Abstract
Long coronavirus disease 2019 (COVID-19) is characterized by persistent COVID-19 symptoms that last for at least 2 months. In the elderly population, apart from the typical symptoms (fatigue, cough, or dyspnea), unspecific symptoms coexist (functional deterioration, cognitive impairment, or delirium) that can mitigate the prevalence of this syndrome in this age group. Its main consequence is the functional decline, leading to sarcopenia, frailty, and disability, in addition to the nutritional and cognitive disorders. Thus, a multicomponent and individualized program (exercise, diet, cognitive stimulation) should be designed for older people with persistent COVID, where new technologies could be useful.
Keywords: Long COVID, Sarcopenia, Function, Frailty, Multicomponent exercise, POSITIVE
Key points
-
•
Long COVID is defined by the World Health Organization (WHO) as a condition that occurs in individuals with a history of probable or confirmed severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, usually 3 months from the onset of coronavirus disease 2019 (COVID-19) with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis.
-
•
The epidemiology and etiopathogenesis are not well known and the available data on long COVID in older people are scarce, which may be because of the different symptoms that this population presents, mainly weakness, confusion, and mood alterations.
-
•
Acute illness, immobilization, lack of physical exercise caused by lockdown and hospitalization, among other things, can result in sarcopenia, leading to worsening functional status in the elderly.
-
•
Early detection of worsening functional status through geriatric comprehensive assessment of old people after acute COVID-19 is essential to provide individualized treatment. Rehabilitation programs based on multicomponent physical activity (Vivifrail) associated with adequate nutrition programs are useful to prevent or improve post–COVID-19 sarcopenia in these patients.
-
•
The use of home remote monitoring system (maintaining and improving the intrinsic capacity involving primary care and caregivers [POSITIVE]) allows clinicians to monitor any worsening of the functional status as well as to prevent, through the prescription of individualized exercise, the development of sarcopenia as a fundamental mechanism in the development of the post–COVID-19 syndrome in the elderly.
Long COVID: is persistent coronavirus disease 2019 a real entity?
To date, more than 260 million cases of coronavirus disease 2019 (COVID-19) infection have been reported worldwide (World Health Organization [WHO] worldwide dashboard). The pandemic is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Persistent symptoms after viral infection is not a novel concept because there is evidence of similar effects seen in severe acute respiratory syndrome and Middle East respiratory syndrome.1 This fact, along with the nonspecificity of much of the symptoms observed in the long term in patients after the acute phase of COVID-19, has raised doubts about the true existence of this syndrome, a controversy that persists. However, there are several bodies of evidence suggesting that it is a true syndrome with relevant consequences for the patients and for health systems.
Although the symptoms of the acute phase of COVID-19 infection are estimated to last 2 weeks for the mildest cases and up to 12 weeks for the most severe cases, this is only indicative, because it is highly dependent on other factors such as age, previous functional status, symptoms, and concomitant diseases.2 The most frequent symptoms include fatigue, dyspnea, myalgia, weakness, headache, and cognitive blunting. In patients with pre-COVID comorbidity, a worsening of preexisting symptoms has been observed.3 Around 50% of patients with acute symptomatic COVID-19 infection progress to a phase of persistence for more than 4 weeks of some of the clinical manifestations, the so-called postacute sequelae of COVID-19 (PASC).4 WHO has developed a clinical case definition of the post–COVID-19 condition by Delphi methodology: a condition that occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19, with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis. Symptoms may be new onset following initial recovery from an acute COVID-19 episode or persist from the initial illness. Symptoms may also fluctuate or relapse over time.5 Among the hypotheses suggested as to the etiopathogenesis of long COVID is the possible persistence of the virus in the host, causing a latent infection, the inflammatory storm, or the existence of autoantibodies that would act against immunomodulatory proteins. However, the cause is still unknown.
Symptoms of long COVID include fatigue or muscle weakness, malaise, dyspnea, headache, and many other neurocognitive conditions described as cognitive impairment, inability to perform everyday physical tasks, and increased likelihood of developing stress, depression, irritability, insomnia, confusion, or frustration.6 However, other symptoms, such as cardiac, dermatologic, digestive, and ear, nose, and throat disorders, are possible, which makes it difficult to accurately assess the syndrome, its clinical manifestations, and pathogenic factors. Along with the definition that is discussed in this article, there are some others that establish different time framework or different sets of symptoms.
