Abstract
Aims
Prophylactic implantable cardioverter‐defibrillators (ICD) reduce mortality in patients with ischaemic heart failure (HF), whereas the effect of ICD in patients with non‐ischaemic HF is less clear. We aimed to investigate the association between concomitant coronary atherosclerosis and mortality in patients with non‐ischaemic HF and the effect of ICD implantation in these patients.
Methods and results
Patients were included from DANISH (Danish Study to Assess the Efficacy of Implantable Cardioverter Defibrillators in Patients with Non‐Ischaemic Systolic Heart Failure on Mortality), randomizing patients to ICD or control. Study inclusion criteria for HF were left ventricular ejection fraction ≤ 35% and increased levels (>200 pg/mL) of N‐terminal pro‐brain natriuretic peptide. Of the 1116 patients from DANISH, 838 (75%) patients had available data from coronary angiogram and were included in this subgroup analysis. We used Cox regression to assess the relationship between coronary atherosclerosis and mortality and the effect of ICD implantation. Of the included patients, 266 (32%) had coronary atherosclerosis. Of these, 216 (81%) had atherosclerosis without significant stenoses, and 50 (19%) had significant stenosis. Patients with atherosclerosis were significantly older {67 [interquartile range (IQR) 61–73] vs. 61 [IQR 54–68] years; P < 0.0001}, and more were men (77% vs. 70%; P = 0.03). During a median follow‐up of 64.3 months (IQR 47–82), 174 (21%) of the patients died. The effect of ICD on all‐cause mortality was not modified by coronary atherosclerosis [hazard ratio (HR) 0.94; 0.58–1.52; P = 0.79 vs. HR 0.82; 0.56–1.20; P = 0.30], P for interaction = 0.67. In univariable analysis, coronary atherosclerosis was a significant predictor of all‐cause mortality [HR, 1.41; 95% confidence interval (CI), 1.04–1.91; P = 0.03]. However, this association disappeared when adjusting for cardiovascular risk factors (age, gender, diabetes, hypertension, smoking, and estimated glomerular filtration rate) (HR 1.05, 0.76–1.45, P = 0.76).
Conclusions
In patients with non‐ischaemic systolic heart failure, ICD implantation did not reduce all‐cause mortality in patients either with or without concomitant coronary atherosclerosis. The concomitant presence of coronary atherosclerosis was associated with increased mortality. However, this association was explained by other risk factors.
Keywords: Non‐ischaemic systolic heart failure, Coronary atherosclerosis, Implantable cardioverter defibrillator, Mortality
Introduction
Heart failure (HF) aetiology is commonly divided into ischaemic and non‐ischaemic, and this division is also reflected in the European guidelines. 1 Prognosis of systolic HF differs due to the aetiology, and it is well established that patients with ischaemic heart disease have poorer prognosis. 2 , 3 For patients with ischaemic HF, the implantation of an implantable cardioverter‐defibrillator (ICD) is associated with a marked reduction in sudden cardiac death (SCD) and all‐cause mortality. 4 , 5 , 6 , 7 For patients with non‐ischaemic systolic HF, no single study has found evidence of benefit of ICD implantation on all‐cause mortality. 8 , 9 , 10 , 11 However, based on meta‐analyses, ICD implantation is recommended as a class I evidence level B treatment for both ischaemic and non‐ischaemic HF in the 2016 European Society of Cardiology Guidelines for the diagnosis and treatment of acute and chronic HF. 1
It is uncertain if concomitant coronary atherosclerosis is related to outcome in patients with non‐ischaemic systolic HF and whether the effect of implanting an ICD is different in patients with non‐ischaemic HF and coronary atherosclerosis. Patients with non‐ischaemic HF and some extent on coronary atherosclerosis may be more similar to patients with ischaemic aetiology of HF than those with no atherosclerosis, and the effect of ICD implantation could potentially differ within these subgroups. Therefore, the aim of this study was to investigate the association between coronary atherosclerosis and all‐cause mortality in patients with non‐ischaemic systolic HF and the effect of ICD implantation in these patients.
