Coronavirus disease 2019 (COVID-19) is a viral infection caused by a strain of coronavirus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), that has afflicted over two hundred million people in a worldwide pandemic. BNT162b2 Pfizer BioNTech mRNA-SarsCoV-2 vaccines are effective in preventing COVID-19 with an excellent safety profile [1], even if cardiac adverse events have been rarely reported, especially acute myocarditis [2], [3]. Early acknowledge of other potential cardiac consequences associated with mRNA COVID-19 vaccines could help health providers to promptly identify and treat adverse events. Takotsubo Syndrome (TTS), also known as stress cardiomyopathy, is a disorder characterized by acute and transient wall motion abnormalities with left ventricular (LV) systolic (and diastolic) dysfunction, often associated with a stressful, emotional, or physical event. Particularly females, in their seventh or eighth decade of life, are more frequently affected [4]. Up to now, few cases of TTS occurred after SARS-CoV-2 vaccines administration have been reported even including different types of vaccines (mRNA-1273-SARS-CoV-2 [5], [6], [7], mRNA-BNT162b2-SarsCoV-2 [8], and ChAdOx1 nCoV-19 vaccine) [9]. The occurrence of TTS in patients affected by congenital Long QT Syndrome (LQTS) has rarely been described [10], [11], [12].
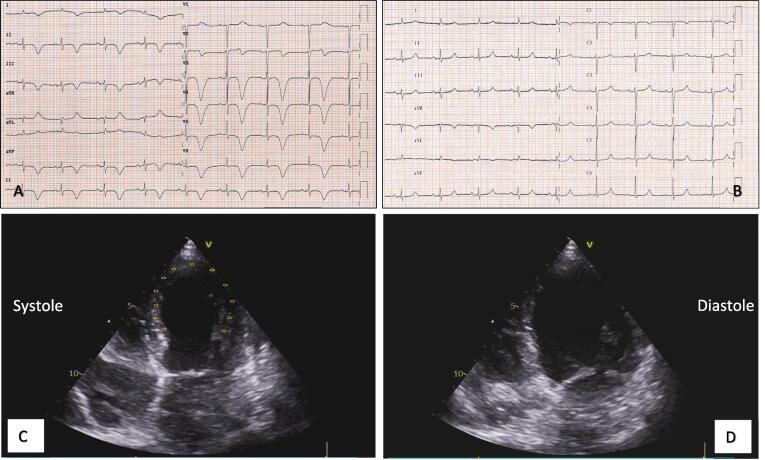
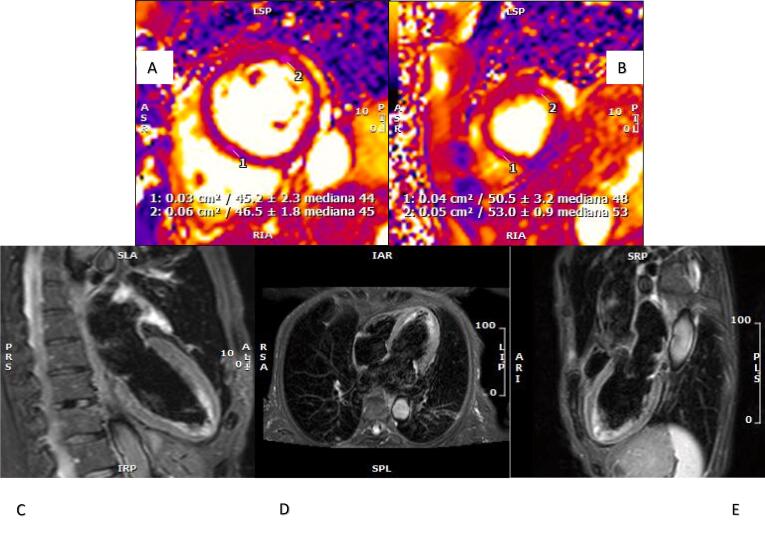
We herein report the case of a 71-year-old female who was admitted to our emergency department (ED) for self-limiting typical chest pain associated with shortness of breath that occurred almost five hours after administration of the first dose of BNT162b2 vaccine and persisted with a worsening trend for ten days. The patient’s previous history included congenital LQTS (mutation in KCHNQ 1 gene), catheter ablation for paroxysmal atrial fibrillation, and known mitral prolapse with mild mitral regurgitation and preserved left ventricular ejection fraction (LVEF) confirmed by transthoracic echocardiography (TTE) performed one month earlier. The patient had no history of SARS-CoV2 infection and had negative SARS-CoV2 serological test two months before vaccine administration. The patient denied any other recent emotional or physical stress. At hospital admission, she presented talking dyspnea, low pulse oxygen saturation (93%), and bilateral diffuse crackles, requiring oxygen support. She showed no fever (temperature 37.2 °C), while she reported intermittent temperature up to 39 °C in previous days. Laboratory data demonstrated mild elevation of high sensitivity cardiac troponin T (TnT-HS) without significant delta of variation (I determination 34 ng/L, II determination 26 ng/L, normal values (NV) < 14 ng/L) and NTproBNP elevation 2261 ng/L (NV < 200 ng/L). Other blood tests, including complete blood count, urea nitrogen, creatinine, and D-Dimer were within normal limits. C-reactive protein (CPR) was mildly elevated (3 mg/dL, NV < 0.5 mg/dL). A 12-lead electrocardiogram (ECG) was performed and showed sinus rhythm with normal atrioventricular conduction, deep and symmetric T-wave inversion in all leads except for aVL and aVF, and