Its epidemiology remains incompletely understood. The numbers vary from one study to another, depending on methodology, whether outpatient or hospital-initiated surveys are used, structure of follow-up programs, comorbidities, length of hospital stay, and with or without intensive care unit stay, among many other factors of variability. Several North American and European studies report an incidence of long COVID of 30% to 90% at 6 months.7
There is increasing published evidence on persistent COVID-19 in the general population. It has been published that patients who have had severe COVID-19 and who have required hospital admission are more likely to have symptoms of persistent COVID-19, especially those who have required oxygen supplementation, treatment with enoxaparin, and with a greater number of symptoms in the acute phase, especially dyspnea, cough, and asthenia.8 Other studies have found that the risk of developing long COVID syndrome is higher in patients with more than 5 symptoms in the acute phase of the infection, and more frequent in women, in obese patients, and in patients with diabetes.9 , 10 In addition, a significant association between the presence of fatigue during the acute phase of infection and azithromycin treatment with long COVID has been published.8
Long COVID in older people: main characteristics
Older people meet several of these clinical characteristics, suggesting that they would have been well represented in the data reports currently available in the literature. However, the available data about long COVID concerning older patients are scarce and inconclusive. Although older people was the segment of the population with a most severe impact (not only death but also clinical severity), it is surprising to see that the reported case series about long COVID show that this condition is affecting mainly middle-aged women,11 raising the potential of underreporting, which seems to be the case according to the report by Groff and colleagues.12 In this systematic review, from 50 studies where age is reported, in only 3 of them is the median or mean age 70 to 74 years, with no studies with median/mean ages of 75 years and older. An alternative explanation could be that the clinical manifestations of the syndrome are different from those usually observed in the general population and not well recognized, or that some of the symptoms could be misinterpreted as general consequences of the acute disease or hospitalization in older people (functional deterioration, cognitive impairment/delirium), not directly linked to the infection by SARS-CoV-2.
Some investigators have published a prevalence of about 9% of patients interviewed in a cohort of 279 old patients, being significantly more frequent among patients with severe versus mild to moderate acute disease.13 Among the most frequent symptoms of long COVID syndrome in older patients are mood disorders, including depression (12.2%), fatigue (8.9%), and anxiety (7.5%), with other symptoms such as cough, dyspnea, myalgia, and loss of smell and taste being less frequent.13 In another study, after interviewing 165 participants with a mean age of 73 years, it was observed that the most frequent symptom was fatigue (53.1%), but breathlessness (51.5%), joint pain (22.2%), and cough (16.7%) were also highly prevalent.8 In addition, clinical data in patients with a mean age of 60 years show that more than 5% of body weight can be lost within 2 weeks of acute COVID-19 infection.14
Functional consequences of coronavirus disease 2019 in older people
Short-term and long-term effects on the musculoskeletal system have been described in older people. Although impairments in other organs and systems, such as the respiratory and cardiovascular systems, can help to explain some of the consequences of COVID-19 in the long term, it seems that the disorders on the musculoskeletal system account for the major part of the functional decline. These consequences come from 3 main areas: the effect of the virus and the disease itself, hospitalization, and confinement.
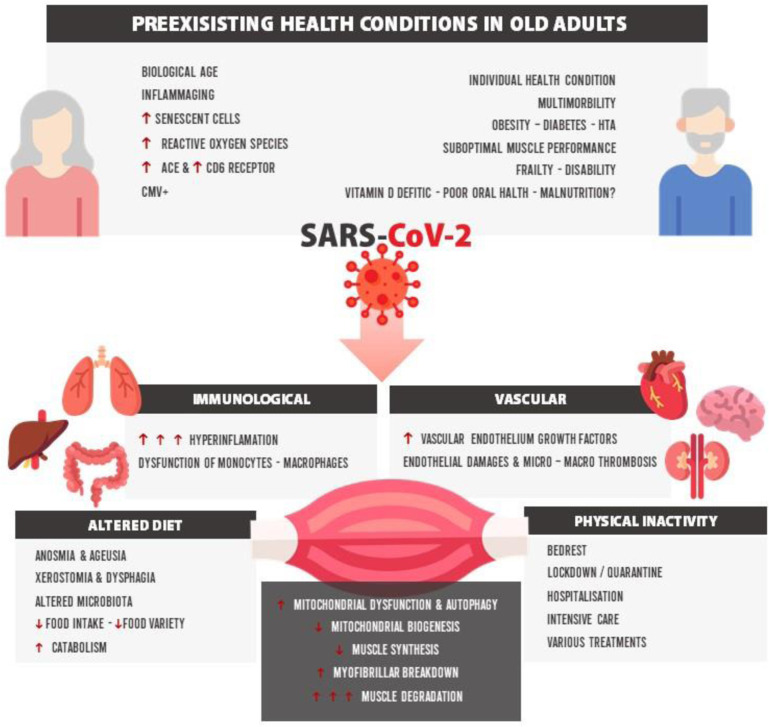
The risk of both sarcopenia and possible cachexia may be much higher in older patients with infection by SARS-CoV-215 , 16 and could play an important role of vulnerability to the post–COVID-19 functional and physical deterioration.17 Furthermore, it has been observed that the strength of biceps brachii and quadriceps femoris were lower in post–COVID-19 survivors (69% and 54% of the predicted normal value, respectively).18 The severity of post–COVID-19 sarcopenia could be explained by different factors such as preexisting health conditions in older adults (eg, biological age, inmunosenescence, and inflammaging), cardiovascular and immunologic status, altered diet (anosmia, ageusia, loss of appetite), physical inactivity, and gut microbiota (Fig. 1 ).15
Fig. 1.
