Abstract
Aims
This study aims to investigate the acute haemodynamic effects of percutaneous transluminal flow regulation (PTCR®) with an inferior vena cava regulator balloon in heart failure patients. Preload reduction in heart failure has been achieved with high potency diuretics. However, no study has been conducted in humans to assess the effect of inferior vena cava intermittent occlusion for preload reduction.
Methods and results
Six patients were included in the study: four men (55 ± 6 years old) and two women (63 ± 4 years old). Baseline evaluations included Doppler echocardiogram, coronary angiogram, and right heart catheterization. Caval balloon was kept inflated for 30 min, and right catheterization and control echocardiogram were performed while the balloon was still inflated. The balloon was then deflated and removed. Right haemodynamic variables were evaluated before balloon insertion and with the inflated balloon. The mean right atrial pressure decreased by 42.59% (P = 0.005); systolic right ventricular pressure decreased by 30.19% (P < 0.003); mean pulmonary arterial pressure decreased by 25.33% (P < 0.043); mean pulmonary capillary wedge pressure decreased by 31.37% (P > 0.016); and cardiac output increased by 9.92% (P < 0.175).
Conclusions
The haemodynamic and echocardiographic changes obtained in our study using PTCR® suggest that this innovative approach can play a beneficial role in the heart failure treatment.
Keywords: Dynamic stenosis, Dynamic regulation, Heart failure, Inferior vena cava, Percutaneous transluminal caval flow regulation PTCR®, Caval flow regulation natural phenomenon
Introduction
Previous studies indicate that hypervolaemia seen in patients with heart failure with reduced ejection fraction (HFrEF) results in increased pulmonary capillary pressure, pulmonary congestion and LV filling pressures. These changes contribute to worsening symptoms and disease progression in patients with HFrEF. 1 Volume overload (hypervolaemia), leads to haemodynamic congestion with increased central filling pressure and eventual development of symptomatic clinical pulmonary congestion. 2 Furthermore, hypervolaemia is present in 90% of hospitalized patients with acute heart failure and chronic re‐acute heart failure. 3 We have previously reported a novel method to reduce preload mechanically in patients with congestive heart failure, using dynamic regulation of flow in the inferior vena cava (PTCR). 4 PTCR significantly reduces pulmonary capillary pressure to normal levels, normalizing pulmonary arterial pressure and maintaining normal cardiac output. 4 We hypothesize that the regulation of hypervolaemia (mechanical regulation of preload) achieved with PTCR could result in a decrease of capillary pulmonary pressure, pulmonary congestion, and decrease in filling pressures, LV and RV volume overload. The purpose of this study was to evaluate the safety and feasibility of the regulation of hypervolaemia with subtotal cyclic balloon occlusion of the inferior vena cava (IVC) (PTCR) as an innovative emerging therapy to treat patients with HFrEF.
Definitions and how the partial caval balloon regulation (percutaneous transluminal caval flow regulation) works?
Caval flow regulation is a natural phenomenon (CFRNP) (Figure 1 ) discovered by our group. This phenomenon, which has a prevalence of 0.5%, regulates IVC flow, reducing hypervolaemia in the cases of HF. CFRNP is guided by respiratory phases producing total occlusion during inspiration and subtotal occlusion during expiration. 4
Figure 1.
