Abstract
Objective:
To evaluate pre- to post-season differences in individual subtests of the Visio-Vestibular Examination (VVE) in healthy middle and high school athletes.
Methods:
This prospective cohort study recruited participants from a private suburban United States secondary school. Participants completed a demographic questionnaire prior to the start of their season. A proxy for head impact exposure was estimated by incorporating previously published head impact frequencies by team and sport. The VVE was completed pre- and post-season and consisted of 9 subtests: smooth pursuit, horizontal/vertical saccades and gaze stability, binocular convergence, left/right monocular accommodation, and complex tandem gait. Generalized estimating equations were employed to assess the relative risk of an abnormal VVE outcome based on testing session (pre- vs. post-season).
Results:
Participants included middle and high school athletes (n=115; female=59 (51.3%); median age at first assessment=14.9 years, [IQR=13.6, 16.0]) during 2017/18 – 2019/20 school years. During pre-season testing, accommodation (10.0%) and complex tandem gait (9.2%) had the largest proportion of abnormal outcomes, while smooth pursuits (10.6%) and convergence (9.5%) had the largest proportion of abnormal outcomes post-season. When assessing the effect of testing session on the relative risk of any abnormal VVE subtest, there were no significant findings (P≥0.25). Additionally, there were no significant effects of testing session when adjusting for estimated head impact exposure for any VVE subtest (P≥0.25).
Conclusions:
Visio-vestibular function as measured by the VVE does not change from pre- to post-season in otherwise healthy adolescent athletes. Our findings suggest that the VVE may be stable and robust to typical neurodevelopment occurring in this dynamic age group and help inform post-injury interpretation of visio-vestibular impairments.
Keywords: oculomotor, vision, concussion, sport, balance, gait
Introduction
Sport- and recreation-related concussions are prevalent injuries among adolescents under 18 years of age with an estimated 1.1–1.9 million concussions occurring annually [1]. Athletic trainers are typically the first health care providers to identify and evaluate concussion[2] and treat approximately 14% of concussions seen by health care providers[1], making the need for comprehensive and easily administered concussion assessment tools compelling. Current consensus guidelines recommend a multi-faceted examination post-injury, including assessment of one or more of the following: physical signs, self-reported symptoms, cognitive function, postural control, behavioral changes, and sleep disturbances [3]. Recent literature has found that physiological deficits following concussion may outlast symptom resolution [4–6], suggesting that our current symptom scales may not be sensitive to assessment of subtle physiological dysfunction. In order to mitigate this, visual and vestibular assessment have begun to play a bigger role in concussion evaluation [7].
The visual and vestibular systems are responsible for maintaining visual stability during head movements and postural control, respectively [8]. Visio-vestibular dysfunction can manifest as self-reported symptoms (dizziness, balance problems, and visual deficits) and physical signs (abnormal oculomotor function, gaze stability, or balance) [9,10]. One method of visio-vestibular assessment is the Visio-Vestibular Examination (VVE), which includes assessments of smooth pursuit, saccades, gaze stability, near point of convergence, monocular accommodation, and tandem gait. The VVE assesses both physical signs of dysfunction, as well as symptom provocation, allowing for insight into real-world functional deficits (e.g., those elicited by school and sport) relative to the clinic setting.
Following concussion, visual and vestibular impairments are evident in up to 69% and 81% of pediatric patients, respectively, in the acute and subacute phases [9,10]. Visio-vestibular deficits are also predictive of prolonged concussion recovery [11]. However, there is a dearth of literature assessing changes in these measures across time and with participation in sports in a healthy adolescent population. Visio-vestibular outcomes may be especially important to understand in uninjured adolescents that are undergoing rapid neurodevelopmental changes [12] including, but not limited to, cerebral blood flow perfusion [13], rapid executive function development, and psychosocial changes [12]. Specifically, baseline visual and vestibular function has been shown to improve with age in uninjured youth football athletes [14]. Previous literature assessing single season changes in measures used in concussion assessment, for example, neurocognitive testing via ImPACT, has shown no change in healthy collegiate athletes [15] and no differences relative to noncontact controls [16]. Studies in uninjured high school athletes, on the other hand, have demonstrated single-season declines in ImPACT scores in contact athletes (football athletes) [17] and a lack of expected improvement in cognitive control with training relative to noncontact controls (soccer athletes) [18]. Specific to visio-vestibular function, White-Schwoch et al.[19] demonstrated no changes in visual function and a slight improvement in vestibular function in youth football athletes across two seasons. Similarly, single-season improvements in total scores from the King-Devick test, a rapid oculomotor number naming test, are evident in youth soccer[20] and high school ice hockey athletes [21]. Additionally, Zonner et al. [22] and Rose et al. [23] demonstrated no pre- to post-season changes in the Vestibular/Oculomotor Screening (VOMS) assessment, though some in-season variance in near point of convergence was noted [22]. This is unsurprising as the VOMS has high internal consistency with a Cronbach’s α=0.92 in adolescents[24]. The VOMS measures similar overlapping domains to VVE (smooth pursuits, saccadic eye movement, gaze stability, and near-point of convergence); however, the VVE was developed to enhance the clinical utility of a visio-vestibular assessment. The VOMS relies on self-report of symptom provocation (Likert scale 0–10) with visual and oculomotor assessment [24] and has a false-positive rate of up 13% in youth athletes [25]. While the VVE similarly records self-reported symptom provocation as a binary outcome (yes/no), the VVE also considers physical signs and established repetition-based cut-offs to assess performance that allow for increased sensitivity in concussion diagnosis [26,27]. Additionally, the VVE incorporates additional exam elements (monocular accommodation and complex tandem gait) for a more comprehensive assessment of the visio-vestibular system [27].
Previous studies examining visio-vestibular function focus on high school football athletes, reducing their generalizability to the larger adolescent athletic population. It is currently unclear what role sport participation, level of play, or regular head impact exposure associated with that sport may play in these concussion metrics in healthy adolescent athletes and how that might be influenced by sex. Therefore, there is a need to further investigate single-season changes in visio-vestibular function in uninjured adolescent athletes in order to elucidate visio-vestibular outcome behavior over time in a physiologically dynamic population. To address this, the primary objective of this study was to compare pre- to post-season assessments of individual elements of the VVE in healthy middle and high school athletes across a variety of sports. Secondary objectives included determining the effect of age, sex, and sport played on these assessments.