Physiopathologic pathways of post–COVID-19 sarcopenia. ACE, angiotensin-converting-enzyme; CMV, cytomegalovirus; CD6, cluster of differentiation 6; HTA, Hypertension.
(Adapted from Piotrowicz, K., Gąsowski, J., Michel, JP. et al. Post-COVID-19 acute sarcopenia: physiopathology and management. Aging Clin Exp Res 33, 2887–2898 (2021). https://doi.org/10.1007/s40520-021-01942-8; under Creative Commons Attribution 4.0 International License.)
The biological basis of the effect of the infection on muscle mass and function derives from several factors linked to the direct damage produced by the virus in several tissues and systems but also to the effect of the response of the organisms, with a special role of the inflammatory response. Muscle hypoxia, the cytokine storm in the presence of a dysfunctional mitochondria, and electrolyte and metabolic disturbances, exacerbated in the presence of increased sedentarism, nutrient deficits, and low physical activity, prompt the development of an impairment in musculoskeletal performance.19, 20, 21
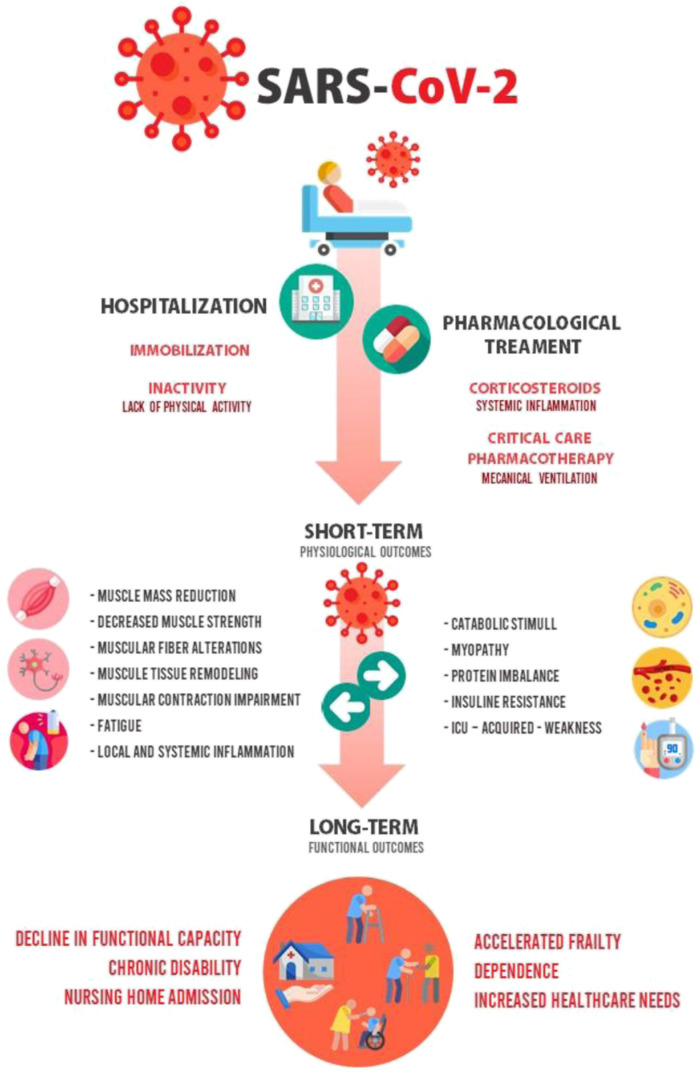
Hospitalization because of COVID-19 is the second factor to be considered, having effects not only caused by the hospitalization but also by some components of the pharmacologic treatment (mainly corticosteroids). Taken jointly, they are associated with the physiologic outcomes previously mentioned (a reduction in muscle mass and strength, alterations of muscular fiber with a subsequent remodeling of muscle tissue, fatigue, and local systemic inflammation), leading to functional outcomes (accelerated frailty, loss of functional status, chronic disability and dependence) that increase the risk of being discharged to a rehabilitation facility or nursing home, mainly in people more than 80 years old,22 increasing the health care needs (Fig. 2 ).23
Fig. 2.
Effects of pharmacologic treatment and in-hospital immobilization on muscular weakness in old people with COVID-19.
(Adapted from Sagarra-Romero L, Viñas-Barros A. COVID-19: Short and Long-Term Effects of Hospitalization on Muscular Weakness in the Elderly. Int J Environ Res Public Health. 2020;17(23):8715. Published 2020 Nov 24. https://doi.org/10.3390/ijerph17238715; Open Access under Creative Commons Attribution License.)
