Abstract
Melanotic neuroectodermal tumor of infancy (MNTI) is a clinically distinctive, benign neoplasm of neural crest origin. The tumor develops usually in the anterior maxilla and rarely in the skull and mandible. This is a report of the interdisciplinary treatment of a rare case of MNTI occurring in the mandible. The patient was initially addressed for examination at the age of 2 months with a rapidly growing tumor of the mandible that had increased double in size in a week. A well-defined lesion in the left mandible shown on MRI and high urine vanillylmandelic acid (VMA) level determined the diagnosis. The complete interdisciplinary treatment included four interventions. Surgery with enucleation and curettage, performed as first intervention, at the age of 2 months. The second intervention lasted from age 7 to age 15 and included a first phase of Orthodontic treatment to monitor normal growth, followed by interceptive Orthodontic treatment and Prosthodontic intervention with interim dentures. The third intervention accomplished after competition of growth and included the pre-prosthetic surgery with an augmentation of the height of the edentulous atrophic mandible and placement of 4 implants. In the fourth intervention the permanent prosthodontic restoration supported by implants was applied.
Keywords: Infant, Enucleation, Mandible, Child, Melanotic, Neuroectodermal, Pigmented, Tumor
Introduction
Melanotic neuroectodermal pigmented tumor of infancy (MNTI) is a rare and benign neoplasm of early infancy with rapid expansive growth, first described in 1918 [1]. The tumor has been referred as congenital melanocarcinoma, retinal anlage tumor, pigmented congenital epulis or melanotic progonoma [2]. It was first proposed to be of neural crest origin in 1966, because of the discovery that many cases were associated with an increase in urinary vanillylmandelic acid (VMA) excretion [3]. This finding also explains the biphasic population of primitive neuroectodermal and melanotic cells, both of which are derived from the neural crest [4, 5]. This lesion is found mainly in children below 1 year of age. A small number of cases have been reported in older children and in adults [6, 7]. It has been found that there is no difference between infant sexes concerning the prevalence of the tumor. In the majority (90%) of the cases the tumor develops in head and neck and, especially in the anterior region of the maxilla, but it can also occur at other locations, including the skull, the mandible, scapula and epididymis, etc. [7, 8]. Clinically, MNTI is a soft and rapidly growing pigmented swelling that can have black, blue, or brown coloration [9]. The mass often destroys underlying bone and may be associated with displacement of developing teeth. Clinical and radiological findings may suggest a diagnosis of MNTI [10, 11], but histopathological examination is required for a definite diagnosis [5, 12, 13]. Currently, MNTI is treated primarily surgically. An extensive resection is often adopted as a surgical approach in the treatment of MNTI to avoid recurrence [14]. However, the optimal extent of surgical resection remains controversial, as the post-operative recurrence rate is as high as 15–27% [6]. The majority of cases of MNTI grow rapidly and are invasive to a certain extent, while a proportion undergo malignant transformation. Therefore, extended resection is often adopted as a surgical approach in the treatment of MNTI despite the risk of leaving extended edentulous areas due to tooth germ subtraction. This report presents a rare case of MNTI in the left mandible and its total treatment from infancy to adulthood. An interdisciplinary treatment among the maxillofacial, prosthetic and orthodontic departments was needed to bring the patient in a stable, functional and esthetic restoration.
Clinical Report
A 2-month-old female infant was referred by a pediatrician for evaluation and treatment of a rapidly growing mass of the left mandible to the Department of Oral and Maxillofacial Surgery of Aristotle University of Thessaloniki Dental School.
