Abstract
Aims
Myocardial infarction (MI) is a type of cardiovascular disease caused by myocardial necrosis. Growing evidences have suggested that circular RNAs (circRNAs) play crucial roles in cardiac hypoxia/reoxygenation (H/R)‐induced injury of MI.
Methods and results
Hypoxia/reoxygenation model of H9C2 cells was established and circ_0001206 expression was detected via quantitative real‐time polymerase chain reaction. Ribonuclease R (RNase R) and Actinomycin D (Act D) assays verified the stability. Cell counting kit‐8 (CCK‐8), western blot, TUNEL, and flow cytometry assays evaluated cell viability and cell apoptosis. RNA pull‐down, RNA binding protein immunoprecipitation (RIP), and luciferase reporter assays explored the mechanisms underlying MI. All experimental data were presented with mean ± standard deviation (SD) and P < 0.05 indicated statistical significance. Circ_0001206 was low‐expressed in H9C2 cells under H/R treatment. Circ_0001206 was formed by cyclization of CRK like proto‐oncogene, adaptor protein (CRKL). Circ_0001206 overexpression promoted cell viability and inhibited cardiomyocyte apoptosis. It was confirmed that circ_0001206 regulated CRKL expression via acting as a competing endogenous RNA (ceRNA) of microRNA‐665 (miR‐665). CRKL played a protective role in MI.
Conclusions
Circ_0001206 regulates miR‐665/CRKL axis to alleviate H/R‐induced cardiomyocyte injury in MI. Our findings suggest that circ_0001206 might be a potential target for MI treatment.
Keywords: circ_0001206, miR‐665, CRKL, Myocardial infarction
Introduction
Myocardial infarction (MI) is mainly caused by myocardial necrosis due to acute and persistent ischaemia and hypoxia in coronary arteries. 1 The opening of infarct related arteries has been confirmed to improve the prognosis of patients within 12 h after the onset of MI; after 12 h, the patients are subjected to standard drug treatment or late reperfusion by percutaneous coronary intervention. 2 As for the mechanisms, emerging studies have proved that cardiomyocyte apoptosis plays a key role in cardiac remodelling as well as in heart failure after MI. Thereby, restraining cardiomyocyte apoptosis could exert beneficial effects on MI‐induced cardiac dysfunction and cardiac remodelling process. 3 Tremendous efforts have been made to investigate the molecular mechanisms concerning apoptotic loss of cardiomyocytes, 4 , 5 but the underlying mechanism about cardiomyocyte apoptosis in MI injury remains largely unclear.
Circular RNAs (circRNAs) have been reported to be hot spots in recent researches about the occurrence and development of diverse diseases. 6 CircRNAs are covalently closed transcripts, which are formed by back‐splicing of precursor messenger RNAs (pre‐mRNA) to link a downstream 5′ splice site to an upstream 3′ splice site. 7 Like linear mRNAs, the well‐expressed, stable circRNAs also indicates tissue and developmental stage‐specific characteristics, which implies a variety of potential biological functions. 8 , 9 In recent years, a handful of circRNAs have been suggested to be closely correlated with the progression of cardiovascular disease. 10 Wang et al. have found that hsa_circ_0004104, a new biomarker for coronary artery disease, is aberrantly expressed in patients with coronary artery disease. 11 CircRNA_010567 has been reported to elevate myocardial fibrosis through the suppression of miR‐141 by targeting TGF‐β1. 12 Likewise, many circRNAs function as modulators to regulate the development of MI, including circFndc3b, 13 ACR, 14 and circNFIB. 15
Mechanically, circRNAs can act as a ceRNA to target miRNA, thereby regulating mRNA expression. 16 Jin and Chen have pointed out that knockdown of circ_0010729 prevents human cardiomyocytes from oxygen–glucose deprivation‐induced injury via targeting miR‐145‐5p. 17 Meanwhile, several studies have proved that some miRNAs regulate the apoptosis of cardiomyocytes to exert protective effects on H/R‐induced MI. 18 However, rare evidence supports protective effect of circRNAs on MI development.
In our study, our aim is to investigate the biological role of circ_0001206 in MI. Functional assays were carried out to confirm the function of circ_0001206 in MI. In addition, the ceRNA mechanism of circ_0001206 in MI was also investigated. Globally, our study might suggest novel targets for MI treatment.
Methods
Myocardial infarction in vivo model
Animal study was performed as described in the Guide for the Care and Use of Laboratory Animals, which is published by the US National Institutes of Health. The animal experiments were subjected to the approval by Zhang Ye People's Hospital Affiliated to Hexi University, and conducted as per the protocols. Eight‐week‐old male mice used in the experiments were obtained from Zhang Ye People's Hospital Affiliated to Hexi University. Control group, MI group and sham group were set up to divide the mice. Anesthetization was performed via intraperitoneally injection with liquid isoflurane (55 mL/kg) into the mice. MI in vivo model (MI group) was established via performing the ligation of left anterior descending coronary artery/reperfusion (LAD/reperfusion) by 5/0 silk suture while mice in the sham group were subjected to the same surgery except for occlusion of LAD. Then, mice were sacrificed at 12 or 24 h after LAD ligation and blood samples were immediately collected. Subsequent to the establishment of MI in vivo model, the lentiviral vectors containing pcDNA3.1‐circ_0001206 (YouBio, Guangzhou, China) or the lentivirus containing control pcDNA3.1 (YouBio) were injected into the ventricular cavity of mice. Subsequently, for further analysis, the heart tissues and blood samples were acquired from mice. The cardiac injury was evaluated by haematoxylin and eosin staining.
Cell culture and treatment
Human cardiomyocytes (H9C2) were acquired from ATCC (Manassas, VA, USA). DMEM (Gibco, Rockville, MD, USA), supplemented with 10% fetal bovine serum and 1% antibiotics was adopted for cultivation. In addition, the RNase R (3 U/μg) was purchased from Epicenter Technologies (Madison, WI, USA), and Act D (1 μM) was bought from Sigma‐Aldrich (St. Louis., MO, USA).
