Abstract
Aims
Iron deficiency (ID) is common in patient with chronic heart failure (HF) and has been widely studied. In contrast, data concerning ID in cardiac amyloidosis (CA) are limited. Amyloidosis is a severe and fatal systemic disease, characterized by an accumulation of amyloid fibrils in various tissues/organs, including nerves, kidneys, gastrointestinal tract, and heart. Amyloid deposits in the heart eventually cause HF. The main subtypes of CA are light chain (AL), hereditary transthyretin (ATTRv), and wild‐type transthyretin (ATTRwt). We performed this study to determine the prevalence, clinical outcome (all‐cause mortality), and determinants of ID among the three main subtypes of CA.
Methods and results
Iron deficiency status were analysed in 816 CA patients enrolled at the French Referral Centre for Cardiac Amyloidosis: 271 (33%) had AL, 164 (20%) ATTRv, and 381 (47%) ATTRwt. ID affected 49% of CA patients, 45% with AL, 58% with ATTRv, and 48% with ATTRwt. We identified ATTR status (ATTRv P = 0.003, ATTRwt P = 0.037), diabetes (P = 0.003), aspirin treatment (P = 0.009), haemoglobin levels (P = 0.006), and altered global longitudinal strain (P = 0.02) as independent ID determinants. There is no difference in all‐cause mortality considering ID status.
Conclusions
Iron deficiency is common in patients with CA, irrespective of the subtype. Patients seem more likely to have ID if diagnosed with ATTR, if diabetic, and/or treated with aspirin. In CA, the benefit of intravenous iron therapy, for ID, on morbidity and mortality needs further study.
Keywords: Amyloidosis, Iron deficiency, Heart failure
Introduction
Worldwide, iron deficiency (ID) is a very common nutritional disorder, even in industrialized nations where it often occurs with cardiovascular disease. 1 ID also frequently occurs with chronic diseases, like chronic heart failure 2 and chronic kidney disease. 3 There are two types of ID: functional ID when serum ferritin levels are between 100 and 299 μg/L and transferrin saturation is below 20%, and absolute ID when serum ferritin levels drop below 100 μg/L. 4 , 5 In patients with functional ID, iron is inadequately distributed for proper tissue function: often from chronic inflammation with iron retention (sequestration), by macrophages and hepatocytes, that is frequently associated with decreased iron absorption. 6 Absolute ID can result from chronic blood loss and inadequate iron intake, or develops from functional ID. 6
Moreover, absolute ID is associated with specific diseases. In heart failure (HF) gastrointestinal iron malabsorption, decreased duodenal iron transfer from gut oedema, and macrophage iron sequestration from inflammation (with high circulating levels of pro‐inflammatory mediators) leads to ID. 7 Approximately 50–62% of HF patients have ID. 2 , 8 , 9 ID occurs irrespective of the left ventricular ejection fraction (LVEF). Indeed, a single‐centre, prospective study reported that 53% of HF patients had ID: among these 50% had HF with reduced (HFrEF), 61% had HF with mildly reduced (HFmrEF), and 64% had HF with preserved ejection fractions (HFpEF). 9 Overall, ID prevalence tends to increases as diastolic function deteriorates. 10 Moreover, ID in HF is a strong, independent predictor of mortality 2 and is associated with reduced exercise capacity, and diminished quality of life. 8 , 9 , 10 Recent studies have reported that iron therapy with carboxymaltose ferric improves quality of life and exercise capacity in HFrEF patients and reduces heart failure re‐hospitalization in patients with acute heart failure (LVEF <50%). 4 , 11 , 12 , 13 , 14 The impact of iron therapy on HFpEF patients with ID is being evaluated in the FAIR HFpEF study (NCT03074591).
Recently, several studies have shown that cardiac amyloidosis (CA) is an underestimated cause of HF. 15 , 16 , 17 A study in elderly patients found that wild‐type TTR amyloidosis (ATTRwt) represented 13% of HFpEF hospitalizations. 16 Amyloidosis is a severe, progressive systemic disease characterized by deposits of misfolded, insoluble, toxic proteins (amyloid fibrils) in the extracellular matrix of various organs, including the heart. 18 Numerous proteins are implicated in CA, among these immunoglobulin light chains (ALs) and transthyretin (TTR) are the most frequent. The three main types of CA are AL, wild‐type TTR amyloidosis (ATTRwt), and hereditary TTR amyloidosis (ATTRv). 19 ATTRv results from a genetic variant of the TTR gene, which produces unstable amyloidogenic TTR. 20 , 21 , 22 In CA, the thickening and increased stiffness of the cardiac walls from amyloid deposits leads to HF, conduction disorders, atrial arrhythmias, and eventually cardiovascular death.
