Abstract
Abacavir (1592U89), a nucleoside reverse transcriptase inhibitor with in vitro activity against human immunodeficiency virus type-1 (HIV-1), has been evaluated for efficacy and safety in combination regimens with other nucleoside analogs, including zidovudine (ZDV) and lamivudine (3TC). To evaluate the potential pharmacokinetic interactions between these agents, 15 HIV-1-infected adults with a median CD4+ cell count of 347 cells/mm3 (range, 238 to 570 cells/mm3) were enrolled in a randomized, seven-period crossover study. The pharmacokinetics and safety of single doses of abacavir (600 mg), ZDV (300 mg), and 3TC (150 mg) were evaluated when each drug was given alone or when any two or three drugs were given concurrently. The concentrations of all drugs in plasma and the concentrations of ZDV and its 5′-glucuronide metabolite, GZDV, in urine were measured for up to 24 h postdosing, and pharmacokinetic parameter values were calculated by noncompartmental methods. The maximum drug concentration (Cmax), the area under the concentration-time curve from time zero to infinity (AUC0–∞), time to Cmax (Tmax), and apparent elimination half-life (t1/2) of abacavir in plasma were unaffected by coadministration with ZDV and/or 3TC. Coadministration of abacavir with ZDV (with or without 3TC) decreased the mean Cmax of ZDV by approximately 20% (from 1.5 to 1.2 μg/ml), delayed the median Tmax for ZDV by 0.5 h, increased the mean AUC0–∞ for GZDV by up to 40% (from 11.8 to 16.5 μg · h/ml), and delayed the median Tmax for GZDV by approximately 0.5 h. Coadministration of abacavir with 3TC (with or without ZDV) decreased the mean AUC0–∞ for 3TC by approximately 15% (from 5.1 to 4.3 μg · h/ml), decreased the mean Cmax by approximately 35% (from 1.4 to 0.9 μg/ml), and delayed the median Tmax by approximately 1 h. While these changes were statistically significant, they are similar to the effect of food intake (for ZDV) or affect an inactive metabolite (for GZDV) or are relatively minor (for 3TC) and are therefore not considered to be clinically significant. No significant differences were found in the urinary recoveries of ZDV or GZDV when ZDV was coadministered with abacavir. There was no pharmacokinetic interaction between ZDV and 3TC. Mild to moderate headache, nausea, lymphadenopathy, hematuria, musculoskeletal chest pain, neck stiffness, and fever were the most common adverse events reported by those who received abacavir. Coadministration of ZDV or 3TC with abacavir did not alter this adverse event profile. The three-drug regimen was primarily associated with gastrointestinal events. In conclusion, no clinically significant pharmacokinetic interactions occurred between abacavir, ZDV, and 3TC in HIV-1-infected adults. Coadministration of abacavir with ZDV or 3TC produced mild changes in the absorption and possibly the urinary excretion characteristics of ZDV-GZDV and 3TC that were not considered to be clinically significant. Coadministration of abacavir with ZDV and/or 3TC was generally well tolerated and did not produce unexpected adverse events.
The typical form of therapy in the treatment of human immunodeficiency virus (HIV) infection uses several potent antiretroviral drugs in combination (4). Combination therapy suppresses HIV replication to levels below the detection limit of sensitive assays for the detection of HIV type 1 (HIV-1) RNA in plasma and prevents the emergence of drug-resistant viruses. However, currently available antiretroviral agents are limited in number and in their mechanisms of action, with cross-resistance often observed between agents. Therefore, the availability of successful treatment options depends on the development of new antiretroviral agents with unique mechanisms of action and resistance profiles as well as limited toxicity and drug interactions.
Abacavir (1592U89) is a 2′-deoxyguanosine analog which has been shown to have potent antiretroviral properties in in vitro studies. Abacavir is phosphorylated by a unique metabolic pathway to carbocyclic guanosine triphosphate, which is a potent inhibitor of HIV-1 reverse transcriptase (8). Abacavir has been demonstrated to have synergistic activity in vitro against HIV-1 when it is used in combination with zidovudine (ZDV), nevirapine, and amprenavir (141W94) in MT4 cells (25). Additive and/or synergistic effects with the other nucleoside analogs (didanosine, zalcitabine, stavudine, and lamivudine [3TC]) were also noted (7). The cross-resistance of abacavir with other nucleoside reverse transcriptase inhibitors has been extensively studied (16, 28). In a study of 943 clinical isolates from HIV-1-infected patients, most of whom had been previously treated with ZDV and/or 3TC, >95% of isolates resistant to ZDV alone, 3TC alone, or one to three other nucleoside reverse transcriptase inhibitors (didanosine, stavudine, or zalcitabine) remained susceptible to abacavir (16).
Early phase I-II trials have shown that abacavir demonstrates favorable safety and desirable pharmacokinetic characteristics that warrant further clinical development. Following administration of a single oral dose of abacavir to HIV-infected adults and children, abacavir is rapidly and well absorbed, with peak concentrations in plasma (Cmax) occurring within 1 to 2 h after dosing (12, 15). Abacavir is rapidly eliminated from the plasma, with an elimination half-life (t1/2) of 1 to 2 h primarily via hepatic metabolism (i.e., glucuronidation or oxidation by alcohol dehydrogenase), with ≤3% of the oral dose excreted unchanged in urine (15, 20).
In vitro studies have shown that abacavir is unlikely to interact with drugs that are metabolized by the human liver microsomal cytochrome P-450 (CYP3A4, CYP2D6, and CYP2C9) enzymes (20). The primary metabolic pathways of abacavir are mediated by microsomal UDP-glucuronyl transferase and cytosolic alcohol dehydrogenase (20), which indicates that the potential for drug interactions in HIV-infected patients is limited. However, it may be important to determine if potential pharmacokinetic interactions may exist between abacavir and coadministered drugs that are extensively eliminated via the glucuronidation pathway, e.g., ZDV.
The observations that abacavir, ZDV, and 3TC act synergistically in vitro and that viral strains resistant to ZDV or 3TC are not cross-resistant to abacavir suggest that a combination of ZDV or 3TC and abacavir may be clinically beneficial. Thus, the present study (CNAA1002) was undertaken prior to phase II-III clinical trials to evaluate the potential pharmacokinetic interactions of abacavir, ZDV, and 3TC administered alone and concurrently in two- and three-drug combinations. If significant pharmacokinetic interactions had been shown to exist, then the results of this study would have assisted in the selection of the doses for the three-drug combination used in phase II-III efficacy and safety assessments.
(Preliminary data from this study were presented in part at the Third International Congress on Drug Therapy in HIV Infection, Birmingham, United Kingdom, November 1996 [26].)
MATERIALS AND METHODS
Study population.
