Abstract
Aims
Sodium‐glucose cotransporter 2 (SGLT2) inhibitors have been shown to be an effective therapy in improving heart failure outcomes. We conducted a meta‐analysis of randomized controlled trials to evaluate the efficacy of SGLT2 inhibitors in heart failure patients with either a reduced or preserved ejection fraction.
Methods and results
We searched MEDLINE and EMBASE for large (≥1000 patients) randomized controlled trials evaluating the effects of SGLT2 inhibitors compared with placebo in the setting of heart failure until September 2021. Our primary outcome was the composite of heart failure hospitalization and cardiovascular death, and secondary outcomes included all‐cause mortality and total heart failure hospitalizations. We pooled hazard ratios and risk ratios and evaluated risk of bias with the Cochrane Collaboration tool. Four randomized controlled trials (DAPA HF, EMPEROR‐Preserved, EMPEROR‐Reduced, and SOLOIST‐WHF) were included (n = 15 684); two of which evaluated patients with a reduced LVEF, one of which evaluated patients with a preserved LVEF, and one of which included both. Treatment with SGLT2 inhibitors resulted in a significant reduction in the composite of CV death and heart failure hospitalization (HR: 0.76, 95% CI: 0.70, 0.82, I 2: 0%, P < 0.00001). This was consistent in sub‐groups of patients with LVEF ≤40% (n = 9199, HR: 0.74, 95% CI: 0.68, 0.81, I 2: 0%) and LVEF >40% (n = 6482, HR: 0.78, 95% CI: 0.68, 0.89, I 2: 0%, P‐for‐interaction: 0.57), as well as in sub‐groups of patients with and without diabetes mellitus at baseline (P‐for‐interaction: 0.81). SGLT2 inhibitors were associated with a significant reduction in cardiovascular death (HR: 0.87, 95% CI: 0.79, 0.97, I 2: 0%, P < 0.00001) and total heart failure hospitalization (RR: 0.71, 95% CI: 0.67, 0.76, I 2: 0%, P < 0.00001); although a potential trend towards reduced all‐cause mortality was noted with SGLT2 inhibitors, no statistically significant difference was observed (HR: 0.91, 95% CI: 0.83, 1.00, I 2: 14%, P = 0.05).
Conclusions
Sodium‐glucose cotransporter 2 inhibitors reduce cardiovascular death and heart failure hospitalization among patients with heart failure, regardless of LVEF status.
Keywords: SGLT2i, Diabetes, HFrEF, HFpEF
Background
There are limited evidence‐based therapeutic options for patients with heart failure (HF) with a preserved left ventricular ejection fraction (LVEF). 1 , 2 Sodium‐glucose cotransporter 2 (SGLT2) inhibitors have been demonstrated to be an effective therapy for reducing HF outcomes across a broad spectrum of patients, including those at a high cardiovascular (CV) risk, renal impairment, and HF patients with a reduced LVEF. 3 , 4 , 5 , 6 , 7 , 8 Recent randomized controlled trials (RCTs) have evaluated the effects of SGLT2 inhibitors in HF patients with a preserved LVEF. 9 , 10
Aims
We performed a systematic review and meta‐analysis of large randomized controlled trials to assess whether SGLT2 inhibitors improved outcomes in all HF patients, including non‐diabetics and those with a preserved LVEF.
Methods
Registration
The protocol for this systematic review and meta‐analysis was not registered. The data underlying this article are available in the article and in its online supplementary material.
Search strategy and selection criteria
We searched MEDLINE and EMBASE from inception until September 2021 using groups of keywords for SGLT2 inhibitors and a validated filter to exclude reports that were not RCTs. 11 A copy of the search strategy can be found in Supporting Information, Table S1 . We screened references of eligible papers and consulted experts to identify additional trials. All studies were screened independently and in duplicate by A. K. P., N. K. D., and M. H. We only included large RCTs (≥1000 patients) evaluating SGLT2 or dual SGLT1/2 inhibitors in the setting of HF (regardless of ejection fraction or other co‐morbidities such as diabetes mellitus).
Outcomes
The primary outcome was the composite of HF hospitalization and cardiovascular death. Secondary outcomes included total HF hospitalizations, all‐cause mortality, and cardiovascular death.
Data extraction and quality assessment
Two reviewers independently extracted data on study characteristics, baseline patient demographics, intervention, and outcomes. Risk of bias was assessed using the Cochrane Collaboration Tool independently and in duplicate. 12 The following domains were evaluated: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, and selective reporting. For analysis and presentation purposes, risk of bias was dichotomized as high (or likely high) or low (or likely low). Reviewers compared results and resolved disagreements by discussion with a third party.