In CA patients besides HF because of oedema in organs, amyloid fibrils also infiltrate the extracellular matrix of the gastrointestinal tract, vessels, and the nervous system. 23 , 24 Nervous system infiltration causes gastroparesis and dysphagia that leads to malnutrition. Vascular vessels infiltration may lead to bleeding and ID. Gastrointestinal amyloid infiltration may aggravate the HF malabsorption and amplify ID associated with HF. We hypothesized that pathophysiological mechanisms observed in CA and associated with HF lead to ID. Thus, amyloid infiltration severity may correlate to ID severity. To our knowledge, ID prevalence and determinants have not yet been assessed in patients with CA.
The aim of our study was to evaluate ID prevalence and its association with clinical, biological, and imaging characteristics in CA patients with AL, ATTRv, and ATTRwt.
Methods
Study population
This retrospective cohort study was conducted in the French Referral Centre for Cardiac Amyloidosis at the Henri Mondor Teaching Hospital (Creteil) from August 2010 to March 2020. All consecutive patients with confirmed CA (with either AL, ATTRv, or ATTRwt amyloidosis) and baseline iron status were prospectively included. Patients had a comprehensive medical evaluation at arrival with clinical and laboratory assessments, including complete blood count, bilirubin, albumin, serum N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), high‐sensitivity cardiac troponin T, and serum ID tests, electrocardiography, echocardiography, and 99mTc‐hydroxy‐methyl‐diphosphonate (HMDP) scintigraphy.
Definition of cardiac amyloidosis and its type
Cardiac amyloidosis was suspected when the interventricular septum thickness (IVST) was >12 mm, measured by echocardiography, in the absence of other known cause of cardiac hypertrophy. AL amyloidosis was diagnosed by an excess of serum and/or urinary free‐light chains (FLCs) and AL deposits assessed by immunohistochemistry or immunofluorescence, with a specific anti‐FLC antibody, on an extra‐cardiac or endomyocardial biopsy. CA severity was assessed using cardiac biomarkers: NT‐proBNP and troponin T. 25 ATTR amyloidosis was diagnosis using the method previously described. 26 Genetic sequencing of patients with ATTR amyloidosis was performed to differentiate ATTRwt and ATTRv subtypes.
Biological tests and assessment of iron deficiency status and anaemia
Iron deficiency was diagnosed using the baseline serum levels of ferritin and transferrin. ID was diagnosed when patients had serum ferritin <100 μg/L (absolute ID), or serum ferritin between 100 and 299 μg/L with transferrin saturation <20% (functional ID). 27 , 28 Anaemia was defined as haemoglobin levels <13 g/dL for men and <12 g/dL for women, as recommended by the World Health Organization (World Health Organization/United Nations University/UNICEF. Iron Deficiency Anaemia, Assessment, Prevention and Control: A Guide for Programme Managers. Geneva: WHO; 2001).
Clinical follow‐up
Patient follow‐up visits were performed according to standard of care. Patients usually consulted every 6–9 months. During these visit, standard clinical evaluations were performed.
Ethical considerations
All patients provided oral consent to be included in the Amyloidosis Network registry. The study was approved by the local ethics committee and data were recorded electronically in accordance with the French CNIL (Commission National de l'Informatique et des Libertés; N°1846564 v0).
Statistical analysis
Continuous variables were expressed as median with interquartile range (IQR) and dichotomous data as numbers with percentages. Frequencies for quantitative variables were compared using the χ 2 test with Pearson's correction. Continuous data were compared using the Mann–Whitney test for two groups and the Kruskal–Wallis test for more than two groups. Follow‐up data were obtained from medical files, or if required, by contacting the patients' families. The study assessed all‐cause mortality in patients with or without ID.
The statistical analyses were performed using the SPSS software (version 19.0 for Windows 2010 SPSS Inc.). A P value below 0.05 was considered as statistically significant.
Logistical regression was used to assess the association between ID and baseline characteristics.
The Kruskal–Wallis test was used to analyse the association between malabsorption and ferritin levels in the CA population. Ferritin levels were compared with albumin tertiles. Similarly, in AL patients, where hepatic dysfunction is frequent and can affect ferritin production, we compared ferritin levels to bilirubin tertiles (T1–T2 vs. T3).
Results
Patient characteristics
From August 2010 to March 2020, of the 2567 patients that presented at the French Referral Centre with suspected CA, 816 patients had CA with iron status and were enrolled in the study: 271 (33%) with AL, 164 (20%) with ATTRv, and 381 (47%) with ATTRwt (Table 1 and Figure 1 ). The median age in the overall population was 76 years (IQR: 67–82): 67 years (IQR: 59–75) for patients with AL, 72 years (IQR: 66–78) with ATTRv, and 81 years (IQR: 76–85) with ATTRwt. Women represented 38% of AL, 34% of ATTRv, and 14% of ATTRwt patients. The baseline characteristics of the overall population and according to subtypes of CA are shown in Table 1 . Overall, patient age, cardiovascular risk factors, clinical laboratory, and echocardiography variables differed significantly between CA subtypes (Table 1 ). Median age, hypertension, and ischaemic heart disease were significantly higher in ATTRwt patients (P < 0.001). While New York Heart Association (NYHA) status (P = 0.003), NT‐proBNP and high‐sensitivity troponin T levels (P < 0.001) were significantly higher in AL patients. ATTRwt patients had more atrial fibrillations (P < 0.001) and received significantly more oral anticoagulant (P < 0.001) than AL and ATTRv patients.
