Abstract
This study investigates eight case reports of spontaneously emerging, brief episodes of vivid altered states of Selfhood (ASoSs) that occurred during mental exercise in six long-term meditators by using a neurophenomenological electroencephalography (EEG) approach. In agreement with the neurophenomenological methodology, first-person reports were used to identify such spontaneous ASoSs and to guide the neural analysis, which involved the estimation of three operational modules of the brain self-referential network (measured by EEG operational synchrony). The result of such analysis demonstrated that the documented ASoSs had unique neurophenomenological profiles, where several aspects or components of Selfhood (measured neurophysiologically and phenomenologically) are affected and expressed differently, but still in agreement with the neurophysiological three-dimensional construct model of the complex experiential Selfhood proposed in our earlier work (Fingelkurts et al. in Conscious Cogn 86:103031. 10.1016/j.concog.2020.103031, 2020).
Keywords: Self-referential brain network (SRN), Default-mode network (DMN), Altered states of Selfhood (ASoS), Subjective sense of Self, First-person perspective, Electroencephalogram (EEG), Alpha rhythm, Operational synchrony, Functional connectivity, Agency, Ownership, Mineness, Embodiment, Narration, Autobiography, Self-consciousness
Introduction
Neurophenomenology was established as a novel research paradigm aiming to unify two different and apparently irreconcilable methodologies (Varela 1996): the neuroscientific experimental approach (quantitative data) and the phenomenological approach (qualitative data) by integrating the lived, experiential data with neuroscientific data (Olivares et al. 2015), where first-person accounts and neurophysiological data mutually inform one another (Varela 1996; Gallagher and Sørensen 2006; Gallagher and Zahavi 2008). In other words, neurophenomenology requires an integration of third-person data (e.g., functional Magnetic Resonance Imaging—fMRI, Electroencephalography—EEG, Magnetoencephalography—MEG, etc.) with first-person accounts (e.g., reports of the participant’s lived experience) (Varela and Shear 1999).
Since its introduction, neurophenomenology has been successfully applied in multiple research studies (Varela et al. 1991; Varela 1997, 1999; Gallagher 1997; Petitot et al. 1999; Lutz 2002; Lutz and Thompson 2003; Petitmengin et al. 2007; Stewart et al. 2010; Froese and Di Paolo 2011; Froese and Fuchs 2012; Berkovich-Ohana et al. 2013, 2020; Berkovich-Ohana 2017). Here, we were interested in applying the neurophenomenological approach to altered states of consciousness (ASC), and more specifically, to the ASC of Selfhood. Even though ASCs have been an integral part of recorded human history (Winkelman 1997), the scientific status of ASCs is still far from uniform and tends to be defined in rather broad terms (Kokoszka 1999; Cofré et al. 2020).
Traditionally, an ASC is defined as a state in which “extraordinary” content is experienced or in which the manner of experiencing is “unusual” (Kokoszka 1999) and being qualitatively different from normal/baseline waking consciousness (Tart 1972). However, such conceptualisation lacks precision and leaves many open questions (Revonsuo 2006; Cofré et al. 2020). For example, “[t]he phenomenal contents of consciousness do not all by themselves directly reveal whether the state of consciousness is an altered state or not. An identical phenomenal content of consciousness (say the experience of seeing an elephant in front of you) may be produced during the baseline state of consciousness (if you are in the zoo) or during an altered state of consciousness (if you are at home and dreaming or under the influence of LSD). The phenomenal content of consciousness (the pattern of subjective experience) does not by itself determine whether the background mechanisms of consciousness function normally or are somehow altered” (Revonsuo 2006; p. 58). Therefore, it has been suggested that the relevant alteration in the background mechanisms of consciousness1 determines the relation between subjective experiences that are actually produced and those that would have been produced in the baseline state of normal wakefulness: the alteration in the background mechanisms of consciousness changes this relationship, leading to an ASC.
Keeping this in mind, one may thus define the normotonic waking consciousness (baseline state of consciousness) as the state where person is awake and perceives the self and environment more or less accurately (Revonsuo 2006). Therefore, in the “baseline state the contents of consciousness are modulated by the physical environment and the physical body and therefore consciousness succeeds in accurately representing them” (Revonsuo 2006; p. 57). Then, for the baseline state of consciousness to count as an ASC, it must be temporally somehow altered in relation to this baseline state of consciousness (for a similar conceptualisation, see Hobson 2001; Boly et al. 2008; Winkelman 2011). Thus, an ASC can be defined as the transitory and typically reversible state of the background mechanisms of subjective experience that process and represent available information (internal and external) inaccurately (or delusionally) so that the resulting subjective experience mismatch the reality in one respect or another (Revonsuo 2006). When such a state manifests, the person is in an ASC regardless of the contents of consciousness and regardless of what circumstances brought about the alteration in the background mechanisms of the person’s brain. Meditative states, aura states during epileptic seizures, dreaming, drug-induced hallucinations, sensory deprivation states and hypnotic suggestions, all are examples of ASCs.2
Analysis of the available literature on these states, suggests that during ASCs a dramatic and profound alteration occurs in the experiential Selfhood. Indeed, alterations in self dominate the phenomenological reports during ASCs: this is the case for dreams when one does not have a body, but rather is present “as an abstract, undefined volume of indeterminate extension or even as an unextended point in space” (Windt 2015; p. 15; see also Windt 2010; Metzinger 2013), as well as during dreamless sleep, when even a minimal form of phenomenal selfhood is lost (Thompson 2015; Windt 2015; Windt et al. 2016), or drug-induced self dissolution (Lebedev et al. 2015; Wittmann 2015; Letheby and Gerrans 2017; Millière 2017; Nour and Carhart-Harris 2017; Deane 2020), or alterations of self features as well as selfless states during meditation (Mañjuśrīmitra et al. 1987; Travis and Pearson 2000; Shear 2007; Josipovic 2010; Berkovich-Ohana et al. 2013; Ataria et al. 2015; Metzinger 2020) and during sensory deprivation (Kjellgren et al. 2008, 2010; Glicksohn et al. 2019; Glicksohn and Ben-Soussan 2020), in hypnotic states (Crawford and Gruzelier 1992; Gruzelier 2000; Kallio and Revonsuo 2003), and during epileptic seizures (Johanson et al. 2008; Blumenfeld 2012). Given this empirical evidence, it is reasonable to speak about the altered states of Selfhood (ASoS). Similar to ASCs, the ASoSs are transitory, reversible and nonpathological.
A broad variety of behavioural, instrumental and pharmacological means are known to induce alterations in self-consciousness, ranging from meditation, special breathing techniques, hypnotic suggestions, sensory deprivation, magnetic/electric brain stimulation, or consumption of psychoactive drugs (Schmidt and Berkemeyer 2018). While mediation- and drug-induced ASoSs have been extensively studied and frequently reported (see for a comprehensive overview, Millière et al. 2018), brain stimulation ASoSs have been largely restricted to the clinical domain. For example, some ASoSs typical for the epileptic seizures, can be elicited by electrically stimulating certain parts of the brain (Penfield 1938; Halgren et al. 1978; Vignal et al. 2007).
Of these, the only ASoSs which can be achieved in a pure form (non-chemically and without one’s volitional effort, or exogenous stimulation) are those that occur spontaneously (for a similar view, see Zahavi and Parnas 1998). In such spontaneously emerging ASoSs, the altered phenomenal contents of self-consciousness that the person experiences are a consequence of the pure altered state that is a result of the background mechanisms of self-consciousness only and not due to confounding factors such as (1) intentional mental effort with biases of particular practice exercise or conceptualisations of the respective traditions, (2) drug-induced neurochemical alterations, or (3) brain activity manipulation by magnetic or electrical stimulation. However, catching and measuring such ASoSs in laboratory controlled settings is challenging. At the same time, possessing the capacity to register and analyse ASoSs in the laboratory (especially in conjunction with neurophenomenological approach) would be very helpful to progress in revealing the nonpathological/normative range of Selfhood variation.
It is known that ASoSs occasionally arise in spontaneous and involuntary manner during meditation practice3 in highly-experienced long-term meditators (Soler et al. 2014; Berkovich-Ohana 2017; Millière et al 2018; Vieten et al. 2018; Penberthy et al. 2020). In this context, the usage of highly experienced meditators is of special interest for the purpose of studying these spontaneous ASoSs. Another important factor to consider is that long-term contemplative practitioners are acutely aware of and sensitive to the variations in their subjective experience, are able to stay within a given experience without being lost in thought or distracted by mind-wandering, and to provide a detailed and reliable first-person reports of such experiences that are lived through (Lutz et al. 2007; Fox et al. 2012; Berkovich-Ohana et al. 2013; Dor-Ziderman et al. 2013; Mrazek et al. 2013; Ataria et al. 2015). This later capability is a fundamental element of the neurophenomenological approach4 (Varela 1996), where the first-person data (phenomenology) “provide additional, valid information about externally uncontrollable aspects of mental activity, and this information can be used to detect significant patterns of dynamic activity at the neural level” (Thompson et al. 2005; pp. 45–46).
As for the “neural” component of the neurophenomenological approach and also to capture the background mechanisms of subjective experience of Selfhood, we used the recently introduced neurophysiological three-dimensional construct model of the complex experiential Selfhood (Fingelkurts and Fingelkurts 2011; Fingelkurts et al. 2016a, b; for a detailed description, see Fingelkurts et al. 2020), which is based on the EEG operational synchrony analysis (Fingelkurts and Fingelkurts 2008, 2015). This triad model of Selfhood has been put forward to account for the phenomenological distinctions between three major aspects of Selfhood, namely first-person agency, embodiment, and reflection/narration, all of which are commensurate with one another (Gallagher 2013; Gallagher and Daly 2018) and thus reflect the multi-faceted nature of self-awareness (Snodgrass and Thompson 1997; Klein and Gangi 2010; Musholt 2013; Millière et al. 2018). Together these three aspects form a unified sense of self (Fingelkurts and Fingelkurts 2011; Fingelkurts et al. 2020).
The triad model of Selfhood
The triad model of Selfhood (Fingelkurts et al. 2016a,b, 2020) is built on neurophysiological evidence that three major spatially separate yet functionally interacting brain subnets (or operational modules—OMs) constitute the brain self-referential network (SRN), also frequently referred to as the default mode network (Raichle et al. 2001; Gusnard 2005; Northoff et al. 2006, 2011; Schilbach et al. 2008; Fingelkurts and Fingelkurts 2011; Fingelkurts et al., 2012; Davey et al. 2016; Northoff 2016). Each OM is a set of brain regions having tight “functional connectivity” among one another within every given OM (Fingelkurts and Fingelkurts 2011; for a further discussion, see Uddin et al. 2009; Andrews-Hanna et al. 2010; Spreng and Grady 2010; Leech et al. 2011). The set of these three OMs include the anterior OM and two symmetrical (left and right) occipito-parieto-temporal OMs (Fig. 1) which can be reliably estimated by means of operational synchrony analysis of the EEG signal (Fingelkurts and Fingelkurts 2008, 2015).
Fig. 1.
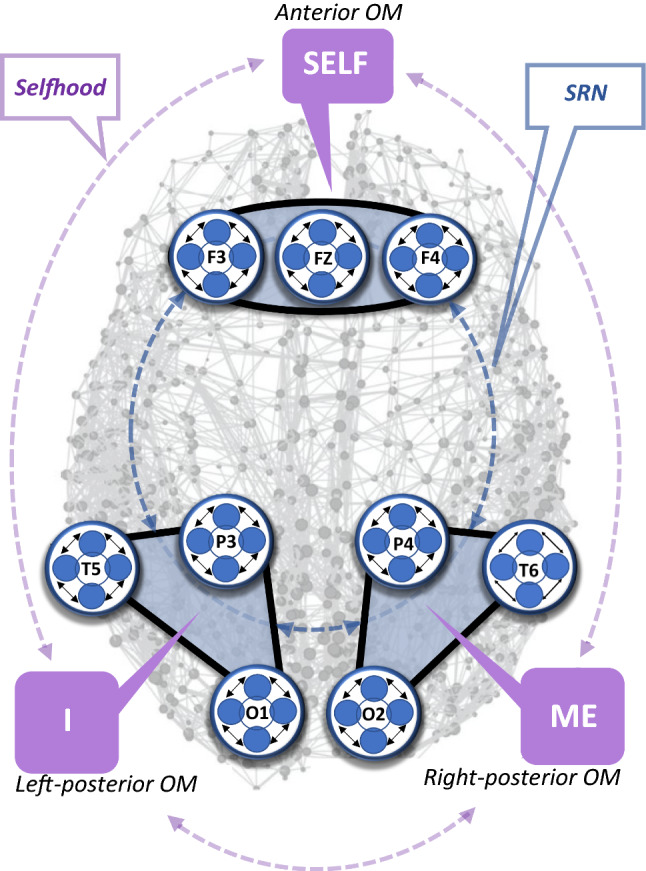
Operational modules (subnets) constituting the self-referential brain network and their relation to the three components of Selfhood. Operational modules (OMs) are indicated as blue-coloured areas that involve operational synchrony among three EEG locations (marked by white circles with EEG electrode IDs) per OM, that are mapped onto a schematic cortex map. A schematic cortex map presents the brain’s functional connections with dark grey spheres marking network nodes and light grey lines representing their functional connections. The network nodes are shown in their respective anatomical coordinates, hence preserving the spatial embedding of the network. Every OM has a clear nested functional hierarchy, where higher levels are physically composed of lower levels (Feinberg 2012). Indeed, every OM is a functional integration of several local brain fields (registered by the correspondent EEG electrodes), which in their turn are the integration of yet smaller local fields of transient functional neuronal assemblies (Fingelkurts and Fingelkurts 2001, 2013; Fingelkurts et al. 2019; see also Freeman and Kozma 2000; Kozma and Freeman 2009; Freeman et al. 2015). Together, three OMs, form a higher level of a functional nested architecture—the self-referential network (marked as a dashed, blue circle line that connects the three OMs). At the phenomenological level they represent three aspects of Selfhood, marked as “Self”, “Me” and “I”; and their dynamic interaction forms the coherent experience of Selfhood (marked as a dashed purple circling line connecting “Self”, “Me” and “I”). Abbreviations: EEG: electroencephalogram; OM: operational module; SRN: self-referential network; Double-pointed black arrows schematically indicate functional couplings of the local fields produced by neuronal assemblies under a given electrode; electrode IDs/positions: F3—left frontal, Fz—frontal midline, F4—right frontal, T5—left temporal, P3—left parietal, O1—left occipital, T6—right temporal, P4—right parietal, O2—right occipital. The figure is modified from Fingelkurts et al. (2020)
According to this triad model of Selfhood (Fingelkurts et al. 2016a, b), the anterior module of the SRN (Fig. 1) is associated with the phenomenal first-person perspective and the phenomenal sense of agency (Fingelkurts et al. 2020). We label it the “witnessing observer” or simply the “Self” in the narrowest sense (Fingelkurts et al. 2020)—as the phenomenal non-conceptual core in the act of knowing itself (Blanke and Metzinger 2009), or in the words of Velmans (2014), a sensed “centre of gravity”, where one having an experience of directly and immediately present as the centre (or a focus) of a phenomenal multimodal perceptual reality (Metzinger 2004, 2008; Revonsuo 2006; Trehub 2007; Blanke and Metzinger 2009). Here, agency is treated as the “sense of ownership” of thoughts, perceptions, and actions relevant to Selfhood (Metzinger 2004, 2008; de Vignemont and Fourneret 2004; Hohwy 2007; Blanke and Metzinger 2009) and the sense that it is “I” who is undergoing the experience in its implicit first-person mode of givenness (Gallagher 2000; Zahavi 2002; Metzinger 2008). Research has shown that such a Self in the act of knowing (a “witnessing observer”) can be enhanced as a symptom in pathological conditions like depression (Fingelkurts and Fingelkurts 2017a), and post-traumatic stress disorder (Fingelkurts and Fingelkurts 2018). It also can be diminished or even disappear completely, as for example, in patients with disorders of consciousness (Fingelkurts et al. 2012, 2016c; Fingelkurts and Fingelkurts 2017b; Huang et al. 2014), and also in non-pathological conditions like dreamless sleep (Thompson 2015; Windt 2015), in specific meditative states (Wahbeh et al. 2018; Josipovic 2019), or under psychedelics like 5-MeO-DMT (Millière 2017, 2020; Deane 2020; Letheby and Gerrans 2017. Further, it can also be voluntary manipulated by trained meditation practitioners (Lutz et al. 2008; Kerr et al. 2011; Fingelkurts et al. 2016a, b, 2020).
The right posterior module of the SRN (Fig. 1) is linked with the experience of self as a normally localized (through interoceptive and exteroceptive sensory processing) within bodily boundaries entity, as well emotional states, and related autobiographical emotional memories (Fingelkurts et al. 2016a, b, 2020). We label this module “representational-emotional agency” or simply “Me”5 (Fingelkurts et al. 2020). The defining feature of the Me-module is that, in contrast to a phenomenal first-person perspective, here only a purely geometrical first-person perspective is present that takes its origin from within the body representation, thus signifying an egocentric spatiotemporal self-model (Blanke and Metzinger 2009). The body here is treated not as just one more object of the physical world, but as a “vehicle” that enables being a self in the world (Varela et al. 1991; Gallagher 2005; Legrand 2006; Hohwy 2010, 2013; Seth et al. 2012; Limanowski and Blankenburg 2013; Apps and Tsakiris 2014). It has been documented that this sense of Me (“bodily self”) can undergo significant alteration or become abnormal during various pathological conditions, as for instance in post-traumatic stress disorder (Fingelkurts and Fingelkurts 2018), depression (Fingelkurts and Fingelkurts 2017a), heautoscopic out-of-body experiences (Blanke and Mohr 2005; Blanke et al. 2002; Ionta et al. 2011), in depersonalization syndrome, where the sense of body ownership is lost (Berlucchi and Aglioti 1997), or in vegetative or minimally conscious states (Fingelkurts et al. 2012). Within a normative continuum, it can be intentionally manipulated by experienced long-term meditators, who are able to achieve a dramatic loss of bodily perceptions—so called “self-boundarylessness” or “bodylessness” (Newberg et al. 2001; Newberg and Iversen 2003; Berkovich-Ohana et al. 2013; Ataria et al. 2015; Fingelkurts et al. 2016b, 2020).
