Abstract
Background
SARS-CoV-2 infection is believed to adversely affect the brain, but the degree of impact on socially relevant cognitive functioning and decision-making is not well-studied, particularly among those less vulnerable to age-related mortality. The current study sought to determine whether infection status and COVID-19 symptom severity are associated with cognitive dysfunction among young and middled-aged adults in the general population, using self-reported lapses in executive control and a standardized decision-making task.
Method
The survey sample comprised 1958 adults with a mean age of 37 years (SD = 10.4); 60.8% were female. Participants reported SARS-CoV-2 infection history and, among those reporting a prior infection, COVID-19 symptom severity. Primary outcomes were self-reported symptoms of cognitive dysfunction assessed via an abbreviated form of the Barkley Deficits in Executive Functioning Scale (BDEFS) and performance on a validated delay-discounting task.
Results
Young and middle-aged adults with a positive SARS-CoV-2 infection history reported a significantly higher number of cognitive dysfunction symptoms (Madj = 1.89, SE = 0.08, CI: 1.74, 2.04; n = 175) than their non-infected counterparts (Madj = 1.63, SE = 0.08, CI: 1.47,1.80; n = 1599; β = 0.26, p = .001). Among those infected, there was a dose-response relationship between COVID-19 symptom severity and level of cognitive dysfunction reported, with moderate (β = 0.23, CI: 0.003–0.46) and very/extremely severe (β = 0.69, CI: 0.22–1.16) COVID-19 symptoms being associated with significantly greater cognitive dysfunction. These effects remained reliable and of similar magnitude after controlling for demographics, vaccination status, mitigation behavior frequency, and geographic region, and after removal of those who had been intubated during hospitalization. Very similar—and comparatively larger—effects were found for the delay-discounting task, and when using only PCR confirmed SARS-CoV-2 cases.
Conclusions
Positive SARS-CoV-2 infection history and moderate or higher COVID-19 symptom severity are associated with significant symptoms of cognitive dysfunction and amplified delay discounting among young and middle-aged adults with no history of medically induced coma.
Keywords: SARS-CoV-2, COVID-19, Brain, Cognition, Executive function, Delay discounting, OFC
1. Introduction
Cognitive dysfunction is one of the potential adverse consequences of SARS-CoV-2 infection, and this risk may extend well below the age margins for increased mortality risk. It is understood that SARS-CoV-2 could impact the brain through a number of non-exclusive, indirect mechanisms including hypoxia, thrombosis, coagulopathy, cytokine storm, and megakaryocyte invasion (Boldrini et al., 2021; Mao et al., 2020; McFadyen et al., 2020; Nauen et al., 2021; Sarubbo et al., 2022; Ritchie and Chan, 2021; Solomon, 2021). Studies of predominantly older, hospitalized patients have revealed cognitive deficits in the areas of memory, spatial navigation, attention, short-term memory, and executive function (Ritchie and Chan, 2021; Solomon, 2021; Jaywant et al., 2021). Further, the cognitive impairments following SARS-Cov-2 infection may persist after the acute phase of infection (Ritchie and Chan, 2021), a phenomenon known as “long covid” (Ladds et al., 2020; Rubin, 2020).
Several studies have reported reliable evidence of cognitive dysfunction among those previously infected with SARS-CoV-2 (Jaywant et al., 2021; Becker et al., 2021; Liu et al., 2021; Almeria et al., 2020; Hampshire et al., 2021). However, some of these studies are limited by non-representative samples and lack of comparison to non-infected controls in the general population. Examination of a population-based sample including asymptomatic and minimally symptomatic individuals, coupled with a control sample of non-infected individuals from the same population facilitates quantification of the reliability and magnitude of SARS-CoV-2 infection impacts on cognition, if they do indeed exist. Beyond the above, relatively little is known about the extent to which cognitive deficits are predicted by age or sex, as demographic moderators. The extent to which SARS-CoV-2 adversely impacts cognitive function among younger and middle-aged adults is relatively unknown. Of particular interest are the executive functions, which are especially susceptible to environmental and systemic insult.
Executive functions are partially supported by the lateral prefrontal cortex, as well as the medial orbitofrontal cortex (mOFC). The mOFC is of particular interest, being the brain subregion most anatomically close to the hypothesized point of SARS-CoV-2 neuroinvasion. Decision-making processes supported by the OFC can be best assessed using decision-making paradigms with heavy temporal and evaluative demands, such as a delay discounting task (McClure et al., 2004; Massar et al., 2015; Peters and D'Esposito, 2016; Sellitto et al., 2010). Delay discounting is a neurobehavioral process reflecting the extent to which future rewards are devalued based on their delay in time (Bickel and Marsch, 2001) and summarized relative balance between the prefrontal cortices and the limbic systems (McClure et al., 2004). Greater delay discounting is reflected in the tendency to choose a lower value option that is immediately available over a higher value option that is delayed in time.
Prior studies have shown that damage to the mOFC is associated with increased delay discounting (Peters and D'Esposito, 2016; Sellitto et al., 2010). Impulsive choice of rewards is mediated by dopaminergic activity within the mOFC (Winstanley et al., 2006), in contrast with choices to avoid punishment, which are mediated by the lateral OFC (Kringelbach and Rolls, 2004). The most anterior aspect of the mOFC has further been proposed as the subregion most clearly involved in processing of abstract rewards (e.g., money), in contrast with the posterior mOFC, which is involved in computation of basic rewards (e.g., food, physical pleasure) (Kringelbach and Rolls, 2004). The anterior mOFC is located immediately superior to the olfactory bulb and nasal mucous membrane, the presumed source of symptoms of anosmia and ageusia reported by some infected individuals (Vaira et al., 2020). This may be a partial explanation for the diverse neuropsychiatric symptoms displayed by many hospitalized patients with severe COVID-19 (Taquet et al., 2021). Recently, an analysis of the UK Biobank data revealed prospective reductions OFC grey matter following infection with SARS-CoV-2, among other findings implicating proximity and connectivity to the olfactory bulb (Douaud et al., 2022).