Hypoxia/reoxygenation injury model
Based on the requirements for experiments, H9C2 cells were subjected to the treatment with H/R for 1, 6, and 24 h, respectively. The cells were subjected to induction by hypoxia in a modular incubator (Thermo Fisher Scientific, Rockford, USA) under the condition of 1% O2, 94% N2 and 5% CO2 for 6 h. Then, reoxygenation was conducted for 3 h in a modular incubator under the condition of 5% CO2 at 37°C.
Total RNA isolation and quantitative real‐time polymerase chain reaction
Total RNAs were subjected to isolation using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) for complementary DNA (cDNA) synthesis in the presence of PrimeScript Reverse Transcriptase Kit (Takara, Shiga, Japan). Gene expression was determined by quantitative real‐time polymerase chain reaction (RT‐qPCR) with SYBR Green PCR Kit (Takara, Japan), calculated in accordance with 2−ΔΔCt method, with GAPDH or U6 used for standardization. The experiment was implemented three times.
Cell transfection
The specific pcDNA3.1 (+) CircRNA Mini Vector to circ_0001206 (pcDNA3.1‐circ_0001206), CRKL (pcDNA3.1‐CRKL) and empty vectors, shRNAs targeting CRKL (sh‐CRKL) and negative control (sh‐NC), as well as miR‐665 mimics and NC mimics were designed and synthesized by GenePharma (Shanghai, China). Plasmids were transfected into cells for 48 h in the presence of Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA). The experiment was conducted three times.
CCK‐8 assay
H9C2 cells were inoculated in 96‐well plates for plasmid transfection and H/R treatment. Subsequently, 10 μL of CCK‐8 reagent (Sigma‐Aldrich) was added for 2 h of incubation. The absorbance at 450 nm was subjected to measurement using a microplate reader. The experiment was conducted three times.
Terminal‐deoxynucleotidyl transferase‐mediated Nick‐end labelling (TUNEL) assay
After 48 h of transfection, the collected cell samples were rinsed in phosphate buffered saline, fixed by 4% paraformaldehyde, and then permeabilized by Triton X‐100 solution. TUNEL reagent (Merck KGaA, Darmstadt, Germany) was used to stain the apoptotic cells, followed by being imaged using an optical microscopy (Olympus, Tokyo, Japan) as requested. The experiment was conducted three times.
Flow cytometry
Cell apoptosis was also detected by flow cytometry after transfection, as per the supplier's guidebook (BD Biosciences, Franklin Lakes, NJ, USA). Samples were double‐stained in Binding Buffer added with Annexin V/PI staining kit (Invitrogen) for 15 min in darkroom. The apoptotic cells were analysed by a flow cytometer. The experiment was conducted three times.
Western blot
Cells were subjected to RIPA lysis buffer for the extraction of total proteins. Afterwards, extracted proteins were treated with 12% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) for separation, followed by transference onto PVDF membranes. Thereafter, membranes, being blocked with 5% defatted milk, were incubated with the specific primary antibodies against β‐actin (Abcam, 1 μg/mL), Bax (Abcam, 1:1000–1:10000), Bcl‐2 (CST, 1:1000), cleaved caspase‐3 (CST, 1:1000), caspase‐3 (CST, 1:1000), cleaved caspase‐9 (CST, 1:1000) and caspase‐9 (CST, 1:1000) overnight at 4°C and HRP‐tagged secondary antibodies for 2 h at room temperature. The protein signals were monitored via the adoption of enhanced chemiluminescence substrate system (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The experiment was conducted three times.
Subcellular fractionation assay
Subcellular fractionation assay determined the subcellular distribution of circ_0001206 using PARIS Kit (Invitrogen) as guided by the manufacturer's protocols. Cells were lysed by the use of cell fractionation buffer and then centrifuged to isolate cytoplasmic and nuclear fractions. The expression of circ_0001206 in both cellular fractions was subjected to RT‐qPCR for expression analysis. The experiment was conducted three times.
Fluorescent in situ hybridization (FISH) assay
For RNA FISH assay, the fixed cell samples were digested, air‐dried, and then treated with circ_0001206‐specific probe (RiboBio, Guangzhou, China) in hybridization buffer. After phosphate buffered saline washing, samples were counterstained in DAPI solution. A fluorescence microscope (Olympus, Tokyo, Japan) was employed to capture images. The experiment was implemented in triplicate.
RNA pull‐down assay
RNA pull‐down assay adopting Pierce Magnetic RNA‐Protein Pull‐Down Kit (Thermo Fisher Scientific, Waltham, MA, USA) was used to detect RNA interaction. Protein lysates were mixed with streptavidin agarose magnetic beads and wild‐type or mutant‐type biotinylated probes specifically for miR‐665 (Bio‐miR‐665‐WT or Bio‐miR‐665‐Mut). The pulled‐down mixture was analysed by RT‐qPCR after RNA purification and RNA isolation. The experiment was performed three times.
RNA‐binding protein immunoprecipitation (RIP)
Magna RIP™ RNA‐Binding Protein Immunoprecipitation Kit was applied for RIP assay, as per the supplier's manual (Millipore, Bedford, MA). Human Ago2 antibody (anti‐Ago2) and normal mouse IgG antibody (anti‐IgG) were acquired from Abcam. Cells were subjected to lysis in RIP lysis buffer, followed by the incubation in RIP buffer containing antibody‐bound magnetic beads. Afterwards, immunoprecipitated RNAs were subjected to extraction for RT‐qPCR analysis. The experiment was implemented three times.
Luciferase reporter assay
Luciferase reporter assay was implemented to explore the association between circ_0001206 and miR‐665 or between CRKL and miR‐665. Wild‐type or mutated sequence of circ_0001206 or CRKL 3′‐untranslated region (3′UTR) fragments covering miR‐665 binding sites was subjected to insertion into the pmirGLO dual‐luciferase vector (Promega, Madison, WI, USA), named as circ_0001206‐WT/Mut and CRKL 3′UTR‐WT/Mut constructs. Then, luciferase reporter vectors were co‐transfected with miR‐665 mimics or NC mimics into cells using Lipofectamine 3000. The luciferase activities were measured using a Dual‐Luciferase Assay kit (Promega). The experiment was conducted three times.