Table 1.
Baseline characteristics of patients according to amyloidosis status
| N (%) | All | AL | ATTRv | ATTRwt | P |
|---|---|---|---|---|---|
| 816 (100) | 271 (33) | 164 (20) | 381 (47) | ||
| Clinical characteristics | |||||
| Age at inclusion, years | 76 (67; 82) | 67 (59; 75) | 72 (66; 78) | 81 (76; 85) | <0.001 |
| Gender, women n (%) | 210 (26) | 103 (38) | 55 (34) | 52 (14) | <0.001 |
| BMI, kg/m2 | 25 (22; 27) | 23 (21; 26) | 24 (22; 27) | 25 (23; 28) | <0.001 |
| CV risk factors | |||||
| Diabetes, n (%) | 151 (19) | 45 (17) | 32 (20) | 74 (19) | 0.615 |
| Hypertension, n (%) | 438 (54) | 107 (40) | 91 (56) | 240 (63) | <0.001 |
| Dyslipidaemia, n (%) | 259 (32) | 73 (27) | 42 (26) | 144 (38) | 0.068 |
| CV characteristics | |||||
| NYHA Class III–IV vs. I–II, n (%) | 342 (42) | 130 (48) | 69 (42) | 143 (38) | 0.003 |
| Heart rate, beats/min | 76 (68; 86) | 82 (73; 94) | 75 (66; 84) | 74 (67; 81) | <0.001 |
| Systolic blood pressure, mmHg | 122 (108; 137) | 112 (101; 127) | 121 (108; 133) | 129 (115; 143) | <0.001 |
| Atrial fibrillation, n (%) | 213 (26) | 30 (11) | 32 (20) | 151 (40) | <0.001 |
| Ischaemic heart disease, n (%) | 150 (18) | 38 (14) | 12 (7) | 100 (26) | <0.001 |
| Echocardiography characteristics | |||||
| LVEF, % | 51 (42; 60) | 55 (45; 62) | 49 (36; 59) | 50 (41; 58) | <0.001 |
| IVST, mm | 17 (15; 19) | 15 (14; 17) | 18 (15; 20) | 18 (15; 20) | <0.001 |
| GL Strain, % | −10 (−8; −13) | −10 (−8; −14) | −10 (−8; −13) | −10 (−8; −13) | 0.452 |
| Blood parameters | |||||
| NT‐proBNP, ng/mL | 3550 (1740; 6735) | 5074 (2257; 10982) | 2525 (1065; 5368) | 3247 (1734; 5802) | <0.001 |
| Troponin T HS, ng/mL | 71 (45; 106) | 83 (53; 136) | 67 (40; 96) | 66 (43; 95) | <0.001 |
| eGFR, mL/min/1.73 m2 | 50 (37; 67) | 55 (38; 75) | 54 (38; 68) | 48 (37; 60) | 0.002 |
| Haemoglobin, g/dL | 13.1 (11.8; 14.1) | 12.7 (10.9; 13.8) | 12.9 (11.8; 13.7) | 13.4 (12.2; 14.4) | <0.001 |
| Mean corpuscular volume, fL | 92 (87; 95) | 91 (87; 95) | 90 (85; 94) | 92 (88; 96) | <0.001 |
| Ferritin, μg/L | 204 (109; 328) | 225 (118; 360) | 139 (72; 283) | 210 (124; 314) | <0.001 |
| Transferrin saturation, % | 18 (13; 24) | 18 (12; 24) | 17 (13; 25) | 18 (13; 24) | 0.560 |
| Iron deficiency treatment a | |||||
| Oral iron during follow‐up, n (%) | 30 (8) | 4 (3) | 7 (8) | 19 (10) | 0.069 |
| I.V. iron during follow‐up, n (%) | 191 (48) | 57 (47) | 44 (47) | 90 (50) | 0.883 |
| Anticoagulant and antiplatelet treatment | |||||
| Aspirin, n (%) | 213 (26) | 75 (28) | 34 (21) | 104 (27) | 0.220 |
| Clopidogrel, n (%) | 46 (6) | 12 (4) | 6 (4) | 28 (7) | 0.135 |
| Oral anticoagulants, n (%) | 434 (53) | 111 (41) | 74 (45) | 249 (65) | <0.001 |
| Heart failure treatment | |||||
| ACE inhibitor, n (%) | 216 (26) | 42 (16) | 54 (33) | 120 (31) | <0.001 |
| ARB, n (%) | 121 (15) | 25 (9) | 29 (18) | 67 (18) | 0.007 |
| Digoxin, n (%) | 14 (2) | 2 (1) | 1 (1) | 11 (3) | 0.055 |
| Selective beta‐blocker, n (%) | 221 (27) | 63 (23) | 48 (29) | 110 (29) | 0.223 |
| Non‐selective beta‐blocker, n (%) | 16 (2) | 5 (2) | 1 (1) | 10 (3) | 0.297 |
| Calcium antagonist, n (%) | 222 (27) | 62 (23) | 45 (27) | 115 (30) | 0.123 |
| Amiodarone, n (%) | 159 (19) | 38 (14) | 31 (19) | 90 (24) | 0.010 |
| Loop diuretic, n (%) | 595 (73) | 197 (73) | 107 (65) | 291 (76) | 0.030 |
| Thiazide diuretic, n (%) | 278 (34) | 69 (25) | 61 (37) | 148 (39) | 0.001 |
| Vasodilator, n (%) | 25 (3) | 8 (3) | 5 (3) | 12 (3) | 0.991 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; GL, global longitudinal; I.V., intravenous; IVST, interventricular septum thickness; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association.