Eligible subjects included HIV-positive, asymptomatic male and female subjects between 18 and 55 years of age with a body weight of 55 to 85 kg. The subjects had tested positive for antibody to HIV-1 (by a positive HIV-1 enzyme-linked immunosorbent assay result, with the positive result confirmed by Western blotting, positive HIV-1 blood culture, or a positive HIV-1 serum antigen test). Subjects were excluded from the study if they had AIDS or a CD4+ cell count of ≤200 cells/mm3; were taking investigational drugs or drugs known to influence the metabolism or disposition of other drugs (e.g., inducers or inhibitors of the P-450 cytochrome system); had a history of hypersensitivity or anaphylactic or idiosyncratic reaction to nucleoside analogues; or had abnormal laboratory test results within 14 days prior to the first day of dosing, including anemia (hemoglobin concentrations, <10 g/dl for women and <11 g/dl for men), neutropenia (neutrophil count, <1,000 c/mm3), thrombocytopenia (platelet count, <75,000/mm3), elevated liver function tests (aspartate aminotransferase [serum glutamic oxalacetic transaminase] or alanine aminotransferase [serum glutamic pyruvic transaminase] levels two or more times above the upper limit of normal), or renal function impairment (estimated creatinine clearance, ≤40 ml/min). Subjects were also excluded from enrollment in the study if they had a history of pancreatitis or hepatitis within the last 3 years, had a malabsorption disorder, were current alcohol, tobacco, or illicit drug users, or were pregnant or nursing. All prescription and over-the-counter medications were withheld for 48 h (or 24 h for antiretroviral agents and prophylactic agents for opportunistic infections) prior to the first day of dosing and until discharge from the study center. The study was approved by a duly constituted institutional review board, and written informed consent was obtained from all participants.
Study design.
The study described here was an open-label, randomized, seven-period crossover study conducted at a single study center. The treatments comprised seven regimens each administered on a separate day (denoted as dosing days 1 to 7). The seven regimens consisted of the following: 600 mg of abacavir, 300 mg of ZDV, 150 mg of 3TC, 600 mg of abacavir plus 300 mg of ZDV, 600 mg of abacavir plus 150 mg of 3TC, 300 mg of ZDV plus 150 mg of 3TC, and 600 mg of abacavir plus 300 mg of ZDV and 150 mg of 3TC. The order of the treatment regimens administered to each subject was randomized on the basis of two 7-by-7 William’s square design. Fourteen subjects were sufficient to produce a balanced design for a meaningful assessment. The dose of abacavir evaluated (600 mg) was the highest single dose used in ongoing clinical trials with adult HIV-infected subjects, while the doses of ZDV (300 mg) and 3TC (150 mg) were those currently approved for use in the treatment of HIV infection. The study drugs were supplied as abacavir caplets containing 100 mg of abacavir free base as the succinate salt, Retrovir capsules containing 100 mg of ZDV, and Epivir tablets containing 150 mg of 3TC.
Subjects reported to the study center the night before dosing day 1 and remained at the center until completion of the 24-h postdosing procedures for dosing day 7. Because of the short t1/2s of all of these compounds, this crossover study was designed with a 48-h washout period between doses to allow for the evaluation of treatment effects with minimal carryover or residual drug concentrations from previous doses. Thus, subjects remained at the study center for a total of 14 days and nights. All subjects fasted for at least 8 h before each treatment regimen and for an additional 4 h postdosing. Standard meals were provided while the subjects were at the study center.
Blood and urine collection.
Blood samples (3 ml for single-drug regimens and 6 ml for two- or three-drug regimens) were collected by venipuncture and were placed into Vacutainer tubes containing powdered dipotassium ethylenediaminetetraacetic acid. A total of 17 blood samples were obtained from each subject during each dosing day: at approximately 30 min before dosing and at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12, 16, and 24 h post dosing. Blood samples were centrifuged within 30 min of collection to separate the plasma. Urine samples were collected during each dosing day at 5 min before dosing and over the intervals of 0 to 4, 4 to 8, 8 to 12, and 12 to 24 h postdosing. Plasma and urine samples were stored at −40°C or lower until they were analyzed.
Abacavir assay.
Plasma abacavir concentrations were determined by a validated reversed-phase high-performance liquid chromatography assay with UV detection over a quantifiable range of 25 to 5,000 ng/ml. Briefly, 0.1 ml of 10% trichloroacetic acid was added to 0.2 ml of plasma samples, and the components were mixed by vortexing and centrifuged at 8,800 × g for 10 min. The supernatant (0.1 ml) was then injected onto a Rainin (4.6 by 250 mm) C-18 Microsorb MV column (Varian, Walnut Creek, Calif.). The mobile phase consisted of 40% methanol in phosphate-triethylamine at a flow rate of 1.0 ml/min. Abacavir was detected by measuring UV absorbance at 284 nm. The approximate retention time for abacavir was 9 min under these conditions. The interday variability (percent coefficient of variation) was <14%, and the bias of the assay was <2%.
ZDV and GZDV assay.
Plasma and urine ZDV and ZDV 5′-glucuronide (GZDV) concentrations were determined by a validated radioimmunoassay by using the commercially available IncStar ZDV-Trac kit (IncStar, Inc., Stillwater, Minn.) with radioimmunoassay detection, as described previously (27). GZDV was hydrolyzed by β-glucuronidase to ZDV prior to radioimmunoassay. GZDV concentrations were calculated as the difference between the concentration of ZDV (including ZDV from hydrolyzed GZDV) and the concentration of ZDV before hydrolysis of GZDV. For the plasma samples, the quantifiable range for ZDV was 0.1 to 270 μg/ml. The interday variability was <15% for ZDV or <20% for GZDV, and the bias was <15% for ZDV or <8% for GZDV. For the urine samples, the quantifiable range for ZDV was 0.27 to 270 μg/ml. The interday variability was <16% for ZDV or <17% for GZDV, and the bias was <15% for ZDV or <14% for GZDV.
3TC assay.
Plasma 3TC concentrations were determined over a quantifiable range of 3 to 5,000 ng/ml (10). Each plasma sample (0.5 ml) was combined with 1% acetic acid containing internal standard. The sample was vortexed and processed through solid-phase extraction with Certify (Varian) cartridges. The analytes were selectively eluted and concentrated prior to analysis by high-performance liquid chromatography. Reverse-phase chromatography was performed with reconstituted samples by using a BDS-Hypersil-C18 (250 by 4.6 mm) 5-μm column with a Supelco LC18 guard column. The mobile phase was 6 mM heptanesulfonic acid in 200 mM acetate buffer (pH 4.75)–methanol (91:9) (vol/vol). The interday variability was <10%, and the bias was <3% of the theoretical level for all quality control levels.
Safety evaluation.
