Abstract
Background
Estimating life expectancy of older adults informs whether to pursue future investigation and therapy. Several models to predict mortality have been developed but often require data not immediately available during routine clinical care. The HOSPITAL score and the LACE index were previously validated to predict 30-day readmissions but may also help to assess mortality risk. We assessed their performance to predict 1-year and 30-day mortality in hospitalized older multimorbid patients with polypharmacy.
Methods
We calculated the HOSPITAL score and LACE index in patients from the OPERAM (OPtimising thERapy to prevent Avoidable hospital admissions in the Multimorbid elderly) trial (patients aged ≥ 70 years with multimorbidity and polypharmacy, admitted to hospital across four European countries in 2016–2018). Our primary and secondary outcomes were 1-year and 30-day mortality. We assessed the overall accuracy (scaled Brier score, the lower the better), calibration (predicted/observed proportions), and discrimination (C-statistic) of the models.
Results
Within 1 year, 375/1879 (20.0%) patients had died, including 94 deaths within 30 days. The overall accuracy was good and similar for both models (scaled Brier score 0.01–0.08). The C-statistics were identical for both models (0.69 for 1-year mortality, p = 0.81; 0.66 for 30-day mortality, p = 0.94). Calibration showed well-matching predicted/observed proportions.
Conclusion
The HOSPITAL score and LACE index showed similar performance to predict 1-year and 30-day mortality in older multimorbid patients with polypharmacy. Their overall accuracy was good, their discrimination low to moderate, and the calibration good. These simple tools may help predict older multimorbid patients’ mortality after hospitalization, which may inform post-hospitalization intensity of care.
Key Points
| The HOSPITAL score and LACE index showed good accuracy to predict mortality in older patients with multiple medications and comorbidities. |
| The HOSPITAL score and LACE index can help clinicians and patients to make decisions about investigations and treatment after hospitalization. |
Introduction
Accurately predicting mortality from readily available clinical data is critical to informing future diagnostic and treatment choices. This is especially important in potentially high-risk individuals such as older patients with multimorbidity, which affects 60% of adults aged ≥ 65 years and is associated with polypharmacy, lower quality of life, higher healthcare resource utilization and mortality, and substantial burden for patients, caregivers, and healthcare systems [1–5]. Increased healthcare use and polypharmacy in older multimorbid patients is partly related to screening procedures and preventive medications, which may not be appropriate for patients whose survival time is likely too short to benefit [6–8]. In this context, the harm and burden of additional tests and medications may outweigh the benefits. Estimating life expectancy may thus be useful for patients and caregivers to make informed decisions about the need for further screening and preventive care. We can hypothesize that avoiding procedures or medications that are no longer appropriate might in turn improve the quality of life of patients with limited life expectancy as well as reduce overuse of care.
Several models have been developed to predict mortality in recent years [9–14]; however, most of them were developed in a single country or a specific population (e.g., palliative care) and have not been externally validated in different older person populations in different countries. Furthermore, none of those models has yet been widely used and implemented in clinical practice, possibly due to their complexity beyond data available during routine clinical care. For example, some models require assessment of functional status [11, 12], which is not routinely collected in electronic medical records. Simpler tools are thus needed.
The HOSPITAL score and the LACE index have been developed and broadly validated in different countries and populations to predict unavoidable hospital readmission (HOSPITAL score) and death or non-elective readmission (LACE index) within 30 days after discharge [15–24]. These models are less complex than most previous scores and are easily computed with routinely available electronic medical records data. Therefore, these models have the potential for increased large-scale implementation.
Given likely similar risk factors for death and readmission, we hypothesized that the HOSPITAL score and the LACE index could accurately predict mortality. The aim of this study was therefore to assess the performance of the HOSPITAL score and the LACE index to predict 1-year and 30-day mortality following an acute care hospitalization in older multimorbid patients with polypharmacy.
Methods
Study Design and Population
We included all patients with completed follow-up from the OPERAM (OPtimising thERapy to prevent Avoidable hospital admissions in the Multimorbid elderly) trial. The design and results of this multicenter European trial, which aimed to reduce inappropriate prescribing as a means of preventing drug-related admissions (DRAs), have been described previously [25, 26]. Enrolled patients were aged ≥ 70 years, had multimorbidity (three or more chronic conditions), and polypharmacy (five or more chronic medications), and were admitted to a medical or surgical ward for an acute care hospitalization. Patients who died before discharge were not included in the OPERAM trial. Participating countries were from four hospitals in Belgium, Ireland, The Netherlands, and Switzerland (patient randomization occurred from December 2016 to October 2018). The OPERAM trial’s primary outcome was any DRA following index hospitalization, while 1-year post-discharge mortality was a secondary outcome. All variables (including those required to calculate the HOSPITAL score and the LACE index) were collected prospectively and systematically in the OPERAM trial according to a predefined protocol. The baseline visit consisted of a face-to-face interview. Follow-up visits were conducted by phone with the patient and/or their relatives and/or their general practitioner. Diagnoses were extracted from electronic medical records.