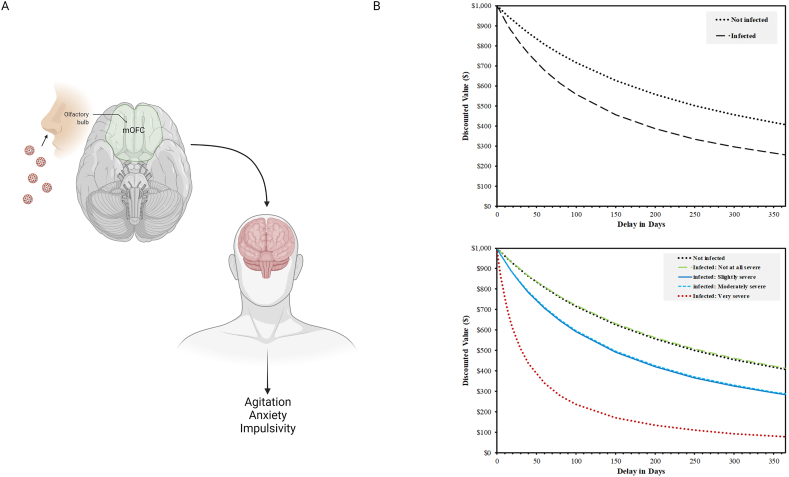
The current study reports findings from a large national survey of adults in the general population, who reported cognitive status, SARS-CoV-2 infection history, and COVID-19 symptom severity. It was hypothesized based on prior research (Jaywant et al., 2021; Becker et al., 2021; Liu et al., 2021; Almeria et al., 2020; Hampshire et al., 2021) that positive SARS-CoV-2 infection history would be associated with greater self-reported cognitive dysfunction, and that severity of COVID-19 symptoms would be positively correlated with severity of cognitive dysfunction, in a dose response manner. Finally, based on the proximity of the mOFC to the hypothesized site of neuroinvasion of SARS-CoV-2 (Fig. 1 panel A), it was expected that deficits would be especially evident on a delay discounting task.
Fig. 1.
Conceptual diagram (A) and delay discounting curves for non-infected and ranges of COVID-19 symptom severity from asymptomatic to “very severe” (B). Image in panel A created with Biorender.com.
2. Methods
2.1. Participants
Participants were recruited as part of the Canadian COVID-19 Experiences Project (CCEP) (Hall et al., 2021a), a multi-study project which includes a national cohort survey of 1958 adults aged 18 to 54. One research objective was to examine differences between fully vaccinated and vaccine-hesitant individuals on a broad set of demographic, psychosocial, and experiential variables. Thus, the cohort was recruited to have an equal proportion of fully vaccinated and vaccine-hesitant Canadians: 50.2% received two vaccine doses, 43.3% had received no doses, and 6.5% received one vaccine dose, but were not intending to receive a second. Employing quota sampling enabled an examination of factors that predict caseness with high statistical power, and also ensured that the use of caseness as a control variable was maximally stringent (e.g., controlling for vaccination status when the sample is a 1:1 ratio of vaccinated to unvaccinated). The mean age was 37.0 (SD = 10.4) and 60.8% were female.
2.2. Procedure
The survey was conducted from 28 September to 21 October 2021, when the predominant SARS-CoV-2 variant in Canada was Delta (4 weeks prior to the appearance of Omicron) (Public Health Agency of Canada, 2020). Participants were contacted by email with an invitation to participate in the survey. A link to the survey was provided for eligible participants, and all measures were completed online following provision of informed consent. A quota target of equal number of vaccinated and vaccine hesitant was applied to obtain a balanced sample with respect to both vaccinated and vaccine-hesitant populations. Within each quota target, the sample was recruited from ten Canadian provinces through an online survey panel (Leger Opinion, the largest nationally representative probability-based panel in Canada). The survey firm and University of Waterloo monitored survey response in the sample of each quota to achieve the final representative sample. This study was reviewed and received ethics clearance from the institutional research ethics board of the University of Waterloo.
2.3. Measures
Executive dysfunction. Symptoms of executive dysfunction were assessed using four “self-restraint” subscale items from the Deficits in Executive Functioning Scale, short form (BDEFS-SF) (Barkley, 2011). Respondents were asked how often they have experienced each the four problems during the past 6 months, including “I am unable to inhibit my reactions or responses to events or to other people”, “I make impulsive comments to others”, “I am likely to do things without considering the consequences for doing them”, and “I act without thinking”. Responses were indicated on a numerical scale where 1 = never or rarely, 2 = sometimes, 3 = often, and 4 = very often. Cronbach's alpha for the 4 items was 0.89, indicating acceptable reliability. The four executive dysfunction items were averaged for this analysis to create a composite executive dysfunction measure.
Delay discounting. To assess delay discounting, participants competed a validated 5-trial delay discounting task wherein they were presented with a series of hypothetical choices between a smaller monetary amount ($500) immediately or a larger amount ($1000) at various time delays (e.g.,1 month, 3 months) (Koffarnus and Bickel, 2014). Delay discounting was calculated as a k value, reflecting the steepness of a hyperbolic devaluation of delayed rewards; higher values of k indicate more impulsive choice.
SARS-CoV-2 infection status: Infection status was assessed using the question “What best describes YOUR experience with [SARS-CoV-2] infection?” where 1 = I have NOT been infected, 2 = I have been infected, and 3 = not stated.
Symptom severity: COVID-19 symptom severity was assessed among those who have been infected by SARS-CoV-2 using two questions. (1) “How do you know that you HAVE BEEN infected with [SARS-CoV-2]?” responses were given the answers of 1 = had symptoms but did not get tested, 2 = had symptoms and tested positive, and 3 = had no symptoms but tested positive. (2) “How severe was your [SARS-CoV-2] illness?” The five-point response scale was 1 = not at all severe, 2 = slightly severe, 3 = moderately severe, 4 = very severe, 5 = extremely severe. Those reporting “had no symptoms but tested positive” were incorporated into the second question as 1 = not at all severe.