The safety and tolerability of the study drugs were evaluated on the basis of clinical adverse experiences, vital signs, clinical laboratory test results, physical examinations, and electrocardiograms. The severity (mild, moderate, or severe), duration, and potential relationship of any adverse events to study drug (unrelated or possibly, probably, or almost certainly related) were assessed by the investigator and were recorded. The AIDS Clinical Trials Group Toxicity Grading Scale was used to evaluate abnormal laboratory values. Vital signs (sitting blood pressure and pulse), routine hematology results (complete blood count with differential, mean corpuscular volume, hemoglobin, and platelet count), clinical chemistry results (electrolyte, aspartate transaminase, alanine transaminase, total bilirubin, creatinine, albumin, glucose, alkaline phosphatase, and serum amylase levels), and urinalysis results (dipstick for protein and blood) were evaluated at screening, just before the administration of each dose, at 24-h postdosing on dosing day 7, and at a follow-up visit.
Pharmacokinetic analysis.
Individual plasma concentration-time data were analyzed by noncompartmental pharmacokinetic methods (WinNonlin Program, version 01.5A; Scientific Consulting Inc., Cary, N.C.). Cmax and the time to Cmax (Tmax) were obtained from direct inspection of the plasma concentration-time profile. Estimates for t1/2 were calculated as ln(2)/λz, where λz is the terminal elimination rate constant and is the first-order rate constant determined by the slope of the linear regression line of the apparent terminal linear portion of the log concentration-versus-time curve. The area under the curve (AUC) from time zero to time t, (AUC0–t) where t is the last time point with a measurable concentration of the compound of interest, was calculated by the linear trapezoidal rule. AUC from time zero to infinity (AUC0–∞) was then determined as AUC0–t + Clast/λz, where Clast is the last measurable concentration of the compound of interest. Apparent clearance from plasma (CL/F) was calculated as dose divided by AUC0–∞.
Statistical analysis.
Data for all subjects who completed the seven regimens were included in the statistical analysis. The primary analysis was performed with log-transformed parameters (AUC0–∞, Cmax, t1/2, and CL/F). Analyses to test for carryover effects were also performed. Differences among the regimens for each analyte (i.e., abacavir, ZDV, GZDV, and 3TC) were analyzed by analysis of variance with the PROC GLM and PROC MIXED procedure (SAS, version 6.12; SAS Institute Inc., Cary, N.C.). The analysis included period and treatment as fixed effects and subject as the random effect. Geometric least-square means (LSM) and the 95% confidence intervals (CIs) for the means were calculated for each parameter after each treatment. The test treatment to reference treatment geometric LSM ratio and the corresponding 90% CI were calculated to assess whether there was a pharmacokinetic interaction between any two treatment regimens. Treatments were considered to have no pharmacokinetic interaction if the 90% CI of the estimated geometric LSM ratio was within the range of 0.80 to 1.25. In cases in which the 90% CI was outside of the range of 0.80 to 1.25, differences between treatments are not necessarily considered “clinically significant.” Nonparametric methods were used to compute the 95% CI of the median Tmax values for each treatment. The 90% CI for the median difference in Tmax between treatments was calculated by using the Wilcoxon signed rank test (6). For Tmax, differences between treatments were not considered statistically significant if the 90% CI of the estimated median difference contained the value zero.
Urinary excretion data (percentage of the ZDV dose recovered in urine as ZDV or GZDV) collected for the 13 subjects who completed all seven regimens were analyzed as untransformed data. The percentage of the dose recovered in urine as ZDV and GZDV was compared among treatments with the SAS PROC MIXED procedure. The test treatment-to-reference treatment LSM ratio and the corresponding 90% CI were calculated to assess whether there was a pharmacokinetic interaction between treatment regimens. No interaction was considered between regimens if the 90% CI of the corresponding LSM ratio was within the range of 0.80 to 1.20.
RESULTS
Fifteen HIV-infected adults (13 men and 2 women) were enrolled in the study, with 13 subjects completing all seven regimens. The subjects had a mean age of 33.1 years (age range, 20 to 40 years), a mean weight of 70.1 kg (weight range, 57.2 to 90.3 kg), and a median CD4+ count of 347 cells/mm3 (CD4+ count range, 238 to 570 cells/mm3). The HIV status of seven enrolled subjects was classified as asymptomatic (Centers for Disease Control and Prevention classification A), and the other eight were symptomatic but did not have AIDS (Centers for Disease Control and Prevention classification B). Ten subjects were black, four subjects were white, and one subject was of other ethnic origin.
Two subjects were prematurely discontinued from the study after successive positive urine dipstick results for blood and protein were recorded. The condition was preexisting in the one subject who withdrew after two regimens (abacavir alone and ZDV alone) but appeared to develop in the second subject who withdrew after three regimens (abacavir alone, ZDV alone, and abacavir-ZDV-3TC).
Pharmacokinetic evaluation.
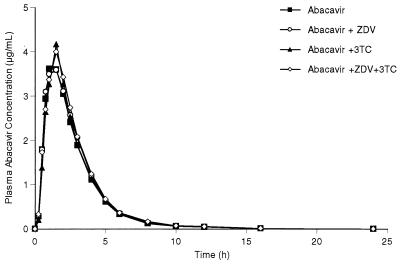
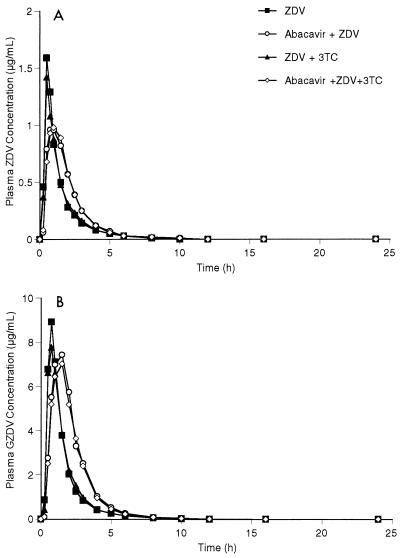
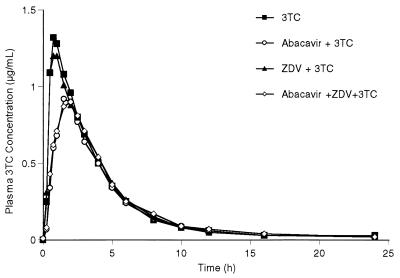
The mean abacavir concentration-versus-time plots are almost superimposable between the four abacavir-containing regimens (Fig. 1). The mean plasma ZDV, GZDV, and 3TC concentration-versus-time plots after the two abacavir-containing regimens have slightly delayed Tmaxs and reduced Cmaxs compared with those for the two regimens without abacavir (Fig. 2 and 3). The mean and percent coefficient of variation pharmacokinetic parameter estimates for abacavir, ZDV, GZDV, and 3TC following each treatment regimen are presented in Table 1.
FIG. 1.
Mean plasma abacavir concentration-versus-time curves following four oral regimens: abacavir, abacavir plus ZDV, abacavir plus 3TC, and abacavir plus ZDV and 3TC.
FIG. 2.
