Abstract
Background
Escherichia coli (E. coli), the main human gut microorganism, is one of the evolved superbugs because of acquiring antimicrobial resistance (AMR) determinants via horizontal gene transfer (HGT).
Purpose
This study aimed to screen isolates of gut commensal E. coli from healthy adult individuals for antimicrobial susceptibility and plasmid-mediated AMR encoding genes.
Methods
Gut commensal E. coli bacteria were isolated from fecal samples that were taken from healthy adult individuals and investigated phenotypically for their antimicrobial susceptibility against diverse classes of antimicrobials using the Kirby Bauer disc method. PCR-based molecular assays were carried out to detect diverse plasmid-carried AMR encoding genes and virulence genes of different E. coli pathotypes (eaeA, stx, ipaH, est, elt, aggR and pCVD432). The examined AMR genes were β-lactam resistance encoding genes (blaCTX-M1, blaTEM, blaCMY-2), tetracycline resistance encoding genes (tetA, tetB), sulfonamides resistance encoding genes (sul1, sulII), aminoglycoside resistance encoding genes (aac(3)-II, aac(6′)-Ib-cr) and quinolones resistance encoding genes (qnrA, qnrB, qnrS).
Results
PCR results revealed the absence of pathotypes genes in 56 isolates that were considered gut commensal isolates. E. coli isolates showed high resistance rates against tested antimicrobial agents belonging to both β-lactams and sulfonamides (42/56, 75%) followed by quinolones (35/56, 62.5%), tetracyclines (31/56, 55.4%), while the lowest resistance rate was to aminoglycosides (24/56, 42.9%). Antimicrobial susceptibility profiles revealed that 64.3% of isolates were multidrug-resistant (MDR). High prevalence frequencies of plasmid-carried AMR genes were detected including blaTEM (64%) sulI (60.7%), qnrA (51.8%), aac(3)-II (37.5%), and tetA (46.4%). All isolates harbored more than one gene with the most frequent genetic profile among isolates was blaTEM-blaCTX-M1-like-qnrA-qnrB-tetA-sulI.
Conclusion
Results are significant in the evaluation of plasmid-carried AMR genes in the human gut commensal E. coli, suggesting a potential human health risk and the necessity of strict regulation of the use of antibiotics in Egypt. Commensal E. coli bacteria may constitute a potential reservoir of AMR genes that can be transferred to other bacterial species.
Keywords: gut microorganisms, antibiotic resistance, plasmid, MDR, resistance genes
Introduction
In recent years, antimicrobial resistance (AMR) has become a global threat to public health.1,2 The urgent AMR crisis and transmission of multidrug-resistant (MDR) bacterial pathogens are major causes of high mortality and morbidity rates worldwide.3 While the reasons for the high rates of AMR, particularly in developing countries, still require further research.4 Many studies reported the extensive dissemination of AMR among Gram-positive and Gram-negative bacteria in Egypt, which requires continuous monitoring of antimicrobial resistance profiles, efficient diagnosis and implementation of effective antibiotic stewardship programs.5–7 AMR encoding genes are the main mechanisms for developing resistance to different classes of antimicrobials among bacteria. These AMR resistance encoding genes may be carried on the bacterial chromosome or located on mobile genetic elements acquired by bacteria such as plasmids.8,9
Commensal microorganisms or microbiota, mainly gut commensal bacteria, can act as a reservoir of AMR genes. Hence, these resistance genes can be transferred from these commensal bacteria to pathogenic bacterial strains.10 Particularly, gut commensal bacteria that are found in huge numbers in the gut are at risk of acquiring genes encoding for AMR traits owing to high exposure to oral antimicrobial therapy, in addition to some parenteral preparations.11 The antimicrobial susceptible gut commensals and/or pathogens will be eradicated. However, the resistant microorganisms of both groups will survive and become predominant in a specified site.8,9 Additionally, some antibiotics are used in sub-therapeutic levels as animal feed additives to promote growth and prevent infections.12,13 Frequent exposure and misuse of antibiotics in both humans and animals has upsurged the emergence and spread of AMR.12,14
Escherichia coli is a bacterium that has an importance in the microbiological world, it has a dual role as a member of the gut microbiota the first bacterial species colonizing the gut.15 In addition, it is one of the most common human and animal pathogens that can cause intestinal and extra-intestinal infections.2,8 Commensal E. coli is normally residing in the gut lumen and rarely causes disease. However, in case of host impaired immunity or breached gastrointestinal barriers, commensal E. coli can cause opportunistic infections in its host such as urinary tract infections.16 Though, these E. coli strains can acquire AMR and virulence traits encoding genes through DNA horizontal transfer of mobile genetic elements (MGEs) including plasmids, transposons and pathogenicity islands and bacteriophages which confer bacteria to cause resistant infectious diseases.17 Furthermore, commensal E. coli might act as a reservoir of MGEs, such as transposons and plasmids, that are carrying AMR and/or virulence traits encoding genes.15 Thus, remarkably, commensal E. coli can act as a donor and/or a recipient of AMR genes in the enterobacterial gene pool; they acquire and pass resistance genes from and to other bacteria by HGT.18 Conjugation, via the assembly of conjugative pili, is identified as the most common mechanism for the transmission of plasmids carrying AMR genes especially those encoding extended-spectrum beta-lactamases and carbapenemases.19,20 Significantly, the gut bacteria can conceal more than one thousand different AMR genes which will be easily transmitted via the fecal-oral route in both humans and animals.21
Regarding infections, E. coli is the most common cause of urinary tract infections (UTIs), although it can infect other anatomical sites in the human body in all age groups.15,22 It can cause meningitis, septicemia, gastrointestinal infections, bloodstream infections, appendicitis, endocarditis, pneumonia and skin abscesses.2,22 In women, gut commensal E. coli, through the ascending route, is the main cause of UTIs due to the short distance between the urethral meatus and anus; that may extend to the bladder wall and kidney.23
Since the emergence of AMR and the issue of resistant commensal gut bacteria in healthy people have yet not been addressed in Egypt as far as we know, the present study was planned to determine the antimicrobial susceptibility profile of gut commensal E. coli isolates from healthy adult individuals. In addition, the study aimed to investigate the harboring of plasmid-carried AMR genes encoding for resistance to diverse classes of antimicrobials by these commensal gut E. coli isolates.
Materials and Methods
Study Population and Fecal Samples
The present study included 72 non-duplicate fecal samples collected from healthy Egyptian adults. These healthy individuals were essentially selected as a must who are in good health and do not suffer from any gastric disorders or other diseases and did not take any antibiotics at least three months before collection of fecal samples. The fecal samples were collected in sterile dedicated containers sealed in stretch film, and then transported to the microbiology laboratory within one hour and kept at 4°C until processing. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Heliopolis University (Ethics Approval Code: HU.REC.H.5-2021).
Fecal Culture and Identification of Gut Commensal E. coli
The fecal samples were processed and cultured on the same day of collection following the procedures of Stanley et al.24 The feces was mixed with sterile normal saline and the fecal suspension was cultured on MacConkey agar medium, then the agar plates were incubated overnight at 37°C. The lactose fermenting colonies with a typical appearance suggestive of E. coli bacteria were picked and subjected to further identification. E. coli isolates were identified using standard microbiological laboratory methods including cultural characteristics on the selective medium; eosin methylene blue (EMB) (Oxoid® Limited, Basingstoke, UK), Gram-staining and biochemical tests according to the previously mentioned identification scheme.25 Identification was confirmed by Vitek 2 automated system using VITEK® 2 GN panel according to the manufacturer’s guidelines (bioMe´rieux, Marcy l’E´toile, France). One E. coli isolate was recovered from each one of the non-repetitive 72 fecal samples that were obtained from each one of the included 72 healthy individuals, who did not take antibiotics during the last three months before collection of the fecal sample. The commensal E. coli was identified by the absence of E. coli pathotypes genes (mentioned in Table 1) that were examined by PCR. The isolates were preserved at − 20°C in glycerol stock media until examined for their antimicrobial susceptibility patterns and PCR studies.