Data synthesis
We used hazard ratios to report effect estimates for the composite of HF hospitalization and cardiovascular death, all‐cause mortality, and cardiovascular death. We used risk ratios to report effect estimates for total HF hospitalizations. All analyses followed the intention‐to‐treat principle.
We assessed clinical and methodological heterogeneity based on study characteristics. We assessed heterogeneity qualitatively by evaluating overlapping of CIs and quantitatively by using the I 2 statistic. 12 We pooled hazard ratios using the generic inverse variance method. 12 We pooled risk ratios using the random‐effects models with Mantel–Haenszel weighting because we expected comparisons to show heterogeneity. HR for the composite of HF hospitalization or cardiovascular death were pooled using the generic inverse‐variance method. All analyses were conducted using RevMan 5.4.1. We considered a P‐value of <0.05 (two‐sided) to be statistically significant.
Subgroups (a priori)
We performed pre‐specified subgroups analysis to compare the effects of SGLT2 inhibitors on the primary outcome (HF hospitalization and cardiovascular death) among patients with reduced vs. preserved LVEF as well as in diabetics vs. non‐diabetics. We performed a standard test for heterogeneity to examine interaction between subgroups.
Results
The electronic search identified 5916 references. After removal of duplicate records as well as title/abstract and full‐text screening, four met eligibility criteria (Supporting Information, Figure S1 ). 7 , 8 , 9 , 10 Details on included studies are presented in Table 1 .
Table 1.
Baseline demographics of patients enrolled in landmark SGLT2 heart failure trials
| DAPA‐HF (n = 4744) | EMPEROR‐Preserved (n = 5988) | EMPEROR‐Reduced (n = 3730) | SOLOIST‐WHF (n = 1222) | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 66 | 72 | 67 | 69 |
| Female sex | 23% | 45% | 24% | 34% |
| White | 70% | 76% | 70% | 93% |
| Black | 5% | 4% | 7% | 4% |
| Asian | 24% | 14% | 18% | 1% |
| Other/unknown | 1% | 6% | 5% | 2% |
| Co‐morbidities/Clinical Characteristics | ||||
| Diabetes mellitus | 42% | 49% | 50% | 100% |
| NYHA class III/IV | 32% | 18% | 25% | 50% |
| Mean/median LVEF, % | 31% | 54% | 27% | 35% |
| LVEF ≤40% | 100% | 0% | 100% | 59% |
| Mean/median BMI, kg/m2 | 28.1 | 29.8 | 27.9 | ~31 |
| Heart failure medications | ||||
| RAAS Inhibitor | 94% | 81% | 89% | 91% |
| Beta‐Blocker | 96% | 86% | 95% | 92% |
| MRA | 71% | 37% | 71% | 64% |
| ARNI | 11% | 2% | 19% | 17% |
Abbreviations: ARNI, angiotensin receptor‐neprolysin inhibitor; BMI, body mass index; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; RAAS, renin–angiotensin–aldosterone system.
Risk of bias assessment
All of the trials were randomized, double‐blinded placebo controlled trials. All studies were judged to be at a low/likely low risk of bias in all domains of the Cochrane Risk of Bias tool, except for SOLOIST‐WHF study which was judged to be at a likely high risk of both Selective Reporting bias due to endpoint switching, as well as Blinding of Outcome/Assessment bias due to a failure to adjudicate events; both were due to a result of loss of sponsor funding. 10
Outcomes
Outcomes for the entire cohort analysed in the present meta‐analysis are presented in Figure 1 . Pooled data from DAPA HF, EMPEROR‐Preserved, EMPEROR‐Reduced, and SOLOIST‐WHF (n = 15 864) demonstrated a significant reduction in the primary composite outcome of CV death and first HF hospitalization (HR: 0.76, 95% CI: 0.70, 0.82, I 2: 0%, P < 0.00001). A sensitivity analysis excluding SOLOIST‐WHF yielded similar results (HR: 0.76, 95% CI: 0.71, 0.82, I 2: 0%, P < 0.00001).
Figure 1.
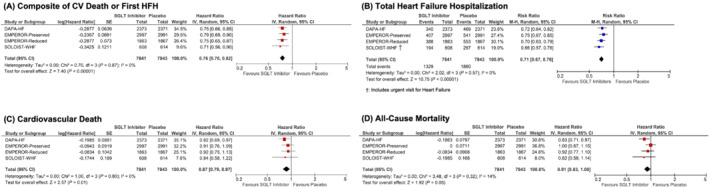
Forest plot demonstrating clinical outcomes between patients on SGLT2 inhibitors vs. placebo in randomized controlled trials in the setting of heart failure. Square markers represent point estimate of HR for individual studies, with square size representing proportional weight given to each study in the meta‐analysis. Horizontal lines indicate 95% CIs. The solid diamonds represent the estimated 95% CI for effect size of all meta‐analysed data. Abbreviations: CI, confidence interval; CV, cardiovascular; HFH, heart failure hospitalization; HR, hazard ratio; RR, risk ratio; SGLT, sodium glucose cotransporter.