Materials and Methods
Study Design and Participants
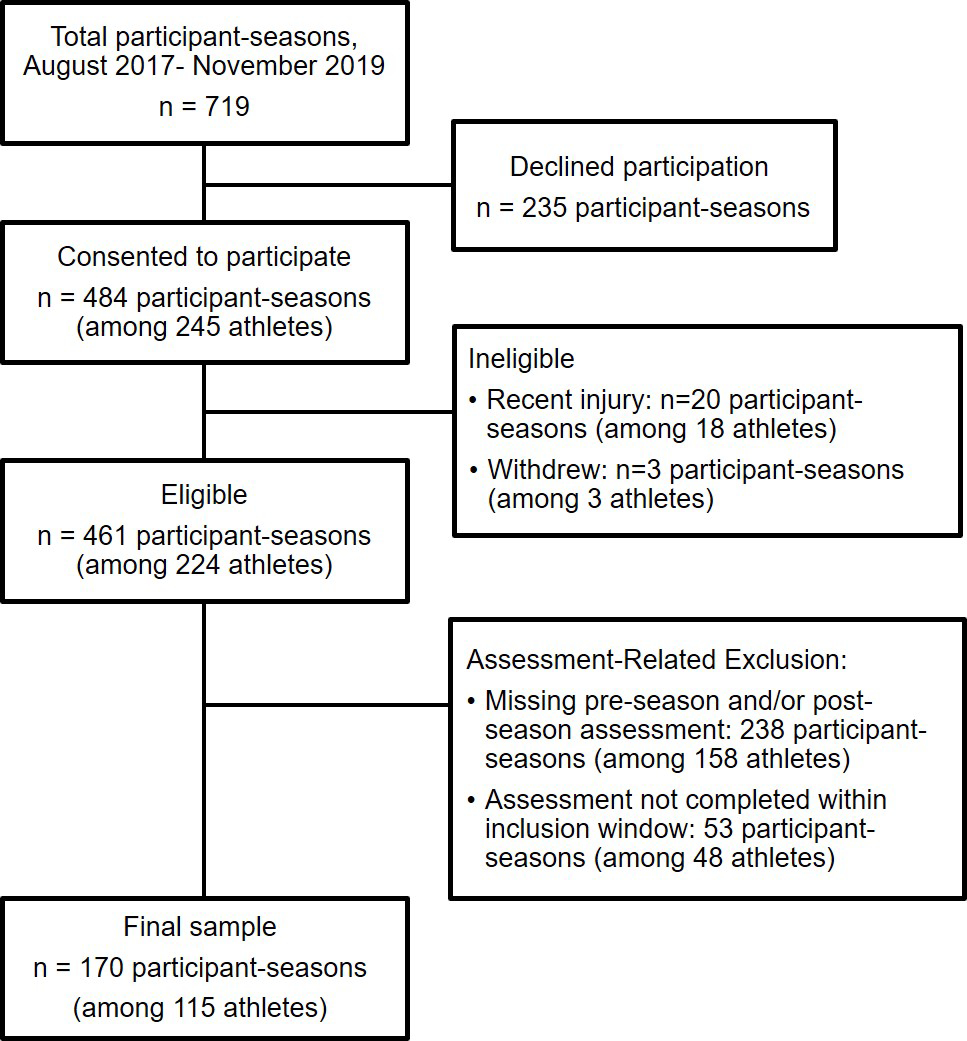
This prospective cohort study recruited healthy athletes age 11–19 from a private suburban middle/high school who completed testing from August 2017 through November 2019 as part of a large, prospective, observational study of clinical and device-based measures of concussion [28]. Participants were recruited via a letter or flyer from the investigative team that was distributed by the athletic department to athletes and their parents, or from general announcements by the study team to athletes, parents, and coaches at meetings, practices, and/or games. Participants recruited as controls in the 2017–2018 school year were offered volunteer hours for each hour participating in the study, which could be applied to the school’s Community Service requirement for graduation. Those who participated in the study from Fall 2018 and onward were offered the choice of volunteer hours or a gift card for their participation ($10 for a baseline test in the academic year, and $20 for each subsequent post-season test in that academic year). Each participant had at least one school-based sport season during the study period, including basketball, field hockey, lacrosse, and soccer, at either the middle school or high school (junior varsity and varsity) level. Participants were included if testing was completed within the following window: pre-season testing in the period 180 days before through 14 days after the first game of their respective scholastic sport seasons and post-season testing within 28 days of the final game of their season (either regular season or post-season game). Exclusion criteria for participation included being less than 30 days removed from physician clearance from a prior concussion. Participants who sustained a concussion during a study season were excluded for that season. Study stage participation is summarized in Figure 1. The study was approved by the Institutional Review Board of the Children’s Hospital of Philadelphia.
Figure 1.
Summary of participant population.
Data Collection
All participants self-reported demographic characteristics including age, sex, race/ethnicity, number of prior concussions, and past medical history (including psychological disorders [anxiety, depression, or bipolar disorder], attention deficit hyperactivity disorder [ADHD], learning disorders [dyslexia, learning disability, speech therapy, or presence of an Individualized Education Plan or 504 plan], motion sickness, and migraine or chronic headaches). The VVE was conducted by trained study coordinators who received standardized training from a sports medicine specialist, including observed practice sessions. The order of VVE subtest performance was uniform across participants. The VVE was a part of a larger battery of clinical (Sport Concussion Assessment Tool 5, Balance Error Scoring System, Immediate Post-Concussion Assessment and Cognitive Testing, King-Devick) and device-based (Biodex Biosway, pupillometry, eye tracking) measures [28] among which the test order was not uniform due to reliance on coordinator and participant availability.
Outcome Measures
The VVE is a battery of 9 subtests which evaluates vestibular function, visual function, and gait. The exam has been shown to be implementable across a variety of practice settings [29,30], with individual components of the VVE demonstrating moderate to substantial agreement among the same provider in the emergency department (ED) setting [26]. The VVE assessment takes 3–5 minutes to complete and includes the following subtests: (1) Smooth pursuits, evaluating the participant’s ability to track horizontally [26]; (2) Horizontal and (3) vertical saccades, where the participant moves their eyes rapidly between two fixed objects in both the horizontal and vertical planes [26,27]; (4) Horizontal and (5) vertical gaze stability (angular vestibular-ocular reflex [VOR]), where the participant’s eyes are fixed and their head moves in either the horizontal or vertical plane [26]; (6) Near point of convergence, evaluating for binocular break (double vision) using a standard Astron accommodative rule (Gulden Ophthalmics, Elkins Park, PA) with a single column 20/30 card [28,31]; (7) Right and (8) left monocular accommodation, evaluating clear to blur distance with one eye open using a standard Astron accommodative rule. An abnormal distance was determined based on age using Hofstetter’s formula where the abnormal distance cut-off increases as a child ages (≥9.8 cm for a child 11 years old, ≥ 10.0 cm for a child 12 years old, etc. up to ≥ 11.7 cm for a child 18 years old) [32]; and (9) Complex tandem gait, where the subject walked in tandem forward for 5 steps with eyes open and for 5 steps with eyes closed, then walked in tandem backward for 5 steps with eyes open and for 5 steps with eyes closed. Errors (steps off a straight line) and the presence of sway were recorded for each of the four conditions. Participants with lower extremity injuries were excluded from this task.
