Abstract
COVID-19 negatively impacts several organs and systems weeks or months after initial diagnosis. Skeletal muscle can be affected, leading to fatigue, lower mobility, weakness, and poor physical performance. Older adults are at increased risk of developing musculoskeletal symptoms during long COVID. Systemic inflammation, physical inactivity, and poor nutritional status are some of the mechanisms leading to muscle dysfunction in individuals with long COVID. Current evidence suggests that long COVID negatively impacts body composition, muscle function, and quality of life. Muscle mass and function assessments can contribute toward the identification, diagnosis, and management of poor muscle health resulting from long COVID.
Keywords: COVID-19, Postacute COVID-19 syndrome, Muscle mass, Aging, Body composition, Muscle strength, Muscle function, Quality of life
Key points
-
•
Long COVID negatively impacts muscle mass, function, and quality of life.
-
•
Longitudinal studies, including long-term assessment of muscle mass and function, can help identify the impact of long COVID on muscle health.
-
•
Respiratory muscle dysfunction may be a marker of muscle wasting and recovery outcome during long COVID.
-
•
Age differences should be explored in future studies to better understand how long COVID affects muscle health across the life course.
Introduction
The World Health Organization (WHO) has recently created a clinical case definition to frame a condition of symptom persistence following a coronavirus disease 2019 (COVID-19).1 The condition, known as post-COVID-19, postacute sequelae of COVID-19, or long COVID, develops in individuals with a history of probable or confirmed SARS-CoV-2 infection in the past 3 months and encompasses a wide range of signs and symptoms that persists for weeks or months and cannot be explained by an alternative diagnosis. Long COVID symptoms can develop de novo after acute COVID-19 or persist from the initial illness. Common symptoms include, but are not limited to shortness of breath, fatigue, weakness, cognitive dysfunction, body aches, sore throat, cough, diarrhea, anosmia, and dysgeusia.2 , 3 It has been estimated that up to 80% of people who recovered from a COVID-19 episode experience at least one long-term symptom.4 , 5
Long COVID has been shown to negatively impact several organs and body systems, including skeletal muscle.6 This organ is essential for movement, balance, posture, daily activities, and a variety of metabolic functions.7 Indeed, more than 60% of individuals presenting with long COVID have reported fatigue, lower mobility, and weakness.8 , 9 Interestingly, a high prevalence of skeletal muscle weakness and low physical performance has been reported in COVID-19 survivors without prior musculoskeletal problems.10 Older adults are at increased risk of developing musculoskeletal symptoms during long COVID,11 , 12 possibly because of the combined effect of viral infection and preexisting age-related declines in muscle mass and function.
The purpose of this narrative review is to describe the potential long-term effects of COVID-19 on muscle health in adults. We used the term muscle health to describe muscle mass and function (ie, strength and performance). Here, we describe muscle health outcomes in people with long COVID presenting with different degrees of disease severity and assessed by different body composition and physical function methods. In addition, we report the impact of long COVID on quality of life (QoL) related to muscle health.
Mechanisms of muscle damage in long COVID
After entering the human body, the spike protein of SARS-CoV-2 binds to the cell membrane receptor angiotensin converter enzyme 2 (ACE2) using the transmembrane protease, serine 2 (TMPRSS2) to deliver its genetic material.13 , 14 Upon cellular entry, the virus replicates and causes disruption of cellular functions, leading to cell death and tissue dysfunction.15 Because ACE2 and TMPRSS2 are expressed in most tissues and organs, SARS-CoV-2 can invade and cause damage to almost all body systems, including the skeletal muscle.16 In addition to direct virus-mediated injury, other factors contributing to muscle damage during a COVID-19 episode include systemic inflammation, electrolyte disturbances, critical ill myopathy, drugs (eg, corticosteroids), and hypoxia.16 Some of these mechanisms and factors are likely to play a role in musculoskeletal damage and its related outcomes in long COVID.