Statistical analyses
Data were presented with means ± SD of at least three separately conducted assays. Group difference was estimated by Student's t test or one‐way/two‐way ANOVA with the application of GraphPad Prism 7. Besides, data were statistically significant when P value was <0.05.
Results
Circ_0001206 alleviates hypoxia/reoxygenation‐induced cardiomyocyte injury in myocardial infarction
It has been reported that CRKL can mitigate H/R‐induced injuries in cardiomyocytes. 19 To identify circRNA associated with MI, we first utilized circBase database to obtain six circRNAs, which were spliced by host gene CRKL with different gene segments (circ_0062400, circ_0001206, circ_0116241, circ_0062401, circ_0001207, and circ_0062402). Then, we assessed the expressions of candidate circRNAs in H9C2 cells undergoing hypoxia for different time. We set the hypoxia time points at 0, 1, 6, and 24 h as previously reported. 20 As shown in Figures S1 A and 1 A , only circ_0001206 expression was significantly down‐regulated in hypoxia‐treated H9C2 cells and MI mouse model, whereas the expression of other circRNAs had witnessed no obvious change. Notably, circ_0001206 expression was the lowest in H9C2 cells after hypoxia treatment for 6 h. Thus, we selected the cells undergoing 6 h treatment in subsequent experiments. We found that humans expressed an ortholog (92% identities) of circ_0001206 through circBase database (http://www.circbase.org/) (Figure S1 B). Moreover, reoxygenation time was determined based on the previous studies. 21 Cells were subjected to treatment with hypoxia for 6 h and reoxygenated for 3 h. To confirm the presence of circ_0001206, we used RNase R to treat H9C2 cells as well as H/R‐treated H9C2 cells, followed by RT‐qPCR to examine the level of linear‐CRKL and circ_0001206. It was found that the level of linear‐CRKL was declined, but that of circ_0001206 remained stable (Figure 1 B ). The results from an Act D assay further revealed that circ_0001206 was more stable than the linear‐CRKL and its half‐life was more than 12 h (Figure 1 C ). Moreover, we designed two sets of primers to validate the presence of circ_0001206. Divergent primers were applied to amplify circ_0001206 exclusively, and convergent primers were used to amplify linear‐CRKL exclusively. The data from agarose gel electrophoresis showed that circ_0001206 was not amplified by divergent primers using genomic DNA (gDNA) but by cDNA (Figure 1 D ). All abovementioned results confirmed the presence of circ_0001206 in H9C2 cells and H/R‐treated H9C2 cells.
Figure 1.
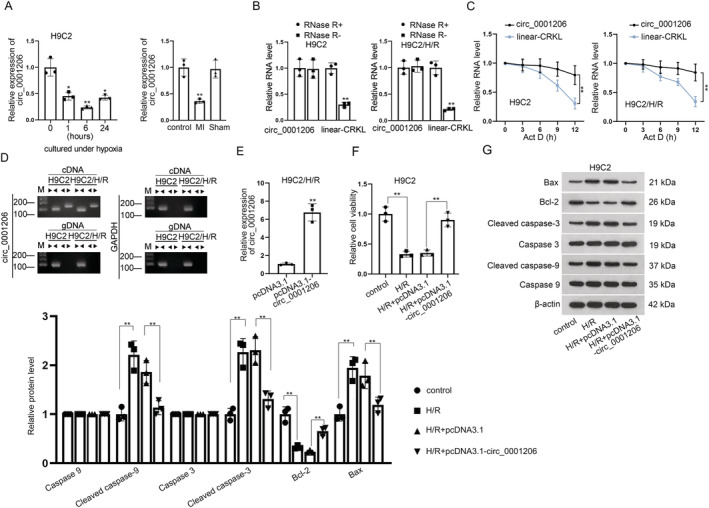
Circ_0001206 alleviates hypoxia/reoxygenation (H/R)‐induced cardiomyocyte injury in myocardial infarction (MI). (A) Expression of circ_0001206 in H9C2 cells was examined via quantitative real‐time polymerase chain reaction (RT‐qPCR) under hypoxia for 0, 1, 6 and 24 h. Expression of circ_0001206 in MI mouse model was detected. (B) RT‐qPCR detected the level of circ_0001206 and linear‐CRKL in RNase R‐treated H9C2 cells. (C) RT‐qPCR examined the stability of circ_0001206 and linear‐CRKL in Act D‐treated H9C2 cells. (D) Circ_0001206 existence was examined in H9C2 cells using RT‐qPCR. Divergent primers amplified circ_0001206 in cDNA but not in genomic DNA (gDNA). (E) RT‐qPCR detected the efficacy of pcDNA3.1‐circ_0001206 in H/R‐treated H9C2 cells. (F) CCK‐8 assay detected the cell viability under H/R treatment in circ_0001206‐overexpressed H9C2 cells. (G) Western blot detected protein levels of Bax, Bcl‐2, cleaved caspase‐3, and cleaved caspase‐9 in H/R‐induced H9C2 cells after the enhancement of circ_0001206 (n = 3). *P < 0.05, **P < 0.01.