The primary outcome from the VVE is the proportion of participants with an abnormal outcome for each of the following individual subtest or combination of related subtests: (1) For smooth pursuit, an abnormality was defined as symptom provocation (eye fatigue, pain, dizziness, headache, or nausea), jerky or jumpy movements, or nystagmus. (2) For horizontal and vertical saccades and gaze stability, an abnormality was defined as symptom provocation (eye fatigue, pain, dizziness, headache, or nausea) on any subtest with 20 or fewer repetitions [27]. (3) For near point of convergence, an abnormality was defined as break at greater than 6 centimeters [32]. (4) For monocular accommodation, an abnormality was based on the age-specific blur distance for either the right or left eye using Hofstetter’s formula.25 (5) For complex tandem gait, an abnormality was defined as a composite score (sum of number of errors and presence of sway) of at least 5 (on a scale of 0–24) [28]. Finally, we created a composite outcome of an abnormality on any of these 5 primary subtests.
Classification of Head Impact Exposure Group
We classified each participant-season into one of five head impact exposure groups based on a study of head impact exposure in a subset of participants from the same population (Supplementary Table 1) [33]. In that study, each participant wore a headband-mounted sensor that measured head impact kinematics during competitive games. Video review of game footage was used to confirm head impacts. Average head impact exposure for a given sport and team (high school/middle school and girls/boys) was determined as the number of head impacts experienced by team members divided by the total athlete exposures among all participants by team and sport. An athlete exposure was defined as an athlete actively participating in a competitive game. Because only high school athletes were included in the Huber et al. study, we made the following assumptions in order to determine groups: 1) middle school soccer, in which heading is prohibited, would likely have head impact rates similar to the high school non-header rates; and 2) among other sports, high school and middle school head impact rates would be similar. From these average exposure measurements, we created head impact groupings by highest to lowest head impact exposure: (1) boys’ high school soccer; (2) girls’ high school soccer; (3) boys’ high school and middle school basketball and lacrosse, boys’ middle school soccer; (4) girls’ high school basketball, girls’ middle school soccer; and (5) girls’ high school and middle school lacrosse and field hockey (Table 1).
Table 1.
Head Impact Exposure Group Classification of 170 Subject-Seasons, n (%)
| Male | Female | ||
|---|---|---|---|
| Soccer | High school | Level 1: 26 (15.3) | Level 2: 29 (17.1) |
| Middle school | Level 3: 46 (27.1) | Level 4: 24 (14.1) | |
| Basketball | High school | ||
| Lacrosse | High school | Level 5: 45 (26.5) | |
| Middle school | |||
| Field Hockey | High school | n/a | |
| Middle school |
Statistical Analysis
We described the demographic characteristics among study participants and season characteristics among participant-seasons using frequencies and proportions for categorical variables and median and interquartile range for continuous variables. We compared the distribution of these characteristics by sex using chi-square test for categorical variables and Wilcoxon rank-sum tests for continuous variables. For each of the 5 primary variables, we compared the proportion of abnormalities from pre-season to post-season assessment using generalized estimating equations (GEE) repeated measures regression with a log-link function. Models accounted for correlation within season (pre- to post-season measurements) and among participants (for participants with measurements in multiple seasons) using an independent covariance structure. From these models, we calculated the unadjusted crude risk ratio (RR) and 95% confidence interval (CI) as well as the adjusted RR and 95% CI, controlling for head impact exposure group, for all participants and then stratified by sex. Analyses were conducted using SAS statistical software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Of the 719 participant-seasons approached for participation, 484 participant-seasons (67.3%; n=245 participants) were enrolled in the study and 170 participant-seasons (23.6%; n=115 participants) were included in the final analysis (Figure 1). Slightly more than half of the participants (51.3%) were female and three-fourths of participants (75.7%) were non-Hispanic white (Table 2). This is comparable to the student body as a whole which reports approximately 20% of their student population as students of color. The median age at first assessment was 14.9 years (IQR: 13.6, 16.0).
Table 2.
Demographic Characteristics for Study Population
| Variable | Total n=115 | Females n=59 | Males n=56 |
|---|---|---|---|
|
| |||
| Age, median (IQR) | 14.9 (13.6, 16.0) | 15.1 (14.1, 16.0) | 14.7 (13.3, 16.0) |
| Race/Ethnicity (%) | |||
| Non-Hispanic White | 87 (75.7) | 44 (74.6) | 43 (76.8) |
| Non-Hispanic Black | 12 (10.4) | 8 (13.6) | 4 (7.1) |
| Hispanic | 5 (4.3) | 2 (3.4) | 3 (5.4) |
| Non-Hispanic Other/Unknown | 11 (9.6) | 5 (8.5) | 6 (10.7) |
| Prior Medical History (%) | |||
| Concussion | 28 (24.3) | 10 (16.9) | 18 (32.1) |
| Psychological Disorders | 12 (10.4) | 7 (11.9) | 5 (8.9) |
| ADHD | 9 (7.8) | 3 (5.1) | 6 (10.7) |
| Learning Disorders | 9 (7.8) | 5 (8.5) | 4 (7.1) |
| Motion Sickness | 7 (6.1) | 5 (8.5) | 2 (3.6) |
| Migraine/Severe Headache | 7 (6.1) | 7 (11.9) | 0 (0.0) |
| Number of Seasons Included (%) | |||
| 1 | 84 (73.0) | 37 (62.7) | 47 (83.9) |
| 2 | 17 (14.8) | 12 (20.3) | 5 (8.9) |
| 3 | 9 (7.8) | 7 (11.9) | 2 (3.6) |
| 4+ | 5 (4.4) | 3 (5.1) | 2 (3.6) |
| Sport (%) | |||
| Soccer | 62 (53.9) | 29 (49.2) | 33 (58.9) |
| Lacrosse | 36 (31.3) | 16 (27.1) | 20 (35.7) |
| Field Hockey | 20 (17.4) | 20 (33.9) | - |
| Basketball | 18 (15.7) | 11 (18.6) | 7 (12.5) |
IQR: Interquartile range; ADHD: Attention deficit/hyperactivity disorder
The majority of participants had only one sport season (73%); 17 participants (14.8%) had 2 seasons, 9 (7.8%) participants had 3 seasons, and only 5 participants (4.3%) contributed 4 or more seasons. Pre-season assessments occurred at a median of 6 days (IQR: −5, 14) prior to the first game and post-season assessments occurred at a median of 10 days (IQR: 2, 16) after the last game. The median days between pre- and post-season assessment was 70 days (IQR: 61, 84). Soccer accounted for 48.2% of all participant-seasons, followed by lacrosse (24.7%), field hockey (14.7%), and basketball (12.4%). Most participant-seasons occurred at the high school level (varsity: 61.8% and junior varsity:14.7%). The distribution of participant-seasons according to head impact group is presented in Table 1; 15.3% were classified as level 1, 17.1% in level 2, 27.1% in level 3, 14.1% in level 4, and 26.5% in level 5.