prolongation of corrected QT (QTc) > 600 ms (Fig. 1A). TTE revealed moderate depression of left ventricular contraction (LVEF 38%) in the presence of hypokinesia of apical and mid-distal walls consistent with the apical ballooning syndrome (Fig. 1C–D). After symptoms relieved, she underwent coronary angiography revealing non-obstructive coronary artery disease. We decided to administer the second dose of COVID-19 vaccine on day 21 after the first dose when the patient clinically recovered, and the echocardiogram revealed an improvement of the LVEF up to 50%. Daily serum TnT-HS monitoring excluded a myocardial injury after the second shot of the mRNA-BNT162b2-SarsCoV-2 vaccine. Finally, a CMR was performed confirming mild systolic dysfunction and complete recovery of mid and apical segments wall motion abnormalities. Nevertheless, signs of edema persisted as pointed out by increased signal of T2-weighted sequences and T2 map sequences. (Fig. 2A–E). At the time of discharge, the QTc interval returned to normal values (440 ms). To the best of our knowledge, this is the first case of a patient with congenital LQTS who developed TTS after receiving the BNT162b2 COVID-19 vaccination. The rare association between TTS and congenital LQTS represents a great insult to myocardial cells repolarization reserve, conferring to patients affected a high risk of life-threatening arrhythmias occurrence [13]. Pathogenetic mechanisms underlying TTS are poorly understood: enhanced sympathetic activity and an excessive catecholamine surge following an emotional or physical trigger seem to play a key role in causing coronary microvascular dysfunction and direct myocardial injury. Furthermore, TTS has been described during viral infections, including Sars-COV2 [14]. In this case, the temporal association between vaccination and the onset of symptoms suggests a close relationship between TTS and the administration of the BNT162b2 vaccine. However, the mechanisms by which COVID-19 vaccination may have induced the TTS phenomenon are not clear. We speculate that the main pathways involved are the intense inflammatory reaction following vaccine administration and an enhanced sympathovagal imbalance towards adrenergic predominance, as described in previous TTS reports after the influenza vaccine [15]. At the same time, robust evidence has demonstrated that psychological factors can impair the immune system’s response to vaccines and are implicated in the prevalence and severity of vaccine-related side effects. The stress of an ongoing pandemic and the uncertainties related to COVID-19 vaccination may therefore have contributed, in a susceptible patient, to TTS occurrence. This case also suggests that TTS should be considered in the differential diagnosis across the spectrum of myocardial injury in patients undergoing COVID19 vaccination. Careful pharmacovigilance programs remain fundamental for evaluating vaccine safety and improving patient outcomes. While the body of evidence related to the possible complications of vaccination is progressively growing, the community benefits of this prevention strategy extensively counterbalance its side effects.
