Abstract
Aims
A method for estimating right ventricular ejection fraction (RVEF) from RV pressure waveforms was recently validated in an experimental model. Currently, cardiac magnetic resonance imaging (MRI) is the clinical reference standard for measurement of RVEF in pulmonary arterial hypertension (PAH). The present study was designed to test the hypothesis that the pressure‐based method can detect clinically significant reductions in RVEF as determined by cardiac MRI in patients with PAH.
Methods and results
RVEF estimates derived from analysis of RV pressure waveforms recorded during right heart catheterization (RHC) in 25 patients were compared with cardiac MRI measurements of RVEF obtained within 24 h. Three investigators blinded to cardiac MRI results independently performed pressure‐based RVEF estimation with the mean of their results used for comparison. Linear regression was used to assess correlation, and a receiver operator characteristic (ROC) curve was derived to define ability of the pressure‐based method to detect a maladaptive RV response, defined as RVEF <35% on cardiac MRI. In 23 patients, an automated adaptation of the pressure‐based RVEF method was also applied as proof of concept for beat‐to‐beat RVEF monitoring. The study cohort was comprised of 16 female and 9 male PAH patients with an average age of 53 ± 13 years. RVEF measured by cardiac MRI ranged from 16% to 57% (mean 37.7 ± 11.6%), and estimated RVEF from 15% to 54% (mean 36.2 ± 11.2%; P = 0.6). Measured and estimated RVEF were significantly correlated (r 2 = 0.78; P < 0.0001). ROC curve analysis demonstrated an area under the curve of 0.94 ± 0.04 with a sensitivity of 81% and specificity of 85% for predicting a maladaptive RV response. As a secondary outcome, with the recognized limitation of non‐coincident measures, Bland–Altman analysis was performed and indicated minimal bias for estimated RVEF (−1.5%) with limits of agreement of ± 10.9%. Adaptation of the pressure‐based estimation method to provide beat‐to‐beat RVEF also demonstrated significant correlation between the median beat‐to‐beat value over 10 s with cardiac MRI (r 2 = 0.66; P < 0.001), and an area under the ROC curve of 0.94 ± 0.04 (CI = 0.86 to 1.00) with sensitivity and specificity of 78% and 86%, respectively, for predicting a maladaptive RV response.
Conclusions
Pressure‐based estimation of RVEF correlates with cardiac MRI and detects clinically significant reductions in RVEF. Study results support potential utility of pressure‐based RVEF estimation for assessing the response to diagnostic or therapeutic interventions during RHC.
Keywords: Right ventricle, Ejection fraction, Cardiac MRI, RV:PA coupling, PAH
Introduction
Right ventricular (RV) function is an important determinant of clinical outcomes in patients with pulmonary hypertension of various aetiologies. 1 , 2 While echocardiography and right heart catheterization (RHC) remain important diagnostic modalities in PH, cardiac magnetic resonance imaging (MRI) remains the clinical reference standard for measurement of RVEF in patients with pulmonary arterial hypertension (PAH). 3 However, this approach is not readily applicable during RHC to quantify acute changes in RVEF in response to diagnostic or therapeutic interventions. Alternatively, ‘single beat’ method using RV pressure waveforms and stroke volume (SV) can be applied to data recorded during RHC for quantification of contractility and afterload as independent entities. 4 , 5 , 6 , 7 , 8 , 9 Within this construct, contractility is measured as end‐systolic ventricular elastance (Ees, in mmHg/mL) and afterload as arterial elastance (Ea, in mmHg/mL). With both expressed in a common term, the ratio Ees/Ea can be used to summarize changes in RV contractility/afterload balance (RV:PA coupling) over time or in response to acute interventions. 4 , 5 , 6 While RV‐PA coupling has been shown to confer prognostic value in PH, 4 , 10 , 11 directly relating Ees/Ea to RVEF is challenging. In this context, being able to directly estimate RVEF from data routinely acquired during diagnostic RHC would be advantageous.
Currently, there are two well‐described catheter‐based methods for clinical measurement of RVEF: (i) intermittent thermodilution and (ii) continuous volume measurement by conductance catheter. While the latter can provide beat‐to‐beat measurements of EF, 12 it is expensive, requires cross‐calibration to other metrics of RV volume, and is technically challenging. In contrast, an automated system for intermittent RVEF measurement using a specialized thermodilution PA catheter is readily available and easy to use. However, prior studies demonstrated that estimation of RVEF during RHC using the thermodilution technique frequently under‐estimated RVEF when compared with the reference standard cardiac MRI 13 and provided invalid data on RV volumes and RVEF in patients with PH. 14 As an alternative, our group recently described a method based entirely on analysis of the RV pressure waveform that can effectively track acute changes in RVEF in animal models. 15 , 16 This methodology raises the possibility of rapidly gauging functional impairment of the RV during RHC and allowing for rapid bedside assessment of a diagnostic or therapeutic intervention.