Predictors: HOSPITAL Score and LACE Index
The HOSPITAL score was computed according to its simplified version, in which the variable ‘procedures’ is omitted and ‘oncologic diagnosis’ is used instead of oncology ward (as oncology wards were not part of the OPERAM trial), resulting in a total of six items for a maximum of 12 points (Table 1) [17, 27]. Furthermore, we used the threshold of ≥ 8 days for prolonged length of stay, which has been validated in Europe, instead of the 5-day cut-off employed in the US [15]. The LACE index was used in its original version, consisting of four variables for a maximum of 19 points (Table 1) [19]. To compute the Charlson Comorbidity Index included in the LACE index, we used the method developed by Quan et al., based on International Classification of Diseases (ICD) codes [28]. Data were missing in 6 (0.3%) patients for previous hospitalizations, 8 patients (0.4%) for previous emergency room visits, 12 patients (0.6%) for serum sodium, and 17 patients (0.9%) for hemoglobin. These missing data points were assigned a score of zero (i.e., coded as normal) in the application of both prediction models, as done in the derivation study of the HOSPITAL score [17].
Table 1.
HOSPITAL score and LACE index
| Variable | Points if positive |
|---|---|
| HOSPITAL score simplified | |
| Hemoglobin level at discharge < 120 g/L | 1 |
| Cancer diagnosis or discharge from an Oncology division | 2 |
| Sodium level at discharge < 135 mmol/L | 1 |
| Any ICD-9 or ICD-10 Procedure during hospitalizationa | NA |
| Index Type of admission: non-elective | 1 |
| Number of hospital Admissions during the previous 12 months | |
| 0–1 | 0 |
| 2–5 | 2 |
| ≥ 5 | 5 |
| Length of stay ≥ 8 days (Europe) or ≥ 5 days (US) | 2 |
| Maximum number of points | 13 |
| LACE index | |
| Length of stay (days) | |
| <1 | 0 |
| 1 | 1 |
| 2 | 2 |
| 3 | 3 |
| 4–6 | 4 |
| 7–13 | 5 |
| ≥ 14 | 7 |
| Acute (emergent) admission | 3 |
| Charlson Comorbidity Index | |
| 0 | 0 |
| 1 | 1 |
| 2 | 2 |
| 3 | 3 |
| ≥ 4 | 5 |
| Emergent department visits < 6 months | |
| 0 | 0 |
| 1 | 1 |
| 2 | 2 |
| 3 | 3 |
| ≥ 4 | 4 |
| Maximum number of points | 19 |
Outcomes
The primary outcome of the present analysis was all-cause mortality within 1 year after discharge of the index hospitalization, while all-cause mortality within 30 days after discharge was the secondary outcome. Mortality was determined based on the follow-up phone call. If no-one among the patients, their relatives/contact persons and their general practitioner could be reached by phone, we contacted their residential place.
Statistical Analyses
We assessed the performance of each model according to its overall accuracy, discrimination and calibration. First, to assess and compare the overall accuracy of the models, we computed the scaled Brier score, as described by Steyerberg et al. (the lower the score, the better) [29, 30]. The overall accuracy refers to the probability that the score correctly classifies the individuals [31]. Second, to assess discrimination (i.e., whether the score separates well lower- vs. higher-risk individuals; 0.5 = no discrimination, 1 = perfect discrimination) [29], we obtained C-statistics for each model and used bootstrapping with 1000 replications to compute 95% confidence intervals (CIs). We further computed the discrimination slope, defined as the absolute difference in mean predicted risk for those with the outcome compared with those without the outcome [29, 32, 33]. To facilitate visualization, we presented boxplots of the predicted risk according to outcome occurrence. We compared discrimination of the HOSPITAL score and the LACE index by assessing the equality of their C-statistics. Finally, to assess calibration (i.e., the agreement between predictions and observed outcomes) [29], we displayed predicted versus observed proportions of death according to (1) lower- and higher-risk categories, and (2) score point categories. We evaluated statistical significance using the Hosmer–Lemeshow goodness-of-fit test (a significant p-value would indicate an overall lack of fit) [29]. We performed all analyses using Stata/MP 16.0 (StataCorp LLC, College Station, TX, USA).
Results
Baseline Characteristics and Mortality Rates
Among the 2008 patients included in the OPERAM trial, 119 withdrew consent and 10 were lost to follow-up, leaving 1879 patients for analysis. Among these, the mean age was 79.4 years (standard deviation [SD] 6.3), with 835 (44.4%) females and a median (interquartile range) number of chronic medications of 9 (7–12). Within 1 year of discharge, 375 (20.0%) patients had died, among whom 94 patients (25.1%; 5.0% overall) within 30 days. Mortality rates during the 12-month follow-up varied by country (Table 2). Baseline characteristics according to death within 1 year of discharge are described in Table 2. Patients who died were older, had higher morbidity (Charlson Comorbidity Index, cancer diagnosis), and received more medications than patients who survived follow-up. They also had a longer length of stay and were less frequently discharged home or to a nursing home. The HOSPITAL score ranged from 0 to 11 points (mean 3.7 [SD 2.0], median 4) and the LACE index ranged from 2 to 19 points (mean 11.2 [SD 2.9], median 11).
Table 2.