Inflammation is advocated as one of the primary factors associated with muscle catabolism in patients with long COVID.10 Systemic inflammation sustained by increased blood levels of interferon gamma, C-reactive protein, interleukin (IL) 6, IL-2, IL-10, and tumor necrosis factor α has been described in people with long COVID.17 , 18 These proinflammatory cytokines are well known for their ability to negatively impact muscle protein metabolism through triggering catabolic pathways and suppressing anabolism.19 The mechanisms underlying the transition from acute to chronic inflammation after a COVID-19 episode are largely unknown. Anomalous microclots enriched in acute-phase inflammatory molecules and resistant to fibrinolysis have been found in blood samples of individuals with long COVID.20 Hence, it is plausible that inflammatory cytokines trapped within microclots may leak into the circulation, thereby maintaining a state of chronic inflammation. SARS-CoV-2 infection was also shown to cause long-term proinflammatory reprogramming of macrophages, possibly via epigenetic modifications.21 In older COVID-19 survivors, SARS-CoV-2–induced long-term inflammation may superimpose to the age-dependent chronic inflammation (inflamm-aging), leading to more severe disruption of muscle metabolic homeostasis.5 Over time, proinflammatory cytokines lead to muscle fiber proteolysis, decreased protein synthesis, hindered capacity of satellite cells to proliferate and differentiate, and eventually fibrosis.15
Muscular issues in long COVID are more likely to occur in patients with more severe diseases who had been admitted to the intensive care unit (ICU).22 , 23 However, Doykov and colleagues24 observed an increase in proinflammatory biomarkers and disruption of muscle metabolic homeostasis 40 to 60 days after initial diagnosis in individuals who had mild and asymptomatic COVID-19.
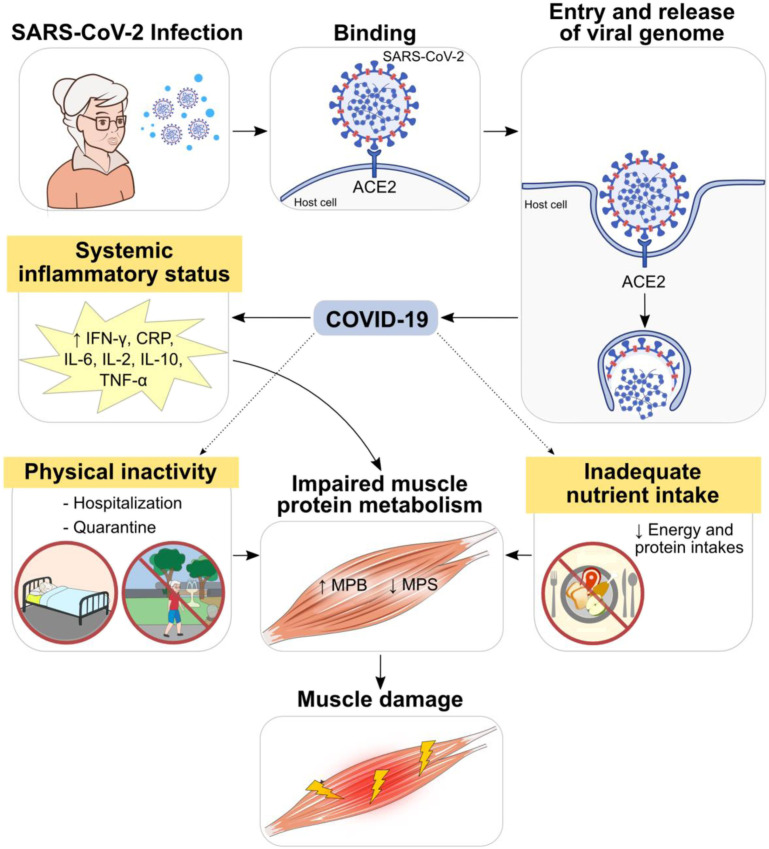
Physical inactivity potentially exacerbated by quarantine and hospitalization has also a major impact on muscle mass and function.6 Lastly, inadequate dietary intake and poor nutritional status, which are common during a COVID-19 episode, negatively impact skeletal muscle during recovery.25 , 26 Bedock and colleagues27 observed that ∼42% of hospitalized patients with COVID-19 were malnourished and the prevalence increased to ∼67% in those admitted to ICU. It has been hypothesized that reduced food intake caused by COVID-19 symptoms (ie, anorexia, diarrhea, vomiting, nausea, abdominal pain, anosmia, and dysgeusia) and increased nutritional needs are main factors leading to malnutrition.25 , 27 , 28 Fig. 1 illustrates some of the mechanisms associated with muscle damage in individuals with long COVID.
Fig. 1.