Table 1.
Target Genes, Sequences of PCR Oligonucleotide Primers and Expected PCR Product Size
| Target Gene | Sequence (5’ – 3’) | Amplicon Size (bp) | Ta/Extension Time | Source |
|---|---|---|---|---|
| Resistance to beta-lactam antibiotics (β-lactamases encoding genes) | ||||
| blaTEM | F: ATAAAATTCTTGAAGAC R: TTACCAATGCTTAATCA |
1075 | 42°C/1 min | [66] |
| blaCTX-M1-like genes | F: TTAATTCGTCTCTTCCAGA R: CAGCGCTTTTGCCGTCTAAG |
1042 | 45°C/1 min | |
| blaCMY-like genes | F: ATGATGAAAAAATCGATATG R: TTATTGCAGTTTTTCAAGAATG |
1146 | 45°C/1 min | |
| Resistance to aminoglycoside antibiotics | ||||
| aac(3)-II | F: TGAAACGCTGACGGAGCCTC R: GTCGAACAGGTAGCACTGAG |
369 | 55°C/30 s | [66] |
| aac(6′)-Ib-cr | F: TTGCGATGCTCTATGAGTGGCTA R: CTCGAATGCCTGGCGTGTTT |
482 | 55°C/30 s | [67] |
| Resistance to quinolones | ||||
| qnrA | F: ATTTCTCACGCCAGGATTTG R: GATCGGCAAAGGTTAGGTCA |
516 | 52°C/40 s | [67] |
| qnrB | F: GATCGTGAAAGCCAGAAAGG R: ATGAGCAACGATGCCTGGTA |
476 | ||
| qnrS | F: GCAAGTTCATTGAACAGGGT R: TCTAAACCGTCGAGTTCGGCG |
428 | ||
| Resistance to tetracyclines | ||||
| tetA | F: GCTACATCCTGCTTGCCT R: CATAGATCGCCGTGAAGA |
210 | 52°C/30 s | [68] |
| tetB | F: TTGGTTAGGGGCAAGTTTTG R: GTAATGGGCCAATAACACCG |
600 | 52°C/40 s | |
| Resistance to sulfonamides | ||||
| sulI | F: TGGTGACGGTGTTCGGCATTC R: GCGAAGGTTTCCGAGAAGGTG |
790 | 56°C/50 s | [66] |
| sulII | F: CGGCATCGTCAACATAACCT R: TGTGCGGATGAAGTCAGCTC |
721 | 56°C/50 s | |
| Type of E. coli pathotype (virulence-associated genes in E. coli) | ||||
| EPEC: eaeA | F: AAACAGGTGAAACTGTTGCC R: CTCTGCAGATTAACCCTCTGC |
454 | 52°C/30 s | [7] |
| EAEC: aggR | F: CTAATTGTACAATCGATGTA R: ATGAAGTAATTCTTGAAT |
308 | 42°C/30 s | |
| EAEC: pCVD432 | F: CTGGCGAAAGACTGTATCAT R: CAATGTATAGAAATCCGCTGTT |
630 | 55°C/40 s | |
| STEC/EHEC: stx | F: GAGCGAAATAATTTATATGTG R: TGATGATGGCAATTCAGTAT |
518 | 42°C/30 s | [69] |
| ETEC: est | F: TTAATAGCACCCGGTACAAGCAGG R: CCTGACTCTTCAAAAGAGAAAATTAC |
147 | 52°C/30 s | |
| ETEC: elt | F: TCTCTATGTGCATACGGAGC R: CCATACTGATTGCCGCAAT |
322 | 52°C/30 s | |
| EIEC: ipaH | F: GTTCCTTGACCGCCTTTCCGATACCGTC R: GCCGGTCAGCCACCCTCTGAGAGTAC |
619 | 58°C/40 s | |
Abbreviations: PCR, polymerase chain reaction; E. coli; Escherichia coli; EPEC, enteropathogenic E. coli; EAEC, enteroaggregative E. coli; STEC, Shiga toxin-producing E. coli; ETEC, enterotoxigenic E. coli; EIEC, enteroinvasive E. coli; EHEC, enterohemorrhagic E. coli; Ta, annealing temperature; bp, base pair.
Antimicrobial Susceptibility Testing
The antimicrobial susceptibility phenotypes of E. coli isolates were determined using the Kirby-Bauer disc diffusion method26 on Mueller-Hinton agar (MHA) (Oxoid® Limited, Basingstoke, UK) following the Clinical and Laboratory Standards Institute (CLSI, 2018) guidelines.27 The following 14 antimicrobials, representing different classes of antimicrobial agents, were tested: ampicillin (10 µg), amoxicillin-clavulanate (20/10 µg), ceftriaxone (30 µg), cefoxitin (30 µg), cefuroxime (30 µg), ceftazidime (30 µg), cefepime (30 µg), aztreonam (30 µg), amikacin (30 µg), gentamicin (10 µg), tetracycline (30 µg), ciprofloxacin (5 µg), levofloxacin (5 µg) and trimethoprim/sulfamethoxazole (1.25/23.75 µg). The antimicrobial discs were the product of Oxoid® Limited, Basingstoke, UK. These antimicrobials were selected considering the target genes to be investigated and that are of clinical importance in treating bacterial infections in humans in Egypt. The inhibition zones formed around the discs, measured in mm, were interpreted as susceptible (S) or resistant (R) to a particular antimicrobial agent according to CLSI breakpoints. According to Magiorakos et al, E. coli isolate was considered as MDR if it is non-susceptible to three or more different antimicrobial classes.28 The standard strain E. coli ATCC 25922 was used as the quality control in the antimicrobial susceptibility testing. The correlation between AMR phenotypic and genotypic patterns of E. coli isolates was assessed.
Identification of ESBLs E. coli Phenotypes
E. coli isolates were screened for potential Extended-Spectrum β-Lactamases (ESBLs) production. E. coli isolates were considered as ESBLs-producer according to CLSI guidelines of being resistant to penicillins, third- and fourth-generation cephalosporins and aztreonam. These isolates were selected for confirmation of ESBLs production according to the CLSI combination disc confirmatory test, or the disc diffusion clavulanate inhibition test, using both cefotaxime and ceftazidime alone and in combination with clavulanate.27 The antimicrobial discs used for this test were ceftazidime (30 μg) and ceftazidime-clavulanate (30 μg/10 μg), cefotaxime (30 μg) and cefotaxime-clavulanate (30 μg/10 μg). The test was carried out using the standard disc diffusion procedure on MHA. The isolate was considered ESBLs-producer if the inhibition zone diameter of both antimicrobial agents’ combination discs was ≥ 5 mm than that of the ceftazidime or cefotaxime disc alone. E. coli ATCC 25922 was used as a negative control for the evaluation of ESBLs production. The accepted quality control limit is ≤ 2-mm increase in zone diameter for antimicrobial agent tested in combination with clavulanate vs the zone diameter when tested alone.
