collagen fragments were also difficult to use due to the small amounts of material produced by the small number of cells and high background protein concentrations from the FBS-supplemented media.
Figure 2.
Matrix remodeling in vitro: (a) Brightfield images of histologic sections show the difference in final size between assays treated with varied concentrations of TGF-β1. The contracted assays were harvested after 12 days, and sections were stained with picrosirius red. Scale bars are 250 μm. (b) Evaluation of mean fluorescence intensity demonstrates consistency in collagen organization across conditions. Quantification of mRNA expression via qPCR evaluated dose-dependent time-course changes in SERPENE1 (c), COL1A1 (d), ACTA2 (e), PLAU (f), PLAT (g), MI67 (h) in response to concentrations of TGF-β1. The dotted lines indicate the zero time point used as reference for relative expression. Statistical significance of qPCR was determined by one-way ANOVA and post-hoc Tukey test for each time point; For ab and bc P < 0.05.
Picrosirius red (PSR) utilizes the same anionic dye as Sircol™ assay kits to visualize collagen in paraffin embedded tissue sections. Under light microscopy, PSR stained collagen appears red and can be used for qualitative evaluation of collagen organization [14]. A variety of quantitative approaches for morphometric assessment of collagen networks implement polarized light to visualize fiber alignment; however, signal strength and hue under linear polarized light are heavily dependent on sample orientation [24]. Fluorescent imaging of PSR stained tissues with standard red filter sets yields a strong red fluorescence signal that is sensitive, collagen-specific and is unaffected by sample orientation [15, 24].
In order to evaluate deposited collagen, contracted cell-ECM spheroids were collected after 12 days of culture. Intermediate time points could not be sectioned due to adhesion of flat fibrin scaffolds to the microplate. Fluorescent micrographs demonstrate relatively homogenous collagen distribution for the interior of the contracted assay with higher deposition around the edge (Fig. 2a). Evaluation of the projected area demonstrated a TGF-β1 dose-dependent increase in final contracted spheroid size (P < 0.05). MFI was measured in order to evaluate relative differences in collagen organization between sections (Fig. 2b). Although this measure cannot provide absolute quantification of collagen content, it indicated relative consistency in organization of collagen networks across different TGF-β1 conditions and the control.
Well-established mechanisms have linked TGF-β1 signaling to exaggerated extracellular deposition of type I collagen in fibrosis [25]. Here, histologic evaluation indicates consistency in ECM deposition between conditions, suggesting that the conveniently visualized contracted spheroid size is correlated with the total amount of collagen accumulated during assay progression.
Alterations in mRNA expression
In order to further evaluate the factors contributing to altered ECM remodeling with TGF-β1 stimulation, qPCR was used to determine mRNA expression for proteins involved in fibrinolysis and collagen deposition.
Quantification of mRNA for SERPENE1, which encodes for the protein plasminogen activator inhibitor-type 1 (PAI-1), demonstrated significant time-dependent and dose-dependent increases in expression in response to TGF-β1 (Fig. 2c) similar to what has been reported in a prior fibrin fibroplasia assay [10, 26]. PAI-1 is the dominant inhibitor of fibrinolysis, and acts by binding to the active sites of urokinase-type and tissue-type plasminogen activators (uPA and tPA). These three regulators have been evaluated extensively in animal models of IPF to evaluate their potential involvement in fibrosis pathogenesis [27]. TGF-β1 mediated increases in PAI-1 contribute to the antifibrinolytic environment during certain stages of wound healing and fibrosis [28]. Additionally, gene polymorphisms of TGF-β1 and PAI-1 have been associated with susceptibility to IPF due in part to dysregulation of the fibrinolytic system [29].
Time-course measurements also show significant increases in expression of the genes for tPA and uPA relative to the initial time point, but the effect of TGF-β1 stimulation is inverted between these two plasminogen activators. uPA demonstrated relative upregulation compared to the control time series, whereas tPA demonstrated a relative downregulation (Fig. 2d and e). Other activators and inhibitors produced by cells can also impact conversion of plasminogen to plasmin [4, 30–33]. Our phenotypic fibrin remodeling assay reflects the aggregate effects of these and other pathways. It is noted that while decreased fibrinolysis is one manifestation of increased PAI-1 levels, the mechanism by which it promotes fibroplasia may be through other pathways such as insulin-like growth factor binding protein 3 (IGFBP3) [34, 35].
