Psoriasis is associated with various systemic disorders, including cardiovascular diseases (CVDs) and metabolic diseases. 1 , 2 Among various co‐morbid diseases, CVDs are particularly important because they could directly affect patients' mortality. 3 Heart failure (HF) is one of the major CVDs with a large disease burden worldwide, and recent studies have shown that psoriasis patients have increased risk of developing HF compared to the general population. 4 , 5 Early biologic therapies are expected to reduce systemic inflammation and alleviate the progression of systemic co‐morbidities. 1 Although anti‐tumour necrosis factor‐α (anti‐TNF‐α) inhibitors have been reported to reduce the risk of cardiovascular events in patients with psoriasis, the relationship between ustekinumab (anti‐interleukin‐12/23 inhibitor) and HF risk remains unclear, and studies comparing the effects of anti‐TNF‐α inhibitors and ustekinumab on the risk of HF are lacking. 1 , 6 In this study, we compared the HF risk between anti‐TNF‐α inhibitors and ustekinumab to aid in selecting biologics in psoriasis patients with HF risk factors.
Through Korean Health Insurance Review and Assessment service database, we included patients with moderate to severe psoriasis (L40) over the age of 20 using ustekinumab or anti‐TNF‐α inhibitors (adalimumab, etanercept, and infliximab) from January 2016 to September 2019, who scored PASI ≥ 10 despite 3 months of conventional treatment. After excluding patients with previously diagnosed HF (I50), a total of 4468 patients (2448 ustekinumab and 2020 anti‐TNF‐α inhibitors) were included. Inverse probability of treatment weighting (IPTW) was used to balance covariates between the two groups (Supporting Information, Table S1 ). The risks of HF in groups treated with ustekinumab or anti‐TNF‐α inhibitors were analysed using a Cox regression hazard model. We adjusted for age, sex, diabetes, hypertension, dyslipidaemia, malignancy, and patients with psoriatic arthritis and severe psoriasis covered by the individual co‐payment beneficiaries programme for rare and intractable disorders in the regression models. Analyses were performed using SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA). The study design was approved by the Ethnic Committee of Seoul St. Mary's Hospital (IRB No. KC20ZISI0189).
After IPTW, the incidence rates of HF were 11.218 per 1000 person‐years in the anti‐TNF‐α inhibitor group and 7.046 per 1000 person‐years in the ustekinumab group, respectively (Table 1A ). The risks of HF were lower in the ustekinumab group (HR, 0.641; 95% CI, 0.415–0.985) than in the anti‐TNF‐α inhibitor group (Table 1A ). Multivariable Cox proportional hazards regression analysis also revealed a lower risk for HF in the ustekinumab group (HR, 0.627; 95% CI, 0.407–0.967) than in the anti‐TNF‐α inhibitor group (Table 1B ). The ustekinumab group demonstrated significantly lower cumulative incidence rates of HF than the anti‐TNF‐α inhibitor group (log‐rank test, P = 0.0436) (Figure 1 ).
Table 1A.
Incidence rates and risks of heart failure in groups treated with ustekinumab or anti‐tumour necrosis factor‐α inhibitors after IPTW
| Number | Event | Person‐years | IR a | HR (95% CI) | P‐value | |
|---|---|---|---|---|---|---|
| HF outcome | ||||||
| Anti‐TNF‐α inhibitor | 2020 | 45.145 | 4024.42 | 11.218 | 1 [reference] | 0.0426 |
| Ustekinumab | 2448 | 38.521 | 5466.82 | 7.046 | 0.641 (0.415–0.985) | |
CI, confidence interval; HF, heart failure; HR, hazard ratio; IPTW, inverse probability of treatment weighting; IR, incidence rate; TNF‐α, tumour necrosis factor‐α.
Per 1000 person‐years.
Table 1B.
The risks of heart failure in ustekinumab‐treated and anti‐tumour necrosis factor‐α inhibitor‐treated groups before inverse probability of treatment weighting
| Number | Event | Person‐years | IR a | HR (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||
| HF outcome | |||||||||
| Anti‐TNF‐α inhibitor | 2020 | 44 | 3995.02 | 11.014 | 1 [reference] | 1 [reference] | 1 [reference] | 1 [reference] | 1 [reference] |
| Ustekinumab | 2448 | 40 | 5507.33 | 7.263 | 0.674 (0.439–1.035) | 0.614 (0.4–0.944) | 0.612 (0.397–0.942) | 0.612 (0.397–0.941) | 0.627 (0.407–0.967) |
CI, confidence interval; HF, heart failure; HR, hazard ratio; IR, incidence rate; TNF‐α, tumour necrosis factor‐α.
Model 1: nonadjusted. Model 2: adjusted for sex and age. Model 3: adjusted sex, age, diabetes mellitus, hypertension, and dyslipidaemia. Model 4: adjusted sex, age, diabetes mellitus, hypertension, dyslipidaemia, and malignancy. Model 5: adjusted sex, age, diabetes mellitus, hypertension, dyslipidaemia, malignancy, and patients with psoriatic arthritis and severe psoriasis covered by the individual co‐payment beneficiaries programme for rare and intractable disorders.
Per 1000 person‐years.
Figure 1.