The current study was therefore designed with two goals: (i) to test the hypothesis that a pressure‐based estimation method can accurately predict clinically significant reductions in RVEF as previously determined by cardiac MRI in patients with PAH and (ii) to assess the potential for continuous, beat‐to‐beat application of the pressure‐based RVEF estimation concept.
Methods
We examined data from 36 consecutive PAH patients who had undergone cardiac MRI measurement of RV and left ventricular (LV) volumes followed by RHC within 24 h between 2016 and 2019. 11 The RHC was performed in the cardiac catheterization laboratory at the University Hospital of Giessen, and the study was approved by the University ethical board (108/2015). During RHC, RV pressure had been acquired by micro manometry, sampled at 500 Hz, subjected to a 10 Hz low pass frequency filter, and saved as ~10 s segments. To limit the potential impact of widely variant conditions during data acquisition, the dataset was limited to patients for whom heart rate at the time of RHC varied from that at cardiac MRI by <25%, yielding a final sample size of 25.
Pressure‐based estimation of right ventricular ejection fraction
Previous investigation demonstrated that EF can be approximated from Ees and Ea as follows: EF = Ees/(Ees + Ea). 15 This can then be simplified to EF = 1 − (ESP/Pmax), where ESP is the RV end‐systolic pressure and Pmax the theoretical maximum pressure generated by the RV during an isovolumic contraction. 15 For the current study, a signal averaged RV pressure waveform was created from several beats and the second derivative squared to create event markers for defining specific points of the RV pressure waveform (Figure 1 ). From these event markers, the pressure intervals used for deriving Pmax from a distribution function (the 4‐parameter Weibull peak fit) applied within the Dynamic Fit Wizard of SigmaPlot (version 13, Systat Software, Inc., San Jose, CA) were defined and a curve delineating Pmax calculated. Three investigators blinded to the cardiac MRI data analysed the same RV pressure sequences with the mean of their RVEF predictions regarded as the final value. Because tricuspid valve insufficiency could potentially affect the initial rise in RV pressure and influence prediction of Pmax, and therefore potentially affect estimated RVEF, the tricuspid regurgitant fraction at cardiac MRI was calculated as follows: (RVSV − LVSV)/RVSV. Additionally, because single beat estimation of Ees assumes the volume intercept of the end‐systolic pressure‐volume relationship (Vo) is negligible, 17 Vo measured at the time of RHC was noted for each patient. To determine Vo, RV volume measured by conductance catheter was cross‐calibrated to volume previously determined by cardiac MRI and acutely altered by transient balloon occlusion of the inferior vena cava to define the end‐systolic pressure‐volume relationship. 11
Figure 1.
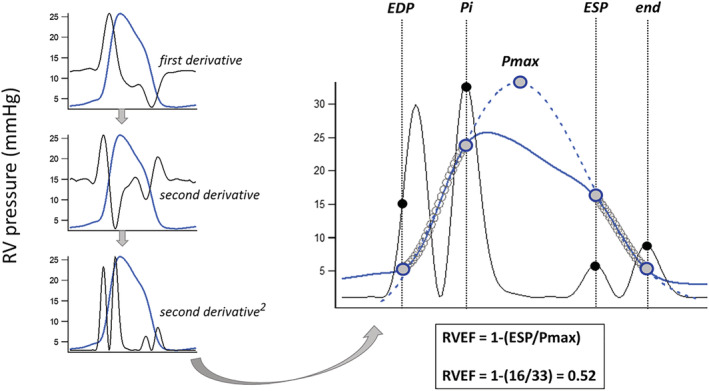
Method derivation for right ventricular ejection fraction (RVEF) estimation from a RV pressure waveform. The signal average for a series of RV pressure waveforms was created (blue line) and its second derivative squared to produce four upright peaks (black lines). These peaks were then used to define the 'up and down’ pressure segments (open circles) for prediction of Pmax, the maximal pressure achieved if the contraction remained isovolumic, as the intervals from half of the first peak (end‐diastolic pressure or EDP) to the second peak (the first inflection point or Pi), and from the third peak (end‐systolic pressure or ESP) to the fourth (end). The third peak approximates the point of maximal time varying elastance (RV pressure/RV volume) with RV pressure at this point regarded as an estimate of true ESP.
Feasibility of continuous estimation of right ventricular ejection fraction
To test the concept that pressure‐based estimation of RVEF can be applied to the RV pressure waveform on a beat‐to‐beat basis, a simplified version of the method was developed using the Pmax prediction feature within the PV module of LabChart (ADInstruments, Australia). Designated by the module as ‘Piso’, the value for each beat over a stable ~8–10 s segment was determined. For the same beats, mPAP was estimated using RVSP and RV pressure at peak positive dP/dt as surrogates for PA systolic and diastolic pressures, respectively. 18 ESP was then derived as follows: (mPAP × 1.65) − 7.79 19 and RVEF calculated for each beat as 1 − (ESP/Pmax). 15 The median RVEF value over the sequence of beats was then used for comparison.