In addition to its effects on the fibrinolytic system, TGF-β1 also has established roles in myofibroblast activation and collagen synthesis [36]. Myofibroblasts are collagen-producing cells that express the contractile protein alpha-smooth muscle actin (αSMA). Increases in myofibroblast activation and myofibroblast resistance to apoptosis have been identified as major contributors to IPF pathogenesis [37]. Evaluation of ACTA2 mRNA demonstrated significant time-course increases in αSMA expression as well as increased expression for the highest concentration of TGF-β1 (Fig. 2f). These time-course changes may be due to a variety of factors including biomechanical feedback, cytokine secretion or downstream signaling of the fibrinolytic system [37–39].
COL1A1 encodes the pro-alpha1(I) chain, which is a primary component of type I collagen. Quantification of mRNA for COL1A1 demonstrated dose-dependent increase in COL1A1 in response to TGF-β1 (Fig. 2g). Collagen expression in pulmonary fibrosis is heavily dependent on myofibroblast activation [40], but increased collagen expression in fibroblasts has also been linked to downstream effects of antifibrinolytic environments [41].
Expression of MKI67 mRNA was evaluated as a marker for proliferation. MKI67 expression was significantly upregulated with higher concentrations of TGF-β1, indicating increased cellular proliferation relative to the control condition (Fig. 2h). The initial decrease in MKI67 expression in all conditions indicates inhibition of proliferation by the fibrin scaffold, as compared to the cell suspension used for time point zero. Pulmonary fibroblasts have previously been shown to proliferate in response to TGF-β1 [42]. Additionally, the 0 ng/ml TGF-β1 conditions contracted within 24 h (Supplementary video 1), and this dense contracted matrix may have inhibited proliferation compared to TGF conditions which had not yet contracted.
TGF-β1 is a key regulator of ECM remodeling and dysregulation of TGF-β function is closely associated with fibrosis [25]. Our assay reveals multiple effects of TGF-β1 on fibroblasts including its ability to impact ECM remodeling through regulation of the fibrinolytic system and upregulated collagen synthesis.
Label-free quantification of fibroplasia
In order to evaluate fibrosis in vitro, conventional approaches generally quantify specific contributors, such as activation of myofibroblasts or concentration of soluble collagen, using multistep post-culture procedures. Here, we tested the extent to which the unexpected, cell-driven ECM contraction and spheroid formation process could be used as a label-free approach to assess fibroplasia.
An image processing pipeline was established in order to automate quantification and to allow consistent human bias-free analysis. Due to transitions in cell-ECM construct appearance over the course of the experiment, the built-in image segmentation software in our live-cell imager could not provide accurate segmentation. To overcome this image analysis challenge, we utilized Ilastic, a freely available image classification tool developed by the European Molecular Biology Laboratory.
Ilastik’s pixel classification tool utilizes a random forest algorithm that can be interactively trained through iterations of user annotations on a small set of training images. To ensure consistent performance over the duration of the experiments, we selected training images with a variety of features taken at different time points throughout the course of the assay. When additional training annotations no longer improved background noise and edge feature fit, the trained pixel classification algorithm was saved for future use. The Supplementary video 1 illustrates the output of this pixel classification algorithm. Ilastik performs semantic segmentation, which returns probability maps that can be converted into masks by thresholding. In order to remove remaining background noise, opening and closing morphological filters were applied to the masks.
The projected area of the final contracted cell-ECM spheroid was the primary readout evaluated in our analysis, which demonstrated a significant dose-dependent increase with TGF-β1 stimulation (Fig. 3a). COL1A1 mRNA quantification and histologic analysis demonstrated increased collagen synthesis and deposition in response to TGF-β1 (Fig. 2a and g). These data and observations demonstrate that greater spheroid size correlates with increased collagen deposition.
Figure 3.