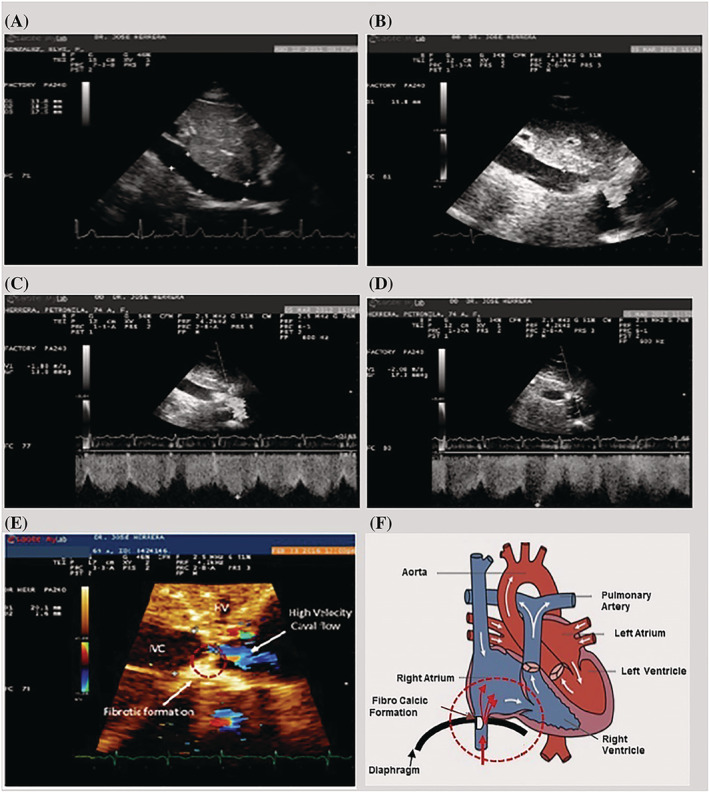
Caval flow regulation natural phenomenon (CFRNP) or dynamic stenosis of the IVC. (A) Normal IVC in long‐axis view. (B) Abnormal IVC and dynamic restriction of the IVC flow. (C) Echo image showing IVC dynamic stenosis in expiration; the flow velocity is 1.8 m/s, which corresponds to 13.8 mmHg gradient. (D) Echo image showing IVC dynamic stenosis during inspiration; the flow velocity is 2.08 m/s, which corresponds to 17.3 mmHg gradient. (E) Important features of caval stenosis. (F) Schematic representation of the dynamic stenosis of the IVC.
Percutaneous transluminal caval flow regulation (PTCR) 5 , 6 (Figure 2 ) is a percutaneous therapy designed to reduce hypervolaemia and therefore total cardiac burden. PTCR achieves regulation of caval flow and hypervolaemia by cyclical regulation of the flow from the IVC to the right atrium (refer to Supporting information, Movie S1 ).
Figure 2.
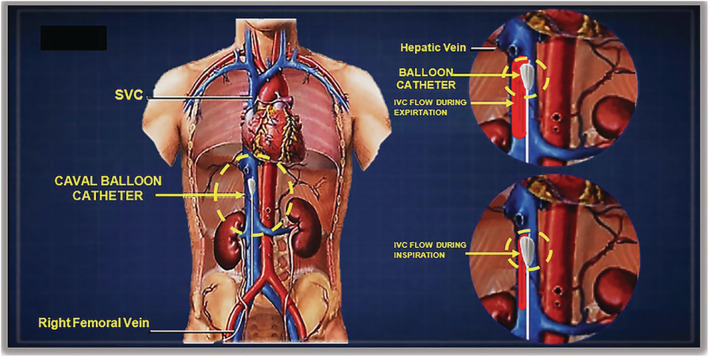
Percutaneous transluminal caval flow regulation PTCR, via femoral vein for treating acute heart failure.
Methods
All patients met the inclusion criteria established by the approved protocol. The inclusion criteria included (i) ≥18 years old patients (both genders) who underwent coronary angiography; (ii) patients with signs of angina and HF who were willing to participate in the study; (iii) patients who showed signs and symptoms of HF and ejection fraction (EF) of <40% and high pro‐brain natriuretic peptide (≥200 pg/dL); (iv) patients who were receiving medical treatment for HF; and (v) all patients were required to sign an informed consent form.
The exclusion criteria included (i) patients on renal dialysis; (ii) patients on ultrafiltration; (iii) patients in hypovolaemic shock; (iv) patients on biventricular artificial stimulation; (v) patients with pleural or pericardial effusion; (vi) patients with valvular heart disease; (vii) patients with acute infection; (viii) patients who received chemotherapy; and patients with abnormal renal function.
Patients' selection
Ten patients met all the protocol requirements, but one patient presented moderate pulmonary congestion after coronary angiography, which needed emergency treatment; hence, balloon caval implantation was aborted. Another patient was excluded because presented with renal complications, and the implantation was aborted because of the contraindication to the use of contrast agent for angiography. Finally, two patients were excluded because they did not sign the informed consent. The flow regulating balloon caval was implanted in the remaining six patients that complied with the criteria and signed the consent forms. They were the first six patients who underwent this innovative procedure.