Fig. 1.
(A) ECG on admission. (B) ECG one month before showing sinus rhythm normal repolarization and normal QTc (450 ms). (C–D) Transthoracic echocardiogram: apical 4-chamber views showing typical apical ballooning.
Fig. 2.
CMR. (A–B) T2 mapping sequences demonstrate longer T2 relaxation time of cardiac apex (B) expressive of myocardial edema. (C–E) T2 STIR sequences in apical four chambers, two chambers, and three chambers view showing apical edema.
Declaration of Competing Interest
The authors report no relationships that could be construed as a conflict of interest.
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Sahin U., Jansen K.U., Gruber W.C. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larson K.F., Ammirati E., Adler E.D., Cooper L.T., Hong K.N., Saponara G., Couri D., Cereda A., Procopio A., Cavalotti C., Oliva F., Sanna T., Ciconte V.A., Onyango G., Holmes D.R., Borgeson D.D. Myocarditis After BNT162b2 and mRNA-1273 Vaccination. Circulation. 2021;144(6):506–508. doi: 10.1161/circulationaha.121.055913. PMID: 34133884; PMCID: PMC8340725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ammirati E., Cavalotti C., Milazzo A., Pedrotti P., Soriano F., Schroeder J.W., Morici N., Giannattasio C., Frigerio M., Metra M., Camici P.G., Oliva F. Temporal relation between second dose BNT162b2 mRNA Covid-19 vaccine and cardiac involvement in a patient with previous SARS-COV-2 infection. Int. J. Cardiol. Heart Vasc. 2021;34 doi: 10.1016/j.ijcha.2021.100774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghadri Jelena-Rima, Wittstein Ilan Shor, Prasad Abhiram, Sharkey Scott, Dote Keigo, Akashi Yoshihiro John, Cammann Victoria Lucia, Crea Filippo, Galiuto Leonarda, Desmet Walter, Yoshida Tetsuro, Manfredini Roberto, Eitel Ingo, Kosuge Masami, Nef Holger M., Deshmukh Abhishek, Lerman Amir, Bossone Eduardo, Citro Rodolfo, Ueyama Takashi, Corrado Domenico, Kurisu Satoshi, Ruschitzka Frank, Winchester David, Lyon Alexander R., Omerovic Elmir, Bax Jeroen J., Meimoun Patrick, Tarantini Guiseppe, Rihal Charanjit, Hassan Shams Y., Migliore Federico, Horowitz John D., Shimokawa Hiroaki, Lüscher Thomas Felix, Templin Christian. International Expert Consensus Document on Takotsubo Syndrome (Part II): Diagnostic Workup, Outcome, and Management. Eur. Heart J. 2018;39(22):2047–2062. doi: 10.1093/eurheartj/ehy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jani C., Leavitt J., Al Omari O., Dimaso A., Pond K., Gannon S., Chandran A.K., Dennis C., Colgrove R. COVID-19 Vaccine-Associated Takotsubo Cardiomyopathy. Am. J. Ther. 2021;28(3):361–364. doi: 10.1097/MJT.0000000000001379. PMID: 34375049. [DOI] [PubMed] [Google Scholar]