Potential mechanisms of muscle damage in patients with long COVID-19. Systemic inflammatory state during the acute phase results in chronic release of proinflammatory cytokines, contributing to imbalances in muscle protein metabolism and impaired muscle health. In addition, physical inactivity due to hospitalization and quarantine as well as inadequate nutrition intake may also negatively impact muscle mass, quality, and function.
(From Servier Medical Art. Servier. Available at https://smart.servier.com/; under Creative Commons Attribution 3.0 Unported License)
Current evidence
A summary of findings specifically related to body composition, muscle function, and QoL related to muscle health is shown in Table 1 .
Table 1.
Summary of findings related to body composition, muscle function, and quality of life related to muscle health in individuals with long COVID
| Body Composition | ||||
|---|---|---|---|---|
| Reference | Body Compartment | Technique | Timeline | Selected Main Findings |
| Tanriverdi et al,32 2021 | FM and FFM | BIA | >3 mo after COVID-19 |
|
| van den Borst et al,29 2021 | FFMI | BIA | 3 mo after COVID-19 |
|
| van Gassel et al,30 2021 | SMA, SMD, and IMAT | CT | 3 mo after hospital discharge |
|
| Farr et al,31 2021 | Diaphragm muscle thickness and thickening ratio | Ultrasound | Admitted to a rehabilitation after COVID-19 |
|
| Muscle Function | ||||
|---|---|---|---|---|
| Reference | Muscle Function Evaluation | Assessment Test | Timeline | Selected Main Findings |
| van Gassel et al,30 2021 | Muscle strength and physical performance | Handgrip strength | 3 mo after COVID-19 |
|
| Tanriverdi et al,32 2021 | Muscle strength and physical performance | Handgrip and quadriceps muscle strength, and 4-m gait speed |
>3 mo after COVID-19 |
|
| van den Borst et al,29 2021 | Physical performance | 6MWD | 3 mo after COVID-19 |
|
| Mittal et al,33 2021 | Muscle strength | Handgrip strength | Not specified. Average: 3 mo |
|
| Bellan et al,34 2021 | Physical performance | SPPB and 2MWT |
4- and 12-mo postdischarge |
|
| Quality of Life | |||
|---|---|---|---|
| Reference | QOL Test | Timeline | Selected Main Findings |
| van den Borst et al,29 2021 | Nijmegen Clinical Screening Instrument | 3 mo after COVID-19 |
|
| van Gassel et al,30 2021 | EQ-5D | 3 mo after COVID-19 |
|
| Cuerda et al,35 2021 | EQ-5D-5 L | Ongoing study: patients are being followed for 12 mo |
|
| Vaes et al,36 2021 | EQ-5D-5 L | 3 and 6 mo after COVID-19 |
|
Abbreviations: 6MWD, 6-minute walking distance; 2MWT, 2-minute walk test; BIA, bioelectrical impedance analysis; COVID-19, coronavirus disease, 2019; CT, computerized tomography; EQ-5D, European Quality of Life Five Dimension; EQ-5D-5L, 5-level European Quality of Life Five Dimension; FFMI, fat-free mass index; FFM, fat-free mass; FM, fat mass; IMAT, intermuscular adipose tissue; QoL, quality of life; SMA, skeletal muscle area; SMD, skeletal muscle radiodensity; SPPB, short physical performance battery.