Values are median (interquartile range).
N = 400.
Figure 1.

Flowchart.
Prevalence of iron deficiency among cardiac amyloidosis patients
Iron deficiency was diagnosed in 400 CA patients (49%): 123 (45%) with AL, 95 (58%) with ATTRv, and 182 (48%) with ATTRwt (Table 2 , Figures 2 and 3 A ). The baseline characteristics of the overall population and according to the presence or not of ID are shown in Table 2 . The median ferritin levels in patients with ID were 113 μg/L compared with 326 μg/L in those without ID. Similarly, the median transferrin saturation levels were 14% in ID patients and 23% in those without ID.
Table 2.
Baseline characteristics of patient according to iron status
| All CA | P value | ||
|---|---|---|---|
| ID status | ID | No ID | |
| N (%) | 400 (49) | 416 (51) | |
| Clinical characteristics | |||
| Age at inclusion, years | 76 (68; 83) | 76 (67; 82) | 0.212 |
| Gender, women n (%) | 125 (31) | 85 (20) | <0.001 |
| BMI, kg/m2 | 25 (22; 27) | 24 (22; 27) | 0.285 |
| CV risk factors | |||
| Diabetes, n (%) | 94 (24) | 57 (14) | <0.001 |
| Hypertension, n (%) | 219 (55) | 219 (53) | 0.297 |
| Dyslipidaemia, n (%) | 137 (34) | 122 (29) | 0.336 |
| CV characteristics | |||
| NYHA Class III–IV vs I–II, n (%) | 179 (48) | 163 (43) | 0.204 |
| Heart rate, beats/min | 75 (68; 85) | 77 (68; 87) | 0.171 |
| Systolic blood pressure, mmHg | 122 (108; 137) | 121 (107; 139) | 0.803 |
| Atrial fibrillation, n (%) | 106 (29) | 107 (28) | 0.812 |
| Ischaemic heart disease, n (%) | 84 (21) | 66 (16) | 0.085 |
| Amyloidosis status | 0.032 | ||
| AL, n (%) | 123 (45) | 148 (55) | |
| ATTRv, n (%) | 95 (56) | 69 (44) | |
| ATTRwt, n (%) | 182 (48) | 199 (52) | |
| Echocardiography characteristics | |||
| LVEF, % | 50 (40; 59) | 52 (43; 60) | 0.069 |
| LVEF class: | 0.129 | ||
| LVEF <40%, n (%) | 86 (23) | 67 (17) | |
| LVEF 40–50%, n (%) | 108 (29) | 112 (29) | |
| LVEF ≥50%, n (%) | 183 (48) | 210 (54) | |
| IVST, mm | 17 (15; 20) | 17 (15; 19) | 0.828 |
| GL strain, % | −10 (−7; −12) | −11 (−8; −13) | 0.022 |
| Biology variables | |||
| NT‐proBNP, ng/mL | 3493 (1810; 6659) | 3655 (1688; 6736) | 0.988 |
| Troponin T HS, ng/mL | 72 (45; 106) | 68 (45; 105) | 0.519 |
| eGFR, mL/min/1.73 m2 | 50 (38; 67) | 51 (37; 67) | 0.996 |
| Haemoglobin, g/dL | 12.9 (11.5; 13.9) | 13.3 (12.0; 14.3) | 0.002 |
| Mean corpuscular volume, μm3 | 90 (85; 94) | 93 (89; 97) | <0.001 |
| Anaemia, n (%) | 174 (44) | 161 (39) | 0.164 |
| Ferritin, μg/L a | 113 (63; 194) | 326 (211; 520) | <0.001 |
| Transferrin saturation, % a | 14 (10; 17) | 23 (20; 28) | <0.001 |
| Anticoagulant and or antiplatelet treatments | |||
| Aspirin, n (%) | 122 (31) | 91 (22) | 0.004 |
| Clopidogrel, n (%) | 26 (7) | 20 (5) | 0.286 |
| Oral anticoagulants, n (%) | 209 (53) | 225 (55) | 0.655 |
| Treatment of CHF | |||
| ACE inhibitor, n (%) | 101 (26) | 115 (28) | 0.465 |
| ARB, n (%) | 68 (17) | 53 (13) | 0.081 |
| Digoxin, n (%) | 8 (2) | 6 (2) | 0.649 |