During pre-season testing, the proportion of all participant-seasons with an abnormality was 10% or less for each of the 5 primary subtests (Table 3). Approximately 3 in 10 had at least one abnormal subtest. The overall proportions of abnormal subtests at post-season testing was similar. For the majority of participant-seasons, subtest results demonstrated no change from pre- to post-season with the proportion that did not change ranging from 87.6% to 90.6% among subtests. Of the participant-seasons in which the subtest results differed from pre- to post-season, similar proportions changed from abnormal to normal and from normal to abnormal. The stability in subtest outcomes from pre- to post-season was ranged from 84.7% to 92.9% in female participants and 87.5% to 94.4% in male participants.
Table 3.
Proportion of Abnormal Visio-Vestibular Examination (VVE) Outcomes at Pre- and Post-Season and Pre- to Post-Season Change.
| Pre-Season Abnormal | Post-Season Abnormal | Pre- to Post-Season Change |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No Change | Normal to Abnormal | Abnormal to Normal | |||||||||
|
| |||||||||||
| Sex | Assessment | N | % | N | % | N | % | N | % | N | % |
| Both | Smooth pursuits | 17 | 10 | 18 | 10.6 | 149 | 87.6 | 11 | 6.5 | 10 | 5.9 |
| Saccades and gaze stability | 12 | 7.1 | 8 | 4.7 | 154 | 90.6 | 6 | 3.5 | 10 | 5.9 | |
| Convergence | 12 | 7.1 | 16 | 9.5 | 152 | 90.5 | 10 | 6.0 | 6 | 3.6 | |
| Accommodation | 17 | 10 | 15 | 8.8 | 154 | 90.6 | 7 | 4.1 | 9 | 5.3 | |
| Tandem walk balance | 15 | 9.2 | 11 | 6.7 | 145 | 89.0 | 7 | 4.3 | 11 | 6.7 | |
| Any of 5 assessments | 52 | 30.6 | 50 | 29.4 | 122 | 71.8 | 23 | 13.5 | 25 | 14.7 | |
|
| |||||||||||
| Female | Smooth pursuits | 13 | 13.3 | 14 | 14.3 | 83 | 84.7 | 8 | 8.2 | 7 | 7.1 |
| Saccades and gaze stability | 9 | 9.2 | 7 | 7.1 | 86 | 87.8 | 5 | 5.1 | 7 | 7.1 | |
| Convergence | 7 | 7.3 | 11 | 11.5 | 84 | 87.5 | 8 | 8.3 | 4 | 4.2 | |
| Accommodation | 9 | 9.2 | 8 | 8.2 | 91 | 92.9 | 3 | 3.1 | 4 | 4.1 | |
| Tandem walk balance | 8 | 8.6 | 6 | 6.5 | 81 | 87.1 | 5 | 5.4 | 7 | 7.5 | |
| Any of 5 assessments | 31 | 31.6 | 31 | 31.6 | 70 | 71.4 | 14 | 14.3 | 14 | 14.3 | |
|
| |||||||||||
| Male | Smooth pursuits | 4 | 5.6 | 4 | 5.6 | 66 | 91.7 | 3 | 4.2 | 3 | 4.2 |
| Saccades and gaze stability | 3 | 4.2 | 1 | 1.4 | 68 | 94.4 | 1 | 1.4 | 3 | 4.2 | |
| Convergence | 5 | 6.9 | 5 | 6.9 | 68 | 94.4 | 2 | 2.8 | 2 | 2.8 | |
| Accommodation | 8 | 11.1 | 7 | 9.7 | 63 | 87.5 | 4 | 5.6 | 5 | 6.9 | |
| Tandem walk balance | 7 | 10 | 5 | 7.1 | 64 | 91.4 | 2 | 2.9 | 4 | 5.7 | |
| Any of 5 assessments | 21 | 29.2 | 19 | 26.4 | 52 | 72.2 | 9 | 12.5 | 11 | 15.3 | |
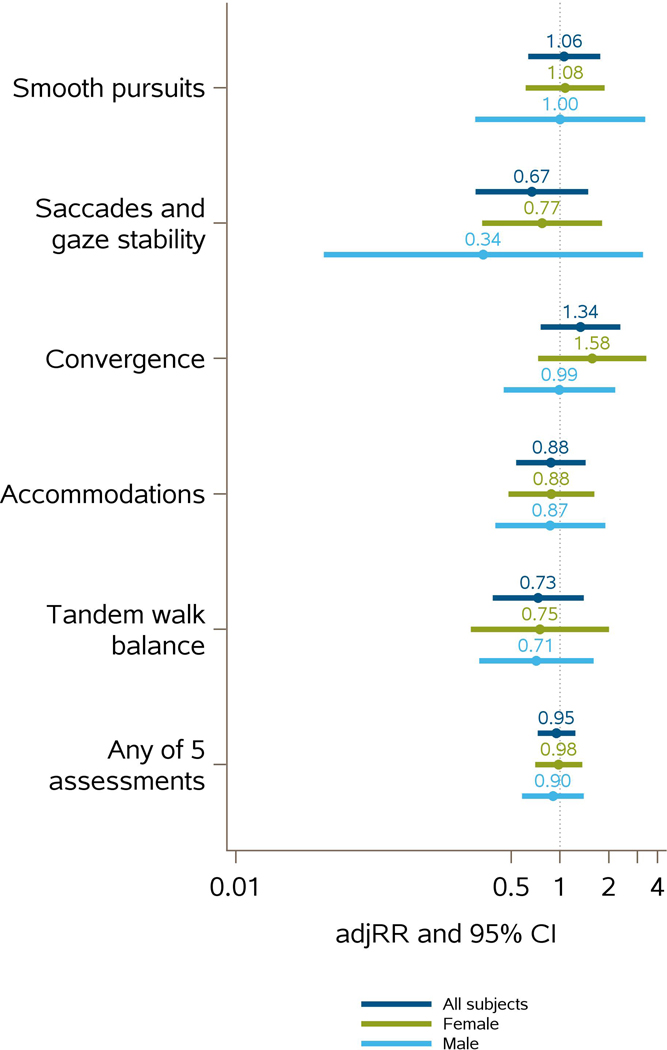
The lack of change in subtest outcomes from pre- to post-season, accounting for within-participant correlation and controlling for head impact exposure group is illustrated in Figure 2. For all participants and with all 5 primary outcomes, the 95% CI for both the crude and adjusted RR included the null value of 1. Similar results were observed for analyses stratified by sex. Data from GEE models are summarized in Supplemental Table 2. To examine the possible effect of including multiple participant-seasons in our analysis, we performed a sensitivity analysis assessing the crude and adjusted RR and 95% CI for all participant-seasons vs. the first participant-season only. The adjusted RR of abnormal VVE for the first participant-season mirrored that of the multiple participant-seasons (Supplementary Table 3).