The left posterior module of the SRN (Fig. 1) is involved in the experience of thinking about and reflecting upon oneself, including momentary narrative thoughts and inner speech, as well as reinterpretation of episodic and semantic memory events related to self—autobiographical story telling (Fingelkurts et al. 2016a,b, 2020). We label it “reflective agency” or simply “I”6 (Fingelkurts et al. 2020). It has been proposed that such narrative self-reflection relies on the uniquely human capability for language (Damasio 1994; Budwig 2000; Gallagher 2000; Craig 2004) and provides the basis for the sense of invariance of Selfhood over time (James 1890; Metzinger 2003; Friston et al. 2017). Research has shown, that such sense of I (“reflective agency”) can be altered, thus presenting either a clinical symptom in several pathologies, like schizophrenia, depression, post-traumatic stress disorder or brain injury (Frith 1992; Gallagher 2003a; Moseley et al. 2013; Fingelkurts and Fingelkurts 2017a, b, 2018), or be a normative variation when voluntary modified through a meditation technique (Fingelkurts et al. 2016b, 2020).
The integration of dynamics of these three SRN OMs enables nonreductive intertwining of the triad of Selfhood aspects (witnessing observer, representational-emotional agency, and reflective agency), thus providing a coherent instantiation of the unique, complex phenomenal pattern—a Self (Fingelkurts and Fingelkurts 2011; Fingelkurts et al. 2016a, b, 2020). Such a neurophysiological three-dimensional construct model of the complex experiential Selfhood treats phenomenological distinctions between different aspects of self not as opposites, but rather as commensurate and complementary with one another (see also Gallagher 2013; Gallagher and Daly 2018).
Recently, it has been documented experimentally that there is a causal link between the three phenomenological aspects of Selfhood and related to them the three OMs of brain SRN (Fingelkurts et al. 2020). In that study, the dynamics of functional integrity of the three SRN OMs were analysed while experienced meditators mentally manipulated (either increase/up-regulate or decrease/down-regulate) every component of the self triad (witnessing observer—“Self”, representational-emotional agency—“Me”, and reflective agency—“I”) in a randomised, independent, and controlled manner. It was shown that up-regulation of the expression of Self, Me, or I resulted in a significant increase in the functional integrity (indexed by EEG operational synchrony) of the corresponding SRN OMs, while conversely, down-regulation of the Self/Me/I expression resulted in a significant decrease in the functional integrity of the respective SRN OMs (Fingelkurts et al. 2020). Further, the observed changes in the functional integrity of the SRN OMs were in keeping with participants’ self-reports of alterations in the phenomenological experience during up- or down-regulation of Selfhood components, and also significantly correlated with phenomenological factors estimated by a set of standardised questionnaires (Fingelkurts et al. 2020).
However, in that study (Fingelkurts et al. 2020) a design-based group analysis was used; hence group averaging may have masked unique individual variation in the phenomenological expression of Selfhood components, that may not always be in synch with the intended target mental state. Further, participants were requested to mentally induce pre-defined states representing either increased or decreased sense of Self, Me, or I through several trials. Such design required a certain mental effort from the participants, which in itself is likely to result in unique phenomenological/neurophysiological manifestation of its own. The first-person reports indicated that nearly all participants experienced spontaneously emerged brief episodes of vivid and intense ASoSs that were independent of the target mental states (unpublished data of the Fingelkurts et al. 2020 study). The present study was built on the rich dataset and insights gained from that previous study; however, it is an original study, since the data used in the present study was not considered in the previous one—it was excluded from the analysis because it did not satisfy the inclusion criteria and was outside the study’s scope (see for details Fingelkurts et al. 2020).
Aim of the study
In the present study, instead of intentionally achieved mental states, we were focusing on the relationship between rare, spontaneously occurring, brief episodes of vivid altered states of Selfhood (as phenomenologically experienced ASoSs) and related to them three OMs of the SRN (measured by EEG operational synchrony). Specifically, and in agreement with the neurophenomenological methodology (Varela 1996; Varela and Shear 1999; Gallagher and Sørensen 2006), first-person reports were used to identify such spontaneous ASoSs and guide the neural analysis, as opposed to a design-based group analysis. We hypothesized that the nuanced phenomenological experiences of ASoSs would associate with expected changes (according to the triad model of Selfhood; Fingelkurts et al. 2016a, b, 2020) in the functional integrity within three OMs of the SRN. In other words, we were interested to see if subjective variability of ASoSs would be tied to specific neurophysiological fluctuations in an integrated and coherent way (see also, Gallagher 2010).
Since the focus of the present study was the rare and spontaneously emerging brief episodes of ASoSs, which are the single time-points during different trials and occurring in different participants, every ASoS was analysed individually, therefore signifying a case study approach7 which is free from group averaging (Shallice 1979; Barabasz and Barabasz 1992).
The results of this study could prove useful not only for the future development of the classification (taxonomies) of altered states affecting Selfhood, but also for a better understanding of the mere nature (the essence) of self-consciousness and its normative boundaries. Further, this line of research could be helpful in interpreting clinical data, since many ASoSs characteristics are also present in different neuropsychopathologies in the form of long-lasting or permanent symptoms (Dittrich 1998; Martin et al. 2014; Fingelkurts and Fingelkurts 2017a, b, 2018).
Materials and methods
Subjects
Six (4 females/2 males; mean age = 54.3, SD = 16.9) long-term meditators with an average of 22 (SD = 7.9) cumulative years of meditation practice, and an average of 3397 (SD = 1895) total hours of meditation practice are reported in the present study. The participants were part of a bigger group that was recruited to investigate the causal link between voluntary manipulated three aspects of Selfhood and functional integrity of the three SRN OMs (Fingelkurts et al. 2020). For the present study the only inclusion criterium was the presence of the spontaneously occurring involuntary,8 brief episodes of vivid and intense ASoSs which did not overlap with the original study’s pre-defined mental states (which was the focus of a previous study, Fingelkurts et al. 2020). In this respect the present study is original and is using the data that was not part of the previous study. All participants of the present study were right-handed and had more than 15 years of education. Exclusion criteria included ASoSs which contents overlapped with the original pre-defined mental states (Fingelkurts et al. 2020), any history of neurologic and/or psychiatric disorder, brain trauma/concussion, epilepsy, serious somatic disorder in the past year, stressful events (e.g. job loss, divorce, bereavement, etc.) in the past 6 months, pregnancy (for the females), or use of medication that may affect brain activity.
The study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and standards established by the BM-Science—Brain and Mind Technologies Research Centre Review Board. Prior to participating in the study and EEG scanning, the experimental procedures were explained and participants signed an informed consent form. The use of the data for scientific studies was authorized by written informed consent of subjects and approval by the Review Board of BM-Science—Brain and Mind Technologies Research Centre. To ensure confidentiality of the participants’ information, all data were anonymized and arbitrary ID-codes were assigned for each individual data set.
Study design
During the experiment, each EEG session began with a 5-min resting-state baseline period with eyes closed, followed by an introspective self-report and a battery of standardised questionnaires (see below). An EEG was then registered again during an eyes-closed 10-min mental exercise session run aimed to achieve a pre-defined target mental state (Fingelkurts et al. 2020). After that, the introspective self-reports were obtained and a battery of standardised questionnaires was administered again.9 In such design the mental state conditions (experimental condition) and rest (control condition) were closely matched to each other, because participants kept their eyes closed during both conditions (rest and experimental task); further, no stimuli were presented in either condition, and no motor responses were required.
Self-reports and questionnaires
The current study used a neurophenomenological design (Varela 1996; Gallagher 2003b; Lutz and Thompson 2003; Berkovich-Ohana et al. 2020), incorporating both EEG recordings and first-person descriptions from the same subjects, that was enhanced by using the replies of participants to a set of standardised questionnaires using Likert scales,10 as was proposed by Gallagher and colleagues (2015). The rest condition with eyes closed and without executing any task served as the start point for participants to produce their baseline subjective reports and answer questionnaires about this baseline condition. The following reports and questionnaire replies were collected immediately11 after every period of 10-min EEG recording while achieving a particular target mental state (Fingelkurts et al. 2020).
In the introspective Self-Report, participants were asked to describe in their own words their subjective experience during the target mental state, focusing on internal process and specific aspects of body awareness and sensations, vigilance, internal speech and narration, and the sense of witnessing. No constraints were specified on the type of reporting; any comments were welcomed. In doing so and in accordance with the recommendations of Gallagher and Sørensen (2006), participants were asked to suspend their own beliefs, metaphysical interpretations and theoretical background assumptions while describing their subjective experiences. Participants were encouraged to report any spontaneously occurring brief episodes of vivid ASoSs, even if they did not coincide with the intended pre-defined target mental states.12 Whenever such ASoS episodes were reported, participants were requested to indicate the time-period during the 10-min EEG recording when such episodes have occurred.13
Additionally, participants filled in a set of standardised questionnaires using Likert-scale type of responses (Norman 2010). For the present study replies on the following questionnaires were also used: (1) Sense of Agency Rating Scale—SOARS (Polito et al. 2013); with “Involuntariness” and “Effortlessness” factors used. (2) Rumination-Reflection Questionnaire—RRQ (Trapnell and Campbell 1999); with “Rumination” and “Reflection” factors used. (3) Five Facet Mindfulness Questionnaire—FFMQ (Baer et al. 2006); with “Observing” factor used. (4) Embodiment Questionnaire—EQ (Aymerich-Franch et al. 2015); with “Ownership”, “Self-Location” and “Agency” factors used. (5) The Sense of Body—SB (an in-house questionnaire, following Pope and Singer 1978; Winget and Kramer 1979); with “Body Image”, “Body Perception” and “Body Orientation” factors used. (6) The Sense of Time (ST) and Thought Speed (TS) (another in-house questionnaire, following Pope and Singer 1978; Winget and Kramer 1979); with “Here and Now”, “Future”, “Past”, “Concatenation of Thoughts”, “Continuity of Thoughts” and “Speed of Thoughts” factors used.
In accordance with the aim of this study, only the results pertaining to rare and brief episodes of spontaneously occurring ASoSs are reported and discussed here.
EEG registration and pre-processing
EEG was recorded during eyes-closed waking state (“rest” or “achieving a target state”) using a 21-channel EEG data acquisition system (Mitsar, St. Petersburg, Russian Federation) from 19 electrodes positioned on the head according to the International 10–20 system (i.e. O1, O2, P3, P4, Pz, C3, C4, Cz, T3, T4, T5, T6, Fz, F3, F4, F7, F8, Fp1, Fp2). The following recording parameters were used: linked earlobes as a reference electrode; 0.5–30 Hz bandpass; 50 Hz notch filter ON; 250 Hz sampling rate; electrooculogram (0.5–70 Hz bandpass); impedance below 10 kΩ.
During the EEG recordings participants were asked to either (1) relax and engage in no specific mental activity with eyes closed (resting state), or (2) engage in achieving a target mental state according to the instructions (Fingelkurts et al. 2020) with eyes closed (experimental state). The presence of an adequate EEG-signal was first determined by visual inspection of the raw signal. Artefacts due to eyes opening, eye movement, significant muscle activity, and movements on EEG channels, as well as drowsy episodes (indexed by slowing of background frequencies by ≥ 1 Hz, vertex sharp waves and slow eye movements) were corrected or eliminated by (a) using spatial filtration technique based on zeroing the activation curves of individual Independent Component Analysis (ICA) components that correspond to these artefacts (Vigário 1997), and (b) excluding epochs with excessive amplitude of EEG (≥ 70 μV) as well as excessive fast (20–30 Hz, ≥ 35 μV) and slow (0.5–1 Hz, ≥ 50 μV) frequency activities.
For every session, artifact-free EEG streams were fragmented into consecutive 1-min epochs, which were bandpass-filtered (Butterworth filter of sixth order) in the alpha (7–13 Hz) frequency band. Forward and backward filtering were used to eliminate phase shifts. The reasons for the alpha frequency choice are described in detail in Fingelkurts et al. (2020).
Deriving SRN OMs and estimating their strength
In the current study (similar to our previous studies on the triad model; Fingelkurts et al. 2012, 2016a, b, c, 2020; Fingelkurts and Fingelkurts 2017a, b, 2018), a set of brain areas that have been previously established belong to SRN (Fingelkurts and Fingelkurts 2011) was used. Such areas were not chosen arbitrary to be part of the SRN. Nine areas (included in the triad model, see Fig. 1) naturally emerged as members of three most stable task-independent EEG spatiotemporal patterns (OMs) with extremely high strength of operational synchrony. This finding has been replicated in two independent studies with participation of subjects from two different nationalities and two different sensory modalities (for detail, see Fingelkurts and Fingelkurts 2011). These nine operationally synchronized cortical areas were used to estimate the operational synchrony strength within the three SRN OMs: anterior OM—formed by F3-Fz-F4 EEG locations; left posterior OM—formed by T5-P3-O1 EEG locations; and right posterior OM—formed by T6-P4-O2 EEG locations (Fig. 1).
Several hierarchical stages of data processing were required in order to estimate operational synchrony strength within every OM. The details of this multistage procedure can be found elsewhere (Fingelkurts and Fingelkurts 2008, 2015). Here only a brief overview of the main steps is provided. During the first step, each local EEG signal was reduced to a naturally existing temporal sequence of nearly stationary (quasi-stationary) segments of varying duration. To uncover these quasi-stationary segments from the complex nonstationary structure of local EEG signals, an adaptive segmentation procedure was used (Fingelkurts and Fingelkurts 2008, 2015). The aim of such segmentation is to divide each local EEG signal into naturally existing quasi-stationary segments by estimating the intrinsic boundaries among segments—rapid transitional periods (RTPs). RTP is defined as an abrupt change in the analytical amplitude of the EEG signal above a particular threshold derived from modelling studies and statistical analysis (Fingelkurts and Fingelkurts 2008, 2015). It has been proposed that each stationary (homogeneous) segment in the local EEG signal corresponds to a temporary stable local microstate—an operation executed by a neuronal assembly (Fingelkurts and Fingelkurts 2001; Fingelkurts et al. 2010, 2013). The temporal coupling (synchronization) of such segments among several local EEG recordings then, reflects synchronization of operations (i.e. operational synchrony) produced by different neuronal assemblies (located in different cortical regions) into the integrated and unified patterns responsible for complex mental operations Fingelkurts and Fingelkurts 2001; Fingelkurts et al. 2010, 2013. see also Freeman and Kozma 2000; Kozma and Freeman 2009; Freeman et al. 2015).
The second step of the analysis constituted estimation of operational synchrony14 within every OM. Operational synchrony estimates the statistical level of RTP temporal coupling between two or more local EEG recordings (Fingelkurts and Fingelkurts 2008, 2015). This measure tends toward zero if there is no synchronization between EEG segments derived from every pair of EEG channels, and has positive or negative values where such synchronization exists. Positive values (above upper stochastic threshold) indicate an “active” process of coupling of EEG segments (synchronization of EEG segments is observed significantly more often than expected by chance as a result of random shuffling of segments during a computer simulation), whereas negative values (below lower stochastic threshold) mark an “active” process of decoupling of segments (synchronization of EEG segments is observed significantly less than expected by chance as a result of random shuffling of segments during a computer simulation) (Fingelkurts and Fingelkurts 2008, 2015). The strength of EEG operational synchrony is proportional to the actual (absolute) value of the measure: the higher this value, the greater the strength of functional connection and correspondently the functional integrity of the OM.
Using the described pair-wise analysis, operational synchrony was identified in several (more than two) channels—synchrocomplexes (SC); these define operational modules—OMs. The criterion for defining an OM is a sequence of the same synchrocomplexes (SC) during every 1-min epoch, whereas a SC is a set of EEG locations in which each location forms a paired combination with valid values of synchrony with all other EEG locations within the same SC; meaning that all pairs of EEG locations in an SC have to have statistically significant synchrony linking them together (Fingelkurts and Fingelkurts 2008, 2015).
Statistics
The participant’s first-person reports were used to guide the EEG operational synchrony analyses. An EEG epoch of 1-min duration was selected around the time-points indicated by the participants for every experienced ASoSs. The strength of functional connectivity within individual OMs was assessed for these 1-min epochs using EEG operational synchrony measure outlined in the previous section. Every individual ASoS-epoch was analysed as a separate event without averaging,15 due to being a unique representative of subjective descriptions given by the participants about ASoS.16 The difference in the strength of operational synchrony from the resting baseline condition to every given ASoSs was presented as a percent change. Likewise, the differences between psychometric tests’ factors were also presented as a percent change from baseline state to a correspondent ASoS.
Since we were testing every ASoS-epoch individually as a unique event, statistical significance estimation was not possible and thus the results of this study should be considered as descriptive (Guetterman 2019).