Baseline characteristics according to death at 1 year
| Characteristic | Dead [n = 375] | Alive [n = 1504] |
|---|---|---|
| Age, years | 81.3 (7.0) | 78.9 (6.0) |
| Female | 162 (43.2) | 673 (44.8) |
| Site of inclusion | ||
| Belgium | 110 (29.3) | 296 (19.7) |
| Ireland | 48 (12.8) | 290 (19.3) |
| The Netherlands | 64 (17.1) | 266 (17.7) |
| Switzerland | 153 (40.8) | 652 (43.4) |
| Morbidity | ||
| Charlson Comorbidity Index, points | 3.5 (2.4) | 3.4 (1.9) |
| Cancer diagnosis | 143 (38.1) | 377 (25.1) |
| Chronic medications, n | 11.4 (4.5) | 9.9 (4.1) |
| Healthcare utilization history | ||
| Hospitalizations <1 year, n | 1.4 (1.6) | 0.9 (1.4) |
| Emergent visits <6 months, n | 3.8 (5.9) | 3.8 (6.8) |
| Index hospitalization | ||
| Non-elective admission | 314 (83.7) | 1124 (74.7) |
| Surgical ward | 54 (14.4) | 328 (21.8) |
| Last available hemoglobin < 120 g/L | 271 (72.3) | 874 (58.1) |
| Last available sodium < 135 mmol/L | 64 (17.1) | 148 (9.8) |
| Length of stay, days | 17.6 (25.0) | 10.6 (9.2) |
| Discharge home | 167 (52.7) | 1044 (69.7) |
| Discharge to a nursing home | 49 (15.5) | 97 (6.5) |
| HOSPITAL score, points | 4.8 (2.0) | 2.5 (1.9) |
| LACE index, points | 12.2 (2.7) | 11.0 (2.8) |
Data are n (%) or mean (SD)
Categorical variables are expressed as n (%) and continuous variables are expressed as mean with standard deviation
Primary Outcome: 1-Year Mortality
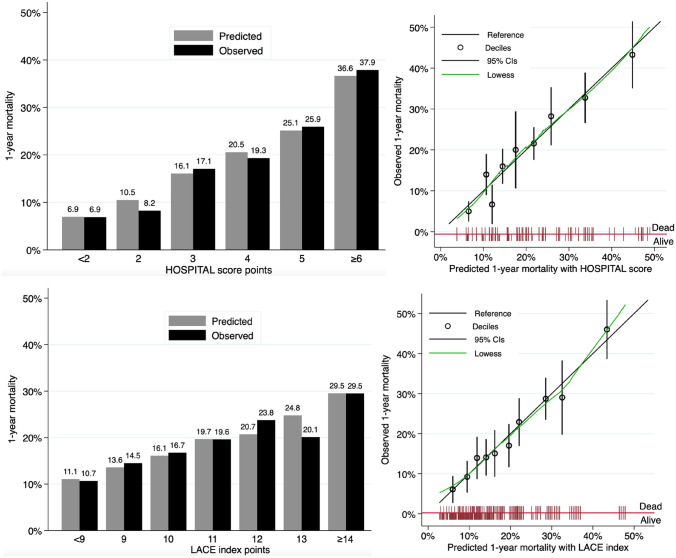
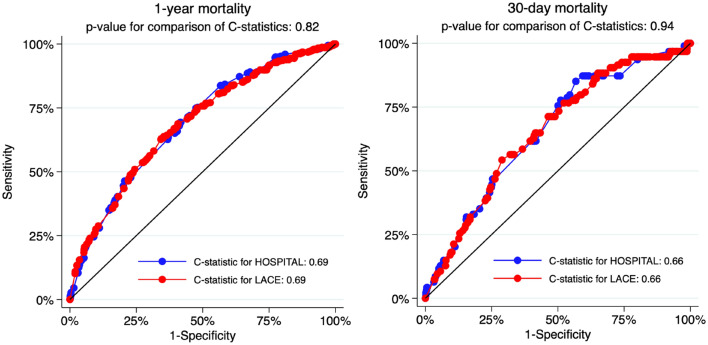
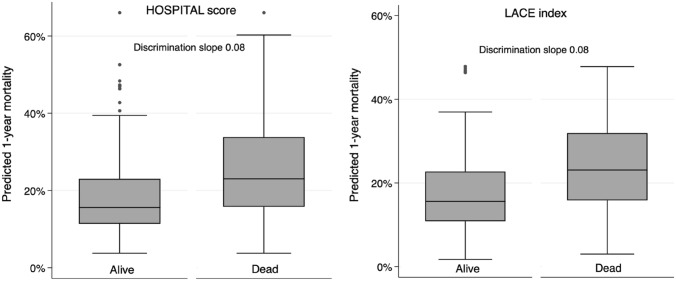
The overall accuracy for prediction of 1-year mortality was good, with a scaled Brier score of 0.08 for both models. Calibration assessment showed no systematic deviation from the reference line, with well-matching predicted and observed proportions overall (Fig. 1, Appendix Table 3). The Hosmer–Lemeshow C-statistic test indicated no overall lack of fit, with a p-value of 0.37 for the HOSPITAL score and 0.95 for the LACE index. The discrimination of the two models was similar, with a C-statistic (95% CI) of 0.69 (0.66–0.72) for the HOSPITAL score and 0.69 (0.66–0.72) for the LACE index (p-value for comparison of the C-statistics: 0.81) (Appendix Fig. 3). The discrimination slope was 0.08 for both models (Fig. 2).