Methods
Study design and patients
The DANISH study (Danish Study to Assess the Efficacy of Implantable Cardioverter Defibrillators in Patients with Non‐Ischaemic Systolic Heart Failure on Mortality) was a randomized controlled multicentre trial investigating the effect of ICD implantation in patients with non‐ischaemic systolic HF. To be included in the study, patients should have left ventricular ejection fraction ≤ 35% and increased levels (>200 pg/mL) of N‐terminal pro‐brain natriuretic peptide (NT‐proBNP). Coronary artery disease (CAD) as the cause of HF had to be ruled out prior to inclusion into the study. This was performed by coronary angiography (96% of patients), computed tomography angiography, or nuclear myocardial perfusion imaging. Patients could have diffuse atherosclerosis, one‐vessel, or two‐vessel disease on the qualifying coronary angiogram if the investigator did not find that the degree of CAD could explain the severely reduced left ventricular ejection fraction. Coronary obstruction was defined as of atherosclerosis of >50% and fractional flow reserve < 0.8 (where available). Patients were randomized to ICD or control. Design and main results have been published previously. No overall effect on all‐cause mortality was found with ICD implantation. 11 , 12
Patients were included in the study only after providing informed consent, and the study was performed according to the principles of the Declaration of Helsinki. The regional scientific ethics committee for the capital region (H‐D‐2007‐0101) and the Danish Data Protection Agency approved the study.
Follow‐up and endpoints
To be included in DANISH, patients should be in optimal medical treatment for HF. No minimum duration of HF was required. The primary endpoint was death from any cause, and the secondary endpoints were cardiovascular (CV) death and SCD. All endpoints were adjudicated according to previously reported criteria by a clinical endpoint committee. 11 The definition of SCD was unexpected death in a previously stable patient, death occurring within an hour of onset or worsening of symptoms, or unwitnessed death, in patients who had no sign of life‐threatening disease or symptoms when seen alive < 72 h before death, and when circumstances suggested sudden death (e.g. when a patient was found in bed). End of follow‐up was 30 June 2016, and patients without an event were right censored at this time.
Statistics
Baseline characteristics of the groups were compared with χ2 test for categorical variables and Wilcoxon two‐sample test for continuous variables. Patients were considered to have coronary atherosclerosis if the invasive cardiologist described diffuse atherosclerosis or coronary stenosis and proportional hazards regression was used to assess the relationship between coronary atherosclerosis and mortality and between ICD implantation and mortality in patients with and without coronary atherosclerosis. Data are presented as hazard ratios with 95% confidence intervals (CIs). Two‐sided P values < 0.05 were considered statistically significant. Multivariable models were adjusted for age, gender, diabetes, hypertension, smoking, and estimated glomerular filtration rate (eGFR). SAS software Version 9.4 (SAS Institute) and R software Version 3.3.1 (R Project for Statistical Computing) were used for analyses.
Results
Coronary angiography data were available in 838 (75%) of the 1116 patients included in DANISH, and all these patients were included in this subgroup analysis. In total, 266 (32%) had coronary atherosclerosis, 216 (81%) of whom were reported by the individual invasive centres as having atherosclerosis without significant stenoses, and 50 (19%) with significant stenosis. Data on stenosis location were not available. Table 1 shows baseline characteristics of patients with and without coronary atherosclerosis. Patients with coronary atherosclerosis were significantly older, more often men and in a higher New York Heart Association class. Furthermore, prevalence of comorbidities (diabetes mellitus, hypertension, atrial fibrillation, and renal impairment) was higher in the group with coronary atherosclerosis. Cause of HF differed between the groups and median NT‐proBNP, median systolic blood pressure, and median QRS duration were higher in patients with coronary atherosclerosis. However, rate of ICD implantation did not differ between groups. Also, no difference was found between groups regarding medical treatment of HF, left ventricular ejection fraction, cardiac resynchronization therapy implantation, or body mass index.
