Abstract
Background
Cystic echinococcosis is a manifestation of a zoonosis caused by larvae of the tapeworm Echinococcus granulosus sensu lato and pterygopalatine fossa cases are extremely rare.
Clinical Presentation and Findings
A 45-year-old Caucasian female with a history of repeated surgeries for HC was referred to our center for treatment of a cystic mass of the pterygopalatine fossa. Multiorgan dissemination was noted on preoperative imaging.
Interventions
An endonasal endoscopic procedure was carried over under general anesthesia and the CE completely removed. Etiology was confirmed by molecular diagnostics. Three weeks after the skull base procedure, the patient underwent a combined abdominal/urological procedure for treatment of other cysts.
Conclusion
This case shows that the pterygopalatine fossa HC are amenable to surgical treatment using the endonasal endoscopic approach. Extensive preoperative workup is essential to assess the extent of the disease.
Keywords: Echinococcus granulosus sensu stricto, Endoscopy, Endonasal, Hydatid cyst
Introduction
Cystic echinococcosis (CE) is manifestations of a zoonosis caused by larval stage of the tapeworm Echinococcus granulosus sensu lato for which humans act as intermediate hosts while d dogs are the most important definitive hosts. The disease presents with symptoms of mass effect and systemic signs secondary to cyst rupture or infection [1]. The disease is, among other countries, endemic in the Mediterranean and has an incidence of 1–200/100,000 in areas of endemicity [1]. The most commonly affected organs are the liver and lungs, rarely other internal organs, even more rare the head and neck region and multiple site involvement is common [2]. Diagnosis is made using imaging methods and serology and treatment is surgical, according to rules of tumor surgery (i.e., aimed at total removal) with varying degrees of radicality and care is taken to avoid cyst rupture and dissemination; therefore, concurrent antiparasitic drugs are administered alongside surgery. Thus far, only a handful of cases of pterygopalatine fossa involvement have been reported in the literature.
Patient Information
General Information and Primary Concerns
The case of a 45-year-old Caucasian female with a history of repeated surgeries for HC was referred to the Department of Neurosurgery, Clinical Hospital Center Croatia from an outside hospital in October 2019 for treatment of a cystic mass of the pterygopalatine fossa.
Medical History and Past Interventions
The patient had no history of serious illness during childhood or adolescence. In 1996, she underwent a conization of the cervix to treat a cervical intraepithelial neoplasia. In 2006, at the age of 32 she was diagnosed with a solitary lung CE, and underwent a right lower lobectomy, without any concomitant antiparasitic treatment. In 2009, at the age of 35 she underwent a mitral valvuloplasty to treat a hydatid cyst. During and after this procedure, she was on multiple albendazole (ABZ) courses, of different dosage and duration. Between ages 35 and 38, she underwent three open neurosurgical (NS) procedures to treat CEs in the left parietal lobe, then cerebellum, and then again a recurrent cyst in the left parietal lobe. Following the procedures, she was started on phenobarbital (PHB) 100 mg 2 × 1. All of the aforementioned procedures were performed in an outside hospital. In October 2012, a right parietal lobe CE developed and the patient was started on 2 × 400 mg ABZ for 28 days, followed by a two-staged gamma-knife (GK) procedure: volume-staging (prescription dose 12 Gy to prescription isodose 45%) followed by second stage after three months (prescription dose 12 Gy to prescription isodose 50%). At that time, the patient underwent a whole body positron emission tomography-computerized tomography (PET-CT) scan which showed no extracranial radiopharmaceutical accumulation. Regular six-month brain MRI scans and clinical follow-up visits were scheduled and the lesions remained radiologically dormant and clinically mute. In 2014, at the age of 40, routine follow-up workup showed progression of the right parietal lobe HC and she underwent a NS procedure and a one-month preoperative and three-month postoperative ABZ treatment of 3 × 400 mg. All surgical specimens were sent to histology and microbiology for analysis and a CE was proven in all of the procedures. The procedure was complicated by a delayed wound infection, for which two wound revision procedures including bone removal and antibiotic treatment were performed in 2017. At the age of 43, the patient underwent a cranioplasty, which was followed by a course of antibiotic treatment. As a consequence of repeated surgeries, several permanent neurological sequealae ensued, namely a left-sided facial hypoesthesia, decreased peripheral vision, discrete truncal ataxia and recurring headaches. Also, the patient was a pack-a-day tobacco smoker and had previously developed an idiosyncratic reaction (DRESS—drug rash with eosinophilia and systemic symptoms syndrome) to a combination to vancomycin and meropenem.