Fig. 1.
Calibration of the HOSPITAL score and LACE index to predict 1-year mortality. CIs confidence intervals. Predicted vs. observed mortality at 1 year after discharge score points (left panels) and deciles (right panels)
Table 3.
Predicted and observed mortality at 30 days and 1 year for the HOSPITAL score and the LACE index, by deciles of equally sized groups, and point categories
| N | 30-Day mortality | 1-Year mortality | |||
|---|---|---|---|---|---|
| Predicted (%) | Observed (%) | Predicted (%) | Observed | ||
| HOSPITAL score deciles | |||||
| Decile 1 | 187 | 1.7 | 1.7 | 6.0 | 6.4 |
| Decile 2 | 188 | 2.3 | 1.6 | 8.2 | 4.8 |
| Decile 3 | 188 | 2.9 | 3.2 | 11.6 | 12.8 |
| Decile 4 | 188 | 3.3 | 0.0 | 13.4 | 12.2 |
| Decile 5 | 188 | 4.3 | 8.0 | 16.3 | 20.2 |
| Decile 6 | 188 | 4.8 | 5.3 | 20.2 | 22.9 |
| Decile 7 | 188 | 5.8 | 5.9 | 23.0 | 19.7 |
| Decile 8 | 188 | 6.3 | 8.4 | 25.0 | 27.7 |
| Decile 9 | 188 | 8.0 | 8.0 | 33.5 | 30.3 |
| Decile 10 | 188 | 10.6 | 9.0 | 42.3 | 42.6 |
| HOSPITAL score points | |||||
| 0 point | 58 | 1.2 | 3.4 | 4.4 | 5.2 |
| 1 point | 218 | 2.1 | 3.2 | 7.6 | 7.6 |
| 2 points | 243 | 2.6 | 0.4 | 10.5 | 10.5 |
| 3 points | 387 | 4.2 | 4.9 | 16.1 | 17.1 |
| 4 points | 368 | 5.0 | 4.3 | 20.5 | 19.3 |
| 5 points | 251 | 6.2 | 6.4 | 25.1 | 25.9 |
| 6 points | 195 | 7.9 | 9.2 | 32.7 | 33.3 |
| 7 points | 95 | 9.0 | 9.5 | 37.5 | 37.9 |
| 8 points | 43 | 11.2 | 7.0 | 44.5 | 44.2 |
| 9 points | 14 | 13.1 | 7.1 | 50.1 | 64.3 |
| 10 points | 3 | 13.2 | 33.3 | 50.8 | 100.0 |
| 11 points | 4 | 20.2 | 25.0 | 66.0 | 50.0 |
| LACE index deciles | |||||
| Decile 1 | 187 | 3.0 | 3.7 | 11.0 | 10.7 |
| Decile 2 | 188 | 3.4 | 1.1 | 11.8 | 13.3 |
| Decile 3 | 188 | 3.8 | 1.1 | 14.2 | 11.2 |
| Decile 4 | 188 | 5.1 | 5.9 | 15.9 | 18.6 |
| Decile 5 | 188 | 5.0 | 4.9 | 20.1 | 23.4 |
| Decile 6 | 188 | 5.5 | 4.3 | 19.4 | 13.8 |
| Decile 7 | 188 | 5.3 | 5.9 | 21.4 | 26.1 |
| Decile 8 | 188 | 5.6 | 5.9 | 26.8 | 24.4 |
| Decile 9 | 188 | 6.6 | 6.9 | 29.0 | 31.9 |
| Decile 10 | 188 | 6.8 | 10.6 | 29.8 | 26.1 |
| LACE index points | |||||
| 2 points | 2 | 1.1 | 0.0 | 2.8 | 0.0 |
| 3 points | 3 | 1.4 | 0.0 | 3.9 | 0.0 |
| 4 points | 4 | 1.8 | 0.0 | 5.3 | 0.0 |
| 5 points | 27 | 2.1 | 3.7 | 6.5 | 7.4 |
| 6 points | 52 | 2.7 | 0.0 | 9.0 | 3.8 |
| 7 points | 92 | 3.1 | 3.3 | 11.0 | 14.1 |
| 8 points | 328 | 3.2 | 2.4 | 11.1 | 10.7 |
| 9 points | 193 | 3.9 | 4.7 | 13.6 | 14.5 |
| 10 points | 257 | 4.3 | 5.4 | 16.1 | 16.7 |
| 11 points | 260 | 4.9 | 5.8 | 19.7 | 19.6 |
| 12 points | 223 | 5.3 | 4.0 | 20.7 | 23.8 |
| 13 points | 184 | 5.9 | 6.0 | 24.8 | 20.1 |
| 14 points | 224 | 6.6 | 5.8 | 29.5 | 29.5 |
| 15 points | 54 | 4.1 | 1.9 | 18.6 | 22.2 |
| 16 points | 98 | 9.5 | 9.2 | 39.8 | 44.9 |
| 17 points | 39 | 4.8 | 7.7 | 23.4 | 28.2 |
| 18 points | 3 | 6.8 | 33.3 | 35.2 | 33.3 |
| 19 points | 16 | 7.2 | 6.2 | 35.7 | 25.0 |
N number of patients in each category
Fig. 3.