Results and discussion
All six participants indicated at least one spontaneously and involuntary occurring vivid ASoS during meditation sessions. A total of 8 unique ASoSs were recorded among 108 trials, that is a sum of 3 target mental states (“Self”, “Me” and “I”) with 2 variant each (Up- and Down-regulation) repeated 3 times for 6 participants. This result indicates that spontaneously occurring ASoSs are indeed rather rare, supporting previous findings in highly experienced, long-term meditators (Soler et al. 2014; Berkovich-Ohana 2017; Millière et al 2018; Vieten et al. 2018; Penberthy et al. 2020).
A summary of the involuntary occurring ASoSs is presented in the Table 1. As it is clear from the table, vast majority (75%) of the registered ASoSs were related to embodiment domain and 25%—to a domain of autobiographic history and narration (the difference is statistically significant: Chi-Square, p = 0.00001). The prevalence of changes in the embodiment phenomenology among other phenomenal features during altered states of Selfhood is consistent with previous reports (Grof 1976; Lindahl et al. 2017; Girn and Christoff 2018; Millière et al. 2018) and could be explained by the fact that it is the most basic and primordial form of self-consciousness that is directly and immediately present to a subject17 (Damasio 1999; Bermúdez 2011; De Vignemont 2011; Seth 2013; Tsakiris 2017). Further, it is also evident that while all ASoSs from the embodiment domain were characterised by some level of disembodiment (see Table 1), there are noticeable nuances in the phenomenological experiences, which points to a conclusion that these ASoSs were essentially different despite the common phenomenological denominator of disembodiment (see also Millière et al. 2018). A similar picture emerged in the autobiographic/narration domain (Table 1).
Table 1.
Spontaneously and involuntary occurring altered states of Selfhood
| 1 | I observed my body from outside |
| 2 | Bodilessness with no location or time |
| 3 | Different parts of my body disappeared completely |
| 4 | My boundaries expanded into a whole room and street |
| 5 | I was both in my body and outside it |
| 6 | I raised and felt the space around |
| 7 | The experience of my life history disappeared |
| 8 | My thoughts stopped |
In light of mentioned in the introduction section findings on the causal link between the three phenomenally distinct aspects of Selfhood (Self, Me and I) and correspondent to them three distinct OMs of brain SRN (Fingelkurts et al. 2020), we expected to see the predictable reflection of the nuanced phenomenology (captured by self-reports and factors derived from the standardised questionnaires) in the variability of neuronal dynamics (represented by OMs functional integrity) accompanying the observed ASoSs. The neurophenomenological profiles (incorporating both EEG OMs and first-person descriptions) for every reported ASoS are presented individually and discussed in detail below.
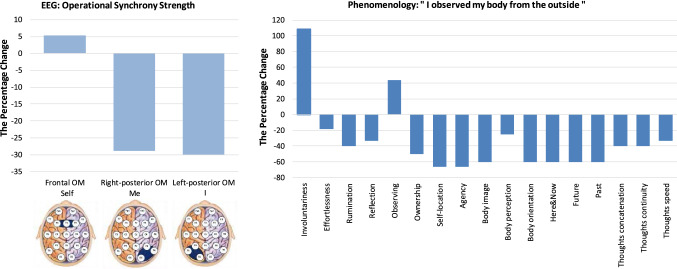
“I observed my body from the outside”
Figure 2 presents the neurophenomenological profile of the ASoS: “I observed my body from the outside”. The participant described this state as: “The boundaries (of the body) however vanished and it felt like I looked/observed my body from the outside. Bodily sensations were decreased and they were not felt in a personal manner”.18 Neurophysiologically, this ASoS was found to be constituted by a slightly enhanced functional integrity of the Self-module of the brain SRN and a pronounced decrease in the functional integrity of both Me- and I- modules of the brain SRN (Fig. 2). According to the triad model of Selfhood and in agreement with a previous study that examined causal link between three aspects of Selfhood and three SRN modules (Fingelkurts et al. 2020), an upregulated Self-module is responsible for the feeling of being a phenomenal spatio-temporal (and often extensionless19) point, that observes and witnesses itself and the world (Revonsuo 2006; Trehub 2007; Damasio 2010; Velmans 2014; Metzinger 2020). It provides the experience of being a witnessing agent (self in the act of knowing)—an epistemic agent that expands its knowledge by directing its own attention at oneself and the world in the present moment (Metzinger 2013; Velmans 2014; see also Gallagher 2000; Revonsuo 2006; Zahavi 2006; Damasio 2010). The fact that the participant has reported to observe her body from the outside clearly pointed to preservation of the phenomenal spatio-temporal centre, despite loss of her body awareness.20 Strong down-regulation of the Me- and I- modules (Fig. 2), that are responsible for embodiment and thoughts/narration respectively (Fingelkurts et al. 2020), is compatible with the subjective feeling of disembodiment and diminished self-reflection.
Fig. 2.
Neurophenomenological profile of the altered state of Selfhood (ASoS) “I observed my body from the outside”. The Y-axis presents percent change from the baseline condition for both neurophysiological/EEG (left part of the Figure) and phenomenological (right part of the Figure) aspects of the profile. The X-axis represents a in the left part of the figure: the functional integrity (indexed by operational synchrony strength) of three SRN OMs corresponding to three phenomenological features of Selfhood: “Self” (witnessing agency), “Me” (body-representational agency) and “I” (reflective/narrative agency). The schematic cortex maps below the graphs indicate the positions of OMs (dark blue shapes) across the cortex; b in the right part of the Figure: the expression of the 17 phenomenological aspects measured by the standardised questionnaires (“Involuntariness” and “Effortlessness” [Sense of Agency Rating Scale—SOARS; Polito et al. 2013]; “Rumination” and “Reflection” [Rumination-Reflection Questionnaire—RRQ; Trapnell and Campbell 1999]; “Observing” [Five Facet Mindfulness Questionnaire—FFMQ; Baer et al. 2006]; “Ownership”, “Self-Location”, and “Agency” [Embodiment Questionnaire—EQ; Aymerich-Franch et al. 2015]; “Body image”, “Body perception”, and “Body orientation” [The Sense of Body—SB; in-house produced questionnaire, following Pope and Singer 1978; Winget and Kramer 1979]; “Here & now”, “The future”, “The past”, “Concatenation of thoughts”, “Thoughts continuity”, and “Speed of thoughts” [The Sense of Time (ST) and Thought Speed (TS); in-house produced questionnaire, following Pope and Singer 1978; Winget and Kramer 1979]). Abbreviations: ASoS altered state of Selfhood, EEG electroencephalogram, OM operational module, SRN self-referential network
The questionnaires data, that were used to facilitate (confirm or correct) interpretations, provided a more nuanced picture of how this ASoS was subjectively perceived. From Fig. 2 it could be seen that the expression of all measured phenomenal aspects diminished except for the aspect “Involuntariness” and “Observing” whose expression increased. In totality, these observations point to the fact that in this ASoS the participant did not experience oneself as a full-fledged embodied entity: the automatic and immediate sense of physical agency decreased, along with a decrease in the first-order experiential sense of ownership (that it is me who owns the body; Gallagher 2000; Tsakiris 2010), body self-location, body orientation, body image and body schema (Gallagher 1986). These changes were corroborated by (1) an increased sense of involuntariness that marks the lack of deliberate control, feeling that body sensations and thoughts are not caused by oneself (Gallagher 2000, 2005) and (2) a sense of increased observing via up-regulation of the witnessing state (Fingelkurts et al. 2020).
Curiously, the participant’s sense of time was transiently suspended in this ASoS (Fig. 2) indicated by a diminished feeling of all three dimensions of time (“Past”, “Present” and “Future”)21—some kind of “timelessness”. A profound alteration in time perception (feeling of timelessness) was systematically reported during various altered states of consciousness (Glicksohn 2001; Shanon 2001; Wackermann et al. 2008; Studerus et al. 2010; Berkovich-Ohana et al. 2013; Ataria et al. 2015; Wittmann 2015). One may speculate that such a loss of the sense of time that goes hand in hand with a feeling of bodilessness is due to a tight connection between the two (Wittmann 2013; Craig 2015). Indeed, it has been proposed that subjective time emerges through the bodily self as an enduring embodied entity across time22 (Wittmann 2013; Berkovich-Ohana and Wittmann 2017).
Further, this ASoS was also accompanied by diminished “Thoughts speed”, “Thoughts concatenation” and “Continuity”, as well as decreased narration (“Reflection” and “Rumination”) and autobiographical reflection (Fig. 2). These findings are in line with previously reported phenomenological records of altered states of self-consciousness, where embodiment distortions were accompanied by the distortions in thought process (Blakemore et al. 2000; Parnas and Handest 2003; Millière 2017, 2020; Ataria et al. 2015; Pollan 2018) and autobiographical/narrative self (Cohen 1964; Grof 1976; Lebedev et al. 2015; Girn and Christoff 2018). Interestingly, as psychedelic observations have shown (Grof 1976; Girn and Christoff 2018), attenuation of the autobiographical self and personal narrative seems to occur following initial changes in bodily self and is associated with changes in perception of time (Schacter et al. 2007; Wang et al. 2017).
“Bodilessness with no location or time”
Figure 3 presents the neurophenomenological profile of the ASoS: “Bodilessness with no location or time”. This state was described as: “Bodily feeling broadened out of the body into bodilessness without location or time… Not many thoughts appeared—very few”. Neurophysiologically, this ASoS was constituted by a similar to an above discussed ASoS changes in the triad of SRN OMs (Fig. 3): a very slightly enhanced functional integrity of the Self-module accompanied by strong decreases in the functional integrity of both Me- and I- modules (with Me-module exhibiting a stronger decrease; which is in contrast to a previously discussed ASoS, in which it was the I-module that showed the largest decrease, see Fig. 2). This particular nuanced quantitative difference between the two ASoSs was probably responsible for the experienced distinguishing qualitative flavour expressed in the phenomenological descriptions of these two ASoSs.
Fig. 3.
Neurophenomenological profile of the altered state of Selfhood (ASoS) “Bodilessness with no location or time”. The legend of this figure is the same as that of the Fig. 2
Despite the fact that the participant reported an absence of “Location” and “Time”, the slight increase in the functional integrity of the Self-module signifies that some phenomenological self-location was still present in this ASoS (as it follows from the previous study, Fingelkurts et al. 2020). This is not particularly strange, as for example, reports of out-of-body experiences (OBEs),23 while describing the experience of disembodiment,24 sometimes mention some “thin/nonexplicit” experience of being an extensionless point not anchored to the body (Alvarado 2000; Lopez and Elzière 2018). Similar experiences were also reported for some “bodiless” dreams when persons do not experience themselves as embodied within the dream (Cicogna and Bosinelli 2001) but rather “as a disembodied point or freely moving center of awareness” (Windt 2010; p. 201). This is also in line with an observation of Dor-Ziderman and colleagues that “even when the [sense of boundaries] disappears, a minimal level of dynamic proprioception continues to exist” (Dor-Ziderman et al. 2016; p. 3). According to Metzinger (2013, 2020), this minimal sensation is sufficient for creating a phenomenological centre of gravity (Velmans 2014) and self-identification that is tied to an individual phenomenological first-personal givenness (Zahavi 2006).
The replies on the questionnaires support the neurophysiological findings for this ASoS (Fig. 3). For example, the expression of phenomenal aspects of “Observing”, “Effortlessness” and “Involuntariness” increased, thus indicating an enhanced sense of involuntary witnessing that occurs without effort (passively) and may signify some level of “distance” between experience and experiencer25 (Lane 2020). This conclusion is corroborated by decreased sense of “Ownership” and all other aspects of embodiment, including geometrical “Self-location” and perception of temporality (Fig. 3). At the same time, the phenomenal sense of physical “Agency” did not change in relation to the restful baseline, as also was a case for “Thoughts continuity” and “Thoughts speed”. Despite this, “Thoughts concatenation” and “Reflection” together with “Rumination” decreased markedly supporting the participant’s statement that there were very few thoughts in this ASoS and implying that such overall experience was not the result of active introspection or thought wandering. It also confirms previous observations that one’s temporally embedded identity facilitates the construction of a personal narrative (Wittmann 2013; Berkovich-Ohana and Wittmann 2017; Fingelkurts et al. 2020), and if one is diminished the other follows (Lebedev et al. 2015; Nour and Carhart-Harris 2017).
“Different parts of my body disappeared completely”
Figure 4 presents the neurophenomenological profile of the ASoS: “Different parts of my body disappeared completely”. The participant described this state as: “Different parts of the body disappeared completely and just some entirety that was breathing or rather that was being breathed in remained. Breathing was a wave and that was all there is. Thoughts were circulating in my head”. Neurophysiologically, this ASoS was constituted by a very slight decrease in the functional integrity of the Self-module and strong decrease of Me-module with an increase in the functional integrity of I-module of the brain SRN (Fig. 4). In accordance with previous results (Fingelkurts et al. 2020), such changes in the functional integrity of the OMs triad implies a strong decrease in the embodiment as pre-reflective, non-conceptual, pre-linguistic, and immediate sense with a geometrical first-person perspective (Gallagher 2000, 2005; Legrand 2006; Metzinger 2008) causing the correspondent decrease in the phenomenal point of view (phenomenal first-person perspective; Metzinger 2004, 2008; Revonsuo 2006; Trehub 2007; Blanke and Metzinger 2009). The phenomenal point of view is, in this conceptualisation, a phenomenal non-conceptual core in the act of knowing itself (Blanke and Metzinger 2009; Velmans 2014), that is experienced as a directly and immediately present centre that witnesses a phenomenal multimodal perceptual reality and itself (witnessing agency, Fingelkurts et al. 2020). Such decreases, however were compensated in this ASoS by an increase in explicit self-reflection flavoured by conceptual and linguistic aspects, thus providing a strong epistemic input (Metzinger 2020), that tried to make sense of the reflected experience (Gallagher and Daly 2018).
Fig. 4.
Neurophenomenological profile of the altered state of Selfhood (ASoS) “Different parts of my body disappeared completely”. The legend of this figure is the same as that of the Fig. 2
The questionnaires data (used to facilitate the phenomenological reports) were in line with neurophysiological findings (Fig. 4). It is noteworthy that expression of such phenomenological aspects as “Thoughts concatenation” and “Reflection” increased markedly, while at the same time “Thoughts speed” and “Thoughts continuity” decreased. Curiously, such an increase in self-reflection was not accompanied by an increase in time perception, the sense of which transiently decreased in this ASooS (Fig. 4), thus indicating that self-reflection was not accompanied by an autobiographical narrative (see Damasio 1999, 2010), but instead was more about making rational sense of the altered ongoing experience. Such a unique dissociation between cognitive thought and witnessing self, alongside a remarkable disembodiment (indexed by a decrease in all embodiment phenomenological aspects, see Fig. 4), is reminiscent of the subjective report of deafblind person taking a relaxing bath, as she appeared to retain an ability to think about what is happening to her (that indicates the ability to form de se thoughts26) despite being in an ASoS of radical disembodiment and self-disintegration (for a detailed discussion, see Millière 2019). These observations are important because they provide evidence that even when one’s sense of body, location and time have faded away, the ability to think about oneself need not necessarily be disrupted also, especially if effortless self-reflections in the form of mind-wandering potentiates the suppression of bodily awareness and self-location, which was apparently the case in this particular ASoS (Fig. 4).
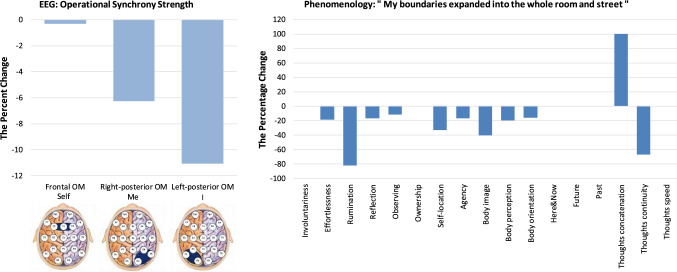
“My boundaries expanded into the whole room and street”
Figure 5 presents the neurophenomenological profile for the ASoS: “My boundaries expanded into the whole room and street”. It has been described by the participant as: “My body boundaries expanded into the whole room and street, then thoughts and images began to pop into my mind”. Neurophysiologically, this ASoS was constituted by a very slight decrease in the functional integrity of Self-module and a rather strong decrease in the functional integrity of the Me- and especially I- modules of the brain SRN (Fig. 5). These changes in the functional integrity of the OMs triad imply that in this particular ASoS, the participant experienced (as it follows from previous findings, Fingelkurts et al. 2020): (1) dramatic disembodiment characterised by body boundaries dissolution, guided by an abrupt detachment from the interoceptive and exteroceptive sense perceptions; (2) dissolution of the normal narrative and self-reflection; and (3) nonsignificant attenuation of witnessing agency. In general, this altered state resembles the so-called transcendental states that are incidents of clear, rational thought-free consciousness, characterized by a self boundaries dissolution, when the knower, the known and the process of knowing become unified in a state of Am-ness (Hebert et al. 2005; see also Travis and Shear 2010). Another similar phenomenon is known as drug-induced ego dissolution (DIED),27 that is a loss of one’s sense of self and self-world boundaries, accompanied by a feeling of “oneness”, typically experienced under the influence of psychedelics (Letheby and Gerrans 2017; Nour and Carhart-Harris 2017).
Fig. 5.