First Oral and Maxillofacial Treatment Intervention
Medical History and Clinical Examination
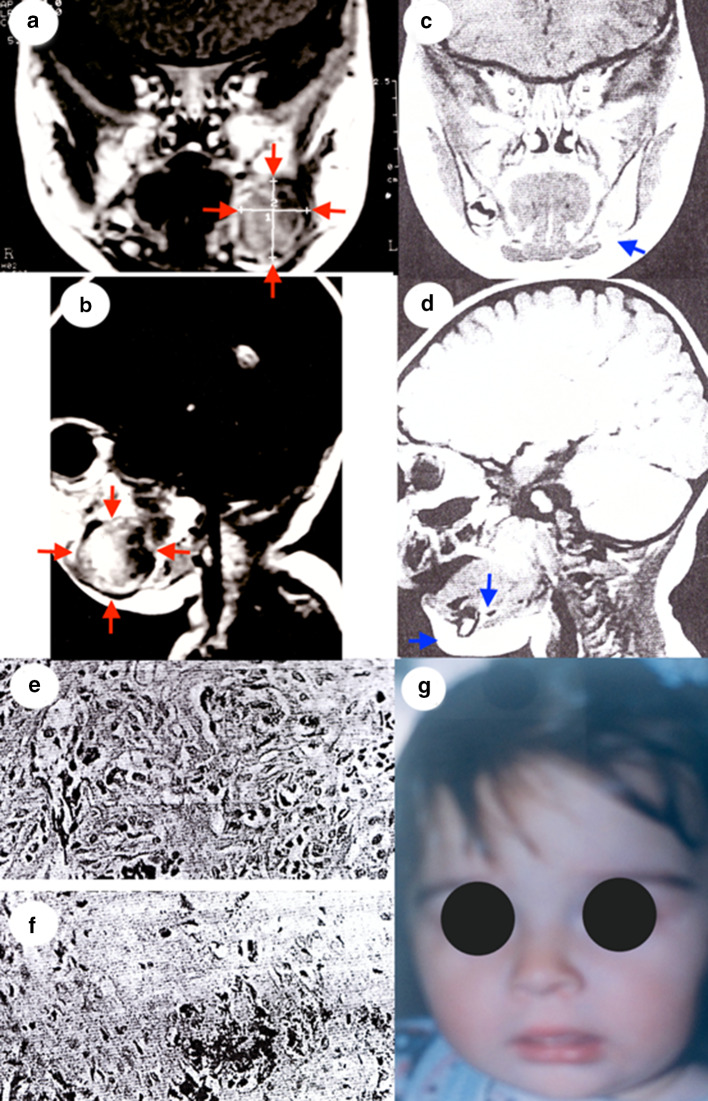
The lesion was noted approximately 5 weeks earlier by her mother who reported progressive enlargement, facial asymmetry and feeding difficulties. According to the mother initially the tumor was consistent with the mucosal color of the surrounding gums; however it became cyanotic as it increased in size. No pain or fever was reported by the infant’s parents. There was no history of medication during pregnancy. Oral examination revealed a 2 × 3 cm sessile, bluish, expansile tumor of the left mandible. There was no tenderness of the tumor mass and it lay firm fixed on the underlying bone. No significant anomalies were detected in the oral mucosa outside of the lesion site. The deciduous teeth were not erupted. No positive cervical lymphadenopathy could be detected. MRI examination revealed an enhancing, hypointense mass on T1- and T2-weighted imaging arising mainly from the left mandibular body invading and eroding it, but there was no invasion into the mouth floor, or the vestibular sulcus mucosa (Fig. 1a, b). Laboratory blood tests were within normal limits except the urine vanillylmandelic acid (VMA) level which was elevated at 10 mg/24 h (normal values range 2.0–7.0 mg/24 h).
Fig. 1.
a Initial MRI (coronal section). b Initial MRI (sagittal section). c 2 years after surgery MRI (coronal section). d 2 years after surgery MRI (sagittal section). e Microscopical examination of the tumor (hematoxylin–eosin stain, original magnification × 400). f Microscopical examination of the tumor (Immunohistochemical Fontana stain, original magnification × 100). g 2 years after surgery extraoral photograph
Second urinary vanillylmandelic acid test confirmed the preceding findings. Based on the clinical and radiographic characteristics of the lesion, a provisional diagnosis of MNTI was rendered.
Surgical Procedure
At the age of 9 weeks old and under general anesthesia surgical excision and enucleation of the mass was performed. The inferior alveolar nerve was left intact. Surgical excision of the tumor and curettage resulted in a reduction in the thickness of the mandibular cortex. Visible, black pigmentation was evident in the tumor mass.