Comparison of the C-statistics for the HOSPITAL score and LACE index for 1-year and 30-day mortality
Fig. 2.
Boxplots and discrimination slope for predicted mortality according to death status at 1 year. The discrimination slopes were calculated as the mean predicted mortality in patients dead minus the mean predicted mortality in patients alive
Secondary Outcome: 30-Day Mortality
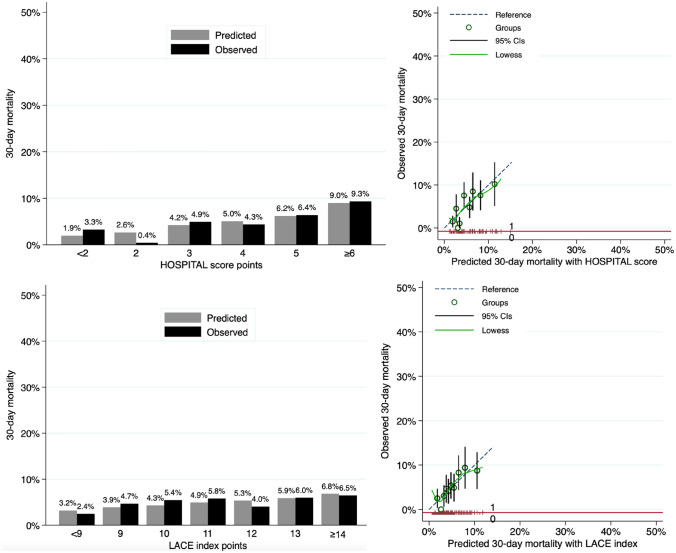
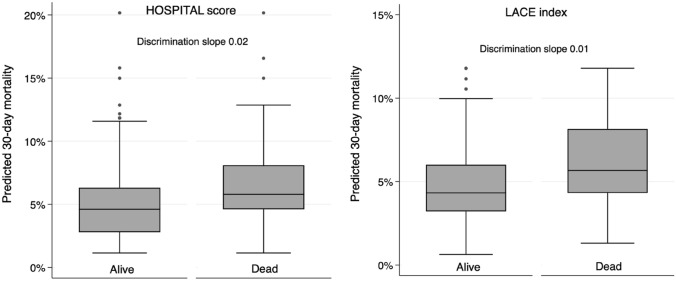
The overall accuracy for the prediction of 30-day mortality was good, with a scaled Brier score of 0.02 for the HOSPITAL score and 0.01 for the LACE index. Calibration assessment showed no systematic deviation from the reference line, with well-matching predicted and observed proportions overall (Appendix Fig. 4, Appendix Table 3). The Hosmer–Lemeshow goodness-of-fit C-statistic p-value was 0.02 for the HOSPITAL score and 0.41 for the LACE index. The discriminatory power of the two models was similar, with a C-statistic (95% CI) of 0.66 (0.61–0.71) for the HOSPITAL score and 0.66 (0.60–0.71) for the LACE index (p-value for comparison of C-statistics: 0.94) (Appendix Fig. 3). The discrimination slope was 0.02 for the HOSPITAL score and 0.01 for the LACE index (Appendix Fig. 5).
Fig. 4.
Calibration of the HOSPITAL score and the LACE index to predict 30-day mortality. CIs confidence intervals. Predicted vs. observed mortality at 30 days after discharge by score points (left panels) and by deciles (right panels)
Fig. 5.
Boxplots and discrimination slope for predicted mortality according to death at 30 days. The discrimination slopes were calculated as the mean predicted mortality in patients dead minus the mean predicted mortality in patients alive
Discussion
In this multicenter trial, the HOSPITAL score and the LACE index showed good overall performance for predicting 30-day and 1-year mortality after an acute medical or surgical hospitalization in older multimorbid patients. Discrimination was moderate and similar for both models (0.69; 0.5 = no discrimination, 1 = perfect discrimination). As mortality prediction tools, the HOSPITAL score and the LACE index are easy to use and may thus help to evaluate life expectancy in older multimorbid patients. The predicted risk of death according to the number of score points can provide useful information to both patients and caregivers when deciding upon further need for screening procedures and preventive medications, whose potential adverse effects may outweigh the expected benefit for patients when the time to benefit is too long.