Neurophenomenological profile of the altered state of Selfhood (ASoS) “My boundaries expanded into the whole room and street”. The legend of this figure is the same as that of the Fig. 2
The questionnaires data largely supported the neurophysiological observations; however, providing additional nuances of how this ASoS was subjectively perceived. From Fig. 5 it could be seen that the expression of all measured phenomenal aspects related to embodiment (that include “Self-location”, “Physical agency”, “Body image”, “Body perception” and “Body orientation”) diminished, thus indicating that alterations in bodily self-experience, guided by disruption in the multimodal integration of sensory signals, resulted in the experience of an altered body image and perception (Giummarra et al. 2008; Blanke and Metzinger 2009; Blanke 2012; Seth 2013). These was accompanied by decreased expression of self- “Reflection”, “Rumination” and “Thoughts continuity”; while “Thoughts concatenation” on the contrary increased (Fig. 5), implying that even though there were groups of thoughts occurring together (“concatenation”), they were semantically discontinuous, and generally were rear, thus resulting in diminished self-reflection and narration. It is possible to further speculate that during this ASoS the subjective experience involves a reduced tendency to be engaged (personally and emotionally) in one’s own thoughts and feelings, such that “the contents of consciousness [were] less filtered through considerations of self-relevance than is usual” (Letheby and Gerrans 2017; p. 7).
Interestingly, neither time perception, nor the feeling of ownership were affected in this ASoS (Fig. 5). Since the autobiographical narrative relies heavily on temporality—either episodic memory of the past or imagined/planned future (Damasio 1999, 2010; Gardiner 2001)—one may speculate that the autobiographical component of the personal narrative remained not altered during this ASoS, while other aspects of the narrative were (as discussed above). This observation pointed to the fact that different components of narrative may have different dynamics28 under certain circumstances. Furthermore, considering the abovementioned strong disembodiment that accompanied this ASoS, and keeping in mind that quite often during the alterations in the sense of Selfhood changes in bodily self and autobiographical self co-occur (Fingelkurts et al. 2020), the present findings indicate that under certain conditions they may have independent dynamics and may in fact de-couple (for a similar conclusion see Sebastián 2020; for further relevant discussion, see Millière et al. 2018). The fact that the Self-module of brain SRN was almost not altered may explain why the subjective sense of “Ownership” was not affected (Fig. 5). Indeed, normally, the Self-module constitutes the sense of a “centre of gravity”, where one is having an experience of directly and immediately present as the centre (or a focus) of a phenomenal multimodal perceptual reality (Metzinger 2004, 2008; Revonsuo 2006; Trehub 2007; Blanke and Metzinger 2009), with the sense of ownership of thoughts, perceptions, and actions relevant to Selfhood (Metzinger 2004, 2008; de Vignemont and Fourneret 2004; Hohwy 2007; Blanke and Metzinger 2009). Keeping this in mind, one may suppose that such only minor alteration in the functional integrity of the Self-module was still sufficient for the presence of these subjective feelings.
“I was both in my body and outside it”
Figure 6 presents the neurophenomenological profile for the ASoS: “I was both in my body and outside it”. The participant described this state as: “Profound experience—I was both in my body and outside it, in this room, and via all the floors (I sensed the rooms below and above me) connected to the air outside”. Neurophysiologically, this ASoS was constituted by a very slight decrease in the functional integrity of the Self-module, a stronger decrease in the functional integrity of the I-module, and a yet stronger increase in the functional integrity of the Me-module of the brain SRN (Fig. 6). Such changes in the functional integrity of the OMs triad, when approached from the findings of the previous study on the causal links between the functional integrity of the three SRN OMs and correspondent to them the three phenomenological aspects of Selfhood (Fingelkurts et al. 2020), could indicate that in this ASoS the participant experienced enhanced embodiment coupled with decreased self-reflection and narration, and only insignificantly altered witnessing agency. At first glance it may look as a contradiction, because the participant described this ASoS as being in the body and outside at the same time, so implying some kind of out-of-body experience (OBE). However, some types of OBE are exactly of this phenomenal nature: “parasomatic” OBE is characterised by the experience of being embodied in a spatial volume, which at the same time share many features of the physical body and simultaneously represented as an indeterminate form of volume (Green 1968; Irwin 1985). Therefore, one may expect to have an enhancement of the sense of embodiment, observed in this particular ASoS (Fig. 6). Indeed, in a recent study of the OBE-specific group, it was documented that some types of OBE display hyper-embodiment (Braithwaite et al. 2017; for a discussion, see Kessler and Braithwaite 2016).
Fig. 6.
Neurophenomenological profile of the altered state of Selfhood (ASoS) “I was both in my body and outside it”. The legend of this figure is the same as that of the Fig. 2
The questionnaires revealed complementary details that enriched the understanding of this ASoS (Fig. 6). First, it was found that explicit body representation (indexed by the phenomenological aspect “Body image”) is not necessary for having some level of self-identification during this ASoS, because voluminous experience of simultaneously being in the body and outside of it is sufficient to produce self-identification (as defined in Metzinger 2013). This is why the sense of “Body perception” did not change. Second, bodily “Ownership” is not a necessary condition either,29 because during this ASoS the enhanced experiences of mental “Self-location” and physical “Agency” were sufficient for creating a geometrical first-person perspective (Fig. 6). At the same time, such experiences were not enough to form a strong, stable and veridical sense of phenomenal first-person givenness (Fingelkurts et al. 2020), as indicated by an insignificantly diminished “Observing” aspect.
This ASoS was further characterised by a marked decrease in the “Self-reflection”, “Rumination” and “Thought”-processes (Fig. 6), confirming a decrease in the functional integrity of the I-module of the brain SRN, which is responsible for self-reflection and narration (Fingelkurts et al. 2020). Curiously, such disruption in thought process and self-reflection did not affect multiple aspects of the experience of time, which remained unaltered in this ASoS (Fig. 6). Considering the conceptualisation of Wittmann (2013, 2015) that continuous visceral and proprioceptive input from the body is the functional anchor of time perception, one may speculate, that while increased body “Agency” and “Self-location” should enhance the experience of time, the decreased sense of body “Ownership” and “Body image” as well as “Body orientation”, on the contrary, should decrease temporal perception, thus resulting in the counterbalance of the opposite tendencies in the temporal experience, leading to the overall experience of unaltered sense of time.
“I raised and felt the space around”
Figure 7 presents the neurophenomenological profile of the ASoS: “I raised and felt the space around”. This state was described by the participant as: “I raised up with my body and felt the space around. I looked at the building from a very high place, as if from an aeroplane. I myself however was in my body. Autobiographical memories and thoughts were present”. Neurophysiologically, this ASoS was constituted by a very slight increase in the functional integrity of the Self-module, medium increase in the functional integrity of the I-module, and a strong increase in the functional integrity of the Me-module of the brain SRN (Fig. 7). Together, these changes in the functional integrity of the OMs triad, may be interpreted (Fingelkurts et al. 2020) as that during this ASoS the participant was experiencing the so-called “somatic” OBE, which is characterised by a changed sense of self-location in comparison to the ordinary, everyday baseline state (Green 1968; Irwin 1985) without losing the sense of body, which was in fact reinforced by a constant self-reflection and analysis, and further accompanied by a slightly enhanced witnessing and self-observation.
Fig. 7.
Neurophenomenological profile of the altered state of Selfhood (ASoS) “I raised and felt the space around”. The legend of this figure is the same as that of the Fig. 2
The questionnaires data largely confirmed the neurophysiological findings by providing additional nuances of how this ASoS was subjectively perceived (Fig. 7). It is noteworthy, that during this ASoS all embodiment aspects (that, in combination, create a sense of owning and inhabiting a physical body which is the locus of one’s experience; Blanke and Metzinger 2009; Blanke 2012; Seth 2013) increased, paralleled by an increase in the sense of “Involuntariness”, “Here and Now”, and further the sense of “Past”, “Thoughts concatenation”, “Reflection”, and “Observing”. Thus, overall, while being in this ASoS, the participant experienced an involuntary increased sense of self and agency, dominated by hyperembodiment and to a lesser extent self-reflection (Fig. 7). Additionally, the participant had an enhanced experience of the present moment and spontaneously emerging past memories, accompanied by slowing of time (indicated by decreased “Thoughts speed”), similar to particular mindful experiences during meditation practices (Berkovich-Ohana et al. 2013; Wittmann 2013; Berkovich-Ohana and Wittmann 2017).
One last note in relation to this ASoS should be in place: given the established potential link between mind-wandering and unhappiness (Killingsworth and Gilbert 2010), it is intriguing that “Rumination”, in fact, decreased during this ASoS (Fig. 7). Ruminative self-processing was previously associated with higher social anxiety, psychological distress, and depression (Trapnell and Campbell 1999; Ben-Artzi and Hamburger 2001; Takano and Tanno 2009), and its lack in this ASoS may seem contradictory given that self-related mind-wandering and sense of the past increased (see Fig. 7). However, this apparent contradiction could be resolved by the fact that mind-wandering, besides self-rumination, also includes self-reflection which is associated with more accurate and extensive self-knowledge and lower psychological distress and increased feeling of well-being (Watson et al. 1996; Trapnell and Campbell 1999). Such self-reflection was increased in this particular ASoS (Fig. 7).
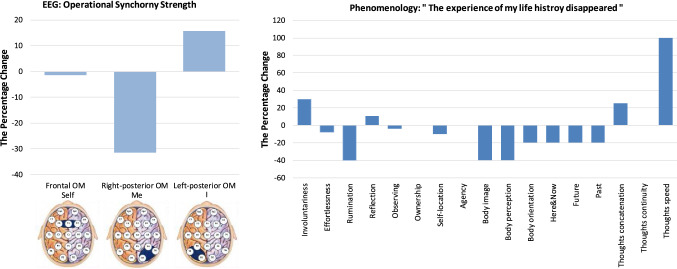
“The experience of my life history disappeared”
Figure 8 presents the neurophenomenological profile of the ASoS: “The experience of my life history disappeared”. The participant described this unusual state as: “In this experience my life history disappeared. I realized it never existed; just a vanishing hint about something that actually did not exist. The experience changed me rather fundamentally”. Neurophysiologically, this ASoS was constituted by a very slight decrease in the functional integrity of the Self-module, strong decrease in the functional integrity of the Me-module and moderate increase in the I-module of the brain SRN (Fig. 8). Based on the previous study (Fingelkurts et al. 2020), such alterations in the SRN OMs may imply that in this ASoS the participant experienced a very slight decrease in witnessing agency, accompanied by a rather pronounced disembodiment, coupled with increased self-reflection.
Fig. 8.
Neurophenomenological profile of the altered state of Selfhood (ASoS) “The experience of my life history disappeared”. The legend of this figure is the same as that of the Fig. 2
The questionnaires data were largely in line with the neurophysiological observations; however, providing interesting nuances. From Fig. 8 it could be seen that the expression of many measured phenomenal aspects related to embodiment (that include “Body image”, “Body perception”, “Body orientation” and “Self-location”) diminished, thus indicating an alteration in bodily self-experience (Giummarra et al. 2008; Blanke and Metzinger 2009; Blanke 2012; Seth 2013), though these were surprisingly not affecting “Ownership”, and “Agency”. It seems that the increased sense of effort (indexed by a decreased “Effortlessness”) maybe was sufficient to produce some sense of self-identification30 (Metzinger 2013, 2020; see also Yufik 2019), thus counterbalancing the effect of body dissolution on the experience of ownership and agency (see Fig. 8).
Further, this ASoS was accompanied by a transient disruption in the subjective experience of time, whereas all aspects of time perception diminished (Fig. 8), thus probably resulting in a feeling of disappearance of the “life history”. In fact, the “autobiographical” self (Damasio 1999, 2010) relies very much on self extended in time, where it is predicated on the retrieval and experience of memories and facts about one’s life (Araujo et al. 2013). Hence, it is only logical to speculate that the disruption in time experience resulted in the disruption of the autobiographical identity and dissolution of life history, as they were temporary not integrated into a personal-level narrative. A similar loss of access to autobiographical memories is often reported during psychedelic drug induced altered states (Millière et al. 2018), with some reporting the disruption of even such fundamental self-knowledge that one is a human being (Johnstad 2019). At the same time, the participant’s phenomenological description clearly pointed that she has had rational thoughts about herself during this ASoS, indicated by her using the phrases like “I realized” or “vanishing hint about” (see above). This is also confirmed by an increase in such phenomenological aspects as “Reflection” and “Thoughts concatenation”, as well as “Thoughts speed” that together were supported by increased functional integrity of the I-module of the brain SRN (Fig. 8). All-in-all, these observations point to the fact that alterations in autobiographical identity and self-referential thoughts may have some degree of independence and may take even opposite directions (although, their dynamics usually co-occur; Andrews-Hanna et al. 2014).
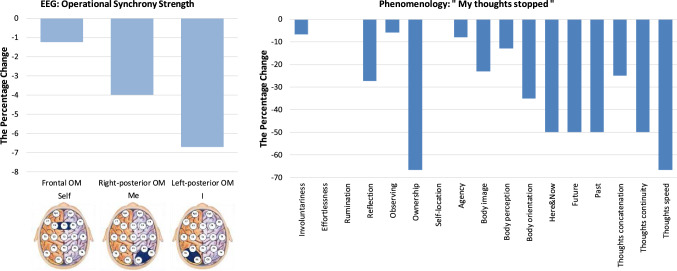
“My thoughts stopped”
Figure 9 presents the neurophenomenological profile of the ASoS: “My thoughts stopped”. This state was described as: “The thoughts stopped. A pleasant openness remained. The body was no more. Nothing to observe”. Neurophysiologically, this ASoS was constituted by a some decrease in the functional integrity of the Self-module, stronger decrease in the functional integrity of the Me-module and even stronger decrease in the I-module of the brain SRN (Fig. 9). Considering the previous study on the relations between the triad SRN OMs and three aspects of Selfhood (Fingelkurts et al 2020), the observed alterations in the SRN OMs triad may signify that in this ASoS the participant had approached31 an experience sometimes described as a “pure awareness” (Stace 1960; Fingelkurts and Fingelkurts 2019), which is a phenomenological state of selfless, objectless and timeless presence (Metzinger 2020), also reminiscent of subjective episodes during dreamless sleep (Thompson 2015; Windt 2015). Generally, such experience, is characterised by an “emptying out” of all phenomenological contents, including thoughts, and a lack of individual first-person perspective.
Fig. 9.
Neurophenomenological profile of the altered state of Selfhood (ASoS) “My thoughts stopped”. The legend of this figure is the same as that of the Fig. 2
The questionnaires data were remarkedly aligned with the neurophysiological observations; however, providing additional interesting nuances. From Fig. 9 it could be seen that the expression of all phenomenological aspects related to discursive thought decreased notably (with “Thought speed” the most). Such alterations may contribute to a participant’s phenomenological experience of decreased self-reflection and thoughts halting,32 with “empty” content (recall the participant’s description that there were “nothing to observe”). As expected, the related aspects of time experience (indexed by “Here & Now”, “Future”, and “Past”) decreased strongly too (Fig. 9), thus indicating that, while in this ASoS, the participant had an experience of being outside of time (Shanon 2001), hence not being located in a temporal frame of reference (Metzinger 2020). Even the sense of “nowness” decreased strongly (see Fig. 9).
Further, during this unique ASoS, the expression of phenomenological aspects contributing to a sense of embodiment (“Body image”, “Body perception”, and “Body orientation”) decreased markedly as well (Fig. 9), pointing to a rather high degree of phenomenological disconnection from the physical body’s sensory and motor systems (Fingelkurts et al. 2020) and leading to a feeling of body dissolution. These changes in the embodiment cause a strong decrease in an immediate sense of a geometrical first-person perspective (Gallagher 2000, 2005; Legrand 2006; Metzinger 2008) resulting in a diminished sense of “Agency”, body “Ownership” and “Here & Now” (see Fig. 9). The alteration in a geometrical first-person perspective is related to a phenomenal point of view (phenomenal first-person perspective; Metzinger 2004, 2008; Revonsuo 2006; Trehub 2007; Blanke and Metzinger 2009). Therefore, one would expect a decrease in the sense of witnessing agency which is conceptualised as a phenomenal non-conceptual core in the act of knowing itself (Blanke and Metzinger 2009; Velmans 2014), that is experienced as a directly and immediately present centre that witnesses/observes a phenomenal multimodal perceptual reality and itself (Fingelkurts et al. 2020).
Indeed, the aspect of “Observing” decreased, though only slightly, in this ASoS (Fig. 9). This observation, together with a finding of a slightly decreased functional integrity of the Self-module of the brain SRN (that is responsible for the witnessing agency) (Fig. 9), may explain why the participant was still able to report this state at all. It seems, that such slight decreases were still sufficient to sustain a some very minimal sense of “self in the act of knowing” (Blanke and Metzinger 2009; Velmans 2014), and thus creating a minimal form of an internal, self-directed first-person perspectivalness; however, already not accompanied with executive control and extended self-reflection thought and autobiographical narrative all temporarily “offline” (Fingelkurts et al. 2020).