Patients were recruited for 1 year according to the protocol, inclusion criteria, and signed informed consent. Baseline Doppler echocardiogram, coronary angiography, and right cardiac catheterization with a Cournand catheter via the brachial vein for evaluating right cardiac pressure (first condition before balloon insertion) were performed. The caval balloon was then inserted via the femoral vein and was advanced into the IVC up to a point just before the hepatic vein drainage.
According to previous calculations of the IVC diameters and inspiratory collapse (breath in) of 20–30% in normal patients, the size of the balloon was calculated based on the following formula:
For example, if the expiratory diameter is 20 mm (100%) and the inspiratory collapse reduces 6 mm (30%), then the balloon diameter must be 14 mm.
In summary, the balloon's diameter is related to the diameter of the IVC during inspiration produced by the inspiratory collapse.
The balloon was kept inflated for 30 min. This technique resulted in intermittent occlusion of the IVC during the respiratory expiratory phase. Measurement of the right heart pressures and Doppler echocardiography were repeated. After 30 min of intermittent IVC occlusion, the balloon was deflated, and the Cournand catheter removed from the patient.
Efficacy and safety evaluations
Co‐primary endpoints were the percentage change of >20% between the baseline pre‐procedure values of the haemodynamic data (mean right atrial pressure, systolic right ventricular pressure, pulmonary artery pressure, and pulmonary capillary wedge pressure) and the values obtained during the procedure.
Safety endpoints included hypovolaemic shock, hypotension, femoral vein bleeding, and serious adverse events, such as thrombosis, migration of the balloon or catheter, and embolization of the balloon or catheter.
Doppler echocardiography
Standard echocardiographic images were obtained using the Philips iE33 ultrasound system (Philips Ultrasound, Inc. Company Profile, Bothell, WA, USA) in lateral decubitus position for parasternal view, apical four‐chamber view, and apical two‐chamber view. Measurements were taken according to the convention of the American Society of Echocardiography (ASE). According to the ASE norms, left ventricular volumes and the EF were calculated using Simpson's biplane method and M‐mode. Left ventricular filling was evaluated by placing a Doppler sample volume or cursor at both ends of the mitral valve; three measurements were acquired and averaged to have a more reliable value. Tricuspid regurgitation was expressed in degrees from I to IV according to maximum regurgitation rates obtained using continuous Doppler. The cardiac output was calculated by obtaining the stroke volume by subtracting the mean value of the final diastolic volume of the left ventricle minus the mean value of the final systolic volume multiplied by the mean value of the heart rate.
Inferior vena cava echocardiography
Inferior vena cava images were obtained in the decubitus position with bent knees; in the subcostal view on the long axis, IVC diameter was measured during expiration and inspiration for obtaining the collapsibility index. The measurements were taken 2 cm away from the right atrium. IVC flow velocity was evaluated using pulsed Doppler at the entry of the IVC into the right atrium.
Right heart catheterization
Catheterization was performed following the Seldinger technique. The Cournand catheter first reached the right brachial vein, then advanced into the right atrium, then the right ventricle, and the pulmonary artery. Catheterization was done before regulating IVC flow with the balloon (first condition) and during the regulation of IVC flow (second condition).
Percutaneous transluminal caval flow regulation
The PTCR was performed after right catheterization. Access was through the right femoral vein following the Seldinger technique, advancing through the IVC, and then reaching the IVC's proximal end before the drainage of the hepatic vein (Figure 2 ). At this point, the balloon was inflated with a 0.9% saline solution to the desired degree of occlusion according to previous calculation and the IVC's diameter during inspiration. The balloon stayed in place floating freely for 30 min, after which right atrial pressure, right ventricular pressure, and capillary wedge pressure (from the right catheterization) were newly measured. Later, another echocardiogram was performed for evaluating echocardiogram variables and for comparing them with the baseline measurements. After the measurements, the balloon was deflated and removed. 5 , 6
The balloon catheter used for the regulation of the IVC was an OTC balloon. This catheter is a double lumen occlusion balloon catheter intended for temporary occlusion of the coronary sinus during a venogram or infusion of contrast media or drug. The technical characteristics of the balloon catheter device used in this investigation were 6 Fr catheters of 2 mm diameter, maximum recommended balloon diameter of 15 mm, maximum balloon capacity of 2 mL, and catheter length of 80 cm.