The primary tumor volume was 2 × 3 × 2 cm. Microscopical examination revealed a well-demarcated neoplasm consisting of a biphasic proliferation of large and small cells. These cells formed nests and cords invested in a fibrous stroma (Fig. 1e, f). The large pigmented cells revealed in the fibrous connective tissue without pathological nuclear fission necrosis or perineural invasion.
The large cells were polygonal and featured ill-defined cytoplasmic membrane, vesicular nuclei with occasionally prominent nucleoli, and variable amounts of intracytoplasmic black pigment, consistent with melanin. The small round cells had scant cytoplasm, and basophilic heterochromatic nuclei. No features of malignancy such as nuclear atypia mitosis or necrosis were appreciated. The diagnosis of MNTI was rendered. One week later the levels of the VMA in urine returned to normal limits.
A 2-years follow-up MRI showed no evidence of recurrence and good development of the remaining mandible (Fig. 1b, d). The extraoral photograph of the face at the same time revealed no disfigurement of the face (Fig. 1g).
Prosthodontic and Orthodontic Treatment Intervention
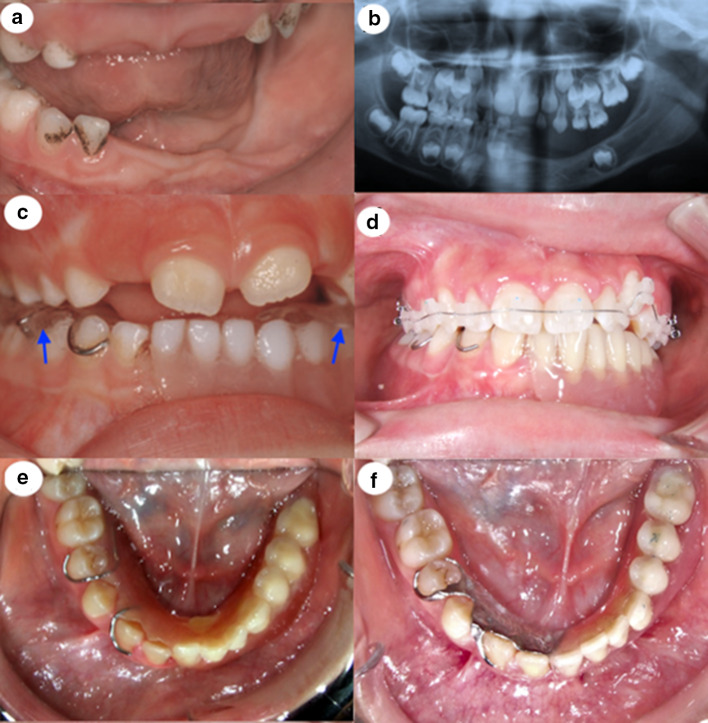
At the age of 7, the patient was referred to the Department of Prosthodontics for consultation on the management of the edentulous area on the left mandibular side. The intraoral examination revealed that all teeth of the left quadrant were missing as well as the right central deciduous incisor. The left alveolar ridge was atrophic (Fig. 2a).
Fig. 2.
a Intraoral photograph at the age of 7 years. b Panoramic X-ray at the age of 7 years. c Intraoral view of the 1st TRPD at the age of 7 years with the posterior bite blocks on the artificial teeth (arrows). d Intraoral view of the 2nd TRPD during the orthodontic treatment. e Intraoral view of the 3rd TRPD by the end of the orthodontic treatment at the age of 14 years. f Intraoral view of the 4th TRPD at the age of 16 years
The panoramic X-ray showed in addition a tooth germ in the form of a premolar in the edentulous area (Fig. 2b). Patient’s facial height was reduced, more pronounced on the left side, creating a light asymmetry that did not seriously deteriorate the appearance of the patient. The patient did not suffer any temporomandibular joint disorders.
Initial prosthodontic intervention included the application of a series of temporary removable partial dentures (TRPD) until the end of the growth period. The goal of this intervention was to improve comfort and esthetics and to prevent further deterioration of the oral structures and function [15].