Reductions in both components of the composite outcome were observed as well. SGLT2 inhibitors significantly reduced the hazard of cardiovascular death (1461 events, HR: 0.87, 95% CI: 0.79, 0.97, I 2: 0%, P = 0.01) and the risk of total HF hospitalizations (3189 events, RR: 0.71, 95% CI: 0.67, 0.76, I 2: 0%, P < 0.00001). Potential trends towards reductions in all‐cause mortality (HR: 0.91, 95% CI: 0.83, 1.00, I 2: 14%, P = 0.05) and composite renal outcomes, which included chronic dialysis, renal transplantation and sustained decline in estimated glomerular filtration rate, among other renal endpoints (HR: 0.72, 95% CI: 0.48, 1.07, I 2: 67%, P = 0.10, Supporting Information, Figure S2 ) were noted with SGLT2 inhibitors, although no statistically significant differences were observed.
Subgroup analyses
Results of the prespecified subgroup analyses are presented in Figure 2 . Two trials included only patients with a reduced LVEF, one trial included only patients with a preserved LVEF, and one trial included both. Similar reductions in the composite outcome of HF hospitalization and CV death were observed in sub‐groups of patients with LVEF ≤40% (n = 9199, HR: 0.74, 95% CI: 0.68, 0.81, I 2: 0%, P < 0.00001) and LVEF >40% (n = 6482, HR: 0.78, 95% CI: 0.68, 0.89, I 2: 0%, P = 0.0001; P‐for‐interaction: 0.57).
Figure 2.
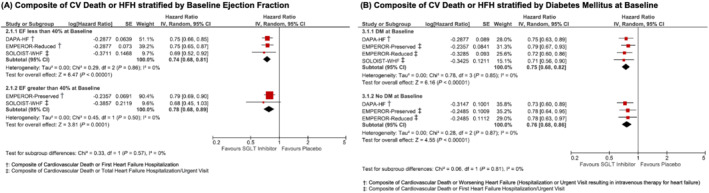
Forest plot demonstrating composite of heart failure hospitalization or cardiovascular death between patients on SGLT2 inhibitors vs. placebo/control in randomized controlled trials stratified by (A) LVEF at baseline and (B) DM status at baseline. Square markers represent point estimate of HR for individual studies, with square size representing proportional weight given to each study in the meta‐analysis. Horizontal lines indicate 95% CIs. The solid diamonds represent the estimated 95% CI for effect size of all meta‐analysed data. Abbreviations: CI, confidence interval; CV, cardiovascular; DM, diabetes mellitus; EF, ejection fraction; HFH, heart failure hospitalization; HR, hazard ratio; RR, risk ratio; SGLT, sodium glucose cotransporter.
Three trials included patients with and without diabetes mellitus, and one trial only included diabetic patients. A similar reduction in the composite outcome of HF hospitalization and CV death was also noted in both diabetics (n = 8155, HR: 0.75, 95% CI: 0.68, 0.82, I 2: 0%, P < 0.00001) and non‐diabetics (n = 7529, HR: 0.76, 95% CI: 0.68, 0.86, I 2: 0%, P < 0.00001; P‐for‐interaction: 0.81).
Conclusions
In this meta‐analysis of large RCTs in the setting of HF, treatment with SGLT2 inhibitors resulted in a significant reduction in cardiovascular death and HF hospitalizations, as well as a potential trend towards a reduction in all‐cause mortality. SGLT2 inhibitors reduced the composite of CV death and HF hospitalizations to a similar degree in patients with a reduced and preserved LVEF, as well as in people with and without diabetes. These data support the notion that SGLT2 inhibitors should be considered broadly in the treatment of HF, irrespective of baseline ejection fraction, glycaemic status or background therapies. The exact molecular mechanisms that underlie the broad benefit observed across ejection fraction remain unclear but may include increased natriuresis, diuresis, EPO release and improved myocardial metabolism, cardio‐renal signalling and changes in cardiac ion‐channels. 13 , 14 , 15 , 16 In patients with and without HF or diabetes, SGLT2 inhibitors have been shown to promote reverse cardiac remodelling. 13 , 14 , 15 , 16 There are several important limitations of this analysis including minor differences in the definition of composite outcomes across studies as well as lack of subgroup data for individual components of the composite outcome. Nevertheless, this investigation represents the most updated analysis to‐date demonstrating the efficacy of SGLT2 inhibitors across the spectrum of HF patients.