Previous studies have developed various scores to predict mortality in hospital settings [9–14]. Some of those scores showed good performance but none of them has yet been broadly implemented to estimate life expectancy. This might be explained by the items included in the models, which may be relatively complex to collect in a standardized way in clinical practice. For example, the Walter Index [12] and the Burden of Illness Score for Elderly Persons (BISEP) [11] include functional status evaluation, which is partly subjective and affected by the method of inquiry about functioning [34]. In addition, this assessment may represent extra workload for healthcare professionals, or additional chart abstraction, which often limits implementation. In contrast, the HOSPITAL score and the LACE index have the advantage of including only items that can be automatically and easily retrieved from electronic medical records, increasing their scalability. The HOSPITAL score has the additional benefit of not requiring assessment of comorbidities, except for active oncological disease, which is unlikely to be frequently omitted in medical records, given the usually highly significant impact of this diagnosis. In contrast, the LACE index requires the calculation of the Charlson Comorbidity Index, whose calculation may be subject to coding quality and underreporting.
In this study, we found lower discrimination for the HOSPITAL score and the LACE index, compared with other survival prognostic tools (C-statistics 0.75–0.90) [10, 12–14]. However, studies developing such prognostic tools were conducted retrospectively, in a single country, in medical (i.e. non-surgical) patients only, or in selected populations, limiting their generalizability to other settings [9–14]. For example, the CARING criteria were developed by retrospective review of Veterans’ charts, so that generalizability to females is unknown [10]. In contrast, the OPERAM trial was a prospective multinational study in unselected older adults with multimorbidity, which enhances our generalizability. It is also noteworthy that the C-statistic dropped from 0.76 in the development cohort to 0.59 only in the validation cohort for the high-risk category of the BISEP model, suggesting that the performance of a prediction model may not be similar in populations or settings that differ from the development cohort [11]. Further studies should compare the HOSPITAL score and the LACE index with other prediction models in similar populations.
Cut-offs are commonly used to categorize a screening or diagnosis test as normal or pathological, although in reality they most often correspond to a continuum between health and illness. Similarly, categories have frequently been created to classify patients at low, moderate, or high risk of death [9–13]. This approach has the disadvantage of attributing a similar prediction to patients with the lowest or those with the highest number of points merged in the same category, although they may actually have a significantly different predicted mortality risk. Estimating a probability for each possible number of points, as we did in this study, and similar to what was done with other prediction models (e.g., Framingham score for cardiovascular mortality risk) [35], may thus be more informative.
Accurately estimating life expectancy is critical towards understanding whether a patient is expected to live long enough to benefit from screening tests or long-term preventative treatments. For example, while it takes approximately 10 years to prevent one death from colorectal or breast cancer from screening 1000 patients [7], time to benefit for statin treatment to prevent myocardial infarction is approximately 2–5 years [6], and 11 months for alendronate treatment to prevent one fragility fracture [8]. Tools predicting mortality, such as the HOSPITAL score or the LACE index, may enable patients to make healthcare choices to better align with their personalized goals, accounting for overall benefit [36].
Whereas previous models were developed to predict mortality over 1 year or longer, we also studied short-term (30-day) mortality. Overall performance was similar for 1-year and 30-day mortality. These models may thus play a role in predicting short-term mortality also, whose clinical implications may be different than for longer-term mortality. This estimation may indeed help to reduce uncertainty about short-term prognosis and consequently inform end-of-life conversations between caregivers and patients. This helps to focus end-of-life care on the patients where appropriate, avoid treatments or procedures whose benefit may be time-limited and that might not be acceptable to the patients, and thus potentially to improve quality of life during the final weeks and months of life of older people.
Limitations and Strengths
We must acknowledge some limitations. First, the Charlson Comorbidity Index used in the LACE index is based on ICD codes, which might be subject to underreporting. Second, patients who died during hospitalization were not included, and we included only patients aged ≥ 70 years with multimorbidity and polypharmacy, limiting generalizability to other populations. Third, the number of outcomes at 30 days was limited. Finally, the population was part of a clinical trial that aimed to optimize prescribing and might thus have influenced mortality; however, this risk is rather unlikely given that the trial was negative and that we adjusted for the intervention arm [26].
This study presents some significant strengths. First, we used prospective data systematically collected along the OPERAM trial. Second, we compared two prediction models and assessed both short- and long-term mortality. Third, there were only few missing data for the predictive variables (< 1% for each). Finally, our study was larger than most previous studies [14], was conducted in four different countries, and included both surgical and medical patients with only a few exclusion criteria (i.e. real-world patients, including patients with dementia, were enrolled) [25], increasing the generalizability of our findings.
Conclusion
In hospitalized older multimorbid patients, the HOSPITAL score and LACE index showed very good overall accuracy (i.e., very low Brier score), good calibration (i.e., well-matching predicted and observed proportions) and moderate discrimination to predict 1-year mortality. Their performance was slightly lower for 30-day mortality. These simple tools may help predict mortality risk in older multimorbid patients after acute hospitalization, which may inform post-hospitalization intensity of care.
Appendix
Declarations
Funding
CEA was supported by an Early Postdoc.Mobility grant from the Swiss National Science Foundation (Grant P2LAP3_184042), and JD was funded by the Swiss National Science Foundation. This work is part of the ‘OPERAM: OPtimising thERapy to prevent Avoidable hospital admissions in the Multimorbid elderly’ project supported by the European Union’s Horizon 2020 research and innovation programme under Grant agreement no. 6342388, and by the Swiss State Secretariat for Education, Research and Innovation (SERI) under contract number 15.0137. The OPERAM study is also funded by the Swiss National Scientific Foundation (SNSF 320030_188549). The opinions expressed and arguments employed herein are those of the authors and do not necessarily reflect the official views of the European Commission and the Swiss government. The funders had no roles in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Open access funding provided by University of Bern.