Clinical Findings
General physical examination revealed that the patient was pale, slightly underweight, had a systolic murmur, breathing sounds were absent at the right base of the lungs, and liver margin was palpable 2 cm below costal margin. Neurological examination revealed the previously described permanent chronic neurological deficits. There were no other abnormalities detected on physical examination.
Timeline
A timeline of surgical procedures is summarized in Fig. 1.
Fig. 1.
Timeline of surgical procedures. HC—hydatid cyst; ABZ—albendazole; NS—neurosurgical procedure; PHB—phenobarbital; SRS—stereotactic radiosurgery; ES—endoscopic surgery; PZQ—praziquantel
Diagnostic Assessment
Laboratory workup of blood samples showed a mild-to-moderate leukocytosis with absolute eosinophil counts ranging from 1200 to 1822 cells/µL during hospital stay. Liver function test levels at admission were: AST 30 U/L, ALT 54 U/L, GGT 319 U/L, AF 137 U/L, LDH 252 U/L, CK 72 U/L. anti-echinococcal blood serology was positive (EIA 34.00 NTU, WB positive). All other routine biochemical and bacteriological workup of blood and cerebrospinal fluid (CSF) samples showed no abnormal findings, including normal ESR and CRP values.
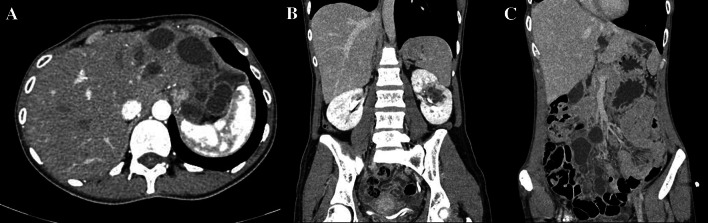
Next, a head CT scan of the abdomen and thorax, transthoracic echocardiogram, and a head magnetic resonance imaging (MRI) were performed. The CT of the abdomen showed that the left liver permeated with HCs, largest of them being 4.5 cm in diameter, a multilocular cyst of 3 cm in diameter in the left kidney and an intraperitoneal pelvic aperture cyst of 5.3 cm in diameter (Fig. 2). The CT of the thorax did not show any cystic lesions, while transthoracic ultrasonography showed a grade III mitral valve insufficiency, without cystic lesions. Brain MRI showed a contrast enhancing multicystic lesion 2.4 × 1.6 cm in the right temporopolar region, with a propagation into the pterygopalatine fossa (Fig. 3).
Fig. 2.
Preoperative CT of the abdomen. Cystic lesions are seen in the liver (a), left kidney (b) and mesentery (c)
Fig. 3.
Pre- and postoperative brain MRI. Preoperative T2-weighted axial image showing a multicystic lesion in the temporopolar region (a) extending into the pterygopalatine fossa, as seen on the coronal image (b). Postoperative T2-weighted axial (c) and coronal (d) images
Due to a rising eosinophilia, asymptomatic leaking of the antigenic material, probably from the extensive abdominal cysts, was suspected, and after an interdisciplinary team meeting (infectious disease specialist, otorhinolaryngologist, neurosurgeon, abdominal surgeon, urologist and anesthesiologist) a stepwise surgical approach was proposed, with endoscopic endonasal removal of the deep cerebral cyst as the first operation.
Therapeutic Intervention
In October 2019, a surgical procedure was performed by a head and neck surgeon and a neurosurgeon. The surgery was done under general anesthesia considered as being a high-risk procedure, due to possibility of intraoperative rupture of one of the extracranial HCs. Thirty-four days prior to surgery the patient were started on ABZ 400 mg 2 × 1 (16 mg/kg), and concommitant praziquantel (PZQ) 600 mg 4 × 1 13 days prior to surgery.