Mitigation Behaviors and Demographics. COVID-19 mitigation behaviors—frequency of mask wearing, observing distancing, and recommended hand hygiene—were assessed via self-report (Hall et al., 2021a). Items were combined and averaged together to form an index variable, wherein higher scores were indicative of more consistent mitigation behavior (Cronbach's alpha = 0.710). Demographic variables were also assessed via self-report, as described in the CCEP protocol (Hall et al., 2021a).
2.4. Statistical analysis
Samples were post-stratified by geographic/language regions: Alberta, British Columbia, Manitoba + Saskatchewan, Ontario, Quebec English, and Quebec French, and Atlantic provinces (Nova Scotia, New Brunswick, Prince Edward Island, Newfoundland and Labrador). For each of the vaccinated and vaccine hesitant group separately, sampling weights were computed using a ranking procedure and calibrated to target marginal joint population distributions of the geographic/language regions, and the gender and age group combinations, based on population figures in the 2016 Canadian census data and the disposition code in the sample, thus allowing generalization to the Canadian population. Survey linear regression models incorporating survey strata and weights were applied to estimate composite executive dysfunction scores and their associations with SARS-CoV-2 infection status and COVID-19 symptom severity. Regression models controlled for respondents’ gender, age group (18–24, 25–39, and 40–54 years), income level (low, medium, high), geographic region (Canadian province), vaccination status (fully, partially, or unvaccinated), and COVID-19 mitigation behavior. Sensitivity analyses examined the impact of further adjustment for education level, stringency of SARS-CoV-2 case identification procedures, and the intensity of medical treatment among participants whom were hospitalized for COVID-19. All models were conducted in SAS with SUDAAN V11. All confidence intervals (CI) and statistical significance were assessed at the 95% confidence level.
3. Results
Baseline characteristics of the sample are presented in Table 1. The majority of the participants were female (60%) and from the 25–39 (40%) or 40–54 (43%) age groups. 84% of participants reported that they had not been infected; those who reported having been infected reported symptoms to be “not at all severe” (3%), “slightly severe” (2.4%), “moderately severe” (2.7%), with relatively few experiencing “very/extremely severe” symptoms (1%). The two cognitive measures were positively correlated (r = 0.17, p < .001).
Table 1.
Sample characteristics.
| Variables | n | % | BDEFS score (unadjusted) |
BDEFS score (adjusted) |
|---|---|---|---|---|
| Mean, 95% CI | Mean, 95% CI | |||
| Gender | ||||
| Male | 768 | 39.22 | - | - |
| Female | 1190 | 60.78 | - | - |
| Age Group | ||||
| 18-24 | 322 | 16.45 | - | - |
| 25-39 | 789 | 40.3 | - | - |
| 40-54 | 847 | 43.26 | - | - |
| Infection Status (Symptom severity) | ||||
| Not infected | 1599 | 83.76 | 1.62 (1.58, 1.66) | 1.62 (1.58, 1.66) |
| Infected (Not at all severe | 57 | 2.99 | 1.72 (1.52, 1.93) | 1.73 (1.54, 1.91) |
| Infected (Slightly severe | 46 | 2.41 | 1.78 (1.44, 2.11) | 1.75 (1.45, 2.05) |
| Infected (Moderately severe) | 51 | 2.67 | 1.83 (1.60, 2.06) | 1.85 (1.63, 2.08) |
| Infected (Very/extremely severe) | 21 | 1.10 | 2.29 (1.82, 2.76) | 2.32 (1.85, 2.78) |
| Not stated | 128 | 6.71 | 1.64 (1.46, 1.81) | 1.63 (1.47, 1.80) |
| Severity not stated | 7 | 0.37 | 2.22 (1.64, 2.81) | 2.16 (1.55, 2.78) |
Note: Each BDEFS mean value is the average of the four BDEFS items. Participants who had no COVID-19 symptoms, but tested positive for SARS-CoV-2, were classified as “not at all severe”. The % values by sex and age groups are unweighted and from all participants included in the survey (N=1958). Due to missing values, the sample for the primary statistical analysis involving BDEFS scores is n=1909. The means for BDEFS scores are weighted and the adjusted % values are adjusted by sex and age group.
3.1. Self-reported executive dysfunction
In demographics-adjusted analyses, those who reported a prior SARS-CoV-2 infection reported a significantly higher number of symptoms of executive dysfunction (Madj = 1.89, SE = 0.08, CI: 1.74, 2.04; n = 175) than their non-infected counterparts (Madj = 1.63, SE = 0.08, CI: 1.47,1.80; n = 1599; β = 0.26, p = .001). Men were likely to experience more executive dysfunction than women (β = 0.15, p < .001); younger adults (25–39 years) were more likely to experience executive dysfunction than middle aged adults (40–54 years; β = 0.30, p < .001).
