Abstract
The coronavirus disease 2019 (COVID-19) has overwhelming healthcare systems globally. To date, a myriad of therapeutic regimens has been employed in an attempt to curb the ramifications of a severe COVID-19 infection. Amidst the ongoing pandemic, the advent and efficacious uptake of COVID-19 vaccination has significantly reduced disease-related hospitalizations and mortality. Nevertheless, many side-effects are being reported after COVID-19 vaccinations and myocarditis is the most commonly reported sequelae post vaccination. Majority of these diseases are associated with COVID-19 mRNA vaccines. Various studies have established a temporal relationship between these complications, yet the causality and the underlying pathogenesis remain hypothetical. In this review, we aim to critically appraise the available literature regarding the cardiovascular side effects of the various mRNA vaccines and the associated pathophysiology.
Keywords: Coronavirus 2019 (COVID-19), SARS-CoV-2, COVID-19 mRNA vaccine, COVID-19 myocarditis, COVID-19 perimyocarditis, Takotsubo syndrome
Graphical Abstract
1. Introduction
The burden that coronavirus disease 2019 (COVID-19) has forced upon global healthcare systems has necessitated concerted efforts towards global immunization programs. As of March 2022, 31 million COVID-19 vaccines have been administered, with 54% of the global population having received at least one dose of a COVID-19 vaccine [1]. While studies have reported strong evidence of immunity after a single dose of a COVID-19 vaccine, vaccination efficacy dramatically increases after two doses, specifically for the alpha and the delta variants [2], [3]. Rare complications of COVID-19 mRNA vaccinations have included several cardiovascular outcomes such as: myocarditis, pericarditis, perimyocarditis, myocardial infarctions (MI), coronary thrombosis, and stress-induced cardiomyopathy (Takotsubo cardiomyopathy) [3]. Various studies have established a temporal relationship between vaccination and cardiovascular complications; nevertheless, causality remains largely inconclusive.
Myocarditis has been the predominant cardiovascular complication post-COVID-19 vaccination [4]. However, this is not the only instance of post-vaccine myocarditis historically, as myocarditis cases have been associated with other vaccinations as well [4]. The Center for Disease Control and Prevention (CDC) and the U.S. Federal Drug Administration (FDA) have been monitoring vaccine safety profile and the data surrounding post-vaccination complications, common or rare, via a plethora of vaccine safety monitoring systems: the Vaccine Adverse Effect Reporting System (VAERS), Vaccine Safety Datalink, V-safe, and the National Healthcare Safety Network. To date, the CDC has confirmed a total of 2337 reports of myocarditis in VAERS for all vaccines (1990 - November 11, 2021), 1969 of which were in people aged 30 and younger who received COVID-19 mRNA vaccines, which include the Pfizer-BioNTech and Moderna vaccines [5], [6]. Following investigation of these reports, the CDC and FDA confirmed that 1005 cases met the criteria for myocarditis, pericarditis, or myopericarditis [5].
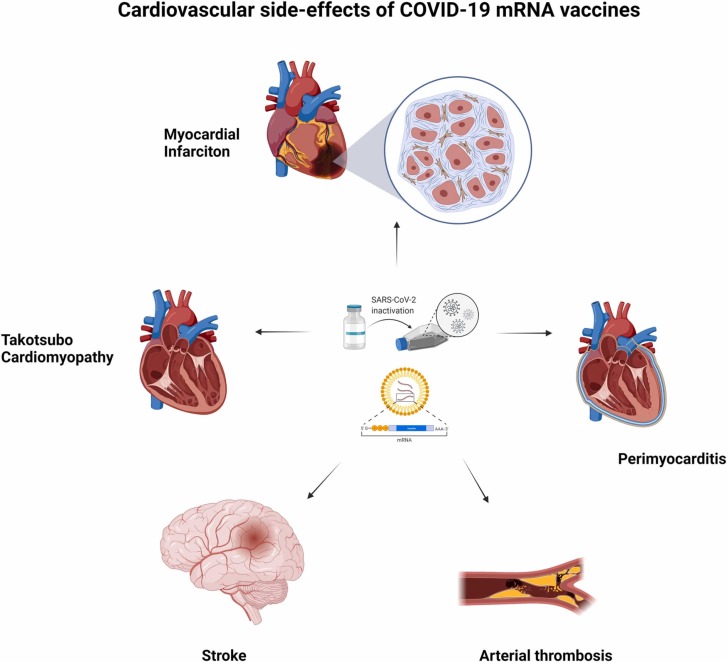
The risk of thrombotic events related to COVID-19 vaccines has been a major concern since reports have surfaced from the U.S., Singapore, India, and Europe. Although the data is limited, studies have already been conducted to differentiate clinical presentation of MI from myocarditis post-COVID-19 vaccinations. In summary, those with post-vaccination MI presented at around 24 h post-vaccination, much earlier than myocarditis at 72 h [7], [8], [9], [10]. More importantly, most developed MI symptoms after the first dose [9], [10]. A potential mechanism for post-vaccination MI could be Kounis syndrome, an allergic hypersensitivity reaction to potentially immunogenic components found within vaccines [9], [11]. The vast spectrum of cardiovascular complications reportedly associated with COVID-19 mRNA vaccinations is delineated by Fig. 1.
Fig. 1.
The various cardiovascular complications that have been reported post-COVID-19 mRNA vaccination if.
2. Methods
Using the digital databases (PubMed/MEDLINE, CINAHL, and Web of Science) to search for relevant material, a scoping review was conducted by two authors (T.A., T.K.) and articles containing cardiovascular complications after COVID-19 vaccine were incorporated in this review in a narrative style. The literature search was don for articles published between 2020 and 2022. In our search, we used the terms(s): “cardiovascular sequelae” OR “cardiovascular complications” OR “cardiovascular ramifications” OR “myocardial infarction” OR “heart failure” OR “Takotsubo cardiomyopathy” OR “myocarditis” OR “pericarditis” AND “COVID-19″ OR “COVID-19 vaccine” OR “COVID-19 vaccination programs”.
3. Main text
3.1. Pathophysiology
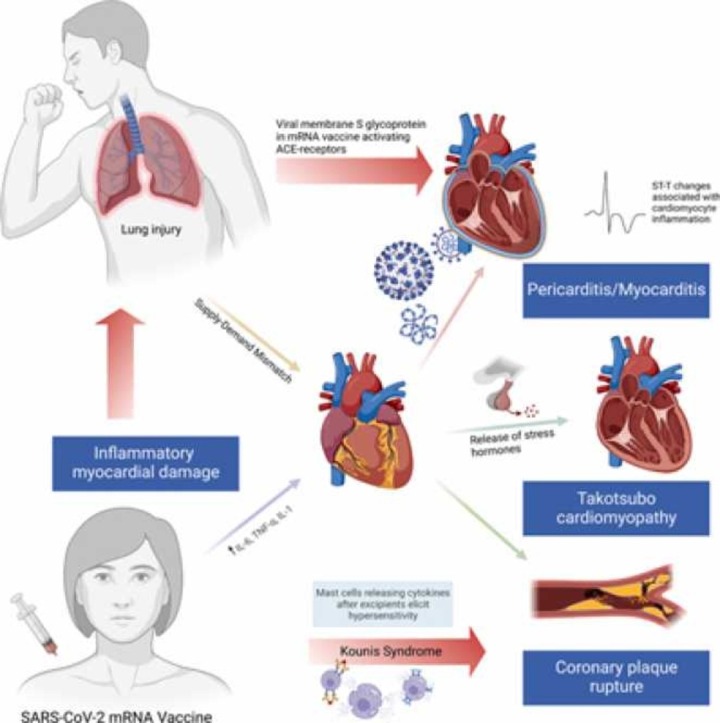
Primary infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) occurs when the virus’ surface spike S glycoprotein binds to angiotensin-converting enzyme 2 (ACE2), allowing it to enter the cell [12], [13]. ACE2 is ubiquitous throughout the body on the apical surface of epithelial cells, which likely explains the sequelae of adverse effects reportedly caused by infection [12]. Interestingly, with higher concentrations of ACE2 in the lung and heart, this potentially accounts for the pulmonary and cardiac damage that typifies a severe systemic COVID-19 infection [12].