(A) Mean plasma ZDV concentration-versus-time curves following four oral regimens: ZDV, abacavir plus ZDV, ZDV plus 3TC, and abacavir plus ZDV and 3TC. (B) Mean plasma GZDV concentrations-versus-time curves following four oral regimens: ZDV, abacavir plus ZDV, ZDV plus 3TC, and abacavir plus ZDV and 3TC.
FIG. 3.
Mean plasma 3TC concentration-versus-time curves following four oral regimens: 3TC, abacavir plus 3TC, ZDV plus 3TC, and abacavir plus ZDV and 3TC.
TABLE 1.
Mean pharmacokinetic parameter estimates following administration of single oral doses of abacavir, ZDV, and 3TC alone or in combination to HIV-infected subjectsa
| Drug (dose [mg]) | Treatment | AUC0–∞ (μg · h/ml) | Cmax (μg/ml) | Tmax (h) | t1/2 (h) |
|---|---|---|---|---|---|
| Abacavir (600) | Abacavir | 11.3 (30) | 4.24 (28) | 1.29 (32) | 1.50 (24) |
| Abacavir + ZDV | 11.7 (29) | 4.49 (30) | 1.35 (40) | 1.47 (23) | |
| Abacavir + 3TC | 11.5 (28) | 4.33 (30) | 1.41 (23) | 1.50 (18) | |
| Abacavir + ZDV + 3TC | 12.2 (33) | 4.56 (37) | 1.39 (33) | 1.50 (22) | |
| ZDV (300) | ZDV | 2.01 (40) | 1.64 (46) | 0.52 (24) | 1.43 (23) |
| ZDV + abacavir | 2.21 (25) | 1.30 (37) | 0.90 (37) | 1.30 (20) | |
| ZDV + 3TC | 1.89 (35) | 1.57 (44) | 0.62 (48) | 1.32 (15) | |
| ZDV + abacavir + 3TC | 2.22 (35) | 1.28 (45) | 1.00 (39) | 1.35 (17) | |
| GZDV | ZDV | 12.2 (26) | 9.41 (24) | 0.71 (20) | 1.40 (19) |
| ZDV + abacavir | 17.3 (32) | 9.21 (39) | 1.31 (32) | 1.31 (20) | |
| ZDV + 3TC | 11.9 (22) | 9.26 (33) | 0.85 (41) | 1.41 (24) | |
| ZDV + abacavir + 3TC | 16.2 (27) | 8.64 (34) | 1.21 (29) | 1.26 (17) | |
| 3TC (150) | 3TC | 5.31 (30) | 1.50 (36) | 0.88 (38) | 3.27 (16) |
| 3TC + abacavir | 4.49 (32) | 0.97 (34) | 1.81 (21) | 3.54 (15) | |
| 3TC + ZDV | 5.23 (26) | 1.37 (34) | 0.87 (42) | 3.20 (12) | |
| 3TC + abacavir + ZDV | 4.79 (42) | 0.97 (42) | 1.60 (40) | 3.58 (22) |
n = 13 for all drug doses. Values in parentheses are percent coefficients of variation.
The 90% CIs of the geometric LSM ratios for all abacavir parameters (except Tmax) for each treatment comparison were well within the range of 0.80 to 1.25, indicating that no pharmacokinetic interactions were found between treatments containing abacavir (Table 2). The 90% CI for Tmax contained the value 0, indicating that there were no significant differences between treatments containing abacavir. No carryover effect was observed for the abacavir parameters.
TABLE 2.
Comparison of pharmacokinetic parameter estimates for abacavir between treatment regimens
| Treatment or treatment comparison | AUC0–∞ (μg · h/ml)
|
Cmax (μg/ml)
|
Tmax (h)a
|
t1/2 (h)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geometric LSMb | 95% CI | LSM ratioc | 90% CI | Geometric LSMb | 95% CI | LSM ratioc | 90% CI | Median | Range | Median difference | 90% CI | Geometric meanb | 95% CI | LSM ratioc | 90% CI | |
| Treatment | ||||||||||||||||
| Abacavir | 10.9 | 9.19–12.9 | 4.10 | 3.42–4.91 | 1.00 | 0.75–2.00 | 1.46 | 1.29–1.66 | ||||||||
| Abacavir + ZDV | 11.3 | 9.56–13.4 | 4.32 | 3.60–5.17 | 1.50 | 0.50–2.50 | 1.44 | 1.27–1.63 | ||||||||
| Abacavir + 3TC | 11.1 | 9.37–13.1 | 4.14 | 3.46–4.96 | 1.50 | 0.75–2.00 | 1.48 | 1.30–1.68 | ||||||||
| Abacavir + ZDV + 3TC | 11.5 | 9.73–13.7 | 4.25 | 3.55–5.09 | 1.50 | 0.75–2.02 | 1.47 | 1.30–1.67 | ||||||||
| Treatment comparison | ||||||||||||||||
| Abacavir + ZDV vs abacavir | 1.04 | 0.98–1.10 | 1.05 | 0.94–1.18 | 0.00 | −0.13–0.25 | 0.98 | 0.93–1.04 | ||||||||
| Abacavir + 3TC vs abacavir | 1.02 | 0.96–1.08 | 1.01 | 0.90–1.13 | 0.25 | 0.00–0.28 | 1.01 | 0.96–1.07 | ||||||||
| Abacavir + ZDV + 3TC vs abacavir | 1.06 | 1.00–1.12 | 1.04 | 0.93–1.16 | 0.125 | −0.13–0.26 | 1.00 | 0.95–1.06 | ||||||||
Untransformed data.
Values for geometric LSMs for all pharmacokinetic parameters are given with the 95% CIs.
Values are ratios of geometric LSM for test treatment to geometric LSM for reference treatment.
The 90% CIs of the geometric LSM ratios for ZDV and GZDV parameters (for ZDV-3TC versus ZDV alone) and 3TC parameters (for ZDV-3TC versus 3TC alone) were within the range of 0.80 to 1.25 (Tables 3, 4, and 5) for all comparisons except for the Cmax comparison between the ZDV-3TC and ZDV treatments (Table 3). The mean Cmax ratio was 0.94, with a 90% CI of 0.77 to 1.15, indicating a slight decrease in Cmax (from 1.52 to 1.43 μg/ml) which was not clinically significant. Thus, no statistically or clinically significant pharmacokinetic interactions were found between ZDV and 3TC.
TABLE 3.