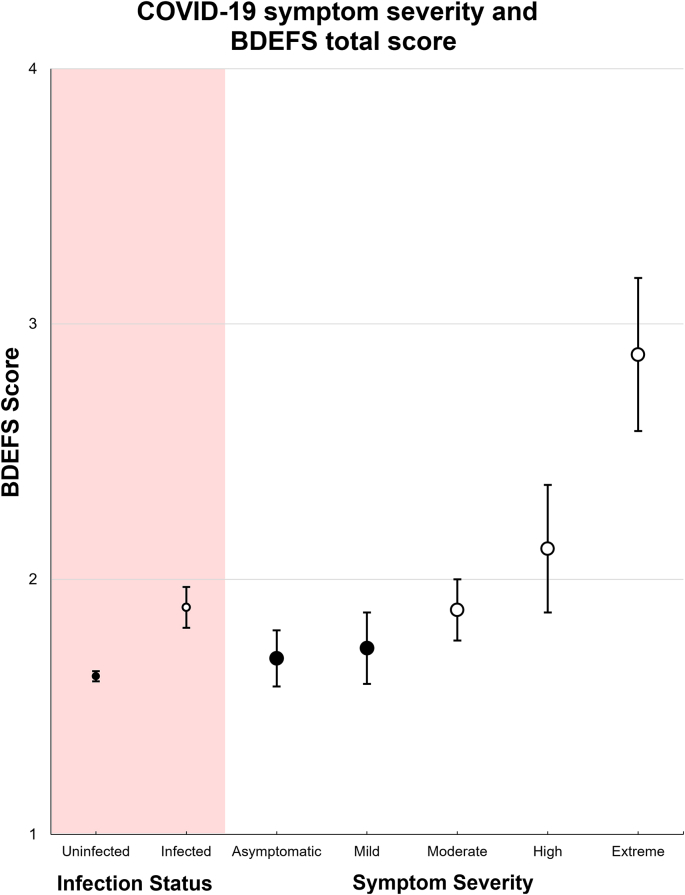
Among those who were infected, there was a dose-response relationship between COVID-19 symptom severity and executive dysfunction. Participants who reported “moderately severe” (Madj = 1.85, 95% CI 1.63–2.08) and “very” or “extremely severe” (Madj = 2.32, 95% CI 1.85–2.78) COVID-19 symptoms were significantly more likely to have higher levels of executive dysfunction compared to non-infected individuals (Madj = 1.62, 95% CI 1.58–1.66). A dose-response relationship between COVID-19 symptom severity and cognitive dysfunction was evident, those with moderate (β = 0.23, CI: 0.003–0.46) and very/extremely severe (β = 0.69, CI: 0.22–1.16) COVID-19 symptoms being associated with significantly greater degrees of executive dysfunction, compared to those not infected and those with asymptomatic infections (Fig. 2). Removing the those who reported having been intubated (n = 5) or hospitalized without intubation (n = 5) did not change the findings. Likewise, following further adjustment for vaccination status, mitigation behaviors, income, and geographical region, those in the very/extremely severe symptom categories continued to report significantly greater symptoms of executive dysfunction than the non-infected reference group (Table 2).
Fig. 2.
Effects of SARS-CoV-2 infection status and COVID-19 symptom severity on BDEFS scores; BDEFS=Barkley Deficits in Executive Functioning Scale. White circles denote estimates that are significantly different from the uninfected reference value.
Table 2.
Fully adjusted models predicting BDEFS scores from SARS-CoV-2 infection status and COVID-19 symptom severity.
| Variable | Frequency | (95% CI) | p |
|---|---|---|---|
| infection status (symptom severity) | |||
| Not infected | 1597 | Ref | Ref |
| Infected (Not at all severe) | 57 | 0.01 (−0.18, 0.21) | 0.881 |
| Infected (Slightly severe) | 46 | 0.11 (−0.17, 0.39) | 0.43 |
| Infected (Moderately severe) | 51 | 0.15 (−0.08, 0.37) | 0.208 |
| Infected (Very severe) | 17 | 0.52 (0.06, 0.98) | 0.026 |
| Infected (Extremely severe) | 4 | 1.13 (0.33, 1.92) | 0.005 |
| Not stated | 128 | −0.05 (−0.22, 0.12) | 0.574 |
| Infected: severity not stated | 7 | 0.49 (−0.20, 1.17) | 0.163 |
| Gender | |||
| Male | 751 | 0.13 (0.06, 0.21) | <0.001 |
| Female | 1156 | Ref | Ref |
| Age group | |||
| 18-24 | 315 | 0.22 (0.11, 0.33) | <0.001 |
| 25-39 | 769 | 0.04 (−0.04, 0.12) | 0.281 |
| 40-54 | 823 | Ref | Ref |
| Income | |||
| Low | 289 | Ref | Ref |
| Moderate | 428 | −0.04 (−0.18, 0.10) | 0.56 |
| High | 1020 | −0.21 (−0.33, −0.09) | <0.001 |
| No answer | 170 | −0.22 (−0.37, −0.06) | 0.006 |
| Geographic Region | |||
| AB | 238 | 0.11 (−0.03, 0.24) | 0.112 |
| BC | 234 | 0.01 (−0.11, 0.14) | 0.819 |
| MB + SK | 117 | 0.16 (−0.02, 0.35) | 0.075 |
| Maritimes | 106 | 0.06 (−0.10, 0.22) | 0.446 |
| ON | 720 | 0.04 (−0.06, 0.15) | 0.409 |
| QC-EN | 129 | 0.11 (−0.04, 0.26) | 0.139 |
| QC-FR | 363 | Ref | Ref |
| Vaccination status | |||
| No shot | 818 | −0.10 (−0.17, −0.02) | 0.015 |
| One shot | 124 | 0.22 (0.04, 0.40) | 0.017 |
| two shots | 965 | Ref | Ref |
| Mitigation behaviour | . | −0.16 (−0.22, −0.10) | <0.001 |
Note: N = 1958; BDEFS=Barkley Deficits in Executive Function Scale; AB = Alberta, BC=British Columbia; MB = Manitoba; SK=Saskatchewan; ON=Ontario; QC = Quebec; Maritimes = Nova Scotia, Prince Edward Island, Newfoundland/Labrador, New Brunswick.
3.2. Delay discounting task performance
In demographics-adjusted analyses, participants infected with SARS-CoV-2 displayed significantly higher delay discounting rates (k = 1.22, SE = 0.48, CI: 0.27, 2.16) than non-infected participants (k = 0.37, SE = 0.08, CI: 0.21, 0.52; β = 0.31, p = .017). With respect to dose-response effects of symptom severity, among infected individuals, those reporting “very severe” COVID-19 symptoms demonstrated significantly higher delay discounting rates than those reporting no infection history, with the remaining severity categories falling between these two values. Discount curves for infected versus non-infected, and among severity levels ranging from asymptomatic and very severe are presented in Fig. 1 panel b. Further adjustment for vaccination status, mitigation behaviors, income and geographical region did not affect the findings (Table 3).
Table 3.