TGF-β1, fetal bovine serum concentration, and seeding density effects: Fibrin assays were evaluated with different conditions for (a) TGF-β1, (b) serum concentration and (c) cell seeding density to evaluate changes in final size. (Statistical significance by ANOVA: P < 0.05; Post-hoc Tukey test: ab, bc, cd = P < 0.05; N = 6 for TGF-β1 conditions and N = 4 for serum and cell seeding).
Evaluation of serum and cell number effects
The effects of serum concentration on matrix remodeling were evaluated by varying volumetric percentage of FBS in the cell culture media. FBS contains a complex mix of growth factors, hormones, cytokines, proteases, zymogens, cofactors, latent TGF-β1 and inhibitors that influence cellular activity. In the context of fibrin remodeling, an important component of FBS is plasminogen which can be activated by fibroblasts into plasmin for cell-mediated fibrinolysis. We evaluated assay media conditions ranging from serum-free to 8% FBS. FBS-free conditions did not induce contraction within the duration of the experiments. In the absence of FBS, cell-ECM constructs also maintained their opaque appearance, indicating minimal fibrin degradation.
The projected area of the final, contracted cell-ECM spheroids exhibited dose-dependent increases in response to FBS (Fig. 3b). These effects may be due, at least in part, to FBS components such as latent TGF-β1 and fibroblast growth factor (FGF). Increased fibroplasia in response to increasing concentrations of FBS is relevant to fibrotic disease. In vivo tissue availability of serum proteins depends largely on vascular permeability, and dysregulated endothelial permeability and vascular leak are associated with pulmonary fibrosis [21, 43].
The initial fibroblast seeding density used in assays is also relevant to IPF. Fibroblasts from fibrotic lungs have particularly proliferative phenotypes, resulting in higher numbers of fibroblasts and myofibroblasts [44]. Over a fibroblast seeding density range of between 1000 and 8000 cells/μl, we observed a cell number-dependent increase in the final projected cell-ECM spheroid area as expected (Fig. 3c).
Fibroblast donor variability
Prior studies have observed altered fibrogenic response in aged and diseased pulmonary fibroblasts compared to fibroblasts from younger and normal donors [45]. PAI-1 production and TGF-β1 signaling have both been implicated in this pathogenic alteration in behavior [46, 47]. We tested primary human pulmonary fibroblasts from two normal donors and two IPF diseased donors. For one of the normal donors, we also compared what happens at a higher passage (p11).
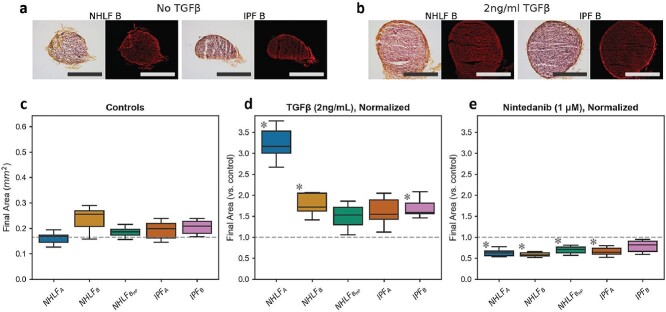
Sections of the final contracted cell-ECM spheroids showed consistency in organization of collagen (Fig. 4a and b). The projected areas of the contracted cell-ECM spheroids in presence of FBS but without TGF-β1 was also relatively consistent across cells from different donors (Fig. 4c). The response to TGF-β1 addition, however, varied significantly (Fig. 4d). NHLFA showed significantly greater response to TGF-β1 compared to NHLFB. The high-passage lineage of NHLFB showed an even smaller, statistically insignificant response. Despite both being considered normal, NHLFA was isolated from a 67-year-old male donor, whereas NHLFB came from a 79-year-old female smoker. Senescent phenotypes induced by age, smoking and high-passage number may explain the varying responsiveness to TGF-β1 observed [48–50]. Nintedanib treatment reduced the final projected area, although one of the two IPF fibroblasts did not reach statistical significance (P < 0.3). In IPF donors, elevated in vivo exposure to TGF-β1 results in a heterogenous population of both fibroblasts and myofibroblasts [51]. This heterogeneity may also contribute to variability in final contracted area.
Figure 4.