Analysis and statistics
Continuous data were compared by t‐test with correlation between paired variables analysed by linear regression. The intraclass correlation coefficient for RVEF estimation among the three blinded investigators was calculated as previously described. 20 To determine the ability of pressure‐based RVEF estimation to detect maladaptive RV changes, a receiver operator characteristic (ROC) curve was used. RV maladaptation was defined as RVEF <35%, as this value was previously shown to be associated with increased RV end‐diastolic and end‐systolic volumes at preserved stroke volume 21 and with increased mortality in patients with PH. 22 , 23 , 24 , 25 Bland–Altman analysis was performed on measured and estimated RVEF data to define bias and limits of agreement recognizing the caveat that measurements were not simultaneous. Measurements were considered potentially interchangeable when the average difference between them (bias) was <10% of the mean measured RVEF values, and the overall error calculated as: (bias standard deviation × 1.96)/mean of all MRI data was ≤30%. 26 Data are presented as mean ± SD or 95% confidence intervals (CI) and for all tests a P value ≤0.05 was considered significant. Statistical analyses were performed using SigmaPlot (version 13, Systat Software, Inc., San Jose, CA) and GraphPad Prism (version 9, GraphPad Software, San Diego, CA).
Results
Patient characteristics and summary RHC data are shown in Table 1 . Across the data set, RVEF measured by cardiac MRI ranged from 16% to 57% (mean 37.7 ± 11.6%) and estimated RVEF from 15% to 54% (mean 36.2 ± 11.2%, P = 0.6). The intraclass correlation coefficient for RVEF estimation was 0.88, a value consistent with good reliability. 20 Individual RVEF measurements were significantly correlated (r 2 = 0.78 P < 0.001) (Figure 2 A ) with 10 patients exhibiting a maladaptive RV response, as defined by a cardiac MRI RVEF <35%. 27 As shown inFigure 2 B , pressure‐based estimation of RVEF was able to predict RVEF <35%, with an area under the curve of 0.94 ± 0.04 (CI = 0.85–1.00), sensitivity of 81% and specificity of 85%. Bland–Altman analysis of non‐coincident data indicated minimal bias (−1.5%; CI = −3.8 to 0.8%) with limits of agreement of −12.5% (CI = −16.4 to −8.5) to 9.5% (CI = 5.4 to 13.4) (Figure 2 A ) and an overall error of 0.29. On average, tricuspid regurgitant fraction measured at cardiac MRI ranged from −17% (suggesting right to left shunt or measurement artefact) to 57%. There was no correlation between tricuspid regurgitant fraction at cardiac MRI and measured (r 2 = 0.05; P = 0.3) or estimated (r 2 = 0.13; P = 0.08) RVEF, or their difference (r 2 = 0.07; P = 0.2). Vo measured at RHC ranged from −100 to 188 mL. There was modest but significant correlation between Vo derived at RHC and both measured (r 2 = 0.32; P = 0.003) and estimated RVEF (r 2 = 0.21; P = 0.02), but not the difference between them (r 2 = 0.1; P = 0.2).
Table 1.
Baseline characteristic of PAH patients including baseline right heart hemodynamic and cardiac MRI data
| Characteristics | PAH (n = 25) |
|---|---|
| Age, years | 53 ± 13 |
| BMI, kg/m2 | 27 ± 6 |
| Female gender, n (%) | 15 (60) |
| PAH aetiology, n (%) | |
| Idiopathic | 20 (80) |
| Hereditary | 1 (4) |
| CTD | 1 (4) |
| Porto‐pulmonary | 3 (12) |
| Medications, n (%) | |
| PDE‐5 inhibitor | 10 (40) |
| Riociguat | 7 (28) |
| Endothelin receptor blocker | 17 (68) |
| Oral prostacyclin (Selexipag) | 4 (16) |
| Inhaled prostacyclin | 4 (16) |
| Systemic prostacyclin | 2 (8) |
| Right heart catheterization data | |
| SpO2 (%) | 91 ± 6 |
| MvO2 (%) | 64 ± 6 |
| Right atrial pressure (mmHg) | 8 ± 5 |
| Cardiac output (L/min) by TD | 4.77 ± 1.2 |
| mPAP (mmHg) | 43 ± 12 |
| PAWP (mmHg) | 10 ± 2 |
| PVR (WU) | 7.3 ± 3.2 |
| Cardiac MRI data | |
| RV stroke volume (mL) | 83.9 ± 24.4 |
| LV stroke volume (mL) | 62.6 ± 19.4 |
| Tricuspid regurgitant fraction (%) | 22.3 ± 22.5 |
BMI, body mass index; CTD, connective tissue disease (CTD); LV, left ventricle; mPAP, mean pulmonary artery pressure; MRI, magnetic resonance imaging; MvO2, mixed venous oxygen saturation; PAH, pulmonary arterial hypertension; PAWP, pulmonary artery wedge pressure; PDE, phosphodiesterase; PVR, pulmonary vascular resistance; RV, right ventricle; SpO2, peripheral oxygen saturation; TD, thermodilution; WU, Woods unit.