While all the different cardiovascular complications have their own pathophysiological pathways, the COVID-19 mRNA vaccines could result in these outcomes through similar mechanisms. For instance, recent studies have implicated vaccines using a nucleoside-modified mRNA that encodes the viral membrane S glycoprotein of SARS-CoV-2. The vaccines instigate the production of antibodies that target the spike S protein, preventing binding to ACE2, thus conferring both cellular and humoral immunity [14], [15]. The exosomes containing the spike proteins were also found to stimulate splenic lymphocytes to secrete interferon (IFN)-γ and tumor necrosis factor (TNF)-α, amplifying the host inflammatory cell response system and instigating a cascade of immunological responses that could affect various organ systems, including the heart [15].
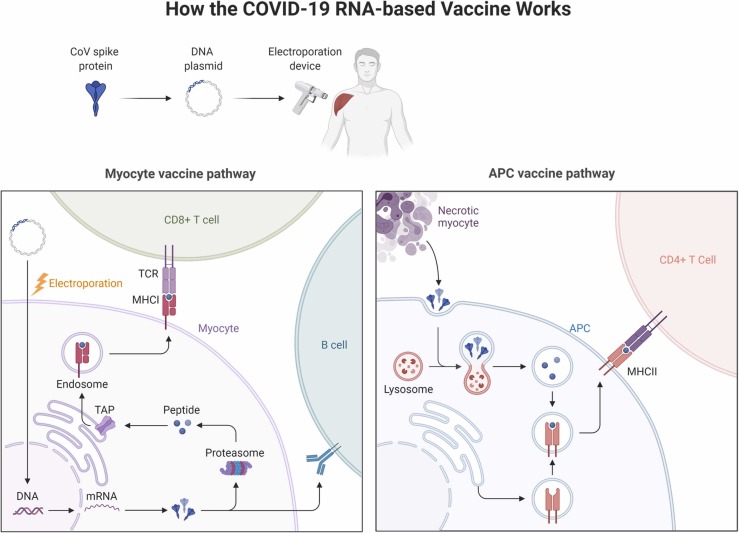
The immunological response induced by the vaccines could explain the inflammatory processes of myocarditis, pericarditis, and perimyocarditis. As briefly mentioned earlier, MI induced by the vaccines could be attributed to Kounis syndrome, which is largely explained by components within the vaccines known as excipients [11]. Excipients are pharmaceutical substances found alongside the active substances in a vaccine. In this case, the COVID-19 vaccine excipients are speculated to be potentially eliciting hypersensitivity reactions amongst some recipients [11]. Different excipients are noted in various vaccine formulations; polyethylene glycol (Pfizer-BioNTech vaccine); polyethylene glycol and tromethamine/trometamol (Moderna vaccine); polysorbate 80 (Johnson & Johnson vaccine); polysorbate 80 and disodium ethylenediaminetetraacetic acid dihydrate (Sputnik V vaccine); and disodium hydrogen phosphate, sodium dihydrogen phosphate monohydrate, and sodium chloride (Sinovac-Coronavac vaccine) [11]. An IgE-mediated hypersensitivity reaction concurrent with an acute coronary syndrome (ACS) or MI is termed Kounis syndrome [16]. This syndrome is accompanied by clinical and laboratory evidence of MI whose more predominant etiologies have been excluded and instead attributed to a hypersensitivity reaction characterized by release of inflammatory mediators such as histamine, neutral proteases (tryptase, chymase, and cathepsin-D), arachidonic acid metabolites, platelet-activating factor (PAF), cytokines, and chemokines released during mast-cell degranulation and by immune cells such as T-cells, B-cells, plasma cells, and macrophages [11], [16], [17]. The mechanism of action of COVID-19 mRNA vaccines is elucidated in Fig. 2.
Fig. 2.
The mechanism of action of COVID-19 mRNA vaccines. TCR: T-cell receptor; MHC-I: major histocompatibility complex I; TAP: trasporter associated with antigen processing; MHC-II: major histocompatibility complex II; APC: antigen presenting cell.
In addition to precipitating ischemic events, there have been reports of broken hearts. A recent case of post-COVID-19 vaccination Takotsubo syndrome was diagnosed and reported [18], [19]. Takotsubo syndrome has been described to be primarily attributed to an identifiable physical or emotional stressor, presents similarly to ACS, and most commonly affects post-menopausal women. It is a result of myocardial wall motion abnormalities in a distribution that is covered by a single coronary artery and nearly always presents with left ventricular wall dysfunction [19], [20], [21]. Its pathophysiology is predominated by catecholamine-mediated dysfunction and its pathogenesis post-COVID-19 vaccination is currently hypothesized to be mediated by the hypersensitivity response seen in Kounis syndrome [19].
3.2. Discussion
The current review aims to consolidate the data surrounding the various adverse cardiovascular events––myocarditis, perimyocarditis, pericarditis, MI, coronary thrombosis, or Takotsubo syndrome––associated with COVID-19 mRNA vaccinations (see Table 1, Table 2, Table 3). The mean age of the participants hovered at 56 ± 8.7 years, with a standard deviation (SD) of 8.4 years. There was a slight male preponderance, with 68.4% males and 31.6% females. More notably, females with any reported adverse cardiovascular event post-vaccination were, on average, older than their male counterparts (62.3 years vs 58.8 years, SD: 8.2 years, 95% confidence interval [CI]: 52.7–70.3 years, P < 0.05).
Table 1.
Details of studies reporting myocarditis cases post-COVID-19 mRNA vaccine. Study cohort characteristics, comorbidities, clinical presentation, diagnostic evaluation, and outcome are all summarized.