Orthodontic evaluation in department of Orthodontics aimed the guidance of patient’s vertical growth, because a lot of anterior and all the posterior mandibular teeth on the left side were missing. To increase the lower face vertical height, in the first interim partial denture were included posterior acrylic bite blocks in both sides. On the left side the bite block was placed on the artificial posterior teeth. They were not extended on the permanent mandibular and maxillary first molar, in order to allow their eruption and thus to enhance the increase in the vertical growth (Fig. 2c).
Adaptations of the first interim denture were done every 3 months to guide the eruption of the permanent teeth. At the age of 10 years old the first temporary removable partial denture was replaced by the second one to accommodate for the growth of the mandible and engage the erupted mandibular permanent teeth. At that time, the permanent first molars were in occlusion, and the incisal edges of the mandibular incisors contacted the lingual surface of the upper incisors at the cingulum. This was an indication to consider that the increase in the vertical height was satisfactory and therefore there was no need for posterior bite blocks incorporated to the second TRPD.
At the age of 14 years old the patient showed with labially positioned left canine because of lack of space and mild maxillary anterior crowding. Interceptive orthodontic treatment was needed to level and align the maxillary teeth and correct the above described malocclusion. Before initiation of orthodontic treatment, a third TRPD was constructed with the additional purpose of providing proper opposing surfaces for the maxillary teeth (Fig. 2d). The occlusal relationship between the TRPD teeth and opposing dentition was closely monitored during orthodontic treatment. After the end of orthodontic treatment, a Hawley retainer was delivered to the patient (Fig. 2e). At the same time it was decided that the existing (third) denture (Fig. 2e) should be replaced once more for stability reasons. The fourth TRPD, was applied when the patient was 15 years old and was fabricated with a cast metal frame maximizing its retention potential (Fig. 2f). All clasps followed natural undercuts; thus no tooth modifications were needed.
Third Oral and Maxillofacial Treatment Intervention
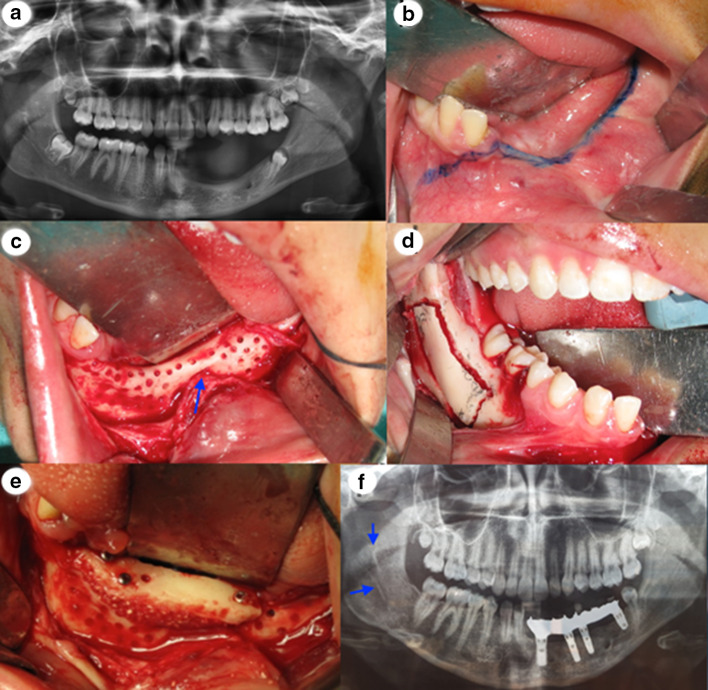
At the age of 17 years old the patient was referred back to the department of Oral and Maxillofacial Surgery in order to augment the height of the atrophic edentulous left side of the mandible. A panoramic radiograph taken at that time showed that the posterior mandible was an excellent donor site for bone harvest and a partially erupted mandibular right third molar, which was removed prior to bone harvest (Fig. 3a). Seven months after the extraction, the bone of the left atrophic mandible was exposed and prepared prior to bone graft harvest. The flap design was an important consideration in bone augmentation and should be well planned prior to surgery. The incision to expose the recipient site was typically made along the residual ridge crest of the mandible, through attached gingiva, as blood vessels do not cross over the ridge crest (lingual vessels). A crest incision maintains vascular supply to the flap. The divergent releasing incision remote to the defect facilitated closure and helped maintain blood supply to the flap. Making the releasing incision one tooth lateral to the graft site is prudent, especially if vertical augmentation is planned (Fig. 3b). The mucoperiosteal reflection was made over a broad area, well beyond the defect margins. This provided a larger flap for advancement over the augmented site. Several perforations of the cortical surface of the bone were performed with a small round bur to release local growth factors and expedite revascularization of the graft (Fig. 3c).