| Selective beta‐blocker, n (%) | 117 (30) | 104 (25) | 0.157 |
| Non‐selective beta‐blocker, n (%) | 7 (2) | 9 (2) | 0.678 |
| Calcium antagonist, n (%) | 114 (29) | 108 (26) | 0.388 |
| Amiodarone, n (%) | 75 (19) | 84 (20) | 0.629 |
| Loop diuretic, n (%) | 304 (77) | 291 (71) | 0.036 |
| Thiazide diuretic, n (%) | 140 (35) | 138 (34) | 0.544 |
| Vasodilator, n (%) | 11 (3) | 14 (3) | 0.620 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; GL, global longitudinal; ID, iron deficiency; I.V., intravenous; IVST, interventricular septum thickness; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association.
Values are median (interquartile range).
P: ID vs no ID in each category: All, AL, ATTRv, ATTRwt.
By definition.
Figure 2.

Prevalence of iron deficiency (ID) with or without anaemia.
Figure 3.

Prevalence of iron deficiency (ID) among amyloid population. (A) Prevalence of iron deficiency with or without anaemia according to the type of cardiac amyloidosis. (B) Prevalence of absolute and functional iron deficiency according to the type of cardiac amyloidosis.
Interestingly, median ferritin levels were significantly lower in ATTRv patients (139 μg/L) compared with those with AL (225 μg/L) and ATTRwt (210 μg/L) (Table 1 ).
Iron deficiency was functional in 67 patients with AL (55%), 40 with ATTRv (42%), and 113 with ATTRwt (62%) (Figure 3 (B)). It is noteworthy that ID was predominantly absolute in ATTRv patients (58%) (Figure 3 B ). The prevalence of anaemia was similar in patients with and without ID. Indeed, 174 patients (44%) with ID had anaemia vs. 161 (39%) without ID (P = 0.164) (Table 2 ).
Relationship between iron deficiency and clinical and biological imaging characteristics
In the overall population, there were no differences between patients with and without ID in terms of NYHA III–IV (48% vs. 43%; P = 0.2), NT‐proBNP levels (3493 pg/mL vs. 3655 pg/mL; P = 0.988), nor high‐sensitivity troponin T (72 ng/mL vs. 68 ng/mL; P = 0.519) (Table 2 , Figure 4 A–C ). In terms of echocardiography characteristics, LVEF (50% in ID patients vs. 52% in non‐ID patients; P = 0.069) and median IVST (17 mm in both groups; P = 0.828) were similar. In contrast, the left ventricular global longitudinal strain (GLS) was increased in patients with ID compared with those without ID (−10% vs. −11%; P = 0.022) (Table 2 ).
Figure 4.

(A) Prevalence of iron deficiency according to New York Heart Association (NYHA) class. (B) Prevalence of iron deficiency according to quartile of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP). (C) Prevalence of iron deficiency according to quartile of troponin T HS.
In ATTRwt subtype, ID patients presented more dyspnoea, with 46% classified NYHA III‐IV compared with 34% of non‐ID patients (P = 0.023) (Table 3 ). There were no other differences in the subtypes concerning symptoms, cardiac biomarkers, or echocardiography attributes.
Table 3.