Consistency in response across donors: (a, b) Histologic sections show final contracted assays for NHLF B and IPF B with picrosirius red staining. For each example, color brightfield images are shown on the left with fluorescent images on the right. Scale bars are 250 μm. (c) Control conditions showed consistency in contraction with no significant differences in final contracted area. (d, e) Final area was normalized to each donor’s mean control area to indicate fold-change in response to (d) TGF-β1 and (e) nintedanib. (Statistical significance by two-way ANOVA P < 0.05; Post-hoc Tukey test asterisk indicates P < 0.05 compared to donor control; N = 5 for all conditions).
Drug response
Here, we tested the response of lung fibroblasts from one normal and one IPF donor using three different drugs that target different pathways (Fig. 5). Pirfenidone has been established to reduce fibroblast proliferation, α-SMA expression and collagen synthesis [52, 53]. Nintedanib is a multiple tyrosine kinase inhibitor with effects on expression of ECM proteins and TGF-β1 induced signaling [54]. Treatment with pirfenidone demonstrated a significant decrease in final area of TGF treated spheroids (P < 0.01). Nintedanib conditions showed significant decreases in final area for all conditions (P < 0.05). The ability of the contacting scar-in-a-drop assay described here to reveal antifibrotic effects of pirfenidone and nintedanib contrasts with our previously reported cell-mediated fibrinolysis assay that did not show significant effects of nintedanib and pirfenidone [7].
Figure 5.

Response to therapeutic stimuli: The IPF therapeutics pirfenidone, nintedanib, and TM5275 were evaluated on NHLF (a, b) and IPF (c, d) cells to determine the effects of these drugs on final assay area. Graphs for each cell type are separated into no TGF-β1 (a, c) and 2 ng/ml TGF-β1 (b, d). (Statistical significance by one-way ANOVA P < 0.05; Post-hoc Tukey test * = P < 0.05 compared to control; N = 5 for all conditions).
Nintedanib and pirfenidone are the two current FDA-approved therapeutics for IPF. In clinical use, however, these drugs do not halt or reverse fibrosis, and merely slow the progression of fibrotic scarring in the lungs [55]. To address the need for alternative treatment strategies, several recent reviews have proposed components of the fibrinolytic system as potential targets for therapeutic intervention [56–58]. Inhibition of PAI-1 is of particular interest, as its increased expression in IPF has been associated with worse clinical outcome [16, 46]. TM5275 is a small molecule inhibitor of PAI-1, which has been shown to minimize the extent of fibrotic remodeling in an animal model of pulmonary fibrosis and trigger apoptosis in TGF-β1 treated (but not untreated) fibroblasts and myofibroblasts [59]. Consistent with these prior observations, TM5275 decreased the spheroid area for all the TGF-β1 treated conditions (P < 0.05) but not in conditions that omitted TGF-β1. This difference in response may be related to PAI-1 upregulation with TGF-β1, where elevated levels could enable TM5275 to inhibit PAI-1 more effectively.
Fibrosis is the aggregate outcome of multiple dysregulated pathways. The ability of the contracting scar-in-a-drop assay to robustly detect effects of three different antifibrotic agents that work through disparate pathways, including the only two FDA-approved drugs, is encouraging for broader drug testing applications in the future.
CONCLUSION
Despite its importance in wound healing and fibrosis, fibrin gels have seen limited applications within fibroplasia assays [9, 10]. Instead, current prevailing assays focus on specific aspects of collagen production or cellular activation. Figure 6 illustrates the steps of wound healing and shows where current phenotypic fibrosis assays stand.
Figure 6.
Fibroplasia assays and the stages of wound healing: Following tissue injury, the process of wound healing can be broken down into clot formation, fibroblast differentiation, ECM remodeling, and contraction. Aberrant progression of these steps can result in tissue fibrosis. Prior approaches such as the Scar-In-A-Jar or Collagen Contraction assays have evaluated specific stages of pathogenesis, whereas the approach developed here (Contracting Scars from Fibrin Drops) recapitulates more of the full biological process.