Figure 2.
Adjusted risk ratios and 95% confidence intervals for participants with all 5 primary outcomes by sex. There were no significant changes in abnormal VVE outcomes from pre- to post-season, accounting for within-participant correlation and controlling for head impact group. An adjusted risk ratio greater than 1 indicates that the change from pre- to post-season was more likely to be from normal to abnormal, while less than one indicates that the change was more likely to be abnormal to normal.
Discussion
This study evaluated single-season changes in the visio-vestibular examination in healthy adolescent athletes. We found no significant changes in the proportion of abnormal outcomes from pre-season to post-season in any VVE subtest when controlling for head impact exposure level, a variable that accounts for differences in exposure by team, sport level (i.e., middle school vs. high school), and sport type. Our findings suggest that, in an otherwise healthy adolescent population, VVE outcomes do not change across a single athletic season.
The lack of pre- to post-season changes in VVE findings aligns with previous literature suggesting that clinical outcomes are largely unaffected by sport participation or sub-concussive head impact exposure [34,35]. Specific to visio-vestibular outcomes, deficits have been demonstrated in the acute and subacute phases following concussion [10,24,36–39]; however, there are few studies that have assessed single-season changes in otherwise healthy adolescent athletes. The proportion of participants with abnormal VVE subtests at both pre- (7.1–10%) and post-season (4.7–10.6%) in the current study are similar to, or lower than, those reported in both collegiate (6–11%)[40] and youth athletes (9–13%)[25] using the VOMS. Previous studies in collegiate athletes have indicated no differences in tandem gait performance [41] following a single season. In collegiate women’s soccer, exposure to fewer headers was associated with improved oculomotor reaction times over the course of a single season, while athletes with greater exposure to headers demonstrated stable oculomotor reaction times [42]. In collegiate men’s lacrosse no associations were found between sub-injurious head impact biomechanics and gaze stability performance [43]. Additionally, head impact frequency and magnitude did not predict pre- to post-season change in oculomotor function in youth and high school football players [22,23]. Our results mirror these and also add adolescent data for both males and females across multiple sport types (soccer, lacrosse, field hockey, basketball), competition level (middle school, junior varsity, varsity) and age ranges (11–19 years old) ranges to the growing body of literature regarding the effect of a single athletic season on clinical concussion metrics.
Though not statistically significant, convergence demonstrated the greatest increase in abnormal tests from pre- to post-season, particularly in girls. Convergence insufficiency is well-described following adolescent mild traumatic brain injury [44], and its complex regulation, involving autonomic control and processing in the dorsolateral prefrontal cortex [45], may make it more sensitive to subtle neurological changes. Previous studies have shown that these changes observed following concussion are associated with longer recovery times than other VVE abnormalities [11]. In both collegiate and high school football athletes, near point of convergence has been shown to worsen during the season, but normalize by post-season testing [22,46]. Though we are unable to infer how our athlete participants performed during the season, our findings support these studies’ findings that near point of convergence appears to normalize over a single season, in that any changes that might have occurred over the course of the season appear to be temporary. Future research should continue to investigate these outcomes in larger samples.
Our null findings may be attributable to several different factors. Two of the VVE outcomes (smooth pursuit and horizontal and vertical saccades and gaze stability) account for participant self-report of symptom provocation, which assists with clinical feasibility and interpretation, though may contribute to a lack in sensitivity to subtler impairments. Recent literature has found that physiological deficits affecting cerebrovascular function [4], white matter integrity [5], and pupillary light reflex [6] are present in asymptomatic athletes suggesting that standard clinical assessments may not detect underlying physiological dysfunction that may occur following a contact sport season. Physiological function may be a critical indicator of brain health in adolescent athletes who are undergoing rapid neurodevelopmental changes. Additionally, the visual [47] and vestibular systems [48] are known to be adaptive to sport where tandem gait assessments have demonstrated moderate practice effects [49]; however, there were no pre- to post-season improvements in the current study. Therefore, it is possible that practice effects in our study population were mitigated by head impact exposure due to the inclusion of both collision (lacrosse) and contact sports (basketball, field hockey, soccer) [50]. Though individual measures of head impact exposure were not included in the current study, our results did not change when controlling for team head impact exposure level, which accounts for the frequency of head impacts by sex, and sport, making this hypothesis less likely [33]. Future research should further investigate the effect of head impact exposure on VVE outcomes at the individual level.
Overall, the clinical meaningfulness of this study is highlighted in the lack of change in VVE outcomes over a single athletic season. It is estimated that almost half of all public high schools and only 40% of private high schools provide full athletic training coverage respectively, with most secondary schools employing only one athletic trainer [51,52]. Though baseline testing is no longer deemed necessary (and often times impractical) [3], it remains a common tool used in secondary school settings, placing testing burden on the athletic trainer. The VVE is a quick, reliable assessment [26] that, if athletic trainers choose to incorporate into their baseline assessment, has flexibility with the timing of administration based on this study’s findings. It may also be a clinically useful visio-vestibular measure and can reduce burden on athletic trainers in adolescent athletic settings which rarely employ more than one athletic trainer [51]. The identification of visual and vestibular deficits with the VVE subtests may also better inform active treatment and targeted rehabilitation [53–57] as well as add valuable data useful in risk stratification [9,11,58] and improvements in diagnostic accuracy [24,27,28,59]. Additionally, based on the lack of change in the VVE, our study suggests that clinicians can be confident that any post-injury abnormalities are not a result of sport participation or physical activity. Importantly, our study provides foundational data regarding VVE outcomes across adolescent populations often underrepresented in the concussion literature, including middle school athletes, female athletes, and non-collision sport athletes.