After anemization of the nasal mucosa using adrenaline-solution (1:1000) soaked cottonoids, the middle turbinate was resected and a left-sided nasoseptal flap harvested. Resection of septal cartilage and bone was performed, followed by a right ethmoidectomy and sphenoidectomy. The reverse roation flap was harvested and sutured into the residual part of the septum [3]. The right inferior turbinate was resected and right endoscopic endonasal medial maxillectomy was performed with removal of its posterior wall, thus creating access to the pterygopalatine fossa and the cystic tumor filling the fossa. The nasal cavity was covered with a 10% NaCl-soaked gauze in order to prevent dissemination of the parasite in case of intraoperative cyst rupture. Thick liquid content of the cyst was aspirated and sent to microbiological analysis. The anterior cyst wall was dilated and then the cyst irrigated over a 20-min period using a 10% NaCl solution. Anatomical structures exposed in the operative field were the contents of the middle cranial fossa, including the temporal lobe and postganglionic fibers of the trigeminal nerve (Fig. 4).
Fig. 4.
Intraoperative view. (a) Aspiration of the HC located in the pterygopalatine fossa with two suction tubes (b). View of the empty cyst after irrigation with 10% NaCl solution—temporal pole (*), 2nd (**) and 3rd (***) branch of the trigeminal nerve exposed in the field (b). (c) Nasoseptal flap placed over the empty cyst covered with oxidized regenerated cellulose (c). Endoscopic view at three months postoperatively (d), endoscopic view 3 months post-surgery, showing the nasoseptal flap in situ covering the content of the pterygopalatine fossa, widely opened maxillary and sphenoid sinus
Twenty-five days later the patient underwent a combined abdominal and urological procedure under general anesthesia for a hepatic lobectomy, resection of 50 cm of the terminal ileum with a termino-terminal anastomosis, and a left nephrectomy. All samples were sent to microbiological and histological analyses.
Light microscopy examination of the aspirated brain cyst content revealed lack of protoscoleces. For final confirmation, DNA was extracted from germinal layer and analyzed by polymerase chain reaction (PCR) and sequencing of four mitochondrial genes: COXI, NAD3, NAD1 and ATP6. After assembling BLAST analysis showed identical sequences to Echinococcus granulossus sensu stricto in all sequenced genes.
Follow-Up and Outcomes
The patient was discharged ten days after the procedure and recovered without any sequelae and to the day of submission of this report the postoperative course remains unremarkable. Albendazole 400 mg 2 × 1 was continued for three months postoperatively. Follow-up laboratory workup and imaging at the most recent visit, at five months postoperatively, showed no abnormalities (Fig. 3).
Discussion
We presented an unusual and challenging case of recurrent, disseminated cystic echinococcosis with a chronic 14-year-long and ongoing course. The case is interesting from a variety of standpoints, including its latest presentation in the pterygopalatine fossa. The case had been reported earlier, prior to its pterygopalatine fossa presentation, from a pharmacological/infectious disease standpoint [4]. Despite its rarity, cystic echinococcosis present a significant health burden in endemic areas. Fatal outcomes are mostly due to anaphylactic shock secondary to cyst rupture or hepatic insufficiency and case fatality rates range from 1.29 to 1.94 per 100,000 cases [5]. Croatia, in particular its southern part, is among the endemic areas with a rare, yet stable incidence [6]. Nevertheless, HCs sporadically appear among our neurosurgical patients and often pose significant challenges, as is the case reported herein. Diagnosis was fairly easy, due to extensive history and routine follow-up imaging, clinical assessments and latter confirmed with sequencing. Nevertheless, in endemic areas, workup should be aimed at eliminating HCs in patients with cystic brain lesions of unknown etiology. The workup should also include an MRI/CT of the thorax and abdomen to identify possible disseminated lesions. If a HC is suspected, ABZ 10–15 mg/kg/day should be administered for at least seven days preoperatively, to decrease the likelihood of dissemination. However, the procedure was considered being high-risk from the point of view of anesthesiology, as anaphylaxis was a reasonable threat at all times during the procedure. The main surgical challenge arose from the fact that the process was in an area not accessed routinely during endoscopic endonasal procedures, i.e., out-of-the-midline. Namely, the majority of endonasal endoscopic procedures performed in our Center are pituitary gland surgeries, or other procedures using an extended midline approach. We opted for an endoscopic endonasal procedure due to its advantages over the open approaches, namely the reduced morbidity, superior visualization and more favorable cosmetic outcome. In general, the non-endoscopic alternatives are grouped into the anterior and lateral approaches, both of which implicate large incisions, bone removal and exposition (including to potential harm) of important anatomic structures [7]. The second challenge was to prevent dissemination of cystic content, which can lead to a lethal anaphylactic shock and recurrence. Spillage prevention is achieved by a total aspiration of the cyst, after which a 10% hypertonic saline solution is instilled and left in place over 20 min to kill E. granulosus scolices and then the puncture site is covered with hypertonic saline soaked cottonoids to inactivate scolices in case of accidental spillage.