Muscle Mass and Other Body Composition Compartments
Body composition was assessed in 4 studies investigating long-term health sequelae of COVID-19 infection29, 30, 31, 32 using the following techniques: bioelectrical impedance analysis (BIA),29 , 32 computed tomography (CT),30 and ultrasound (US).31
After 4 to 5 weeks of COVID-19 infection, Tanriverdi and colleagues32 assessed fat mass and fat-free mass (FFM) using BIA in people who recovered from mild (n = 25) and moderate (n = 23) disease severity. The 2 body compartments were not different between individuals who suffered mild or moderate disease, although a sex-specific comparison was not presented.32 Another study assessed the body composition of patients discharged after COVID-19 using BIA.29 Patients were classified according to disease severity (mild: n = 27; moderate: n = 51; severe: n = 26; critical: n = 20).29 Fat-free mass index (FFMI) was calculated as FFM/height2 and classified as low in 19% of the patients (7/27; 5/51; 7/26; 4/20).29 No differences in FFMI or in the number of patients with low FFMI were observed among the 4 groups.29
In a prospective cohort study, 46 patients who were admitted to the ICU and received mechanical ventilation were assessed 3 months after hospital discharge.30 Thoracic CT scans at the 12th vertebra were used to quantify skeletal muscle area, skeletal muscle radiation attenuation (an index of muscle quality), and intermuscular adipose tissue (IMAT).30 Patients were categorized based on their performance on the 6-min walk distance (6MWD) test as having normal (n = 24) or low physical performance (<80% of predicted, n = 22).30 Both skeletal muscle area and skeletal muscle radiation did not differ between patient subgroups; however, IMAT was higher in those with low physical performance.30 Physical performance remained significantly associated with IMAT after adjusting for age, sex, handgrip strength, and diffusing capacity for carbon monoxide.30
US images of the diaphragm muscle were assessed in 21 patients admitted to rehabilitation after severe COVID-19 and compared with 11 non–COVID-19 controls who needed ventilator support during hospitalization.31 Diaphragm muscle thickness was not different between cases and controls, but the thickening ratio (ie, maximal inspiration/end-expiration) was reduced in patients who had been diagnosed with COVID-19, suggesting reduced diaphragm function.31
Muscle Function
Muscle strength
Muscle strength was evaluated by handgrip strength testing in 3 studies assessing the health impact in individuals diagnosed with long COVID.30 , 32 , 33 In a prospective cohort study, handgrip strength was assessed in 46 COVID-19 survivors 3 months after ICU discharge.30 Individuals who tested lower than predicted on the 6MWD test showed a trend for lower handgrip strength than those with a normal 6MWD test result.30
Mittal and colleagues33 compared handgrip strength of 52 patients with type 2 diabetes (T2D) who had mild to moderate COVID-19 and T2D patients who did not have COVID-19 (n = 56). Although no difference in strength was found between groups, handgrip strength was significantly reduced when patients with COVID-19 were categorized into high and low fatigue scores.33 These findings indicate that neither patient group had significant dynapenia, but patients with T2D who had COVID-19 had higher fatigue and lower muscle strength than those who did not have COVID-19.
Handgrip strength and quadriceps muscle strength were assessed in a cross-sectional study including patients recovering from mild (n = 25) and moderate (n = 23) interstitial pneumonia after at least 12 weeks from COVID-19 diagnosis.32 The prevalence of quadriceps and handgrip weakness was not different between groups (mild: 35%; moderate: 43.5%; P = .597).32
Physical performance
The prevalence of impaired physical performance in long COVID was evaluated in 4 studies including participants with different degrees of disease severity.29 , 30 , 32 , 34 Direct measures of physical performance included walking tests (ie, 6MWD, 4-m gait speed test, and 2-min walk test)29 , 30 , 32 and the short physical performance battery (SPPB).34
In a prospective observational study of mild to critical COVID-19 cases (n = 124), 22% of patients presented with low performance on the 6MWD test (ie, <80% of predicted) at 10 weeks after hospital discharge.29 Although the prevalence of low 6MWD performance was numerically greater in individuals with moderate (28%) and severe (32%) compared with mild (12%) and critical (5%) disease, no statistical difference was found among groups, possibly due to the small sample size.29 Using the same criteria to define impaired physical performance, another study observed that 48% of patients who survived critical COVID-19 had low performance on the 6MWD test at 3 months after hospital discharge.30 Notably, the greater proportion of functional impairment observed in the latter study was likely driven by patients who required mechanical ventilation during ICU stay. Tanrivedi and colleagues32 compared physical performance between groups of disease severity, and found that survivors of mild (n = 25) and moderate (n = 23) COVID-19 had similar 4-m gait speed.
The longest study evaluating physical performance in long COVID assessed 238 and 198 individuals after 4 and 12 months postdischarge, respectively.34 Using a cutoff of 10 on the SPPB, low physical performance was found in 22.3% and 18.7% of patients at 4 and 12 months of follow-up, respectively. Highly functioning individuals (ie, those who scored 10 or more on the SPPB) also performed a 2-min walk test at both time points; 31.5% and 7.1% of patients had a poor physical function at 4 months and 12 months follow-up, respectively. Furthermore, the proportion of patients with low performance on either the SPPB or the 2-min walk test) was smaller at 12 months (25.8%) than at 4 months (52.8%).34 This finding suggests that although some patients improved their physical function as they recovered, a significant proportion of individuals was still experiencing detrimental effects of COVID-19 after 12 months of hospital discharge.