Comparison of pharmacokinetic parameter estimates for ZDV between treatment regimens
| Treatment or treatment comparison | AUC0–∞ (μg · h/ml)
|
Cmax (μg/ml)
|
Tmax (h)a
|
t1/2 (h)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geometric LSMb | 95% CI | LSM ratioc | 90% CI | Geometric LSMb | 95% CI | LSM ratioc | 90% CI | Median | Range | Median difference | 90% CI | Geometric meanb | 95% CI | LSM ratioc | 90% CI | |
| Treatment | ||||||||||||||||
| ZDV | 1.89 | 1.59–2.24 | 1.52 | 1.21–1.91 | 0.50 | 0.25–0.75 | 1.39 | 1.26–1.55 | ||||||||
| Abacavir + ZDV | 2.15 | 1.81–2.55 | 1.22 | 0.97–0.53 | 1.00 | 0.50–1.50 | 1.29 | 1.16–1.43 | ||||||||
| ZDV + 3TC | 1.80 | 1.52–2.14 | 1.43 | 1.14–1.80 | 0.50 | 0.27–1.50 | 1.30 | 1.18–1.45 | ||||||||
| Abacavir + ZDV + 3TC | 2.11 | 1.78–2.51 | 1.17 | 0.93–1.47 | 1.00 | 0.50–1.50 | 1.34 | 1.20–1.48 | ||||||||
| Treatment comparison | ||||||||||||||||
| Abacavir + ZDV vs ZDV | 1.14 | 1.05–1.24 | 0.80 | 0.66–0.98 | 0.38 | 0.25–0.50 | 0.92 | 0.85–1.01 | ||||||||
| ZDV + 3TC vs ZDV | 0.96 | 0.88–1.04 | 0.94 | 0.77–1.15 | 0.01 | 0.00–0.25 | 0.94 | 0.86–1.02 | ||||||||
| Abacavir + ZDV + 3TC vs ZDV | 1.12 | 1.03–1.22 | 0.77 | 0.63–0.94 | 0.50 | 0.25–0.75 | 0.96 | 0.88–1.03 | ||||||||
Untransformed data.
Values for geometric LSMs for all pharmacokinetic parameters are given with the 95% CIs.
Values are ratios of geometric LSM for test treatment to geometric LSM for reference treatment.
TABLE 4.
Comparison of pharmacokinetic parameter estimates for GZDV between treatment regimens
| Treatment or treatment comparison | AUC0–∞ (μg · h/ml)
|
Cmax (μg/ml)
|
Tmax (h)a
|
t1/2 (h)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geometric LSMb | 95% CI | LSM ratioc | 90% CI | Geometric LSMb | 95% CI | LSM ratioc | 90% CI | Median | Range | Median difference | 90% CI | Geometric meanb | 95% CI | LSM ratioc | 90% CI | |
| Treatment | ||||||||||||||||
| GZDV | 11.8 | 10.2–13.7 | 9.11 | 7.46–11.1 | 0.75 | 0.50–1.00 | 1.38 | 1.23–1.54 | ||||||||
| Abacavir + ZDV | 16.5 | 14.2–19.1 | 8.57 | 7.02–10.5 | 1.50 | 0.75–2.02 | 1.28 | 1.15–1.44 | ||||||||
| ZDV + 3TC | 11.7 | 10.1–13.6 | 8.79 | 7.19–10.7 | 0.75 | 0.50–1.50 | 1.38 | 1.23–1.54 | ||||||||
| Abacavir + ZDV + 3TC | 15.6 | 13.4–18.1 | 8.15 | 6.67–9.95 | 1.00 | 0.75–2.00 | 1.24 | 1.11–1.39 | ||||||||
| Treatment comparison | ||||||||||||||||
| Abacavir + ZDV vs ZDV | 1.40 | 1.30–1.50 | 0.94 | 0.81–1.09 | 0.62 | 0.38–0.87 | 0.93 | 0.88–0.99 | ||||||||
| ZDV + 3TC vs ZDV | 0.99 | 0.93–1.06 | 0.97 | 0.83–1.12 | 0.13 | 0.00–0.25 | 1.00 | 0.94–1.06 | ||||||||
| Abacavir + ZDV + 3TC vs ZDV | 1.32 | 1.24–1.42 | 0.90 | 0.77–1.04 | 0.50 | 0.25–0.75 | 0.90 | 0.85–0.96 | ||||||||
Untransformed data.
Values for geometric LSMs for all pharmacokinetic parameters are given with the 95% CIs.
Values are ratios of geometric LSM for test treatment to geometric LSM for reference treatment.
TABLE 5.
Comparison of pharmacokinetic parameter estimates for 3TC between treatment regimens
| Treatment or treatment comparison | AUC0–∞ (μg · h/ml)
|
Cmax (μg/ml)
|
Tmax (h)a
|
t1/2 (h)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geometric LSMb | 95% CI | LSM ratioc | 90% CI | Geometric LSMb | 95% CI | LSM ratioc | 90% CI | Median | Range | Median difference | 90% CI | Geometric meanb | 95% CI | LSM ratioc | 90% CI | |
| Treatment | ||||||||||||||||
| 3TC | 5.09 | 4.18–6.21 | 1.40 | 1.12–1.75 | 0.75 | 0.50–1.50 | 3.23 | 2.94–3.55 | ||||||||
| Abacavir + 3TC | 4.29 | 3.52–5.23 | 0.91 | 0.73–1.14 | 2.00 | 1.03–2.50 | 3.50 | 3.18–3.85 | ||||||||
| ZDV + 3TC | 5.06 | 4.16–6.17 | 1.31 | 1.05–1.63 | 1.00 | 0.50–1.50 | 3.20 | 2.91–3.52 | ||||||||
| Abacavir + ZDV + 3TC | 4.39 | 3.60–5.34 | 0.89 | 0.71–1.11 | 1.50 | 0.75–2.50 | 3.51 | 3.19–3.86 | ||||||||
| Treatment comparison | ||||||||||||||||
| Abacavir + 3TC vs 3TC | 0.84 | 0.75–0.95 | 0.65 | 0.56–0.75 | 0.89 | 0.64–1.14 | 1.08 | 1.01–1.17 | ||||||||
| ZDV + 3TC vs 3TC | 0.99 | 0.88–1.12 | 0.93 | 0.81–1.08 | 0.00 | −0.25–0.25 | 0.99 | 0.92–1.07 | ||||||||
| Abacavir + ZDV + 3TC vs 3TC | 0.86 | 0.77–0.97 | 0.64 | 0.55–0.73 | 0.75 | 0.375–1.00 | 1.09 | 1.01–1.17 | ||||||||
Untransformed data.
Values for geometric LSMs for all pharmacokinetic parameters are given with the 95% CIs.
Values are ratios of geometric LSM for test treatment to geometric LSM for reference treatment.
The 90% CIs of the geometric LSM ratios for the Cmax of ZDV for the abacavir-ZDV or abacavir-ZDV-3TC versus ZDV treatment comparisons were below the range of 0.80 to 1.25 (Table 3). Mean Cmax of ZDV decreased by approximately 20% (from 1.52 to 1.22 or 1.17 μg/ml), and the Tmax of ZDV was delayed by approximately 0.5 h (from 0.5 to 1.0 h) when abacavir was given concurrently with or without 3TC. There were no differences in the AUC0–∞ or t1/2 of ZDV between any treatments.