| Case Series | Myocarditis Following COVID-19 mRNA Vaccine: A Case Series and Incidence Rate Determination | Acute myocardial infarction and myocarditis following COVID-19 vaccination | Myocarditis and pericarditis in association with COVID-19 mRNA-vaccination: cases from a regional pharmacovigilance center | Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military | Patients With Acute Myocarditis Following mRNA COVID-19 Vaccination | Myocarditis and Pericarditis After Vaccination for COVID-19 | Case Report | Acute Myocarditis after COVID-19 vaccination: A case report |
|---|---|---|---|---|---|---|---|---|
| Study | Perez et al. [7] | Aye et al. [9] | Istampoulouoglou et al. [24] | Montgomery et al. [25] | Kim et al. [26] | Diaz et al. [27] | Study | Schmitt et al. [31] |
| Characteristics | Characteristics | |||||||
| Cases, n | 7 | 42 | 5 | 23 | 4 | 20 | Case, n | 1 |
| Male, % | 6 (86%) | 38 (91%) | 3 (60%) | 23 (100%) | 3 (75%) | 15 (75%) | Gender | Male |
| Medianage (range), years | 44 (22–71) | 21 (17–30) | 34 ( 20–44) | 25 (20–51) | 38.3 (23–70) | 36 (26.3–48.3) | Age | 19 |
| Vaccine type | Pfizer-BioNTech: 3 (42%) Moderna: 4 (57%) | Pfizer BioNTech: 35 (83%) Moderna: 6 (14%) | Moderna: 4 (80%) Comirnaty: 1 (20%) | Pfizer BioNTech: 7 (30%) Moderna: 16 (70%) | Pfizer BioNTech: 2 (50%) Moderna: 2 (50%) | Pfizer BioNTech: 9 (45%) Moderna: 11 (55%) | Vaccine type | Second dose of Pfizer-BioNTech |
| Comorbidities | Comorbidities | |||||||
| Hypertension | 5 (72%) | 4 (10%) | – | – | 1 (25%) | 5 (25%) | Hypertension | – |
| Hyperlipidemia | 3 (42%) | 4 (10%) | – | – | – | – | Hyperlipidemia | – |
| Diabetes mellitus | – | – | – | 1 (25%) | 2 (10%) | Diabetes Mellitus | – | |
| Smoking | 3 (42%) | 2 (5%) | – | – | 1 (25%) | – | Smoking | – |
| Previous hx of CAD | 2 (29%) | – | – | – | – | 1 (5%) | Previous hx of CAD | – |
| FHx of CAD | 1 (14%) | – | – | – | – | – | FHx of CAD | – |
| Prior COVID-19 infection | 1 (14%) | – | – | 3 (13%) | – | – | Prior COVID-19 infection | – |
| COVID-19 PCR positive | – | – | – | 0 | – | – | COVID-19 PCR positive | No |
| Presentation | Presentation | |||||||
| Time between last vaccine and symptoms onset, median days (range) | 3 (1–13) | 3 (2–3) | 15.4 (3–28) | 2 (0.5–4) | 2.75 (1–5) | 3.5 (3–10.8) | Time between last vaccine and symptoms onset (days) | 1 |
| Symptoms post-second dose | 1 (14%) | 35 (83%) | 2 (40%) | 20 (87%) | 4 (100%) | 16 (80%) | Symptoms post-second dose | Yes |
| Chest pain | – | – | 4 (80%) | 100% | 1 (25%) | – | Chest pain | No |
| Other symptoms (e.g., myalgia, fatigue, fever) | – | – | 2 (40%) | – | 4 (100%) | – | Other symptoms (e.g., myalgia, fatigue, fever) | Yes |
| Diagnostic Evaluation | Diagnostic Evaluation | |||||||
| Elevated troponin | – | – | 4 (60%) | 23 (100%) | 4 (100%) | 19 (95%) | Elevated troponin | Yes |
| Elevated BNP or NT-proBNP | 4/5 (80%) | – | – | – | 2 (50%) | – | Elevated BNP or NT-proBNP | Yes |
| Elevated CRP | – | – | – | – | 3 (75%) | – | Elevated CRP | Yes |
| Abnormal ECG | 5/7 (71%) | 27 (64%) | 3 (60%) | 19 (83%) | 4 (100%) | 9 (45%) | Abnormal ECG | Yes; PR depression in inferior leads and a persistent concave ST elevation without reciprocal depression |
| Abnormal MRI | 3/6 (50%) | 32 (76%) | 3 (60%) | 8 (35%) | 4 (100%) | – | Abnormal MRI | Yes |
| Abnormal echocardiogram | 3/6 (50%) | – | – | 4 (17%) | – | – | Abnormal echocardiogram | – |
| LVEF < 50% | 3/6 (60%) | – | 1 (20%) | 4 (17%) | 1 (25%) | 5 (25%) | LVEF < 50% | No |
| Outcome | Outcome | |||||||
| Symptoms resolved | 7 (100%) | – | 5 (100%) | 16 (69%) | 100% | 13 (65%) | Symptoms resolved | Yes |
| Median length of hospitalization, days (range) | 2 (2–4) | – | 6 (3–10) | – | – | 2 (2–3) | Length of hospitalization | – |
| Treatment regimen | Combination of steroids (2/7, 29%), NSAIDs(2/7, 29%), and colchicine (5/7, 71%) which is known to modulate multiple anti-inflammatory pathways[16]. None received IVIG. ACE inhibitors and β-blockers were prescribed for patients with LV dysfunction. | Discharged on NSAIDs 24/42 (57%), colchicine 13/42 (31%), aspirin 3/42 (7%), β-blockers 6/42 (14%) | Cardioprotective therapy with an ACE inhibitor (60%) followed by a β-blocker (40%) | Cardiac symptoms resolved within 1 week of onset (16 patients). Seven patients continued to have chest discomfort at the time of this report; follow-up is ongoing. | NSAIDs and colchicine, with 1 patient receiving corticosteroids | NSAIDs 75%, and colchicine 45% | Follow-up and medications received for myocarditis | At 1 month follow-up, the patient remained asymptomatic, with normal ECG and echocardiogram. No medication was prescribed. |
Hx: history, CAD: coronary artery disease, FHx: family history, PCR: polymerase chain reaction, brain natriuretic peptide (BNP), N-terminal prohormone BNP (NT-proBNP), CRP: C-reactive protein, ECG: electrocardiogram, MRI: magnetic resonance imaging, LVEF: left ventricular ejection fraction, NSAIDs: nonsteroidal anti-inflammatory drugs, IVIG: intravenous immunoglobulin, ACE: angiotensin converting enzyme, LV: left ventricle.
Table 2.
Studies of pericarditis & perimyocarditis cases post-COVID-19 mRNA vaccine with study cohort characteristics, comorbidities, clinical presentation, diagnostic evaluation, and outcomes all summarized.
| Case Series | Pericarditis | Myocarditis and pericarditis in association with COVID-19 mRNA-vaccination: cases from a regional pharmacovigilance center | Myocarditis and Pericarditis After Vaccination for COVID-19 | Perimyocarditis | Myocarditis and pericarditis in association with COVID-19 mRNA-vaccination: cases from a regional pharmacovigilance center |
|---|---|---|---|---|---|
| Author | Istampoulouoglou et al. [24] | Diaz et al. [27] | Istampoulouoglou et al. [24] | ||
| Characteristics | |||||
| Cases, n | 3 | 37 | 9 | ||
| Male, % | 2 (67%) | 27 (73%) | 7 (78%) | ||
| Median age (range), years | 61 (33–71) | 59 (46–69) | 57 (17–88) | ||
| Vaccine type | Pfizer-BioNTech: 1 (33.33%)Moderna: 1 (33.33%) | mRNA-1273: 12 (32%)BNT162b2: 23 (62%)Ad26. COV2. S: 2 (5%) | Moderna: 6 (67%)Comirnaty: 3 (33%) | ||
| Comorbidities | |||||
| Hypertension | 1 (33%) | 18 (48.6%) | 3 (33%) | ||
| Hyperlipidemia | – | – | – | ||
| Diabetes Mellitus | – | 4 (10.8%) | – | ||
| Smoking | – | – | – | ||
| Previous hx of CAD | – | 4 (10.8%) | 2 (22%) | ||
| FHx of CAD | – | – | – | ||
| Prior COVID-19 infection | – | – | – | ||
| COVID-19 PCR positive | – | – | – | ||
| Presentation | |||||
| Median time between last vaccine and symptoms onset, days (range) | 6.5 (1–14) | 20 (6.0–41.0) | 4.67 (<1–17) | ||
| Symptoms post second-dose | – | 22 (59.5%) | 7 (78%) | ||
| Chest pain | 1 (33%) | – | 56% | ||
| Other symptoms (e.g., myalgia, fatigue, fever) | 1 (33%) | – | 20% | ||
| Diagnostic Evaluation | |||||
| Elevated troponin | 2 (67%) | 0 | 8/9 (89%) | ||
| Elevated BNP or NT-proBNP | – | – | – | ||
| Elevated CRP | – | – | – | ||
| Abnormal ECG | 2 (67%) | 14 (37.8%) | 7/8 (88%) | ||
| Abnormal MRI | – | – | 6/7 (86%) | ||
| Abnormal echocardiogram | – | – | – | ||
| LVEF < 50% | – | 3 (8.1%) | – | ||
| Outcome | |||||
| Symptoms resolved | 2 (67%) | 7 (18.9%) | 3 (33%) | ||
| Median length of hospitalization, days (range) | 7.5 (0–11) | 1 (1–2) | 6 (3–10) | ||
| Treatment regimen | t | For the management of pericarditis, all three patients received anti-inflammatory therapy with ibuprofen and colchicine. | Treated with NSAIDs 48.6%, colchicine 54.1%, and systemic steroids 10.8% | The most used treatment was a cardioprotective therapy with an ACE inhibitor or a β-blocker (each 44%), followed by a combination of these two drugs or ibuprofen as anti-inflammatory therapy (each 33%). |
Hx: history, CAD: coronary artery disease, FHx: family history, PCR: polymerase chain reaction, brain natriuretic peptide (BNP), N-terminal prohormone BNP (NT-proBNP), CRP: C-reactive protein, ECG: electrocardiogram, MRI: magnetic resonance imaging, LVEF: left ventricular ejection fraction, NSAIDs: nonsteroidal anti-inflammatory drugs, ACE: angiotensin converting enzyme, LV: left ventricle.