Thus far, only a handful of cases of pterygopalatine/infratemporal fossa HC cases have been reported in the literature. Baglam et al. reported of a successfully treated infratemporal HC in a 30-year-old-female and identified 5 previously reported cases [8]. In the pterygopalatine fossa, only 5 cases have been described, including the publication by Gökmen et al., who reported on a successful treatment of a solitary pterygopalatine fossa HC in a 36-year-old man, using the same approach as in our case [9]. What differs in our case is the fact that the HCs in our patient were multiple, the disease had a chronic-recurrent course (including also repeated neurosurgical and radiosurgical interventions) and it was deemed high risk due to other-than-surgical reasons.
Conclusion
Pterygopalatine fossa is an extremely rare localization for a HC. This case shows that the lesion is amenable to surgical treatment using the endonasal endoscopic approach. When suspected, a thorough laboratory and imaging workup is indispensable in order to identify the extent of dissemination and ensure maximum safety during the procedure.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wen H, Vuitton L, Tuxun T, Li J, Vuitton DA, Zhang W, McManus DP. Echinococcosis: advances in the 21st century. ClinMicrobiol Rev. 2019;32:e00075–e118. doi: 10.1128/CMR.00075-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilgen C, Oner K, OvuI I, Kirazlı T. Vertebral hydatid disease presenting as a parapharyngeal and neck mass: a case report. Otolaryngol Head Neck Surg. 2002;126:89–90. doi: 10.1067/mhn.2002.121319. [DOI] [PubMed] [Google Scholar]
- 3.Caicedo-Granados E, Carrau R, Snyderman C, Prevedello D, Fernandez-Miranda J, Gardner P, et al. Reverse rotation flap for reconstruction of donor site after vascular pedicled nasoseptal flap in skull base surgery. Laryngoscope. 2010;120(8):1550–1552. doi: 10.1002/lary.20975. [DOI] [PubMed] [Google Scholar]
- 4.Skuhala T, Trkulja V, Runje M, et al. Combined albenazole-praziquantel treatment in recurrent brain echinococcosis: case report. Iran J Parasitol. 2019;14(3):492–496. [PMC free article] [PubMed] [Google Scholar]
- 5.Khachatryan A. Analysis of lethality in echinococcal disease. Korean J Parasitol. 2017;55(5):549–553. doi: 10.3347/kjp.2017.55.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skuhala T, Trkulja V, Runje M, Vukelic D, Desnica B. Albendazole sulphoxide concentrations in plasma and hydatid cyst and prediction of parasitological and clinical outcomes in patients with liver hydatidosis caused by Echinococcus granulosus. Croat Med J. 2014;55(2):146–155. doi: 10.3325/cmj.2014.55.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uehara M, Tominaga K, Asahina I. Surgical approach to the pterygopalatine fossa-comparison between anterior approach and lateral approach. J Craniofac Surg. 2013;24(2):536–539. doi: 10.1097/SCS.0b013e3182646b44. [DOI] [PubMed] [Google Scholar]
- 8.Baglam T, Karatas E, Durucu C, Sirikci A, Kara F, Kanlikama M. Primary hydatid cyst of the infratemporal fossa. J CraniofacSurg. 2009;20(4):1200–1201. doi: 10.1097/SCS.0b013e3181acdd5d. [DOI] [PubMed] [Google Scholar]
- 9.Gökmen M, Beton S, Meço C. Endonasal endoscopic management of pterigopalatine fossa hydatid cyst. J CraniofacSurg. 2019;30(8):e757–e760. doi: 10.1097/SCS.0000000000005758. [DOI] [PubMed] [Google Scholar]