Abstract
Aims
We hypothesized that left atrial (LA) remodelling and function are associated with poor exercise capacity as prognostic marker in chronic heart failure (CHF) across a broad range of left ventricular ejection fraction (LVEF).
Methods and results
One hundred seventy‐one patients with CHF were analysed [age 65 ± 11 years, 136 males (80%); 86 heart failure with reduced ejection fraction (HFrEF), 27 heart failure with mid‐range ejection fraction (HFmrEF), 58 heart failure with preserved ejection fraction (HFpEF)]. All patients underwent echocardiography and maximal cardiopulmonary exercise testing and were classified according to a prognostic cut‐off of peak VO2 (pVO2; 14 mL/kg/min). Seventy‐seven (45%) patients reached pVO2 < 14 and 94 (55%) pVO2 ≥ 14 mL/kg/min. Between the two groups, there was a considerable difference in both left atrial volume (LAVi, 53 ± 24 vs. 44 ± 18 mL/m2, P = 0.005) and function (LA reservoir strain 12 ± 5 vs. 20 ± 10%, P < 0.0001). Receiver‐operating characteristic curves identified LA reservoir strain (area under the curve: 0.73 [0.65–0.80], P < 0.0001) as strong predictor for impaired pVO2 among all echocardiographic variables; LA reservoir strain < 23% had 37% specificity but a very high sensitivity (96%) in identifying a severely reduced pVO2. In logistic regression analysis, LA reservoir strain < 23% was associated with a highly increased risk of pVO2 < 14 mL/kg/min (odds ratio 16.0 [4.7–54.6]; P < 0.0001). The multivariate analysis showed that a reduced LA reservoir strain was associated with pVO2 < 14 mL/kg/min after adjustment for age, body mass index (BMI), and clinical variables, that is, New York Heart Association class, atrial fibrillation, haemoglobin, and creatinine (b 0.22 [95% confidence interval, CI, 0.12–0.31]; P < 0.0001), and after adjustment for echocardiographic variables, that is, LVEF or left ventricular global longitudinal strain (LVGLS) and tricuspid annular plane systolic excursion (TAPSE) (b 0.16 [95% CI 0.08–0.24]; P < 0.0001). Patients with HFrEF, HFmrEF, and HFpEF were separately analysed. Among LA reservoir strain, LAVi, LVEF, LVGLS, and TAPSE, LA reservoir strain was the only one significantly associated with pVO2 in all subgroups (after adjustment for sex and BMI, P = 0.003, 0.04, and 0.01, respectively).
Conclusions
In patients with CHF, an impaired LA reservoir function is independently associated with a severely reduced pVO2. LA dysfunction represents a marker of poor prognosis across LVEF borders in the CHF population.
Keywords: Left atrial strain, Cardiopulmonary exercise test, Exercise capacity, Heart failure, Ejection fraction, Prognosis
Introduction
Contemporary classification of heart failure (HF) is ubiquitously based on the use of left ventricular ejection fraction (LVEF), and three groups are currently recognized in clinical practice and research. 1 Indeed, LVEF is an established powerful predictor of outcome in HF patients, especially in those with reduced EF. In the subgroup with an LVEF above 45%, patients have a much lower risk of cardiovascular events than those with lower EF, but LVEF is not useful in further risk stratification of patients, 2 so the contribution of systolic function in prognostic assessment across the full spectrum of HF could be questioned. However, the identification of a universal marker of poor prognosis in the HF population would be of utmost importance, because the HF syndrome comprehends disorders with a variety of pathophysiological mechanisms; moreover, patients could switch from one EF‐based group to another in the course of the disease, so that appeals have been recently made to shift from an HF classification system based on LVEF alone. 3
The relationship between left atrial (LA) function and prognosis has not yet been described in HF patients across the full spectrum of EF. However, LA function has been found to be closely related to functional capacity in patients with HF both with reduced and preserved EF. 4 , 5 , 6 , 7 In the present study, we analysed the influence of LA function on exercise capacity, as recognized marker of prognosis in chronic HF, in a cohort of HF patients with a broad spectrum of EF. Indeed, LA function plays a role in exercise capacity through its influence on LV filling, because it buffers flow and pressure fluctuations during the cardiac cycle, so that on the one hand it affects LV output and on the other hand it influences pressures in the pulmonary circulation. We hypothesized that in an HF population, LA function could be a marker of poor exercise capacity regardless of LVEF.
Methods
Two hundred forty‐seven patients with a diagnosis of HF, evaluated between August 2016 and December 2019 in an outpatient setting or during hospitalization for HF in two European HF clinics, were enrolled. All patients were stable and fully recompensated before inclusion into this study. One hundred thirty‐one consecutive patients with a diagnosis of HF with preserved ejection fraction (HFpEF) or mid‐range ejection fraction (HFmrEF) were evaluated at the Charité University Hospital, Berlin, Germany, and prospectively enrolled in the German HFpEF Registry (data previously published 7 ); 116 consecutive patients with HF evaluated at the Cardiac Rehabilitation Centre, Veruno, Italy, in the same time frame were retrospectively enrolled.
Inclusion criteria of the German HFpEF Registry have been previously published. 7 Inclusion criteria for patients included at the Veruno Centre were (i) known LV systolic dysfunction (LVEF ≤ 50%); (ii) age ≥ 18 years; and (iii) New York Heart Association (NYHA) functional class ≥ II. For both populations, patients were ineligible in the presence of acute coronary syndrome or cardiac surgery/percutaneous intervention during the past 3 months, haemodynamic relevant pericardial disease, significant mitral annular calcification, congenital heart disease, previous cardiac transplantation, restrictive cardiomyopathy, severe chronic obstructive pulmonary disease, severe kidney disease, or severe liver disease. After patients were evaluated for inclusion, those with more than moderate valve disease, unsuitable LA echocardiographic analyses, or sub‐maximal exercise testing were excluded. The study complies with the Declaration of Helsinki. The ethics committee of Charité University Hospital approved the research project. As for the patients retrospectively enrolled at Veruno Cardiac Rehab Centre, an informed consent to the treatment of anonymized clinical data was signed by all patients, according to the institutional policy. Thus, written informed consent was obtained from all study subjects.
For every study participant, clinical data were collected: demographics, body mass index (BMI), cardiovascular risk factors, chronic ischaemic heart disease, history of paroxysmal or permanent atrial fibrillation (AFib), NYHA functional class, and medications. Blood samples were collected for laboratory testing, including haemoglobin, creatinine, and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP).
Echocardiography
All patients underwent comprehensive 2D echocardiography at rest using commercially available ultrasound systems (Philips EPIQ 7, Philips Medical Systems, Andover, MA, or Vivid 7/Vivid S6, GE Vingmed Healthcare). In addition, images were obtained at a frame rate of 50 to 80 frame/s for speckle‐tracking echocardiographic analysis. A minimum of three cardiac cycles (for patients in sinus rhythm) or five cardiac cycles (for patients in AFib) were acquired. All sonographers were trained in accordance with a pre‐specified standard operation procedure. All 2D, Doppler, and strain measurements were performed offline, at the Charité Academic Echocardiography core laboratory using a customized software package (TomTec Image Arena, Unterschleissheim, Germany) and at the Veruno echocardiographic laboratory using the EchoPAC Workstation Software (GE Healthcare). All analyses were performed according to ASE/EACVI recommendations by a single investigator, with over‐reading by a second investigator. All researchers were blinded to the clinical characteristics of the patients.