Data are presented as n (%) or mean ± SD unless otherwise stated.
Figure 2.
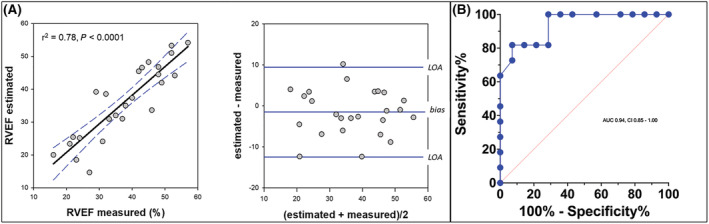
(A) Scatter plot demonstrating correlation between estimated RV ejection fraction (RVEF in percent) and that measured by cardiac magnetic resonance imaging (cardiac MRI). Data are presented with 95% confidence intervals of the linear regression function (hatched line). Bland–Altman plot showing the mean difference between methods (bias) and limits of agreement (LOA) between estimated and measured RVEF. (B) Receiver operating characteristic curve for pressure‐based estimation of RVEF in patients with cardiac MRI RVEF <35%.
An example of the pressure‐based RVEF method adapted for application on a beat‐to‐beat basis is shown in Figure 3 A . Of the 25 patients in the full data set, Pmax could not be adequately derived in 2 due to marked respiratory variation of the RV pressure waveform. Within the remaining 23, median estimated RVEF over the beat‐to‐beat interval ranged from 16% to 52% with corresponding cardiac MRI RVEF of 16–57%. Figure 3 B shows significant correlation between measured and estimated RVEF along with a Bland Altman plot demonstrating a bias of −3.7% (CI = −6.7 to −0.75%) with limits of agreement from 9.7 (CI = 4.6 to 14.8) to −17.1% (CI = −22.3 to −12.0) and an overall error of 0.34. Figure 3 C shows the ROC curve for prediction of an MRI RVEF ≤35% by the continuous estimation method and demonstrates an AUC of 0.94 ± 0.04 (CI = 0.86 to 1.00) with sensitivity of 78% and specificity of 86%.
Figure 3.
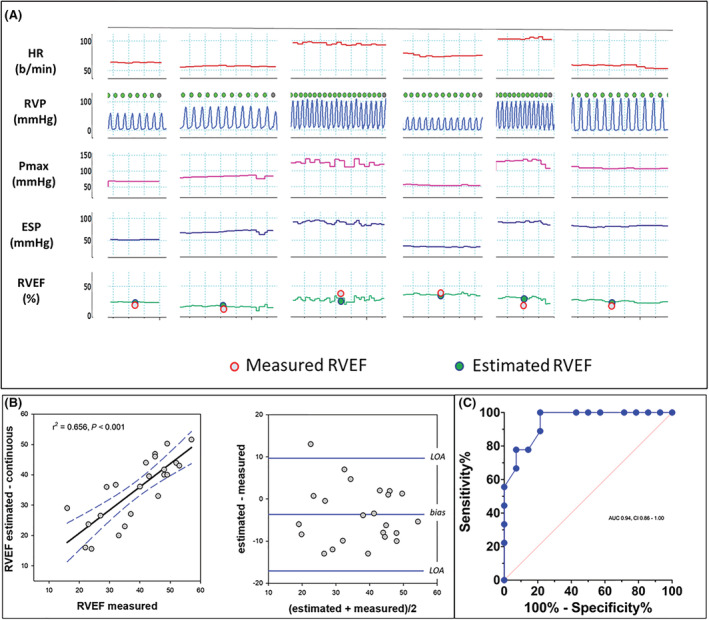
(A) An example of beat‐to‐beat right ventricular ejection fraction (RVEF) derived from six intervals of right ventricular pressure (RVP) recorded during clinically indicated right heart catheterization. Among the patients, heart rate ranged from ~50 to 125 b.p.m. and RV systolic pressure from ~25 to 110 mmHg. Bottom row compares the median value for estimated RVEF over the interval to RVEF measured by cardiac magnetic resonance imaging (cardiac MRI). (B, C) Scatter and Bland–Altman plots along with the receiver operating characteristic curve for predicting RVEF <35% measured by cardiac MRI using beat‐to‐beat estimation of RVEF.
Discussion
Results from the current study demonstrate a strong correlation over a wide range values between RVEF estimated from RV pressure recorded during RHC and that measured by cardiac MRI within the preceding 24 h. Additionally, we show that pressure‐based RVEF estimation can reliably predict a maladaptive RV response evident on cardiac MRI in PAH patients.