Summary, conclusions and limitations
The present study reports on eight unique ASoSs, that spontaneously occurred during mediation in six experienced long-term meditators. Since these ASoSs were the single time-points during different trials, they could be considered as phenomenological snapshots at given moments in time. Following the guidelines of the neurophenomenological framework (Varela 1996; Gallagher and Sørensen 2006; Gallagher and Zahavi 2008; Berkovich-Ohana et al. 2020), the estimation of the OMs triad of the brain SRN, that was done using EEG measurement, was guided by the participants’ subjective reports. The result of such analysis demonstrated that documented 8 ASoSs had rather unique neurophenomenological profiles (Figs. 2, 3, 4, 5, 6, 7, 8, 9), despite that all of them may be broadly divided in only two clusters: (1) changes in embodiment and (2) changes in self-reflection and narration (see Table 1; ASoSs 1–6 and 7–8 respectively). In other words, grossly similar phenomenological experiences had very different subtle nuances that were captured by the neurophenomenological profiles that reveal transient perturbation of different components of Selfhood during any given ASoS. Therefore, one may conclude that experiences such as “disembodiment”/“OBE”, loss of “autobiographic identity” and “self-reflection”, or slight alterations of “witnessing observer”, each is far from being the unequivocal phenomenon and all can take different forms where various aspects or components of Selfhood are affected and expressed differently (for a similar deduction, see Gallagher and Daly 2018; Millière et al. 2018; Fingelkurts et al. 2020).
Figure 10 presents the dynamics of all three Selfhood components (Self, Me, and I) during eight ASoSs side by side. This representation allows to have an overview of all ASoSs at once and get new insights while comparing various ASoSs using range of common thresholds. It is straightforward that all ASoSs had a rather unique expression of Self–Me–I components. At the same time, and surprisingly, there were two different phenomenological states which shared an identical Self–Me–I phenotype—the two ASoSs in question were: (1) “My boundaries expanded into a whole room and street” and (2) “My thoughts stopped” (Fig. 10). Thus, one may conclude that similar neurophysiological changes in the OMs triad were associated with both states. A closer look at these states (see analyses above: “My boundaries expanded into the whole room and street” and “My thoughts stopped” sections) reveals, that while participants in their subjective reports have focused on rather different phenomenological features (embodiment vs autobiographical narrative) of their experience, a detailed evaluation of the questionnaire replies revealed that the key features in the phenomenology had similar changes (diminished “Reflection”, “Observing”, “Physical agency”, “Body image”, “Body perception”, “Body orientation”, “Thoughts continuity”), thus supporting the similarity between the SRN OMs triads. These results highlight the importance and the utility of combining phenomenological descriptions and questionnaires with EEG neuroimaging measurement within the same experimental design when characterizing complex experiential Selfhood. Based on this analysis one may further suggest that a concrete altered state of Selfhood is determined not only by the direction of functional change in Selfhood components, but also by the magnitude of this change and a mutual relation between these components.
Fig. 10.
Dynamics of the three components of Selfhood (measured by the EEG functional integrity of OMs triad) during eight spontaneous altered states of Selfhood (ASoSs). The colour code is proportional to the percent change from the baseline condition in every component during a particular ASoS. Cold colour shades indicate decrease, while the warm colour shades indicate increase. Abbreviations: ASoS altered state of Selfhood, EEG electroencephalogram, OM operational module
Analysis of Fig. 10 data reinforces the view that self-consciousness is not a simple fixed unidimensional construct, but rather a fluid complex multidimensional pattern emerging from the dynamic interaction of differentially expressed characteristic aspects/features/qualities/components that jointly constitute Selfhood (Gallagher 2013; see also Metzinger 2014; Zahavi 2014; Musholt 2015; Millière et al. 2018; Fingelkurts et al. 2020). Furthermore, every aspect of Selfhood is not a simple unidimensional feature either, but instead a higher-order emergent complex multidimensional construct in its own right. Such a proposition has been made in our previous work (Fingelkurts et al. 2020) and is clearly confirmed in the present study. Indeed, every studied component of Selfhood comprises several low-level components. For example, the Me-component subsumes body image, body perception, body orientation, ownership, geometrical first-person perspective and physical agency; the I-component includes reflection, rumination, narrative, autobiography, thoughts’ structure and speed; the Self-component comprises phenomenal centre, phenomenal first-person perspective, epistemic certitude, witnessing observer. While, usually, the sub-components belonging to the same characteristic component behave in a similar manner (Fingelkurts et al. 2020), under certain conditions like during ASoSs, some of them may have different (even opposite) dynamics, as was observed in the present study (see the previous sections).
By the same token, taken together, the analysis of 8 ASoS profiles indicates that changes in the triad of SRN OMs are largely in line with our previous study that established a causal link between three phenomenological aspects of Selfhood and brain OMs triad (Fingelkurts et al. 2020). Thus, the findings of the present study contribute to new converging evidence for a more comprehensive understanding of the ASoSs complexity.
Limitations
Since this study analyzed a subset of the data from a previous larger study (Fingelkurts et al. 2020), its limitations apply to the present one. These limitations are: (1) a small sample size as it was restricted to participants with a history of long-term meditation practice, (2) uncertainty in regards to generalizability of results to a meditation naïve general population, (3) analysis restricted to EEG alpha range only. Additionally, and specifically to the present study, no statistical evaluation was possible since the focus of this study was on brief spontaneously occurring, unique episodes of ASoSs that represent single snapshots of the phenomenological experience, hence making this a descriptive study. At the same time, such studies are crucially important because they often lead to unexpected hypotheses (Zhou et al. 2016) and provide a unique opportunity to address previously unasked questions (Pöppel et al. 2013). Another potential limitation of the present study was the way the ASoS time-stamps were indicated: they were not reported on the spot, but re-called after completing the 10-min experimental condition. Therefore, there is some possibility that the timings of ASoSs were assessed imprecisely. We have discussed this possibility in the footnote 13 and conclude that this is unlikely due to several reasons (see footnote 13). Thus, we are confident that the self-reported ASoS time stamps were indeed reliable.
Despite these limitations, the strength of this study is that it used a kind of enhanced neurophenomenological study (adding questionnaires and Likert scales to the usual pairing of EEG and phenomenology), following the proposal by Gallagher et al. (2015). Further, the findings from this study increase our knowledge on the normal variation of self-consciousness as could be observed in the spectrum of various ASoSs. Additionally, the results presented here provide a rich framework for new studies, specifically in the field of neuropsychopathology, where mechanisms similar to those that bring about the expression of a particular ASoS may be present as a given symptom or dysfunction contributing to a particular pathology.
Acknowledgements
The authors thank the participants of this study for dedicating their time and unique contemplative expertise, and for their contribution in advancing the scientific understanding of experiential Selfhood. The authors would like further to thank C. Neves (Computer Science specialist) for programming, technical, and IT support and D. Skarin for English editing.
Author contributions
AnAF: Conceptualization, Methodology, Investigation, Resources, Data curation, Formal analysis, Writing—Original Draft, Visualization, Project administration. AlAF: Conceptualization, Methodology, Investigation, Resources, Formal analysis, Writing—Review and Editing, Visualization. TK-T: Conceptualization, Methodology, Writing—Review and Editing, Funding acquisition.
Funding
The authors received no funding for the research; however, some limited financial support was provided by private donations. These private persons had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Data availability
The data are not publicly available, due to privacy or ethical restrictions.
Declarations
Conflict of interest
None of the authors have potential conflicts of interest with respect to the research, authorship, and/or publication of this article to be disclosed.
Footnotes
By “the background mechanisms of consciousness” we consider, following Revonsuo (2006), the “immediately lower-level [in relation to consciousness] entities [at the level of brain organization] on which consciousness as a whole is ontologically dependent, meaning that consciousness could not exist without them to be present” (Fingelkurts et al. 2013; p. 14). However, phenomenologically only contents are experienced and not the content-formation process itself (background mechanisms of consciousness), so called “phenomenal transparency” (Metzinger 2003; 2014).
For the sake of completeness, it should be noted that ordinary visual illusions do not count as evidence for an ASC, even though they also misrepresent reality, because they are not temporary or reversible: particular types of stimuli invariably elicit illusions (Revonsuo 2006). Further, momentarily fleeting misperceptions due to noisy signal are obviously not an ASC either. The same goes for the neuropsychopathology: during such disorders, the alterations of self-consciousness have a long-term or permanent presence (Parnas et al. 2005; Beck 2008) and therefore such disorders do not count as evidence of ASC (Revonsuo 2006), even though they may share similar mechanisms (Dittrich 1998). Therefore, pragmatically, ASCs are the nonpathological states (Kokoszka 1999).
It is important to keep in mind that meditation and related contemplative practices, by themselves, do not represent altered states of consciousness, but rather facilitate them (Newberg and Yaden 2018).
In the neurophenomenological research paradigm, the ability to become aware of lived experience is considered a special skill, that requires a certain way of reflection toward the one’s own subjective experience, and that it can and should be trained and learned (Froese et al. 2011). This is why subjects in such studies are usually phenomenologically trained (Thompson et al. 2005). This training is rather effortful and time-consuming. At the same time, experienced, long-term meditation practitioners have been repeatedly proposed as subjects suitable for such inquiry, because due to their long-term practice they already acquired the needed skill (“pre-trained”) that enables the systematic gathering of reliable phenomenological reports (Varela et al. 1991; Varela and Shear 1999; Bitbol 2019; Kordeš et al. 2019; Berkovich-Ohana et al. 2020).
The other names found in literature are “minimal self” (Gallagher 2000; Gallagher 2005), “proto-self” (Panksepp 2005; Panksepp and Northoff 2009), or “bodily self” (Damasio 1999; Legrand 2006; Blanke 2012).
In the literature other terms have been used: “narrative self” (Gallagher 2000), “conceptual self” (Neisser 1988; Demiray and Bluck 2011), “autonoetic self” (Gardiner 2001; Klein 2016), or “autobiographical self” (Damasio 1999, 2010).
Case studies have been crucially important in shaping the psychophysiology science since its initiation (Zhou et al. 2016; Pöppel et al. 2013). Further, a case study approach is frequently utilized to investigate meditation-induced alterations in the subjective experience (see, for example, Lehmann et al. 2001; Engström and Söderfeldt 2010; Hagerty et al. 2013; Ataria 2015; Modestino 2016; Berkovich-Ohana 2017).
Subjects felt as if the experience ‘comes to them’. They did not purposely make the experience happen.
This sequence was repeated 3 times for 3 target mental states (“Self”, “Me” and “I”) with 2 variant each (Up- and Down-regulation), thus resulting in 108 trials for 6 participants.
The questionnaires were used to facilitate (confirm or correct) the interpretations across the different types of data (see also Gallagher et al. 2015).
This strategy guarantees a sufficient trustworthiness of reports (Windt 2013) and also minimizes reliance on episodic recall inference, misremembering or confabulation (Jack and Roepstorff 2002; Windt 2013).
The exact meditation technique routinely used by these participants was not important, because during an experiment the participants were instructed to voluntary reach particular pre-defined mental states (see Fingelkurts et al. 2020) instead of practicing a specific meditation. So, they did not meditate in the traditional sense of the practice (Nash and Newberg 2013).
We used a mixed visual analogue scale (VAS; Costa et al. 2016) with discrete scale, where VAS was complimented with temporal markers in seconds and minutes to help the participants to locate time-period of ASoS. One may argue that such post-hoc ASoS timing may be imprecise. While this is certainly a possibility, it is an established fact that the ability to accurately detect the temporal order of events is a stable human trait (Grabot and van Wassenhove 2017). Further, the temporal accuracy is higher for new, unusual or remarkable events that appear within a given time period (James 1890; Block and Zakay 1997), which was exactly the case in the present study. Taking these observations together, one can have trust in the reliability of the temporal stamps for the ASoSs. Moreover, the fact that participants of the present study were experienced mediators adds additional assurance that the ASoSs timing was assessed reliably, since long-term experienced meditators are known for having enhanced skill to provide detailed and accurate first-person descriptions of their experiences (Lutz et al. 2007; Fox et al. 2012; Berkovich-Ohana et al. 2013; Dor-Ziderman et al. 2013; Ataria et al. 2015). It is also important to note that meditation and related practices, by themselves, do not represent ASCs, but rather facilitate them (Newberg and Yaden 2018).
A brief note has to be made here: it is sometimes claimed that EEG analysis performed at the sensor level is prone to volume conduction and it may present an obstacle in interpreting EEG data in terms of brain functional connectivity. The operational synchrony measure used in the current study has been specifically tested through previous modelling experiments to address this issue. These tests show that operational synchrony values are sensitive to morpho-functional organization of the cortex as opposed to volume conduction, EEG signal power, and/or choice of the reference electrode (for further details, we refer the reader to Fingelkurts and Fingelkurts 2008, 2015).
The usefulness and advantage of such an approach was illustrated in the seminal work of Lutz (2002) and Lutz et al. (2002), which received much attention (Gallagher 2003b; Bayne 2004; Overgaard 2004).
Within each of these phenomenological ASoSs there were patterns of subjective variability that uniquely characterize and distinguish distinct types of ASoSs.
Indeed, it has been argued, that in normal conditions, bodily proprioceptive and interoceptive processing, due to their constant presence, ground a basic sense of self, anchoring it in a body-centered spatial frame of reference (Metzinger 2004, 2008), thus making a tight link between self and one’s own body (Damasio 1999; Craig 2002; Seth 2013; Tsakiris 2017). It is also thought, that such dependence likely evolved evolutionary to help organisms maintain homeostasis (Herbert and Pollatos 2012; Craig 2013; Damasio and Carvalho 2013), thus intimately connected to self-regulation and eventually self-awareness (Seth 2013; Farb et al. 2015). Interpedently, the interoceptive dysregulation was systematically reported in relation to many “self-involved” disorders, including dissociative disorders (Michal et al. 2014; Sedeño et al. 2014), post-traumatic stress disorder (Wald and Taylor 2008; Fingelkurts and Fingelkurts 2018), affective disorders and depression (Paulus and Stein 2010; Fingelkurts and Fingelkurts 2017a), somatoform disorders (Schaefer et al. 2012), and addiction (Naqvi and Bechara 2010).
This and further reports of the ASoSs of the current study are admittedly concrete and without fine-grained and often esoteric or mystic phenomenological descriptions typical for the Advaita and Tibetan Buddhist traditions (Rgyal-ba-g'yung-drung et al. 2017; Rinpoche and Namgyal 2011). This is so because participants were encouraged to suspend their own beliefs, metaphysical interpretations and theoretical background assumptions while describing their experiences during ASoSs, thus avoiding the so-called “theory contamination” (see also Nash and Newberg 2013).
The feeling as an extensionless point in space was often reported during various bodiless states (Kjellgren et al. 2008; Berkovich-Ohana et al. 2013; Ataria et al. 2015; Windt 2010; Wittmann 2015).
This is in line with the observations done by Blanke and Metzinger (2009) and also Fingelkurts et al (2020) that bodily agency while a causally enabling, is not a constitutive condition, for phenomenal self.
Interestingly, functional disintegration of the posterior parts of the SRN was previously reported to be associated with decreased mental time travel (Speth et al. 2016), while strong SRN functional connectivity at rest was related to increased tendency for mental time travel (Godwin et al. 2017; Karapanagiotidis et al. 2017).
Such a proposition is compatible with the notion of “temporal thickness” (Friston 2018; Limanowski and Friston 2018), according to which a brain continuously predicts future states by embodying the tonic alertness within a certain temporal span with simultaneous creation of counterfactual depth—representation of possible future states of knowledge (Friston 2018).
One potentially interesting direction for future research is to use hypnosis as an instrumental means to produce—in controlled manner—an OBE—(or near-death-experience [NDE]) like phenomenology while monitoring the EEG. For some examples of such studies, see Palmieri et al. 2014; Facco et al. 2019; Martial et al. 2019.
Disembodiment is normally characterized by a loss of the geometrical self-location, which is the experience of oneself as located within one’s body that serves as a spatial (embodied) frame of reference (Metzinger 2013; see also Fingelkurts et al. 2020).
This experience is quite similar to the episodes of clear, effortless mindfulness with distant perception of the physical body and the environment with an entirely quiet mind (as for example in Vedic, Buddhist and Chinese traditions; Travis and Shear 2010; Ataria et al. 2015; Finnigan 2018).
In philosophy of mind, de se thoughts are referred to thoughts that involve the first-person concept and are naturally expressed using the first-person pronoun (García-Carpintero 2015). There is a spectrum of de se thoughts. For example, one may explicitly reflect on one’s personality traits or one’s life trajectory (so called narrative selfhood) or just has thoughts that include more mundane instances of mind-wandering about the current ongoing experience that is happening to oneself.
Curiously, and in agreement with the current results, the main neurophysiological correlate of the DIED is a decreased functional integrity of the SRN (Carhart-Harris et al. 2016), which is also reflected in the increased entropic brain activity (Lebedev et al. 2016) as well as increase in the neurophysiological signal diversity (Schartner et al. 2017).
Such a possibility is discussed in detail by Millière et al. (2018; pp. 6–7): “There are at least two ways in which narrative self-consciousness may be disrupted. First, the rate of occurrence of self-referential thought and mental time travel may be dramatically reduced, or altogether suppressed, during a certain time interval. […] While the temporary cessation of self-referential thoughts is one way in which narrative self-consciousness may be altered, it may also be disrupted by a total loss of access to autobiographical memories and self-related beliefs. […] However, the experience of losing access to these memories and beliefs might differ from the mere cessation of de se thought from a phenomenological point of view”.
Lack of body ownership causing the moments of OBE was also reported before during meditation practice (Ataria 2015).
Indeed, the subjective sense of effort presupposes some level of intentionality (Posner and Rothbart 1998) and is sufficient to form at least a minimal sense of ownership and agency (Millière and Metzinger 2020; Metzinger 2020; see also Ataria et al. 2015).