Baseline characteristics of patient according to iron status by amyloidosis status
| Type of amyloidosis | AL | P | ATTRv | P | ATTRwt | P | |||
|---|---|---|---|---|---|---|---|---|---|
| ID status | ID | No ID | ID | No ID | ID | No ID | |||
| N (%) | 123 (45) | 148 (55) | 95 (58) | 69 (42) | 182 (48) | 199 (52) | |||
| Clinical characteristics | |||||||||
| Age at inclusion, years | 68 (55; 75) | 67 (60; 74) | 0.845 | 74 (68; 79) | 71 (63; 78) | 0.131 | 82 (76; 86) | 81 (76; 84) | 0.124 |
| Gender, women n (%) | 61 (49) | 42 (28) | <0.001 | 33 (35) | 22 (32) | 0.702 | 31 (17) | 21 (11) | 0.066 |
| BMI, kg/m2 | 24 (22; 27) | 23 (21; 27) | 0.248 | 25 (22; 27) | 24 (21; 27) | 0.879 | 25 (22; 28) | 25 (23; 28) | 0.979 |
| CV risk factors | |||||||||
| Diabetes, n (%) | 22 (18) | 23 (16) | 0.605 | 24 (25) | 8 (12) | 0.029 | 48 (26) | 26 (13) | 0.001 |
| Hypertension, n (%) | 43 (35) | 64 (43) | 0.165 | 59 (62) | 32 (46) | 0.045 | 117 (64) | 123 (62) | 0.617 |
| Dyslipidaemia, n (%) | 31 (25) | 42 (28) | 0.590 | 24 (25) | 18 (26) | 0.948 | 82 (45) | 62 (31) | 0.029 |
| CV characteristics | |||||||||
| NYHA Class III–IV vs. I–II, n (%) | 60 (53) | 70 (56) | 0.650 | 41 (45) | 28 (44) | 0.988 | 78 (46) | 65 (34) | 0.023 |
| Heart rate, beats/min | 81 (71; 94) | 82 (74; 93) | 0.543 | 75 (67; 82) | 74 (65; 84) | 0.700 | 73 (67; 80) | 75 (66; 81) | 0.361 |
| Systolic blood pressure, mmHg | 112 (101; 128) | 111 (100; 126) | 0.639 | 126 (108; 139) | 115 (107; 130) | 0.078 | 128 (114; 140) | 130 (116; 145) | 0.093 |
| Atrial fibrillation, n (%) | 12 (11) | 18 (13) | 0.584 | 21 (23) | 11 (19) | 0.551 | 73 (44) | 78 (42) | 0.704 |
| Ischaemic heart disease, n (%) | 20 (17) | 18 (12) | 0.176 | 9 (10) | 3 (4) | 0.141 | 55 (30) | 45 (23) | 0.173 |
| Echocardiography characteristics | |||||||||
| LVEF, % | 53 (44; 62) | 55 (46; 62) | 0.420 | 48 (35; 59) | 50 (37; 58) | 0.549 | 50 (40; 58) | 51 (42; 58) | 0.189 |
| IVST, mm | 15 (14; 17) | 15 (13; 17) | 0.254 | 17 (15; 21) | 18 (16; 20) | 0.700 | 17 (15; 20) | 18 (16; 20) | 0.213 |
| GL strain, % | 10 (8; 13) | 11 (9; 14) | 0.320 | 10 (8; 12) | 10 (8; 14) | 0.150 | 10 (7; 12) | 10 (8; 13) | 0.116 |
| Biology variables | |||||||||
| NT‐proBNP, ng/mL | 4960 (2036; 10 541) | 5 227 (2394; 13 099) | 0.634 | 2553 (1253; 5404) | 2150 (799; 5440) | 0.296 | 3310 (1929; 6368) | 3233 (1556; 5605) | 0.388 |
| Troponin T HS, ng/mL | 83 (46; 123) | 84 (56; 151) | 0.358 | 65 (41; 99) | 67 (30; 95) | 0.536 | 70 (46; 98) | 62 (41; 93) | 0.050 |
| eGFR, mL/min/1.73 m2 | 58 (41; 77) | 52 (35; 72) | 0.162 | 51 (38; 65) | 58 (36; 79) | 0.157 | 47 (35; 58) | 48 (37; 61) | 0.500 |
| Haemoglobin, g/dL | 12.4 (10.9; 13.6) | 12.8 (10.8; 13.9) | 0.320 | 12.7 (11.5; 13.6) | 13.0 (11.8; 13.9) | 0.259 | 13.2 (11.9; 14.2) | 13.6 (12.6; 14.7) | 0.001 |
| Mean corpuscular volume, μm3 | 90 (85; 94) | 96 (88; 97) | 0.001 | 88 (84; 92) | 92 (89; 96) | <0.001 | 91 (86; 94) | 94 (90; 97) | <0.001 |
| Anaemia, n (%) | 59 (48) | 76 (51) | 0.579 | 44 (46) | 26 (38) | 0.278 | 71 (39) | 59 (30) | 0.054 |
| Ferritin, μg/L a | 118 (59; 185) | 343 (252; 578) | <0.001 | 81 (56; 141) | 308 (183; 482) | <0.001 | 126 (75; 208) | 307 (205; 511) | <0.001 |
| Transferrin saturation, % a | 13 (9; 17) | 22 (18:27) | <0.001 | 14 (10; 17) | 25 (21; 31) | <0.001 | 14 (11; 18) | 23 (20; 28) | <0.001 |
| Anticoagulant and or antiplatelet treatments | |||||||||