Macromolecular crowding agent-based approaches, so-called scar-in-a-jar assays, have recently been the focus of several publications [60–64]. The scar-in-a-jar assays are possible to perform in high-throughput format [61] but have difficulty detecting effects of some drugs when the readout is based on ECM production [64]. The readouts are always based on methods that requires additional procedures such as staining or immunoassays. Our method, on the other hand, is label-free and provides a direct visual readout of fibroplasia. Our ATPS bioprinting method uses macromolecules, particularly dextran, for ATPS-based fibrin printing; this may appear to mimic the scar-in-a-jar assay. The polymers in our assay, however, are quickly washed out after the 30 min cross-linking reaction, although the presence of some residual dextran is not completely ruled out. Our assay is also different in allowing contraction of the cell-produced ECM.
Wound closure involves contraction at the macroscopic scale and fibrosis involves mechanical activation of cytokines and mechanotransduction making assays that provide readouts of mechanical function of cells important. The collagen contraction assay is the classic assay of this type and has been used extensively over the years [65]. We have also reported a microscale format collagen contraction assay based on ATPS bioprinting of collagen microdroplets [66]. These assays, however, are also not sensitive to effects of antifibrotic drugs such as pirfenidone and the well-to-well variability can be quite large [65, 66]. Furthermore, collagen is known to inhibit fibroplasia by limiting collagen production by cells [12]. Our contracting scar-in-a-drop assay is beneficial in starting from a collagen-free gel to allow uninhibited cellular collagen deposition followed by contraction once fibrin has sufficiently degraded [12]. Our integrated approach enables this assay to replicate, in vitro, more of the biological wound-healing process compared to prior models, showing the cumulative impact of multiple steps of an abnormal scarring process.
In summary, this paper reports a unique microscale fibrosis assay that induces fibroplasia in fibrin gels, uninhibited by presence of pre-existing collagen, that in later stages undergo a dramatic ECM contraction. The convenience of direct visual readouts of fibroplasia coupled with high sensitivity to multiple antifibrotic drugs makes this assay promising for drug testing applications. Given the variety of diseases that involve fibroplasia and mechanotransduction such as fibrotic diseases, cancer and cardiovascular diseases; this phenotypic assay provides broad utility beyond IPF. This paper focuses on evaluating the projected area of cell-ECM spheroids after contraction. Given the central role of cell and tissue mechanics in fibrosis, there may also be opportunities to analyse contraction dynamics to gain additional information and readouts from this assay in the future.
Supplementary Material
Contributor Information
Stephen Robinson, Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory School of Medicine, Atlanta, GA, USA; The Parker H. Petit Institute of Bioengineering and Bioscience, Georgia Institute of Technology, Atlanta, GA, USA.
Eric Parigoris, Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory School of Medicine, Atlanta, GA, USA; The Parker H. Petit Institute of Bioengineering and Bioscience, Georgia Institute of Technology, Atlanta, GA, USA.
Jonathan Chang, Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory School of Medicine, Atlanta, GA, USA; The Parker H. Petit Institute of Bioengineering and Bioscience, Georgia Institute of Technology, Atlanta, GA, USA.
Louise Hecker, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Emory University School of Medicine, Atlanta, GA, USA.
Shuichi Takayama, Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory School of Medicine, Atlanta, GA, USA; The Parker H. Petit Institute of Bioengineering and Bioscience, Georgia Institute of Technology, Atlanta, GA, USA.
Funding
The National Institute of Health (R21 AG061687 and R01 HL136141). This material is also based upon work supported by the National Science Foundation Graduate Research Fellowship Program (DGE-1650044 to. E.P. and J.C.).