There are limitations to the current study. Our participants were sampled from a population of student athletes at a suburban, private secondary school, limiting the generalizability of our study findings to more diverse populations. Additionally, 23.6% of participant-seasons approached for participation in the study were included in our final analysis and therefore our results may not be representative of the general athlete population at this school. We did not collect information on reasons students declined to participate. The pre-season demographic questionnaire did not include past medical history questions regarding accommodative dysfunction, convergence insufficiency, or developmental coordination disorder. While our study found a low prevalence of VVE abnormalities, we are unable to infer if these conditions factored into those who presented with abnormal VVE outcomes. Though the order of VVE subtest performance was uniform across participants, the VVE was a part of a larger battery of clinical and device-based measures [28] among which the test order was not uniform based on coordinator and participant availability which may have influenced VVE performance. All students at this school are required to participate in athletics, therefore their competitive level and ability may be more variable than other settings. While we controlled for head impact exposure level, a variable based on sport type, team and estimated head impacts [33], we did not quantify individual-level, in-season head impact exposure. Additionally, the current study estimated the head impact exposure category for middle school athletes, as Huber et al.[33] included only high school athletes. Given the diverse population of athletes included in this sample, it will be important in future work to quantify head impact at an individual level across a broad age range and during practices to correlate with clinical and objective measures. Finally, there are many unmeasured exposures over the course of an athletic season (including participation in other sports and recreational activities outside the school setting); however, the fact we did not measure significant changes in any of the elements of the VVE underscores its robustness to these additional potential head impacts.
Conclusion
In summary, there is a critical need for sensitive and clinically-relevant concussion assessments that are robust in the setting of dynamic neurodevelopment occurring during childhood and adolescence. Our study indicates that visio-vestibular function, as measured by the VVE, does not change over the course of a single-season, even when controlling for team head impact level in healthy adolescent athletes. The VVE is a practical, quick, and easily administered assessment across clinical settings [30,60] and the findings of this study suggest that there may be flexibility in the timing of baseline test administration. This study provides formative data that may better inform post-injury interpretation of visio-vestibular deficits and provides insight into the clinical meaningfulness of lack of short-term changes in visio-vestibular function.
Supplementary Material
Acknowledgements
We thank Ronni S. Kessler, MS, Fairuz Mohammed, MS, MPH, Ari Fish, BS, Shelly Sharma, BA, Taylor Valerio, Anne Mozel and Julia Vanni, BS, of Children’s Hospital of Philadelphia for their contribution to data collection for this study. In addition, the authors thank the students and parents from the Shipley School for their participation. The authors appreciate the support from the Shipley School administration and athletic department: Steve Piltch, Michael Turner, Mark Duncan, Katelyn Taylor, Dakota Carroll, Kimberly Shaud, and Kayleigh Jenkins.
Footnotes
Conflict of Interest: The authors have no conflicts to disclose
References
- 1.Bryan MA, Rowhani-Rahbar A, Comstock RD, Rivara F. Sports-and recreation-related concussions in US youth. Pediatrics. 2016;138(1). [DOI] [PubMed] [Google Scholar]
- 2.Broglio SP, Cantu RC, Gioia GA, Guskiewicz KM, Kutcher J, Palm M, et al. National Athletic Trainers’ Association position statement: management of sport concussion. J Athl Train [Internet]. 2014. Apr [cited 2019 Mar 31];49(2):245–65. Available from: 10.4085/1062-6050-49.1.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCrory P, Meeuwisse W, Dvořák J, Aubry M, Bailes J, Broglio S, et al. Consensus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med [Internet]. 2017. Jun [cited 2019 Mar 31];51(11):838–47. Available from: 10.1136/bjsports-2017-097699 [DOI] [PubMed] [Google Scholar]
- 4.Svaldi DO, McCuen EC, Joshi C, Robinson ME, Nho Y, Hannemann R, et al. Cerebrovascular reactivity changes in asymptomatic female athletes attributable to high school soccer participation. Brain Imaging Behav [Internet]. 2017. [cited 2019 Mar 31];11(1):98–112. Available from: 10.1007/s11682-016-9509-6 [DOI] [PubMed] [Google Scholar]
- 5.Kuzminski SJ, Clark MD, Fraser MA, Haswell CC, Morey RA, Liu C, et al. White Matter Changes Related to Subconcussive Impact Frequency during a Single Season of High School Football. Am J Neuroradiol [Internet]. 2018. Feb [cited 2020 Feb 11];39(2):245–51. Available from: 10.3174/ajnr.A5489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph JR, Swallow JS, Willsey K, Almeida AA, Lorincz MT, Fraumann RK, et al. Pupillary changes after clinically asymptomatic high-acceleration head impacts in high school football athletes. J Neurosurg [Internet]. 2019. Nov [cited 2020 May 26];1–6. Available from: 10.3171/2019.7.{JNS191272} [DOI] [PubMed] [Google Scholar]
- 7.Ventura RE, Balcer LJ, Galetta SL. The concussion toolbox: the role of vision in the assessment of concussion. Semin Neurol [Internet]. 2015. Oct [cited 2019 Mar 31];35(5):599–606. Available from: 10.1055/s-0035-1563567 [DOI] [PubMed] [Google Scholar]
- 8.Cullen Kathleen E.. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 2014;23(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corwin DJ, Wiebe DJ, Zonfrillo MR, Grady MF, Robinson RL, Goodman AM, et al. Vestibular Deficits following Youth Concussion. J Pediatr [Internet]. 2015. May [cited 2019 Mar 31];166(5):1221–5. Available from: 10.1016/j.jpeds.2015.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Master CL, Scheiman M, Gallaway M, Goodman A, Robinson RL, Master SR, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila) [Internet]. 2016. Mar [cited 2019 Mar 31];55(3):260–7. Available from: 10.1177/0009922815594367 [DOI] [PubMed] [Google Scholar]