The way in which hospitalization because of acute COVID-19 infection contributes to this deleterious outcome of COVID-19 has been studied both in general hospitalization by any cause and in specific hospitalization by COVID-19. Results from the Gruppo Lavoro Italiano Sarcopenia-Trattamento e Nutrizione (GLISTEN) study, which could be extrapolated to admission for acute COVID-19 infection, show that there is a 38.4% excess risk of sarcopenia associated with hospital stays of 11 days or more,24 an usual length in these patients.
Moreover, a recent Norwegian article with a follow-up of 6 months after hospitalization because of COVID-19 has shown a loss of mobility in one-third of the participants, as well as a decreased performance of activities of daily living in 11% of the individuals in the study.25 In addition, there was the higher risk of persistent functional deterioration, mortality, and readmissions (up to 24-fold, 5-fold, and 4-fold higher, respectively) at 3 months associated with the presence of severe dependence at discharge (Barthel Index <40).26
However, this outcome is not restricted to hospitalization in acute care settings. In a recent published study of older people living in long-term care facilities, 29.4% of patients infected by COVID-19 had worsened their previous functional status, even with all physical environmental modifications done for the patients’ safety.27
The COVID-19 pandemic has led governments to implement unprecedented measures to try to control the spread of the virus. Quarantine, confinement, and social isolation have been widely used measures, especially in the elderly population, where COVID-19 mortality has been higher. Lockdown in older people with and without COVID-19, disregarding its cause, has been related to a large decrease of vigorous and moderate intensity physical activity,28, 29, 30, 31, 32, 33 especially in men,33 and walking time,29 , 30 , 33 which can accelerate the loss of muscle mass (sarcopenia), increased body fat, and worsening function. According to the WHO recommendations on physical activity, older adults should participate in 150 min/wk of moderate-intensity or 75 min/wk of vigorous-intensity activity.34 These thresholds not only have not been reached during the COVID-19 quarantine but also sedentary behavior has increased (up to 2 h/d of sitting time),30 , 33 , 35 , 36 taking into account that confinement and social isolation interrupted not only the spontaneous physical activity but also any supervised exercise program in these older people. As recently published, physical activity was reduced to one-third of baseline, and sedentary time increased from 5 to 8 h/d according to a survey of people older than 55 years during the first wave of the pandemic.37 Consequently, up to 10% of this population could have developed muscle function loss,38, 39, 40 especially those people who had recovered from SARS-CoV-2 infection,38 , 39 accelerating the risk of frailty and sarcopenia.41 , 42
In addition, physical inactivity in older people caused by COVID-19 lockdown has a negative impact on nutrition behavior, with an increased risk to 2- to 4-fold for developing nutritional deficits.43 Nutritional changes, especially in low-income populations, contribute to worsening sarcopenia and function. Data published in adult and old populations show a reduction in the consumption of vegetables, legumes, and fruits and an increase in the consumption of rice, meat, dairy products, and fast food during the first period of confinement.44 , 45 In older people, to be male, social isolation or greater feelings of loneliness, poor housing conditions, as well as a higher prevalence of chronic morbidities are risk factors for developing unhealthier lifestyles or mental health declines during confinement. In contrast, having a good adherence to the Mediterranean diet or doing physical activity before the confinement were protective for the development of unhealthier lifestyles during confinement. It must be highlighted that most of these changes reversed after the end of confinement.45 The insufficient consumption of essential nutrients for the maintenance of muscle mass, especially amino acids such as leucine, together with the increase in obesity, inflammatory mechanisms characteristic of COVID-19, and the lack of physical activity, could result in sarcopenic obesity.15 Moreover, these changes in nutritional habits and physical activity may generate changes in gut microbiota, which is known to be related to the severity of COVID-19.46
In addition, the role of some of the drugs used in the treatment of acute COVID-19 infection may also act on skeletal muscle, producing sarcopenia in patients with prolonged treatments, in addition to the effect in the acute phase of the disease previously mentioned.
The geriatric care model and its application to the specific case of persistent coronavirus disease: coordination of care and comprehensive care
As is the usual case in geriatric medicine, functional decline in older people with long COVID should be the cornerstone of the approach to these patients, along with the persistent dyspnea, fatigue, or musculoskeletal pain. In addition, altered diet because of gastrointestinal complaints, anosmia, and dysgeusia could precipitate or impair a sarcopenia condition, leading to a lower functional status, a worsening in instrumental and basic activities of daily living, and a higher risk of falls.23 In addition, this loss of function could worsen psychological health (depression, anxiety, a lower quality of sleep) and increase the need of social resources (home assistance or new institutionalizations),22 , 23 promoting the scenario where an integrated, comprehensive, coordinated, and continued care is needed.
Geriatric medicine has been characterized by a comprehensive care, coordinating the required care of older people according to their needs. Thanks to the information obtained through the geriatric comprehensive assessment and the availability of the different geriatric care levels (outpatient office, rehabilitation unit, geriatric day hospital, institutionalized and noninstitutionalized long-term care), it is possible to provide the best personalized care.