Conflict of interest
Carole E. Aubert, Nicolas Rodondi, Samuel W. Terman, Martin Feller, Claudio Schneider, Jolanda Oberle, Olivia Dalleur, Wilma Knol, Denis O’Mahony, Drahomir Aujesky, and Jacques Donzé declare that they have no conflicts of interest.
Availability of data, material and code
The original data from the trial cannot be made available but the codes are available upon reasonable request.
Authors’ contributions
Conception of the project: CEA, NR, JD. Data collection: NR, DOM, OD, WK, JO. Data analysis and interpretation: CEA, JD, SWT. Drafting of the manuscript: CEA. Critical revision of the manuscript: All authors.
Ethics approval
The OPERAM trial was approved by all local Ethics Committees. No additional approval was requested for this subanalysis.
Consent for publication
All authors approved the current manuscript for publication.
Contributor Information
Carole E. Aubert, Email: caroleelodie.aubert@insel.ch
Nicolas Rodondi, Email: nicolas.rodondi@insel.ch.
Samuel W. Terman, Email: sterman@umich.edu
Martin Feller, Email: martin.feller@extern.insel.ch.
Claudio Schneider, Email: claudio.scheider@insel.ch.
Jolanda Oberle, Email: jolanda.oberle@insel.ch.
Olivia Dalleur, Email: olivia.dalleur@uclouvain.be.
Wilma Knol, Email: w.knol@umcutrecht.nl.
Denis O’Mahony, Email: denis.omahony@ucc.ie.
Drahomir Aujesky, Email: drahomir.aujesky@insel.ch.
Jacques Donzé, Email: jacques.donze@rhne.ch.
References
- 1.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 2.Fortin M, Bravo G, Hudon C, Lapointe L, Almirall J, Dubois MF, et al. Relationship between multimorbidity and health-related quality of life of patients in primary care. Qual Life Res. 2006;15(1):83–91. doi: 10.1007/s11136-005-8661-z. [DOI] [PubMed] [Google Scholar]
- 3.Bahler C, Huber CA, Brungger B, Reich O. Multimorbidity, health care utilization and costs in an elderly community-dwelling population: a claims data based observational study. BMC Health Serv Res. 2015;15:23. doi: 10.1186/s12913-015-0698-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Librero J, Peiro S, Ordinana R. Chronic comorbidity and outcomes of hospital care: length of stay, mortality, and readmission at 30 and 365 days. J Clin Epidemiol. 1999;52(3):171–179. doi: 10.1016/S0895-4356(98)00160-7. [DOI] [PubMed] [Google Scholar]
- 5.Payne RA. The epidemiology of polypharmacy. Clin Med (Lond) 2016;16(5):465–469. doi: 10.7861/clinmedicine.16-5-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes HM, Min LC, Yee M, Varadhan R, Basran J, Dale W, et al. Rationalizing prescribing for older patients with multimorbidity: considering time to benefit. Drugs Aging. 2013;30(9):655–666. doi: 10.1007/s40266-013-0095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O'Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441. [DOI] [PMC free article] [PubMed]
- 8.van de Glind EM, Willems HC, Eslami S, Abu-Hanna A, Lems WF, Hooft L, et al. Estimating the time to benefit for preventive drugs with the statistical process control method: an example with alendronate. Drugs Aging. 2016;33(5):347–353. doi: 10.1007/s40266-016-0344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Bari M, Balzi D, Roberts AT, Barchielli A, Fumagalli S, Ungar A, et al. Prognostic stratification of older persons based on simple administrative data: development and validation of the "Silver Code," to be used in emergency department triage. J Gerontol A Biol Sci Med Sci. 2010;65(2):159–164. doi: 10.1093/gerona/glp043. [DOI] [PubMed] [Google Scholar]
- 10.Fischer SM, Gozansky WS, Sauaia A, Min SJ, Kutner JS, Kramer A. A practical tool to identify patients who may benefit from a palliative approach: the CARING criteria. J Pain Symptom Manag. 2006;31(4):285–292. doi: 10.1016/j.jpainsymman.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Inouye SK, Bogardus ST, Jr, Vitagliano G, Desai MM, Williams CS, Grady JN, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70–83. doi: 10.1097/00005650-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Walter LC, Brand RJ, Counsell SR, Palmer RM, Landefeld CS, Fortinsky RH, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285(23):2987–2994. doi: 10.1001/jama.285.23.2987. [DOI] [PubMed] [Google Scholar]
- 13.Richardson P, Greenslade J, Shanmugathasan S, Doucet K, Widdicombe N, Chu K, et al. PREDICT: a diagnostic accuracy study of a tool for predicting mortality within one year: who should have an advance healthcare directive? Palliat Med. 2015;29(1):31–37. doi: 10.1177/0269216314540734. [DOI] [PubMed] [Google Scholar]
- 14.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aubert CE, Folly A, Mancinetti M, Hayoz D, Donze J. Prospective validation and adaptation of the HOSPITAL score to predict high risk of unplanned readmission of medical patients. Swiss Med Wkly. 2016;146:w14335. [DOI] [PubMed]