| Aspirin, n (%) | 37 (31) | 38 (26) | 0.350 | 27 (29) | 7 (10) | 0.004 | 58 (32) | 46 (24) | 0.068 |
| Clopidogrel, n (%) | 5 (4) | 7 (5) | 0.825 | 5 (5) | 1 (1) | 0.191 | 16 (9) | 12 (6) | 0.322 |
| Oral anti‐coagulants, n (%) | 45 (38) | 66 (45) | 0.241 | 41 (44) | 33 (48) | 0.637 | 123 (68) | 126 (64) | 0.499 |
| Treatment of CHF | |||||||||
| ACE inhibitor, n (%) | 16 (13) | 26 (18) | 0.343 | 27 (29) | 27 (39) | 0.178 | 58 (32) | 62 (32) | 0.961 |
| ARB, n (%) | 11 (9) | 14 (10) | 0.935 | 21 (23) | 8 (12) | 0.071 | 36 (20) | 31 (16) | 0.313 |
| Digoxin, n (%) | 1 (1) | 1 (1) | 0.881 | 1 (1) | 0 (0) | 0.388 | 4 (2) | 7 (4) | 0.427 |
| Selective beta‐blocker, n (%) | 28 (23) | 35 (24) | 0.952 | 29 (31) | 19 (28) | 0.615 | 60 (33) | 50 (26) | 0.111 |
| Non‐selective beta‐blocker, n (%) | 3 (3) | 2 (1) | 0.490 | 0 (0) | 1 (1) | 0.244 | 4 (2) | 6 (3) | 0.601 |
| Calcium antagonist, n (%) | 23 (19) | 39 (26) | 0.165 | 34 (37) | 11 (16) | 0.004 | 57 (31) | 58 (30) | 0.715 |
| Amiodarone, n (%) | 14 (12) | 24 (16) | 0.288 | 18 (19) | 13 (19) | 0.934 | 43 (24) | 47 (24) | 0.936 |
| Loop diuretic, n (%) | 91 (76) | 106 (72) | 0.437 | 42 (61) | 65 (70) | 0.230 | 148 (81) | 143 (73) | 0.054 |
| Thiazide diuretic, n (%) | 33 (28) | 36 (24) | 0.554 | 33 (36) | 28 (41) | 0.508 | 74 (41) | 74 (38) | 0.563 |
| Vasodilator, n (%) | 3 (3) | 5 (3) | 0.674 | 4 (4) | 1 (1) | 0.299 | 4 (2) | 8 (4) | 0.297 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; GL, global longitudinal; ID, iron deficiency; I.V., intravenous; IVST, interventricular septum thickness; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association. P: ID vs. no ID in each category: All, AL, ATTRv, ATTRwt.
Values are median (interquartile range).
By definition.
In the overall population, the C‐reactive protein (CRP) levels were significantly higher in patients with functional ID, 4.3 ng/mL, compared with 2.8 ng/mL in those with absolute ID (P = 0.001) (supporting information, Table S1 ).
Determinants of iron deficiency in cardiac amyloidosis
In multivariate analysis, by logistic regression (Table 4 , Figure 5 ), the following variables were found to be significantly associated with ID: female sex, amyloidosis subtype (ATTRv/ATTRwt vs. AL), diabetes status, aspirin treatment, haemoglobin levels, and altered GLS.
Table 4.
Univariate and multivariate logistic regression of the iron deficiency determinants in cardiac amyloidosis
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Variables | N | Hazard ratio | CI | P value | Hazard ratio | CI | P value |
| Women | 816 | 1.770 | 1.287; 2.434 | <0.001 | 1.919 | 1.360; 2.707 | <0.001 |
| Amyloidosis type | 271 | AL reference | AL reference | ||||
| ATTRv | 164 | 1.657 | 1.120; 2.451 | 0.012 | 1.849 | 1.227; 2.785 | 0.003 |
| ATTRwt | 381 | 1.100 | 0.805; 1.504 | 0.548 | 1.438 | 1.022; 2.023 | 0.037 |
| Diabetes | 816 | 1.935 | 1.346; 2.780 | <0.001 | 1.752 | 1.204; 2.548 | 0.003 |
| Aspirin | 808 | 1.581 | 1.153; 2.168 | 0.004 | 1.555 | 1.119; 2.161 | 0.009 |
| Haemoglobin | 816 | 0.892 | 0.827; 0.961 | 0.003 | 0.895 | 0.826; 0.969 | 0.006 |
| Absolute GLS | 816 | 0.970 | 0.941; 1.000 | 0.049 | 0.963 | 0.933; 0.994 | 0.020 |
CI, confidence interval; GLS, global longitudinal strain.
Figure 5.
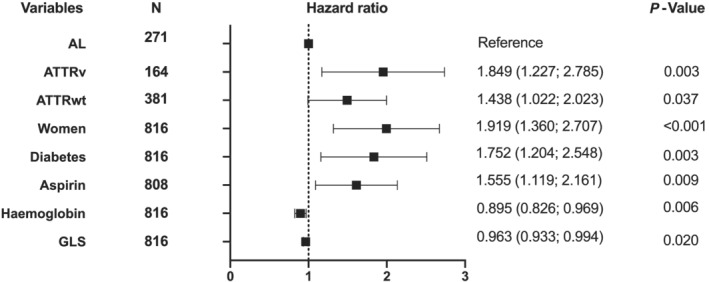
Iron deficiency's determinants in cardiac amyloidosis.