Table 3.
Studies of MI (myocardial infarction), Coronary Thrombosis & Takotsubo syndrome cases post-COVID-19 mRNA vaccine.
| Case Series - AMI | Acute Myocardial Infarction Within 24 h After COVID-19 Vaccination | Acute myocardial infarction and myocarditis following COVID-19 vaccination | Case Report - Coronary thrombosis | Acute Coronary Tree Thrombosis After Vaccination for COVID-19 | Case Report - Takotsubo syndrome | Takotsubo syndrome after receiving the COVID-19 vaccine |
|---|---|---|---|---|---|---|
| Author | G. Sung et al. [10] | Aye et al. [9] | Author | Tajstra et al. [18] | Author | Fearon et al. [19] |
| Characteristics | Characteristics | Characteristics | ||||
| Cases, n | 2 | 35 | Case | 1 | Case | 1 |
| Male sex, % | 1 (50%) | 28 (80%) | Gender | Male | Gender | Female |
| Median age (range), y | 55 (42–68) | 65 (59–74) | Age | 86 | Age | 73 |
| Vaccine type | mRNA-1273 vaccine: 2 (100%) | Pfizer BioNTech:30 (86%)Moderna: 1 (1%) | Vaccine type | Pfizer–BioNTech vaccine | Vaccine type | Moderna COVID-19 vaccination |
| Comorbidities | Comorbidities | Comorbidities | ||||
| Hypertension | 1 (50%) | 22 (63%) | Hypertension | – | Hypertension | – |
| Hyperlipidemia | 2 (100%) | 19 (54%) | Hyperlipidemia | – | Hyperlipidemia | – |
| Diabetes mellitus | – | 18 (51%) | Diabetes Mellitus | – | Diabetes mellitus | – |
| Smoking | 1 (50%) | 12 (34%) | Smoking | – | Smoking | – |
| Previous hx of CAD | 1 (50%) | 9 (26%) | Previous hx of CAD | Yes | Previous hx of CAD | – |
| FHx of CAD | 1 (50%) | – | FHx of CAD | – | FHx of CAD | – |
| Prior COVID-19 infection | – | – | Prior COVID-19 infection | – | Prior COVID-19 infection | – |
| COVID-19 PCR positive | – | – | COVID-19 PCR positive | – | COVID-19 PCR positive | – |
| Presentation | Presentation | Presentation | ||||
| Time between last vaccine and symptoms onset, median days (range) | 2 (1–3) | 1 (1–2) days | Time between last vaccine and symptoms onset (days) | < 1 (30 min) | Time between last vaccine and symptoms onset (days) | < 1 day (17 h after vaccination) |
| Symptoms post-second dose | 0 | 6/18 (33%) | Symptom post-second dose | No | Symptoms post-second dose | |
| Chest pain | – | – | Chest pain | No | Chest pain | Yes |
| Other symptoms (e.g., myalgia, fatigue, fever) | – | – | Other symptoms (e.g., myalgia, fatigue, fever) | Yes | Other symptoms (e.g., myalgia, fatigue, fever) | No |
| Diagnostic Evaluation | Diagnostic Evaluation | Diagnostic Evaluation | ||||
| Elevated troponin | 2 (100%) | – | Elevated troponin | – | Elevated troponin | Yes |
| Elevated BNP or NT-proBNP | – | – | Elevated BNP or NT-proBNP | – | Elevated BNP or NT-proBNP | – |
| Elevated CRP | – | – | Elevated CRP | – | Elevated CRP | – |
| Abnormal ECG | 1 (50%) | 20 (57%) | Abnormal ECG | – | Abnormal ECG | Electrocardiogram had ST wave changes concerning for inferolateral ischemia and new poor anterior R wave progression |
| Abnormal MRI | – | – | Abnormal MRI | – | Abnormal MRI | – |
| Abnormal echocardiogram | – | – | Abnormal Echocardiogram present | Yes, acute ST-segment elevation myocardial infarction of the inferior wall | Abnormal echocardiogram | – |
| LVEF < 50% | – | – | LVEF < 50% | – | LVEF < 50% | Yes |
| Outcome | Abnormal coronary angiography | Occlusions/distal embolization found in 3 arteries | Outcome | |||
| Underwent PCI | 2 (100%) | 21 (60%) | Outcome | Symptoms resolved | Yes | |
| Symptoms resolved | 2 (100%) | – | Patient underwent PCI | Primary PCI of the RCA with manual aspiration thrombectomy was performed, along with coronary balloon angioplasty and glycoprotein IIb/IIIa receptor inhibitor (eptifibatide) administration, resulting in coronary flow improvement. | Length of hospitalization | 8 |
| Median length of hospitalization, days (range) | 4.5 (2–7) | – | Symptoms resolved | On January 30, 2021, the patient died.(3 days after symptoms developed) | ||
| Treatment regimen | Initiated on guideline-directed medical therapy | Discharged on:25/26 (96%) Aspirin, 22/29 (76%) on P2Y12 inhibitor, 20/26 (77%) on β-blockers, 14/26 (54%) on ACE inhibitor, 28/35 (80%) on statins, direct anticoagulants 5/35 (14%), CCBs 4/35 (11%), nitrates 3/35 (9%), diuretics 8/35 (23%) | Length of hospitalization | 3 |
Hx: history, CAD: coronary artery disease, FHx: family history, PCR: polymerase chain reaction, brain natriuretic peptide (BNP), N-terminal prohormone BNP (NT-proBNP), CRP: C-reactive protein, ECG: electrocardiogram, MRI: magnetic resonance imaging, LVEF: left ventricular ejection fraction, ACE: angiotensin converting enzyme, PCI: percutaneous coronary intervention, RCA: right coronary artery, CCB: calcium channel blockers.