Fig. 3.
a Panoramic X-ray at the age of 16 years. b The incision line to expose the recipient side of the mandible. c Perforation of the cortical bone in the recipient site. The positions of the mental nerve are close to the residual mandibular ridge (arrow). d Intraoral photograph of the osteotomy in the donor site. e Fixation of the block graft to the ridge with three screws. f Panoramic radiograph showing the donor side (arrow) 7 months after the bone block harvest and the placement of the 4 implants
In the donor side the incision was made similar to the one used in sagittal split osteotomy, because we wanted to take a bone block graft. Following the incision, a mucoperiosteal flap was reflected to expose the lateral ramus and body of the mandible. The masseter muscle was reflected laterally with a larger retractor to form a large open pocket. The limits of harvesting bone from the ramus and retromolar area are well dictated by clinical access, in addition to the coronoid process, molar teeth and mandibular canal. Four osteotomies were made to harvest a bone block graft (external oblique, superior ramus, anterior body and inferior) (Fig. 3d). The bone volume needed to reconstruct the site determines the length of the osteotomies. A rectangular-like piece of bone 4 mm in thickness was harvested from the donor site area. This morphology was well suited for veneer grafting to gain additional ridge width.
The osseous recipient bed was prepared to improve the fit and contact of the bone block graft to the ridge. The block graft was fixed to the ridge with three screws (Fig. 3e). After 7 months the fixations screws were removed and four implants were placed by the same maxillofacial surgeon team. This time period allowed the bone graft to remodel and be incorporated adequately, while also enabling implant placement in satisfying prosthetic positions. A panoramic X-ray taken 7 months after the bone block harvest and the placement of the 4 implants with the metal framework of the final restoration showed that the second premolar, that by chance «escaped» the tumor exertion, was completely erupted distally to the final restoration (Fig. 3f).
Fourth Prosthodontic Treatment Intervention
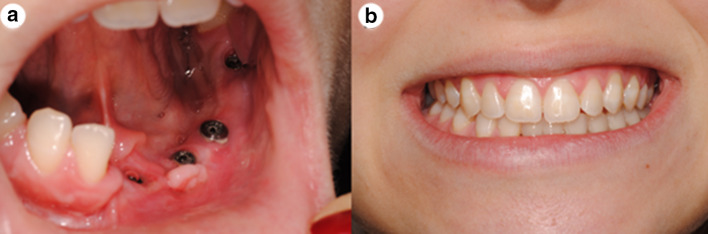
Six months later a permanent fixed prosthetic restoration was placed by the department of Prosthodontics (Fig. 4a). The final result was satisfactory both from functional as from esthetic aspects (Fig. 4b).
Fig. 4.
a Good hard and soft tissue contours 15 days after revelation of the implants. b Definite restoration
Discussion
The melanotic neuroectodermal tumor of infancy (MNTI) is a clinically distinctive, benign neoplasm of neuroectodermal origin [3]. Most often it manifests in infants less than 1 year old, although there have also been cases reporting its occurrence in older children [6]. The lesion arises predominantly in the head and neck region (93%) and most commonly in the anterior maxilla (68–80%). Followed by skull (15.6%) and the mandible (7.8%) and 14.3% have been found in other anatomical areas [6].
The origin of the tumor from the neural crest has been established and is widely accepted. It is based on evidence indicating features of immature neuroblast and melanin producing cells, both of which are embryologically derived from the neural crest [5].
Although MNTI is a clinically distinctive, benign neoplasm in most cases, it can still exhibit rapid local growth, infiltrating both bone and soft tissue of surrounding structures [6, 16].