The use of the word “approached” here is done on purpose. It stresses the point that the participant did not in fact reach this state of “pure awareness”. If that state were to occur, then the complete absence of all phenomenological content would render the participant incapable of reporting such an episode. This is because during these episodes the self-referential mechanisms of forming an event in the subject’s inner life narrative would be suspended (Metzinger 2020). Therefore, the subjective experience during the full-fledged “pure awareness” ASS could not be reported, only the process of entering into it or of emerging out of it can.
This, could be conceptualized as a longer time production (indexed by a slower rate of functioning of the internal timer), subjectively experienced as if the time seems to be moving slower or stopped (Glicksohn 2001; Wittmann 2013; Berkovich-Ohana and Wittmann 2017).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alvarado CS. Out-of-body experiences. In: Cardeña E, Lynn SJ, Krippner S, editors. Varieties of anomalous experiences: examining the scientific evidence. Washington: American Psychological Association; 2000. pp. 183–218. [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps MA, Tsakiris M. The free-energy self: a predictive coding account of self-recognition. Neurosci Biobehav Rev. 2014;41:85–97. doi: 10.1016/j.neubiorev.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo HF, Kaplan J, Damasio A. Cortical midline structures and autobiographical-self processes: an activation-likelihood estimation meta-analysis. Front Hum Neurosci. 2013;7:548. doi: 10.3389/fnhum.2013.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataria Y. Where do we end and where does the world begin? The case of insight mediation. Philos Psychol. 2015;28(8):1128–1146. [Google Scholar]
- Ataria Y, Dor-Ziderman Y, Berkovich-Ohana A. How does it feel to lack a sense of boundaries? A case study of a long-term mindfulness meditator. Conscious Cogn. 2015;37:133–147. doi: 10.1016/j.concog.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Aymerich-Franch L, Petit D, Ganesh G, Kheddar A (2015) Embodiment of a humanoid robot is preserved during partial and delayed control. In: Proceedings of the IEEE international workshop on advanced robotics and its social impacts (ARSO 2015), Lyon, France. 10.1109/ARSO.2015.7428218
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:7–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- Barabasz A, Barabasz M. Research designs and considerations. recent research and future directions. In: Fromm E, Nash M, editors. Contemporary hypnosis research. New York: Guilford Press; 1992. pp. 173–200. [Google Scholar]
- Bayne T. Closing the gap? Some questions for neurophenomenology. Phenomenol Cogn Sci. 2004;3:349–364. [Google Scholar]
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Ben-Artzi E, Hamburger YA. Private self-consciousness sub-scales: correlates with neuroticism, extraversion, and self-discrepancy. Imagin Cogn Pers. 2001;21:21–31. [Google Scholar]
- Berkovich-Ohana A. A case study of a meditation-induced altered state: increased overall gamma synchronization. Phenomenol Cogn Sci. 2017;16:91–106. [Google Scholar]
- Berkovich-Ohana A, Wittmann M. A typology of altered states according to the consciousness state space (CSS) model. J Conscious Stud. 2017;24(3–4):37–61. [Google Scholar]
- Berkovich-Ohana A, Dor-Ziderman Y, Glicksohn J, Goldstein A. Alterations in the sense of time, space and body in the mindfulness-trained brain: a neurophenomenologically-guided MEG study. Front Psychol. 2013;4:912. doi: 10.3389/fpsyg.2013.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkovich-Ohana A, Dor-Ziderman Y, Trautwein F-M, Schweitzer Y, Nave O, Fulder S, Ataria Y. The hitchhiker’s guide to neurophenomenology—the case of studying self boundaries with meditators. Front Psychol. 2020;11:1680. doi: 10.3389/fpsyg.2020.01680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlucchi G, Aglioti S. The body in the brain: neural bases of corporeal awareness. Trends Neurosci. 1997;20:560–564. doi: 10.1016/s0166-2236(97)01136-3. [DOI] [PubMed] [Google Scholar]
- Bermúdez JL. Bodily awareness and self-consciousness. In: Gallagher S, editor. The Oxford handbook of the self. Oxford: Oxford University Press; 2011. pp. 157–179. [Google Scholar]
- Bitbol M. Consciousness, being and life: phenomenological approaches to mindfulness. J Phenomenol Psychol. 2019;50:127–161. [Google Scholar]
- Blakemore SJ, Smith J, Steel R, Johnstone EC, Frith CD. The perception of self-produced sensory stimuli in patients with auditory hallucinations and passivity experiences: evidence for a breakdown in self-monitoring. Psychol Med. 2000;30:1131–1139. doi: 10.1017/s0033291799002676. [DOI] [PubMed] [Google Scholar]
- Blanke O. Multisensory brain mechanisms of bodily self-consciousness. Nat Rev Neurosci. 2012;13:556–571. doi: 10.1038/nrn3292. [DOI] [PubMed] [Google Scholar]
- Blanke O, Metzinger T. Full-body illusions and minimal phenomenal selfhood. Trends Cogn Sci. 2009;13:7–13. doi: 10.1016/j.tics.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Blanke O, Mohr C. Out-of-body experience, heautoscopy, and autoscopic hallucination of neurological origin Implications for neurocognitive mechanisms of corporeal awareness and self-consciousness. Brain Res Brain Res Rev. 2005;50:184–199. doi: 10.1016/j.brainresrev.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Blanke O, Ortigue S, Landis T, Seeck M. Stimulating illusory own-body perceptions. Nature. 2002;419:269–270. doi: 10.1038/419269a. [DOI] [PubMed] [Google Scholar]
- Block RA, Zakay D. Prospective and retrospective duration judgments: a meta-analytic review. Psychon Bull Rev. 1997;4:184–197. doi: 10.3758/BF03209393. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H. Impaired consciousness in epilepsy. Lancet Neurol. 2012;11:814–826. doi: 10.1016/S1474-4422(12)70188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Phillips C, Tshibanda L, Vanhaudenhuyse A, Schabus M, Dang-Vu T, Moonen G, Hustinx R, Maquet P, Laureys S. Intrinsic brain activity in altered states of consciousness: how conscious is the default mode of brain function? Ann N Y Acad Sci. 2008;1129:119–129. doi: 10.1196/annals.1417.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite JJ, Watson DG, Dewe H. Predisposition to out-of-body experience (OBE) is associated with aberrations in multisensory integration: psychophysiological support from a “rubber hand illusion” study. J Exp Psychol Hum Percept Perform. 2017;43(6):1125–1143. doi: 10.1037/xhp0000406. [DOI] [PubMed] [Google Scholar]
- Budwig N. Language and the construction of self: developmental reflections. In: Budwig N, Uzgiris IC, Wertsch JV, editors. Communication: an arena of development. Stamford: Ablex; 2000. pp. 195–214. [Google Scholar]
- Carhart-Harris RL, Muthukumaraswamy S, Roseman L, Kaelen M, Droog W, Murphy K, Tagliazucchi E, Schenberg EE, Nest T, Orban C, Leech R, Nutt DJ. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci USA. 2016;113:4853–4858. doi: 10.1073/pnas.1518377113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicogna P, Bosinelli M. Consciousness during dreams. Conscious Cogn. 2001;10(1):26–41. doi: 10.1006/ccog.2000.0471. [DOI] [PubMed] [Google Scholar]
- Cofré R, Herzog R, Mediano PAM, Piccinini J, Rosas FE, Perl YS, Tagliazucchi E. Whole-brain models to explore altered states of consciousness from the bottom up. Brain Sci. 2020;10(9):626. doi: 10.3390/brainsci10090626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. The beyond within. New York: Atheneum; 1964. [Google Scholar]
- Costa RM, Pestana J, Costa D, Wittmann M. Altered states of consciousness are related to higher sexual responsiveness. Conscious Cogn. 2016;42:135–141. doi: 10.1016/j.concog.2016.03.013. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Human feelings: why are some more aware than others? Trends Cogn Sci. 2004;8:239–241. doi: 10.1016/j.tics.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Craig AD. An interoceptive neuroanatomical perspective on feelings, energy, and effort. Behav Brain Sci. 2013;36:685–686. doi: 10.1017/S0140525X13001489. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? An interoceptive moment with your neurobiological self. Princeton: Princeton University Press; 2015. [Google Scholar]
- Crawford HJ, Gruzelier JH. A midstream view of the neuropsychophysiology of hypnosis: recent research and future directions. In: Fromm E, Nash M, editors. Contemporary hypnosis research. New York: Guilford Press; 1992. pp. 227–266. [Google Scholar]
- Damasio AR. Descartes’ error: emotion, reason and the human brain. New York: Grosset/Putnam; 1994. [Google Scholar]
- Damasio AR. The feeling of what happens: body and emotion in the making of consciousness. San Diego: Harcourt Brace; 1999. [Google Scholar]
- Damasio AR. When self comes to mind. New York: Pantheon Books; 2010. [Google Scholar]
- Damasio AR, Carvalho GB. The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neurosci. 2013;14(2):143–152. doi: 10.1038/nrn3403. [DOI] [PubMed] [Google Scholar]
- Davey CG, Pujol J, Harrison BJ. Mapping the self in the brain's default mode network. Neuroimage. 2016;132:390–397. doi: 10.1016/j.neuroimage.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Deane G. Dissolving the self: active inference, psychedelics, and ego-dissolution. Philos Mind Sci. 2020;1(I):2. doi: 10.33735/phimisci.2020.I.39. [DOI] [Google Scholar]
- Demiray B, Bluck S. The relation of the conceptual self to recent and distant autobiographical memories. Memory. 2011;19(8):975–992. doi: 10.1080/09658211.2011.626427. [DOI] [PubMed] [Google Scholar]
- de Vignemont F. Bodily immunity to error. In: Prosser S, Recanati F, editors. Immunity to error through misidentification: new essays. Cambridge: Cambridge University Press; 2011. pp. 1–27. [Google Scholar]
- de Vignemont F, Fourneret P. The sense of agency: a philosophical and empirical review of the “Who” system. Conscious Cogn. 2004;13:1–19. doi: 10.1016/S1053-8100(03)00022-9. [DOI] [PubMed] [Google Scholar]
- Dittrich A. The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry. 1998;31:80–84. doi: 10.1055/s-2007-979351. [DOI] [PubMed] [Google Scholar]
- Dor-Ziderman Y, Berkovich-Ohana A, Glicksohn J, Goldstein A. Mindfulness-induced selflessness: a MEG neurophenomenological study. Front Hum Neurosci. 2013;7:582. doi: 10.3389/fnhum.2013.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor-Ziderman Y, Ataria Y, Fulder S, Goldstein A, Berkovich-Ohana A. Self-specific processing in the meditating brain: a MEG neurophenomenology study. Neurosci Conscious. 2016;2016(1):niw019. doi: 10.1093/nc/niw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström M, Söderfeldt B. Brain activation during compassion meditation: a case study. J Altern Complement Med. 2010;16:597–599. doi: 10.1089/acm.2009.0309. [DOI] [PubMed] [Google Scholar]
- Facco E, Casiglia E, Al Khafaji BE, Finatti F, Duma GM, Mento G, Pederzoli L, Tressoldi P. The neurophenomenology of out-of-body experiences induced by hypnotic suggestions. Int J Clin Exp Hypn. 2019;67:39–68. doi: 10.1080/00207144.2019.1553762. [DOI] [PubMed] [Google Scholar]
- Farb N, Daubenmier J, Price CJ, Gard T, Kerr C, Dunn BD, Klein AC, Paulus MP, Mehling WE. Interoception, contemplative practice, and health. Front Psychol. 2015;6:763. doi: 10.3389/fpsyg.2015.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg TE. Neuroontology, neurobiological naturalism, and consciousness: a challenge to scientific reduction and a solution. Phys Life Rev. 2012;9(1):13–34. doi: 10.1016/j.plrev.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA. Operational architectonics of the human brain biopotential field: towards solving the mind-brain problem. Brain Mind. 2001;2(3):261–296. [Google Scholar]
- Fingelkurts AA, Fingelkurts AA. Brain–mind operational architectonics imaging: technical and methodological aspects. Open Neuroimaging J. 2008;2:73–93. doi: 10.2174/1874440000802010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA. Persistent operational synchrony within brain default-mode network and self-processing operations in healthy subjects. Brain Cogn. 2011;75:79–90. doi: 10.1016/j.bandc.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA. Dissipative many-body model and a nested operational architectonics of the brain. Phys Life Rev. 2013;10:103–105. doi: 10.1016/j.plrev.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA. Operational architectonics methodology for EEG analysis: theory and results. NeuroMethods. 2015;91:1–59. doi: 10.1007/7657_2013_60. [DOI] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA. Three-dimensional components of selfhood in treatment-naive patients with major depressive disorder: a resting-state qEEG imaging study. Neuropsychologia. 2017;99:30–36. doi: 10.1016/j.neuropsychologia.2017.02.020. [DOI] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA. Longitudinal dynamics of 3-dimensional components of selfhood after severe traumatic brain injury: a qEEG case study. Clin EEG Neurosci. 2017;48(5):327–337. doi: 10.1177/1550059417696180. [DOI] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA. Alterations in the three components of Selfhood in persons with post-traumatic stress disorder symptoms: a pilot qEEG neuroimaging study. Open Neuroimaging J. 2018;12:42–54. doi: 10.2174/1874440001812010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA. Placing pure experience of Eastern tradition into the neurophysiology of Western tradition. Cogn Neurodyn. 2019;13(1):121–123. doi: 10.1007/s11571-018-9506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA, Neves CFH (2010) Natural world physical, brain operational, and mind phenomenal space–time. Phys Life Rev 7:195–249 [DOI] [PubMed]
- Fingelkurts AA, Fingelkurts AA, Bagnato S, Boccagni C, Galardi G. DMN operational synchrony relates to self-consciousness: evidence from patients in vegetative and minimally conscious states. Open Neuroimaging J. 2012;6:55–68. doi: 10.2174/1874440001206010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA, Neves CFH. Consciousness as a phenomenon in the operational architectonics of brain organization: criticality and self-organization considerations. Chaos Solitons Fractals. 2013;55:13–31. [Google Scholar]
- Fingelkurts AA, Fingelkurts AA, Bagnato S, Boccagni C, Galardi G. The chief role of frontal operational module of the brain default mode network in the potential recovery of consciousness from the vegetative state: A preliminary comparison of three case reports. Open Neuroimaging J. 2016;10(Suppl-1, M4):41–51. doi: 10.2174/1874440001610010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA, Kallio-Tamminen T. Long-term meditation training induced changes in the operational synchrony of default mode network modules during a resting state. Cogn Process. 2016;17(1):27–37. doi: 10.1007/s10339-015-0743-4. [DOI] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA, Kallio-Tamminen T. Trait lasting alteration of the brain default mode network in experienced meditators and the experiential selfhood. Self Identity. 2016;15(4):381–393. [Google Scholar]
- Fingelkurts AA, Fingelkurts AA, Neves CFH, Kallio-Tamminen TK. Brain–mind operational architectonics: at the boundary between quantum physics and Eastern metaphysics. Phys Life Rev. 2019;31:122–133. doi: 10.1016/j.plrev.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA, Kallio-Tamminen T. Selfhood triumvirate: from phenomenology to brain activity and back again. Conscious Cogn. 2020;86:103031. doi: 10.1016/j.concog.2020.103031. [DOI] [PubMed] [Google Scholar]
- Finnigan B. Is consciousness reflexively self-aware? A Buddhist analysis. Ratio. 2018;31(4):389–401. [Google Scholar]
- Fox KC, Zakarauskas P, Dixon M, Ellamil M, Thompson EC. Meditation experience predicts introspective accuracy. PLoS ONE. 2012;7(9):e45370. doi: 10.1371/journal.pone.0045370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman W, Kozma R. Local–global interactions and the role of mesoscopic (intermediate-range) elements in brain dynamics. Behav Brain Sci. 2000;23(3):401–401. [Google Scholar]
- Freeman WJ, Kozma R, Li G, Quiroga RQ, Vitiello G, Zhang T. Advanced models of cortical dynamics in perception. Adv Cogn Neurodyn. 2015;4:127–136. [Google Scholar]
- Friston K. Am I self-conscious? (Or does self-organization entail self-consciousness?) Front Psychol. 2018;9:579. doi: 10.3389/fpsyg.2018.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Rosch R, Parr T, Price C, Bowman H. Deep temporal models and active inference. Neurosci Biobehav Rev. 2017;90:486–501. doi: 10.1016/j.neubiorev.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD. The cognitive neuroscience of schizophrenia. Hillsdale: Erlbaum; 1992. [Google Scholar]
- Froese T, Di Paolo EA. The enactive approach: theoretical sketches from cell to society. Pragmat Cogn. 2011;19(1):1–36. [Google Scholar]
- Froese T, Fuchs T. The extended body: a case study in the neurophenomenology of social interaction. Phenomenol Cogn Sci. 2012;11:205–235. [Google Scholar]
- Froese T, Gould C, Barrett A. Re-viewing from within: a commentary on first- and second-person methods in the science of consciousness. Constr Found. 2011;6:254–269. [Google Scholar]
- Gallagher S. Body image and body schema: a conceptual clarification. J Mind Behav. 1986;7(4):541–554. [Google Scholar]
- Gallagher S. Mutual enlightenment: recent phenomenology in cognitive science. J Conscious Stud. 1997;4(3):195–214. [Google Scholar]