3.3. Myocarditis
We included a total of 7 studies (6 case series and 1 case report) between August and November 3rd, 2021: a total of 102 cases of myocarditis post-mRNA vaccination (Table 1). The vaccinations received in the studies were as follows: 57 received the Pfizer BioNTech vaccine, 44 received Moderna, and 1 Comirnaty. Most cases were male, including the single case report, with an age range of 19–71 years old. The most common comorbidities were hypertension, smoking, and a previous history of coronary artery disease, all of which demonstrated a worse prognosis. Interestingly, patients with comorbidities, specifically hypertension and active smoking, exhibited significantly lower antibody titers following COVID-19 vaccination, which could contribute to an increased likelihood of post-vaccine complications [22]. Another study sheds further light by promoting smoking cessation prior to vaccination to ameliorate the efficacy of the BioNTech Pfizer vaccine [23].
Of the 102 cases with myocarditis, four were PCR-positive after vaccination. One study, pooling cases of myocarditis from the USA, Israel, Singapore, and a few other countries, demonstrated that 83% of the patients developed myocarditis symptoms only after the second vaccine dose [9]. Additionally, a retrospective case series analyzing data from the Mayo Clinic COVID-19 Vaccine Registry elucidated that all seven cases occurred after the second dose [7]. Other studies that have reported myocarditis symptoms occurring post-second dose of COVID-19 mRNA vaccines reported rates hovering between 40% and 100%. Among the included studies, the median time between vaccination and onset of symptoms was 1–3 days [24]. However, one study reported a mean time of onset of 15.4 days, with a range of 3–28 days [24]. The study included case reports from Switzerland using the Regional Pharmacovigilance Center (RPVC) internal database for cases of myocarditis, perimyocarditis, and pericarditis temporally related to COVID-19 mRNA vaccination [24].
Most cases of myocarditis post-vaccination presented with elevated cardiac troponin levels along with simultaneous elevations in C-reactive protein (CRP), brain natriuretic peptide (BNP) or N-terminal prohormone BNP (NT-proBNP) [7], [9], [24]. Interestingly, abnormal electrocardiogram findings were seen in a few patients. Fourteen myocarditis cases presented with a left ventricular ejection fraction (LVEF) of < 50% [7], [8], [9], [10], [25], [26]. Abnormal cardiac magnetic resonance imaging (MRI) with late gadolinium enhancement and edema were observed in 51 cases [7], [9], [24], [25], [26], [27]. Late gadolinium enhancement was found in 41% of the patients in the subepicardial layer, 14% in the mid-myocardial layer, and 24% in both layers [9]. The same study found edema in 21% of cases within the subepicardial layer, 17% within the mid-myocardial layer, and 24% within both layers [9]. These findings further substantiate the presence of epicardial and myocardial inflammation in the aftermath of vaccination.
Almost all of the cases experienced a prompt recovery, with no residual cardiac dysfunction. However, in one study, seven patients continued to experience chest discomfort [25]. While their follow-up is ongoing, median length of stay for all myocarditis cases was around 2–3 days with a range of 2–10 days [25]. Treatment of myocarditis cases amongst the studies included non-steroidal anti-inflammatory drugs (NSAIDs), colchicine, aspirin and steroids [25], [26], [27]. Beta blockers and ACE inhibitors were administered in some cases for left ventricular dysfunction as a cardioprotective therapy to prevent positive remodeling of the heart [7], [24].
As of March 2022, the CDC reported an incidence of 4.8 cases of myocarditis per one million post-COVID-19 mRNA vaccines, with a predominance in young males [27]. The curtailed duration between COVID-19 vaccination and symptom onset is supplanted by the elevated incidence of myocarditis [27]. Notably, many studies have acknowledged limitations in these statistics, hypothesizing missed cases of post-vaccine myocarditis that would underestimate the reported incidence [28].
The incidence of myocarditis following the mRNA vaccines was higher after the second dose, especially in adolescent and young adult males within a week of vaccination [7]. The CDC collected this using data from the U.S.A database of healthcare encounters consisting of more than 900 hospitals between March 2020 and March 2022, revealing that patients infected with COVID-19 had 15.7 times the risk of myocarditis compared to noninfected patients [8]. The risk ratio varied across age (7.0 for aged 16–39 and >30.0 for those < 16 and > 75 years old) and gender (13.8 for males, 17.8 for females) [8]. In another study from Israel, the risk ratio of myocarditis associated with mRNA vaccination and COVID-19 infection were compared (3.24 vs 18.28, respectively), showing that the risk of myocarditis is higher with infection than vaccination [8]. In regards to prognosis in this cohort, no long-term data is yet available.
3.4. Pericarditis
Cases of post-vaccination pericarditis were reported in studies from Switzerland and the United States, both of which compared the incidence with that of post-COVID-19 myocarditis [24], [27]. A total of 40 pericarditis cases were recorded, with 75% males and a median age of 59–60 years old. While pericarditis presents similarly to myocarditis, pericarditis had a higher incidence in men with old age. Hypertension, diabetes mellitus and a previous history of coronary artery disease were the top three comorbidities.
The mean time from vaccination to onset of symptoms was 6.5 and 20 days [24], [27]. It can be explained by difficulty in diagnosis of pericarditis due to less specific symptoms. In the Switzerland study, three cases were reported, with one presenting with unclear symptoms and vague diagnostic criteria score due to self-reporting [24]. This less-than-optimal clinical evaluation could be attributed to recall bias, which would contribute to limitations within the studies and lead to difficulty in evaluating temporality between the vaccine and its complications.
In the United States, 59.5% of pericarditis occurred after the second dose of the vaccine [27]. Three patients had an LVEF of < 50%, increased troponin levels, and abnormal ECGs. Length of hospitalization ranged from 0 to 11 days. Treatment included a regimen of NSAIDs, colchicine, and steroids. However, complete resolution of symptoms was reported in only 18.9% of pericarditis cases [27].
3.5. Perimyocarditis
In a study, cases of perimyocarditis were identified and reported in association with the COVID-19 vaccines [24]. Data from a regional pharmacovigilance center in Switzerland included perimyocarditis diagnosed in line with the diagnostic criteria of the European Society of Cardiology [24]. Nine cases of perimyocarditis were reported with a median age of 57 years (range 17–88). Seven of the cases were males (78%) with a median age of 23 years (range 17–86) [24]. Six patients (67%) received the Moderna vaccine, and three received Comirnaty [24]. Hypertension was reported in three patients (33%) and a previous history of CAD in two (22%). The mean time from vaccination to symptom onset was 4.7 days, with seven (78%) cases presenting after second dose [24]. Five patients (56%) presented with chest pain and two (22%) presented with nonspecific symptoms such as myalgia, fever, and other flu-like symptoms [24]. Abnormal troponin levels were observed in eight patients (89%), significant ECG findings in seven (77%), and noteworthy MRI findings in six (67%). Notably, symptoms fully resolved in only three patients (33%), among which the median length of stay was 6 days. The treatment regimen for these cases included an ACE inhibitor (administered to 4 patients) or a β-blocker (4 patients), followed by combination therapy with an ACE inhibitor and β-blocker or ibuprofen as anti-inflammatory therapy [24].
3.6. Myocardial infarction
A total of two case series with a total of 37 cases documenting cases of MI post-COVID-19 mRNA vaccination were studied as demonstrated in Table 3 [9], [10]. Males were predominantly affected making up 78% of cases with a mean age of 55 years in one study and 65 in the other, interestingly this gender distribution was noted to be similar to the demographic most vulnerable to COVID-induced myocarditis [9], [10]. Comorbidities found within the two studies included hypertension (62%), hyperlipidemia (57%), diabetes mellitus (51%), and active smoking (35%), all amongst the most significant risk factors for the complication of MI [9], [10].