Despite non‐coincidence of RVEF measurements, study results are generally consistent with a previous proof‐of‐concept animal study comparing pressure‐based estimation of RVEF to simultaneous measurement by conductance catheter. 15 In that prior investigation, Bland–Altman comparison of 69 simultaneous measurements acquired from 15 swine demonstrated similar correlation (r2 = 0.73, P < 0.0001), bias (3%) and limits of agreement from −9% to +13%. 15 Study results do not, however, suggest that pressure‐based estimation of RVEF represents an alternative to cardiac MRI. The potential clinical value of the current study relates to application of the method at the bedside during a routine RHC when diagnostic or therapeutic interventions are made, such as administration of an acute pulmonary vasodilator, systemic vasopressor, inotropic agent, or fluid challenge. Accordingly, the current investigation also explored the feasibility of adapting the pressure‐based method to provide continuous, automated estimation of RVEF when RV pressure is measured. In 23 PAH patients, the median beat‐to‐beat RVEF estimation over 8–10 s segments correlated with cardiac MRI and demonstrated potential value for predicting a cardiac MRI RVEF <35%. Although encouraging, these preliminary results require controlled and rigorous validation and method refinement.
Pressure‐based estimation of RVEF, like single beat methods for defining RV:PA coupling, relies on the derivation of Pmax and ESP. The former represents the pressure generated within the RV if ejection never occurred (i.e. if the contraction remained isovolumic). 28 , 29 Importantly, however, for the current study derivation of these variables was different from more conventional single beat methods. In contrast to the common approach of predicting Pmax from a sinusoidal function 4 , 5 , 9 , 28 using customized software and an a priori estimation of Pmax, the current method applies a distribution function. This does not require an empiric estimation of Pmax and should theoretically improve reproducibility among investigators. Similarly, various surrogates for ESP have been proposed, including peak RV systolic pressure, 30 mPAP, 4 , 10 a mathematical correction equation for mPAP [ESP = (mPAP*1.65) − 7.79], 19 and more recently one approximating the point of maximal RV elastance. 15 Of these proposed surrogates for ESP, the latter two methods provided the best accuracy and precision when compared with a reference standard derived from simultaneous measurement of RV pressure and volume. 31 In the current study, a previously described approach for defining ESP as the point of maximal RV elastance was applied. 15 , 31 This method was developed using simultaneous RV pressure and volume measurements over a range of loading conditions in swine and regards the point of maximal RV pressure/RV volume ratio as end‐systole 32 (Figure 4 ). For the proof‐of‐concept evaluation of a simplified continuous beat‐to‐beat assessment of RVEF, we estimated ESP using the correction equation for mPAP [ESP = (mPAP*1.65) − 7.79]. 19
Figure 4.
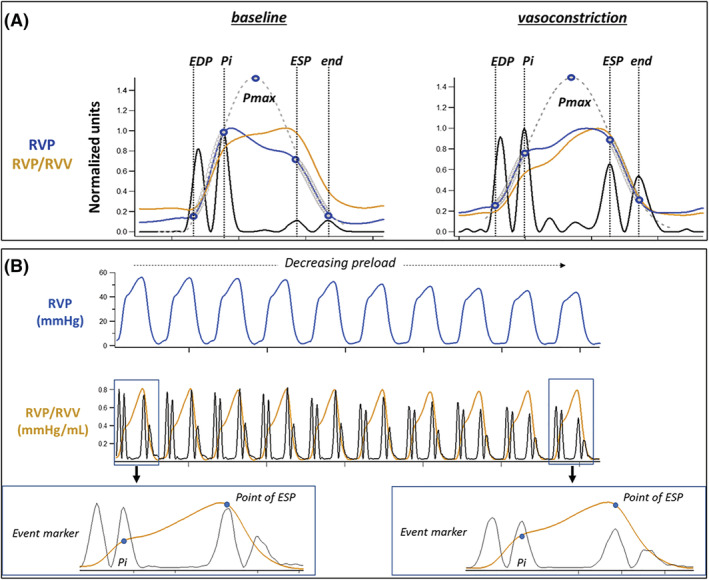
Panel (A) demonstrates that the relationship between maximal time varying elastance (RVP/RVV) and ESP defined using the pressure‐based single beat method remains constant with increased afterload despite a shift in the timing of peak RVP from early to late systole. Panel (B) demonstrates that that the relationship between maximal time varying elastance and single beat definition of ESP also remains constant when decreasing preload by caval occlusion. Data were acquired in a swine model 15 under protocols approved by Institutional Animal Care and use Committee and in compliance with the NIH Guide for the Care and Use of Laboratory Animals. Ea, pulmonary arterial elastance; Ees, end systolic elastance; ESP, end systolic pressure; Pi, first inflection point on RVP; Pmax, maximal pressure attained during isovolumic contraction; RVP, right ventricle pressure; RVV, right ventricle volume.