- 6.Boscolo Berto M., Spano G., Wagner B., Bernhard B., Häner J., Huber A.T., Gräni C. Takotsubo Cardiomyopathy After mRNA COVID-19 Vaccination. Heart Lung Circulat. 2021;30(12):e119–e120. doi: 10.1016/j.hlc.2021.06.521. Epub 2021 Jul 15. PMID: 34330629; PMCID: PMC8279960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fearon C., Parwani P., Gow-Lee B., Abramov D. Takotsubo syndrome after receiving the COVID-19 vaccine. J. Cardiol. Cases. 2021;24(5):223–226. doi: 10.1016/j.jccase.2021.08.012. Epub 2021 Sep 15. PMID: 34539938; PMCID: PMC8440167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toida R., Uezono S., Komatsu H., Toida T., Imamura A., Fujimoto S., Kaikita K. Takotsubo cardiomyopathy after vaccination for coronavirus disease 2019 in a patient on maintenance hemodialysis. CEN Case Rep. 2021:1–5. doi: 10.1007/s13730-021-00657-z. Epub ahead of print. PMID: 34731486; PMCID: PMC8564792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crane P., Wong C., Mehta N., Barlis P. Takotsubo (stress) cardiomyopathy after ChAdOx1 nCoV-19 vaccination. BMJ Case Rep. 2021;14(10):e246580. doi: 10.1136/bcr-2021-246580. PMID: 34625447; PMCID: PMC8504353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grilo L.S., Pruvot E., Grobéty M., Castella V., Fellmann F., Abriel H. Takotsubo cardiomyopathy and congenital long QT syndrome in a patient with a novel duplication in the Per-Arnt-Sim (PAS) domain of hERG1. Heart Rhythm. 2010;7(2):260–265. doi: 10.1016/j.hrthm.2009.09.026. Epub 2009 Sep 24. PMID: 20004623. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki O., Nishioka T., Akima T., Tabata H., Okamoto Y., Akanuma M., Uehata A., Takase B., Katsushika S., Isojima K., Ohtomi S., Yoshimoto N. Association of takotsubo cardiomyopathy and long QT syndrome. Circ J. 2006;70(9):1220–1222. doi: 10.1253/circj.70.1220. PMID: 16936440. [DOI] [PubMed] [Google Scholar]
- 12.El-Battrawy I., Behnes M., Borggrefe M., Akin I. Association of a congenital long QT syndrome type 1 with Takotsubo cardiomyopathy. Clin. Case Rep. 2016;4(8):789–792. doi: 10.1002/ccr3.567. PMID: 27525086; PMCID: PMC4974430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taguchi M., Sasa T., Izuhara M., Shioji K., Iwamuro A., Uegaito T., Matsuda M. Ventricular Fibrillation Induced by Takotsubo Syndrome with Congenital Long QT Syndrome. Intern. Med. 2020;59(6):789–792. doi: 10.2169/internalmedicine.3484-19. Epub 2019 Nov 29. PMID: 31787694; PMCID: PMC7118393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Templin C., Manka R., Cammann V.L., Szawan K.A., Gotschy A., Karolyi M., Winnik S., Moch H., Meyer P., Moreo A., Bossone E., D'Ascenzo F., Secco G.G., Mancone M., Infusino F., Gaido L., Giammaria M., Wittstein I.S., Varga Z., Ghadri J.R. Takotsubo Syndrome in Coronavirus Disease 2019. Am. J. Cardiol. 2021;138:118–120. doi: 10.1016/j.amjcard.2020.10.005. Epub 2020 Oct 9. PMID: 33045199; PMCID: PMC7546657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanza G.A., Barone L., Scalone G., Pitocco D., Sgueglia G.A., Mollo R., Nerla R., Zaccardi F., Ghirlanda G., Crea F. Inflammation-related effects of adjuvant influenza A vaccination on platelet activation and cardiac autonomic function. J. Intern. Med. 2011;269(1):118–125. doi: 10.1111/j.1365-2796.2010.02285.x. Epub 2010 Oct 22 PMID: 20964738. [DOI] [PubMed] [Google Scholar]