Because the management of the functional decline is the main pillar of the treatment of these patients, promoting self-governance and social participation in the community has a protective effect on physical function, increasing the levels of physical activity.33, 34, 35, 36 The benefits of physical activity are widely known: greater muscle mass; better balance and cognitive function; and lower comorbidities, falls, frailty, sarcopenia, and disability in older people.22 , 23 , 33 , 34 , 38, 39, 40, 41, 42, 43 In addition, related to SARS-CoV-2, physical activity improves immune function,47 having a potential protective effect in the older population.
Some programs of functional rehabilitation or telerehabilitation have been proposed, as well as respiratory rehabilitation. First of all, it is important to tailor the multicomponent exercise program not only to the patient but also to where it is going to take place. For older adults who exercise at home, body-weight exercises are crucial, being safe and effective, with chairs, walls, and floor being used to perform them. A Brazilian telerehabilitation program following discharge after COVID-19 stratified the patients according to their Barthel Index to assess different types of physical therapy and time of follow-up. In addition, they also evaluated the need for a nonphysical rehabilitation program: speech-language pathologist for dysphagia, cardiac rehabilitation if oxygen therapy was needed or for worsened dyspnea, occupational therapy if there was fine motor control or cognition decline, physiatrist if pain rated greater than 5, dietitian if weight loss, and psychologist if the patient was anxious or depressed.48 Other options that have been proved effective in older people at home include the Vivifrail program,49 which is also useful in older people in nursing homes with COVID-19.50 Another arm of intervention is pulmonary rehabilitation, showing an improvement of pulmonary function, quality of life, and anxiety levels after a 6-week program.51, 52, 53
To prevent or improve post–COVID-19 sarcopenia in these patients, it is important to ensure an adequate intake of proteins, vitamins, and minerals, oral nutritional supplements (ONS) being included in the diet, especially when the diet alone may not be sufficient. Thus, the recommendation is to provide at least 400 kcal/d with ONS, with 30 g of protein or more for, at minimum, 30 days.54 If a high risk of malnutrition exists, these requirements increase up to 600 kcal/d.55 Although changes in gut microbiota are known to be linked to immune response, and prebiotics and probiotics seem to have a benefit in frail people, the evidence is still unclear in post–COVID-19 older people.56
Cognitive consequences related to infection by SARS-CoV-2 may affect physical activity and sarcopenia57 in long COVID, making cognitive training programs potentially useful.58 , 59 A few programs to fight loneliness have been developed, especially in nursing homes. The Telephone Outreach in the COVID-19 Outbreak (TOCO) is a pilot telephone program implemented by medical students of Yale University in which once per week they call older people living in nursing homes. Residents and volunteers share stories of their lives, observing a positive experience.60
As mentioned earlier, an increase of social resources is needed as a consequence of higher functional limitations and disability after COVID-19. An early evaluation is important to assess the future needs of the patient, in which the comprehensive geriatric assessment could be helpful, working hand in hand with social workers.
The role of technology in long COVID syndrome in old people
During the months of confinement and social isolation caused by COVID-19, mobile health (mHealth) and digital health (eHealth) technologies offered a means by which older people could engage in physical activity. However, there was low to moderate evidence that interventions delivered via mHealth or eHealth approaches may be effective in increasing physical activity in older adults in the short term.61
POSITIVE (an acronym for maintaining and improving the intrinsic capacity involving primary care and caregivers) technology is a European Union Institute of Innovation and Technology project (EIT-Health–funded project).62 Its main objective is to make available a home remote monitoring system that allows better management and treatment of frailty, in order to maintain or improve the intrinsic capacity of the elderly, through a telematic platform of services. The home monitoring system consists of a series of questionnaires and 3 sensors that measure variables such as gait speed, power in the lower extremities, and involuntary weight loss. In addition, POSITIVE has a system of personalized prescription of physical exercise based on the Vivifrail program, as well as a nutritional plan and monitoring of functional status.63 The use of this technology will allow clinicians to monitor any worsening of the functional status as well as to prevent, through the prescription of individualized exercise, the development of sarcopenia as a fundamental mechanism in the development of the symptoms of post–COVID-19 syndrome in older people.
Clinics care points
-
•
A proactive search for long COVID syndrome in older people in the acute phase of the infection is recommended, taking into account the high prevalence and the difficulty of identifying the syndrome in some old patients.
-
•
Functional decline and its consequences should be the focus of attention in the integrated and coordinated care of older people with long COVID syndrome, establishing the level of geriatric care needed for each person, according to the comprehensive geriatric assessment.
-
•
A multicomponent and individualized intervention program should be prescribed, taking into account not only the characteristics of the patient but also the place to do it. Several programs of personalized physical exercise to be performed in different settings of care are accessible. A protein intake of at least 30 g/d should be provided for a minimum of 30 days. The use of prebiotics and probiotics is still controversial.
-
•
A cognitive and social approach should be done to avoid cognitive and mood disorders.
-
•
Technologies currently available in the market can be useful in monitoring the evolution of these patients and in the provision of multicomponent interventions.