Results of the study need to be interpreted in the context of potential limitations. First, Bland–Altman analysis was performed on RVEF measurements made hours apart and under what appeared to be different hemodynamic conditions for some patients. For example, one patient with a heart rate of 62 and systemic SV of 31 mL during cardiac MRI, had a heart rate of 106 and SV of 53 mL during RHC. Data analysis was therefore focused upon patients in whom heart rate measured during cardiac MRI and RHC varied by <25%. Importantly, the results of Bland–Altman analysis need to be considered in light of non‐coincident measurement. Similar to end‐diastolic volume, stroke volume, and cardiac output, RVEF is constantly changing with respiration and beat‐to‐beat heart rate oscillation. Therefore, even if measured serially and simultaneously, inherent variability of both the reference standard (cardiac MRI) and the pressure‐based EF prediction method will affect precision (limits of agreement). 33 As such, while the modest bias suggests accuracy of the EF prediction method relative to cardiac MRI, the limits of agreement are relatively wide. Nonetheless, when the limits of agreement (calculated as the bias standard deviation × 1.96) are considered relative to the mean of all the cardiac MRI RVEF measurements, overall error can be calculated as: limits of agreement (10.9%)/mean (37.7%) = 0.29. While seemingly high, this value falls within a range commonly regarded as clinically acceptable for hemodynamic monitors. 33
Second, because the method for predicting Pmax involves linking the initial rise in RV pressure during isovolumic contraction and early ejection with the later decline during isovolumic relaxation, it is possible that alteration in morphology of the initial pressure rise due to significant tricuspid insufficiency could affect prediction of Pmax and ultimately estimation of RVEF. Alternatively, because RVEF measured by cardiac MRI reflects the total change in RV volume during systole—which is bidirectional in the setting of significant tricuspid insufficiency—disparity in measured and estimated RVEF may be enhanced by progressively severe tricuspid regurgitation if the estimated RVEF is primarily reflective of only forward ejection. Interestingly, there was no demonstrable correlation between the tricuspid regurgitant fraction at cardiac MRI and either measured or estimated RVEF, or their difference. Third, a recognized limitation of single beat methods for defining RV:PA coupling, and for estimating RVEF, is the assumption that Vo, the volume intercept of the end‐systolic pressure/volume relationship is negligible. 17 Recent data have underscored that this is rarely true especially in the setting of PH. 17 Results from the current study demonstrated that while both measured and estimated RVEF were correlated with Vo, their difference was not. Ultimately these data indicate that despite the wide range of Vo measured at RHC, the resulting impact of Vo on estimated RVEF relative to cardiac MRI was not as pronounced as anticipated. Finally, as with any waveform analysis algorithm, signal quality is a critical feature and the current investigation involved only high‐fidelity pressure data obtained with micromanometers. While a previous report demonstrated a favourable comparison between RVEF estimated using RV pressure measured from fluid‐filled catheters and that measured on the same day by cardiac MRI, this study included only six patients. 15 The fluid‐filled catheter system is vulnerable to hydrostatic influences and is often limited by poor fidelity and pressure damping. As an alternative, solid state pressure wires have been increasingly utilized in clinical studies to overcome these limitations. 34 , 35
In summary, a method for estimating RVEF entirely from the RV pressure waveform significantly correlated with RVEF measured by cardiac MRI and was able to reliably predict a maladaptive RV response in patients with PAH. In addition, proof‐of‐concept data suggest it may be possible to apply the method on a beat‐to‐beat basis at the bedside. Overall, the current study supports potential utility of pressure‐based RVEF estimation as a tool for assessing the response to diagnostic or therapeutic interventions during RHC. Further studies within a larger cohort of PH patient are needed to examine the applicability of the current pressure‐based method using conventional fluid‐filled catheters.
Conflict of interest
PMH: Consulting for Philips and Cardiage LLC.
Funding
This study is funded by NIH 2T32GM086287‐11 (A.E.); NIH NHLBI K25 HL33481NIH (V.K.); CRC 1213 SFB Project B08 (M.J.R. and K.T.).
Ethical approval
IRB approval was given by the University Hospital Giessen 108/15.
Heerdt, P. M. , Singh, I. , Elassal, A. , Kheyfets, V. , Richter, M. J. , and Tello, K. (2022) Pressure‐based estimation of right ventricular ejection fraction. ESC Heart Failure, 9: 1436–1443. 10.1002/ehf2.13839.