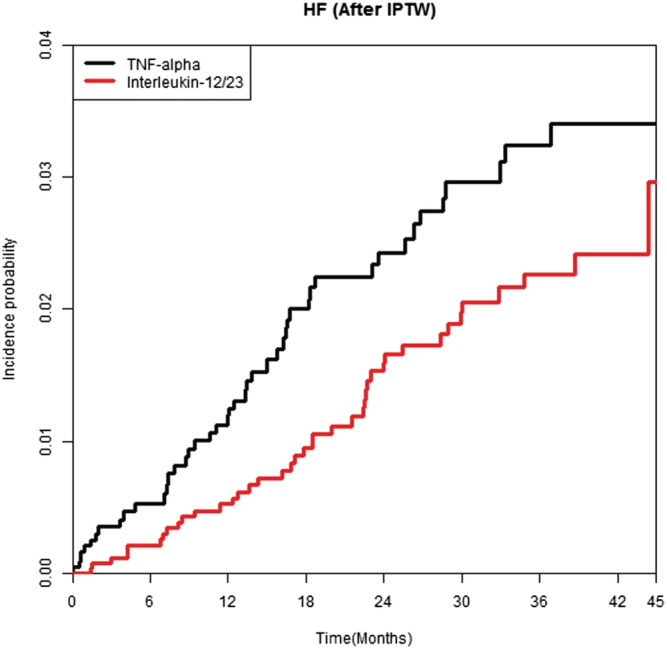
Weighted cumulative incidence curves of heart failure after IPTW in patients treated with ustekinumab or anti‐tumour necrosis factor‐α inhibitors.
In this study, ustekinumab reduced the risk of HF in patients with moderate to severe psoriasis compared to anti‐TNF‐α inhibitors. Anti‐TNF‐α inhibitors have been suggested to have a cardioprotective effect and are recommended for treatment of psoriasis in patients with cardiovascular risk factors. 7 Meanwhile, there are several conflicting reports regarding anti‐TNF‐α inhibitors and risk of HF, and several previous reports have suggested that anti‐TNF‐α inhibitors might be associated with new‐onset or exacerbation of HF. Moreover, ustekinumab initially triggered concerns about increased CVD risk, and there is limited evidence of ustekinumab on HF risk; however, several recent RCTs and meta‐analyses have reported no increased risk of HF. 3 , 8 Recently, elevated pro‐inflammatory cytokines including TNF‐α, IFN‐γ, IL‐1β, IL‐6, and IL‐17 are suggested to play an important role in HF development by inducing cardiac hypertrophy and fibrosis. 3 Although still unclear, biologics might reduce the HF risk in psoriasis through the action of reducing overall inflammation. There are several limitations to this study that the diagnosis was conducted based on the HIRA claim data and the information on severity of the diseases was lacking. Despite these limitations, the strengths of our study are its nationally representative study population and that we controlled metabolic disorders which could act as a confounding factor for HF development. Our study is meaningful in that we directly compared the cardioprotective effects of ustekinumab and anti‐TNF‐α inhibitors in patients with moderate to severe psoriasis. Furthermore, our results suggest that, in patients with moderate to severe psoriasis at risk of HF, ustekinumab might be a good option compared to anti‐TNF‐α inhibitors. Further investigations are needed to confirm and clarify the exact mechanism.
Funding
None.
Author contributions
J.H.H. and C.H.B. contributed in the design and conduct of the study; H.E.P., Y.H.K., and J.‐H.J. in the collection, management, analysis, and interpretation of the data; J.H.H., H.E.P., Y.M.P., J.H.L., and C.H.B. in the preparation, review, or approval of the manuscript; and C.H.B. in the decision to submit the manuscript for publication.
Supporting information
Table S1. Baseline clinical characteristics of the study population.
Han, J. H. , Park, H. E. , Kim, Y. H. , Jung, J.‐H. , Lee, J. H. , Park, Y. M. , and Bang, C. H. (2022) Comparison of the risk of heart failure in psoriasis patients using anti‐TNF α inhibitors and ustekinumab. ESC Heart Failure, 9: 1502–1504. 10.1002/ehf2.13855.
References
- 1. Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol 2020; 182: 840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA 2020; 323: 1945–1960. [DOI] [PubMed] [Google Scholar]
- 3. Koppikar S, Colaco K, Harvey P, Akhtari S, Chandran V, Gladman DD, Cook R, Eder L. Incidence of and risk factors for heart failure in patients with psoriatic disease—a cohort study. Arthritis Care Res (Hoboken) 2021. 10.1002/acr.24578 [DOI] [PubMed] [Google Scholar]
- 4. Polachek A, Touma Z, Anderson M, Eder L. Risk of cardiovascular morbidity in patients with psoriatic arthritis: a meta‐analysis of observational studies. Arthritis Care Res (Hoboken) 2017; 69: 67–74. [DOI] [PubMed] [Google Scholar]
- 5. Khalid U, Ahlehoff O, Gislason GH, Kristensen SL, Skov L, Torp‐Pedersen C, Hansen PR. Psoriasis and risk of heart failure: a nationwide cohort study. Eur J Heart Fail 2014; 16: 743–748. [DOI] [PubMed] [Google Scholar]
- 6. Amin M, Lee EB, Tsai TF, Wu JJ. Psoriasis and co‐morbidity. Acta Derm Venereol 2020; 100: adv00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol 2019; 80: 27–40. [DOI] [PubMed] [Google Scholar]
- 8. Thatiparthi A, Martin A, Liu J, Egeberg A, Wu JJ. Biologic treatment algorithms for moderate‐to‐severe psoriasis with comorbid conditions and special populations: a review. Am J Clin Dermatol 2021; 22: 425–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline clinical characteristics of the study population.