- Gallagher S. Philosophical conceptions of the self: implications for cognitive science. Trends Cogn Sci. 2000;4:14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- Gallagher S. Self-narrative in schizophrenia. In: Kircher T, David A, editors. The self in neuroscience and psychiatry. Cambridge: Cambridge University Press; 2003. pp. 336–357. [Google Scholar]
- Gallagher S. Phenomenology and experimental design. J Conscious Stud. 2003;10(9–10):85–99. [Google Scholar]
- Gallagher S. How the body shapes the mind. Oxford: Clarendon Press; 2005. [Google Scholar]
- Gallagher S. Phenomenology and non-reductionist cognitive science. In: Gallagher S, Schimcking D, editors. Handbook of phenomenology and cognitive science. New York: Springer; 2010. pp. 21–34. [Google Scholar]
- Gallagher S. A pattern theory of self. Front Hum Neurosci. 2013;7:443. doi: 10.3389/fnhum.2013.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S, Daly A. Dynamical relations in the self-pattern. Front Psychol. 2018;9:664. doi: 10.3389/fpsyg.2018.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S, Sørensen JB. Experimenting with phenomenology. Conscious Cogn. 2006;15:119–134. doi: 10.1016/j.concog.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Gallagher S, Zahavi D. The phenomenological mind: an introduction to philosophy of mind and cognitive science. New York: Routledge; 2008. [Google Scholar]
- Gallagher S, Janz B, Reinerman L, Trempler J, Bockelman P. A neurophenomenology of awe and wonder: towards a non-reductionist cognitive science. London: Palgrave-Macmillan; 2015. [Google Scholar]
- García-Carpintero M. De se thought. Oxford: Oxford Handbooks Online; 2015. [Google Scholar]
- Gardiner JM. Episodic memory and autonoetic consciousness: a first-person approach. Philos Trans R Soc Lond Ser B Biol Sci. 2001;356:1351–1361. doi: 10.1098/rstb.2001.0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girn M, Christoff K. Expanding the scientific study of self-experience with psychedelics. J Conscious Stud. 2018;25(11–12):131–154. [Google Scholar]
- Giummarra MJ, Gibson SJ, Georgiou-Karistianis N, Bradshaw JL. Mechanisms underlying embodiment, disembodiment and loss of embodiment. Neurosci Biobehav Rev. 2008;32(1):143–160. doi: 10.1016/j.neubiorev.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Glicksohn J. Temporal cognition and the phenomenology of time: a multiplicative function for apparent duration. Conscious Cogn. 2001;10:1–25. doi: 10.1006/ccog.2000.0468. [DOI] [PubMed] [Google Scholar]
- Glicksohn J, Ben-Soussan TD. Immersion, absorption, and spiritual experience: some preliminary findings. Front Psychol. 2020;11:2118. doi: 10.3389/fpsyg.2020.02118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glicksohn J, Berkovich-Ohana A, Mauro F, Ben-Soussan TD. Individual EEG alpha profiles are gender-dependent and indicate subjective experiences in whole-body perceptual deprivation. Neuropsychologia. 2019;125:81–92. doi: 10.1016/j.neuropsychologia.2019.01.018. [DOI] [PubMed] [Google Scholar]
- Godwin CA, Hunter MA, Bezdek MA, Lieberman G, Elkin-Frankston S, Romero VL, Witkiewitz K, Clark VP, Schumacher EH. Functional connectivity within and between intrinsic brain networks correlates with trait mind wandering. Neuropsychologia. 2017;103:140–153. doi: 10.1016/j.neuropsychologia.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Grabot L, van Wassenhove V. Time order as psychological bias. Psychol Sci. 2017;28:670–678. doi: 10.1177/0956797616689369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CE. Out-of-body experiences. Oxford: Institute of Psychological Research; 1968. [Google Scholar]
- Grof S. Realms of the human unconscious: observations from LSD research. New York: Plume Books; 1976. [Google Scholar]
- Gruzelier JH. Redefining hypnosis: theory, methods and integration. Contemp Hypn. 2000;17:51–70. [Google Scholar]
- Guetterman TC. Basics of statistics for primary care research. Family Med Community Health. 2019;7(2):e000067. doi: 10.1136/fmch-2018-000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA. Being a self: considerations from functional imaging. Conscious Cogn. 2005;14:679–697. doi: 10.1016/j.concog.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Hagerty MR, Isaacs J, Brasington L, Shupe L, Fetz EE, Cramer SC. Case study of ecstatic meditation: fMRI and EEG evidence of self-stimulating a reward system. Neural Plast. 2013;2013:653572. doi: 10.1155/2013/653572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren E, Walter RD, Cherlow DG, Crandall PH. Mental phenomena evoked by electrical stimulation of the human hippocampal formation and amygdala. Brain. 1978;101:83–117. doi: 10.1093/brain/101.1.83. [DOI] [PubMed] [Google Scholar]
- Hebert R, Lehmann D, Tan G, Travis F, Arenander A. Enhanced EEG alpha time-domain phase synchrony during transcendental meditation: implications for cortical integration theory. Signal Process. 2005;85:2213–2232. [Google Scholar]
- Herbert BM, Pollatos O. The body in the mind: on the relationship between interoception and embodiment. Top Cogn Sci. 2012;4:692–704. doi: 10.1111/j.1756-8765.2012.01189.x. [DOI] [PubMed] [Google Scholar]
- Hobson A. The dream drugstore: chemically altered states of consciousness. Cambridge: MIT Press; 2001. [Google Scholar]
- Hohwy J. The sense of self in the phenomenology of agency and perception. Psyche (stuttg) 2007;13:1–20. [Google Scholar]
- Hohwy J (2010) The hypothesis testing brain: some philosophical applications. In: ASCS09: proceedings of the 9th conference of the Australasian society for cognitive science, Sydney, Australia pp 135–144
- Hohwy J. The predictive mind. Oxford: Oxford University Press; 2013. [Google Scholar]
- Huang Z, Dai R, Wu X, Yang Z, Liu D, Hu J, Gao L, Tang W, Mao Y, Jin Y, Northoff G. The self and its resting state in consciousness: an investigation of the vegetative state. Hum Brain Mapp. 2014;35:1997–2008. doi: 10.1002/hbm.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionta S, Heydrich L, Lenggenhager B, Mouthon M, Fornari E, Chapuis D, Gassert R, Blanke O. Multisensory mechanisms in temporo-parietal cortex support self-location and first-person perspective. Neuron. 2011;70:363–374. doi: 10.1016/j.neuron.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Irwin HJ. Flight of mind: a psychological study of the out-of-body experience. Metuchen: Scarecrow Press; 1985. [Google Scholar]
- Jack AI, Roepstorff A. Introspection and cognitive brain mapping: from stimulus–response to script–report. Trends Cogn Sci. 2002;6:333–339. doi: 10.1016/s1364-6613(02)01941-1. [DOI] [PubMed] [Google Scholar]
- James W. The principles of psychology. New York: Henry Holt and Company; 1890. [Google Scholar]
- Johanson M, Valli K, Revonsuo A, Wedlund JE. Alterations in the contents of consciousness in partial epileptic seizures. Epilepsy Behav. 2008;13:366–371. doi: 10.1016/j.yebeh.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Johnstad PG (2019) How to disappear completely: entheogen-induced experiences of self-dissolution. Downloaded from: https://www.researchgate.net/publication/337944144_How_to_disappear_completely_Entheogen-induced_experiences_of_self-dissolution
- Josipovic Z. Duality and non-duality in meditation research. Conscious Cogn. 2010;19:1119–1121. doi: 10.1016/j.concog.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Josipovic Z. Nondual awareness: consciousness-as-such as non-representational reflexivity. Prog Brain Res. 2019;244:273–298. doi: 10.1016/bs.pbr.2018.10.021. [DOI] [PubMed] [Google Scholar]
- Kallio S, Revonsuo A. Hypnotic phenomena and altered states of consciousness: a multilevel framework of description and explanation. Contemp Hypn. 2003;20:111–164. [Google Scholar]
- Karapanagiotidis T, Bernhardt BC, Jefferies E, Smallwood J. Tracking thoughts: exploring the neural architecture of mental time travel during mind-wandering. Neuroimage. 2017;147:272–281. doi: 10.1016/j.neuroimage.2016.12.031. [DOI] [PubMed] [Google Scholar]
- Kerr CE, Josyula K, Littenberg R. Developing an observing attitude: an analysis of meditation diaries in an MBSR clinical trial. Clin Psychol Psychother. 2011;18:80–93. doi: 10.1002/cpp.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler K, Braithwaite JJ. Deliberate and spontaneous sensations of disembodiment: capacity or flaw? Cogn Neuropsychiatry. 2016;21(5):412–428. doi: 10.1080/13546805.2016.1203769. [DOI] [PubMed] [Google Scholar]
- Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330:932–932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- Kjellgren A, Lyden F, Norlander T. Sensory isolation in flotation tanks: altered states of consciousness and effects on well-being. Qual Rep. 2008;13(4):636–656. [Google Scholar]
- Kjellgren A, Buhrkall H, Norlander T. Psychotherapeutic treatment in combination with relaxation in a flotation tank: effects on “burn-out syndrome”. Qual Rep. 2010;15(5):1243–1269. [Google Scholar]
- Klein SB. Autonoetic consciousness: reconsidering the role of episodic memory in future-oriented self-projection. Q J Exp Psychol. 2016;69(2):381–401. doi: 10.1080/17470218.2015.1007150. [DOI] [PubMed] [Google Scholar]
- Klein SB, Gangi CE. The multiplicity of self: neuropsychological evidence and its implications for the self as a construct in psychological research. Ann N Y Acad Sci. 2010;1191:1–15. doi: 10.1111/j.1749-6632.2010.05441.x. [DOI] [PubMed] [Google Scholar]
- Kokoszka A. Altered states of consciousness: a comparison of profoundly and superficially altered states. Imagin Cogn Pers. 1999;19(2):165–184. [Google Scholar]
- Kordeš U, Oblak A, Smrdu M, Demšar E. Ethnography of meditation: an account of pursuing meditative practice as a tool for researching consciousness. J Conscious Stud. 2019;26:184–237. [Google Scholar]
- Kozma R, Freeman WJ. The KIV model of intentional dynamics and decision making. Neural Netw. 2009;22(3):277–285. doi: 10.1016/j.neunet.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Lane TJ. The minimal self hypothesis. Conscious Cogn. 2020;85:103029. doi: 10.1016/j.concog.2020.103029. [DOI] [PubMed] [Google Scholar]
- Lebedev AV, Lövdén M, Rosenthal G, Feilding A, Nutt DJ, Carhart-Harris RL. Finding the self by losing the self: neural correlates of ego-dissolution under psilocybin. Hum Brain Mapp. 2015;36(8):3137–3153. doi: 10.1002/hbm.22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedev AV, Kaelen M, Lövdén M, Nilsson J, Feilding A, Nutt D, Carhart-Harris RL. LSD-induced entropic brain activity predicts subsequent personality change. Hum Brain Mapp. 2016;37:3203–3213. doi: 10.1002/hbm.23234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31:3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand D. The bodily self: the sensori-motor roots of pre-reflective self-consciousness. Phenomenol Cogn Sci. 2006;5:89–118. [Google Scholar]
- Lehmann D, Faber P, Achermenn P, Jeanmonod D, Gianotti LR, Pizzagalli D. Brain sources of EEG gamma frequency during volitionally meditation-induced, altered states of consciousness, and experience of the self. Psychiatry Res Neuroimaging. 2001;108:111–121. doi: 10.1016/s0925-4927(01)00116-0. [DOI] [PubMed] [Google Scholar]
- Letheby C, Gerrans P. Self unbound: ego dissolution in psychedelic experience. Neurosci Conscious. 2017;2017(1):nix016. doi: 10.1093/nc/nix016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limanowski J, Blankenburg F. Minimal self-models and the free energy principle. Front Hum Neurosci. 2013;7:547. doi: 10.3389/fnhum.2013.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limanowski J, Friston K. Seeing the dark: grounding phenomenal transparency and opacity in precision estimation for active inference. Front Psychol. 2018;9:643. doi: 10.3389/fpsyg.2018.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl JR, Fisher NE, Cooper DJ, Rosen RK, Britton WB. The varieties of contemplative experience: a mixed-methods study of meditation-related challenges in Western Buddhists. PLoS ONE. 2017;12:e0176239. doi: 10.1371/journal.pone.0176239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C, Elzière M. Out-of-body experience in vestibular disorders—a prospective study of 210 patients with dizziness. Cortex. 2018;104:193–206. doi: 10.1016/j.cortex.2017.05.026. [DOI] [PubMed] [Google Scholar]
- Lutz A. Toward a neurophenomenology of generative passages: a first empirical case study. Phenomenol Cogn Sci. 2002;1:133–167. [Google Scholar]
- Lutz A, Thompson E. Neurophenomenology: integrating subjective experience and brain dynamics in the neuroscience of consciousness. J Conscious Stud. 2003;10:31–52. [Google Scholar]
- Lutz A, Lachaux J-P, Martinerie J, Varela FJ. Guiding the study of brain dynamics by using first-person data: synchrony patterns correlate with ongoing conscious states during a simple visual task. Proc Natl Acad Sci U S A. 2002;99:1586–1591. doi: 10.1073/pnas.032658199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Dunne J, Davidson R. Meditation and the neuroscience of consciousness: an introduction. In: Zelazo P, Moscovitch M, Thompson E, editors. The Cambridge handbook of consciousness. Cambridge: Cambridge University Press; 2007. pp. 499–551. [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn Sci. 2008;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mañjuśrīmitra (translated by Lipman K, Norbu N) (1987) Primordial experience: an introduction to rDzogs-chen meditation. Shambhala, Boston
- Martial C, Mensen A, Charland-Verville V, Vanhaudenhuyse A, Rentmeister D, Bahri MA, Cassol H, Englebert J, Gosseries O, Laureys S, Faymonville M-E. Neurophenomenology of near-death experience memory in hypnotic recall: a within-subject EEG study. Sci Rep. 2019;9:14047. doi: 10.1038/s41598-019-50601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Wittmann M, Franck N, Cermolacce M, Berna F, Giersch A. Temporal structure of consciousness and minimal self in schizophrenia. Front Psychol. 2014;5:1175. doi: 10.3389/fpsyg.2014.01175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzinger T. Phenomenal transparency and cognitive self-reference. Phenomenol Cogn Sci. 2003;2(4):353–393. [Google Scholar]
- Metzinger T. Being no one: the self-model theory of subjectivity. Cambridge: MIT Press; 2004. [Google Scholar]
- Metzinger T. Empirical perspectives from the self-model theory of subjectivity: a brief summary with examples. In: Banerjee R, Chakrabarti BK, editors. Progress in brain research. Amsterdam: Elsevier B.V; 2008. pp. 215–245. [DOI] [PubMed] [Google Scholar]
- Metzinger T. Why are dreams interesting for philosophers? The example of minimal phenomenal selfhood, plus an agenda for future research. Conscious Res. 2013;4:746. doi: 10.3389/fpsyg.2013.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzinger T. How does the brain encode epistemic reliability? Perceptual presence, phenomenal transparency, and counterfactual richness. Cogn Neurosci. 2014;5(2):122–124. doi: 10.1080/17588928.2014.905519. [DOI] [PubMed] [Google Scholar]
- Metzinger T. Minimal phenomenal experience: Meditation, tonic alertness, and the phenomenology of “pure” consciousness. Philos Mind Sci. 2020;1(I):7. doi: 10.33735/phimisci.2020.I.46. [DOI] [Google Scholar]
- Michal M, Reuchlein B, Adler J, Reiner I, Beutel ME, Vögele C, Schächinger H, Schulz A. Striking discrepancy of anomalous body experiences with normal interoceptive accuracy in depersonalization-derealization disorder. PLoS ONE. 2014;9(2):e89823. doi: 10.1371/journal.pone.0089823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millière R. Looking for the self: phenomenology, neurophysiology and philosophical significance of drug-induced ego dissolution. Front Hum Neurosci. 2017 doi: 10.3389/fnhum.2017.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millière R. Are there degrees of self-consciousness? J Conscious Stud. 2019;26(3–4):252–282. [Google Scholar]
- Millière R. The varieties of selflessness. Philos Mind Sci. 2020;1(I):8. doi: 10.33735/phimisci.2020.I.48. [DOI] [Google Scholar]
- Millière R, Metzinger T. Radical disruptions of self-consciousness: editorial introduction. Philos Mind Sci. 2020;1(I):1. doi: 10.33735/phimisci.2020.I.50. [DOI] [Google Scholar]
- Millière R, Carhart-Harris RL, Roseman L, Trautwein F-M, Berkovich-Ohana A. Psychedelics, meditation, and self-consciousness. Front Psychol. 2018;9:1475. doi: 10.3389/fpsyg.2018.01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modestino EJ. Neurophenomenology of an altered state of consciousness: an fMRI case study. Explore. 2016;12(2):128–135. doi: 10.1016/j.explore.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Moseley P, Fernyhough C, Ellison A. Auditory verbal hallucinations as atypical inner speech monitoring, and the potential of neurostimulation as a treatment option. Neurosci Biobehav Rev. 2013;37:2794–2805. doi: 10.1016/j.neubiorev.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrazek MD, Franklin MS, Phillips DT, Baird B, Schooler JW. Mindfulness training improves working memory capacity and GRE performance while reducing mind wandering. Psychol Sci. 2013;24:776–781. doi: 10.1177/0956797612459659. [DOI] [PubMed] [Google Scholar]