References
- 1. Vonk Noordegraaf A, Chin KM, Haddad F, Hassoun PM, Hemnes AR, Hopkins SR, Kawut SM, Langleben D, Lumens J, Naeije R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: An update. Eur Respir J 2019; 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sanz J, Sanchez‐Quintana D, Bossone E, Bogaard HJ, Naeije R. Anatomy, function, and dysfunction of the right ventricle: JACC state‐of‐the‐art review. J Am Coll Cardiol 2019; 73: 1463–1482. [DOI] [PubMed] [Google Scholar]
- 3. Peacock AJ, Vonk Noordegraaf A. Cardiac magnetic resonance imaging in pulmonary arterial hypertension. Eur Respir Rev 2013; 22: 526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh I, Rahaghi FN, Naeije R, Oliveira RKF, Systrom DM, Waxman AB. Right ventricular‐arterial uncoupling during exercise in heart failure with preserved ejection fraction: Role of pulmonary vascular dysfunction. Chest 2019; 156: 933–943. [DOI] [PubMed] [Google Scholar]
- 5. Singh I, Oliveira RKF, Naeije R, Rahaghi FN, Oldham W, Systrom DM, Waxman AB. Pulmonary vascular Distensibility and early pulmonary vascular remodeling in pulmonary hypertension. Chest 2019; 156: 724–732. [DOI] [PubMed] [Google Scholar]
- 6. Spruijt OA, de Man FS, Groepenhoff H, Oosterveer F, Westerhof N, Vonk‐Noordegraaf A, Bogaard HJ. The effects of exercise on right ventricular contractility and right ventricular‐arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med 2015; 191: 1050–1057. [DOI] [PubMed] [Google Scholar]
- 7. Rajaratnam A, Rehman S, Sharma P, Singh VK, Saul M, Vanderpool RR, Gladwin MT, Simon MA, Morris A. Right ventricular load and contractility in HIV‐associated pulmonary hypertension. PLoS One 2021; 16: e0243274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Contijoch F, Wong D, Igata S, Mizzell AM, Auger W, DeMaria AN, Blanchard D, Waheed A, Bachman TN, Simon MA, Pinsky MR, Madani M. Association between preoperative dynamic measures of vascular load and postoperative hemodynamics in patients with chronic thromboembolic pulmonary hypertension after pulmonary Thromboendarterectomy. Ann Am Thorac Soc 2021; 18: 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh I, Rahaghi FN, Naeije R, Oliveira RKF, Vanderpool RR, Waxman AB, Systrom DM. Dynamic right ventricular‐pulmonary arterial uncoupling during maximum incremental exercise in exercise pulmonary hypertension and pulmonary arterial hypertension. Pulm Circ 2019; 9 2045894019862435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singh I, Oliveira RK, Heerdt PM, Brown MB, Urbina MF, Waxman AB, Systrom DM. Dynamic right ventricular function response to incremental exercise in pulmonary hypertension. Pulm Circ 2020; 10: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Richter MJ, Peters D, Ghofrani HA, Naeije R, Roller F, Sommer N, Gall H, Grimminger F, Seeger W, Tello K. Evaluation and prognostic relevance of right ventricular‐arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med 2020; 201: 116–119. [DOI] [PubMed] [Google Scholar]
- 12. Schreuder JJ, van der Veen FH, van der Velde ET, Delahaye F, Alfieri O, Jegaden O, Lorusso R, Jansen JR, van Ommen V, Finet G, Wellens HJ. Beat‐to‐beat analysis of left ventricular pressure‐volume relation and stroke volume by conductance catheter and aortic Modelflow in cardiomyoplasty patients. Circulation 1995; 91: 2010–2017. [DOI] [PubMed] [Google Scholar]
- 13. Bootsma IT, Boerma EC, de Lange F, Scheeren TWL. The contemporary pulmonary artery catheter. Part 1: Placement and waveform analysis. J Clin Monit Comput 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoeper MM, Tongers J, Leppert A, Baus S, Maier R, Lotz J. Evaluation of right ventricular performance with a right ventricular ejection fraction thermodilution catheter and MRI in patients with pulmonary hypertension. Chest 2001; 120: 502–507. [DOI] [PubMed] [Google Scholar]
- 15. Heerdt PM, Kheyfets V, Charania S, Elassal A, Singh I. A pressure‐based single beat method for estimation of right ventricular ejection fraction: Proof of concept. Eur Respir J 2020; 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elassal A, Steppan J, Charania S, Santhanam L, Singh I, Heerdt PM. Pressure‐based estimation of right ventricular ejection fraction: Validation as a clinically relevant target for drug development in a rodent model of pulmonary hypertension. J Pharmacol Toxicol Methods 2021; 112: 107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trip P, Kind T, van de Veerdonk MC, Marcus JT, de Man FS, Westerhof N, Vonk‐Noordegraaf A. Accurate assessment of load‐independent right ventricular systolic function in patients with pulmonary hypertension. J Heart Lung Transplant 2013; 32: 50–55. [DOI] [PubMed] [Google Scholar]