- 11.Master CL, Master SR, Wiebe DJ, Storey EP, Lockyer JE, Podolak OE, et al. Vision and vestibular system dysfunction predicts prolonged concussion recovery in children. Clin J Sport Med [Internet]. 2018. [cited 2020 May 22];28(2):139–45. Available from: 10.1097/{JSM}.0000000000000507 [DOI] [PubMed] [Google Scholar]
- 12.Konrad K, Firk C, Uhlhaas PJ. Brain development during adolescence: neuroscientific insights into this developmental period. Dtsch Arztebl Int [Internet]. 2013. Jun [cited 2019 Mar 31];110(25):425–31. Available from: 10.3238/arztebl.2013.0425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satterthwaite TD, Shinohara RT, Wolf DH, Hopson RD, Elliott MA, Vandekar SN, et al. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proc Natl Acad Sci U S A [Internet]. 2014. Jun [cited 2019 Mar 31];111(23):8643–8. Available from: 10.1073/pnas.1400178111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White-Schwoch T, Krizman J, McCracken K, Burgess JK, Thompson EC, Nicol T, et al. Baseline profiles of auditory, vestibular, and visual functions in youth tackle football players. Concussion. 2019;4(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller JR, Adamson GJ, Pink MM, Sweet JC. Comparison of preseason, midseason, and postseason neurocognitive scores in uninjured collegiate football players. Am J Sports Med [Internet]. 2007. Aug [cited 2019 Mar 31];35(8):1284–8. Available from: 10.1177/0363546507300261 [DOI] [PubMed] [Google Scholar]
- 16.McAllister TW, Flashman LA, Maerlender A, Greenwald RM, Beckwith JG, Tosteson TD, et al. Cognitive effects of one season of head impacts in a cohort of collegiate contact sport athletes. Neurology [Internet]. 2012. May [cited 2019 Mar 31];78(22):1777–84. Available from: 10.1212/{WNL}.0b013e3182582fe7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breedlove KM, Breedlove EL, Robinson M, Poole VN, Iii JRK, Rosenberger P, et al. Detecting Neurocognitive and Neurophysiological Changes as a Result of Subconcussive Blows in High School Football Athletes. Athl Train Sport Heal Care. 2014;6(X):1–9. [Google Scholar]
- 18.Koerte IK, Nichols E, Tripodis Y, Schultz V, Lehner S, Igbinoba R, et al. Impaired cognitive performance in youth athletes exposed to repetitive head impacts. J Neurotrauma [Internet]. 2017. Aug [cited 2019 Mar 31];34(16):2389–95. Available from: 10.1089/neu.2016.4960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White-Schwoch T, Krizman J, McCracken K, Burgess JK, Thompson EC, Nicol T, et al. Performance on auditory, vestibular, and visual tests is stable across two seasons of youth tackle football. Brain Inj [Internet]. 2020;34(2):236–44. Available from: 10.1080/02699052.2019.1683899 [DOI] [PubMed] [Google Scholar]
- 20.Bretzin AC, Anderson M, Moran RN, Covassin T. Long-term test-retest evaluation of the King-Devick test in youth soccer athletes. J Neurol Sci [Internet]. 2020;416(May 2020):116951. Available from: 10.1016/j.jns.2020.116951 [DOI] [PubMed] [Google Scholar]
- 21.Dhawan PS, Leong D, Tapsell L, Starling AJ, Galetta SL, Balcer LJ, et al. King-Devick Test identifies real-time concussion and asymptomatic concussion in youth athletes. Neurol Clin Pract [Internet]. 2017. Dec;7(6):464–73. Available from: http://journals.lww.com/01586158-900000000-99617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zonner SW, Ejima K, Fulgar CC, Charleston CN, Huibregtse ME, Bevilacqua ZW, et al. Oculomotor Response to Cumulative Subconcussive Head Impacts in US High School Football Players: A Pilot Longitudinal Study. JAMA Ophthalmol. 2019;137(3):265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose SC, Yeates KO, Fuerst DR, Ercole PM, Nguyen JT, Pizzimenti NM. Head Impact Burden and Change in Neurocognitive Function during a Season of Youth Football. J Head Trauma Rehabil. 2019;34(2):87–95. [DOI] [PubMed] [Google Scholar]
- 24.Mucha A, Collins MW, Elbin RJ, Furman JM, Troutman-Enseki C, DeWolf RM, et al. A Brief Vestibular/Ocular Motor Screening ({VOMS}) assessment to evaluate concussions: preliminary findings. Am J Sports Med [Internet]. 2014. Oct [cited 2019 Mar 31];42(10):2479–86. Available from: 10.1177/0363546514543775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran RN, Covassin T, Elbin RJ, Gould D, Nogle S. Reliability and Normative Reference Values for the Vestibular/Ocular Motor Screening (VOMS) Tool in Youth Athletes. Am J Sports Med. 2018;46(6):1475–80. [DOI] [PubMed] [Google Scholar]
- 26.Corwin DJ, Arbogast KB, Swann C, Haber R, Grady MF, Master CL. Reliability of the visio-vestibular examination for concussion among providers in a pediatric emergency department. Am J Emerg Med [Internet]. 2020;38(9):1847–53. Available from: 10.1016/j.ajem.2020.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storey EP, Corwin DJ, McDonald CC, Arbogast KB, Pfeiffer MR, Marguiles SS, et al. Assessment of saccades and gaze stability in the diagnosis of pediatric concussion. [In Press]. Clin J Sport Med. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corwin DJ, McDonald CC, Arbogast KB, Mohammed FN, Metzger KB, Pfeiffer MR, et al. Clinical and Device-based Metrics of Gait and Balance in Diagnosing Youth Concussion. Med Sci Sports Exerc. 2020;52(3):542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arbogast KB, Curry AE, Metzger KB, Kessler RS, Bell JM, Haarbauer-Krupa J, et al. Improving Primary Care Provider Practices in Youth Concussion Management. Clin Pediatr (Phila). 2017;56(9):854–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corwin DJ, Propert KJ, Zorc JJ, Zonfrillo MR, Wiebe DJ. Use of the vestibular and oculomotor examination for concussion in a pediatric emergency department. Am J Emerg Med [Internet]. 2019;37(7):1219–23. Available from: 10.1016/j.ajem.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheiman M, Cotter S, Kulp MT, Mitchell GL, Cooper J, Gallaway M, et al. Treatment of accommodative dysfunction in children: Results from a randomized clinical trial. Optom Vis Sci. 2011;88(11):1343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavrich JB. Convergence insufficiency and its current treatment. Curr Opin Ophthalmol. 2010;21(5):356–60. [DOI] [PubMed] [Google Scholar]
- 33.Huber CM, Patton DA, McDonald CC, Jain D, Simms K, Lallo VA, et al. Sport- and Gender-Based Differences in Head Impact Exposure and Mechanism in High School Sports. Orthop J Sport Med [Internet]. 2021. Mar 1;9(3):232596712098442. Available from: 10.1177/2325967120984423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belanger HG, Vanderploeg RD, McAllister T. Subconcussive Blows to the Head: A Formative Review of Short-term Clinical Outcomes. J Head Trauma Rehabil [Internet]. 2016. Jun [cited 2019 Mar 31];31(3):159–66. Available from: 10.1097/{HTR}.0000000000000138 [DOI] [PubMed] [Google Scholar]