PCR-Based Molecular Experiments
DNA Extraction and PCR Oligonucleotide Primers
Total crude DNA was extracted from all tested isolates using the boiling method. Bacterial cell suspensions in 50 μL of molecular biology-grade water were subjected to boiling at 100°C for 10 min, followed by removal of cellular debris by centrifugation at 15,000 ×g for 30s. The supernatant was collected and stored at −20°C for use as template DNA for PCR. Aliquots of 2 μL of template DNA were used for PCR assays to identify E. coli pathotypes. For detection of plasmid-carried AMR genes, plasmid DNA was extracted from E. coli isolates using Gene JET™ Plasmid Miniprep Kit (Thermo Scientific, Waltham, USA) according to the manufacturers’ instructions.29 The extraction of plasmid DNA from all isolates was analyzed as the extracted DNA solution was subjected to electrophoresis on a 0.7% agarose gel (Bioline, UK) against 1 kb DNA ladder marker (TianGen, China). Extracted DNA was collected and stored in small aliquots at – 20°C and used as a template in PCR experiments. The sequences of the previously published PCR oligonucleotide primers used in this study, synthesized by Invitrogen (UK), are listed in Table 1. The lyophilized powder of each primer was reconstituted using nuclease-free water to achieve a concentration of 100 pmol/µL and then was adjusted to the working concentration of 10 pmol/µL.
PCR Assays for Detection of Antimicrobial Resistance Genes and Identification of E. coli Pathotypes
E. coli isolates were examined by PCR for selected plasmid-carried AMR genes (available at GenBank database) encoding for resistance to diverse classes of antimicrobial drugs and different E. coli pathotypes genes (Table 1). PCR reaction mixtures were prepared in total volumes of 20 μL. Each reaction contained 2 μL of template DNA, 1 μL (equivalent to 10 pmol concentration) of each primer and 10 μL of GoTaq® Green Master 2× Ready Mix (Promega, Madison, USA), then the volume was completed to 20 μL by adding 6 μL of nuclease-free water. The PCR amplification programs included initial denaturation for 5 min at 95°C, then 35 cycles of denaturing at 95°C for 30 seconds, annealing for 30 seconds and extension at 72°C, followed by a final extension at 72°C for 7 min. The appropriate annealing temperature for each pair of primers and the time for the extension step for each PCR amplicon are mentioned in Table 1.
DNA fragments of PCR products were detected using TAE agarose gel (0.8%) (Bioline, London, UK) electrophoresis in 1 × TAE buffer containing ethidium bromide for DNA visualization on a UV light source. A suitable GeneRuler DNA molecular weight marker (Thermo Scientific, USA) was used for sizing the PCR products.
Data Analysis
Data were analyzed using Eviews 8 software. Data were presented in tables as relative percentages and frequencies outputs for resistance patterns to different antimicrobial agents and gene variables.
Results
Identification of Gut Commensal E. coli Isolates
In this study, 72 non-repetitive E. coli isolates were recovered from the 72 fecal samples of healthy individuals (one E. coli isolate from each fecal sample that was taken from each individual). Out of these 72 E. coli isolates, 56 isolates (56/72, 77.8%) were identified as gut commensals. These 56 E. coli isolates showed the typical phenotypic characteristics of E. coli including the cultural characteristics, on McConkey and EMB media and specific biochemical reactions, as well as VITEK® 2 automated system. In addition, PCR results revealed the absence of E. coli pathotypes virulence genes (eaeA, stx, ipaH, est, elt, aggR and pCVD432) in these 56 isolates. Twelve E. coli isolates other than these 56 isolates were excluded due to the presence of any of these pathotypes virulence genes.30–32
Antimicrobial Susceptibility Patterns and Frequency of MDR Isolates
The antimicrobial susceptibility pattern of the 56 E. coli gut commensal isolates against 14 antimicrobial agents is presented in Table 2. Among these isolates, 78.6% (44/56) of isolates showed resistance to all of the tested antimicrobial agents, while 21.4% (12/56) showed no resistance to any one of them (ie, sensitive to all tested antimicrobials). Generally, E. coli isolates showed high resistance rates against tested antimicrobial agents. According to the antimicrobial classes, the highest resistance frequency was against tested antimicrobial agents belonging to both β-lactams and sulfonamides (42/56, 75%) followed by quinolones (35/56, 62.5%), tetracyclines (31/56, 55.4%), while the lowest resistance rate was to aminoglycosides (24/56, 42.9%).
Table 2.
Distribution of Antimicrobial Resistance Among Gut Commensal E. coli Isolates
| Antimicrobial Agent | Sensitive No. (%)a | Resistant No. (%)a |
|---|---|---|
| Ampicillin | 14 (25) | 42 (75) |
| Amoxicillin-clavulanate | 19 (33.9) | 37 (66.1) |
| Cefoxitin | 26 (46.4) | 30 (53.6) |
| Ceftriaxone | 15 (26.8) | 41 (73.2) |
| Cefuroxime | 16 (28.6) | 40 (71.4) |
| Ceftazidime | 16 (28.6) | 40 (71.4) |
| Cefepime | 17 (30.4) | 39 (69.6) |
| Aztreonam | 16 (28.6) | 40 (71.4) |
| Ciprofloxacin | 21 (37.5) | 35 (62.5) |
| Levofloxacin | 21 (37.5) | 35 (62.5) |
| Gentamicin | 32 (57.1) | 24 (42.9) |
| Amikacin | 38 (67.9) | 18 (32.1) |
| Tetracycline | 25 (44.6) | 31 (55.4) |
| Trimethoprim/sulfamethoxazole | 14 (25) | 42 (75) |
Note: aPercentage correlated to the total number of commensal isolates (n = 56).
Abbreviation: E. coli, Escherichia coli.
In detail, the highest resistance rate of 75% was recorded against each ampicillin (indicator for amoxicillin resistance as well) and trimethoprim/sulfamethoxazole, followed by the resistance to ceftriaxone (73.2%), then each cefuroxime, ceftazidime and aztreonam (71.4%) and cefepime (69.6%). There was also a significant frequency of resistance to amoxicillin-clavulanate (66.1%), tested fluoroquinolones (62.5%), tetracycline (55.4%) and cefoxitin (64%). Lower resistance rates were determined to amikacin (32.1%) and gentamicin (42.9%). Of the 44 isolates that showed resistance to antimicrobial agents, 81.8% (36/44) of these isolates or 64.3% (36/56) of total isolates included in this study were MDR which were resistant to at least three antimicrobials belonging to three different antimicrobial classes.
Distribution of ESBLs-Producing E. coli Phenotypes
A total of 71.4% (40/56) of E. coli isolates were considered as potential ESBLs producers according to CLSI guidelines of being resistant to ceftriaxone, ceftazidime, cefepime or aztreonam. However, 67.9% (38/56) isolates showed positive results of the confirmatory combination disc method for detection of ESBL production based on cefotaxime and ceftazidime susceptibility alone or in combination with clavulanate.
Plasmid Separation and Frequencies of Target Plasmid-Carried Antimicrobial Resistance Genes by PCR Assays
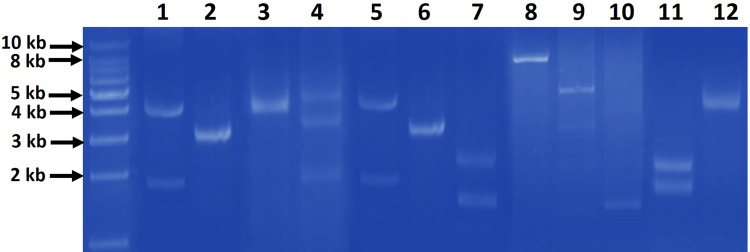
Agarose gel electrophoresis of the plasmid DNA extracts revealed that there were detectable plasmids in 44 (78.6%) isolates while 12 isolates (21.4%) had no plasmids. Isolated intact plasmids showed different patterns among isolates which comprised from one to three bands of different sizes (Figure 1).
Figure 1.