Left ventricular endocardial longitudinal strain was measured with an algorithm designed for the LV in apical four‐chamber and two‐chamber view, and an average of the two values was calculated; the biplane longitudinal strain was considered for the analyses as global longitudinal strain (GLS). LV endocardial border was contoured at LV end‐diastole and end‐systole and manually adjusted when required. When there were dropout or poor tracking in two or more segments out of six, LV strain was not measured.
In apical four‐chamber view, LA maximal volume and LA strain were measured. The onset of QRS was used as the referent point, and the average of three consecutive measurements was considered. LA endocardial border was manually contoured at LV end‐diastole and end‐systole, with visual tracking quality and manual adjustment when required. When there were dropout or poor tracking due to inadequate image quality in one out of three segments (LA septum, LA lateral wall, or LA roof), LA strain was not measured and patients without measurable LA strain were excluded from the final study population. Overall, a total of 238/247 (96%) LA speckle tracings were suitable for strain analysis. LA enlargement was defined as an LA maximal volume index (LAVi) higher than 34 mL/m2. The three components of LA function were evaluated: reservoir (the LA filling phase, corresponding to LV systole), conduit (the passive LA empting phase, from mitral valve opening to P‐wave), and contractile (the active LA empting phase, from the onset of P‐wave to mitral valve closure). The value of reservoir strain was considered normal when >23%, as defined in a large multicentre study. 8
Measurement's reproducibility has been estimated for LA reservoir strain and LVGLS by means of interclass correlation coefficient (ICC). The intra‐observer variability was excellent for both reservoir strain (ICC 0.92 [95% confidence interval, CI, 0.83–0.96]) and LVGLS (ICC 0.95 [95% CI 0.93–0.96]).
Cardiopulmonary exercise test
All patients performed a symptom‐limited cardiopulmonary exercise test (CPET) using a cycle ergometer protocol within a 1 week time interval from echocardiography. The protocol consisted in cycling at 60 rpm, starting at a workload of 20 W, with a stepwise 20 W increment every 2 min. Heart rate and blood pressure were monitored at rest and during exercise. By means of a ventilatory expired gas analysis system, breath‐by‐breath oxygen consumption (VO2), carbon dioxide production (VCO2), and minute ventilation (VE) were registered and averaged over a 30 s interval. Test was terminated due to symptoms onset, ventricular arrhythmia, ST segment depression ≥ 2.0 mm, and drop in systolic blood pressure ≥ 20 mmHg. All oral medications were continued before and through CPET.
Peak VO2 (pVO2) was defined as the highest averaged VO2 during the last stage of exercise. Percentage values of predicted pVO2 were calculated using the Wasserman formula. The ventilatory response to exercise was estimated by VE/VCO2 slope. The maximal respiratory exchange ratio (RER) was calculated as the VCO2/VO2 ratio during the last stage of exercise. The ability to perform maximal exercise testing (RER > 1.0) was considered a mandatory inclusion criterion. Therefore, patients with maximal RER < 1.0 were excluded from the study analyses. 9
Statistical analysis
Data are presented as mean ± standard deviation (SD) or absolute values and percentages, as appropriate. Exercise capacity was evaluated by measuring pVO2 and VE/VCO2. Patients were divided into two groups according to their exercise capacity, using a validated pVO2 cut‐off of prognostic value in HF patients' populations (pVO2 14 mL/kg/min). 4 , 10 , 11 , 12 Student's t‐test and χ 2 test were used to compare continuous and categorical variables between groups, respectively. To determine the association between continuous variables, Pearson's correlation coefficient was used. Receiver‐operating characteristic (ROC) curve analysis was performed to assess the area under the curve (AUC) for the most relevant echocardiographic variables measured, and DeLong's test was performed to compare AUC values. The independent association of LA strain with pVO2 was studied with regression analysis. All variables were considered on a continuous scale. Two different models were used. In addition to LA strain, in the clinical model, we considered as independent variables age and BMI as non‐cardiac factors known to have an influence on pVO2 values 12 and clinical and laboratory variables significantly associated with pVO2 at univariate analysis. In the echocardiographic model, LVEF or LVGLS as measure of LV systolic function and tricuspid annular plane systolic excursion (TAPSE) as measure of right ventricular function were used. Then, univariate and multivariate logistic regression analyses were performed to assess the predictability of VO2 < 14 mL/kg/min for LAVi and LA strain, in the overall population and in subgroups with normal and dilated LAVi. Finally, we analysed separately patients with HF with reduced ejection fraction (HFrEF), HFmrEF, and HFpEF, to validate the study results in LVEF subgroups. All tests were two‐tailed. A P‐value < 0.05 was considered statistically significant. Analyses were performed using SPSS Version 20.0 (SPSS, Chicago, IL).
Results
Of the 247 patients with HF evaluated for enrolment in the study and analysed, 54 patients were excluded due to sub‐maximal exercise testing, 10 due to absence of CPET, 3 due to resting heart rate < 45 or >100 b.p.m., 1 due to severe valve disease, and 8 due to unsuitable LA strain analysis. Thus, 171 patients formed the final population. Mean age was 65 ± 11 years, 136 (80%) were males, and 42 (25%) were obese (BMI ≥ 30 kg/m2). Of the 171 patients, 86 were classified HFrEF (LVEF < 40%), 27 HFmrEF (LVEF 40–49%), and 58 HFpEF (LVEF ≥ 50%). Overall, mean pVO2 was 16 ± 11 mL/kg/min; 77 patients (45%) reached a pVO2 < 14 mL/kg/min (mean value 11 ± 2), and 94 (55%) a pVO2 ≥ 14 mL/kg/min (mean value 18 ± 4). Clinical and echocardiographic characteristics of patients divided according to pVO2 are shown in Table 1 . On average, patients with more severely reduced exercise capacity were older and had higher BMI, lower systolic blood pressure, higher prevalence of NYHA class III, higher NT‐proBNP, worse renal function and higher prevalence of loop diuretics medications, slightly lower haemoglobin, and higher AFib prevalence. On the contrary, between the two groups, there was not a significant difference in terms of sex, cardiovascular comorbidities, resting heart rate, and beta‐blockers intake.
Table 1.