Quality of Life Related to Muscle Health
QoL is related to several factors, and not only muscle health or physical performance. However, in this article, we focused only on studies assessing QoL related to muscle health. QoL of patients diagnosed with long COVID has been reported in 4 studies that assessed muscle function.29 , 30 , 35 , 36 Two studies used the Euro-QoL-5D (EQ-5D) questionnaire.30 , 36 This instrument evaluates 5 dimensions of a person’s QoL (ie, mobility, self-care, daily activities, pain, and anxiety/depression).30 , 35 Van Gassel and colleagues30 assessed the QoL of 46 patients 3 months after hospital discharge using the EQ-5D. Health-related QoL was lower in people with low performance on the 6MWD test (n = 22) compared with those presenting with a normal test result (n = 24). The second study is an ongoing multicenter observational study including 176 COVID-19 survivors.35 Preliminary results showed that 71.2% of participants were unable to move or presented with moderate impairment and 75.5% reported problems to perform daily activities.35
QoL of 239 patients with long COVID was assessed 3 and 6 months after the onset of COVID-19-related symptoms using the 5-level EuroQol-5 Dimensions version (EQ-5D-5 L).36 This questionnaire is similar to the EQ-5D but with higher sensitivity36; 61.9% of the patients reported they were receiving physiotherapy and 11.7% rehabilitation between 3 and 6 months of follow-up. However, 62% of the patients still presented with moderate-to-extreme problems performing daily activities, and 49% experienced moderate-to-severe pain/discomfort.36
Lastly, QoL was assessed using the Nijmegen Clinical Screening Instrument in 124 patients with symptoms persisting for more than 6 weeks who attended a COVID-19 aftercare facility.29 Patients were divided into 4 groups (mild: n = 27; moderate: n = 51; severe: n = 26; and critical: n = 20). Functional impairment was observed in 64% of the patients, fatigue in 69%, and reduced QoL in 72%.29
Discussion
Long COVID has recently only been recognized; therefore, available evidence on the impact of this condition on muscle health is limited. Overall, studies suggest that long COVID negatively impacts body composition, muscle function, and QoL.
Body composition in individuals with long COVID was not found to differ based on disease severity.29 , 30 , 32 However, this finding may be attributed to limitations of the body composition techniques used and small sample size, among others. Furthermore, the lack of body composition assessments during the acute phase and at hospital discharge hinders our understanding of whether people did experience changes in body composition, and at which disease phase. In one study, IMAT was found to be greater in patients with low physical performance.30 This measurement is an estimate of skeletal muscle “quality” and has been associated with COVID-19 severity37 and physical function after recovery.38 Notably, long COVID was associated with a reduction of the thickening ratio of the diaphragm muscle, which can be related to diaphragm dysfunction.31 This is an interesting marker in COVID-19 survivors, as it relates to fatigue after the acute phase.39 In fact, 4 ongoing clinical trials are investigating how abnormal diaphragm muscle thickening ratio, which is related to muscle contractility, can impact QoL of individuals with long COVID.40, 41, 42, 43
Muscle function of people with long COVID was shown to improve over 12 months.34 The negative effects of long COVID on muscle function were obvious after 4 months and 12 months posthospital discharge,32 , 34 although one study showed improvements at 1-year follow-up.34 Inconsistency between the studies might be related to the degree of disease severity, age, and the presence of comorbidities.44 , 45 Similarly to the findings observed by Huan and colleagues,9 some studies hereby included reported worse physical performance in patients who recovered from severe COVID-19, when compared with those who had suffered a mild or moderate disease.30 , 32 , 33 Notably, the presence of comorbidities also plays a critical role in long COVID. Cox and colleagues46 observed that critically ill patients with low muscle mass who were recovering from sepsis experienced worse physical function 6 months after hospital discharge, when compared with critically ill patients with normal muscle mass. The presence of other comorbidities, such as obesity and pulmonary disease, has also been associated with symptoms of long COVID, including poor muscle function,47 and is therefore an important variable to assess in future studies investigating long COVID.