Statistical analysis
Due to the small sample size of six patients, the statistical analyses used were hypothesis tests applied for the paired data, specifically nonparametric tests such as sign tests and median statistical tests for determining the significant differences between the average values of the haemodynamic and echocardiographic variables pre‐established in the protocol before and after receiving caval flow regulation treatment with a balloon catheter.
All analyses were performed using the StatGraphics Plus statistical computation package (Corporate Headquarters, The Plains, VA, USA), with a significance level of 5% (alpha = 0.05).
Ethical consideration
The study protocol was approved by the institutional review board and ethics committee of the Ascardio. Each patient signed an informed consent form to comply with the principles of the Declaration of Helsinski on human research. The study was developed in Western Cardiological Association Ascardio, a national reference cardiovascular centre in Barquisimeto, Venezuela.
Results
Of the six patients who underwent coronary angiography, four had HF from ischaemic causes and two from non‐ischaemic causes. Baseline parameters were considered the first condition, and the second condition involved parameters measured during PTCR while reducing the preload. The evaluation of haemodynamic parameters in the first and second conditions showed a significant reduction in the mean right atrial pressure from 9 mmHg pre‐balloon first condition to 5.17 mmHg during partial occlusion balloon second condition, which indicated a −42% reduction (P ≤ 0.005) (Table 1 ). Systolic right ventricular pressure showed a −30.19% reduction (from 44.17 mmHg pre‐balloon first condition to 30.83 mmHg during partial occlusion balloon second condition) (P ≤ 0.003) (Table 1 ). The mean pulmonary artery pressure showed a −25.33% reduction from 37.50 mmHg pre‐balloon first condition to 28.00 mmHg (P ≤ 0.043) during partial occlusion balloon second condition. The mean pulmonary capillary pressure showed a −31.37% reduction from 25.50 mmHg pre‐balloon first condition to 17.50 mmHg (P = 0.016) during partial occlusion balloon second condition (Table 1 ). The cardiac output showed a slight 9.92% increase from 4.09 L/min pre‐balloon first condition to 4.50 L/min during partial occlusion balloon second condition, and it was not statistically significant (P = 0.175) (Table 1 ) (Figure 3 ). Regarding the echocardiographic data, a significant reduction was observed in the end‐diastolic diameter from 6.43 cm pre‐balloon first condition to 5.76 cm during balloon partial occlusion second condition, with a percentage change of −10.45% (P ≤ 0.009) (Table 1 ). The end‐systolic diameter showed a decrease from 5.19 cm pre‐balloon first condition to 4.78 cm during balloon partial occlusion second condition, with a percentage change of −7.84% (P ≤ 0.010) (Table 1 ). The end‐diastolic volume showed a decrease from 187.67 mL pre‐balloon first condition to 160.82 mL during balloon partial occlusion second condition, with a −14.31% of change (P ≤ 0.036) (Table 1 ). The end left ventricle systolic volume (LVSV) showed a decrease from 123.23 mL pre‐balloon first condition to 101.60 mL during balloon partial occlusion second condition, with −17.54% of change (P ≤ 0.041) (Table 1).
Table 1.