Demographic, clinical, and echocardiographic characteristics in the overall population and according to exercise capacity < 14 or ≥14 mL/kg/min; P‐value for comparison of each variable between the two groups (peak VO2 < 14 or ≥14 mL/kg/min); P‐value for Pearson's correlation between each variable and linear peak VO2 in the overall population
| Overall population (n = 171) | Peak VO2 < 14 mL/kg/min (n = 77) | Peak VO2 ≥ 14 mL/kg/min (n = 94) | P‐value for comparison | P‐value for correlation with linear peak VO2 | |
|---|---|---|---|---|---|
| Age, years | 65 ± 11 | 68 ± 10 | 63 ± 11 | 0.001 | 0.002 |
| Male sex, n (%) | 136 (80) | 59 (77) | 77 (82) | 0.4 | 0.4 |
| Body mass index, kg/m2 | 27 ± 5 | 28 ± 5 | 26 ± 4 | 0.01 | 0.003 |
| NYHA class III, n (%) | 23 (13) | 18 (23) | 5 (5) | <0.0001 | <0.0001 |
| Hypertension, n (%) | 105 (61) | 48 (62) | 57 (61) | 0.6 | 0.8 |
| Hypercholesterolaemia, n (%) | 79 (46) | 37 (48) | 42 (42) | 0.7 | 0.7 |
| Diabetes, n (%) | 36 (21) | 17 (22) | 19 (20) | 0.7 | 0.7 |
| Ischaemic heart disease, n (%) | 93 (54) | 45 (58) | 48 (51) | 0.4 | 0.4 |
| Atrial fibrillation, n (%) | 30 (18) | 21 (27) | 9 (10) | 0.004 | 0.003 |
| Haemoglobin, g/dL | 13.4 ± 1.6 | 13.1 ± 1.6 | 13.6 ± 1.6 | 0.05 | 0.003 |
| Creatinine, mg/dL | 1.1 ± 0.4 | 1.2 ± 0.4 | 1.1 ± 0.3 | 0.01 | <0.0001 |
| NT‐proBNP, pg/mL | 1473 ± 1962 | 1900 ± 2355 | 1055 ± 1382 | 0.03 | 0.002 |
| Loop diuretics, n (%) | 134 (78) | 70 (91) | 64 (68) | <0.0001 | <0.0001 |
| Beta‐blockers, n (%) | 145 (85) | 69 (90) | 76 (81) | 0.2 | 0.1 |
| ACE inhibitors, n (%) | 121 (71) | 45 (58) | 76 (81) | 0.005 | 0.02 |
| Mineralocorticoid receptor antagonists, n (%) | 41 (24) | 16 (21) | 25 (26) | 0.5 | 0.6 |
| SBP, mmHg | 120 ± 22 | 117 ± 22 | 124 ± 23 | 0.05 | 0.003 |
| HR at rest, b.p.m. | 68 ± 11 | 67 ± 9 | 69 ± 11 | 0.3 | 0.6 |
| Peak VO2, mL/kg/min | 16 ± 11 | 11.4 ± 1.8 | 18.5 ± 3.6 | <0.0001 | — |
| % predicted peak VO2 | 66 ± 21 | 55 ± 18 | 76 ± 20 | <0.0001 | <0.0001 |
| Peak RER | 1.09 ± 0.08 | 1.08 ± 0.08 | 1.09 ± 0.08 | 0.2 | 0.9 |
| VE/VCO2 slope | 37 ± 8 | 40.5 ± 9.4 | 33.6 ± 6.0 | <0.0001 | <0.0001 |
| LV EDVi, mL/m2 | 85 ± 36 | 88 ± 35 | 83 ± 36 | 0.4 | 0.01 |
| LVMi, g/m2 | 120 ± 31 | 122 ± 29 | 119 ± 32 | 0.5 | 0.08 |
| LVEF, % | 42 ± 16 | 38 ± 17 | 44 ± 18 | 0.01 | <0.0001 |
| LVGLS, % | −11.6 ± 6.1 | −9.8 ± 6.2 | −12.9 ± 5.9 | 0.001 | <0.0001 |
| SV‐LVOT‐i, mL/m2 | 29 ± 7 | 27 ± 6 | 32 ± 7 | 0.008 | 0.1 |
| Diastolic dysfunction | 156 (91%) | 72 (94%) | 84 (89%) | 0.1 | 0.07 |
| I degree | 39 (23%) | 16 (21%) | 23 (24%) | ||
| II degree | 36 (21%) | 22 (29%) | 14 (15%) | ||
| III degree | 23 (13%) | 11 (14%) | 12 (13%) | ||
| Indeterminate | 58 (34%) | 23 (30%) | 35 (37%) | ||
| LAVi, mL/m2 | 48 ± 21 | 53 ± 24 | 44 ± 18 | 0.005 | 0.002 |
| LA reservoir strain, % | 16 ± 9 | 12 ± 5 | 20 ± 10 | <0.0001 | <0.0001 |
| LA conduit strain, % | 9 ± 5 | 7 ± 3 | 11 ± 5 | <0.0001 | <0.0001 |
| LA contractile strain, % | 9 ± 5 | 6 ± 3 | 10 ± 5 | <0.0001 | <0.0001 |
| E, cm/s | 77 ± 25 | 83 ± 28 | 74 ± 23 | 0.05 | 0.1 |
| A, cm/s | 66 ± 24 | 66 ± 26 | 66 ± 22 | 0.9 | 0.9 |
| E/A | 1.4 ± 0.9 | 1.5 ± 1.1 | 1.3 ± 0.9 | 0.2 | 0.1 |
| E/e′ | 12.4 ± 5.1 | 12.8 ± 4.6 | 12.1 ± 5.6 | 0.5 | 0.08 |
| MR, n (%) | 136 (80) | 62 (81) | 74 (79) | 0.6 | 0.6 |
| TR, n (%) | 120 (70) | 61 (79) | 59 (63) | 0.01 | 0.01 |
| SPAP, mmHg | 35 ± 12 | 38 ± 13 | 32 ± 9 | 0.01 | 0.001 |
| TAPSE, mm | 20 ± 5 | 18 ± 5 | 21 ± 4 | <0.0001 | <0.0001 |
| TAPSE/SPAP, mm/mmHg | 0.60 ± 0.24 | 0.51 ± 0.22 | 0.68 ± 0.23 | <0.0001 | <0.0001 |
EDVi, end‐diastolic volume indexed to body surface area; HR, heart rate; LAVi, left atrial volume indexed to body surface area; LV, left ventricular; LVEF, left ventricular ejection fraction (biplane); LVGLS, left ventricular global longitudinal strain (biplane); LVMi, left ventricular mass indexed to body surface area; MR, mitral regurgitation; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; RER, respiratory exchange ratio; SBP, systolic blood pressure; SPAP, systolic pulmonary artery pressure; SV‐LVOT‐i, stroke volume measured at left ventricular outflow tract indexed to body surface area; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; VE/VCO2, minute ventilation/carbon dioxide production ratio; VO2, oxygen consumption.
Systolic function according to LVEF and LVGLS but not LV dimensions, LV mass, and diastolic dysfunction degree was significantly different between the two groups. Moreover, patients with reduced exercise capacity showed lower right ventricular function (TAPSE), higher systolic pulmonary artery pressure (SPAP), and lower TAPSE/SPAP (Table 1 ).
Left atrial function and exercise capacity
In the overall population, 130 patients (76%) showed a dilated LAVi, and 131 (77%) a reduced LA function according to LA reservoir strain. In patients with pVO2 < 14 mL/kg/min, LAVi and all LA strain parameters were different and worse in comparison with patients with pVO2 ≥ 14 mL/kg/min (reservoir strain 11 ± 5% vs. 20 ± 10%, conduit strain 7 ± 3% vs. 11 ± 5%, contractile strain 6 ± 3% vs. 10 ± 5%; P < 0.0001 for all). In continuous regression analysis, LA volume and all strain parameters were associated with pVO2 (Table 2 , Figure 1 ) and, similarly, with % predicted pVO2 (LAVi, P = 0.006; reservoir strain, P < 0.0001; contractile strain, P = 0.01; conduit strain, P < 0.0001). Also, for VE/VCO2 (LAVi, P = 0.02; reservoir strain, P < 0.0001; contractile strain, P = 0.006; conduit strain, P = 0.01), a significant association was found.