To investigate the role of circ_0001206, we up‐regulated the expression of circ_0001206 by transfecting pcRNA3.1 targeting circ_0001206 into H/R‐treated H9C2 cells. As shown in RT‐qPCR, circ_0001206 expression was significantly increased after transfection (Figure 1 E ). Afterwards, functional assays were performed. In CCK‐8 assays, the cell viability was reduced in H/R‐treated H9C2 cells, while this effect was reversed after co‐transfection of pcDNA3.1‐circ_0001206 (Figure 1 F ). It was unearthed by TUNEL assay and flow cytometry analysis that cell apoptosis rate in H/R group was promoted relative to that in the control group, while circ_0001206 overexpression inhibited the effect of H/R on cell apoptosis (Figure S1 C and S1 D). Western blot analysed the impacts of circ_0001206 increase on the levels of apoptosis‐related proteins in H/R‐treated H9C2 cells. It was revealed by the data that Bax, cleaved caspase‐3 and cleaved caspase‐9 levels were enhanced while Bcl‐2 level was decreased after the treatment of H/R in H9C2 cells; however, circ_0001206 up‐regulation could abolish this effect caused by H/R (Figure 1 G ). For further verification, we performed in vivo experiments. The lentiviral vector of pcDNA3.1‐circ_0001206 was injected into the mice ventricular chamber for MI mouse model construction. The efficiency of pcDNA3.1‐circ_0001206 was verified (Figure S2 A). Via haematoxylin and eosin staining, we found that, 12 or 24 h after reperfusion, the enhancement of circ_0001206 significantly attenuated cardiac injuries related to MI (Figure S2 B) and led to the infarct size reduction (Figure S2 C). Furthermore, we performed CCK‐8 and TUNEL assays, finding that circ_0001206 overexpression enhanced proliferation and decreased apoptosis of the cardiomyocytes from mice 12 or 24 h after reperfusion (Figure S2 D– S2 E). Collectively, circ_0001206 alleviates H/R‐induced cardiomyocyte injury in MI.
CRKL plays a protective role in hypoxia/reoxygenation‐induced cardiomyocyte injury
Next, we probed into the potential mechanism of circ_0001206 in MI. Subcellular fractionation and FISH assays were implemented, in order to detect the subcellular localization of circ_0001206 in H9C2 cells and H9C2/H/R cells. As indicated in Figure 2 A and 2 B , circ_0001206 was mainly located in the cytoplasm, implying the post‐transcriptional role of circ_0001206 in MI. It has been reported that circRNA affects the development of MI via regulating its host gene in MI. 22 Thus, we speculated that circ_0001206 might regulate its host gene CRKL to affect the progression of MI. Via RT‐qPCR and western blot analyses, we found that CRKL expression was significantly declined in H9C2/H/R cells, and this effect was then reversed after circ_0001206 enhancement (Figure 2 C ). We performed RT‐qPCR to detect the overexpression efficiency of pcDNA3.1‐CRKL (Figure 2 D ). To confirm the role of CRKL in MI, we up‐regulated CRKL expression level and found that overexpression of CRKL restored the reduced cell viability caused by H/R treatment (Figure 2 E ). Meanwhile, the enhanced apoptosis rate induced by H/R treatment was also offset after co‐transfection of pcDNA3.1‐CRKL (Figure S3 A and S3 B). Moreover, western blot analysis further indicated that CRKL overexpression counteracted the effect of H/R treatment on the lessened protein levels of Bax, cleaved caspase‐3 and cleaved caspase‐9 as well as the enhanced Bcl‐2 level (Figure 2 F ). Collectively, CRKL plays a protective role in MI.
Figure 2.
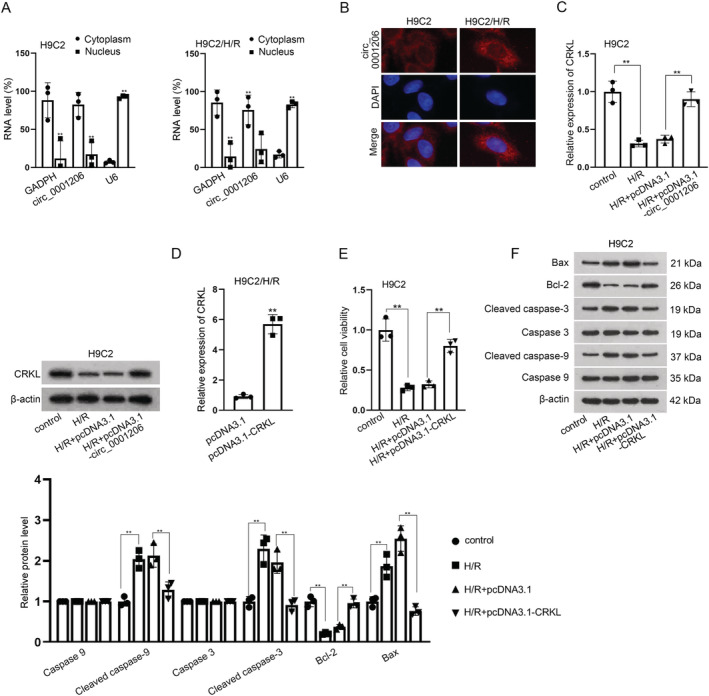
CRKL plays a protective role in H/R‐induced cardiomyocyte injury. (A,B) Subcellular fractionation and FISH assays were conducted to identify the cellular location of circ_0001206. (C) CRKL expression was detected in H/R‐induced H9C2 cells upon circ_0001206 overexpression using quantitative real‐time polymerase chain reaction (RT‐qPCR) and western blot. (D) The efficacy of pcDNA3.1‐CRKL was tested by RT‐qPCR in hypoxia/reoxygenation (H/R)‐treated H9C2 cells. (E) Cell viability was tested in H/R‐induced H9C2 cells upon CRKL overexpression. (F) Western blot detected the protein levels of Bax, Bcl‐3, cleaved caspase‐3, and cleaved caspase‐9 in H/R‐induced H9C2 cells transfected with pcDNA3.1‐CRKL (n = 3). **P < 0.01.