Haemodinamic and echocardiographic data results
| Variables | Cases | Mean value | % of change | P value ≤ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||||||||||
| GV | TD | DB | AR | DV | AM | |||||||||||
| Basal pre‐occlusion | During balloon occlusion | Basal pre‐occlusion | During balloon occlusion | Basal pre‐occlusion | During balloon occlusion | Basal pre‐occlusion | During balloon occlusion | Basal pre‐occlusion | During balloon occlusion | Basal pre‐occlusion | During balloon occlusion | Basal pre‐occlusion | During balloon occlusion | |||
| Haemodynamic data results | ||||||||||||||||
| 1.‐mRAP (mmHg) | 5 | 2 | 10 | 6 | 6 | 3 | 13 | 12 | 12 | 6 | 8 | 2 | 9.00 | 5.17 | −42.59 | 0.005 |
| 2.‐sRVP (mmHg) | 16 | 10 | 34 | 17 | 52 | 42 | 61 | 43 | 40 | 35 | 62 | 38 | 44.17 | 30.83 | −30.19 | 0.003 |
| 3.‐mPAP (mmHg) | 24 | 22 | 48 | 28 | 39 | 36 | 47 | 35 | 20 | 19 | 47 | 28 | 37.50 | 28.00 | −25.33 | 0.043 |
| 4.‐mPWP (mmHg) | 16 | 9 | 23 | 17 | 32 | 24 | 27 | 19 | 20 | 19 | 35 | 17 | 25.50 | 17.50 | −31.37 | 0.016 |
| 5.‐CO (l/min) | 2.61 | 2.31 | 4.48 | 5.24 | 3.86 | 4.09 | 3 | 4.4 | 6.106 | 6.141 | 4.48 | 4.79 | 4.09 | 4.50 | 9.92 | 0.175 |
| Echocardiographic data results | ||||||||||||||||
| 1.‐LVEDD, cm | 6.07 | 5.61 | 7.12 | 5.67 | 6.95 | 6 | 5.89 | 5.43 | 7.03 | 6.55 | 5.52 | 5.29 | 6.43 | 5.76 | −10.45 | 0.009 |
| 2.‐LVESD, cm | 4.99 | 4.14 | 5.17 | 4.5 | 6.01 | 5.8 | 4.3 | 3.83 | 6.09 | 5.87 | 4.57 | 4.55 | 5.19 | 4.78 | −7.84 | 0.01 |
| 3.‐LVEDV, ml | 114 | 94.9 | 194 | 148 | 215 | 219 | 134 | 97 | 317 | 271 | 152 | 135 | 187.67 | 160.82 | −14.31 | 0.036 |
| 4.‐LVSV, ml | 57.5 | 52.3 | 103 | 68.4 | 163 | 161 | 89 | 52 | 231 | 182 | 95.9 | 94 | 123.23 | 101.62 | −17.54 | 0.041 |
| 5.‐EF% Simpson | 45 | 40 | 41 | 55 | 25 | 27.6 | 34 | 56.2 | 27 | 33 | 28.91 | 33.3 | 33.49 | 40.85 | 21.99 | 0.115 |
| 6.‐EA ratio. | 1.38 | 1.24 | 2.5 | 2.07 | 2.5 | 2.03 | Atrial fibrillation | 0.8 | 0.8 | 1.43 | 0.7 | 1.72 | 1.37 | −20.56 | 0.051 | |
| 7.‐E/e′´ lateral | 15 | 12.9 | 22.2 | 9.19 | 12.9 | 9.97 | 19.5 | 19.37 | 14.5 | 20.5 | 10.7 | 7.1 | 15.80 | 13.17 | −16.64 | 0.343 |
| 8.‐Mitral TVI, cm | 22.5 | 12.6 | 20.6 | 19.4 | 13.6 | 9.31 | Atrial fibrillation | 17.5 | 14.8 | 21.5 | 16.7 | 19.14 | 11.60 | −39.38 | 0.035 | |
| 9.‐LA diameter cm | 4.49 | 4.14 | 5 | 4.63 | 5 | 4.38 | 4.55 | 4.29 | 5.2 | 4.2 | 4.9 | 4.9 | 4.86 | 4.42 | −8.92 | 0.026 |
| 10.‐IVC flow velocity. m/s | 0.8 | 1.28 | 0.63 | 1.68 | 0.75 | 1.03 | 1.07 | 1.4 | 0.8 | 1.45 | 0.5 | 1.21 | 0.76 | 1.34 | 76.92 | 0.036 |
Statistical significance was defined as P ≤ 0.05.
Haemodinamic variables: 1.‐mRAP, mean right atrial pressure; 2.‐sRVP, systolic right ventricular pressure; 3.‐mPAP, mean pulmonary artery pressure; 4.‐mPCWP, mean pulmonary capillary wedge pressure; 5.‐C.O., cardiac output.