Table 2.
Linear regression analysis for association between LA parameters, clinical or other echocardiographic variables, and peak VO2 (mL/kg/min): P‐value, b coefficient (95% confidence interval)
| LA reservoir strain, % | LA contractile strain, % | LA conduit strain, % | LA volume index, mL/m2 | |
|---|---|---|---|---|
| Univariate | <0.0001; 0.21 (0.14–0.28) | <0.0001; 0.29 (0.16–0.44) | <0.0001; 0.32 (0.18–0.46) | 0.002; −0.05 (−0.08 to −0.02) |
| Model 1 | <0.0001; 0.22 (0.12–0.31) | 0.001; 0.33 (0.15–0.52) | <0.0001; 0.35 (0.17–0.53) | 0.1; −0.03 (−0.09 to 0.01) |
| Model 2 | <0.0001; 0.16 (0.08–0.24) | 0.003; 0.23 (0.08–0.37) | 0.007; 0.20 (0.06–0.35) | 0.06; −0.03 (−0.06 to 0.001) |
LA, left atrial; VO2, oxygen consumption.
Model 1: adjusted for age, body mass index, New York Heart Association class, atrial fibrillation/sinus rhythm, haemoglobin, and creatinine. Model 2: adjusted for left ventricular ejection fraction and tricuspid annular plane systolic excursion.
Figure 1.
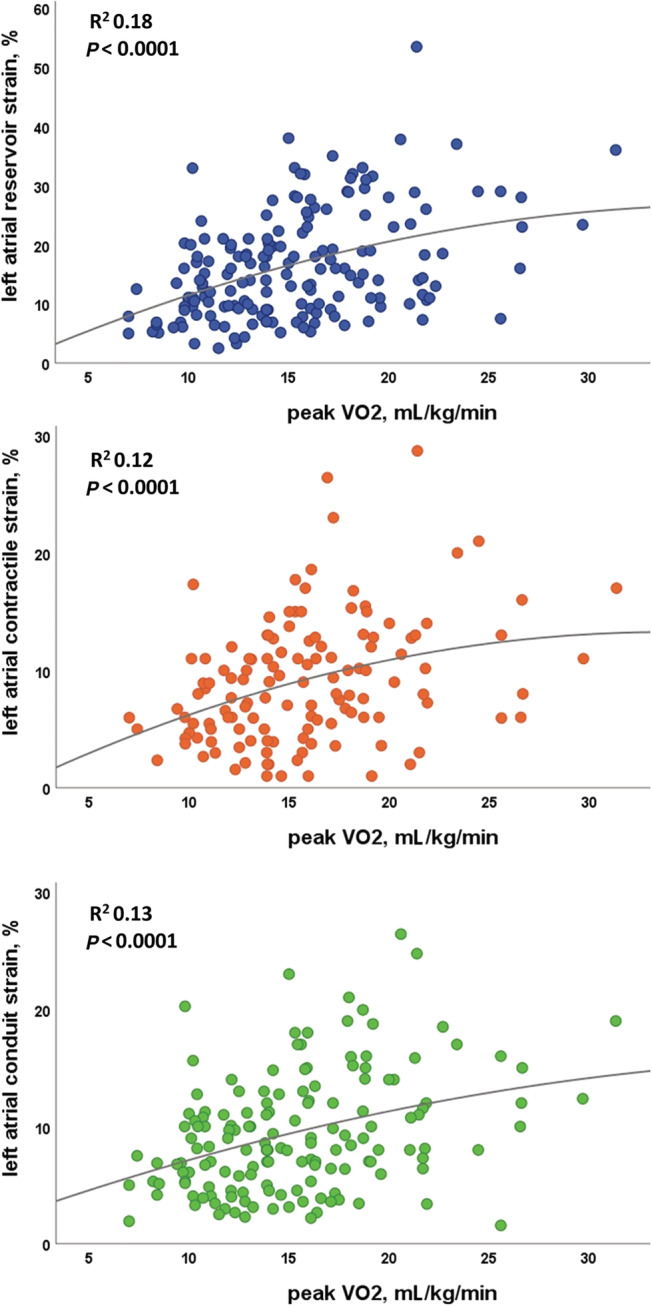
Linear correlations between left atrial strain and peak VO2 values in the overall study population.
At ROC analysis of LA reservoir strain (0.73), LA contractile strain (0.72), TAPSE (0.68), and LVGLS (0.67) (Figure 2 ) showed the highest AUC (P‐value for comparison with AUC for LA reservoir strain > 0.05) for pVO2 < 14 mL/kg/min among other echocardiographic variables tested (AUC for LVEF 0.62, LAVi 0.63, SPAP 0.62); in particular, an impaired LA reservoir strain (<23%) had a low specificity (37%) but a very high sensitivity (96%) in identifying a severely reduced pVO2. In fact, in patients with pVO2 > 14 mL/kg/min, LA parameters showed a wide range of values, whereas when pVO2 is <14 mL/kg/min, LAVi varies from normal to severely increased but LA strain is almost invariably reduced (Figure 3 ).
Figure 2.
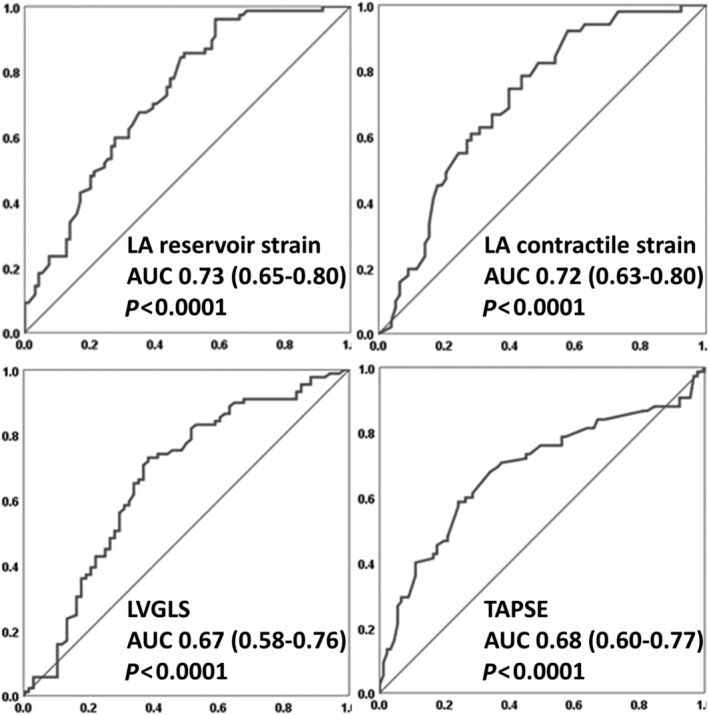
Receiver‐operating characteristic curves analysis of the parameters with the greatest value of area under the curve (AUC). LA, left atrial; LVGLS, left ventricular global longitudinal strain; TAPSE, tricuspid annular plane systolic excursion.