Fully adjusted models predicting delay discounting rates from SARS-CoV-2 infection status and COVID-19 symptom severity.
| Variable | Frequency | (95% CI) | p |
|---|---|---|---|
| COVID19 infection status | |||
| Not infected | 1638 | Ref | Ref |
| Infected (Not at all severe) | 57 | −0.10 (−0.39, 0.19) | 0.495 |
| Infected (Slightly severe) | 46 | 0.22 (−0.11, 0.56) | 0.19 |
| Infected (Moderately severe) | 52 | 0.30 (−0.17, 0.76) | 0.21 |
| Infected (Very severe) | 18 | 1.24 (0.29, 2.19) | 0.011 |
| Not stated | 134 | −0.07 (−0.28, 0.14) | 0.505 |
| Infected: severity not stated | 7 | 0.53 (−0.77, 1.82) | 0.425 |
| Gender | |||
| Male | 767 | −0.10 (−0.21, 0.00) | 0.058 |
| Female | 1189 | Ref | Ref |
| Age group | |||
| 18-24 | 322 | −0.03 (−0.17, 0.12) | 0.703 |
| 25-39 | 788 | 0.01 (−0.11, 0.13) | 0.843 |
| 40-54 | 846 | Ref | Ref |
| Income | |||
| Low | 305 | Ref | Ref |
| Moderate | 436 | 0.02 (−0.17, 0.21) | 0.836 |
| High | 1029 | −0.28 (−0.42, −0.13) | <0.001 |
| No answer | 186 | −0.30 (−0.53, −0.08) | 0.007 |
| Geographic region | |||
| AB | 245 | 0.06 (−0.13, 0.25) | 0.512 |
| BC | 243 | −0.03 (−0.20, 0.15) | 0.751 |
| MB + SK | 119 | 0.25 (0.04, 0.45) | 0.019 |
| NS, PEI, NL, NB | 111 | 0.15 (−0.08, 0.37) | 0.2 |
| ON | 737 | 0.07 (−0.07, 0.22) | 0.324 |
| QC-EN | 134 | −0.01 (−0.23, 0.20) | 0.901 |
| QC-FR | 367 | Ref | Ref |
| Vaccination status | |||
| No shot | 847 | 0.19 (0.07, 0.31) | 0.002 |
| One shot | 127 | 0.33 (0.04, 0.62) | 0.024 |
| Two shots | 982 | Ref | Ref |
| Mitigation behaviour | . | −0.03 (−0.12, 0.06) | 0.507 |
Note: N = 1958; BDEFS=Barkley Deficits in Executive Function Scale; AB = Alberta, BC=British Columbia; MB = Manitoba; SK=Saskatchewan; ON=Ontario; QC = Quebec; NS=Nova Scotia, PEI=Prince Edward Island, NL=Newfoundland/Labrador, NB=New Brunswick.
In general, males had marginally steeper discount rates than females (β = −0.10, p = .066), and individuals reporting high incomes had significantly lower discounting rates than individuals reporting low income (β = −0.30, p < .001). No significant age differences in k values were observed (see supplementary materials). No two-way interactions were observed between sex and infection status predicting delay discounting were observed (Wald F = 0.09, p = .91), or between age and infection status predicting delay discounting (Wald F = 0.90, p = .46). Likewise, the three-way interaction term between sex, age and infection status in predicting delay discounting was non-significant (Wald F = 1.37, p = .22).
3.3. Comparative analysis
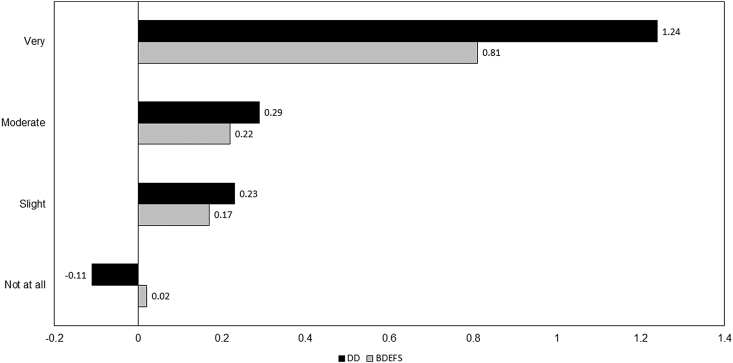
In order to compare the strength of association between COVID-19 symptom severity and the two cognitive indicators, the predictors were standardized and entered into the same fully adjusted predictive model separately as focal predictors. The findings are presented in Fig. 3. The magnitude of the standardized beta weight predicting very severe COVID-19 symptoms from delay discounting task performance was 53.09% larger than that predicting very severe COVID-19 symptoms from BDEFS scores.
Fig. 3.
Comparative magnitude of associations between COVID-19 symptom severity and cognitive indicators; BDEFS=Barkley Deficits in Executive Functioning Scale; DD = delay discounting task.
3.4. Sensitivity analyses
Because education can impact both executive function and delay discounting, we examined whether additional adjustment for education might alter the pattern of findings. However, when education was added as a covariate to the predictive models, those who reported a prior SARS-CoV-2 infection still reported significantly higher BDEFS scores than their non-infected counterparts (β = 0.26, CI: 0.09, 0.42, p = .002); likewise, very/extremely severe COVID-19 symptoms predicted significantly higher BDEFS scores (β = 0.72, CI: 0.23, 1.20, p = .004). In terms of delay discounting task performance, participants with a positive SARS-CoV-2 infection history displayed significantly higher k values than their non-infected counterparts (β = −0.28, CI: -0.54, −0.02, p = .033), and this was primarily driven by significantly higher k values among those with “very severe” COVID-19 symptoms (β = 1.25, CI: 0.31, 2.20, p = .010).
Further sensitivity analyses were undertaken after removing unconfirmed COVID-19 cases from the sample (i.e., those who reported having symptoms but did not get tested) and limiting analyses to only those with PCR confirmation. The pattern and significance of the findings in this case was essentially identical to the primary analyses (see supplemental materials).