- 35.Gysland SM, Mihalik JP, Register-Mihalik JK, Trulock SC, Shields EW, Guskiewicz KM. The relationship between subconcussive impacts and concussion history on clinical measures of neurologic function in collegiate football players. Ann Biomed Eng [Internet]. 2012. Jan [cited 2019 Mar 31];40(1):14–22. Available from: 10.1007/s10439-011-0421-3 [DOI] [PubMed] [Google Scholar]
- 36.Heitger MH, Anderson TJ, Jones RD, Dalrymple-Alford JC, Frampton CM, Ardagh MW. Eye movement and visuomotor arm movement deficits following mild closed head injury. Brain. 2004;127(3):575–90. [DOI] [PubMed] [Google Scholar]
- 37.Johnson B, Zhang K, Hallett M, Slobounov S. Functional neuroimaging of acute oculomotor deficits in concussed athletes. Brain Imaging Behav [Internet]. 2015. Sep [cited 2019 Mar 31];9(3):564–73. Available from: 10.1007/s11682-014-9316-x [DOI] [PubMed] [Google Scholar]
- 38.Johnson B, Hallett M, Slobounov S. Follow-up evaluation of oculomotor performance with fMRI in the subacute phase of concussion. Neurology. 2015;85(13):1163–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suh M, Kolster R, Sarkar R, McCandliss B, Ghajar J, Cognitive, et al. Deficits in predictive smooth pursuit after mild traumatic brain injury. Neurosci Lett [Internet]. 2006. Jun [cited 2019 Mar 31];401(1–2):108–13. Available from: 10.1016/j.neulet.2006.02.074 [DOI] [PubMed] [Google Scholar]
- 40.Kontos AP, Sufrinko A, Elbin RJ, Puskar A, Collins MW. Reliability and associated risk factors for performance on the vestibular/ocular motor screening ({VOMS}) tool in healthy collegiate athletes. Am J Sports Med [Internet]. 2016. Jun [cited 2019 Mar 31];44(6):1400–6. Available from: 10.1177/0363546516632754 [DOI] [PubMed] [Google Scholar]
- 41.Caccese JB, Best C, Lamond LC, Difabio M, Kaminski TW, Watson DAN, et al. Effects of Repetitive Head Impacts on a Concussion Assessment Battery. Med Sci Sports Exerc. 2019;51(7):1355–61. [DOI] [PubMed] [Google Scholar]
- 42.Gallagher VT, Murthy P, Stocks J, Vesci B, Colegrove D, Mjaanes J, et al. Differential Change in Oculomotor Performance among Female Collegiate Soccer Players versus Non-Contact Athletes from Pre- to Post-Season. Neurotrauma Reports. 2020;1(1):169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyashita TL, Ullucci PA. Correlation of Head Impact Exposures With Vestibulo-Ocular Assessments. J Sport Rehabil. 2020;29(3):310–4. [DOI] [PubMed] [Google Scholar]
- 44.Storey EP, Master SR, Lockyer JE, Podolak OE, Grady MF, Master CL. Near Point of Convergence after Concussion in Children. Optom Vis Sci. 2017;94(1):96–100. [DOI] [PubMed] [Google Scholar]
- 45.Gamlin PDR. Neural mechanisms for the control of vergence eye movements. Ann N Y Acad Sci. 2002;956:264–72. [DOI] [PubMed] [Google Scholar]
- 46.Kawata K, Rubin LH, Lee JH, Sim T, Takahagi M, Szwanki V, et al. Association of football subconcussive head impacts with ocular near point of convergence. {JAMA} Ophthalmol [Internet]. 2016. Jul [cited 2019 Mar 31];134(7):763–9. Available from: 10.1001/jamaophthalmol.2016.1085 [DOI] [PubMed] [Google Scholar]
- 47.Stine CD, Arterburn MR, Stern NS. Vision and sports: a review of the literature. J Am Optom Assoc [Internet]. 1982. Aug [cited 2019 Oct 22];53(8):627–33. Available from: https://www.ncbi.nlm.nih.gov/pubmed/7130603 [PubMed] [Google Scholar]
- 48.Paillard T Plasticity of the postural function to sport and/or motor experience. Neurosci Biobehav Rev. 2017;72:129–52. [DOI] [PubMed] [Google Scholar]
- 49.Howell DR, Brilliant AN, Meehan WP. Tandem gait test-retest reliability among healthy child and adolescent athletes. J Athl Train. 2019;54(12):1254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rice SG, of Pediatrics Council on Sports Medicine AA, Fitness. Medical conditions affecting sports participation. Pediatrics [Internet]. 2008. Apr [cited 2019 May 6];121(4):841–8. Available from: 10.1542/peds.2008-0080 [DOI] [PubMed] [Google Scholar]
- 51.Pryor RR, Casa DJ, Vandermark LW, Stearns RL, Attanasio SM, Fontaine GJ, et al. Athletic training services in public secondary schools: A benchmark study. J Athl Train. 2015;50(2):156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pike A, Pryor RR, Mazerolle SM, Stearns RL, Casa DJ. Athletic trainer services in US private secondary schools. J Athl Train. 2016;51(9):717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aligene K, Lin E. Vestibular and balance treatment of the concussed athlete. NeuroRehabilitation. 2013;32(3):543–53. [DOI] [PubMed] [Google Scholar]
- 54.Alsalaheen BA, Mucha A, Morris LO, Whitney SL, Furman JM, Camiolo-Reddy CE, et al. Vestibular rehabilitation for dizziness and balance disorders after concussion. J Neurol Phys Ther [Internet]. 2010. Jun [cited 2019 Mar 31];34(2):87–93. Available from: 10.1097/{NPT}.0b013e3181dde568 [DOI] [PubMed] [Google Scholar]
- 55.Gagnon I, Grilli L, Friedman D, Iverson GL. A pilot study of active rehabilitation for adolescents who are slow to recover from sport-related concussion. Scand J Med Sci Sports [Internet]. 2016. Mar [cited 2019 Mar 31];26(3):299–306. Available from: 10.1111/sms.12441 [DOI] [PubMed] [Google Scholar]
- 56.Thiagarajan P, Ciuffreda KJ. Effect of oculomotor rehabilitation on accommodative responsivity in mild traumatic brain injury. J Rehabil Res Dev [Internet]. 2014;51(2):175–92. Available from: http://www.rehab.research.va.gov/jour/2014/512/pdf/JRRD-2013-01-0027.pdf [DOI] [PubMed] [Google Scholar]
- 57.Ventura RE, Balcer LJ, Galetta SL, Rucker JC. Ocular motor assessment in concussion: Current status and future directions. J Neurol Sci [Internet]. 2016;361:79–86. Available from: 10.1016/j.jns.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 58.Ellis MJ, Cordingley D, Vis S, Reimer K, Leiter J, Russell K. Vestibulo-ocular dysfunction in pediatric sports-related concussion. J Neurosurg Pediatr [Internet]. 2015. Sep;16(3):248–55. Available from: https://thejns.org/view/journals/j-neurosurg-pediatr/16/3/article-p248.xml [DOI] [PubMed] [Google Scholar]
- 59.Corwin DJ, Arbogast KB, Haber RA, Pettijohn KW, Zonfrillo MR, Grady MF, et al. Characteristics and Outcomes for Delayed Diagnosis of Concussion in Pediatric Patients Presenting to the Emergency Department. J Emerg Med [Internet]. 2020;59(6):795–804. Available from: 10.1016/j.jemermed.2020.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Master CL, Grady MF. Office-based management of pediatric and adolescent concussion. Pediatr Ann [Internet]. 2012. Sep [cited 2019 Mar 31];41(9):1–6. Available from: 10.3928/00904481-20120827-08 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.