Figure 3.
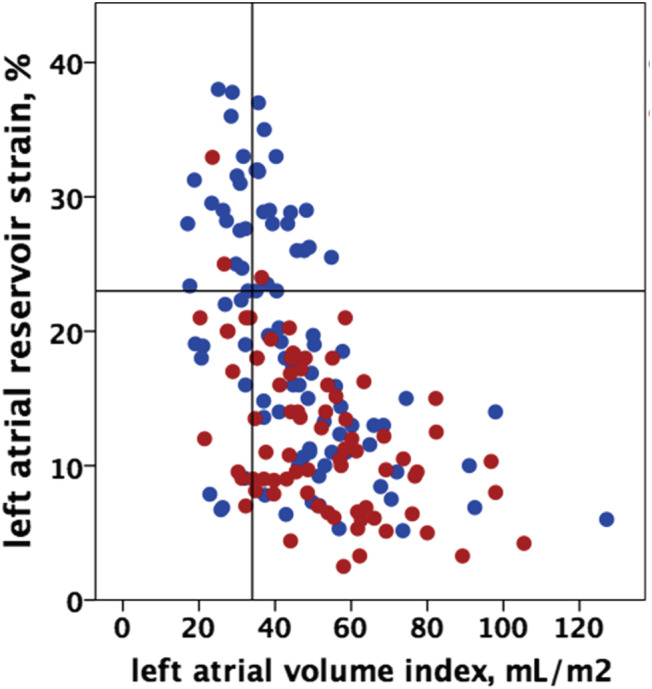
Distribution of left atrial volume index and left atrial reservoir function values in patients with peak VO2 < 14 (red dots) or ≥14 mL/kg/min (blue dots).
Then, we tested the independent role of LA strain through two multivariate analysis models. In the clinical model, LA reservoir, contractile, and conduit strain were all associated with pVO2 after adjustment for age, BMI, AFib, creatinine, haemoglobin, and NYHA class (Table 2 ). Among other covariates, also BMI (in the model with LA reservoir strain, P < 0.0001, B −0.32 [−0.49 to −0.15]; in the model with LA contractile strain, P = 0.001, B −0.36 [−0.58 to −0.15]; in the model with LA conduit strain, P < 0.0001, B −0.38 [−0.57 to −0.19]) and NYHA class (P = 0.002, B −3 [−4.8 to −1.1]; P = 0.001, B −3.9 [−6.1 to −1.6]; P = 0.005; B −3 [−5.1 to −0.9], respectively) were independently associated with pVO2. Moreover, in the 93 patients for which NT‐proBNP was measured, LA reservoir and conduit strain were independently associated with pVO2 after adjustment for age, BMI, AFib, creatinine, haemoglobin, and LogNT‐proBNP (P = 0.02 and P = 0.03, respectively; P = 0.08 for LA contractile strain). In the echocardiographic model, LA strain was associated with pVO2 after adjustment for LVEF and TAPSE (Table 2 ). Also, LVEF was independently associated with pVO2 in the model with LA reservoir, contractile, and conduit strain (P = 0.02, B 0.05 [0.008–0.09]; P = 0.02, B 0.06 [0.009–0.10]; P = 0.01; B 0.06 [0.01–0.10], respectively), whereas TAPSE was associated with pVO2 only in the model adjusted for LA conduit strain (P = 0.02, B 0.17 [0.02–0.32]). We obtained the same results when LVGLS was considered instead of LVEF (for LA reservoir, contractile, and conduit strain, P < 0.0001, P = 0.003, and P = 0.01, respectively) or TAPSE/SPAP as measure of right ventricular‐arterial coupling instead of TAPSE (P < 0.0001, P = 0.002, and P = 0.002, respectively). Another analysis considering as covariates LA reservoir strain, LVEF, TAPSE, and SPAP separately showed the same results (P < 0.0001 for LA reservoir strain, P > 0.1 for LVEF and TAPSE, P = 0.01 for SPAP). Similarly, LA strain was associated with % predicted pVO2 in the clinical multivariate model (for LA reservoir and conduit strain, P < 0.0001; for LA contractile strain, P = 0.004). On the contrary, LA reservoir strain was independently associated with VE/VCO2 in the echocardiographic model but not in the clinical model (P = 0.004).
Left atrial reservoir strain in patients with normal and increased left atrial volume
In the overall population, LA volume and reservoir strain were both normal in 20 patients (12%); 20 (12%) had dilated LAVi but normal reservoir strain; 20 (12%) had normal LAVi but reduced reservoir strain; 110 (64%) had dilated LAVi and reduced reservoir strain (Figure 4 ). In the ROC analysis, the highest AUC was obtained when LA reservoir strain and LAVi were considered together, but it was not significantly different from the AUC for LA reservoir strain alone (0.75 vs. 0.73 and 0.63 for LA strain and LAVi alone, P = 0.6 and P = 0.04, respectively).
Figure 4.
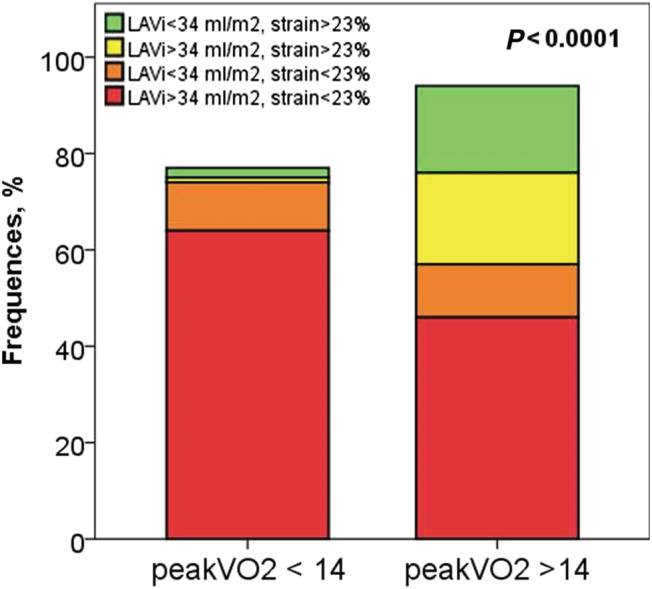
Prevalence of patients with normal/dilated left atrial volume (LAVi) and normal/reduced left atrial reservoir strain in patients with peak VO2 < 14 or ≥14 mL/kg/min.
The logistic regression analysis showed that a dilated LA (>34 mL/mq) was associated with a two‐fold risk of pVO2 < 14 mL/kg/min (P = 0.02, odds ratio, OR, 2.4 [1.1–5.1]) whereas a reduced LA reservoir strain (<23%) was associated with a 16‐fold risk of pVO2 < 14 mL/kg/min (P < 0.0001, OR 16.0 [4.7–54.6]). Moreover, a reduced LA reservoir strain was associated with a highly increased risk of pVO2 < 14 mL/kg/min in patients with both a normal LAVi (P = 0.01, OR 8.2 [1.5–44]) and a dilated LAVi (P = 0.002, OR 26.4 [3.4–204]). In patients with normal LAVi, a value of LA reservoir strain < 23% was the only parameter independently associated with pVO2 < 14 mL/kg/min after adjustment for LVEF and TAPSE (P = 0.02, OR 7.4 [1.3–42]) at multivariate analysis, whereas in patients with a dilated LAVi, LA reservoir strain < 23% (P = 0.006, OR 18.5 [2.3–148]) and also TAPSE (P = 0.01, OR 0.9 [0.81–0.97]) were independently associated with pVO2 < 14 mL/kg/min after the same adjustment.