Circ_0001206 regulates CRKL through acting as a ceRNA to target miR‐665
Given that the location of circ_0001206 in the cytoplasm, we supposed that circ_0001206 might regulate CRKL expression via acting as a ceRNA to interact with miRNAs. We applied starBase (http://starbase.sysu.edu.cn) and Circinteractome (https://circinteractome.nia.nih.gov/) databases to predict potential miRNAs which was combined with circ_0001206 and CRKL in common and only found one shared miRNA, miR‐665 (Figure 3 A ). To further validate the correlation among circ_0001206, miR‐665, and CRKL, we conducted RIP and RNA pull‐down assays. It was found that circ_0001206, miR‐665, and CRKL were largely enriched in Anti‐Ago2 groups, suggesting the co‐existence of the three RNAs in RNA‐induced silencing complex (RISC) (Figure 3 B ). We further validated that both circ_0001206 and CRKL were significantly abundant in bio‐miR‐665‐WT groups, while being scarce in bio‐miR‐665‐Mut groups (Figure 3 C ). The binding sites between circ_0001206/CRKL and miR‐665 were predicted via starBase (Figure 3 D ). After detecting the efficiency of miR‐665 mimics (Figure 3 E ), we performed luciferase reporter assays and found that miR‐665 mimics considerably decreased the luciferase activity of wild‐type circ_0001206 and CRKL 3'UTR, but had no effects on that of mutant‐type circ_0001206 and CRKL 3'UTR (Figure 3 F ). Collectively, circ_0001206 regulates CRKL by targeting miR‐665.
Figure 3.
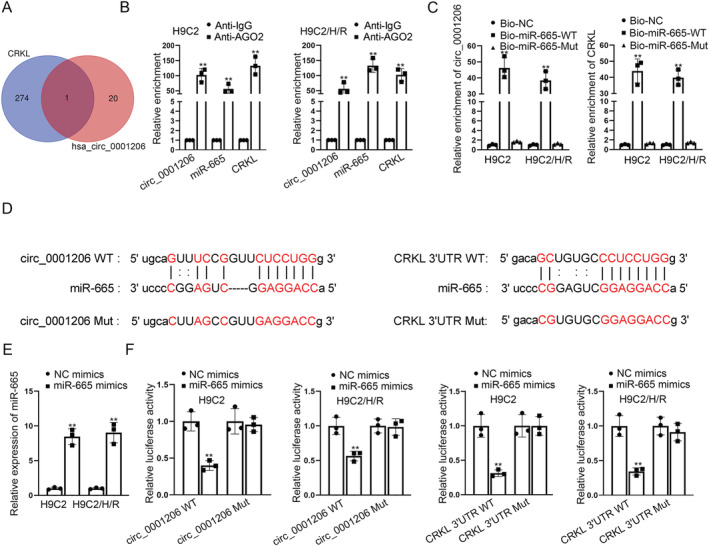
Circ_0001206 regulates CRKL through acting as a ceRNA to target miR‐665. (A) starBase and Circinteractome databases predicted the mutual miRNAs binding with circ_0001206 and CRKL. (B). RIP assay confirmed the coexistence of circ_0001206, miR‐665, and CRKL in RISC. (C) RNA pull‐down assay verified the correlation between circ_0001206 and miR‐665, or CRKL and miR‐665 in H9C2 cells. (D) The putative binding sites between circ_0001206/CRKL and miR‐665 were predicted via starBase. (E) The overexpression efficacy of miR‐665 mimics was tested by RT‐qPCR in H/R‐induced H9C2 cells. (F) Luciferase reporter assays assessed the luciferase activity of circ_0001206 WT/Mut and CRKL 3′UTR WT/Mut when miR‐665 was overexpressed. **P < 0.01.
Circ_0001206 affects hypoxia/reoxygenation‐induced cardiomyocyte injury myocardial infarction thorough targeting miR‐665 to modulate CRKL expression
Rescue experiments further investigated whether circ_0001206 regulates CRKL via ceRNA mode to target miR‐665 in MI. We enhanced the expression of miR‐665 and found that up‐regulation of CRKL expression induced by circ_0001206 overexpression could be down‐regulated after the up‐regulation of miR‐665 (Figure 4 A and 4 B ). Besides, we silenced CRKL expression by transfection of shRNAs targeting CRKL (sh‐CRKL#1/#2) into H9C2 cells treated with H/R, and RT‐qPCR was applied to verify the knockdown efficacy of sh‐CRKL#1/2 (Figure 4 C ). Subsequently, it was found in CCK‐8 assays that miR‐665 overexpression or CRKL silencing offset the elevated cell viability induced by circ_0001206 up‐regulation in H9C2/H/R cells (Figure 4 D ). The data from TUNEL assay and flow cytometry analysis revealed that circ_0001206 overexpression inhibited the cell apoptosis, which was then totally reversed by co‐transfection of miR‐665 mimics or sh‐CRKL#1 (Figure S3 C and S3 D). Moreover, it was revealed that the decreased protein levels of Bax, cleaved caspase‐3 and caspase‐9 as well as the enhanced protein level of Bcl‐2 caused by pcDNA3.1‐circ_0001206 could be completely restored after miR‐665 overexpression or CRKL knockdown, based on the results of western blot (Figure 4 E ). All these data suggested that circ_0001206 affected MI thorough targeting miR‐665 to modulate CRKL expression.
Figure 4.