Long COVID negatively affects QoL,29 , 30 , 35 , 36 especially in individuals with impaired physical performance.30 Some individuals still reported fatigue and difficulties in performing daily activities 6 months after hospital discharge and rehabilitation.29 , 36 According to Rios and colleagues48 and Rives-Lange,49 malnutrition is a possible contributor to low QoL during long COVID. The relationship between QoL and nutritional status has been previously investigated in other conditions and is associated with impaired functional status and delayed recovery.50 However, reduced QoL is not entirely explained by reduced physical performance, QoL also depends on mental and cognitive factors.51
Although older adults are at greater risk for the detrimental effects of long COVID on muscle health, little is known regarding age-related differences, and whether the sequelae are worse in older compared with younger adults. Age differences should be explored in future studies to better understand how long COVID affects muscle health across the lifespan.
Finally, despite the limited literature, long COVID directly or indirectly impacts muscle health. Muscle mass and function assessments can contribute toward the identification, diagnosis, and management of poor muscle health resulting from long COVID, consequently informing the design of targeted interventions.52 Early approaches to optimize muscle health throughout disease trajectory and the recovery period are essential and should involve a multidisciplinary team of health professionals.
Summary
Despite the relatively low number of studies and the presence of methodological limitations, available evidence suggests long COVID negatively impacts muscle health and QoL. These sequelae may be amplified in older adults due to preexisting age-related declines in muscle health. Acute and long-term assessments of these parameters are needed to optimize patient care. The mechanisms by which long COVID impacts muscle health are multifactorial and involve a combination of systemic inflammation, physical inactivity, poor nutritional status, and inadequate dietary intake. Other factors such as age, comorbidities, and degree of disease severity may also contribute to negative musculoskeletal outcomes during long COVID.
Overall, the evidence to date suggests long COVID negatively impacts body composition, muscle function, and QoL. Recovery and rehabilitation services with adequate nutrition, mental and social support should be explored as potential multimodal interventions to improve muscle health of these patients.
Clinics care points
-
•
Clinicians should be aware of the prevalence and deleterious impact of poor muscle health in individuals with long COVID.
-
•
People with long COVID can present with skeletal muscle symptoms 1 year after diagnosis.
-
•
Muscle health in long COVID is related to low quality of life
Disclosure
The authors have nothing to disclose.
Footnotes
C.M. Prado has received honoraria and/or paid consultancy from Abbott Nutrition, Nutricia, Nestlé Health Science, Fresenius Kabi, and Pfizer. The other authors declare that they have no known conflicts of interest.
References
- 1.World Health Organization. (2021). A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. World Health Organization. https://apps.who.int/iris/handle/10665/345824. License: CC BY-NC-SA 3.0 IGO Available at: Accessed January 28, 2022.
- 2.Greenhalgh T., Knight M., A'Court C., et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 3.NICE Covid-19 rapid guideline: managing the long-term effects of covid-19. https://www.nice.org.uk/guidance/NG188 Available at: Accessed January 28, 2022. [PubMed]
- 4.Lopez-Leon S., Wegman-Ostrosky T., Perelman C., et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soraas A., Kalleberg K.T., Dahl J.A., et al. Persisting symptoms three to eight months after non-hospitalized COVID-19, a prospective cohort study. PLoS One. 2021;16(8):e0256142. doi: 10.1371/journal.pone.0256142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piotrowicz K., Gasowski J., Michel J.P., et al. Post-COVID-19 acute sarcopenia: physiopathology and management. Aging Clin Exp Res. 2021;33(10):2887–2898. doi: 10.1007/s40520-021-01942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tieland M., Trouwborst I., Clark B.C. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. 2018;9(1):3–19. doi: 10.1002/jcsm.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karaarslan F., Guneri F.D., Kardes S. Long COVID: rheumatologic/musculoskeletal symptoms in hospitalized COVID-19 survivors at 3 and 6 months. Clin Rheumatol. 2022;41(1):289–296. doi: 10.1007/s10067-021-05942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akbarialiabad H., Taghrir M.H., Abdollahi A., et al. Long COVID, a comprehensive systematic scoping review. Infection. 