The 90% CIs of the geometric LSM ratios for the AUC0–∞ for GZDV for the abacavir-ZDV or abacavir-ZDV-3TC versus ZDV treatment comparisons were above the range of 0.80 to 1.25 (Table 4). The mean AUC0–∞ for GZDV increased by approximately 32 to 40% (from 11.8 to 16.5 or 15.6 μg · h/ml), and the Tmax for GZDV was delayed by approximately 0.5 h (from 0.75 to 1.5 or 1.0 h) when abacavir was given concurrently with ZDV with or without 3TC. The 90% CI of the geometric LSM ratio for the Cmax of GZDV between any two treatment comparisons was within the range of 0.80 to 1.25 for all treatment comparisons except for the abacavir-ZDV-3TC versus ZDV treatment comparison (Table 4). The mean Cmax decreased slightly (from 9.11 to 8.15 μg/ml) for the abacavir-ZDV-3TC versus ZDV treatment comparison, but this difference was not clinically significant. There were no significant differences in the t1/2 of GZDV between any of the treatments. No carryover effect was observed for pharmacokinetic parameters for ZDV or GZDV.
The 90% CIs of the geometric LSM ratios for the AUC0–∞ and Cmax parameters for 3TC for the abacavir-3TC or abacavir-ZDV-3TC versus 3TC treatment comparisons were below the range of 0.80 to 1.25 (Table 5). The mean AUC0–∞ for 3TC decreased by approximately 15% (from 5.09 to 4.29 or 4.39 μg · h/ml) and the mean Cmax of 3TC decreased by approximately 35% (from 1.40 to 0.91 or 0.89 μg/ml) when abacavir was given concurrently with 3TC with or without ZDV. Abacavir caused a 1-h delay in the Tmax for 3TC (from 0.75 to 1.5 or 2.0 h), and no difference was observed in t1/2 estimates for 3TC between any treatments. No carryover effect was observed for AUC parameters for 3TC, but a borderline carryover effect was observed for Cmax and t1/2 estimates for 3TC.
Urinary excretion data.
The arithmetic LSM (and the corresponding 95% CIs) for the percentage of the dose recovered in urine as ZDV were 13.3 (11.4 to 15.3), 11.5 (9.4 to 13.5), 14.4 (12.3 to 16.6), and 13.4 (11.3 to 15.5) for the ZDV alone, abacavir-ZDV, ZDV-3TC, and abacavir-ZDV-3TC treatment regimens, respectively. The arithmetic LSM (and the corresponding 95% CIs for the percentage of the dose recovered in urine as GZDV were 74.4 (66.2 to 82.6), 69.3 (60.7 to 77.9), 76.1 (67.1 to 85.1), and 79.6 (70.6 to 88.6) for the ZDV alone, abacavir-ZDV, ZDV-3TC, and abacavir-ZDV-3TC treatment regimens, respectively. The 90% CIs of the LSM ratios for each treatment comparison (versus ZDV alone) were within the range of 0.80 to 1.20 for all treatment comparisons for GZDV. In contrast, for ZDV, both the abacavir-ZDV versus ZDV and the ZDV-3TC versus ZDV comparisons were outside the range of 0.80 to 1.20. The percentage of the dose excreted as ZDV in the urine decreased by 14% in the presence of abacavir but increased by 8% in the presence of 3TC. Overall, the extent of urinary recoveries of ZDV and GZDV was not significantly different when ZDV was coadministered with 3TC and/or abacavir. No carryover effect was observed.
Safety.
Abacavir was well tolerated when it was administered alone or in combination with ZDV and/or 3TC, with no serious adverse events or deaths. All adverse events following treatment with abacavir-containing regimens were recorded as mild or moderate in intensity. The number of subjects and the number of drug-related adverse events following abacavir, ZDV, and 3TC treatments alone were 4 and 8, 3 and 3, and 1 and 1, respectively. The adverse events with abacavir alone were headache, nausea, lymphadenopathy, hematuria, musculoskeletal chest pain, neck stiffness, and fever. No hypersensitivity reaction was observed among the subjects who received abacavir. The number of subjects and the number of drug-related adverse events following treatments with abacavir-ZDV, abacavir-3TC, ZDV-3TC, and abacavir-ZDV-3TC were 2 and 3, 3 and 4, 1 and 1, and 7 and 9, respectively. The adverse events reported following the addition of ZDV or 3TC to the abacavir regimen were similar to those reported for abacavir given alone, while adverse events associated with the three-drug regimen were mainly gastrointestinal events, including nausea, diarrhea, colic, epigastric pain, and vomiting. Median values from clinical chemistry and hematology studies were recorded during treatment but were unaffected by administration of study drugs.
DISCUSSION
The results of this study indicate that neither ZDV nor 3TC coadministration with abacavir affected abacavir pharmacokinetics. The abacavir pharmacokinetic parameter estimates obtained in this study are consistent with those obtained previously from a single-dose study with HIV-infected adults (15). In contrast, the pharmacokinetics of ZDV and/or 3TC were moderately affected by coadministration with abacavir, and the observed changes were consistent with a delay in the absorption of the two nucleosides from the gastrointestinal tract. This degree of pharmacokinetic interaction is not believed to be clinically significant. Results also confirm previous findings that the pharmacokinetics of ZDV or 3TC were not affected by coadministration with each other (11, 19). The urinary excretion data for ZDV and GZDV similarly indicated a lack of pharmacokinetic interaction between ZDV and 3TC.
Abacavir appeared to delay the absorption of ZDV (as indicated by the 0.5-h delay in Tmax and the 20% decrease in the mean Cmax for ZDV). However, abacavir did not significantly affect either the extent of absorption or the elimination of ZDV, as indicated by an absence of significant changes in the AUC0–∞ for ZDV, and t1/2, respectively. Studies by Shelton et al. (22) and Unadkat et al. (30) have reported that food caused a 45% decrease in the mean Cmax, more than a 1-h delay in Tmax, and a 10 to 24% decrease in the mean AUC0–∞ for ZDV. Thus, the effect of abacavir on ZDV is similar to the changes in ZDV pharmacokinetics caused by food intake, but the magnitude of the changes is smaller. The 20% decrease in the mean Cmax of ZDV caused by abacavir is not considered to be clinically significant.
The coadministration of ZDV and abacavir appears to increase the overall plasma exposure to GZDV (as indicated by the increase in the mean AUC0–∞ for GZDV up to 40%) but had little effect on the Cmax of GZDV. Because GZDV is not pharmacologically active and has little toxicological potential compared with ZDV (5), the 40% increase in the mean AUC0–∞ for GZDV should have little clinical relevance. Furthermore, the urinary excretion data for ZDV and GZDV showed no clinically significant differences in the urinary recoveries of these compounds, indicating that the overall amount of ZDV absorbed and the metabolism of ZDV by glucuronidation were unchanged in the presence of abacavir. These results also support the finding of a lack of a clinically significant interaction between abacavir and ZDV. The urinary recovery results obtained in our study for ZDV (11 to 14%) and GZDV (69 to 80%) are also consistent with values reported previously (3).