Table 1.
Characteristics of the patients at baseline
| Patients without coronary atherosclerosis (N = 572) | Patients with coronary atherosclerosis (N = 266) | P value | |
|---|---|---|---|
| Age (IQR), year | 61 (54–68) | 67 (61–73) | <0.0001 |
| Male gender, no. (%) | 402 (70) | 206 (77) | 0.03 |
| Body mass index (IQR), kg/m2 | 27.1 (23.9–30.5) | 26.5 (23.9–30.2) | 0.27 |
| NT‐proBNP (IQR), pg/mL | 1120 (533–2198) | 1424 (654–2742) | 0.02 |
| Estimated GFR (IQR), mL/min/1.73 m2 | 75 (60–94) | 73 (54–90) | 0.01 |
| QRS duration (IQR), ms | 144 (112–166) | 151 (120–167) | 0.04 |
| Left ventricular ejection fraction (IQR), % | 25 (20–30) | 25 (20–30) | 0.93 |
| Blood pressure (IQR), mmHg | |||
| Systolic | 121 (109–135) | 128 (114–142) | <0.001 |
| Diastolic | 73 (65–81) | 74 (66–83) | 0.38 |
| NYHA class, no. (%) | |||
| II | 326 (57) | 128 (48) | 0.02 |
| III | 238(42) | 136 (51) | |
| IV | 8 (1) | 2 (1) | |
| Coexisting conditions, no. (%) | |||
| Diabetes mellitus | 97 (17) | 79 (30) | <0.0001 |
| Hypertension | 146 (26) | 114 (43) | <0.001 |
| Permanent atrial fibrillation | 101 (18) | 63 (24) | 0.03 |
| Ever smoker | 453 (79) | 207 (78) | 0.65 |
| Means of exclusion of ischemic cause of heart failure, no. (%) | |||
| Myocardial scintigraphy | 2 (1) | 0 (0) | 0.34 |
| CT angiogram | 18 (3) | 2 (1) | 0.03 |
| Coronary angiography | 556 (97) | 264 (99) | 0.06 |
| Cause of heart failure, no. (%) | |||
| Idiopathic | 457 (80) | 191 (72) | <0.01 |
| Valvular | 20 (4) | 7 (3) | |
| Hypertension | 50 (9) | 41 (15) | |
| Other | 45 (8) | 27 (10) | |
| Medications, no. (%) | |||
| ACE‐inhibitor or ARB | 553 (97) | 255 (96) | 0.56 |
| Beta blocker | 525 (92) | 248 (93) | 0.47 |
| Aldosterone receptor antagonist | 325 (57) | 143 (54) | 0.41 |
| Amiodarone | 37 (6) | 13 (5) | 0.37 |
| Device therapy, no. (%) | |||
| Cardiac resynchronization therapy | 343 (60) | 164 (62) | 0.64 |
| Implantable cardioverter‐defibrillator | 276 (48) | 137 (52) | 0.38 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin‐receptor blocker; CT, computed tomography; GFR, glomerular filtration rate; IQR, interquartile range; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association.
During a median follow‐up of 64.3 months [interquartile range (IQR) 47–82], 174 (21%) of the patients included in this subgroup analysis died. Treatment with ICD did not affect all‐cause mortality in patients with or without coronary atherosclerosis [hazard ratio (HR) 0.94; 0.58–1.52; P = 0.79 vs. HR 0.82; 0.56–1.20; P = 0.30], P for interaction = 0.67. In univariable analysis, coronary atherosclerosis significantly predicted of all‐cause mortality (HR, 1.41; 95% CI 1.04–1.91; P = 0.03), (Figure 1 ). This did not change when adjusting for hypertension (HR, 1.42; 95% CI 1.04–1.95; P = 0.03) or smoking (HR, 1.41; 95% CI 1.04–1.91; P = 0.03) However, the association between coronary atherosclerosis and all‐cause mortality disappeared when adjusting for other CV risk factors (age, gender, diabetes, or eGFR) as well as in a multiple analysis adjusting for multiple CV risk factors (age, gender, diabetes, hypertension, smoking, and eGFR) (HR 1.05, 0.76–1.45, P = 0.76). Unadjusted and adjusted hazard ratios are shown in Figure 2 .