References
- 1. Clark RA. Fibrin and wound healing. Ann N Y Acad Sci 2001;936:355–67. [DOI] [PubMed] [Google Scholar]
- 2. Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol 2004;36:1031–7. [DOI] [PubMed] [Google Scholar]
- 3. Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci 2010;123:4195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cesarman-Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. Br J Haematol 2005;129:307–21. [DOI] [PubMed] [Google Scholar]
- 5. Mutsaers SE, Bishop JE, McGrouther G, Laurent JG. Mechanisms of tissue repair: from wound healing to fibrosis. Int J Biochem Cell Biol 1997;29:5–17. [DOI] [PubMed] [Google Scholar]
- 6. Herouy Y, Trefzer Z, Hellstern MO, et al. Plasminogen activation in venous leg ulcers. Br J Dermatol 2000;143:930–6. [DOI] [PubMed] [Google Scholar]
- 7. Robinson S, Chang J, Parigoris E, et al. Aqueous two-phase deposition and fibrinolysis of fibroblast-laden fibrin micro-scaffolds. Biofabrication 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clark RA, McCoy GA, Folkvord JM, McPherson JM. TGF-beta 1 stimulates cultured human fibroblasts to proliferate and produce tissue-like fibroplasia: a fibronectin matrix-dependent event. J Cell Physiol 1997;170:69–80. [DOI] [PubMed] [Google Scholar]
- 9. Tuan TL, Song A, Chang S, et al. In vitro fibroplasia: matrix contraction, cell growth, and collagen production of fibroblasts cultured in fibrin gels. Exp Cell Res 1996;223:127–34. [DOI] [PubMed] [Google Scholar]
- 10. Tuan TL, Wu H, Huang EY, et al. Increased plasminogen activator inhibitor-1 in keloid fibroblasts may account for their elevated collagen accumulation in fibrin gel cultures. Am J Pathol 2003;162:1579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grassl ED, Oegema TR, Tranquillo RT. Fibrin as an alternative biopolymer to type-I collagen for the fabrication of a media equivalent. J Biomed Mater Res 2002;60:607–12. [DOI] [PubMed] [Google Scholar]
- 12. Nien YD, Han YP, Tawil B, et al. Fibrinogen inhibits fibroblast-mediated contraction of collagen. Wound Repair Regen 2003;11:380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parigoris E, Lee S, Mertz D, et al. Cancer cell invasion of mammary organoids with basal-in phenotype. Adv Healthc Mater 2021;10:e2000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 1979;11:447–55. [DOI] [PubMed] [Google Scholar]
- 15. Wegner KA, Keikhosravi A, Eliceiri KW, Vezina CM. Fluorescence of Picrosirius red multiplexed with immunohistochemistry for the quantitative assessment of collagen in tissue sections. J Histochem Cytochem 2017;65:479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kotani I, Sato A, Hayakawa H, et al. Increased procoagulant and antifibrinolytic activities in the lungs with idiopathic pulmonary fibrosis. Thromb Res 1995;77:493–504. [DOI] [PubMed] [Google Scholar]
- 17. Imokawa S, Sato A, Hayakawa H, et al. Tissue factor expression and fibrin deposition in the lungs of patients with idiopathic pulmonary fibrosis and systemic sclerosis. Am J Respir Crit Care Med 1997;156:631–6. [DOI] [PubMed] [Google Scholar]
- 18. Laurens N, Koolwijk P, de Maat MP. Fibrin structure and wound healing. J Thromb Haemost 2006;4:932–9. [DOI] [PubMed] [Google Scholar]
- 19. Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev 2008;14:199–215. [DOI] [PubMed] [Google Scholar]
- 20. Leipnitz G, Miyashita C, Heiden M, et al. Reference values and variability of plasminogen in healthy blood donors and its relation to parameters of the fibrinolytic system. Haemostasis 1988;18:61–8. [DOI] [PubMed] [Google Scholar]
- 21. Dvorak HF. Vascular permeability to plasma, plasma proteins, and cells: an update. Curr Opin Hematol 2010;17:225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gharaee-Kermani M, Hu B, Phan SH, Gyetko MR, et al. Recent advances in molecular targets and treatment of idiopathic pulmonary fibrosis: focus on TGFbeta signaling and the myofibroblast. Curr Med Chem 2009;16:1400–17. [DOI] [PubMed] [Google Scholar]
- 23. Walsh BJ, Thornton SC, Penny R, Breitab SN. Microplate reader-based quantitation of collagens. Anal Biochem 1992;203:187–90. [DOI] [PubMed] [Google Scholar]