The approximately 40% increase in the mean AUC0–∞ for GZDV by abacavir (together with unchanged urinary excretion data and t1/2) suggest that abacavir (or a metabolite) may reduce the renal clearance of GZDV. Because the elimination of both GZDV and abacavir are dependent on renal excretion, the reduced renal clearance of GZDV could be due to a competitive inhibition of tubular secretion by abacavir or a metabolite(s). An increased AUC0–∞, together with reduced renal clearance and reduced urinary recovery of GZDV, has been reported for other drug combinations, including ZDV and didanosine administered concurrently (2, 17).
Abacavir appeared to delay the absorption and slightly decrease the extent of plasma exposure to 3TC (as indicated by the 35% decrease in the mean Cmax, the 1-h delay in the mean Tmax, and the 15% decrease in the mean AUC0–∞) but had no significant effect on the t1/2 of 3TC. A study by Angel et al. (1) has reported that the effect of a high-fat meal on a single dose of 50 mg of 3TC reduced the mean Cmax by 47%, delayed Tmax by over 2 h, but did not significantly affect AUC0–∞ or the fraction of the dose excreted in urine. Thus, the effect of abacavir on 3TC pharmacokinetics appears to be similar to that caused by food intake, but the magnitude was smaller. The 15% decrease in the mean AUC0–∞ is not considered clinically significant and may be attributed to a decrease in the extent of oral bioavailability or an increase in the renal clearance of 3TC. The carryover effect noted for the 3TC parameters between treatments could be due to the short interval (48 h) between the administration of each dose and the relatively sensitive assay for 3TC concentrations in plasma. This effect is not considered significant.
Abacavir was generally well tolerated when it was administered alone or in combination with ZDV and/or 3TC. Coadministration of ZDV or 3TC with abacavir did not increase the frequency or severity of adverse events that were reported, while the three-drug regimen was primarily associated with the occurrence of gastrointestinal adverse events. The favorable safety profile of abacavir reported in this study is consistent with those observed in previous studies (12, 15). No hypersensitivity reaction was observed among subjects who received abacavir.
Potential interactions of the nucleoside analogs or their metabolites must also be investigated at the intracellular level for any changes in intracellular phosphorylation, which in turn may affect antiviral activity. Abacavir, ZDV, and 3TC exert their antiviral effects only after intracellular sequential phosphorylation by cellular kinases to the active metabolite, the triphosphate derivative (7, 9, 13). Because the three drugs are activated by different metabolic pathways, it is unlikely that there will be interferences of the intracellular metabolic activation of any one drug by the other two drugs. This is supported by early results of durable anti-HIV activity observed in clinical trials of abacavir combined with ZDV, 3TC, and other antiretroviral agents in antiretroviral agent-naive and -experienced patients (14, 18, 21, 23, 24, 29).
In conclusion, this single-dose study indicates that, with a 600-mg dose of abacavir, there were no clinically significant pharmacokinetic interactions between abacavir, ZDV, and 3TC. This study also shows that abacavir used at twice the recommended dose in combination with ZDV and/or 3TC does not produce increased drug toxicity. Studies are ongoing to evaluate the antiretroviral efficacy and safety of long-term therapy with this triple-drug combination.
ACKNOWLEDGMENTS
We gratefully acknowledge the assistance of Veronica Parker for data management; Michael J. O’Mara, David M. Morris, and Elizabeth H. Culverhouse for performing the bioanalytical studies; Mark Liu for performing the statistical analyses; Yu Lou for critical review of the manuscript; and Belinda Ha for manuscript and writing assistance.
REFERENCES
- 1.Angel J B, Hussey E K, Hall S T, Donn K H, Morris D M, McCormack J P, Montaner J S G, Ruedy J. Pharmacokinetics of 3TC (GR 109714X) administered with and without food to HIV-infected patients. Drug Invest. 1993;6:70–74. [Google Scholar]
- 2.Barry M, Howe J L, Ormesher S, Back D J, Breckenridge A M, Bergin C, Mulcahy F, Beeching N, Nye F. Pharmacokinetics of zidovudine and dideoxyinosine alone and in combination in patients with the acquired immunodeficiency syndrome. Br J Clin Pharmacol. 1994;37:421–426. doi: 10.1111/j.1365-2125.1994.tb05708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blum M R, Liao S H T, Good S S, De Miranda P. Pharmacokinetics and bioavailability of zidovudine in humans. Am J Med. 1988;85(Suppl. 2A):189–194. [PubMed] [Google Scholar]
- 4.Carpenter C C, Fischl M A, Hammer S M, Hirsch M S, Jacobsen D M, Katzenstein D A, Montaner J S, Richman D D, Saag M S, Schooley R T, Thompson M A, Vella S, Yeni P G, Volberding P A. Antiretroviral therapy for HIV infection in 1997. Updated recommendations of the International AIDS Society-USA Panel. JAMA. 1997;277:1962–1969. [PubMed] [Google Scholar]
- 5.Collins J M, Unadkat J D. Clinical pharmacokinetics of zidovudine. An overview of current data. Clin Pharmacokinet. 1989;17:1–9. doi: 10.2165/00003088-198917010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Conover W J. Practical non-parametric statistics. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1980. Some methods based on ranks; pp. 213–343. [Google Scholar]
- 7.Daluge S M, Good S S, Faletto M B, Miller W H, St. Clair M H, Boone L R, Tisdale M, Parry N R, Reardon J E, Dornsife R E, Averett D R, Krenitsky T A. 1592U89, a novel carbocyclic nucleoside analog with potent, selective anti-human immunodeficiency virus activity. Antimicrob Agents Chemother. 1997;41:1082–1093. doi: 10.1128/aac.41.5.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faletto M B, Miller W H, Garvey E P, St. Clair M H, Daluge S M, Good S S. Unique intracellular activation of the potent anti-human immunodeficiency virus agent 1592U89. Antimicrob Agents Chemother. 1997;41:1099–1107. doi: 10.1128/aac.41.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furman P A, Fyfe J A, St. Clair M H, Weinhold K, Rideout J L, Freeman G A, Lehrman S N, Bolognesi D P, Broder S, Mitsuya H, et al. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci USA. 1986;83:8333–8337. doi: 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harker A J, Evans G L, Hawley A E, Morris D M. High-performance liquid chromatographic assay for 2′-deoxy-3′-thiacytidine in human serum. J Chromatogr B Biomed Appl. 1994;657:227–232. doi: 10.1016/0378-4347(94)80092-8. [DOI] [PubMed] [Google Scholar]