Funding
CIBER-ISCIII (CB16/10/00464), co-funded by FEDER. A Way to Make Europe.
Acknowledgments
Disclosure
The authors have nothing to disclose.
References
- 1.Malik P., Patel K., Pinto C., et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-A systematic review and meta-analysis. J Med Virol. 2022;94(1):253–262. doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landi F., Carfì A., Benvenuto F., et al. Predictive factors for a new positive nasopharyngeal swab among patients recovered from COVID-19. Am J Prev Med. 2021;60(1):13–19. doi: 10.1016/j.amepre.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernández-de-Las-Peñas C., Florencio L.L., Gómez-Mayordomo V., et al. Proposed integrative model for post-COVID symptoms. Diabetes Metab Syndr. 2021;15(4):102159. doi: 10.1016/j.dsx.2021.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenhalgh T., Knight M., A’Court C., et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 5.Douglas H., Georgiou A., Westbrook J. Social participation as an indicator of successful aging: an overview of concepts and their associations with health. Aust Health Rev. 2017;41(4):455–462. doi: 10.1071/AH16038. [DOI] [PubMed] [Google Scholar]
- 6.Jimeno-Almazán A., Pallarés J.G., Buendía-Romero Á., et al. Post-COVID-19 syndrome and the potential benefits of exercise. Int J Environ Res Public Health. 2021;18(10) doi: 10.3390/ijerph18105329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naeije R., Caravita S. Phenotyping long COVID. Eur Respir J. 2021;58(2) doi: 10.1183/13993003.01763-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tosato M., Carfì A., Martis I., et al. Prevalence and predictors of persistence of COVID-19 symptoms in older adults: a single-center study. J Am Med Dir Assoc. 2021;22(9):1840–1844. doi: 10.1016/j.jamda.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sudre C.H., Murray B., Varsavsky T., et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman E.L., Savelieff M.G., Hayek S.S., et al. COVID-19 and diabetes: a collision and collusion of two diseases. Diabetes. 2020;69(12):2549–2565. doi: 10.2337/dbi20-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis H.E., Assaf G.S., McCorkell L., et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groff D., Sun A., Ssentongo A.E., et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4(10):e2128568. doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.P S., Madhavan S., Pandurangan V. Prevalence, pattern and functional outcome of post COVID-19 syndrome in older adults. Cureus. 2021;13(8):e17189. doi: 10.7759/cureus.17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Filippo L., De Lorenzo R., D’Amico M., et al. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: a post-hoc analysis of a prospective cohort study. Clin Nutr. 2021;40(4):2420–2426. doi: 10.1016/j.clnu.2020.10.043. Edinb Scotl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piotrowicz K., Gąsowski J., Michel J.-P., et al. Post-COVID-19 acute sarcopenia: physiopathology and management. Aging Clin Exp Res. 2021;33(10):2887–2898. doi: 10.1007/s40520-021-01942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welch C., Greig C., Masud T., et al. COVID-19 and acute sarcopenia. Aging Dis. 2020;11(6):1345–1351. doi: 10.14336/AD.2020.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paneroni M., Simonelli C., Saleri M., et al. Muscle strength and physical performance in patients without previous disabilities recovering from COVID-19 pneumonia. Am J Phys Med Rehabil. 2021;100(2):105–109. doi: 10.1097/PHM.0000000000001641. [DOI] [PubMed] [Google Scholar]
- 19.Ayres J.S. A metabolic handbook for the COVID-19 pandemic. Nat Metab. 2020;2(7):572–585. doi: 10.1038/s42255-020-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno Fernández-Ayala D.J., Navas P., López-Lluch G. Age-related mitochondrial dysfunction as a key factor in COVID-19 disease. Exp Gerontol. 2020;142:111147. doi: 10.1016/j.exger.2020.111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirwan R., McCullough D., Butler T., et al. Sarcopenia during COVID-19 lockdown restrictions: long-term health effects of short-term muscle loss. GeroScience. 2020;42(6):1547–1578. doi: 10.1007/s11357-020-00272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrmann M.L., Hahn J.-M., Walter-Frank B., et al. COVID-19 in persons aged 70+ in an early affected German district: risk factors, mortality and post-COVID care needs-a retrospective observational study of hospitalized and non-hospitalized patients. PLoS One. 2021;16(6):e0253154. doi: 10.1371/journal.pone.0253154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sagarra-Romero L., Viñas-Barros A. COVID-19: short and long-term effects of hospitalization on muscular weakness in the elderly. Int J Environ Res Public Health. 2020;23:17. doi: 10.3390/ijerph17238715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martone A.M., Bianchi L., Abete P., et al. The incidence of sarcopenia among hospitalized older patients: results from the Glisten study. J Cachexia Sarcopenia Muscle. 2017;8(6):907–914. doi: 10.1002/jcsm.