There have also been rare cases of malignant MNTI reported in the literature accounting for approximately 6.5% of the cases [16]. The few cases of so-called malignant MNTI that have recurred or metastasized have probably represented neuroblastomas, which are the most common early childhood malignancy and the fourth most common malignancy in the head and neck area. Other infancy tumors with aggressive behavior and possible blue coloration are rhabdomyosarcomas, which have a predilection for the head and neck area in children, and hemangiomas or lymphangiomas, which indeed may present with a bluish color and/or often appear within a few months after birth, with rapid development [5, 6]. The MNTI is never congenital but will emerge sometime within the first year of life and usually at less than 6 months of age, or 2 months, or less like our case. The rapid growth raises the concern of a malignancy. It is not uncommon to see the mass double in size in 1 week as in our case. About 10% to 15% of MNTIs will elaborate vanillylmandelic acid (VMA or 3-methoxy-4-hydroxy mandelic acid), which is a soluble metabolic breakdown product of norepinephrine. It is indicative of the neuroectodermal cell origin of this tumor [14]. VMA levels from a 24-h urine collection may be compared to normal values but have no real diagnostic value. Increased VMA levels may imply a neuroectodermal origin, but normal VMA levels do not rule out a neuroectodermal cell origin, because not all the cells are involved in norepinephrine synthesis. VMA levels do not seem to correlate with biologic behavior, but aneuploidy has been associated with an increased risk of recurrence [12, 16, 17].
Microscopically MNTIs are biphasic tumors (two types of cells) and composed of larger epithelioid, (polygonal) melanin-containing cells (intracellular melanin granules) and smaller round, neuroblast-like cells, arranged in nest or cord arrangements, set in a variably vascularized fibrous stroma [5, 6, 12]. Both cell types are positive for neurospecific enolase, vimentin, and s-100 protein. The larger cells are cytokeratin positive and HMB (human melanoma block)-45 positive, features that are noted in pigmented retinal epithelium. The smaller cells may be positive for synaptophysin and chromogranin, which are neurocrine markers and CD56La neuron cell-adhesion molecule [5, 17].
According to the longest retrospective review data of Rachidi et al. [6], the age of diagnosis appears to be a prognostic indicator of disease recurrence. They found that infants receiving a diagnosis within the first 2 months of life were more likely to have recurrence within 6 months and had a shorter free-disease survival. Infants who received a diagnosis at the age of 4.5 or older had minimal risk of recurrence and infants who received a diagnosis within 2 to 4.5 months of age had an intermediate chance of recurrence [5, 6]. However, a recent, relatively large series of 11 patients from a French cohort questioned that observation [17].
Radiotherapy in combination with resection or radiotherapy in combination with chemotherapy and resection have only been used in a limited number of cases 45. Neoadjuvant chemotherapy and surgical resection are usually reserved for inoperable tumors, when clear surgical margins are not achievable [5, 12].
In conclusion, we have reported an extremely rare case of a mandibular melanotic neuroectodermal tumor of infancy that was successfully treated with enucleation and curettage as first intervention. The second intervention was performed from the Prosthodontics and Orthodontics Departments to improve comfort and esthetics, as well as to prevent further deterioration of the oral structures and function. The third intervention was performed, again, by the same surgeons when the patient was 17 years old and included block bone harvesting from the right mandible area to augment the height of the atrophic left area of the mandible. After 6 months dental implants were placed in the grafted area. During the fourth and final prosthetic intervention the definite restoration was supported by the four implants placed previously in the grafted area.
Funding
No funding was received to assist with the preparation of this manuscript.