- 24. Borges LF, Taboga SR, Gutierrez PS. Simultaneous observation of collagen and elastin in normal and pathological tissues: analysis of Sirius-red-stained sections by fluorescence microscopy. Cell Tissue Res 2005;320:551–2. [DOI] [PubMed] [Google Scholar]
- 25. Verrecchia F, Mauviel A. Transforming growth factor-beta and fibrosis. World J Gastroenterol 2007;13:3056–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dennler S, Itoh S, Vivien D, et al. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J 1998;17:3091–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Swaisgood CM, French EL, Noga C, et al. The development of bleomycin-induced pulmonary fibrosis in mice deficient for components of the fibrinolytic system. Am J Pathol 2000;157:177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Idell S, Zwieb C, Boggaram J, et al. Mechanisms of fibrin formation and lysis by human lung fibroblasts: Influence of TGF-beta and TNF-alpha. Am J Physiol 1992;263:L487–94. [DOI] [PubMed] [Google Scholar]
- 29. Li XX, Li N, Ban CJ, et al. Idiopathic pulmonary fibrosis in relation to gene polymorphisms of transforming growth factor-beta1 and plasminogen activator inhibitor 1. Chin Med J (Engl) 2011;124:1923–7. [PubMed] [Google Scholar]
- 30. Kucharewicz I, Kowal K, Buczko W, Bodzenta-Łukaszk A. The plasmin system in airway remodeling. Thromb Res 2003;112:1–7. [DOI] [PubMed] [Google Scholar]
- 31. Levi M, Roem D, Kamp AM, et al. Assessment of the relative contribution of different protease inhibitors to the inhibition of plasmin in vivo. Thromb Haemost 1993;69:141–6. [PubMed] [Google Scholar]
- 32. Bouma BN, Mosnier LO. Thrombin activatable fibrinolysis inhibitor (TAFI)--how does thrombin regulate fibrinolysis? Ann Med 2006;38:378–88. [DOI] [PubMed] [Google Scholar]
- 33. Douglas SA, Lamothe SE, Singleton TS, et al. Human cathepsins K, L, and S: Related proteases, but unique fibrinolytic activity. Biochim Biophys Acta Gen Subj 2018;1862:1925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ozcan S, Alessio N, Acar MB, et al. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging (Albany NY) 2016;8:1316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rana T, Jiang C, Liu G, et al. PAI-1 regulation of TGF-beta1-induced alveolar type II cell senescence, SASP secretion, and SASP-mediated activation of alveolar macrophages. Am J Respir Cell Mol Biol 2020;62:319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem 2001;276:17058–62. [DOI] [PubMed] [Google Scholar]
- 37. Phan SH. The myofibroblast in pulmonary fibrosis. Chest 2002;122:286S–9S. [DOI] [PubMed] [Google Scholar]
- 38. Huang X, Yang N, Fiore V, et al. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 2012;47:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bernstein AM, Twining SS, Warejcka DJ, et al. Urokinase receptor cleavage: a crucial step in fibroblast-to-myofibroblast differentiation. Mol Biol Cell 2007;18:2716–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Pathol 1994;145:114–25. [PMC free article] [PubMed] [Google Scholar]
- 41. Tuan TL, Hwu P, Ho W, et al. Adenoviral overexpression and small interfering RNA suppression demonstrate that plasminogen activator inhibitor-1 produces elevated collagen accumulation in normal and keloid fibroblasts. Am J Pathol 2008;173:1311–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khalil N, Xu YD, O’Connor R, Duronio V. Proliferation of pulmonary interstitial fibroblasts is mediated by transforming growth factor-beta1-induced release of extracellular fibroblast growth factor-2 and phosphorylation of p38 MAPK and JNK. J Biol Chem 2005;280:43000–9. [DOI] [PubMed] [Google Scholar]
- 43. Probst CK, Montesi SB, Medoff BD. Vascular permeability in the fibrotic lung. Eur Respir J 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vancheri C. Idiopathic pulmonary fibrosis: an altered fibroblast proliferation linked to cancer biology. Proc Am Thorac Soc 2012;9:153–7. [DOI] [PubMed] [Google Scholar]
- 45. Pardo A, Selman M. Lung fibroblasts, aging, and idiopathic pulmonary fibrosis. Ann Am Thorac Soc 2016;13:S417–21. [DOI] [PubMed] [Google Scholar]