Among patients with dilated LA, most patients with moderately or severely enlarged LA (21/25, 84%, and 71/74 patients, 96%, respectively) had LA reservoir strain < 23%, whereas in the 31 patients with mild LA enlargement, LA reservoir strain showed high predictive value for reduced exercise capacity (AUC 0.85 [0.71–0.99]). In this subgroup, also regression analysis showed the independent association of LA reservoir strain with pVO2 at univariate analysis and after adjustment for LVEF and TAPSE (P = 0.01 and P = 0.02, respectively).
Value of left atrial function in patients with heart failure with reduced ejection fraction, mid‐range ejection fraction, and preserved ejection fraction
We focused on key indexes of ventricular systolic function and LA remodelling, and interestingly, we found that LA reservoir strain, LAVi, LVEF, LVGLS, and TAPSE were all significantly related to each other in the overall population (P < 0.05), with the strongest correlations between LVEF and LVGLS (r −0.91, P < 0.0001), LA reservoir strain and LAVi (r −0.50, P < 0.0001), and LVGLS and LA reservoir strain (r −0.39, P < 00001). Patients with HFrEF, HFmrEF, and HFpEF were then analysed separately. Mean pVO2 was 14 ± 4, 16 ± 4, and 17 ± 5 mL/kg/min, respectively (P for trend 0.002, with a significant difference between mean pVO2 value between HFrEF and HFpEF). When the three groups were analysed separately, the association between LA strain and LVGLS persisted only for patients with HFrEF (P < 0.0001). Moreover, the association between pVO2 and key echocardiographic variables has been tested. Neither LV mass index (LVMi) nor E/e′, diastolic dysfunction degree, and SPAP were linearly associated with pVO2 (P for correlation > 0.05); the association was statistically significant for LVEF only in HFrEF patients, for LVGLS in HFrEF and HFmrEF, and for TAPSE in HFrEF and HFpEF (P < 0.05). Interestingly, among all the variables described, LA strain was the only parameter associated with pVO2 at linear regression analysis in all the subgroups, both at univariate analysis and after adjustment for age and BMI (Table 3 , Figure 5 ). Finally, when the association between LA reservoir strain and LVGLS and pVO2 was studied at linear regression analysis, LA strain but not LVGLS was independently associated (P = 0.02 in HFrEF and HFmrEF, P = 0.01 in HFpEF).
Table 3.
Association between exercise capacity (pVO2, mL/kg/min) and key echocardiographic parameters in patients with HFrEF, HFmrEF, and HFpEF
| HFrEF (n = 86) | HFmrEF (n = 27) | HFpEF (n = 58) | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | P‐value for association with pVO2 at linear regression analysisa | Mean ± SD | P‐value for association with pVO2 at linear regression analysisa | Mean ± SD | P‐value for association with pVO2 at linear regression analysisa | |
| LA reservoir strain, % | 14 ± 9 | 0.003 | 19 ± 8 | 0.04 | 18 ± 9 | 0.01 |
| LAVi, mL/m2 | 54 ± 22 | 0.1 | 41 ± 16 | 0.04 | 44 ± 20 | 0.8 |
| LVEF, % | 28 ± 7 | 0.002 | 45 ± 3 | 0.1 | 62 ± 6 | 0.08 |
| LVGLS, % | −7 ± 2 | 0.002 | −13 ± 3 | 0.06 | −19 ± 3 | 0.1 |
| TAPSE, mm | 19 ± 5 | 0.002 | 19 ± 4 | 0.6 | 20 ± 4 | 0.01 |
HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LA, left atrial; LAVi, left atrial volume indexed to body surface area; LVEF, left ventricular ejection fraction; LVGLS, left ventricular global longitudinal strain; pVO2, maximal oxygen consumption; SD, standard deviation; TAPSE, tricuspid annular plane systolic excursion.
Adjusted for age and body mass index.
Figure 5.
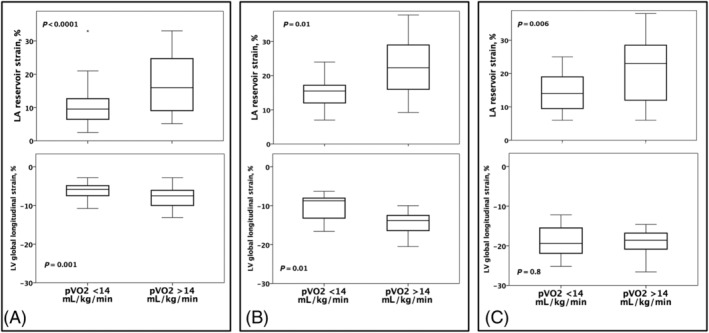
Box plots of left atrial (LA) reservoir strain and left ventricular (LV) global longitudinal strain in patients with heart failure with reduced ejection fraction (A), mid‐range ejection fraction (B), and preserved ejection fraction (C).
Discussion
The main results of the present study are the following: (i) LA function was reduced in HF patients across the whole spectrum of LVEF; (ii) LA reservoir strain has a powerful and independent ability, among the echocardiographic parameters, in identifying HF patients with severely reduced exercise capacity; and (iii) LA reservoir strain but not left or right ventricular systolic function was associated with exercise capacity in all the subgroups of patients with HFrEF, HFmrEF, and HFpEF.
Although EF reduction is the main functional feature of HFrEF and HFmrEF patients, previous studies questioned the correlation between LV systolic function and exercise capacity, rather highlighting the role of diastolic function impairment. 13 , 14 HFrEF and HFpEF are characterized by different degrees of systolic and diastolic dysfunction, but the contribution of their respective impairment is not completely understood, especially in relation to exercise intolerance. In the present cohort, a strong association was found between pVO2 and LV systolic function, especially in HFrEF, but neither E/e′ nor the diastolic dysfunction grading could predict a reduced pVO2. Interestingly, neither rest E/e′ nor peak exercise E/e′ was significantly associated with exercise capacity in a previous CPET study. 15
LAVi, as a marker of global LV systolic and diastolic dysfunction with important prognostic power, 16 has been shown to correlate frequently 17 but not invariably 7 , 18 with exercise tolerance in HF patients. However, it is unlikely that the increased LA volume might completely reflect the complex phenomenon of LA remodelling and, recently, functional LA parameters were introduced to better extrapolate the LA contribution to the severity of the disease. Actually, in our study, LA reservoir function and exercise capacity were closely and independently associated, both in the global HF population and in all EF subgroups. In particular, the value added by LA reservoir strain is highly relevant in patients with normal LAVi and with mildly dilated LA. In fact, when LA is moderately to severely dilated, also LA strain is usually reduced, supporting the notion that LA dysfunction in more sensitive and discriminative than structural parameters. 19 The association between LA function and exercise capacity has already been documented in previous studies of both HFrEF 4 , 6 and HFpEF populations. 20 , 21 In these studies, the authors explained this association as a consequence of the role of LA in contributing to adequate LV filling and in mitigating increased filling pressure. 22 To our knowledge, this is the first study that considered the whole HF population across LVEF borders.