Finally, due to the known adverse effects of medically-induced coma on cognitive function, additional sensitivity analyses were undertaken to ensure that cognitive findings were not attributable to hospitalization and intubation. Removal of 5 cases reporting being placed on mechanical ventilator did not change the effects of SARS-CoV-2 infection status (β = 0.23, CI: 0.01,0.45, p = .043) or COVID-19 symptom severity (β = 0.95, CI: 0.20,1.71, p = .014) on delay discounting rate. Likewise, when limiting the “infected” group to only those whom reported having their infection confirmed by a positive PCR test, the effect of SARS-CoV-2 infection remained significant, and somewhat larger in magnitude (β = 0.40; CI: 0.07, 0.72, p = .016).
4. Discussion
In this population-representative cohort of community-dwelling adults, those with a positive history of SARS-CoV-2 infection reported more symptoms of cognitive dysfunction than those with no such history. This effect was evident on both self-reported symptoms of executive dysfunction and on a validated decision-making task. A dose-response relationship between COVID-19 symptom severity and magnitude of cognitive dysfunction was evident such that increasing infection severity was associated with greater symptoms of cognitive dysfunction for both self-reported symptoms and task performance. Importantly, reliable effects of positive SARS-CoV-2 infection history and COVID-19 symptom severity on cognitive dysfunction were evident—on both measures—even in this sample of individuals not typically subject to age-related cognitive decline (ages 18 to 54) and not exposed to medically induced coma via hospital-based treatment for severe COVID-19. Our findings were similar to a prior report of executive dysfunction as correlated with COVID-19 symptom severity in a large population sample (Hampshire et al., 2021), but extend them to include self-reported symptoms of interpersonal significance, and a standardized decision making paradigm previously linked to the site of hypothesized neuroinvasion of the SARS-CoV-2 virus (the mOFC; Fig. 1 panel A). It is noteworthy that, in our data, the strength of the relationship between very severe COVID-19 symptoms and cognitive outcomes was approximately 50% larger in the indicator most closely associated with OFC function. This finding may be an important link to structural anomalies observed in this region in a recent brain imaging study involving SARS-CoV-2 infection (Douaud et al., 2022).
There are several hypothesized mechanisms by which SARS-CoV-2 infection could produce cognitive dysfunction, including hypoxia, thrombosis, coagulopathy, cytokine storm, and megakaryocyte invasion (Nauen et al., 2021; Ritchie and Chan, 2021; Solomon, 2021). The current investigation cannot distinguish among these neurophysiological mechanisms, or others that may yet be identified. Further, the current findings do not preclude the possibility that symptoms of cognitive dysfunction are influenced by reporting biases among those who are continuing to experience emotional distress following the measurement period. Given that the effects of negative mood on symptom reporting is causally established (Howren and Suls, 2011; Reimers et al., 2009), and given that mood impacts of the COVID-19 pandemic are well-documented (Czeisler et al., 2020; Xiong et al., 2020; Duan et al., 2020; Daly et al., 2020; Hall et al., 2021b), this possibility cannot be definitively excluded. However, at least one earlier population-based study has found similar dose-response effects using performance-based measures of cognitive function (i.e., cognitive tasks rather than reported symptoms). (Hampshire et al., 2021).
It is not clear why there appeared to be a stronger link between SARS-CoV-2 infection and cognitive dysfunction in younger adults as compared with middle-aged adults. It is possible that such deficits were more salient to younger adults, given that a higher proportion would be in educational programs wherein lapses in attention and concentration may have been more impactful. It is noteworthy that there was no evidence of age moderation on the corresponding task-related cognitive indicator, which may support the hypothesis that the age effect is a reporting bias rather than reflective of a stronger pathophysiological process among younger adults as compared to middle-aged adults.
In either case, it is not clear how consequential symptoms of cognitive dysfunction would be expected to be, even if reliable across studies. It is not uncommon for other types of viral infections to cause symptoms of cognitive dysfunction, including the seasonal flu, herpes, MERS, Zika, and Varicella (chickenpox) (Goenka et al., 2014; Kim et al., 2017; de Araújo et al., 2016; Berger and Houff, 2008; Gilden et al., 2000). Documenting the stability and functional impact of any SARS-CoV-2 infection impairments in cognition will be important. However, in the meantime, reductions in unnecessary exposure to SARS-CoV-2 infection may be an important public health strategy even for young and middle-aged adults, despite the limited mortality risk.
Finally, given that the predominant SARS-CoV-2 variant during the time of the survey was Delta, the findings are applicable only to the Delta and earlier variants. Moreover, the retrospective nature of the study does not allow us to determine with confidence which infections were attributable to Delta versus earlier variants. We also cannot conclude that the same associations would be observed with the Omicron variant, in particular because of the lower COVID-19 symptom severity apparent with Omicron in comparison with earlier variants, at least based on early data (Christie, 2021; Abdullah et al., 2021; Sheikh et al., 2021). In the current (pre-Omicron) sample, we found that only moderate and higher COVID-19 symptom severities were associated with significantly elevated symptoms of executive dysfunction. Further analyses of follow-up waves of the CCEP data will enable examination of the relative impact of the Omicron variant on cognitive outcomes.
4.1. Strengths and limitations
There are several strengths of the current study. One strength is the use of a population-representative sample, consisting of infected individuals of a wide range of disease symptom severities—ranging from asymptomatic to severe symptoms requiring hospitalization—as well as non-infected controls. Another strength is the use of a validated measure of interpersonally relevant dimensions of cognitive dysfunction; our findings therefore augment those of other studies employing more sensitive but less ecologically valid performance-based measures of cognitive function. Finally, the finding of similar effects on a decision-making task increases confidence that the findings were not a function of self-report methodology alone, and provide an important conceptual link to evaluative processing within the OFC. The later identifies a plausible mechanism by which SARS-CoV-2 infection could produce neuropsychiatric symptoms--especially agitation, anxiety and confusion--widely documented among hospitalized patients early in the pandemic before wide vaccine availability.