Figure 1.
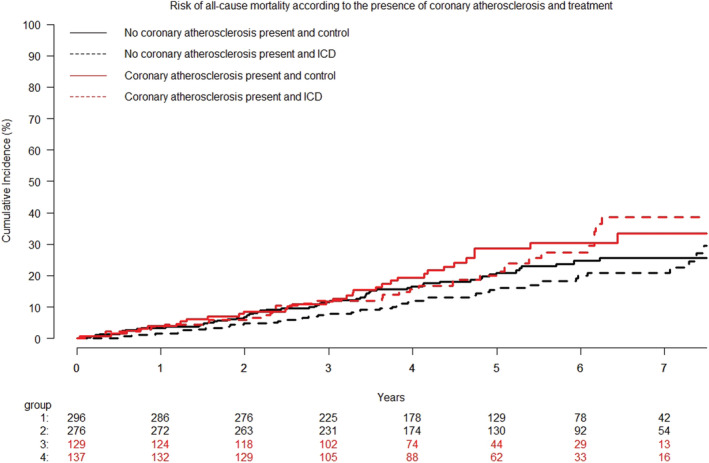
Cumulative incidence curves of all‐cause mortality according to ICD treatment and the presence of coronary atherosclerosis. Patients with coronary atherosclerosis had an increased risk of all‐cause mortality hazard ratio 1.41 (95% confidence interval, 1.04–1.91); P = 0.03. ICD, implantable cardioverter defibrillators.
Figure 2.
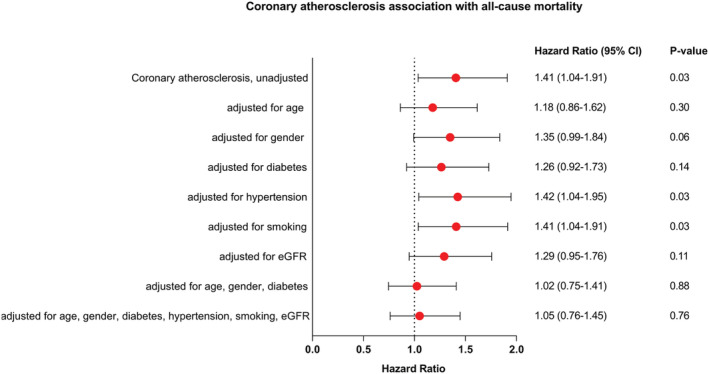
Coronary atherosclerosis association with all‐cause mortality. eGFR denotes estimated glomerular filtration rate. CI, confidence interval.
No difference was found in CV death between patients with and without coronary atherosclerosis [HR 0.92 (95% CI, 0.63–1.36); P = 0.69] (Figure 3 ) The risk of SCD also did not differ between the two groups [HR 0.71 (95% CI, 0.37–1.35); P = 0.29] (Figure 4 ). There was no significant statistical interaction between treatment effect of ICD and presence of coronary atherosclerosis on CV death (P for interaction = 0.53) or SCD (P for interaction = 0.11) (Figures 3 and 4 ). Finally, there was no indication of a difference in results when patients with coronary atherosclerosis were divided into those with and without obstructive coronary artery disease on the qualifying coronary angiogram. Descriptive data on cause of death stratified by level of coronary obstruction are shown in Table 2 .
Figure 3.