- 46. Huang WT, Akhter H, Jiang C. Plasminogen activator inhibitor 1, fibroblast apoptosis resistance, and aging-related susceptibility to lung fibrosis. Exp Gerontol 2015;61:62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sueblinvong V, Neujahr DC, Mills ST, et al. Predisposition for disrepair in the aged lung. Am J Med Sci 2012;344:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mu XC, Higgins PJ. Differential growth state-dependent regulation of plasminogen activator inhibitor type-1 expression in senescent IMR-90 human diploid fibroblasts. J Cell Physiol 1995;165:647–57. [DOI] [PubMed] [Google Scholar]
- 49. Martens JW, Sieuwerts AM, Bolt-de Vries J, et al. Aging of stromal-derived human breast fibroblasts might contribute to breast cancer progression. Thromb Haemost 2003;89:393–404. [PubMed] [Google Scholar]
- 50. Serrano M, Lin AW, McCurrach ME, et al. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997;88:593–602. [DOI] [PubMed] [Google Scholar]
- 51. Habiel DM, Hogaboam CM. Heterogeneity of fibroblasts and myofibroblasts in pulmonary fibrosis. Curr Pathobiol Rep 2017;5:101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Conte E, Gili E, Fagone E, et al. Effect of pirfenidone on proliferation, TGF-beta-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur J Pharm Sci 2014;58:13–9. [DOI] [PubMed] [Google Scholar]
- 53. Staab-Weijnitz CA, Fernandez IE, Knüppel L, et al. FK506-binding protein 10, a potential novel drug target for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2015;192:455–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rangarajan S, Kurundkar A, Kurundkar D, et al. Novel mechanisms for the antifibrotic action of nintedanib. Am J Respir Cell Mol Biol 2016;54:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ahluwalia N, Shea BS, Tager AM. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am J Respir Crit Care Med 2014;190:867–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Menou A, Duitman J, Crestani B. The impaired proteases and anti-proteases balance in idiopathic pulmonary fibrosis. Matrix Biol 2018;68-69:382–403. [DOI] [PubMed] [Google Scholar]
- 57. Adnot S, Breau M, Houssaini A. PAI-1: A new target for controlling lung-cell senescence and fibrosis? Am J Respir Cell Mol Biol 2020;62:271–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schuliga M, Jaffar J, Harris T, et al. The fibrogenic actions of lung fibroblast-derived urokinase: a potential drug target in IPF. Sci Rep 2017;7:41770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huang WT, Vayalil PK, Miyata T, et al. Therapeutic value of small molecule inhibitor to plasminogen activator inhibitor-1 for lung fibrosis. Am J Respir Cell Mol Biol 2012;46:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen CZ, Peng YX, Wang ZB, et al. The scar-in-a-jar: studying potential antifibrotic compounds from the epigenetic to extracellular level in a single well. Br J Pharmacol 2009;158:1196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Good RB, Eley JD, Gower E, et al. A high content, phenotypic 'scar-in-a-jar' assay for rapid quantification of collagen fibrillogenesis using disease-derived pulmonary fibroblasts. BMC Biomed Eng 2019;1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fan C, Lim LK, Wu Z, et al. In vitro model of human cutaneous hypertrophic scarring using macromolecular crowding. J Vis Exp 2020;159. [DOI] [PubMed] [Google Scholar]
- 63. Holm Nielsen S, Willumsen N, Leeming DJ, et al. Serological assessment of activated fibroblasts by alpha-smooth muscle actin (alpha-SMA): a noninvasive biomarker of activated fibroblasts in lung disorders. Transl Oncol 2019;12:368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ronnow SR, Dabbagh RQ, Genovese F, et al. Prolonged scar-in-a-jar: an in vitro screening tool for anti-fibrotic therapies using biomarkers of extracellular matrix synthesis. Respir Res 2020;21:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yamanishi C, Robinson S, Takayama S. Biofabrication of phenotypic pulmonary fibrosis assays. Biofabrication 2019;11:032005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yamanishi C, Parigoris E, Takayama S. Kinetic analysis of label-free microscale collagen gel contraction using machine learning-aided image analysis. Front Bioeng Biotechnol 2020;8:582602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.