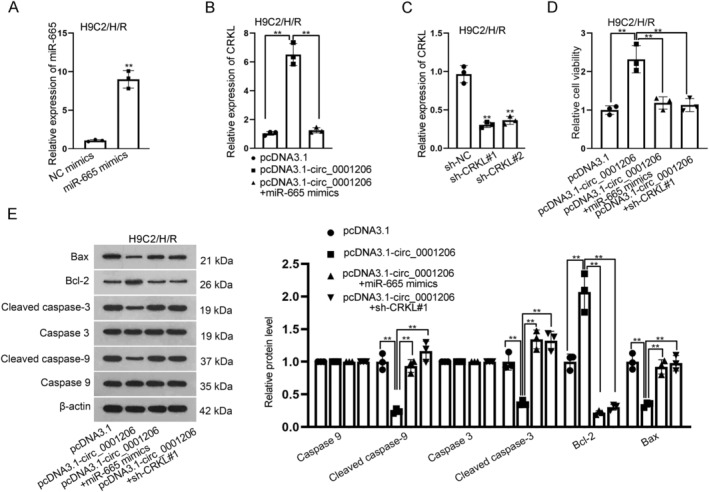
Circ_0001206 affects hypoxia/reoxygenation (H/R)‐induced cardiomyocyte injury myocardial infarction (MI) thorough targeting miR‐665 to modulate CRKL expression. (A). quantitative real‐time polymerase chain reaction (RT‐qPCR) detected the effectiveness of miR‐665 mimics in H/R‐treated H9C2 cells. (B). RT‐qPCR examined CRKL expression in H/R‐treated H9C2 cells after the transfection of pcDNA3.1, pcDNA3.1‐circ_0001206 or pcDNA3.1‐circ_0001206 + miR‐665 mimics. (C). RT‐qPCR detected the interference efficiency of sh‐CRKL#1/#2 in H/R‐treated H9C2 cells. (D). CCK‐8 assay detected the cell viability in H/R‐treated H9C2 cells after the transfection of pcDNA3.1, pcDNA3.1‐circ_0001206, pcDNA3.1‐circ_0001206 + miR‐665 mimics or pcDNA3.1‐circ_0001206 + sh‐CRKL#1. (E). Western blot examined Bax, Bcl‐3, cleaved caspase‐3 and cleaved caspase‐9 levels in H/R‐treated H9C2 cells after the transfection of pcDNA3.1, pcDNA3.1‐circ_0001206, pcDNA3.1‐circ_0001206 + miR‐665 mimics or pcDNA3.1‐circ_0001206 + sh‐CRKL#1 (n = 3). **P < 0.01.
Discussion
Recently, it has been reported that aberrant expressions of many circRNAs are implicated in the pathogenesis of cardiovascular diseases, including MI. 23 Circ‐Ttc3 affects cardiac function after MI via targeting miR‐15b. 24 However, circRNAs have been rarely reported to modulate H/R‐induced apoptosis and other injuries in myocardial cells. In our study, it was found that circ_0001206 expression was down‐regulated in MI model. Moreover, circ_0001206 overexpression obviously reduced the cell apoptosis and promoted the proliferation of H/R‐treated H9C2 cells. Therefore, circ_0001206 could alleviate cardiomyocyte apoptosis and promote proliferation in MI, which might offer a novel target for MI treatment.
There is compelling evidence that circRNAs can modulate its host gene to exert functions in the development and progression of various diseases. 25 Consistently, our study also investigated the regulatory mechanism between circ_0001206 and its host gene, CRKL. CRKL has been found to play vital roles in the initiation and development of multiple diseases. 26 , 27 Intriguingly, CRKL can assuage H/R‐induced injuries in cardiomyocytes. 19 In line with that previous study, our research found that CRKL was lowly expressed in H9C2 cells treated with hypoxia. More importantly, CRKL up‐regulation could suppress cardiomyocyte apoptosis and promote proliferation in H/R‐treated H9C2 cells, which further verified the protective role of CRKL in the development of MI.
Functionally, circRNAs can serve as a ceRNA to regulate mRNA expression via targeting miRNA. 28 In our study, we confirmed that miR‐665 was the certain miRNA binding to both circ_0001206 and CRKL. As reported previously, a handful of miRNAs are implicated in H/R‐induced apoptosis and other injuries in myocardial cells. 29 MiR‐1 deteriorates H/R‐induced apoptosis of cardiomyocytes through directly regulating Bcl‐2 expression. 30 MiR‐34a‐5p accelerates intestinal ischaemia/reperfusion (I/R) induced by hindering SIRT1‐mediated inhibition of reactive oxygen species accumulation and apoptosis. 31 MiR‐499 suppresses H/R‐induced cardiomyocytes injury via modulating SOX6 expression. 32 Consistent with the findings in these studies, our study showed that CRKL was the target gene of miR‐665, and miR‐665 might play a destructive role in MI development. Interestingly, miR‐665 plays a tumour‐promoting role in tumorigenesis and development, 33 which further supports the deteriorative role of miR‐665 in MI development.
Finally, our study carried out rescue experiments to confirm that circ_0001206 inhibited cardiomyocyte apoptosis and promoted proliferation in MI by targeting miR‐665 to modulate CRKL expression. Taken together, the results suggest that circ_0001206 might be a potential drug target for treating MI. Further research about human clinical trials is needed for the investigation into clinical application of circ_0001206 to develop effective treatment strategies for MI patients.
Conflict of interest
None.
Funding
This research was supported by Gansu Natural Science Foundation Project (No: 21JR1RG306).
Supporting information
Figure S1. CircRNAs expression in MI model and TUNEL assay and flow cytometry analysis. A. RT‐qPCR examined the expressions of circRNAs in H/R‐induced H9C2 cells and MI mouse model. B. CircBase database predicted the pairwise alignment of the human and murine circ_0001206 sequences. C‐D. TUNEL assay and flow cytometry analysis detected cell apoptosis in circ_0001206‐overexpressed H9C2 cells treated with H/R. **P < 0.01.
Figure S2. The overexpression of circ_0001206 alleviates cardiac injury post‐MI in MI mice. A. RT‐qPCR detected the overexpression efficacy of pcDNA3.1‐circ_0001206 in mice. B. H&E staining assessed the effect of circ_0001206 on cardiac injury 12 h or 24 h after reperfusion. C. The infarct size was assessed 12 h or 24 h after reperfusion. D. CCK‐8 assay detected the proliferation of cells from mice 12 h or 24 h after reperfusion. E. TUNEL assay detected the apoptosis of cells from mice 12 h or 24 h after reperfusion. **P < 0.01.
Figure S3. TUNEL assays and flow cytometry analysis. A‐B. TUNEL assay and flow cytometry analysis testified cell apoptosis in H/R‐induced H9C2 cells transfected with pcDNA3.1‐CRKL. C‐D. TUNEL assay and flow cytometry analysis examined the apoptosis rate in H/R‐treated H9C2 cells after the transfection of pcDNA3.1, pcDNA3.1‐circ_0001206, pcDNA3.1‐circ_0001206 + miR‐665 mimics or pcDNA3.1‐circ_0001206 + sh‐CRKL#1. **P < 0.01.