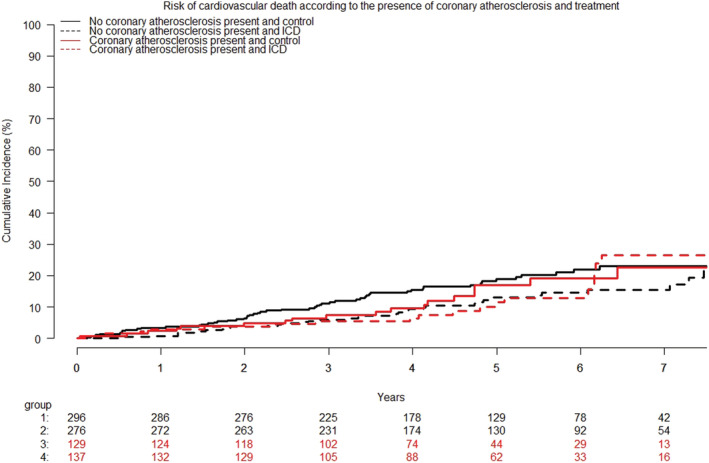
Cumulative incidence curves of the risk of cardiovascular death according to ICD treatment and the presence of coronary atherosclerosis. The presence of coronary atherosclerosis was not associated with the risk of cardiovascular death HR 0.92 (95% CI, 0.63–1.36); P = 0.69. ICD, implantable cardioverter defibrillators.
Figure 4.
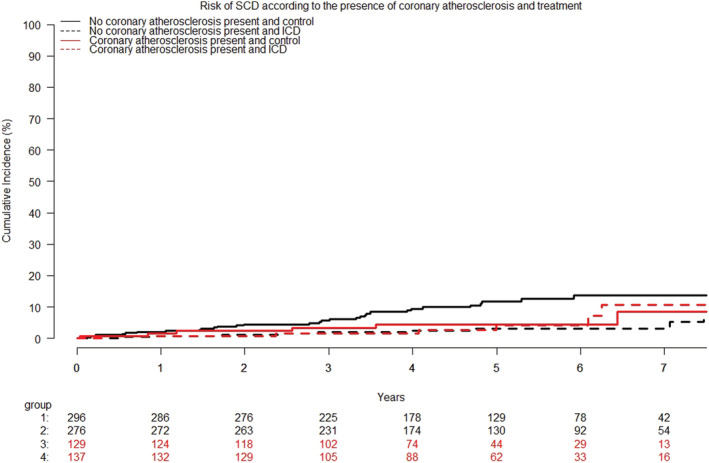
Cumulative incidence curves of the risk of sudden cardiac death according to ICD treatment and the presence of coronary atherosclerosis. The presence of coronary atherosclerosis was not associated with the risk of cardiovascular death hazard ratio 0.71 (95% confidence interval, 0.37–1.35); P = 0.29. ICD, implantable cardioverter defibrillators; SCD, sudden cardiac death.
Table 2.
Cause of death stratified by level of coronary obstruction
| No coronary atherosclerosis (N = 572) | Coronary atherosclerosis (N = 216) | One‐vessel disease (N = 37) | Multi‐vessel disease (N = 13) | |
|---|---|---|---|---|
| All‐cause mortality, no. (%) | 108 (19) | 52 (24) | 9 (24) | 5 (38) |
| Cardiovascular death, no. (%) | 90 (16) | 28 (13) | 5 (14) | 3 (23) |
| Sudden cardiac death, no. (%) | 39 (7) | 9 (4) | 1 (3) | 2 (15) |
One‐vessel or multi‐vessel disease was defined as coronary obstruction of >50% and fractional flow reserve < 0.8 (where available) in one or more coronary arteries, respectively.
Discussion
The current study is a substudy to DANISH investigating 838 patients with available coronary angiography data. The study demonstrated that in patients with non‐ischaemic systolic HF, ICD implantation was not associated with changes in mortality risk, independent of concomitant coronary atherosclerosis. The concomitant presence of coronary atherosclerosis was significantly associated with increased all‐cause mortality. However, in multivariable analyses, this association was no longer significant after adjusting for traditional CV risk factors associated with atherosclerosis and well known to predict mortality.