Echocardiographic variables: 1.‐LVEDD, left ventricular end‐diastolic diameter; 2.‐LVESD, left ventricular end‐systolic diameter, 3.‐LVDV, left ventricular diastolic volume; 4.‐LVSV, left ventricular systolic volume, 5.‐EF, ejection fraction, 6.‐EA RATIO, EA mitral waves; 7.‐E/e′ LATERAL, E mitral wave/e′ mitral annulus; 8.‐MITRAL TVI, time velocity integral; 9.‐LA diameter, left atrium diameter; 10.‐IVC flow velocity, inferior vena cava flow velocity.
Figure 3.
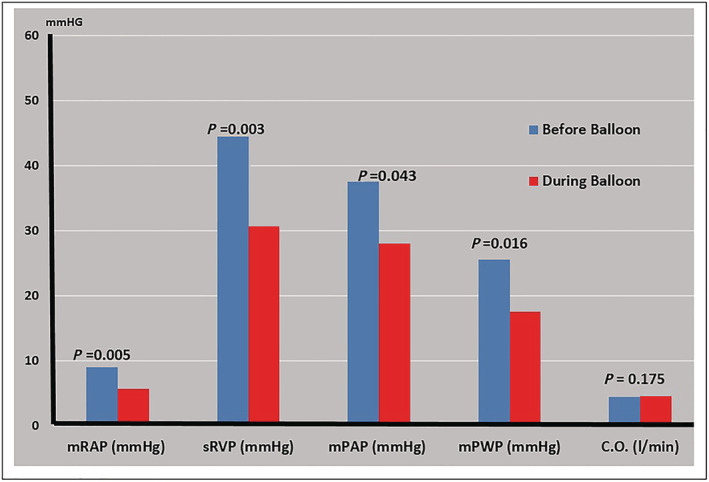
Haemodynamic data results.
The left ventricle ejection fraction slightly increased from 33.49% pre‐balloon first condition to 40.85%, during balloon partial occlusion second condition, with 21.99% of change increase, with no statistical significance (P = 0.115) (Table 1 ). The E/e′ decreased from 15.80 pre‐balloon first condition to 13.17 during balloon partial occlusion second condition, which indicated a −16.64% reduction but with no statistical significance (P ≤ 0.343) (Table 1 ); the E/A ratio of the mitral flow decreased from 1.72 pre‐balloon first condition to 1.37 during balloon partial occlusion second condition (P ≤ 0.051) (Table 1 ). The mitral time velocity integral (TVI) decreased from 19.14 cm pre‐balloon first condition to 11.60 cm during balloon partial occlusion second condition (P ≤ 0.035) (Table 1 ). The diameter AP of the left atrium reduced from 4.86 cm pre‐balloon first condition to 4.42 cm during balloon partial occlusion second condition (P ≤ 0.026) (Table 1 ). IVC flow velocity increased from 0.76 m/s pre‐balloon first condition to 1.34 m/s during balloon partial occlusion second condition with a % of change 76.92% (P ≤ 0.036) (Table 1 ).
Discussion
This study reports the use of PTCR for the first time in humans. This study demonstrated the beneficial impact of PTCR in six patients with congestive HFrEF. PTCR allowed the regulation of preload with the balloon located in the IVC, and this regulation was synchronized with the respiratory phases. This procedure was associated with beneficial haemodynamic changes. PTCR resulted in a significant decrease in the mean right atrium pressure, systolic right ventricular pressure, mean pulmonary artery pressure, and mean pulmonary capillary wedge pressure but with no significant changes in cardiac output. The echocardiographic evaluation showed a significant reduction in the diastolic diameter of the left ventricle (−10.45% average) and the end‐diastolic volume (−14.31% average) (Table 1 ). This demonstrated that the left ventricle is very sensitive to reduction in the preload, as evidenced by the subrogated products of the left ventricular filling pressures (EA and E/e′ ratio). 7 , 8 Furthermore, this reduction in the preload was associated with a −8.92% reduction in the left atrial diameter (P ≤ 0.026) (Table 1 ), leading to a decreased left atrium pressure and pulmonary capillary wedge pressure. There was a significant decrease in mitral time‐velocity integrals during PTCR, 9 which may be explained by the preload regulation caused by the procedure. However, cardiac output did not change because the heart rate increased 22% from 72 beats/minute to 88 beats/minute during IVC flow regulation, thus avoiding the fall in cardiac output. 10
It is worth noting that these results were obtained while performing mild PTCR in which the flow velocity in the IVC during caval flow regulation increased up to 1.34 m/s, and the balloon was inflated in the IVC for just 30 min. This novel PTCR procedure may be useful for both ischaemic and non‐ischaemic cardiomyopathies. It may also be useful for treating patients with congestive HF and impaired renal function, where diuretics fail to improve pulmonary congestion as it is the case for 20–50% of patients with congestive HF who develop resistance or tolerance to diuretics. 11 Further studies are needed for evaluating this technique in a larger cohort of patients with congestive HF (Figure 4 ).