One of the studies pooled data from a retrospective cohort of cases of myocarditis and MI found in patients post-COVID-19 mRNA vaccination in Singapore, and included these cases in a systematic review of similar cases from the USA, Israel, India, and other countries [9]. The median time noted between vaccination and onset of symptoms was approximately 1–2 days, with 33% of documented cases occurring post-second vaccination in one of the studies alluding to the differentiation of MI cases post vaccination occurring post first dose of the vaccine in comparison to myocarditis cases occurring more commonly post second dose of the vaccine [9]. Troponin levels were elevated in most cases, with ST-elevations found in 57% of patients in one of the studies [9]. The ST elevations were noted in anterior leads in 20% of cases, lateral leads in 6%, and inferior leads in 20%. Mean LVEF was 50% (range 40–55%), with 89% presenting with regional wall motion abnormalities on transthoracic echocardiogram (TTE) [9]. Within the same study, 66% of cases received culprit-vessel percutaneous coronary intervention (PCI): 37% left anterior descending (LAD) artery, 20% right coronary artery (RCA), 5% left circumflex (LCX) artery, and 5% other vessels [9], [10].
The patients in the retrospective cohort of 35 patients were discharged on NSAIDs, colchicine, direct oral anticoagulants, β blockers, and statins, which resulted in a prompt abatement of their clinical symptoms [9]. Effective treatment with the aforementioned regimen lends further evidence to Kounis syndrome as a plausible pathophysiological explanation for the observed inflammatory phenomenon [14]. The other study reported two patients that underwent PCI to the LCX [10]. Both patients remained symptom free upon discharge and the median hospital stay was 4.5 days [10].
One hypothesis for the development of MI post-COVID-19 vaccine states that vaccines induce an autoimmune reaction response against platelets, a clinically similar phenomenon to autoimmune heparin induced thrombocytopenia. [29], [30] Other studies have postulated that the stress and emotional turmoil that can accompany receipt of the COVID-19 vaccine amongst adults could lead to a demand-ischemia, resulting in the unduly cardiac events amongst the recipients [9], [31]. While the pathogenesis of MI post-COVID-19 mRNA vaccination remains elusive, Kounis syndrome is hypothesized to be a contributing factor or even etiology [9], [10], [14]. Older patients with underlying comorbidities such as hypertension and a previous history of CAD are more vulnerable to high stressors, and this could explain the cases of MI post-vaccination [31]. Note that these theories are different from those of myocarditis induced by COVID-19 vaccines, which were mostly attributed to non-inflammatory responses between vaccine and cardiac cell proteins [32].
A recent paper reported the case of a 86-year-old male who collapsed within 30 min after receiving the vaccination, with ECG showing ST-elevations in the inferior wall. Investigations revealed a triple coronary artery thrombosis after the first dose of the Pfizer–BioNTech vaccine, which was soon followed by hemodynamic instability [18]. Coronary angiography showed distal blockage in the left anterior descending artery, the first diagonal branch, and right coronary artery [18]. The patient was treated with primary percutaneous coronary intervention of the right coronary artery with aspiration thrombectomy, coronary balloon angioplasty, and administration of a eptifibatide, glycoprotein IIb/IIIa receptor inhibitor [18]. The patient remained hospitalized for three days and expired shortly thereafter [18]. It is of significance to note that the patient in the study had a previous history of prostate cancer for which he received treatment in 2006 through surgical resection and radiotherapy [18]. At the time of vaccination, he was on an androgen receptor inhibitor, and his medical history included paroxysmal atrial fibrillation for which he was taking apixaban twice daily [18]. In this context, a concoction of risk factors could have precipitated his clinical deterioration, contributing to a hypercoagulable state and thus leading to coronary thrombosis. Nevertheless, this would not explain the acute presentation post-vaccination. While not much can be ascertained from a single case report, the curtailed intervening period between vaccination and onset of symptoms alludes to a link between the two, with Kounis syndrome remaining the predominant unifying theory for the pathogenesis at hand.
3.7. Takotsubo syndrome
Many cases of Takotsubo cardiomyopathy have been reported in association with COVID-19 infection [21]. Yet perhaps more interesting is that, since the commencement of COVID-19 vaccination regimens, there has been an increasing incidence of Takotsubo syndrome. A recent study reported the case of a 73-year-old woman with a history of chronic kidney disease (CKD) and hypertension who was admitted 17 h after Moderna’s COVID-19 vaccination on suspicion of non-ST elevation myocardial infarction: a troponin of 0.22 ng/mL, creatinine 2.0 mg/dL, BNP > 70,000 pg/mL, procalcitonin 0.61 ug/L, no signs of infection, and ECG findings indicative of inferolateral ischemia and poor R wave progression [19]. TTE demonstrated LV apical ballooning, an EF of 20%, with mild mitral regurgitation, and severe right ventricular dysfunction with functional tricuspid regurgitation [19]. She was diagnosed with Takotsubo syndrome and was subsequently treated for acute heart failure [19], [20]. Repeat TTE at three days showed marked improvement in her biventricular function and an EF of 35–40%, and by discharge on day 8 the heart failure had resolved.
While the ACE2 receptor has been implicated in the pathogenesis of COVID-19-induced myocardial injury leading to Takotsubo syndrome, the mechanism underlying the vaccine-induced entity of Takotsubo syndrome is not expected to herald comparable levels of cardiac injury [19]. Rather than direct damage to the heart as in infection, it was postulated that the vaccine may stimulate a systemic inflammatory response that merely sensitizes patients to catecholaminergic effects, resulting in an imbalance between sympathetic and parasympathetic drives that manifests as Takotsubo cardiomyopathy [19], [21], [33], [34]. Furthermore, it has been postulated that the overwhelming emotional turmoil and stress fomented by the uptake of COVID-19 vaccination can herald even greater catecholamine release, which can precipitate the myocardial stunning that is observed in Takotsubo cardiomyopathy.
4. Summary
To summarize, several studies reporting cases of cardiovascular side effects secondary to mRNA COVID-19 vaccines have been discussed. These include myocarditis, perimyocarditis, pericarditis, MIs, and Takotsubo syndrome [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32]. Most pertinently, the time between vaccination and the onset of symptoms varied between complications. The reported cases shared certain predisposing risk factors, such as hypertension, active smoking and a previous history of CAD [14], [15], [16], [17], [18], [19], [20], [21]. The reported cases were mainly males with age variation. Notably, myocarditis predominantly affected younger males while pericarditis was observed in older males [24], [27]. While myocarditis is the highest reported cardiovascular ramification, other serious complications are also being increasingly reported [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [27], [31], [32], [33]. Nevertheless, the strength of association between the mRNA COVID-19 vaccines and cardiovascular complications is temporal, and any causality or underlying pathogenesis remain unclear and the subject of a pathophysiological conundrum.