- 16.Cooksley T, Nanayakkara PW, Nickel CH, Subbe CP, Kellett J, Kidney R, et al. Readmissions of medical patients: an external validation of two existing prediction scores. QJM Mon J Assoc Physicians. 2016;109(4):245–248. doi: 10.1093/qjmed/hcv130. [DOI] [PubMed] [Google Scholar]
- 17.Donze J, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632–638. doi: 10.1001/jamainternmed.2013.3023. [DOI] [PubMed] [Google Scholar]
- 18.Donze JD, Williams MV, Robinson EJ, Zimlichman E, Aujesky D, Vasilevskis EE, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496–502. doi: 10.1001/jamainternmed.2015.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Walraven C, Dhalla IA, Bell C, Etchells E, Stiell IG, Zarnke K, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551–557. doi: 10.1503/cmaj.091117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damery S, Combes G. Evaluating the predictive strength of the LACE index in identifying patients at high risk of hospital readmission following an inpatient episode: a retrospective cohort study. BMJ Open. 2017;7(7):e016921. [DOI] [PMC free article] [PubMed]
- 21.Heppleston E, Fry CH, Kelly K, Shepherd B, Wright R, Jones G, et al. LACE index predicts age-specific unplanned readmissions and mortality after hospital discharge. Aging Clin Exp Res. 2021;33(4):1041–1048. doi: 10.1007/s40520-020-01609-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson R, Hudali T. The HOSPITAL score and LACE index as predictors of 30 day readmission in a retrospective study at a university-affiliated community hospital. PeerJ. 2017;5:e3137. [DOI] [PMC free article] [PubMed]
- 23.Burke RE, Schnipper JL, Williams MV, Robinson EJ, Vasilevskis EE, Kripalani S, et al. The HOSPITAL score predicts potentially preventable 30-day readmissions in conditions targeted by the hospital readmissions reduction program. Med Care. 2017;55(3):285–290. doi: 10.1097/MLR.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibrahim AM, Koester C, Al-Akchar M, Tandan N, Regmi M, Bhattarai M, et al. HOSPITAL Score, LACE Index and LACE+ Index as predictors of 30-day readmission in patients with heart failure. BMJ Evid Based Med. 2020;25(5):166–167. doi: 10.1136/bmjebm-2019-111271. [DOI] [PubMed] [Google Scholar]
- 25.Adam L, Moutzouri E, Baumgartner C, Loewe AL, Feller M, M'Rabet-Bensalah K, et al. Rationale and design of OPtimising thERapy to prevent avoidable hospital admissions in multimorbid older people (OPERAM): a cluster randomised controlled trial. BMJ Open. 2019;9(6):e026769. [DOI] [PMC free article] [PubMed]
- 26.Blum MR, Sallevelt B, Spinewine A, O'Mahony D, Moutzouri E, Feller M, et al. Optimizing therapy to prevent avoidable hospital admissions in multimorbid older adults (OPERAM): cluster randomised controlled trial. BMJ. 2021;374:n1585. [DOI] [PMC free article] [PubMed]
- 27.Aubert CE, Schnipper JL, Williams MV, Robinson EJ, Zimlichman E, Vasilevskis EE, et al. Simplification of the HOSPITAL score for predicting 30-day readmissions. BMJ Qual Saf. 2017;26(10):799–805. doi: 10.1136/bmjqs-2016-006239. [DOI] [PubMed] [Google Scholar]
- 28.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 29.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128–138. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arkes HR, Dawson NV, Speroff T, Harrell FE, Jr, Alzola C, Phillips R, et al. The covariance decomposition of the probability score and its use in evaluating prognostic estimates. SUPPORT Investigators. Med Decis Mak. 1995;15(2):120–131. doi: 10.1177/0272989X9501500204. [DOI] [PubMed] [Google Scholar]
- 31.Alberg AJ, Park JW, Hager BW, Brock MV, Diener-West M. The use of "overall accuracy" to evaluate the validity of screening or diagnostic tests. J Gen Intern Med. 2004;19(5 Pt 1):460–465. doi: 10.1111/j.1525-1497.2004.30091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pencina MJ, D'Agostino RB., Sr Evaluating discrimination of risk prediction models: the C statistic. JAMA. 2015;314(10):1063–1064. doi: 10.1001/jama.2015.11082. [DOI] [PubMed] [Google Scholar]
- 33.Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35(29):1925–1931. doi: 10.1093/eurheartj/ehu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gill TM, Robison JT, Tinetti ME. Difficulty and dependence: two components of the disability continuum among community-living older persons. Ann Intern Med. 1998;128(2):96–101. doi: 10.7326/0003-4819-128-2-199801150-00004. [DOI] [PubMed] [Google Scholar]
- 35.D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 36.Smith AK, Williams BA, Lo B. Discussing overall prognosis with the very elderly. N Engl J Med. 2011;365(23):2149–2151. doi: 10.1056/NEJMp1109990. [DOI] [PMC free article] [PubMed] [Google Scholar]