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walle-Hansen M.M., Ranhoff A.H., Mellingsæter M., et al. Health-related quality of life, functional decline, and long-term mortality in older patients following hospitalisation due to COVID-19. BMC Geriatr. 2021;21(1):199. doi: 10.1186/s12877-021-02140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrillo-Garcia P., Garmendia-Prieto B., Cristofori G., et al. Health status in survivors older than 70 years after hospitalization with COVID-19: observational follow-up study at 3 months. Eur Geriatr Med. 2021;12(5):1091–1094. doi: 10.1007/s41999-021-00516-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vallecillo G., Anguera M., Martin N., et al. Effectiveness of an acute care for elders unit at a long-term care facility for frail older patients with COVID-19. Geriatr Nurs. 2021;42(2):544–547. doi: 10.1016/j.gerinurse.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sepúlveda-Loyola W., Rodríguez-Sánchez I., Pérez-Rodríguez P., et al. Impact of social isolation due to COVID-19 on health in older people: mental and physical effects and recommendations. J Nutr Health Aging. 2020;24(9):938–947. doi: 10.1007/s12603-020-1500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castañeda-Babarro A., Arbillaga-Etxarri A., Gutiérrez-Santamaría B., et al. Physical Activity Change during COVID-19 Confinement. Int J Environ Res Public Health. 2020;17(18):6878. doi: 10.3390/ijerph17186878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trabelsi K., Ammar A., Masmoudi L., et al. Sleep quality and physical activity as predictors of mental wellbeing variance in older adults during COVID-19 lockdown: ECLB COVID-19 international online survey. Int J Environ Res Public Health. 2021;18(8) doi: 10.3390/ijerph18084329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki Y., Maeda N., Hirado D., et al. Physical activity changes and its risk factors among community-dwelling Japanese older adults during the COVID-19 epidemic: associations with subjective well-being and health-related quality of life. Int J Environ Res Public Health. 2020;17(18) doi: 10.3390/ijerph17186591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okely J.A., Corley J., Welstead M., et al. Change in physical activity, sleep quality, and psychosocial variables during COVID-19 lockdown: evidence from the lothian birth cohort 1936. Int J Environ Res Public Health. 2020;1:18. doi: 10.3390/ijerph18010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasaki S., Sato A., Tanabe Y., et al. Associations between socioeconomic status, social participation, and physical activity in older people during the COVID-19 pandemic: a cross-sectional study in a northern Japanese city. Int J Environ Res Public Health. 2021;18(4) doi: 10.3390/ijerph18041477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization . Global recommendations on physical activity for health. WHO Press; Geneva Switzerland: 2010. [PubMed] [Google Scholar]
- 35.Sañudo B, Fennell C, Sánchez-Oliver AJ. Objectively-assessed physical activity, sedentary behavior, smartphone use.
- 36.Fernández-García Á.I., Marin-Puyalto J., Gómez-Cabello A., et al. Impact of the home confinement related to COVID-19 on the device-assessed physical activity and sedentary patterns of Spanish older adults. Biomed Res Int. 2021;2021:5528866. doi: 10.1155/2021/5528866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ammar A., Trabelsi K., Brach M., et al. Effects of home confinement on mental health and lifestyle behaviours during the COVID-19 outbreak: insights from the ECLB-COVID19 multicentre study. Biol Sport. 2021;38(1):9–21. doi: 10.5114/biolsport.2020.96857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Hara J. Mayo Clinic News Network; 2020. Rehabilitation after COVID-19. Available at. [Google Scholar]
- 39.Moro T., Paoli A. When COVID-19 affects muscle: effects of quarantine in older adults. Eur J Transl Myol. 2020;30(2):9069. doi: 10.4081/ejtm.2019.9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.da Rocha A.Q., Lobo P.C.B., Pimentel G.D. Muscle function loss and gain of body weight during the COVID-19 pandemic in elderly women: effects of one year of lockdown. J Nutr Health Aging. 2021;25(8):1028–1029. doi: 10.1007/s12603-021-1663-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartley P., Costello P., Fenner R., et al. Change in skeletal muscle associated with unplanned hospital admissions in adult patients: a systematic review and meta-analysis. PLoS One. 2019;14(1):e0210186. doi: 10.1371/journal.pone.0210186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bell K.E., von Allmen M.T., Devries M.C., et al. Muscle disuse as a pivotal problem in sarcopenia-related muscle loss and dysfunction. J Frailty Aging. 2016;5(1):33–41. doi: 10.14283/jfa.2016.78. [DOI] [PubMed] [Google Scholar]