5. Future perspectives
This review incorporated numerous studies reporting cardiovascular sequelae of COVID-19 mRNA vaccines. Although post-vaccination myocarditis is mild and self-resolving, other serious complications such as thrombosis can incur adverse outcomes in the patients. The aim should be close monitoring for those who present with similar symptoms after COVID-19 mRNA vaccination. Nonetheless, we cannot rule out whether mRNA vaccines were the sole cause of the cardiovascular outcomes. Future studies are necessary to confirm the true relationship and strength of association between the COVID-19 mRNA vaccines and cardiovascular complications reported in this review. Further research is warranted on the extent to which a SARS-CoV-2 infection can itself elicit adverse downstream cardiovascular effects [18], [19], [20], [21], [22], [23], [25], [26], [27], [28]. While the race towards the development of novel, all-encompassing vaccinations runs astride the pharmaceutical giants, it is pivotal to factor in the ramifications that these vaccines can elicit. Nevertheless, the true challenge arises from a concoction of the paucity of data that exists, and the inability to identify the true etiologies of these cardiovascular outcomes in the aforementioned patient cohorts.
6. Conclusion
This review highlights various cardiovascular complications associated with COVID-19 mRNA vaccines in order to emphasize that early recognition of similar presentations can lead to prompt treatment and improved patient outcomes. While the severity of these cases cannot be understated, the present review reinforces that the benefits and effectiveness of the COVID-19 mRNA vaccines trump their risk of the aforementioned sequelae.
Funding
This research received no external funding.
Institutional review board statement
Not applicable.
CRediT authorship contribution statement
T.A.: Conceptualization, Validation, Writing – original draft, Writing – review & editing, Supervision, Project administration. H.UHV: Conceptualization. S.R.: Methodology, Writing – original draft. J.M.: Methodology, Visualization. E.M.: Methodology. T.K.: Methodology, Software, Validation, Writing – original draft. A.A: Software, Formal analysis. N.A.: Software, Formal analysis, Writing – original draft. V.RN: Software, Validation. A.H.A: Validation. : Validation. R.A.: Validation. A.R.:M.A. Formal analysis, Writing – review and editing. D.R.N.: Formal analysis. V.G.: Writing – original draft. E. A.: Writing – original draft. A.S.: Writing – review & editing. Y.S.: Visualization, Supervision. J.G.: Visualization. M.C.A.: Supervision. J.L., X.X. and Y.Y. All authors have read and agreed to the published version of the manuscript.”
Contributions made my newly added authors
Varman Gunasaegaram: Helped in drafting major proportions of the manuscript, re-creating the tables included, conducting literature search, revising the manuscript critically for intellectual content. Enaam Alzadjali: Created the newly included graphical abstract, provided the software required for doing so, helped re-draft the manuscript extensively, and helped in reviewing and editing the paper in line with reviewer comments. Arthi Subramanian: Re-drafted the manuscript, helped in graphical abstract curation, helped in redrafting the tables included, and critically reviewed and edited the final revised draft. Abida Rahman: redrafted the revised manuscript, addressed reviewer comments, created figure and table legends, and revised the original draft critically for intellectual content.
Acknowledgments
Not applicable.
Conflicts of interest statement
The authors declare no conflict of interest.
Data Availability
No data was used for the research described in the article.
References
- 1.Ritchie H., Mathieu E., Rodés-Guirao L., 2021. et al. Coronavirus (COVID-19) vaccinations - statistics and research. Our World in Data. 〈https://ourworldindata.org/covid-vaccinations〉. Published March 5, 2020. Accessed 4 December 2021.
- 2.Hill E.M., Keeling M.J. Comparison between one and two dose SARS-CoV-2 vaccine prioritization for a fixed number of vaccine doses. J. R. Soc. Interface. 2021;18(182):20210214. doi: 10.1098/rsif.2021.0214. doi: 10.1098/rsif.2021.0214. Epub 2021 Sep 1. PMID: 34465208; PMCID: PMC8437233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Stowe J., Tessier E., Groves N., Dabrera G., Myers R., Campbell C.N.J., Amirthalingam G., Edmunds M., Zambon M., Brown K.E., Hopkins S., Chand M., Ramsay M. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N. Engl. J. Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. doi: 10.1056/NEJMoa2108891. Epub 2021 Jul 21. PMID: 34289274; PMCID: PMC8314739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckart R.E., Love S.S., Atwood J.E., Arness M.K., Cassimatis D.C., Campbell C.L., Boyd S.Y., Murphy J.G., Swerdlow D.L., Collins L.C., Riddle J.R., Tornberg D.N., Grabenstein J.D., Engler R.J. Department of defense smallpox vaccination clinical evaluation team. incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J. Am. Coll. Cardiol. 2004;44(1):201–205. doi: 10.1016/j.jacc.2004.05.004. doi: 10.1016/j.jacc.2004.05.004. PMID: 15234435. [DOI] [PubMed] [Google Scholar]
- 5.Myocarditis and pericarditis after mrna COVID-19 vaccination. Centers for Disease Control and Prevention. 〈https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html〉. Accessed 4 December 2021.
- 6.Fact check-graph showing increase in myo/pericarditis after COVID-19 vaccine rollout does not confirm link. Reuters. 〈https://www.reuters.com/article/factcheck-coronavirus-usa-idUSL1N2SA1EN〉. Published November 19, 2021. Accessed 4 December 2021.
- 7.Perez Y., Levy E.R., Joshi A.Y., Virk A., Rodriguez-Porcel M., Johnson M., Roellinger D., Vanichkachorn G., Huskins W.C., Swift M.D. Myocarditis following COVID-19 mRNA vaccine: a case series and incidence rate determination. Clin. Infect. Dis. 2021:ciab926. doi: 10.1093/cid/ciab926. doi: 10.1093/cid/ciab926. Epub ahead of print. PMID: 34734240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boehmer T.K., Kompaniyets L., Lavery A.M., Hsu J., Ko J.Y., Yusuf H., Romano S.D., Gundlapalli A.V., Oster M.E., Harris A.M. Association between COVID-19 and myocarditis using hospital-based administrative data - United States, March 2020-January 2021. MMWR Morb. Mortal. Wkly Rep. 2021;70(35):1228–1232. doi: 10.15585/mmwr.mm7035e5. doi: 10.15585/mmwr.mm7035e5. PMID: 34473684; PMCID: PMC8422872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aye Y.N., Mai A.S., Zhang A., Lim O.Z.H., Lin N., Ng C.H., Chan M.Y., Yip J., Loh P.H., Chew N.W.S. Acute myocardial infarction and myocarditis following COVID-19 vaccination. QJM. 2021:hcab252. doi: 10.1093/qjmed/hcab252. doi: 10.1093/qjmed/hcab252. Epub ahead of print. PMID: 34586408; PMCID: PMC8522388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sung J.G., Sobieszczyk P.S., Bhatt D.L. Acute myocardial infarction within 24 h after COVID-19 vaccination. Am. J. Cardiol. 2021;156:129–131. doi: 10.1016/j.amjcard.2021.06.047. doi: 10.1016/j.amjcard.2021.06.047. Epub 2021 Jul 12. PMID: 34364657; PMCID: PMC8272970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kounis N.G., Koniari I., Mplani V., Kouni S.N., Plotas P., Tsigkas G. Acute myocardial infarction within 24 h after COVID-19 vaccination: is kounis syndrome the culprit? Am. J. Cardiol. 