Representative agarose gel (0.7%) electrophoresis of the different patterns of extracted plasmids from gut commensal E. coli isolates. The patterns comprised of one band (lanes 2, 3, 6, 8, 10, 12), two bands (lanes 1, 5, 7, 9, 11) and three bands (lane 4) of different sizes. First most left lane, 1 kb DNA molecular weight marker.
Overall, based on PCR assays targeting genes encoding resistance to different antimicrobial agents/classes, the most widespread genes among isolates were blaTEM and sulI with frequencies of 66.1% and 60.7%, respectively, followed by qnrA with a frequency of 51.8% (Table 3). In addition, PCR results revealed the absence of E. coli pathotypes virulence genes (eaeA, stx, ipaH, est, elt, aggR and pCVD432) in these 56 isolates.
Table 3.
Distribution of AMR Genes with Corresponding AMR Phenotypes Detected in E. coli Isolates
| Target Genes Encoding Diverse Antimicrobial Classes | Prevalence of Target Genes Among E. coli Isolates (n = 56) No. (%)a | AMR Testing of E. coli Isolates with Corresponding Resistance Genes No. (%)a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β-Lactams Resistance Genes | Ampicillin | Amoxicillin-Clavulanate | Aztreonam | Cefoxitin | Ceftriaxone | Cefuroxime | Ceftazidime | Cefepime | |
| blaTEM | 37 (66.1) | 37 (100) | 33 (89.1) | 35 (94.5) | 26 (70.2) | 36 (97.3) | 35 (94.5) | 35 (94.5) | 34 (91.9) |
| blaCTX-M1-like genes | 24 (42.8) | 24 (100) | 19 (79.1) | 22 (91.6) | 17 (70.8) | 24 (100) | 24 (100) | 24 (100) | 24 (100) |
| blaCMY-like genes | 6 (10.7) | 6 (100) | 6 (100) | 6 (100) | 4 (66.6) | 6 (100) | 6 (100) | 6 (100) | 5 (83.3) |
| Aminoglycoside Resistance Genes | Gentamicin | Amikacin | |||||||
| aac(3)-II | 24 (42.9) | 24 (100) | 18 (75) | ||||||
| aac(6′)-Ib-cr | 14 (25) | 14 (100) | 12(85.7) | ||||||
| Quinolones Resistance Genes | Ciprofloxacin | Levofloxacin | |||||||
| qnrA | 31 (55.4) | 31 (100) | 31 (100) | ||||||
| qnrB | 17 (30.4) | 16 (94.1) | 16 (94.1) | ||||||
| qnrS | 0 (0) | 0 (0) | 0 (0) | ||||||
| aac(6)-Ib-cr | 14 (25) | 13 (92.8) | 13 (92.8) | ||||||
| Tetracyclines Resistance Genes | Tetracycline | ||||||||
| tetA | 27 (48.2) | 27 (100) | |||||||
| tetB | 14 (25) | 14 (100) | |||||||
| Sulfonamides Resistance Genes | Trimethoprim/Sulfamethoxazole | ||||||||
| sulI | 34 (60.7) | 34 (100) | |||||||
| sulII | 18 (32.1) | 18 (100) | |||||||
Note: aPercentage correlated to the total number of commensal isolates (n = 56).
Abbreviations: E. coli, Escherichia coli; AMR, antimicrobial resistance.
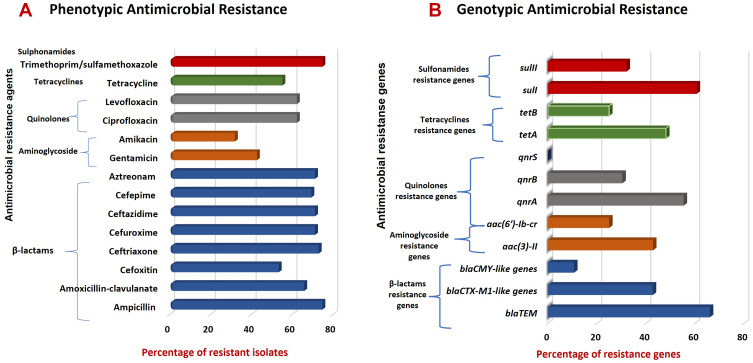
The distribution of AMR genes with corresponding AMR phenotypes among E. coli isolates is also demonstrated in Table 3. This matching revealed similar phenotypic susceptibility patterns of the isolates versus coexisting quinolones resistance genes, tetracyclines resistance genes and sulfonamides resistance genes. While there was a different distribution pattern for β-lactams resistance genes and resistance patterns to amoxicillin-clavulanate, aztreonam, cefoxitin, ceftriaxone, cefuroxime, ceftazidime and cefepime and between aminoglycoside resistance genes and amikacin (Table 3 and Figure 2).
Figure 2.
Prevalence of AMR-phenotypes and associated genes in commensal E. coli isolates. (A) AMR phenotypes among E. coli isolates; (B) AMR-associated genes harbored by E. coli resistant phenotypes.
The 44 isolates that showed resistance to any of the tested antimicrobials (44/56, 78.6%) harbored more than one AMR encoding gene while 12 isolates showed no carrying of any resistance genes as they showed no harboring of plasmids and exhibited full phenotypic sensitivity pattern to all tested antimicrobial agents. The most frequent genetic profile among isolates was blaTEM-blaCTX-M1-like-qnrA-qnrB-tetA-sulI in 21.4% of isolates, followed by blaTEM-aac(3)-II-aac(6′)-Ib-cr-qnrA-tetA-tetB-sulI that was found in 14.3% of isolates (Table 4).
Table 4.
Genotypic Profiles of AMR Genes Among E. coli Isolates
| Genotypic Profile | Frequencya |
|---|---|
| blaTEM-blaCTX-M1-like-qnrA-qnrB-tetA-sulI | 12/56, 21.4% |
| blaTEM-aac(3)-II- aac(6′)-Ib-cr-qnrA-tetA-tetB-sulI | 8/56, 14.3% |
| blaCTX-M1-like-aac(3)-II-qnrA-tetB-sulI-sulII | 4/56, 7.1% |
| blaTEM-blaCTX-M1-like-aac(3)-II-aac(6′)-Ib-cr-qnrA-sulI | 4/56, 7.1% |
| blaTEM-qnrA-aac(3)-II-tetA-sulII | 3/56, 5.4% |
| blaTEM-blaCMY-like-qnrB-aac(3)-II-sulI-sulII | 3/56, 5.4% |
| blaTEM-blaCMY-like-sulI-sulII | 3/56, 5.4% |
| blaTEM-blaCTX-M1-like-sulI | 2/56, 3.6% |
| tetA-tetB-sulII | 2/56, 3.6% |
| blaTEM-blaCTX-M1-like-tetA | 1/56, 1.8% |
| blaTEM-aac(3)-II-aac(6′)-Ib-cr-qnrB | 1/56, 1.8% |
| blaCTX-M1-like-aac(3)-II-aac(6′)-Ib-cr-qnrB-tetA-sulII | 1/56, 1.8% |
| No resistance genes | 12/56, 21.4% |
Note: aPercentage correlated to the total number of commensal isolates (n = 56).
Abbreviations: E. coli, Escherichia coli; AMR, antimicrobial resistance.