2021;49(6):1163–1186. doi: 10.1007/s15010-021-01666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Docherty A.B., Harrison E.M., Green C.A., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welch C., Greig C., Masud T., et al. COVID-19 and acute sarcopenia. Aging Dis. 2020;11(6):1345–1351. doi: 10.14336/AD.2020.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benton D.J., Wrobel A.G., Xu P., et al. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature. 2020;588(7837):327–330. doi: 10.1038/s41586-020-2772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease Inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Disser N.P., De Micheli A.J., Schonk M.M., et al. Musculoskeletal consequences of COVID-19. J Bone Joint Surg Am. 2020;102(14):1197–1204. doi: 10.2106/JBJS.20.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finsterer J., Scorza F.A. SARS-CoV-2 myopathy. J Med Virol. 2021;93(4):1852–1853. doi: 10.1002/jmv.26550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malik P., Patel K., Pinto C., et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-A systematic review and meta-analysis. J Med Virol. 2022;94(1):253–262. doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., Li S., Liu J., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webster J.M., Kempen L.J.A.P., Hardy R.S., et al. Inflammation and skeletal muscle wasting during cachexia. Front Physiol. 2020;11:597675. doi: 10.3389/fphys.2020.597675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pretorius E., Vlok M., Venter C., et al. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. 2021;20(1):172. doi: 10.1186/s12933-021-01359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theobald S.J., Simonis A., Georgomanolis T., et al. Long-lived macrophage reprogramming drives spike protein-mediated inflammasome activation in COVID-19. EMBO Mol Med. 2021;13(8):e14150. doi: 10.15252/emmm.202114150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schefold J.C., Wollersheim T., Grunow J.J., et al. Muscular weakness and muscle wasting in the critically ill. J Cachexia Sarcopenia Muscle. 2020;11(6):1399–1412. doi: 10.1002/jcsm.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soares M.N., Eggelbusch M., Naddaf E., et al. Skeletal muscle alterations in patients with acute Covid-19 and post-acute sequelae of Covid-19. J Cachexia Sarcopenia Muscle. 2022;13(1):11–22. doi: 10.1002/jcsm.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doykov I., Hällqvist J., Gilmour K.C., et al. The long tail of Covid-19' - the detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Res. 2020;9:1349. doi: 10.12688/f1000research.27287.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barazzoni R., Bischoff S.C., Breda J., et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr (Edinburgh, Scotland) 2020;39(6):1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerard M., Mahmutovic M., Malgras A., et al. Long-term Evolution of malnutrition and loss of muscle strength after COVID-19: a major and Neglected Component of long COVID-19. Nutrients. 2021;13(11):3964. doi: 10.3390/nu13113964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bedock D., Bel Lassen P., Mathian A., et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin Nutr ESPEN. 2020;40:214–219. doi: 10.1016/j.clnesp.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wischmeyer P.E. Nutrition therapy in sepsis. Crit Care Clin. 2018;34(1):107–125. doi: 10.1016/j.ccc.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Borst B., Peters J.B., Brink M., et al. Comprehensive health assessment 3 Months after recovery from acute Coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2021;73(5):e1089–e1098. doi: 10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Gassel R.J.J., Bels J., Remij L., et al. Functional outcomes and their association with physical performance in mechanically ventilated Coronavirus disease 2019 survivors at 3 Months following hospital discharge: a cohort study. Crit Care Med. 2021;49(10):1726–1738. doi: 10.1097/CCM.0000000000005089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farr E., Wolfe A.R., Deshmukh S., et al. Diaphragm dysfunction in severe COVID-19 as determined by neuromuscular ultrasound. Ann Clin Transl Neurol. 2021;8(8):1745–1749. doi: 10.1002/acn3.51416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanriverdi A., Savci S., Kahraman B.O., et al. Extrapulmonary features of post-COVID-19 patients: muscle function, physical activity, mood, and sleep quality. Ir J Med Sci. 2021:1–7. doi: 10.1007/s11845-021-02667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittal J., Ghosh A., Bhatt S.P., et al. High prevalence of post COVID-19 fatigue in patients with type 2 diabetes: a case-control study. Diabetes Metab Syndr. 