2021;S0002–9149(21):00956-5–5–5. doi: 10.1016/j.amjcard.2021.09.032. doi: 10.1016/j.amjcard.2021.09.032. Epub ahead of print. PMID: 34702550; PMCID: PMC8541841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azevedo R.B., Botelho B.G., Hollanda J.V.G., Ferreira L.V.L., Junqueira de Andrade L.Z., Oei S.S.M.L., Mello T.S., Muxfeldt E.S. Covid-19 and the cardiovascular system: a comprehensive review. J. Hum. Hypertens. 2021;35(1):4–11. doi: 10.1038/s41371-020-0387-4. doi: 10.1038/s41371-020-0387-4. Epub 2020 Jul 27. PMID: 32719447; PMCID: PMC7384729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salamanna F., Maglio M., Landini M.P., Fini M. Body localization of ACE-2: on the trail of the keyhole of SARS-CoV-2. Front. Med. 2020;7 doi: 10.3389/fmed.2020.594495. doi: 10.3389/fmed.2020.594495. PMID: 33344479; PMCID: PMC7744810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Jr., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. doi: 10.1056/NEJMoa2034577. Epub 2020 Dec 10. PMID: 33301246; PMCID: PMC7745181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinagra G., Merlo M., Porcari A. Exploring the possible link between myocarditis and mRNA COVID-19 vaccines. Eur. J. Intern. Med. 2021;92:28–30. doi: 10.1016/j.ejim.2021.08.018. doi: 10.1016/j.ejim.2021.08.018. Epub 2021 Aug 28. PMID: 34518081; PMCID: PMC8397534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biteker M. Current understanding of Kounis syndrome. Expert Rev. Clin. Immunol. 2010;6(5):777–788. doi: 10.1586/eci.10.47. doi: 10.1586/eci.10.47. PMID: 20828286. [DOI] [PubMed] [Google Scholar]
- 17.Kounis N.G. Coronary hypersensitivity disorder: the kounis syndrome. Clin. Ther. 2013;35(5):563–571. doi: 10.1016/j.clinthera.2013.02.022. doi: 10.1016/j.clinthera.2013.02.022. Epub 2013 Mar 13. PMID: 23490289. [DOI] [PubMed] [Google Scholar]
- 18.Tajstra M., Jaroszewicz J., Gąsior M. Acute coronary tree thrombosis after vaccination for COVID-19. JACC Cardiovasc Interv. 2021;14(9):e103–e104. doi: 10.1016/j.jcin.2021.03.003. doi: 10.1016/j.jcin.2021.03.003. PMID: 33958175; PMCID: PMC8092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fearon C., Parwani P., Gow-Lee B., Abramov D. Takotsubo syndrome after receiving the COVID-19 vaccine. J. Cardiol. Cases. 2021;24(5):223–226. doi: 10.1016/j.jccase.2021.08.012. doi: 10.1016/j.jccase.2021.08.012. Epub 2021 Sep 15. PMID: 34539938; PMCID: PMC8440167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sattar Y., Siew K.S.W., Connerney M., Ullah W., Alraies M.C. Management of Takotsubo syndrome: a comprehensive review. Cureus. 2020;12(1) doi: 10.7759/cureus.6556. doi: 10.7759/cureus.6556. PMID: 32042529; PMCID: PMC6996473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almas T., Khedro T., Haadi A., Ahmed R., Alshaikh L., Al-Awaid A.H., Panhwar M.S., Hassan Virk H.U. COVID-19-induced takotsubo cardiomyopathy: venturing beyond the obvious. Ann. Med Surg. 2021;65 doi: 10.1016/j.amsu.2021.102291. doi: 10.1016/j.amsu.2021.102291. PMID: 33981423; PMCID: PMC8082200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe M., Balena A., Tuccinardi D., Tozzi R., Risi R., Masi D., Caputi A., Rossetti R., Spoltore M.E., Filippi V., Gangitano E., Manfrini S., Mariani S., Lubrano C., Lenzi A., Mastroianni C., Gnessi L. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab. Res. Rev. 2021 doi: 10.1002/dmrr.3465. doi: 10.1002/dmrr.3465. Epub ahead of print. PMID: 33955644; PMCID: PMC8209952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nomura Y., Sawahata M., Nakamura Y., Kurihara M., Koike R., Katsube O., Hagiwara K., Niho S., Masuda N., Tanaka T., Sugiyama K. Age and smoking predict antibody titres at 3 months after the second dose of the BNT162b2 COVID-19 vaccine. Vaccine. 2021;9(9):1042. doi: 10.3390/vaccines9091042. doi: 10.3390/vaccines9091042. PMID: 34579279; PMCID: PMC8472889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Istampoulouoglou I., Dimitriou G., Späni S., Christ A., Zimmermanns B., Koechlin S., Stoeckmann O., Winterhalder C., Marono D., Toma V., Leuppi-Taegtmeyer A.B. Myocarditis and pericarditis in association with COVID-19 mRNA-vaccination: cases from a regional pharmacovigilance centre. Glob. Cardiol. Sci. Pract. 2021;2021(3) doi: 10.21542/gcsp.2021.18. doi: 10.21542/gcsp.2021.18. PMID: 34805376; PMCID: PMC8587334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montgomery J., Ryan M., Engler R., Hoffman D., McClenathan B., Collins L., Loran D., Hrncir D., Herring K., Platzer M., Adams N., Sanou A., Cooper L.T., Jr. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US Military. JAMA Cardiol. 2021;6(10):1202–1206. doi: 10.1001/jamacardio.2021.2833. doi: 10.1001/jamacardio.2021.2833. PMID: 34185045; PMCID: PMC8243257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H.W., Jenista E.R., Wendell D.C., Azevedo C.F., Campbell M.J., Darty S.N., Parker M.A., Kim R.J. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021;6(10):1196–1201. doi: 10.1001/jamacardio.2021.2828. doi: 10.1001/jamacardio.2021.2828. PMID: 34185046; PMCID: PMC8243258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz G.A., Parsons G.T., Gering S.K., Meier A.R., Hutchinson I.V., Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326(12):1210–1212. doi: 10.1001/jama.2021.13443. doi: 10.1001/jama.2021.13443. PMID: 34347001; PMCID: PMC8340007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace M., Oliver S. , 2021. Covid-19 mrna vaccines in adolescents and young adults. CDC. 〈https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021–06/05-COVID-Wallace-508.pdf?ftag=MSF0951a18〉. Published June 23, 2021. Accessed 4 December 2021.
- 29.Greinacher A., Thiele T., Warkentin T., Weisser K., Kyrle P., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wise J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ. 2021:n699. doi: 10.1136/bmj.n699. [DOI] [PubMed] [Google Scholar]
- 31.Boivin Z., Martin J. Untimely myocardial infarction or COVID-19 vaccine side effect. Cureus. 2021;13(3) doi: 10.7759/cureus.13651. doi: 10.7759/cureus.13651. PMID: 33824804; PMCID: PMC8012173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segal Y., Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell. Mol. Immunol. 2018;15(6):586–594. doi: 10.1038/cmi.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh K., Marinelli T., Horowitz J.D. Takotsubo cardiomyopathy after anti-influenza vaccination: catecholaminergic effects of immune system. Am. J. Emerg. Med. 2013;31(11):1627. doi: 10.1016/j.ajem.2013.06.039. e1-4. doi: 10.1016/j.ajem.2013.06.039. Epub 2013 Jul 26. PMID: 23891597. [DOI] [PubMed] [Google Scholar]
- 34.Schmitt P., Demoulin R., Poyet R., Capilla E., Rohel G., Pons F., Jégo C., Sidibe S., Druelle A., Brocq F.X., Dutasta F., Cellarier G.R. Acute myocarditis after COVID-19 vaccination: a case report. Rev. Med. Interne. 2021;42(11):797–800. doi: 10.1016/j.revmed.2021.10.003. doi: 10.1016/j.revmed.2021.10.003. Epub 2021 Oct 19. PMID: 34740463; PMCID: PMC8523482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.