Discussion
Resistance to antimicrobial drugs is largely believed to be a consequence of human activities, for instance, the extensive and/or misuse of antibiotics. While many studies revealed the presence of significant numbers of AMR encoding genes within the genomes of human bacterial flora as well as environmental bacteria.1,10,15,33,34 In particular, the resistance of E. coli to diverse antimicrobial classes owing to either acquired or extrinsic mechanisms is already a major public health problem.1 Gut commensal E. coli is one of the most important reservoirs of AMR genes leading to treatment failure of infections in both human and veterinary medicine.35 Presently, it is important to determine the bacterial AMR patterns and virulence to reduce the risk of complications and/or avoid therapy failure of infections especially those caused by MDR strains.36 Hence, we aimed in the current study to investigate gut commensal E. coli isolates from healthy individuals for antimicrobial susceptibility and plasmid-mediated AMR genes.
Here, we report a high rate (64.3%) of MDR E. coli bacteria isolated from the feces of clinically healthy individuals. These E. coli bacteria carry various AMR genes without the existence of pathotype virulence-associated genes including EPEC: eaeA, EAEC: aggR, EAEC: pCVD432, STEC/EHEC: stx, ETEC: est, ETEC: elt and EIEC: ipaH. In most cases, a high rate of AMR is associated, either directly or indirectly, with decreased virulence and fitness.37 However, in other studies, highly virulent microorganisms showed high resistance profiles as well.12,38 Although, it is increasingly evident that the presence of both virulence and AMR traits is likely of greater benefit to the microorganism.37 Virulence factors are necessary to overcome host defense mechanisms, and the development of AMR is essential to enable pathogenic bacteria to overcome antimicrobial drugs and to adapt to and survive in competitive and demanding environments.37 Notably, although AMR is not itself a virulence factor, it is a key factor in developing infections in certain situations. Thus, it may be considered a virulence-like factor in specific ecological niches where the antimicrobial drug-resistant bacteria are particularly able to colonize.39 Unfortunately, the extensive use of antibiotics has changed the natural evolution of bacteria by reducing the susceptible bacterial populations and increasing resistant ones.40 Moreover, the genetic background of resistant bacteria allows them to persist in the presence of minimal concentrations of antibiotics.41
In this study, out of 72 E. coli isolates recovered from fecal samples of healthy adult individuals, 56 isolates (77.8%) were identified as gut commensals. These isolates were further analyzed in the current study for antimicrobial susceptibility profiles and mechanisms. Thirty-six (64.3%) of these 56 isolates were MDR that were resistant to at least one antimicrobial agent from three different antimicrobial classes. This finding is concordant with the findings of similar studies, carried out in different countries, that investigated antimicrobial susceptibility of gut E. coli in humans, waterfowls and broiler chickens.2,4,8,12,42 Many factors contribute to AMR with a complicated inter-relationship that spans across different sectors other than healthcare, such as agriculture and industry. In developing countries including Egypt, the main identified reasons that are leading to AMR in commensal E. coli include poverty, overcrowding, socioecological behaviors, highly contaminated waste effluents, food and supply chain safety issues and inadequate surveillance systems.43,44 Nevertheless, the principal driver of MDR in these countries is the misuse and over-prescription of antibiotics.45 Consequently, commensal E. coli typically present in the guts of humans, animals, birds as well as the environmental E. coli strains are likely to develop resistance to multiple antimicrobial agents through natural selection when antimicrobial drugs are ingested for the treatment of bacterial infectious diseases.44
The present study exhibits a high prevalence of AMR among gut commensal E. coli isolates to the most clinically prescribed antimicrobial drugs that are acting with different mechanisms of action. The highest resistance rate of 75% was recorded against each ampicillin and trimethoprim/sulfamethoxazole, followed by ceftriaxone (73.2%), then each cefuroxime, ceftazidime and aztreonam of 71.4% and cefepime of 69.6%. In addition, there was also a high frequency of resistance to amoxicillin-clavulanate (66.1%), fluoroquinolones (62.5%), tetracycline (55.4%) and cefoxitin (64%). However, lower resistance rates to amikacin among isolates were determined (32.1%) and gentamicin (42.9%). These findings were in accordance with the results of many previous studies investigating the AMR profiles of gut commensal E. coli but with variable rates from that recorded in the current study. A similar study from Vietnam where gut E. coli bacteria, isolated from healthy adults, showed resistance to streptomycin (80.6%), tetracycline (67.0%), ampicillin (65.0%), and trimethoprim/sulfamethoxazole (48.5%).2 Moreover, a study that included many countries in South Asia and sub-Saharan Africa regions revealed that commensal E. coli isolates were resistant to ampicillin, trimethoprim/sulfamethoxazole, streptomycin, tetracycline and norfloxacin with frequencies 65%, 66%, 43%, 56%, 17%, respectively.4 Another study from the same geographical area of this study, Egypt, reported high AMR rates among gut E. coli isolates from healthy broilers to different antimicrobials including penicillin, erythromycin, trimethoprim/sulfamethoxazole, tetracycline, ceftazidime and amoxicillin/clavulanic acid with frequencies 98.2%, 96.4%, 64.3%, 50%, 41.1%, 26.8%, respectively.46 Additionally, another study from Upper Egypt investigated virulence and AMR traits in E. coli isolates from broiler chickens showed a high rate of resistance to the majority of the examined antimicrobial agents including 100% resistance rate against each gentamicin, amoxicillin, and quinolones. These isolates showed also considerable resistance rates to oxytetracycline and streptomycin (88%), sulfamethoxazole and trimethoprim (84%) and cefotaxime (76%).46 These high levels of AMR in E. coli strains from the farm, including resistance to clinically valuable antimicrobials, suggest that E. coli might play a significant role as a reservoir for AMR genes and to be a key source for the transfer of AMR traits to other major human pathogens. Consequently, the need for more strict surveillance and improved farming practices (including the regulation of antibiotic usage) is required, which can reduce the carriage of antibiotic-resistant bacteria in foods and thereby minimize the likelihood of HGT of mobile antibiotic resistance genes to other bacteria in the human gut.46