- 18. Reynolds DW, Bartelt N, Taepke R, Bennett TD. Measurement of pulmonary artery diastolic pressure from the right ventricle. J Am Coll Cardiol 1995; 25: 1176–1182. [DOI] [PubMed] [Google Scholar]
- 19. Tello K, Richter MJ, Axmann J, Buhmann M, Seeger W, Naeije R, Ghofrani HA, Gall H. More on single‐beat estimation of right Ventriculo‐arterial coupling in pulmonary arterial hypertension. Am J Respir Crit Care Med 2018; 198: 816–818. [DOI] [PubMed] [Google Scholar]
- 20. Koo TK, Li MY. A guideline of selecting and reporting Intraclass correlation coefficients for reliability research. J Chiropr Med 2016; 15: 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vanderpool RR, Rischard F, Naeije R, Hunter K, Simon MA. Simple functional imaging of the right ventricle in pulmonary hypertension: Can right ventricular ejection fraction be improved? Int J Cardiol 2016; 223: 93–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baggen VJ, Leiner T, Post MC, van Dijk AP, Roos‐Hesselink JW, Boersma E, Habets J, Sieswerda GT. Cardiac magnetic resonance findings predicting mortality in patients with pulmonary arterial hypertension: A systematic review and meta‐analysis. Eur Radiol 2016; 26: 3771–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brewis MJ, Bellofiore A, Vanderpool RR, Chesler NC, Johnson MK, Naeije R, Peacock AJ. Imaging right ventricular function to predict outcome in pulmonary arterial hypertension. Int J Cardiol 2016; 218: 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van de Veerdonk MC, Kind T, Marcus JT, Mauritz GJ, Heymans MW, Bogaard HJ, Boonstra A, Marques KM, Westerhof N, Vonk‐Noordegraaf A. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011; 58: 2511–2519. [DOI] [PubMed] [Google Scholar]
- 25. Vanderpool RR, Pinsky MR, Naeije R, Deible C, Kosaraju V, Bunner C, Mathier MA, Lacomis J, Champion HC, Simon MA. RV‐pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart 2015; 101: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berlin DA, Manoach S, Oromendia C, Heerdt PM. Automated expiratory ventilation assistance through a small endotracheal tube can improve venous return and cardiac output. Intensive Care Med Exp 2019; 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tello K, Dalmer A, Axmann J, Vanderpool R, Ghofrani HA, Naeije R, Roller F, Seeger W, Sommer N, Wilhelm J, Gall H, Richter MJ. Reserve of Right Ventricular‐Arterial Coupling in the setting of chronic overload. Circ Heart Fail 2019; 12: e005512. [DOI] [PubMed] [Google Scholar]
- 28. Sunagawa K, Yamada A, Senda Y, Kikuchi Y, Nakamura M, Shibahara T, Nose Y. Estimation of the hydromotive source pressure from ejecting beats of the left ventricle. IEEE Trans Biomed Eng 1980; 27: 299–305. [DOI] [PubMed] [Google Scholar]
- 29. Brimioulle S, Wauthy P, Ewalenko P, Rondelet B, Vermeulen F, Kerbaul F, Naeije R. Single‐beat estimation of right ventricular end‐systolic pressure‐volume relationship. Am J Physiol Heart Circ Physiol 2003; 284: H1625–H1630. [DOI] [PubMed] [Google Scholar]
- 30. Metkus TS, Mullin CJ, Grandin EW, Rame JE, Tampakakis E, Hsu S, Kolb TM, Damico R, Hassoun PM, Kass DA, Mathai SC, Tedford RJ. Heart rate dependence of the pulmonary resistance x compliance (RC) time and impact on right ventricular load. PLoS One 2016; 11: e0166463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singh I, Oakland H, Elassal A, Heerdt PM. Defining end‐systolic pressure for single‐beat estimation of right ventricle‐pulmonary artery coupling: Simple… But not really. ERJ Open Res 2021; 7: 00219–02021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sagawa KML, Suga H, Sunagawa K. Cardiac Contraction and the Pressure‐Volume Relationship. New York: Oxford University Press; 1988. [Google Scholar]
- 33. Odor PM, Bampoe S, Cecconi M. Cardiac output monitoring: Validation studies‐how results should be presented. Curr Anesthesiol Rep 2017; 7: 410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Richter MJ, Yogeswaran A, Husain‐Syed F, Vadasz I, Rako Z, Mohajerani E, Ghofrani HA, Naeije R, Seeger W, Herberg U, Rieth A, Tedford RJ, Grimminger F, Gall H, Tello K. A novel non‐invasive and echocardiography‐derived method for quantification of right ventricular pressure‐volume loops. Eur Heart J Cardiovasc Imaging 2021; jeab038. [DOI] [PubMed] [Google Scholar]
- 35. Jain CC, Borlaug BA. Performance and interpretation of invasive hemodynamic exercise testing. Chest 2020; 158: 2119–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]