- 43.Visser M., Schaap L.A., Wijnhoven H.A.H. Self-reported impact of the COVID-19 pandemic on nutrition and physical activity behaviour in Dutch older adults living independently. Nutrients. 2020;12(12) doi: 10.3390/nu12123708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sidor A., Rzymski P. Dietary choices and habits during COVID-19 lockdown: experience from Poland. Nutrients. 2020;12(6) doi: 10.3390/nu12061657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.García-Esquinas E., Ortolá R., Gine-Vázquez I., et al. Changes in health behaviors, mental and physical health among older adults under severe lockdown restrictions during the COVID-19 pandemic in Spain. Int J Environ Res Public Health. 2021;18(13) doi: 10.3390/ijerph18137067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarkar A., Harty S., Moeller A.H., et al. The gut microbiome as a biomarker of differential susceptibility to SARS-CoV-2. Trends Mol Med. 2021;27(12):1115–1134. doi: 10.1016/j.molmed.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gjevestad G.O., Holven K.B., Ulven S.M. Effects of exercise on gene expression of inflammatory markers in human peripheral blood cells: a systematic review. Curr Cardiovasc Risk Rep. 2015;9(7):34. doi: 10.1007/s12170-015-0463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leite V.F., Rampim D.B., Jorge V.C., et al. Persistent symptoms and disability after COVID-19 hospitalization: data from a comprehensive telerehabilitation program. Arch Phys Med Rehabil. 2021;102(7):1308–1316. doi: 10.1016/j.apmr.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romero-García M., López-Rodríguez G., Henao-Morán S., et al. Effect of a multicomponent exercise program (VIVIFRAIL) on functional capacity in elderly ambulatory: a non-randomized clinical trial in mexican women with dynapenia. J Nutr Health Aging. 2021;25(2):148–154. doi: 10.1007/s12603-020-1548-4. [DOI] [PubMed] [Google Scholar]
- 50.Courel-Ibáñez J., Pallarés J.G., García-Conesa S., et al. Supervised exercise (vivifrail) protects institutionalized older adults against severe functional decline after 14 weeks of COVID confinement. J Am Med Dir Assoc. 2021;22(1):217–219.e2. doi: 10.1016/j.jamda.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gautam A.P., Arena R., Dixit S., et al. Pulmonary rehabilitation in COVID-19 pandemic era: the need for a revised approach. Respirol Carlton Vic. 2020;25(12):1320–1322. doi: 10.1111/resp.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boukhris M., Hillani A., Moroni F., et al. Cardiovascular implications of the COVID-19 pandemic: a global perspective. Can J Cardiol. 2020;36(7):1068–1080. doi: 10.1016/j.cjca.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu K., Zhang W., Yang Y., et al. Respiratory rehabilitation in elderly patients with COVID-19: a randomized controlled study. Complement Ther Clin Pract. 2020;39:101166. doi: 10.1016/j.ctcp.2020.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barazzoni R., Bischoff S.C., Breda J., et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr Edinb Scotl. 2020;39(6):1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cawood A.L., Walters E.R., Smith T.R., et al. A review of nutrition support guidelines for individuals with or recovering from COVID-19 in the community. Nutrients. 2020;12(11) doi: 10.3390/nu12113230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jayanama K., Theou O. Effects of probiotics and prebiotics on frailty and ageing: a narrative review. Curr Clin Pharmacol. 2020;15(3):183–192. doi: 10.2174/1574884714666191120124548. [DOI] [PubMed] [Google Scholar]
- 57.Alonso-Lana S., Marquié M., Ruiz A., et al. Cognitive and neuropsychiatric manifestations of COVID-19 and effects on elderly individuals with dementia. Front Aging Neurosci. 2020;12:588872. doi: 10.3389/fnagi.2020.588872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bernini S., Stasolla F., Panzarasa S., et al. Cognitive telerehabilitation for older adults with neurodegenerative diseases in the COVID-19 era: a perspective study. Front Neurol. 2020;11:623933. doi: 10.3389/fneur.2020.623933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salawu A., Green A., Crooks M.G., et al. A proposal for multidisciplinary tele-rehabilitation in the assessment and rehabilitation of COVID-19 survivors. Int J Environ Res Public Health. 2020;17(13) doi: 10.3390/ijerph17134890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Dyck L.I., Wilkins K.M., Ouellet J., et al. Combating heightened social isolation of nursing home elders: the telephone outreach in the COVID-19 outbreak program. Am J Geriatr Psychiatry. 2020;28(9):989–992. doi: 10.1016/j.jagp.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGarrigle L., Todd C. Promotion of physical activity in older people using mhealth and ehealth technologies: rapid review of reviews. J Med Internet Res. 2020;22(12):e22201. doi: 10.2196/22201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barrio Cortes J., Guevara Guevara T., Aguirre Cocha K.P., et al. [Positive Project: maintenance and improvement of intrinsic capacity involving primary care and caregivers through a home monitoring system and a telematic services platform. Rev Esp Salud Publica. 2021;8:95. [PubMed] [Google Scholar]
- 63.Casas-Herrero A., Anton-Rodrigo I., Zambom-Ferraresi F., et al. Effect of a multicomponent exercise programme (VIVIFRAIL) on functional capacity in frail community elders with cognitive decline: study protocol for a randomized multicentre control trial. Trials. 2019;20(1):362. doi: 10.1186/s13063-019-3426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]