2021;15(6):102302. doi: 10.1016/j.dsx.2021.102302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellan M., Soddu D., Balbo P.E., et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4(1):e2036142. doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuerda C., Sanchez Lopez I., Gil Martinez C., et al. Impact of COVID-19 in nutritional and functional status of survivors admitted in intensive care units during the first outbreak. Preliminary results of the NUTRICOVID study. Clin Nutr. 2021 doi: 10.1016/j.clnu.2021.11.017. S0261-5614(21)00526-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaes A.W., Goertz Y.M.J., Van Herck M., et al. Recovery from COVID-19: a sprint or marathon? 6-month follow-up data from online long COVID-19 support group members. ERJ Open Res. 2021;7(2) doi: 10.1183/23120541.00141-2021. 00141-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viddeleer A.R., Raaphorst J., Min M., et al. Intramuscular adipose tissue at level Th12 is associated with survival in COVID-19. J Cachexia Sarcopenia Muscle. 2021;12(3):823–827. doi: 10.1002/jcsm.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Besutti G., Pellegrini M., Ottone M., et al. The impact of chest CT body composition parameters on clinical outcomes in COVID-19 patients. PLOS ONE. 2021;16(5):e0251768. doi: 10.1371/journal.pone.0251768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Z., de Vries H.J., Vlaar A.P.J., et al. Diaphragm pathology in critically ill patients with COVID-19 and postmortem findings from 3 medical Centers. JAMA Intern Med. 2021;181(1):122–124. doi: 10.1001/jamainternmed.2020.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ClinicalTrials.gov. National Library of Medicine (U.S.). Respiratory Muscles After Hospitalisation for COVID-19 (REMAP-COVID-19) .Identifier NCT04854863. Available from: https://clinicaltrials.gov/ct2/show/NCT04854863?cond=NCT04854863&draw=2&rank=1
- 41.ClinicalTrials.gov. National Library of Medicine (U.S.). Diaphragm Ultrasound Evaluation During Weaning From Mechanical Ventilation in the Positive COVID-19 Patient. Identifier NCT05019313. Available from:https://clinicaltrials.gov/ct2/show/NCT05019313?cond=NCT05019313&draw=2&rank=1
- 42.ClinicalTrials.gov. National Library of Medicine (U.S.).Pulmonary Function in Patients Recovering From COVID19 Infection : a Pilot Study (EFRUPIC). Identifier NCT05074927. Available from: https://clinicaltrials.gov/ct2/show/NCT05074927?cond=NCT05074927&draw=2&rank=1
- 43.ClinicalTrials.gov. National Library of Medicine (U.S.). Respiratory Muscle Function, Dyspnea, Exercise Capacity and Quality of Life in Severe COVID19 Patients. Identifier NCT04853940. Available from: https://clinicaltrials.gov/ct2/show/NCT04853940?cond=NCT04853940&draw=2&rank=1
- 44.Kirwan R., McCullough D., Butler T., et al. Sarcopenia during COVID-19 lockdown restrictions: long-term health effects of short-term muscle loss. GeroScience. 2020;42(6):1547–1578. doi: 10.1007/s11357-020-00272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y., Mao B., Liang S., et al. Association between age and clinical characteristics and outcomes of COVID-19. Eur Respir J. 2020;55(5):2001112. doi: 10.1183/13993003.01112-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cox M.C., Booth M., Ghita G., et al. The impact of sarcopenia and acute muscle mass loss on long-term outcomes in critically ill patients with intra-abdominal sepsis. J Cachexia Sarcopenia Muscle. 2021;12(5):1203–1213. doi: 10.1002/jcsm.12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aminian A., Bena J., Pantalone K.M., et al. Association of obesity with postacute sequelae of COVID-19. Diabetes Obes Metab. 2021;23(9):2183–2188. doi: 10.1111/dom.14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rios T.C., de Oliveira L.P.M., da Costa M.L.V., et al. A poorer nutritional status impacts quality of life in a sample population of elderly cancer patients. Health Qual Life Outcomes. 2021;19(1):90. doi: 10.1186/s12955-021-01735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rives-Lange C., Zimmer A., Merazka A., et al. Evolution of the nutritional status of COVID-19 critically-ill patients: a prospective observational study from ICU admission to three months after ICU discharge. Clin Nutr. 2021 doi: 10.1016/j.clnu.2021.05.007. S0261-5614(21)00257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norman K., Kirchner H., Lochs H., et al. Malnutrition affects quality of life in gastroenterology patients. World J Gastroenterol. 2006;12(21):3380–3385. doi: 10.3748/wjg.v12.i21.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geense W.W., de Graaf M., Vermeulen H., et al. Reduced quality of life in ICU survivors - the story behind the numbers: a mixed methods study. J Crit Care. 2021;65:36–41. doi: 10.1016/j.jcrc.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 52.Prado C.M., Anker S.D., Coats A.J.S., et al. Nutrition in the spotlight in cachexia, sarcopenia and muscle: avoiding the wildfire. J Cachexia Sarcopenia Muscle. 2021;12(1):3–8. doi: 10.1002/jcsm.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]