Acknowledgement
We appreciate the supports of our experimenters.
Wang, D. , Tian, L. , Wang, Y. , Gao, X. , Tang, H. , and Ge, J. (2022) Circ_0001206 regulates miR‐665/CRKL axis to alleviate hypoxia/reoxygenation‐induced cardiomyocyte injury in myocardial infarction. ESC Heart Failure, 9: 998–1007. 10.1002/ehf2.13725.
Contributor Information
Hanbo Tang, Email: tanghanbo75@126.com.
Junbo Ge, Email: ge_jbge1@163.com.
References
- 1. White HD, Thygesen K, Alpert JS, Jaffe AS. Clinical implications of the third universal definition of myocardial infarction. Heart (British Cardiac Society) 2014; 100: 424–432. [DOI] [PubMed] [Google Scholar]
- 2. Yang HT, Xiu WJ, Zheng YY, Liu F, Gao Y, Ma X, Yang YN, Li XM, Ma YT, Xie X. Invasive reperfusion after 12 hours of the symptom onset remains beneficial in patients with ST‐segment elevation myocardial infarction: Evidence from a meta‐analysis of published data. Cardiol J 2019; 26: 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang Y, Ju C, Hu J, Huang K, Yang L. PRMT4 overexpression aggravates cardiac remodeling following myocardial infarction by promoting cardiomyocyte apoptosis. Biochem Biophys Res Commun 2019; 520: 645–650. [DOI] [PubMed] [Google Scholar]
- 4. Qian L, Van Laake LW, Huang Y, Liu S, Wendland MF, Srivastava D. miR‐24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J Exp Med 2011; 208: 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fan F, Sun A, Zhao H, Liu X, Zhang W, Jin X, Wang C, Ma X, Shen C, Zou Y, Hu K, Ge J. MicroRNA‐34a promotes cardiomyocyte apoptosis post myocardial infarction through down‐regulating aldehyde dehydrogenase 2. Curr Pharm Des 2013; 19: 4865–4873. [DOI] [PubMed] [Google Scholar]
- 6. Shao Y, Chen Y. Roles of circular RNAs in neurologic disease. Front Mol Neurosci 2016; 9: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013; 495: 333–338. [DOI] [PubMed] [Google Scholar]
- 8. Guo JU, Agarwal V, Guo H, Bartel DP. Expanded identification and characterization of mammalian circular RNAs. Genome Biol 2014; 15: 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol 2014; 32: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Altesha MA, Ni T, Khan A, Liu K, Zheng X. Circular RNA in cardiovascular disease. J Cell Physiol 2019; 234: 5588–5600. [DOI] [PubMed] [Google Scholar]
- 11. Wang L, Shen C, Wang Y, Zou T, Zhu H, Lu X, Li L, Yang B, Chen J, Chen S, Lu X, Gu D. Identification of circular RNA Hsa_circ_0001879 and Hsa_circ_0004104 as novel biomarkers for coronary artery disease. Atherosclerosis 2019; 286: 88–96. [DOI] [PubMed] [Google Scholar]
- 12. Zhou B, Yu JW. A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR‐141 by targeting TGF‐beta1. Biochem Biophys Res Commun 2017; 487: 769–775. [DOI] [PubMed] [Google Scholar]
- 13. Garikipati VNS, Verma SK, Cheng Z, Liang D, Truongcao MM, Cimini M, Yue Y, Huang G, Wang C, Benedict C, Tang Y, Mallaredy V, Ibetti J, Grisanti L, Schumacher SM, Gao E, Rajan S, Wilusz JE, Goukassian D, Houser SR, Koch WJ, Kishore R. Circular RNA CircFndc3b modulates cardiac repair after myocardial infarction via FUS/VEGF‐A axis. Nat Commun 2019; 10: 4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou LY, Zhai M, Huang Y, Xu S, An T, Wang YH, Zhang RC, Liu CY, Dong YH, Wang M, Qian LL, Ponnusamy M, Zhang YH, Zhang J, Wang K. The circular RNA ACR attenuates myocardial ischemia/reperfusion injury by suppressing autophagy via modulation of the Pink1/FAM65B pathway. Cell Death Differ 2019; 26: 1299–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu Y, Pan W, Yang T, Meng X, Jiang Z, Tao L, Wang L. Upregulation of circular RNA CircNFIB attenuates cardiac fibrosis by sponging miR‐433. Front Genet 2019; 10: 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang F, Zhang R, Zhang X, Wu Y, Li X, Zhang S, Hou W, Ding Y, Tian J, Sun L, Kong X. Comprehensive analysis of circRNA expression pattern and circRNA‐miRNA‐mRNA network in the pathogenesis of atherosclerosis in rabbits. Aging 2018; 10: 2266–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jin Q, Chen Y. Silencing circular RNA circ_0010729 protects human cardiomyocytes from oxygen‐glucose deprivation‐induced injury by up‐regulating microRNA‐145‐5p. Mol Cell Biochem 2019; 462: 185–194. [DOI] [PubMed] [Google Scholar]
- 18. Huang Z, Wu S, Kong F, Cai X, Ye B, Shan P, Huang W. MicroRNA‐21 protects against cardiac hypoxia/reoxygenation injury by inhibiting excessive autophagy in H9c2 cells via the Akt/mTOR pathway. J Cell Mol Med 2017; 21: 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang ZS, Yang DY, Fu YB, Zhang L, Zhao QP, Li G. Knockdown of CkrL by shRNA deteriorates hypoxia/reoxygenation‐induced H9C2 cardiomyocyte apoptosis and survival inhibition via Bax and downregulation of P‐Erk1/2. Cell Biochem Funct 2015; 33: 80–88. [DOI] [PubMed] [Google Scholar]