The resistance to antimicrobials in bacteria can occur by several mechanisms. One of the main mechanisms is modification or degradation of the antimicrobial drug by the production of enzymes such as β-lactamases, especially ESBLs.47 The spread of ESBLs in Enterobacteriaceae has become an ever-increasing problem. In E. coli, as a member of Enterobacteriaceae harboring ESBLs genes, multidrug resistance due to ESBLs production is rapidly becoming a threat to humans.48 In developing countries, low levels of sanitation provide opportunities for the transfer of AMR genes in Enterobacteriaceae, including blaCTX-M, between humans, animals and the natural environment.49 The most striking finding in the current study is the widespread of ESBLs production among commensal E. coli isolates where a total of 71.4% of E. coli isolates was considered as potential ESBLs producers which considered a high percentage even though compared to pathogenic E. coli isolated from different clinical infections. A study from Egypt that performed molecular characterization of extended-spectrum β-lactamase-producing uropathogenic E. coli revealed that 59.7% of isolates was positive ESBLs producers.48 Further, a study reported that 50.5% of isolates were classified as ESBLs-producers from both clinical and environmental E. coli isolates.50 ESBLs can be broadly divided into three groups, TEM, SHV and CTX-M.51 ESBL CTX-M has emerged as the predominant type in both humans and animals, commensal, and pathogenic organisms, in addition to community and healthcare-associated infections. In the current work blaTEM gene was the most predominant β-lactamase gene (66.1%) followed by blaCTX-M1-like gene (42.8%) and blaCMY-like genes (10.7%). This result was consistent with the antimicrobial susceptibility results of amoxicillin but slightly differ for other β-lactam antibiotics as illustrated in Table 2. The phenotype-genotype variation may be due to targeting specific β-lactams resistance genes or other resistance mechanisms that were not included in this study.42,51 The other main bacterial resistance mechanisms to antimicrobials include prevention of the antimicrobial molecule from reaching toxic levels inside the cell by efflux pumps and modification of the antibiotic target site.52,53 These resistance mechanisms can arise through mutations in chromosomal genes or by the acquisition of AMR genes from other bacteria via HGT which is likely the main genetic mechanism of the dissemination of the AMR and virulence encoding genes.19 HGT may occur through DNA transformation, plasmid conjugation or transduction among bacteria in the high bacterial load environments such as soil as well as the gut in humans and animals.53–55
AMR genes can be carried on different mobile genetic elements, of all, plasmids play an important role in transferring the resistance genes among different bacterial species.56 In the present study, plasmid DNA extracts revealed that there were detectable plasmids in 78.6% of isolates and showed different patterns among isolates which comprised from one to three bands of different sizes. This result was comparable to other studies where 80.7% and 60% of E. coli and other bacterial species isolates harbored different plasmids carrying AMR genes.56,57 The demonstration of harboring plasmids with different sizes among isolates that were resistant to multiple antimicrobials indicates that plasmid-mediated MDR is significant among bacterial pathogens, particularly gut bacteria. Various patterns of AMR genes of other different antimicrobial classes were detected in the present study. The detection of aminoglycoside resistance genes aac(3)-II, (42.9%) and aac(6)-Ib-cr (25%) was the same pattern for phenotypic resistance to gentamicin but differed for amikacin. This result was different from a study in China where aac(3)-II and aac(6’)-Ib-cr in E. coli isolates from waterflows were 27.7% and 72.7%, respectively, also Zhao et al detected aac(6)-Ib-cr by 7.5% in E. coli isolates from rabbit farms, which was less frequent than our study.12,58 The cr variant of aac(6′)-Ib, ie, (aac(6)-Ib-cr), encodes an aminoglycoside acetyltransferase and also confers resistance to ciprofloxacin by N-acetylation of its piperazinyl amine.59 Our results revealed that 92.8% of all E. coli isolates showed phenotypic resistance to both ciprofloxacin and levofloxacin carriers aac(6)-Ib-cr gene. Here quinolones resistance genes qnrA and qnrB were detected in percentages of 55.4% and 30.4%, respectively, while qnrS gene was not found at all. This was less than detected by Zhang et al in China where qnrB and qnrS were detected in percentages 57.2% and 99.4%, respectively.12 This difference may be due to the less frequent use of fluoroquinolone in Egypt and/or variable consumption of antimicrobials across countries. In our study, the frequency of tetracyclines and sulfonamide resistance genes were tetA (48.2%) and tetB (25%), sulI (60.7%) and sulII (32.1%), respectively. These rates were in contrast to Zhang et al where tetA, tetB and sulII were detected in higher percentages of 94.2%, 44.4% and 91.6%, respectively. On the other hand, Zhao et al reported the presence of all sulfonamides resistance genes in the E. coli isolates without the coexistence of any tetracyclines resistance genes, which may be attributed to different antibiotics used across different countries and subsequent development of resistance.12,58 Additionally, a study from Egypt reported the presence of tetA (55%) and tetB (40%) in a similar range to our study.42 The observed variation could be attributed to the difference in species, or due to different patterns of antibiotic usage across different countries.
In the current work, the presence of multiple resistance genes were detected in 44 (78.6%) isolates, of these isolates, 21.4% and 14.3% carried resistance genes for most common antimicrobial classes; blaTEM-blaCTX-M1-like-qnrA-qnrB-tetA-sulI and blaTEM-aac(3)-II- aac(6′)-Ib-cr-qnrA-tetA-tetB-sulI, respectively. Similarly, Amer et al from Egypt reported multi-resistance genes in 85% of isolates, and 60% of isolates showed the carriage of 4 to 7 resistance genes.42 Additionally, a study from Saudi Arabia reported the presence of multi-resistance genes in 99% of E. coli isolated plasmids where 15.8% harbored plasmid resistance determinants blaCTX-M2, blaCTX-M9, blaCTX-M8/25, rmtB, qnrA, qnrB and qnrS.57 E. coli had been reported as a contributor to the dissemination of antibiotic resistance genes in the natural environment.60 A key contributor to the dissemination of these clones is the frequent coexistence of blaCTX-M with genes conferring resistance to other antimicrobial classes like fluoroquinolones and aminoglycosides, a situation that might lead to high rates of co-selection.61 The high prevalence of resistance genes has been observed in various reports of animal and human studies.2,8,18,62 The presence of numerous antibiotic resistance characteristics on the plasmid is associated with an increase in the presence of pathogenicity features.63 Unfortunately, antibiotic exposure by commensal bacteria certainly increases the risk of the formation of resistant strains that can pass the resistance genes to virulent strains.57
Conclusion
The development of MDR bacteria is an emerging problem worldwide, especially in developing countries. The results of the current study revealed a high risk of fecal carriage of antimicrobial-resistant E. coli bacteria from healthy adults. E. coli isolates showed high resistance rates against diverse antimicrobial agents. Antimicrobial susceptibility profiles revealed that 64.3% of isolates were MDR. In addition, E. coli isolates in this study showed high rates of harboring plasmid-carried AMR genes encoding resistance to different antimicrobials. One reason could be due to the over usage or improper use of antimicrobial drugs in Egypt where antimicrobials can be purchased over the counter without a prescription and there is non-compliance with drug law regulations.64 Alternatively, it could be due to improper use of antimicrobials in veterinary medicine and for food animals which can be considered a potential reservoir of antimicrobial-resistant bacteria that is transmitted to humans.42,46,65 Therefore, it is important to regularly determine the antimicrobial resistance patterns and virulence of microbes to reduce the risk of complications and/or avoid treatment failure in infections caused by MDR E. coli strains and the necessity of strict regulation of antibiotics use in Egypt. In addition, based on the results of this study, further investigation of antimicrobial profiles of other gut commensal bacteria is warranted.
Funding Statement
This research is self-funded and received no external funding or any specific grant from funding agencies.
Data Sharing Statement
Data and material are available from the corresponding author upon request.
Ethics Approval and Informed Consent
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Heliopolis University (Ethics Approval Code: HU.REC.H.5-2021). Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Disclosure
The authors declare no conflicts of interest for this work.