In accordance with previous findings, 23 we found a strong direct association between LA and LV systolic function in the overall population and in the subgroup with HFrEF, but not in the HFmrEF and HFpEF patients. Consistently, mean LA reservoir strain values are even lower in HFrEF. However, other two major elements are involved in reducing LA function in HF. First, the LA is characterized by myocyte apoptosis, fibroblast proliferation, and fibrosis, 24 that is tissue alterations that affect directly the LA independently of the degree of LV dysfunction, also referred as intrinsic atrial myopathy. Second, LA reservoir function is highly affected by the global haemodynamic overload. Indeed, in a cohort of HFrEF patients, LA reservoir function was found to be strongly impaired in decompensated HF but significantly recovered in a subset of patients in the weeks after decongestive therapy. 25
The LA function conveys the complex interplay between LV systolic function, filling pressure, and pathological processes typical of HF that causes the atrial myopathy and in its turn is a key element for right ventricular‐pulmonary circulation coupling. 26 Therefore, the strong association found between LA function and exercise intolerance suggests LA reservoir strain as marker with prognostic value into the whole spectrum of HF and consequently it might be considered as potential therapeutic target. In fact, LA functional assessment provides information on the effectiveness of HF therapies in mitigating symptoms through the LA unloading and the beneficial effect on the LA dysfunction progression. 27 LA volume value as a therapeutic target has been questioned, because LA dilatation could persist despite improvement in LV filling pressure. 28 Instead, LA function is severely impaired during decompensation and improves after decongestive therapy. Such LA functional improvement has been associated with outcome in an HFrEF cohort. 25
Limitations
The major limitation of the present study is the use of two different echocardiographic vendors and relative software for strain analysis. However, inter‐vendor differences in LA strain measurements may be of relatively little importance according to previous analyses. 29 , 30 Moreover, we used a value > 23% to define normal LA reservoir function, as suggested in a large multicentre study, 8 but a definite cut‐off of normality has still not been identified and we did not have a control group. However, we used the LA reservoir strain mainly as a continuous variable to overcome this limitation.
Although recommendations suggest the biplane Simpson's method to assess LA volume, it has been measured only in apical four‐chamber view.
Another limitation is the small size of the HFmrEF subgroup, which is underrepresented in the present study. Moreover, we did not analyse separately patients with AFib due to the small sample size. However, we performed ROC analyses and multivariate regression analyses after the exclusion of patients with AFib, and the study results were confirmed.
Finally, a limitation of the study is the lack of prognostic data. Exercise capacity is a well‐recognized prognostic marker in chronic HF. However, prognosis assessment would have strengthened the study results.
Conclusions
In a cohort of stable HFrEF, HFmrEF, and HFpEF patients, LA function assessed through LA reservoir strain is compromised and associated with exercise intolerance independently from known determinants of exercise capacity. Therefore, LA function assessed in addition to clinical and echocardiographic parameters could represent a marker of disease severity and portend clinical utility in the prognostic evaluation and therapy of HF patients across the full spectrum of LVEF.
Conflict of interest
None declared.
Funding
Funding Open Access funding enabled and organized by Projekt DEAL.
Acknowledgements
All authors contributed as co‐authors.
Maffeis, C. , Rossi, A. , Cannata, L. , Zocco, C. , Belyavskiy, E. , Radhakrishnan, A. K. , Feuerstein, A. , Morris, D. A. , Pieske‐Kraigher, E. , Pieske, B. , Edelmann, F. , and Temporelli, P. L. (2022) Left atrial strain predicts exercise capacity in heart failure independently of left ventricular ejection fraction. ESC Heart Failure, 9: 842–852. 10.1002/ehf2.13788.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016; 37: 2129–2200.27206819 [Google Scholar]
- 2. Solomon SD, Anavekar N, Skali H, McMurray JJV, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA, for the Candesartan in Heart Failure Reduction in Mortality (CHARM) Investigators . Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005; 112: 3738–3744. [DOI] [PubMed] [Google Scholar]
- 3. Lam CSP, Voors AA, Piotr P, McMurray JJV, Solomon SD. Time to rename the middle child of heart failure: heart failure with mildly reduced ejection fraction. Eur Heart J 2020; 0: 1–3. [DOI] [PubMed] [Google Scholar]
- 4. Terzi S, Dayi SU, Akbulut T, Sayar N, Bilsel T, Tangurek B, Akgoz H, Kose H, Yilmazer S, Yesilcimen K. Value of left atrial function in predicting exercise capacity in heart failure with moderate to severe left ventricular systolic dysfunction. Int Heart J 2005; 46: 123–131. [DOI] [PubMed] [Google Scholar]
- 5. Bytyçi I, Bajraktari G, Ibrahimi P, Berisha G, Rexhepaj N, Henein MY. Left atrial emptying fraction predicts limited exercise performance in heart failure patients. Int J Cardiol Heart Vessel 2014; 24: 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D'Andrea A, Caso P, Romano S, Scarafile R, Cuomo S, Salerno G, Riegler L, Limongelli G, Di Salvo G, Romano M, Liccardo B, Iengo R, Ascione L, Del Viscovo L, Calabrò P, Calabrò R. Association between left atrial myocardial function and exercise capacity in patients with either idiopathic or ischemic dilated cardiomyopathy: a two‐dimensional speckle strain study. Int J Cardiol 2009; 132: 354–363. [DOI] [PubMed] [Google Scholar]
- 7. Maffeis C, Morris DA, Belyavskiy E, Kropf M, Radhakrishnan AK, Zach V, Rozados da Conceicao C, Trippel TD, Pieske‐Kraigher E, Rossi A, Pieske B, Edelmann F. Left atrial function and maximal exercise capacity in heart failure with preserved and mid‐range ejection fraction. ESC Heart Fail 2021; 8: 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morris DA, Takeuchi M, Krisper M, Kohncke C, Bekfani T, Carstensen T, Hassfeld S, Dorenkamp M, Otani K, Takigiku K, Izumi C, Yuda S, Sakata K, Ohte N, Tanabe K, Osmanoglou E, Kuhnle Y, Dungen HD, Nakatani S, Otsuji Y, Haverkamp W, Boldt L‐H. Normal values and clinical relevance of left atrial myocardial function analysed by speckle‐tracking echocardiography: multicentre study. Eur Heart J Cardiovasc Imaging 2015; 16: 364–372. [DOI] [PubMed] [Google Scholar]
- 9. von Roeder M, Rommel CP, Kowallick JT, Blazek S, Besler C, Fengler K, Lotz J, Hasenfuß G, Lücke C, Gutberlet M, Schuler G, Schuster A, Lurz P. Influence of left atrial function on exercise capacity and left ventricular function in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging 2017; 10: e005467. [DOI] [PubMed] [Google Scholar]