- 20. Li Y, Lu J, Bao X, Wang X, Wu J, Li X, Hong W. MiR‐499‐5p protects cardiomyocytes against ischaemic injury via anti‐apoptosis by targeting PDCD4. Oncotarget 2016; 7: 35607–35617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang B, Zhou M, Li C, Zhou J, Li H, Zhu D, Wang Z, Chen A, Zhao Q. MicroRNA‐92a inhibition attenuates hypoxia/reoxygenation‐induced myocardiocyte apoptosis by targeting Smad7. PLoS ONE 2014; 9: e100298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu X, Wang M, Li Q, Liu W, Song Q, Jiang H. CircRNA ACAP2 induces myocardial apoptosis after myocardial infarction by sponging miR‐29. Minerva Med 2020. [DOI] [PubMed] [Google Scholar]
- 23. Wang S, Zhan J, Lin X, Wang Y, Wang Y, Liu Y. CircRNA‐0077930 from hyperglycaemia‐stimulated vascular endothelial cell exosomes regulates senescence in vascular smooth muscle cells. Cell Biochem Funct 2020; 38: 1056–1068. [DOI] [PubMed] [Google Scholar]
- 24. Cai L, Qi B, Wu X, Peng S, Zhou G, Wei Y, Xu J, Chen S, Liu S. Circular RNA Ttc3 regulates cardiac function after myocardial infarction by sponging miR‐15b. J Mol Cell Cardiol 2019; 130: 10–22. [DOI] [PubMed] [Google Scholar]
- 25. Zhou J, Zhang S, Chen Z, He Z, Xu Y, Li Z. CircRNA‐ENO1 promoted glycolysis and tumor progression in lung adenocarcinoma through upregulating its host gene ENO1. Cell Death Dis 2019; 10: 885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Franke FC, Müller J, Abal M, Medina ED, Nitsche U, Weidmann H, Chardonnet S, Ninio E, Janssen KP. The tumor suppressor SASH1 interacts with the signal adaptor CRKL to inhibit epithelial‐mesenchymal transition and metastasis in colorectal cancer. Cell Mol Gastroenterol Hepatol 2019; 7: 33–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yanagi H, Wang L, Nishihara H, Kimura T, Tanino M, Yanagi T, Fukuda S, Tanaka S. CRKL plays a pivotal role in tumorigenesis of head and neck squamous cell carcinoma through the regulation of cell adhesion. Biochem Biophys Res Commun 2012; 418: 104–109. [DOI] [PubMed] [Google Scholar]
- 28. Li Y, Huo C, Lin X, Xu J. Computational identification of cross‐talking ceRNAs. Adv Exp Med Biol 2018; 1094: 97–108. [DOI] [PubMed] [Google Scholar]
- 29. Liu LF, Liang Z, Lv ZR, Liu XH, Bai J, Chen J, Chen C, Wang Y. MicroRNA‐15a/b are up‐regulated in response to myocardial ischemia/reperfusion injury. J Geriatr Cardiol: JGC 2012; 9: 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhai C, Tang G, Peng L, Hu H, Qian G, Wang S, Yao J, Zhang X, Fang Y, Yang S, Zhang X. Inhibition of microRNA‐1 attenuates hypoxia/re‐oxygenation‐induced apoptosis of cardiomyocytes by directly targeting Bcl‐2 but not GADD45Beta. Am J Transl Res 2015; 7: 1952–1962. [PMC free article] [PubMed] [Google Scholar]
- 31. Wang G, Yao J, Li Z, Zu G, Feng D, Shan W, Li Y, Hu Y, Zhao Y, Tian X. miR‐34a‐5p inhibition alleviates intestinal ischemia/reperfusion‐induced reactive oxygen species accumulation and apoptosis via activation of SIRT1 signaling. Antioxid Redox Signal 2016; 24: 961–973. [DOI] [PubMed] [Google Scholar]
- 32. Shi Y, Han Y, Niu L, Li J, Chen Y. MiR‐499 inhibited hypoxia/reoxygenation induced cardiomyocytes injury by targeting SOX6. Biotechnol Lett 2019; 41: 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu Y, Yang C, Yang S, Cheng F, Rao J, Wang X. miR‐665 promotes hepatocellular carcinoma cell migration, invasion, and proliferation by decreasing hippo signaling through targeting PTPRB. Cell Death Dis 2018; 9: 954. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. CircRNAs expression in MI model and TUNEL assay and flow cytometry analysis. A. RT‐qPCR examined the expressions of circRNAs in H/R‐induced H9C2 cells and MI mouse model. B. CircBase database predicted the pairwise alignment of the human and murine circ_0001206 sequences. C‐D. TUNEL assay and flow cytometry analysis detected cell apoptosis in circ_0001206‐overexpressed H9C2 cells treated with H/R. **P < 0.01.
Figure S2. The overexpression of circ_0001206 alleviates cardiac injury post‐MI in MI mice. A. RT‐qPCR detected the overexpression efficacy of pcDNA3.1‐circ_0001206 in mice. B. H&E staining assessed the effect of circ_0001206 on cardiac injury 12 h or 24 h after reperfusion. C. The infarct size was assessed 12 h or 24 h after reperfusion. D. CCK‐8 assay detected the proliferation of cells from mice 12 h or 24 h after reperfusion. E. TUNEL assay detected the apoptosis of cells from mice 12 h or 24 h after reperfusion. **P < 0.01.
Figure S3. TUNEL assays and flow cytometry analysis. A‐B. TUNEL assay and flow cytometry analysis testified cell apoptosis in H/R‐induced H9C2 cells transfected with pcDNA3.1‐CRKL. C‐D. TUNEL assay and flow cytometry analysis examined the apoptosis rate in H/R‐treated H9C2 cells after the transfection of pcDNA3.1, pcDNA3.1‐circ_0001206, pcDNA3.1‐circ_0001206 + miR‐665 mimics or pcDNA3.1‐circ_0001206 + sh‐CRKL#1. **P < 0.01.