Conflict of interest
Arjun K. Pandey, Nitish K. Dhingra, Makoto Hibino, and Vijay Gupta have no conflict of interest. Subodh Verma holds a Tier 1 Canada Research Chair in Cardiovascular Surgery. He has also received grants and personal fees for speaker honoraria and advisory board participation from AstraZeneca, Bayer, Boehringer Ingelheim, Janssen, Amgen, HLS, Merck, Novartis, Sun Pharmaceuticals, Toronto Knowledge Translation Working Group, Phase Bio. He also serves as President of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not‐for‐profit physician organization.
Supporting information
Table S1. Search Strategy for MEDLINE Database.
Figure S1. PRISMA Flow Diagram.
Figure S2. Forest plot demonstrating composite renal outcomes between patients on SGLT2 inhibitors versus placebo/control in randomized controlled trials.
Pandey, A. K. , Dhingra, N. K. , Hibino, M. , Gupta, V. , and Verma, S. (2022) Sodium‐glucose cotransporter 2 inhibitors in heart failure with reduced or preserved ejection fraction: a meta‐analysis. ESC Heart Failure, 9: 942–946. 10.1002/ehf2.13805.
References
- 1. Pagel PS, Tawil JN, Boettcher BT, Izquierdo DA, Lazicki TJ, Crystal GJ, Freed JK. Heart failure with preserved ejection fraction: a comprehensive review and update of diagnosis, pathophysiology, treatment, and perioperative implications. J Cardiothorac Vasc Anesth 2021; 35: 1839–1859. [DOI] [PubMed] [Google Scholar]
- 2. Del Buono MG, Iannaccone G, Scacciavillani R, Carbone S, Camilli M, Niccoli G, Borlaug BA, Lavie CJ, Arena R, Crea F, Abbate A. Heart failure with preserved ejection fraction diagnosis and treatment: an updated review of the evidence. Prog Cardiovasc Dis 2020; 63: 570–584. [DOI] [PubMed] [Google Scholar]
- 3. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RH, Bhatt DL. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. The Lancet 2019; 393: 31–39. [DOI] [PubMed] [Google Scholar]
- 4. Seidu S, Kunutsor SK, Cos X, Gillani S, Khunti K. SGLT2 inhibitors and renal outcomes in type 2 diabetes with or without renal impairment: a systematic review and meta‐analysis. Prim Care Diabetes 2018; 12: 265–283. [DOI] [PubMed] [Google Scholar]
- 5. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta‐analysis of the EMPEROR‐reduced and DAPA‐HF trials. The Lancet. 2020; 396: 819–829. [DOI] [PubMed] [Google Scholar]
- 6. McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZ, Dagogo‐Jack S, Pratley R, Greenberg M, Wang S, Huyck S, Gantz I. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta‐analysis. JAMA Cardiol 2021; 6: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McMurray JJ, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M. Dapagliflozin in patients with heart failure and reduced ejection fraction. New England Journal of Medicine 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 8. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W. Cardiovascular and renal outcomes with empagliflozin in heart failure. New England Journal of Medicine. 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 9. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner–La Rocca HP, Choi DJ, Chopra V, Chuquiure‐Valenzuela E, Giannetti N. Empagliflozin in heart failure with a preserved ejection fraction. New England Journal of Medicine 2021. [Google Scholar]
- 10. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH. Sotagliflozin in patients with diabetes and recent worsening heart failure. New England Journal of Medicine. 2021; 384: 117–128. [DOI] [PubMed] [Google Scholar]
- 11. McKibbon KA, Wilczynski NL, Haynes RB, Team H. Retrieving randomized controlled trials from Medline: a comparison of 38 published search filters. Health Information & Libraries Journal 2009; 26: 187–202. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JP. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0. [updated 2011]. 2011. [Google Scholar]
- 13. Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co‐transporter 2 (SGLT2) inhibitors: a state‐of‐the‐art review. JACC: Basic to translational science 2020; 5: 632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, Zuo F, Quan A, Farkouh ME, Fitchett DH, Goodman SG. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA‐HEART CardioLink‐6 randomized clinical trial. Circulation 2019; 140: 1693–1702. [DOI] [PubMed] [Google Scholar]
- 15. Mazer CD, Hare GM, Connelly PW, Gilbert RE, Shehata N, Quan A, Teoh H, Leiter LA, Zinman B, Jüni P, Zuo F. Effect of empagliflozin on erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation 2020; 141: 704–707. [DOI] [PubMed] [Google Scholar]
- 16. Wanner C, Marx N. SGLT2 inhibitors: the future for treatment of type 2 diabetes mellitus and other chronic diseases. Diabetologia 2018; 61: 2134–2139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search Strategy for MEDLINE Database.
Figure S1. PRISMA Flow Diagram.
Figure S2. Forest plot demonstrating composite renal outcomes between patients on SGLT2 inhibitors versus placebo/control in randomized controlled trials.