References
- 1.Stephens C, Arismendi T, Wright M, et al. F plasmids are the major carriers of antibiotic resistance genes in human-associated commensal Escherichia coli. mSphere. 2020;5(4). doi: 10.1128/msphere.00709-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoang PH, Awasthi SP, Do Nguyen P, et al. Antimicrobial resistance profiles and molecular characterization of Escherichia coli strains isolated from healthy adults in Ho Chi Minh City, Vietnam. J Vet Med Sci. 2017;79(3):479–485. doi: 10.1292/jvms.16-0639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giacomini E, Perrone V, Alessandrini D, Paoli D, Nappi C, Esposti LD. Evidence of antibiotic resistance from population-based studies: a narrative review. Infect Drug Resist. 2021;14:849–858. doi: 10.2147/IDR.S289741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ingle DJ, Levine MM, Kotloff KL, Holt KE, Robins-Browne RM. Dynamics of antimicrobial resistance in intestinal Escherichia coli from children in community settings in South Asia and sub-Saharan Africa. Nat Microbiol. 2018;3(9):1063–1073. doi: 10.1038/s41564-018-0217-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Kholy A, Baseem H, Hall GS, Procop GW, Longworth DL, El Kholy A. Antimicrobial resistance in Cairo, Egypt 1999 – 2000: a survey of five hospitals. The Journal of Antimicrobial Chemotherapy. 2003;51:625–630. doi: 10.1093/jac/dkg101 [DOI] [PubMed] [Google Scholar]
- 6.Fahim NAE. Prevalence and antimicrobial susceptibility profile of multidrug-resistant bacteria among intensive care units patients at Ain Shams University Hospitals in Egypt-a retrospective study. J Egypt Public Health Assoc. 2021;96(1):7. doi: 10.1186/s42506-020-00065-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali MMM, Ahmed SF, Klena JD, Mohamed ZK, Moussa TAA, Ghenghesh KS. Enteroaggregative Escherichia coli in diarrheic children in Egypt: molecular characterization and antimicrobial susceptibility. J Infect Dev Ctries. 2014;8(5):589–596. doi: 10.3855/jidc.4077 [DOI] [PubMed] [Google Scholar]
- 8.Dyar OJ, Hoa NQ, Trung NV, et al. High prevalence of antibiotic resistance in commensal Escherichia coli among children in rural Vietnam. BMC Infect Dis. 2012;12. doi: 10.1186/1471-2334-12-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Von Baum H, Marre R. Antimicrobial resistance of Escherichia coli and therapeutic implications. Int J Med Microbiol. 2005;295(6–7):503–511. doi: 10.1016/j.ijmm.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 10.Blake DP, Hillman K, Fenlon DR, Low JC. Transfer of antibiotic resistance between commensal and pathogenic members of the Enterobacteriaceae under ileal conditions. J Appl Microbiol. 2003;95(3):428–436. doi: 10.1046/j.1365-2672.2003.01988.x [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Covington A, Pamer EG. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunol Rev. 2017;279(1):90–105. doi: 10.1111/imr.12563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Chen S, Rehman MU, et al. Distribution and association of antimicrobial resistance and virulence traits in Escherichia coli isolates from healthy waterfowls in Hainan, China. Ecotoxicol Environ Saf. 2021;220:112317. doi: 10.1016/j.ecoenv.2021.112317 [DOI] [PubMed] [Google Scholar]
- 13.Alekshun MN, Levy SB. Commensals upon us. Biochem Pharmacol. 2006;71(7):893–900. doi: 10.1016/j.bcp.2005.12.040 [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Wu XT, He Q, et al. Complete sequence of a tet(X4)-harboring IncX1 plasmid, pYY76-1-2, in Escherichia coli from a cow sample in China. Antimicrob Agents Chemother. 2019;63(12). doi: 10.1128/AAC.01528-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poirel L, Madec J-Y, Lupo A, et al. Antimicrobial resistance in Escherichia coli. In: Antimicrobial Resistance in Bacteria from Livestock and Companion Animals. ASM Press; 2018:289–316. doi: 10.1128/9781555819804.ch13 [DOI] [Google Scholar]
- 16.Kaper JB, Nataro JP, Mobley HLT. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2(2):123–140. doi: 10.1038/nrmicro818 [DOI] [PubMed] [Google Scholar]
- 17.Bien J, Sokolova O, Bozko P. Role of uropathogenic Escherichia coli virulence factors in development of urinary tract infection and kidney damage. Int J Nephrol. 2012;2012:1–15. doi: 10.1155/2012/681473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puvača N, de Frutos RL. Antimicrobial resistance in Escherichia coli strains isolated from humans and pet animals. Antibiotics. 2021;10(1):1–19. doi: 10.3390/antibiotics10010069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Von Wintersdorff CJH, Penders J, Van Niekerk JM, et al. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front Microbiol. 2016;7:173. doi: 10.3389/fmicb.2016.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye Q, Wu Q, Zhang S, et al. Characterization of extended-spectrum β-lactamase-producing Enterobacteriaceae from retail food in China. Front Microbiol. 2018;9:1709. doi: 10.3389/FMICB.2018.01709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Y, Yang X, Qin J, et al. Metagenome-wide analysis of antibiotic resistance genes in a large cohort of human gut microbiota. Nat Commun. 2013;4(1):1–7. doi: 10.1038/ncomms3151 [DOI] [PubMed] [Google Scholar]
- 22.Mohr KI. History of antibiotics research. Curr Top Microbiol Immunol. 2016;398:237–272. doi: 10.1007/82_2016_499 [DOI] [PubMed] [Google Scholar]
- 23.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269–284. doi: 10.1038/nrmicro3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanley IJ, Kajumbula H, Bazira J, Kansiime C, Rwego IB, Asiimwe BB. Multidrug resistance among Escherichia coli and Klebsiella pneumoniae carried in the gut of out-patients from pastoralist communities of Kasese district, Uganda. PLoS One. 2018;13(7):e0200093. doi: 10.1371/journal.pone.0200093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ewing W. Edwards and Ewing’s Identification of Enterobacteriaceae. Int J Syst. 1986;(Edition 4):536. doi: 10.1099/00207713-36-4-581 [DOI] [Google Scholar]
- 26.Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4_ts):493–496. doi: 10.1093/AJCP/45.4_TS.493 [DOI] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, M07Ed11. 11th ed. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2018:2018. [Google Scholar]
- 28.Magiorakos A-P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 29.Pronobis MI, Deuitch N, Peifer M. The miraprep: a protocol that uses a miniprep kit and provides maxiprep yields. PLoS One. 2016;11(8):e0160509. doi: 10.1371/journal.pone.0160509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waturangi DE, Hudiono F, Aliwarga E. Prevalence of pathogenic Escherichia coli from salad vegetable and fruits sold in Jakarta. BMC Res Notes. 2019;12(1):1–9. doi: 10.1186/s13104-019-4284-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahdavi Broujerdi S, Roayaei Ardakani M, Rezatofighi SE. Characterization of diarrheagenic Escherichia coli strains associated with diarrhea in children, Khouzestan, Iran. J Infect Dev Ctries. 2018;12(8):649–656. doi: 10.3855/jidc.9538 [DOI] [PubMed] [Google Scholar]
- 32.Ifeanyi CIC, Ikeneche NF, Bassey BE, Al-Gallas N, Ben Aissa R, Boudabous A. Diarrheagenic Escherichia coli pathotypes isolated from children with diarrhea in the Federal Capital Territory Abuja, Nigeria. J Infect Dev Ctries. 2015;9(2):165–174. doi: 10.3855/jidc.5528 [DOI] [PubMed] [Google Scholar]