In terms of limitations, by virtue of the survey format, it was not possible to validate infection status by direct PCR testing. Use of self-reported PCR testing status may lead to some error in estimation of effect size and statistical significance of tests, vis-a-vis misreporting of confirmed infection status. This is a limitation of other large scale survey studies of COVID-19 and cognitive dysfunction, however (Hampshire et al., 2021). Further, the cross-sectional dataset limits our ability to assess directionality, as does the lack of precise timing information on infection in relation to measurement of cognitive function. The latter may also lead to some influence of current infection on cognitive testing scores and self-reported cognitive dysfunction symptoms. With future waves of data collection, it will be possible to examine time since infection as a moderator of COVID-19 brain health outcomes, and to track the longevity of any cognitive effects observed.
Future studies using other datasets should examine the extent to which the dose-response and age gradients observed here are replicable across samples. Finally, additional studies examining neurological impacts at the level of the brain itself will be required, using brain imaging paradigms to quantify structural and functional impacts of SARS-CoV-2 infection. In particular studies are needed that follow individuals forward from the point of infection to examine changes over time, in a prospective manner; the findings of one early study of this nature suggests that localized and global brain changes should be examined closely (Douaud et al., 2022).
4.2. Conclusions
In summary, the current study used a population-representative sample consisting of a balanced proportion of vaccinated and unvaccinated individuals to estimate the association between SARS-CoV-2 infection and symptoms of cognitive dysfunction. Findings indicated that individuals previously infected with SARS-CoV-2 reported significantly greater symptoms of cognitive dysfunction than non-infected individuals. Further, among those reporting a positive infection history, a dose-response relationship between COVID-19 symptom severity and cognitive dysfunction was evident, such that those with moderate to severe symptoms were more likely to experience symptoms of cognitive dysfunction. The above pattern was evident for both self-reported symptoms of cognitive dysfunction and performance on a decision-making task. Taken together with findings from other studies, cognitive dysfunction appears to be a correlate of SARS-CoV-2 infection, particularly among those with at least moderate COVID-19 symptom severity. If such cognitive effects prove to be long-lasting, this may be one additional piece of evidence in support of public health messaging around the importance of vaccination and limiting unnecessary exposure to severe COVID-19.
Research ethics statement
This study protocol was reviewed by and received approval from the University of Waterloo Office of Research Ethics.
Funding statement
Funding for this study was provided by a grant from the Canadian Institutes of Health Research (GA3-177733) to P. Hall (PI), G. Fong (co-PI) and S. Hitchman (co-I).
Data availability statement
Data will be available upon reasonable request to either of the corresponding authors.
Author contributions
PH, GF, and SH conceived the study, planned and oversaw the statistical analyses, and wrote the final draft. GM planned and completed all statistical analyses and contributed to the writing of the final draft. MNS, AH, JM, and WB contributed to the writing of the final draft.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
We thank Anne C.K. Quah and Thomas Agar for their assistance with survey design and management.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2022.100454.
Contributor Information
Peter A. Hall, Email: pahall@uwaterloo.ca.
Geoffrey T. Fong, Email: geoffrey.fong@uwaterloo.ca.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abdullah F., Myers J., Basu D., et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, South Africa. Int. J. Infect. Dis. 2021 doi: 10.1016/j.ijid.2021.12.357. Published online December. S120197122101256X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeria M., Cejudo J.C., Sotoca J., Deus J., Krupinski J. Cognitive profile following COVID-19 infection: clinical predictors leading to neuropsychological impairment. Brain Behav. Immun - Health. 2020;9:100163. doi: 10.1016/j.bbih.2020.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley R.A. Guilford Press; 2011. Barkley Deficits in Executive Functioning Scale (BDEFS) [Google Scholar]
- Becker J.H., Lin J.J., Doernberg M., et al. Assessment of cognitive function in patients after COVID-19 infection. JAMA Netw. Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.30645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J.R., Houff S. Neurological complications of herpes simplex virus type 2 infection. Arch. Neurol. 2008;65(5) doi: 10.1001/archneur.65.5.596. [DOI] [PubMed] [Google Scholar]
- Bickel W.K., Marsch L.A. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96(1):73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Boldrini M., Canoll P.D., Klein R.S. How COVID-19 affects the brain. JAMA Psychiatr. 2021;78(6):682. doi: 10.1001/jamapsychiatry.2021.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Agency of Canada . 2020. COVID-19 Daily Epidemiology Update.https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html?stat=rate&measure=total_last14&map=pt Published April 19, 2020. (Accessed 2 January 2022) [Google Scholar]
- Christie B. Covid-19: early studies give hope omicron is milder than other variants. BMJ. 2021;375:n3144. doi: 10.1136/bmj.n3144. [DOI] [PubMed] [Google Scholar]
- Czeisler M.É., Lane R.I., Petrosky E., et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic — United States, June 24–30, 2020. Morb. Mortal. Wkly. Rep. 2020;69(32):1049–1057. doi: 10.15585/mmwr.mm6932a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M., Sutin A.R., Robinson E. Longitudinal changes in mental health and the COVID-19 pandemic: evidence from the UK Household Longitudinal Study. Psychol. Med. 2020:1–10. doi: 10.1017/S0033291720004432. Published online November 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araújo T.V.B., Rodrigues L.C., de Alencar Ximenes R.A., et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect. Dis. 2016;16(12):1356–1363. doi: 10.1016/S1473-3099(16)30318-8. [DOI] [PubMed] [Google Scholar]
- Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., McCarthy P., Lange F., Andersson J.L., Griffanti L., Duff E., Jbabdi S. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022 Mar 7:1–7. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L., Shao X., Wang Y., et al. An investigation of mental health status of children and adolescents in China during the outbreak of COVID-19. J. Affect. Disord. 2020;275:112–118. doi: 10.1016/j.jad.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden D.H., Kleinschmidt-DeMasters B.K., LaGuardia J.J., Mahalingam R., Cohrs R.J. Neurologic complications of the reactivation of Varicella–Zoster virus. N. Engl. J. Med. 2000;342(9):635–645. doi: 10.1056/NEJM200003023420906. [DOI] [PubMed] [Google Scholar]
- Goenka A., Michael B.D., Ledger E., et al. Neurological manifestations of influenza infection in children and adults: results of a national British surveillance study. Clin. Infect. Dis. 2014;58(6):775–784. doi: 10.1093/cid/cit922. [DOI] [PubMed] [Google Scholar]
- Hall P., Fong G., Hitchman S. The Canadian COVID-19 experiences survey: study protocol. medRxiv. 2021;12(24):21268387. doi: 10.1101/2021.12.24.21268387. [DOI] [Google Scholar]
- Hall P.A., Sheeran P., Fong G.T., et al. Biobehavioral aspects of the COVID-19 pandemic: a review. Psychosom. Med. 2021;83(4):309–321. doi: 10.1097/PSY.0000000000000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Suls J. The symptom perception hypothesis revised: depression and anxiety play different roles in concurrent and retrospective physical symptom reporting. J. Pers. Soc. Psychol.. 20110110;100(1):182. doi:10.1037/a0021715. [DOI] [PubMed]
- Hampshire A., Trender W., Chamberlain S.R., Jolly A.E., Grant J.E., Patrick F., Mazibuko N., Williams S.C., Barnby J.M., Hellyer P., Mehta M.A. Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine. 2021;39(Sep 1):101044. doi: 10.1016/j.eclinm.2021.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaywant A., Vanderlind W.M., Alexopoulos G.S., Fridman C.B., Perlis R.H., Gunning F.M. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology. 2021;46(13):2235–2240. doi: 10.1038/s41386-021-00978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E., Heo J.H., Kim H ok, et al. Neurological complications during treatment of Middle East respiratory syndrome. J. Clin. Neurol. 2017;13(3):227. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus M.N., Bickel W.K. A 5-trial adjusting delay discounting task: accurate discount rates in less than one minute. Exp. Clin. Psychopharmacol. 2014;22(3):222–228. doi: 10.1037/a0035973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach M.L., Rolls E.T. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ladds E., Rushforth A., Wieringa S., et al. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv. Res. 2020;20(1):1144. doi: 10.1186/s12913-020-06001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.H., Wang Y.R., Wang Q.H., et al. Post-infection cognitive impairments in a cohort of elderly patients with COVID-19. Mol. Neurodegener. 2021;16(1):48. doi: 10.1186/s13024-021-00469-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massar S.A.A., Libedinsky C., Weiyan C., Huettel S.A., Chee M.W.L. Separate and overlapping brain areas encode subjective value during delay and effort discounting. Neuroimage. 2015;120:104–113. doi: 10.1016/j.neuroimage.2015.06.080. [DOI] [PubMed] [Google Scholar]
- McClure S.M., Laibson D.I., Loewenstein G., Cohen J.D. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McFadyen J.D., Stevens H., Peter K. The emerging threat of (Micro)Thrombosis in COVID-19 and its therapeutic implications. Circ. Res. 2020;127(4):571–587. doi: 10.1161/CIRCRESAHA.120.317447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauen D.W., Hooper J.E., Stewart C.M., Solomon I.H. Assessing brain capillaries in coronavirus disease 2019. JAMA Neurol. 2021;78(6):760–762. doi: 10.1001/jamaneurol.2021.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., D'Esposito M. Effects of medial orbitofrontal cortex lesions on self-control in intertemporal choice. Curr. Biol. 2016;26(19):2625–2628. doi: 10.1016/j.cub.2016.07.035. [DOI] [PubMed] [Google Scholar]
- Reimers S., Maylor E.A., Stewart N., Chater N. Associations between a one-shot delay discounting measure and age, income, education and real-world impulsive behavior. Pers. Indiv. Differ. 2009;47(8):973–978. doi: 10.1016/j.paid.2009.07.026. [DOI] [Google Scholar]
- Ritchie K., Chan D. The emergence of cognitive COVID. World Psychiatr. 2021;20(1):52–53. doi: 10.1002/wps.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. As their numbers grow, COVID-19 “long Haulers” stump experts. JAMA. 2020;324(14):1381–1383. doi: 10.1001/jama.2020.17709. [DOI] [PubMed] [Google Scholar]
- Sarubbo F., El Haji K., Vidal-Balle A., Bargay Lleonart J. Neurological consequences of COVID-19 and brain related pathogenic mechanisms: a new challenge for neuroscience. Brain Behav. Immun - Health. 2022;19:100399. doi: 10.1016/j.bbih.2021.100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellitto M., Ciaramelli E., di Pellegrino G. Myopic discounting of future rewards after medial orbitofrontal damage in Humans. J. Neurosci. 2010;30(49):16429–16436. doi: 10.1523/JNEUROSCI.2516-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh A., Kerr S., Woolhouse M., McMenamin J., Robertson C. Severity of Omicron variant of concern and vaccine effectiveness against symptomatic disease: national cohort with nested test negative design study in Scotland. 2021. https://www.research.ed.ac.uk/en/publications/severity-of-omicron-variant-of-concern-and-vaccine-effectiveness- Published online December 22. [DOI] [PMC free article] [PubMed]
- Solomon T. Neurological infection with SARS-CoV-2 — the story so far. Nat. Rev. Neurol. 2021;17(2):65–66. doi: 10.1038/s41582-020-00453-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatr. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A., Salzano G., Deiana G., De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020;130(7):1787. doi: 10.1002/lary.28692. 1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley C.A., Theobald D.E.H., Dalley J.W., Cardinal R.N., Robbins T.W. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cerebr. Cortex. 2006;16(1):106–114. doi: 10.1093/cercor/bhi088. [DOI] [PubMed] [Google Scholar]
- Xiong J., Lipsitz O., Nasri F., et al. Impact of COVID-19 pandemic on mental health in the general population: a systematic review. J. Affect. Disord. 2020;277:55–64. doi: 10.1016/j.jad.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available upon reasonable request to either of the corresponding authors.