Figure 4.
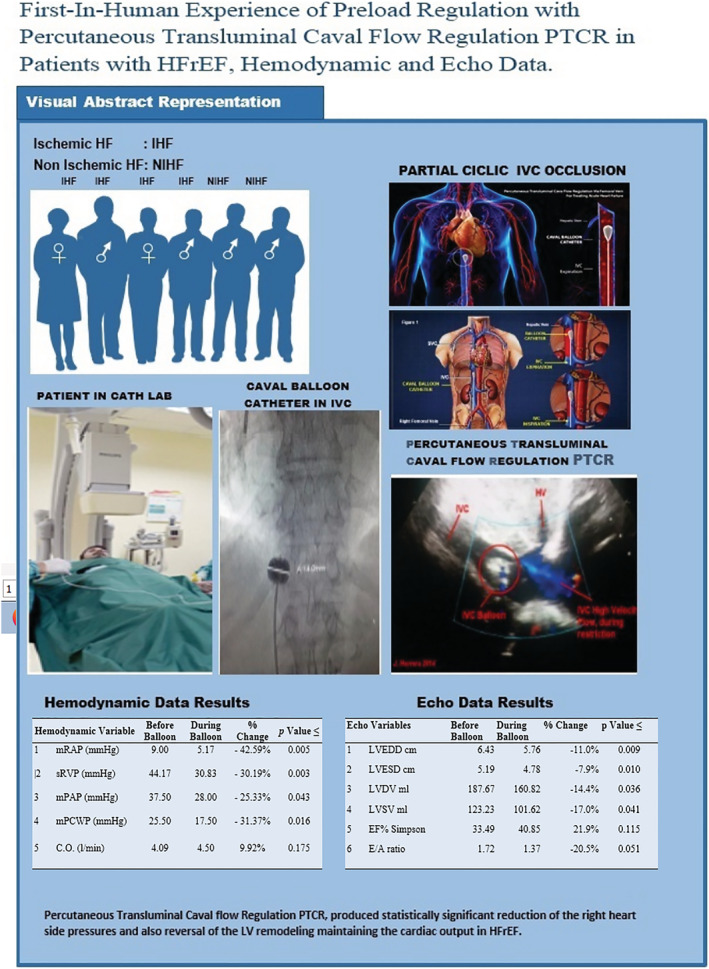
Central figure, visual abstract.
These transient acute haemodynamic changes (Table 1 ) validate our proof of concept concerning this innovative procedure because the procedure leads to the normalization of right cardiac pressures; this could imply an important clinical benefit, such as decreased pulmonary congestion, which is indicative of all forms of HF (acute, chronic, systolic, and diastolic).
The acute echocardiographic changes (Table 1 ) reflect a reversal of the remodelling of the left ventricle and left atrium owing to a decrease in the diameters and therefore the total cardiac burden, bringing the heart closer to its original design with a slight improvement in the EF while maintaining average cardiac output without causing dehydration or electrolyte disturbances.
The main limitation of this study is the small number of patients evaluated. It is therefore recommended to perform further studies involving a larger number of patients.
No complications related to the procedure were observed in our study. There was no impairment of kidney or liver function in the short‐term or long‐term follow‐ups of 2 years.
In conclusion, the haemodynamic and echocardiographic experiences in six patients with HF treated using PTCR with a cyclical reduction in the cardiac preload were reported. The haemodynamic and echocardiographic changes observed in these patients suggest that this innovative approach could help treat patients with HF. Further studies are needed for evaluating this new innovative approach.