- 33.Simjee S, McDermott P, Trott DJ, Chuanchuen R. Present and future surveillance of antimicrobial resistance in animals: principles and practices. Antimicrob Resist Bact Livest Companion Anim. 2018;595–618. doi: 10.1128/9781555819804.ch28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sommer MOA, Dantas G, Church GM. Functional characterization of the antibiotic resistance reservoir in the human microflora. Science. 2009;325(5944):1128–1131. doi: 10.1126/science.1176950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marshall BM, Levy SB. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev. 2011;24(4):718–733. doi: 10.1128/CMR.00002-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cabal A, Gómez-Barrero S, Porrero C, et al. Assessment of virulence factors characteristic of human Escherichia coli pathotypes and antimicrobial resistance in O157: H7 and non-O157: H7 isolates from livestock in Spain. Appl Environ Microbiol. 2013;79(13):4170–4172. doi: 10.1128/AEM.00537-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beceiro A, Tomás M, Bou G. Antimicrobial resistance and virulence: a successful or deleterious association in the bacterial world? Clin Microbiol Rev. 2013;26(2):185–230. doi: 10.1128/CMR.00059-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massella E, Giacometti F, Bonilauri P, et al. Antimicrobial resistance profile and expec virulence potential in commensal Escherichia coli of multiple sources. Antibiotics. 2021;10(4):1–28. doi: 10.3390/antibiotics10040351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lye DC, Earnest A, Ling ML, et al. The impact of multidrug resistance in healthcare-associated and nosocomial Gram-negative bacteraemia on mortality and length of stay: cohort study. Clin Microbiol Infect. 2012;18(5):502–508. doi: 10.1111/j.1469-0691.2011.03606.x [DOI] [PubMed] [Google Scholar]
- 40.Andersson DI, Levin BR. The biological cost of antibiotic resistance. Curr Opin Microbiol. 1999;2(5):489–493. doi: 10.1016/s1369-5274(99)00005-3 [DOI] [PubMed] [Google Scholar]
- 41.Andersson DI, Hughes D. Persistence of antibiotic resistance in bacterial populations. FEMS Microbiol Rev. 2011;35(5):901–911. doi: 10.1111/j.1574-6976.2011.00289.x [DOI] [PubMed] [Google Scholar]
- 42.Amer MM, Mekky HM, Amer AM, Fedawy HS. Antimicrobial resistance genes in pathogenic Escherichia coli isolated from diseased broiler chickens in Egypt and their relationship with the phenotypic resistance characteristics. Vet World. 2018;11(8):1082–1088. doi: 10.14202/vetworld.2018.1082-1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iskandar K, Molinier L, Hallit S, et al. Drivers of antibiotic resistance transmission in low-and middle-income countries from a “one health” perspective—a review. Antibiotics. 2020;9(7):1–23. doi: 10.3390/antibiotics9070372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nji E, Kazibwe J, Hambridge T, et al. High prevalence of antibiotic resistance in commensal Escherichia coli from healthy human sources in community settings. Sci Rep. 2021;11(1):1–11. doi: 10.1038/s41598-021-82693-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nadimpalli M, Delarocque-Astagneau E, Love DC, et al. Combating global antibiotic resistance: emerging one health concerns in lower- and middle-income countries. Clin Infect Dis. 2018;66(6):963–969. doi: 10.1093/cid/cix879 [DOI] [PubMed] [Google Scholar]
- 46.Mohamed MA, Shehata MA, Rafeek E. Virulence genes content and antimicrobial resistance in Escherichia coli from broiler chickens. Vet Med Int. 2014;2014:1–6. doi: 10.1155/2014/195189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karen B. Past and present perspectives on β-lactamases. Antimicrob Agents Chemother. 2021;62(10):e01076–18. doi: 10.1128/AAC.01076-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hassuna NA, Khairalla AS, Farahat EM, Hammad AM, Abdel-Fattah M. Molecular characterization of Extended-spectrum β lactamase- producing E. coli recovered from community-acquired urinary tract infections in Upper Egypt. Sci Rep. 2020;10(1):2772. doi: 10.1038/s41598-020-59772-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bevan ER, Jones AM, Hawkey PM. Global epidemiology of CTX-M β-lactamases: temporal and geographical shifts in genotype. J Antimicrob Chemother. 2017;72(8):2145–2155. doi: 10.1093/jac/dkx146 [DOI] [PubMed] [Google Scholar]
- 50.El-Shaer S, Abdel-Rhman SH, Barwa R, Hassan R. Genetic characterization of extended-spectrum β-lactamase- and carbapenemase-producing Escherichia coli isolated from Egyptian hospitals and environments. PLoS One. 2021;16:1–21. doi: 10.1371/journal.pone.0255219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pitout JDD, Laupland KB. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8(3):159–166. doi: 10.1016/S1473-3099(08)70041-0 [DOI] [PubMed] [Google Scholar]
- 52.Blair JMA, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJV. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13(1):42–51. doi: 10.1038/nrmicro3380 [DOI] [PubMed] [Google Scholar]
- 53.McInnes RS, McCallum GE, Lamberte LE, van Schaik W. Horizontal transfer of antibiotic resistance genes in the human gut microbiome. Curr Opin Microbiol. 2020;53:35–43. doi: 10.1016/j.mib.2020.02.002 [DOI] [PubMed] [Google Scholar]
- 54.Forsberg KJ, Reyes A, Wang B, Selleck EM, Sommer MOA, Dantas G. The shared antibiotic resistome of soil bacteria and human pathogens. Science (80-). 2012;337(6098):1107–1111. doi: 10.1126/science.1220761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.San Millan A. Evolution of plasmid-mediated antibiotic resistance in the clinical context. Trends Microbiol. 2018;26(12):978–985. doi: 10.1016/j.tim.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 56.Beige F, Baseri Salehi M, Bahador N, Mobasherzadeh S. Plasmid mediated antibiotic resistance in isolated bacteria from burned patients. Jundishapur J Microbiol. 2014;8(1):4–7. doi: 10.5812/jjm.13567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shabana II, Al-Enazi AT. Investigation of plasmid-mediated resistance in E. coli isolated from healthy and diarrheic sheep and goats. Saudi J Biol Sci. 2020;27(3):788–796. doi: 10.1016/j.sjbs.2020.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao X, Yang J, Ju Z, Chang W, Sun S. Molecular characterization of antimicrobial resistance in Escherichia coli from rabbit farms in Tai’an, China. Biomed Res Int. 2018;2018:1–7. doi: 10.1155/2018/8607647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park CH, Robicsek A, Jacoby GA, Sahm D, Hooper DC. Prevalence in the United States of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob Agents Chemother. 2006;50(11):3953–3955. doi: 10.1128/AAC.00915-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao J, Dang H. Coastal seawater bacteria harbor a large reservoir of plasmid-mediated quinolone resistance determinants in Jiaozhou Bay, China. Microb Ecol. 2012;64(1):187–199. doi: 10.1007/s00248-012-0008-z [DOI] [PubMed] [Google Scholar]
- 61.Bonnet R. Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother. 2004;48(1):1–14. doi: 10.1128/AAC.48.1.1-14.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang H, Rehman MU, Zhang S, et al. High prevalence of CTX-M belonging to ST410 and ST889 among ESBL producing E. coli isolates from waterfowl birds in China’s tropical island, Hainan. Acta Trop. 2019;194:30–35. doi: 10.1016/j.actatropica.2019.03.008 [DOI] [PubMed] [Google Scholar]
- 63.Pitout J. Extraintestinal pathogenic Escherichia coli: a combination of virulence with antibiotic resistance. Front Microbiol. 2012;3. doi: 10.3389/fmicb.2012.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kasim K, Hassan H. Self medication problem in Egypt: a review of current and future perspective. Int J Curr Res Rev. 2018. doi: 10.7324/ijcrr.2018.1048 [DOI] [Google Scholar]
- 65.Moawad AA, Hotzel H, Neubauer H, et al. Antimicrobial resistance in Enterobacteriaceae from healthy broilers in Egypt: emergence of colistin-resistant and extended-spectrum β-lactamase-producing Escherichia coli. Gut Pathog. 2018;10(1):1–12. doi: 10.1186/s13099-018-0266-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adamus-Białek W, Baraniak A, Wawszczak M, et al. The genetic background of antibiotic resistance among clinical uropathogenic Escherichia coli strains. Mol Biol Rep. 2018;45(5):1055–1065. doi: 10.1007/s11033-018-4254-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao C-H, Hsueh P-R, Jacoby GA, Hooper DC. Risk factors and clinical characteristics of patients with qnr-positive Klebsiella pneumoniae bacteraemia. J Antimicrob Chemother. 2013;68(12):2907–2914. doi: 10.1093/jac/dkt295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Del Castillo JRE. Tetracyclines. Antimicrob Ther Vet Med. 2013;257–268. doi: 10.1002/9781118675014.CH15 [DOI] [Google Scholar]
- 69.Toma C, Lu Y, Higa N, et al. Multiplex PCR assay for identification of human diarrheagenic Escherichia coli. J Clin Microbiol. 2003;41(6):2669–2671. doi: 10.1128/JCM.41.6.2669-2671.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]