- Musholt K. A philosophical perspective on the relation between cortical midline structures and the self. Front Hum Neurosci. 2013;7:536. doi: 10.3389/fnhum.2013.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musholt K. Thinking about oneself: from nonconceptual content to the concept of a Self. Cambridge: MIT Press; 2015. [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214(5–6):435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash JD, Newberg A. Toward a unifying taxonomy and definition for meditation. Front Psychol. 2013;4:806. doi: 10.3389/fpsyg.2013.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisser U. Five kinds of self-knowledge. Philos Psychol. 1988;1:35–59. [Google Scholar]
- Newberg A, Iversen J. The neural basis of the complex mental task of meditation: neurotransmitter and neurochemical considerations. Med Hypotheses. 2003;61:282–291. doi: 10.1016/s0306-9877(03)00175-0. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Yaden DB. A Neurotheological perspective on altered states of consciousness. J Conscious Stud. 2018;25(11–12):204–225. [Google Scholar]
- Newberg A, Alavi A, Baime M, Pourdehnad M, Santanna J, d’Aquili EG. The measurement of regional cerebral blood flow during the complex cognitive task of meditation: a preliminary SPECT study. Psychiatry Res. 2001;106:113–122. doi: 10.1016/s0925-4927(01)00074-9. [DOI] [PubMed] [Google Scholar]
- Norman G. Likert scales, levels of measurement and the “laws” of statistics. Adv Health Sci Educ. 2010;15(5):625–632. doi: 10.1007/s10459-010-9222-y. [DOI] [PubMed] [Google Scholar]
- Northoff G. Is the self a higher-order or fundamental function of the brain? The ‘basis model of self-specificity’ and its encoding by the brain's spontaneous activity. Cogn Neurosci. 2016;7:203–222. doi: 10.1080/17588928.2015.1111868. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain. A meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Northoff G, Qin P, Feinberg TE. Brain imaging of the self—conceptual, anatomical and methodological issues. Conscious Cogn. 2011;20:52–63. doi: 10.1016/j.concog.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Nour MM, Carhart-Harris RL. Psychedelics and the science of self-experience. Br J Psychiatry. 2017;210(3):177–179. doi: 10.1192/bjp.bp.116.194738. [DOI] [PubMed] [Google Scholar]
- Olivares FA, Vargas E, Fuentes C, Martínez-Pernía D, Canales-Johnson A. Neurophenomenology revisited: second-person methods for the study of human consciousness. Front Psychol. 2015;6:673. doi: 10.3389/fpsyg.2015.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard M. On the naturalising of phenomenology. Phenomenol Cogn Sci. 2004;3:365–379. [Google Scholar]
- Palmieri A, Calvo V, Kleinbub JR, Meconi F, Marangoni M, Barilaro P, Broggio A, Sambin M, Sessa P. “Reality” of near-death-experience memories: evidence from a psychodynamic and electrophysiological integrated study. Front Hum Neurosci. 2014;8:429. doi: 10.3389/fnhum.2014.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J (2005) On the embodied neural nature of the core emotional affects. J Conscious Stud 5:158–184
- Panksepp J, Northoff G (2009) The trans-species core SELF: the emergence of active cultural and neuro-ecological agents through self-related processing within subcortical-cortical midline networks. Conscious Cogn 18:193–215 [DOI] [PubMed]
- Parnas J, Handest P. Phenomenology of anomalous self-experience in early schizophrenia. Compr Psychiatry. 2003;44(2):121–134. doi: 10.1053/comp.2003.50017. [DOI] [PubMed] [Google Scholar]
- Parnas J, Møller P, Kircher T, Thalbitzer J, Jansson L, Handest P, Zahavi D. EASE: examination of anomalous self-experience. Psychopathology. 2005;38:236–258. doi: 10.1159/000088441. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct Funct. 2010;214(5–6):451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penberthy JP, Hodge AS, Hook JN, Delorme A, Pehlivanova M, Vieten C. Meditators and nonmeditators: a descriptive analysis over time with a focus on unusual and extraordinary experiences. J Yoga Physiother. 2020;8(3):555744. doi: 10.19080/JYP.2020.08.555744. [DOI] [Google Scholar]
- Penfield W. The cerebral cortex in man. I. The cerebral cortex and consciousness. Arch Neurol Psychiatry. 1938;40:417–442. [Google Scholar]
- Petitmengin C, Navarro V, Le Van Quyen M. Anticipating seizure: pre-reflective experience at the center of neuron-phenomenology. Conscious Cogn. 2007;16(3):746–764. doi: 10.1016/j.concog.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Petitot J, Varela FJ, Pachoud B, Roy J-M. Naturalizing phenomenology: issues in contemporary phenomenology and cognitive science. Stanford: Stanford University Press; 1999. [Google Scholar]
- Polito V, Barnier AJ, Woody EZ. Developing the sense of agency rating scale (SOARS): an empirical measure of agency disruption in hypnosis. Conscious Cogn. 2013;22:684–696. doi: 10.1016/j.concog.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Pollan M. How to change your mind: what the new science of psychedelics teaches us about consciousness, dying, addiction, depression, and transcendence. New York: Penguin Press; 2018. [DOI] [PubMed] [Google Scholar]
- Pope KS, Singer JL. The stream of consciousness. New York: Plenum; 1978. [Google Scholar]
- Pöppel E, Bao Y, Han S, Sozinov AA, Ushakov DV, Kovalev AI, Chernorizov AM, Gya M, Zaytseva Y. Unasked questions and unused answers in psychology. Psychol Russia State Art. 2013;6:4–18. [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philos Trans R Soc Lond Ser B Biol Sci. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revonsuo A. Inner presence: consciousness as a biological phenomenon. Cambridge: MIT Press; 2006. [Google Scholar]
- Rgyal-ba-g'yung-drung B-S, Gurung GS, Brown D. The Pith instructions for the stages of the practice sessions of the a khrid system of Bon Rdzogs Chen (great completion) meditation. 2. Occidental: Bright Alliance; 2017. [Google Scholar]
- Rinpoche K, Namgyal L. The ninth Karmapa's ocean of definitive meaning. New York: Snow Lion Publications, Incorporated; 2011. [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007;8:657. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Egloff B, Witthöft M. Is interoceptive awareness really altered in somatoform disorders? Testing competing theories with two paradigms of heartbeat perception. J Abnorm Psychol. 2012;121(3):719–724. doi: 10.1037/a0028509. [DOI] [PubMed] [Google Scholar]
- Schartner MM, Carhart-Harris RL, Barrett AB, Seth AK, Muthukumaraswamy SD. Increased spontaneous MEG signal diversity for psychoactive doses of ketamine, LSD and Psilocybin. Sci Rep. 2017;7:46421. doi: 10.1038/srep46421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn. 2008;17:457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Schmidt TT, Berkemeyer H. The altered states database: psychometric data of altered states of consciousness. Front Psychol. 2018;9:1028. doi: 10.3389/fpsyg.2018.01028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastián MÁ. Perspectival self-consciousness and ego-dissolution: an analysis of (some) altered states of consciousness. Philos Mind Sci. 2020;1(I):9. doi: 10.33735/phimisci.2020.I.44. [DOI] [Google Scholar]
- Sedeño L, Couto B, Melloni M, Canales-Johnson A, Yoris A, Baez S, Esteves S, Velásquez M, Barttfeld P, Sigman M, Kichic R, Ibanez A. How do you feel when you can't feel your body? Interoception, functional connectivity and emotional processing in depersonalization-derealization disorder. PLoS ONE. 2014;9(6):e98769. doi: 10.1371/journal.pone.0098769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth AK. Interoceptive inference, emotion, and the embodied self. Trends Cogn Sci. 2013;17:565–573. doi: 10.1016/j.tics.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Seth AK, Suzuki K, Critchley HD. An interoceptive predictive coding model of conscious presence. Front Psychol. 2012;2:395. doi: 10.3389/fpsyg.2011.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. Case-study approach in neuropsychological research. J Clin Neuropsychol. 1979;1:183–211. [Google Scholar]
- Shanon B. Altered temporality. J Conscious Stud. 2001;8(1):35–58. [Google Scholar]
- Shear J. Eastern methods for investigating mind and consciousness. In: Schneider S, Velmans M, editors. The Blackwell companion to consciousness. Malden: Wiley; 2007. pp. 697–710. [Google Scholar]
- Snodgrass JG, Thompson RL. The self across psychology: self-recognition, self-awareness, and the self-concept. New York: New York Academy of Sciences; 1997. [Google Scholar]
- Soler J, Cebolla A, Feliu-Soler A, Demarzo MMP, Pascual JC, Baños R, García-Campayo J. Relationship between meditative practice and self-reported mindfulness: the MINDSENS composite index. PLoS ONE. 2014;9(1):e86622. doi: 10.1371/journal.pone.0086622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth J, Speth C, Kaelen M, Schloerscheidt AM, Feilding A, Nutt DJ, Carhart-Harris RL. Decreased mental time travel to the past correlates with default-mode network disintegration under lysergic acid diethylamide. J Psychopharmacol. 2016;30:344–353. doi: 10.1177/0269881116628430. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory-of-mind and their relationship to the default mode network. J Cogn Neurosci. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Stace WT. Mysticism and philosophy. New York: TarcherPerigee; 1960. [Google Scholar]
- Stewart J, Gapenne O, Di Paolo EA. Enaction: towards a new paradigm for cognitive science. Cambridge: The MIT Press; 2010. [Google Scholar]
- Studerus E, Gamma A, Vollenweider FX. Psychometric evaluation of the altered states of consciousness rating scale (OAV) PLoS ONE. 2010;5:e12412. doi: 10.1371/journal.pone.0012412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano K, Tanno Y. Self-rumination, self-reflection, and depression: self-rumination counteracts the adaptive effect of self-reflection. Behav Res Ther. 2009;47:260–264. doi: 10.1016/j.brat.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Tart CT. Altered states of consciousness. Oxford: Doubleday; 1972. [Google Scholar]
- Thompson E. Dreamless sleep, the embodied mind, and consciousness—the relevance of a classical Indian debate to cognitive science. In: Metzinger T, Windt JM, editors. Open MIND: 37(T) Frankfurt am Main: MIND Group; 2015. [Google Scholar]
- Thompson E, Lutz A, Cosmelli D. Neurophenomenology: an introduction for neurophilosophers. In: Brook A, Akins K, Brook AK, editors. Cognition and the brain: the philosophy and neuroscience movement. Cambridge: Cambridge University Press; 2005. pp. 40–97. [Google Scholar]
- Trapnell PD, Campbell JD. Private self-consciousness and the five-factor model of personality: distinguishing rumination from reflection. J Pers Soc Psychol. 1999;76(2):284–304. doi: 10.1037//0022-3514.76.2.284. [DOI] [PubMed] [Google Scholar]
- Travis F, Pearson C. Pure consciousness: distinct phenomenological and physiological correlates of “consciousness itself”. Int J Neurosci. 2000;100:77–89. [PubMed] [Google Scholar]
- Travis F, Shear J. Focused attention, open monitoring and automatic self-transcending: categories to organize meditations from Vedic, Buddhist and Chinese traditions. Conscious Cogn. 2010;19(4):1110–1118. doi: 10.1016/j.concog.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Trehub A. Space, self, and the theater of consciousness. Conscious Cogn. 2007;16:310–330. doi: 10.1016/j.concog.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Tsakiris M. My body in the brain: a neurocognitive model of body-ownership. Neuropsychologia. 2010;48:703–712. doi: 10.1016/j.neuropsychologia.2009.09.034. [DOI] [PubMed] [Google Scholar]
- Tsakiris M. The material me: unifying the exteroceptive and interoceptive sides of the bodily self. In: de Vignemont F, Alsmith AJT, editors. The subject’s matter: self-consciousness and the body. Cambridge: MIT Press; 2017. pp. 335–362. [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F. Neurophenomenology: a methodological remedy for the hard problem. J Conscious Stud. 1996;3:330–349. [Google Scholar]
- Varela FJ. The naturalization of phenomenology as the transcendence of nature: searching for generative mutual constraints. Alt Rev Phénoménol. 1997;5:355–381. [Google Scholar]
- Varela FJ. The specious present: a neurophenomenology of time consciousness. In: Petitot J, Varela FJ, Pachoud B, Roy J-M, editors. Naturalizing phenomenology: issues in contemporary phenomenology and cognitive science. Stanford: Stanford University Press; 1999. pp. 266–317. [Google Scholar]
- Varela F, Shear J. First-person methodologies: what, why, how? J Conscious Stud. 1999;6:1–14. [Google Scholar]
- Varela FJ, Thompson E, Rosch E. The embodied mind: cognitive science and human experience. Cambridge: The MIT Press; 1991. [Google Scholar]
- Velmans M. Conscious agency and the preconscious/unconscious Self. In: Menon S, Sinha A, Sreekantan BV, editors. Interdisciplinary perspectives on consciousness and the self. New Delhi: Springer; 2014. pp. 11–25. [Google Scholar]
- Vieten C, Wahbeh H, Cahn BR, MacLean K, Estrada M, Mills P, Murphy M, Shapiro S, Radin D, Josipovic Z, Presti DE, Delorme A. Future directions in meditation research: recommendations for expanding the field of contemplative science. PLoS ONE. 2018;13(11):e0205740. doi: 10.1371/journal.pone.0205740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigário RN. Extraction of ocular artefacts from EEG using independent component analysis. Electroencephalogr Clin Neurophysiol. 1997;103:395–404. doi: 10.1016/s0013-4694(97)00042-8. [DOI] [PubMed] [Google Scholar]
- Vignal JP, Maillard L, McGonigal A, Chauvel P. The dreamy state: hallucinations of autobiographic memory evoked by temporal lobe stimulations and seizures. Brain. 2007;130:88–99. doi: 10.1093/brain/awl329. [DOI] [PubMed] [Google Scholar]
- Wackermann J, Wittmann M, Hasler F, Vollenweider FX. Effects of varied doses of psilocybin on time interval reproduction in human subjects. Neurosci Lett. 2008;435:51–55. doi: 10.1016/j.neulet.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Wahbeh H, Sagher A, Back W, Pundhir P, Travis F. A systematic review of transcendent states across meditation and contemplative traditions. Explore. 2018;14(1):19–35. doi: 10.1016/j.explore.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Wald J, Taylor S. Responses to interoceptive exposure in people with posttraumatic stress disorder (PTSD): a preliminary analysis of induced anxiety reactions and trauma memories and their relationship to anxiety sensitivity and PTSD symptom severity. Cogn Behav Ther. 2008;37(2):90–100. doi: 10.1080/16506070801969054. [DOI] [PubMed] [Google Scholar]
- Wang H-T, Poerio G, Murphy C, Bzdok D, Jefferies E, Smallwood J. Dimensions of experience: exploring the heterogeneity of the wandering mind. Psychol Sci. 2017;29:56–71. doi: 10.1177/0956797617728727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PJ, Morris RJ, Ramsey A, Hickman SE, Waddell MG. Further contrasts between self-reflectiveness and internal state awareness factors of private self-consciousness. J Psychol. 1996;130:183–192. doi: 10.1080/00223980.1996.9915000. [DOI] [PubMed] [Google Scholar]
- Windt JM. The immersive spatiotemporal hallucination model of dreaming. Phenomenol Cogn Sci. 2010;9(2):295–316. [Google Scholar]
- Windt JM. Reporting dream experience: why (not) to be skeptical about dream reports. Front Hum Neurosci. 2013;7:708. doi: 10.3389/fnhum.2013.00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windt JM (2015) Just in time—dreamless sleep experience as pure subjective temporality. In: Metzinger TK, Windt JM (eds) Open MIND. Frankfurt am Main: MIND Group. 10.15502/9783958571174
- Windt JM, Nielsen T, Thompson E. Does consciousness disappear in dreamless sleep? Trends Cogn Sci. 2016;20(12):871–882. doi: 10.1016/j.tics.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Winget C, Kramer M. Dimensions of dreams. Gainesville: University Press of Florida; 1979. [Google Scholar]
- Winkelman M. Altered states of consciousness and religious behavior. In: Glazier S, editor. Anthropology of religion: a handbook of method and theory. Westport: Greenwood Press; 1997. pp. 393–428. [Google Scholar]
- Winkelman M. A paradigm for understanding altered consciousness: the integrative mode of consciousness. In: Cardeña E, Winkelman MJ, editors. Altering consciousness: multidisciplinary perspectives. Westport: Praeger; 2011. pp. 23–41. [Google Scholar]
- Wittmann M. The inner sense of time: how the brain creates a representation of duration. Nat Rev Neurosci. 2013;14:217–223. doi: 10.1038/nrn3452. [DOI] [PubMed] [Google Scholar]
- Wittmann M. Modulations of the experience of self and time. Conscious Cogn. 2015;38:172–181. doi: 10.1016/j.concog.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Yufik YM. The understanding capacity and information dynamics in the human brain. Entropy. 2019;21:308. doi: 10.3390/e21030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahavi D. First-person thoughts and embodied self-awareness: some reflections on the relation between recent analytic philosophy and phenomenology. Phenomenol Cogn Sci. 2002;1:7–26. [Google Scholar]
- Zahavi D. Subjectivity and selfhood. Cambridge: The MIT Press; 2006. [Google Scholar]
- Zahavi D. Self and other: exploring subjectivity, empathy, and shame. Oxford: Oxford University Press; 2014. [Google Scholar]
- Zahavi D, Parnas J. Phenomenal consciousness and self-awareness: a phenomenological critique of representational theory. J Conscious Stud. 1998;5:687–705. [Google Scholar]
- Zhou B, Pöppel R, Wang L, Yang T, Zaytseva Y, Ba Y. Seeing without knowing: operational principles along the early visual pathway. PsyCh J. 2016;5:145–160. doi: 10.1002/pchj.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not publicly available, due to privacy or ethical restrictions.