Declaration
Conflict of interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Krombecher E. Zur Histogenese und Morphologie der Adamantinome und sonstiger Kiefergeschwulste. Beitr Pathol Anat Allg Pathol. 1918;64:165–197. [Google Scholar]
- 2.Lurie H. Congenital melanocarcinoma, melanotic adamantinoma, retinal anlage tumor, progonoma, and pigmented epulis of infancy. Cancer. 1961;14:1090–1108. doi: 10.1002/1097-0142(196109/10)14:5<1090::AID-CNCR2820140529>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Borello E, Gorlin R. Melanotic neuroectodermal tumor of infancy: a neoplasm of neural crest origin. Cancer. 1966;19:196–206. doi: 10.1002/1097-0142(196602)19:2<196::AID-CNCR2820190210>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Kalcheim C. Neural crest emigration: from start to stop. Genesis. 2018;56:6–7. doi: 10.1002/dvg.23090. [DOI] [PubMed] [Google Scholar]
- 5.Soles B, Wilson A, Lucas D, Heider A. Melanotic neuroectodermal tumor of infancy. Arch Pathol Lab Med. 2018;142:1358–1363. doi: 10.5858/arpa.2018-0241-RA. [DOI] [PubMed] [Google Scholar]
- 6.Rachidi S, Sood A, Patel K. Melanotic neuroectodermal tumor of infancy: a systematic review. J Oral Maxillofac Surg. 2015;73:1946–1956. doi: 10.1016/j.joms.2015.03.061. [DOI] [PubMed] [Google Scholar]
- 7.Kruse-Lösler B, Gaertner C, Bürger H, Seper L, Joos U, Kleinheinz J. Melanotic neuroectodermal tumor of infancy: systematic review of the literature and presentation of a case. Oral Surg Oral Med Oral Pathol. 2006;102:204–216. doi: 10.1016/j.tripleo.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Stowens D, Lin T. Melanotic progonoma of the brain. Hum Pathol. 1974;5:105–113. doi: 10.1016/S0046-8177(74)80104-8. [DOI] [PubMed] [Google Scholar]
- 9.Unsal H, Yalcin M. Melanotic neuroectodermal tumor of infancy in the Maxilla. J Craniofac Surg. 2018;29:28–30. doi: 10.1097/SCS.0000000000003993. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki C, Maeda M, Matsushima N, Takamura M, Matsubara T, Taki W, Takeda K. Melanotic neuroectodermal tumor of infancy in the skull: CT and MRI features. J Neuroradiol. 2007;34:212–213. doi: 10.1016/j.neurad.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Haque S, McCarville MB, Sebire N, McHugh K. Melanotic neuroectodermal tumour of infancy: CT and MR findings. Pediatr Radiol. 2012;42:699–705. doi: 10.1007/s00247-011-2339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piperi E, Rake S, Tosios K, Vasilopoulou E, Rake A, Sandler N, Isaacson T, Sklavounou A, Koutlas I. Mandibular melanotic neuroectodermal tumor of infancy treated conservatively with enucleation. J Craniofac Surg. 2010;21:685–688. doi: 10.1097/SCS.0b013e3181d7f0c5. [DOI] [PubMed] [Google Scholar]
- 13.Maroun C, Khalifeh I, Alam E, Akl P, Saab R, Moukarbel R. Mandibular melanotic neuroectodermal tumor of infancy: a role for neoadjuvant chemotherapy. Eur Arch Otorhinolaryngol. 2016;273:4629–4635. doi: 10.1007/s00405-016-4066-6. [DOI] [PubMed] [Google Scholar]
- 14.Beogo R, Nikiema Z, Traore S, Bouletreau P. Maxillary melanotic neuroectodermal tumor of infancy management: is conservative surgery the best approach? J Craniofac Surg. 2013;24:338–340. doi: 10.1097/SCS.0b013e31828a7c4c. [DOI] [PubMed] [Google Scholar]
- 15.Kotsiomiti E, Kolokitha OE, Lazaridis N. Interim prosthodontic management of surgery-induced dental agenesis: a clinical report of 8 years of treatment. J Prosthodont. 2013;22:408–412. doi: 10.1111/jopr.12013. [DOI] [PubMed] [Google Scholar]
- 16.de Oliveira MG, Thompson L, Chaves A, Rados P, da Silva Lauxen I, Filho M. Management of melanotic neuroectodermal tumor of infancy. Ann Diagn Pathol. 2004;8:207–212. doi: 10.1053/j.anndiagpath.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Marx R, Stern D. Oral and maxillofacial pathology: a rationale for diagnosis and treatment. 2. Chicago: Quintessence Publishing Co, Inc; 2012. [Google Scholar]