Conflict of interest
Igor F. Palacios declares having a relationship with the medical industry Abiomed. Juan A. Marqués declares having a relationship with the medical industry Merck Sharp & Dohme MSD.
Funding
This work was funded by Asociación Cardiologica de Occidente Ascardio.
Supporting information
Movie S1. Supporting information.
Acknowledgements
The authors appreciate the valuable cooperation of the engineers, Amilcar Gomez, PhD, and Joel Jiménez, and Professor Raul Herrera in the preparation of this manuscript.
Herrera, J. E. , Herrera, J. A. , Finizola, B. , García, E. , Velasco, L. E. , Torres, W. R. , D'empaire, G. , Octavio, J. A. , Marqués, J. A. , Levine, R. A. , and Palacios, I. F. (2022) First‐in‐human experience of preload regulation with percutaneous transluminal caval flow regulation in heart failure with reduced ejection fraction patients. ESC Heart Failure, 9: 1118–1126. 10.1002/ehf2.13780.
Subject Term: Cardiology and vascular medicine (Medical Devices to treat heart failure)
References
- 1. Androne AS, Hryniewicz K, Hudaihed A, Mancini D, Lamanca J, Katz SD. Relation of unrecognized hypervolemia in chronic heart failure to clinical status, hemodynamics, and patient outcomes. Am J Cardiol 2004; 93: 1254–1259. [DOI] [PubMed] [Google Scholar]
- 2. Wayne LM. Fluid volume overload and congestion in heart failure: time to reconsider pathophysiology and how volume is assessed. Circ Heart Fail 2016; 9: e002922. [DOI] [PubMed] [Google Scholar]
- 3. Constanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, Jaski BE, Fang JC, Feller ED, Haas GJ, Anderson AS. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007; 49: 675–683. [DOI] [PubMed] [Google Scholar]
- 4. Herrera JE, Herrera JA, Marques JE, Mendoza I. A novel method to reduce preload mechanically in patients with congestive heart failure, the dynamic restriction of flow in the inferior vena cava. Eur J Heart Fail Supplements 2011; 10: 4. https://onlinelibrary.wiley.com/doi/epdf/10.1093/eurjhf/hsr005 [Google Scholar]
- 5. Herrera JE, Herrera JA, Palacios IF. TCT‐428 first percutaneous transluminal caval flow restriction in one patent with heart failure. J Am Coll Cardiol 2014; 64: B125–B126. [Google Scholar]
- 6. Herrera JE, Cubeddu R, Torres W, Velasco L, Finizola B, D'Empaire G, Octavio JA, Garcia E, Herrera JA, Levine R, Palacios IF. TCT‐733 acute hemodynamic effects percutaneous transluminal caval flow intermittent restriction with balloon in ischemic and non‐ischemic heart failure patients. J Am Coll Cardiol 2015; 66: B299. [Google Scholar]
- 7. Bastos MB, Burkhoff D, Maly J, Daemen J, den Uil CA, Ameloot K, Lenzen M, Mahfoud F, Zijlstra F, Schreuder JJ, van Mieghem NM. Invasive left ventricle pressure–volume analysis: overview and practical clinical implications. Eur Heart J 2020; 41: 1286–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pelà G, Regolisti G, Coghi P, Cabassi A, Basile A, Cavatorta A, Manca C, Borghetti A. Effects of the reduction of preload on left and right ventricular myocardial velocities analyzed by Doppler tissue echocardiography in healthy subjects. Eur J Echocardiogr 2004; 5: 262–271. [DOI] [PubMed] [Google Scholar]
- 9. Popović ZB, Desai MY, Buakhamsri A, Puntawagkoon C, Borowski A, Levine BD, Tang WWH, Thomas JD. Predictors of mitral annulus early diastolic velocity: impact of long‐axis function, ventricular filling pattern, and relaxation. Eur J Echocardiogr 2011; 12: 818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bamber JH, Nolas V. The influence of vena cava occlusion on predictive haemodynamic parameters. Anaesthesia 2015; 70: 1209–1210. [DOI] [PubMed] [Google Scholar]
- 11. Gupta R, Testani J, Collins S. Diuretic resistance in heart failure. Curr Heart Fail Rep 2019; 16: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Supporting information.