- 10. Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV, American Heart Association Exercise , Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology , Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease , Interdisciplinary Council on Quality of Care and Outcomes Research . Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 2010; 122: 191–225. [DOI] [PubMed] [Google Scholar]
- 11. Nadruz W, West E, Sengelov M, Santos M, Groarke JD, Forman DE, Claggett B, Skali H, Shah AM. Prognostic value of cardiopulmonary exercise testing in heart failure with reduced, midrange, and preserved ejection fraction. J Am Heart Assoc 2017; 6: e006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malhotra R, Bakken K, D'Elia E, Lewis GD. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail 2016; 4: 607–616. [DOI] [PubMed] [Google Scholar]
- 13. Little WC. Diastolic dysfunction beyond distensibility: adverse effects of ventricular dilatation. Circulation 2005; 112: 2888–2890. [DOI] [PubMed] [Google Scholar]
- 14. Ohara T, Iwano H, Thohan V, Kitzman DW, Upadhya B, Pu M, Little WC. Role of diastolic function in preserved exercise capacity in patients with reduced ejection fractions. J Am Soc Echocardiogr 2015; 28: 1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pugliese NR, Fabiani I, Santini C, Rovai I, Pedrinelli R, Natali A, Dini FL. Value of combined cardiopulmonary and echocardiography stress test to characterize the haemodynamic and metabolic responses of patients with heart failure and mid‐range ejection fraction. Eur Heart J Cardiovasc Imaging 2019; 20: 828–836. [DOI] [PubMed] [Google Scholar]
- 16. Rossi A, Temporelli PL, Quintana M, Dini FL, Ghio S, Hillis GS, Klein AL, Marsan NA, Prior DL, Man YC, Poppe KK, Doughty RN, Whalley GA, MeRGE Heart Failure Collaborators . Independent relationship of left atrial size and mortality in patients with heart failure: an individual patient meta‐analysis of longitudinal data (MeRGE Heart Failure). Eur J Heart Fail 2009; 11: 929–936. [DOI] [PubMed] [Google Scholar]
- 17. Rossi A, Cicoira M, Bonapace S, Golia G, Zanolla L, Franceschini L, Vassanelli C. Left atrial volume provides independent and incremental information compared with exercise tolerance parameters in patients with heart failure and left ventricular systolic dysfunction. Heart 2007; 93: 1420–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malagoli A, Rossi L, Bursi F, Zanni A, Sticozzi C, Piepoli MF, Villani GQ. Left atrial function predicts cardiovascular events in patients with chronic heart failure with reduced ejection fraction. J Am Soc Echocardiogr 2019; 32: 248–256. [DOI] [PubMed] [Google Scholar]
- 19. Morris DA, Belyavskiy E, Aravind‐Kumar R, Kropf M, Frydas A, Braunauer K, Marquez E, Krisper M, Lindhorst R, Osmanoglou E, Boldt LH, Blaschke F, Haverkamp W, Tschöpe C, Edelmann F, Pieske B, Pieske‐Kraigher E. Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc Imaging 2018; 11: 1405–1415. [DOI] [PubMed] [Google Scholar]
- 20. Kusunose K, Motoki H, Popovic ZB, Thomas JD, Klein AL, Marwick TH. Independent association of left atrial function with exercise capacity in patients with preserved ejection fraction. Heart 2012; 98: 1311–1317. [DOI] [PubMed] [Google Scholar]
- 21. Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, Klein DA, Dixon D, Baldridge A, Rasmussen‐Torvik LJ, Maganti K, Shah SJ. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging 2016; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Inciardi RM, Rossi A, Bergamini C, Benfari G, Maffeis C, Greco C, Drago A, Guazzi M, Ribichini FL, Cicoira M. Mitral regurgitation, left atrial structural and functional remodeling and the effect on pulmonary hemodynamics. Eur J Heart Fail 2020; 22: 499–506. [DOI] [PubMed] [Google Scholar]
- 23. Frydas A, Morris DA, Belyavskiy E, Radhakrishnan AK, Kropf M, Tadic M, Roessig L, Lam CSP, Shah SJ, Solomon SD, Pieske B, Pieske‐Kraigher E. Left atrial strain as sensitive marker of left ventricular diastolic dysfunction in heart failure. ESC Heart Fail 2020; 7: 1956–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Triposkiadis F, Pieske B, Butler J, Parissis J, Giamouzis G, Skoularigis J, Brutsaert D, Boudoulas H. Global atrial failure in heart failure. Eur J Heart Fail 2016; 18: 1307–1320. [DOI] [PubMed] [Google Scholar]
- 25. Deferm S, Martens P, Verbrugge FH, Bertrand PB, Dauw J, Verhaert D, Dupont M, Vandervoort PM, Mullens W. LA mechanics in decompensated heart failure: insights from strain echocardiography with invasive hemodynamics. JACC Cardiovasc Imaging 2020; 13: 1107–1115. [DOI] [PubMed] [Google Scholar]
- 26. Sugimoto T, Bandera F, Generati G, Alfonzetti E, Bussadori C, Guazzi M. Left atrial function dynamics during exercise in heart failure. Pathophysiological implications on the right heart and exercise ventilation inefficiency. J Am Coll Cardiol Img 2017; 10: 1253–1264. [DOI] [PubMed] [Google Scholar]
- 27. Peigh G, Shah SJ, Patel RB. Left atrial myopathy in atrial fibrillation and heart failure: clinical implications, mechanisms, and therapeutic targets. Curr Heart Fail Rep 2021; 18: 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state‐of‐the‐art review. J Am Coll Cardiol 2019; 73: 1961–1977. [DOI] [PubMed] [Google Scholar]
- 29. Pathan F, D'Elia N, Nolan MT, Marwick TH, Negishi K. Normal ranges of left atrial strain by speckle‐tracking echocardiography: a systematic review and meta‐analysis. J Am Soc Echocardiogr 2017; 30: 59–70. [DOI] [PubMed] [Google Scholar]
- 30. Sugimoto T, Robinet S, Dulgheru R, Bernard A, Ilardi F, Contu L, Addetia K, Caballero L, Kacharava G, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Hagendorff A, Hristova K, Lopez T, de la Morena G, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, van de Veire N, von Bardeleben RS, Vinereanu D, Zamorano JL, Go YY, Marchetta S, Nchimi A, Rosca M, Calin A, Moonen M, Cimino S, Magne J, Cosyns B, Galli E, Donal E, Habib G, Esposito R, Galderisi M, Badano LP, Lang RM, Lancellotti P, NORRE Study , Lancellotti P, Dulgheru R, Kou S, Sugimoto T, Bernard A, Ilardi F, Marchetta S, Nchimi A, Robinet S, Go YY, Barone D, Baroni M, de Diego JJG, Hagendorff A, Hristova K, de la Morena G, Lopez T, Zamorano JL, Cardim N, Popescu BA, Kacharava G, Gonjilashvili N, Kurashvili L, Akhaladze N, Mgaloblishvili Z, Oliva MJ, González‐Carrillo J, Athanassopoulos GD, Vinereanu D, Rimbas R, Ciobanu AO, Badano LP, Peluso D, Jose SP, van de Veire N, de Sutter J, Penicka M, Kotrc M, Voigt JU, Ozyigit T, Carbonero JDR, Salustri A, von Bardeleben RS, Lang RM, Addetia K. Echocardiographic reference ranges for normal left atrial function parameters. Eur Heart J Cardiovasc Imaging 2018; 19: 630–638. [DOI] [PubMed] [Google Scholar]