In a recent registry study by Braga et al, the authors found significant association between non‐obstructive coronary artery disease and death from any cause in patients with reduced ejection fraction, also when adjusting for baseline comorbidities. 13 However, 13% of patients with non‐obstructive coronary artery disease had a history of myocardial infarction and would likely not have been included in the DANISH trial. It is uncertain whether results would have been similar if patients with history of AMI were excluded from the study by Braga et al.
The presence of atherosclerosis in patients with systolic HF of non‐ischaemic aetiology did not indicate an increased effect of ICD implantation. An explanation may be that the increased risk of malignant arrhythmia is mainly caused by large areas of ischemic scar tissue with border zone ischaemia and re‐entry pathways in the infarct region. 14 , 15 , 16 Therefore, atherosclerosis without history of prior myocardial infarction may not presuppose the same benefit of ICD implantation.
In perspective, the effect of ICD implantation in patients with systolic HF on current state of the art optimized guideline directed medical therapy has been called into question. Evidence‐based medications for the treatment of systolic HF have improved over the last two decades, and correspondingly, the rates of sudden death have declined consistently and substantially over time among ambulatory patients with systolic HF who were enrolled in clinical trials. These declining rates of sudden death may undermine the benefits of ICD implantation. 17
Some limitations of the current study should be addressed. This is a post hoc analysis, and randomization to ICD or control was not stratified by atherosclerosis burden. Also, details on the performed coronary angiography were only available from the invasive centres for 75% of the patients included in DANISH. We had limited statistical power to assess whether there was in a difference in outcomes for patients with obstructive versus non‐obstructive coronary atherosclerosis.
In conclusion, no association was found between ICD implantation and all‐cause mortality either in patients with non‐ischemic systolic HF with or without concomitant coronary atherosclerosis. The concomitant presence of coronary atherosclerosis was univariably associated with increased all‐cause mortality. However, this association was explained by the presence of other well‐established CV risk factors.
Conflict of interest
Christina Byrne, Ole Ahlehoff, Marie Bayer Elming, Frants Pedersen, Hans Eiskjær, Anna Margrethe Thøgersen, Jens Haarbo, Lars Videbæk, Lars Køber, and Jens Jakob Thune report no conflicts of interest related to the present manuscript. Jens Cosedis Nielsen is supported by a grant from the Novo Nordisk Foundation (NNF16OC0018658). Jesper Hastrup Svendsen has received research grants outside the present study and personal speaker fee from Medtronic. Steen Pehrson has received personal speaker fee from Abbott.
Funding
This work is supported by unrestricted grants from Medtronic, St. Jude Medical, TrygFonden, and the Danish Heart Foundation.