- 11.Horton C M, Yuen G, Mikolich D M, Fisher A, Rana K, Mydlow P, Dudley M N. Pharmacokinetics (PK) of oral lamivudine administered alone and with oral zidovudine (ZDV) in asymptomatic patients with human immunodeficiency virus (HIV) infection. Clin Pharmacol Ther. 1994;55:198. (abstr. PIII-64). [Google Scholar]
- 12.Hughes W, McDowell J A, Shenep J, Flynn P, Kline M W, Yogev R, Symonds W, Lou Y, Hetherington S. Safety and single-dose pharmacokinetics of abacavir (1592U89) in human immunodeficiency virus type 1-infected children. Antimicrob Agents Chemother. 1999;43:609–615. doi: 10.1128/aac.43.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kewn S, Veal G J, Hoggard P G, Barry M G, Back D J. Lamivudine (3TC) phosphorylation and drug interactions in vitro. Biochem Pharmacol. 1997;54:589–595. doi: 10.1016/s0006-2952(97)00189-5. [DOI] [PubMed] [Google Scholar]
- 14.Kost R, Cao Y, Vesanen M, Talal A, Hurley A, Schluger R, Monard S, Rogers M, Johnson J, Smiley L, Ho D, Markowitz M. Abstracts of the 5th Conference on Retroviruses and Opportunistic Infections. 1998. Combination therapy with abacavir (1592), 141W94, and AZT/3TC in subjects acutely and chronically infected with HIV, abstr. 363; p. 147. [Google Scholar]
- 15.Kumar P N, Sweet D E, McDowell J A, Symonds W, Lou Y, Hetherington S, LaFon S. Safety and pharmacokinetics of abacavir (1592U89) following oral administration of escalating single doses in human immunodeficiency virus type-1 infected adults. Antimicrob Agents Chemother. 1999;43:603–608. doi: 10.1128/aac.43.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellors J W, Hertogs K, Peeters F, Lanier R, Miller V, Graham N, Larder B, Stoffels P, Pauwels R. Abstracts of the 5th Conference on Retroviruses and Opportunistic Infections. 1998. Susceptibility of clinical HIV-1 isolates to 1592U89, abstr. 687; p. 208. [Google Scholar]
- 17.Morse G D, Shelton M J, Ho M, Bartos L, DeRemer M, Ragni M. Pharmacokinetics of zidovudine and didanosine during combination therapy. Antivir Res. 1995;27:419–424. doi: 10.1016/0166-3542(95)00032-h. [DOI] [PubMed] [Google Scholar]
- 18.Prins J, Jurriaans S, Roos M, De Wolf F, Miedema F, Lange J. Abstracts of the 5th Conference on Retroviruses and Opportunistic Infections. 1998. An attempt at maximally suppressive anti-HIV therapy, abstr. 385; p. 150. [Google Scholar]
- 19.Rana K Z, Horton C M, Yuen G J, Pivarnik P E, Mikolich D M, Fisher A E, Mydlow P K, Dudley M N. Program and abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. Effect of lamivudine on zidovudine pharmacokinetics in asymptomatic HIV-infected individuals, abstr. A62; p. 83. [Google Scholar]
- 20.Ravitch J R, Bryant B J, Reese M J, Boehlert C C, Walsh J S, McDowell J P, Sadler B M. Abstracts of the 5th Conference on Retroviruses and Opportunistic Infections. 1998. In vivo and in vitro studies of the potential for drug interactions involving the anti-retroviral 1592 in humans, abstr. 634; p. 199. [Google Scholar]
- 21.Saag, M. S., A. Sonnerborg, R. A. Torres, D. Lancaster, B. G. Gazzard, R. T. Schooley, C. Romero, D. Kelleher, W. Spreen, S. LaFon, and the Abacavir Phase 2 Clinical Team. Antiretroviral effect and safety of abacavir and abacavir in combination with zidovudine in HIV-infected adults. AIDS 12:F203–F209. [DOI] [PubMed]
- 22.Shelton M J, Portmore A, Blum M R, Sadler B M, Reichman R C, Morse G D. Prolonged but not diminished, zidovudine absorption induced by a high-fat breakfast. Pharmacotherapy. 1994;14:671–677. [PubMed] [Google Scholar]
- 23.Staszewski, S., C. Katlama, T. Harrer, P. Massip, P. Yeni, A. Cutrell, S. M. Tortell, P. R. Harrigan, H. Steel, E. R. Lanier, and G. Pearce. A dose-ranging study to evaluate the safety and efficacy of three doses of abacavir (1592U89) alone or in combination with zidovudine and lamivudine in antiretroviral treatment naive subjects. AIDS 12:F197–F202. [DOI] [PubMed]
- 24.Staszewski S, Katlama C, Harrer T, Massip P, Yeni P, Cutrell A, Tortell S M, Steel H M, Lanier E R, Pearce G. Abstracts of the 5th Conference on Retroviruses and Opportunistic Infections. 1998. Preliminary long-term open-label data from patients using abacavir (1592) containing antiretroviral treatment regimens, abstr. 658; p. 203. [Google Scholar]
- 25.St. Clair M H, Millard J, Rooney J, Tisdale M, Parry N, Sadler B M, Blum M R, Painter G. In vitro antiviral activity of 141W94 (VX-478) in combination with other antiretroviral agents. Antivir Res. 1996;29:53–56. doi: 10.1016/0166-3542(95)00916-7. [DOI] [PubMed] [Google Scholar]
- 26.Symonds W T, McDowell J, Chittick G, Moss J, Bye A. The safety and pharmacokinetics of GW1592U89, zidovudine, and lamivudine (3TC) alone and in combination after single-dose administration in HIV-infected patients. AIDS. 1996;10(Suppl. 2):S23. [Google Scholar]
- 27.Tadepalli S M, Puckett L, Jeal S, Kanics L, Quinn R P. Differential assay of zidovudine and its glucuronide metabolite in serum and urine with a radioimmunoassay kit. Clin Chem. 1990;36:897–900. [PubMed] [Google Scholar]
- 28.Tisdale M, Alnadaf T, Cousens D. Combination of mutations in human immunodeficiency virus type 1 reverse transcriptase required for resistance to the carbocyclic nucleoside 1592U89. Antimicrob Agents Chemother. 1997;41:1094–1098. doi: 10.1128/aac.41.5.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torres R, Saag M, Lancaster D, Sonnerborg A, Feinberg J, Thompson M, Lang W, Schooley R, Mulder J, D’Aquila R, Santin M, Lafon S. Abstracts of the 5th Conference on Retroviruses and Opportunistic Infections. 1998. Antiviral effects of abacavir (1592) following 36 weeks of therapy, abstr. 659; p. 203. [Google Scholar]
- 30.Unadkat J D, Collier A C, Crosby S S, Cummings D, Opheim K E, Corey L. Pharmacokinetics of oral zidovudine (azidothymidine) in patients with AIDS when administered with and without a high-fat meal. AIDS. 1990;4:229–232. doi: 10.1097/00002030-199003000-00008. [DOI] [PubMed] [Google Scholar]