Byrne, C. , Ahlehoff, O. , Elming, M. B. , Pedersen, F. , Pehrson, S. , Nielsen, J. C. , Eiskjær, H. , Videbæk, L. , Svendsen, J. H. , Haarbo, J. , Thøgersen, A. M. , Køber, L. , and Thune, J. J. (2022) Effect of implantable cardioverter‐defibrillators in patients with non‐ischaemic systolic heart failure and concurrent coronary atherosclerosis. ESC Heart Failure, 9: 1287–1293. 10.1002/ehf2.13810.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long‐term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000; 342: 1077–1084. [DOI] [PubMed] [Google Scholar]
- 3. Gheorghiade M, Sopko G, De Luca L, Velazquez EJ, Parker JD, Binkley PF, Sadowski Z, Golba KS, Prior DL, Rouleau JL, Bonow RO. Navigating the crossroads of coronary artery disease and heart failure. Circulation 2006; 114: 1202–1213. [DOI] [PubMed] [Google Scholar]
- 4. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigators. N Engl J Med 1996; 335: 1933–1940. [DOI] [PubMed] [Google Scholar]
- 5. Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter unsustained tachycardia trial investigators. N Engl J Med 1999; 341: 1882–1890. [DOI] [PubMed] [Google Scholar]
- 6. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML, Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346: 877–883. [DOI] [PubMed] [Google Scholar]
- 7. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp‐Channing N, Davidson‐Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH, Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) Investigators . Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med 2005; 352: 225–237. [DOI] [PubMed] [Google Scholar]
- 8. Bänsch D, Antz M, Boczor S, Volkmer M, Tebbenjohanns J, Seidl K, Block M, Gietzen F, Berger J, Kuck KH. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the cardiomyopathy trial (CAT). Circulation 2002; 105: 1453–1458. [DOI] [PubMed] [Google Scholar]
- 9. Strickberger SA, Hummel JD, Bartlett TG, Frumin HI, Schuger CD, Beau SL, Bitar C, Morady F, AMIOVIRT Investigators . Amiodarone versus implantable cardioverter‐defibrillator: randomized trial in patients with nonischemicdilated cardiomyopathy and asymptomaticnonsustained ventricular tachycardia—AMIOVIRT. J Am Coll Cardiol 2003; 41: 1707–1712. [DOI] [PubMed] [Google Scholar]
- 10. Kadish A, Dyer A, Daubert JP, Quigg R, Estes NAM, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH, Defibrillators in Non‐Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators . Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004; 350: 2151–2158. [DOI] [PubMed] [Google Scholar]
- 11. Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjær H, Brandes A, Thøgersen AM, Gustafsson F, Egstrup K, Videbæk R, Hassager C, Svendsen JH, Høfsten DE, Torp‐Pedersen C, Pehrson S. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016; 375: 1221–1230. [DOI] [PubMed] [Google Scholar]
- 12. Thune JJ, Pehrson S, Nielsen JC, Haarbo J, Videbæk L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjær H, Brandes A, Thøgersen AM, Egstrup K, Hastrup‐Svendsen J, Høfsten DE, Torp‐Pedersen C, Køber L. Rationale, design, and baseline characteristics of the DANish randomized, controlled, multicenter study to assess the efficacy of implantable cardioverter defibrillators in patients with non‐ischemic systolic heart failure on mortality (DANISH). Am Heart J 2016; 179: 136–141. [DOI] [PubMed] [Google Scholar]
- 13. Braga JR, Austin PC, Ross HJ, Tu JV, Lee DS. Importance of nonobstructive coronary artery disease in the prognosis of patients with heart failure. JACC: Heart Failure 2019; 7: 493–501. [DOI] [PubMed] [Google Scholar]
- 14. Zipes DP, Wellens HJ. Sudden cardiac death. Circulation 1998; 98: 2334–2351. [DOI] [PubMed] [Google Scholar]
- 15. Stevenson WG, Friedman PL, Sager PT, Saxon LA, Kocovic D, Harada T, Wiener I, Khan H. Exploring postinfarction reentrant ventricular tachycardia with entrainment mapping. J Am Coll Cardiol 1997; 29: 1180–1189. [DOI] [PubMed] [Google Scholar]
- 16. Roes SD, Borleffs CJW, van der Geest RJ, Westenberg JJM, Marsan NA, Kaandorp TAM, Reiber JHC, Zeppenfeld K, Lamb HJ, de Roos A, Schalij MJ, Bax JJ. Infarct tissue heterogeneity assessed with contrast‐enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter‐defibrillator. Circ Cardiovasc Imaging 2009; 2: 183–190. [DOI] [PubMed] [Google Scholar]
- 17. Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, Dargie HJ, Granger CB, Kjekshus J, Køber L, Latini R, Maggioni AP, Packer M, Pitt B, Solomon SD, Swedberg K, Tavazzi L, Wikstrand J, Zannad F, Zile MR, McMurray JJV. Declining risk of sudden death in heart failure. N Engl J Med 2017; 377: 41–51. [DOI] [PubMed] [Google Scholar]