Impact on outcome
During a mean follow‐up 17 months, a total of 299 deaths occurred (Figure S1 ) illustrates the freedom from all‐cause mortality depending on the presence or not of ID. The difference in mortality was not significant different in CA patients with or without ID (log rank = 0.785).
Discussion
We found a high prevalence of ID in the CA patients (49%), which is comparable with that found in HF patients (50–62%). 2 , 8 , 9 ID was more frequent in ATTRv amyloidosis patients compared with those with ATTRwt and AL. The prevalence of ID was significantly associated with diabetes, aspirin treatment, altered GLS, and lower haemoglobin levels.
High prevalence of iron deficiency in cardiac amyloidosis
Iron deficiency incidence in CA is similar to that observed in HF patients and is not specific to a CA subtype. Thus, ID cannot be used for diagnosis. Multiple assessments, including electrocardiography, transthoracic echocardiogram, magnetic resonance imagery, and biological assessments (clonal expressions), should be used to diagnose CA. It is crucial that CA be diagnosis rapidly so that appropriate treatment, specific to the amyloidosis subtype, can be implemented. CA is a severe and debilitating disease with limited treatments to improve prognosis and symptoms.
In HF, several mechanisms cause inflammation and malabsorption of iron that results in ID. 6 Functional ID results from chronic inflammation with iron sequestration by macrophages. 6 Elevated levels of interleukin‐6 are associated with inflammatory iron sequestration in HF and increased iron metabolism. 29 HF is also associated with structural and functional changes in the gut that result in peritoneal oedema and malabsorption, causing absolute ID. 30 We analysed albumin levels classified by tertiles according to ID status. Patients with ID had significantly decreased albumin levels, affirming the malabsorption hypothesis (Figure S2 , T1 vs. T2 vs. T3, P = 0.022).
In our study, we observed a more pronounced systemic inflammation (higher levels of CRP) in CA patients with functional ID than those with absolute ID. Thus, in patients with absolute ID, we suspect that other mechanisms specific to amyloidosis, such as gastrointestinal bleeding, might be involved. 23 Recently, a study confirmed, by duodenal biopsy, submucosal amyloid deposits of AL and TTR fibrils in systemic amyloidosis. 24 As the duodenum is the predominant site of iron absorption, this infiltration may limit iron absorption in the intestine, leading to ID. In addition, intestinal bleeding, a major cause of ID, is associated with amyloidosis. 31 Amyloid deposits on blood vessel walls may cause erosive changes and mucosal friability leading to intestinal bleeding. 31 Aspirin treatment can accentuate this gastrointestinal frailty. 32
The AL amyloidosis, contrary to ATTR, is often associated with hepatic amyloid infiltration. 33 This liver involvement includes hyperbilirubinemia and hepatic insufficiency. 34 In our AL CA population, we analysed bilirubin levels classified by tertiles: patients with ID had higher bilirubin levels (data not shown, T1 vs. T2 vs. T3, P = 0.031).
Variations of iron deficiency prevalence and severity among cardiac amyloidosis types
Iron deficiency severity was different in the CA subtypes. ID was more severe (with lower ferritin levels) in ATTRv while HF severity appears to be less severe in the same patients (with lower NT‐proBNP levels). The increased severity of ID resulting from the prolonged exposure of the intestinal tract to amyloid protein in ATTRv amyloidosis due to early onset because of its genetic aetiology. This extended exposure can lead to chronic intestinal bleeding in ATTRv patients that exacerbates ID. ATTRv amyloidosis is also associated with gastroparesis, leading to malnutrition, attributed to autonomic dysfunction by nerve infiltrations. 23 In contrast, these gastrointestinal autonomic dysfunctions are poorly described in AL and ATTRwt amyloidosis.
Clinical and imaging variables associated with iron deficiency in amyloidosis
Several characteristics were significantly associated with ID, including diabetes, aspirin treatment, and GLS. Diabetes was present in 24% of patients with ID compared with 14% in those without ID (P < 0.001). The association between ID and diabetes has already been described. 2 , 9 , 35 Indeed, Praveen et al. reported that 14/89 (16%) diabetic patients had ID 35 and identified a significantly increased inflammatory response in these patients. They suggest that chronic inflammation in diabetic patients leads to macrophage iron sequestration (functional ID) and intestinal malabsorption (absolute ID).
Aspirin treatment was identified as a determinant of ID in our CA population (HR = 1.55, P = 0.009). As previously described, chronic use of aspirin increases the risk of gastrointestinal bleeding. A randomized control trial reported a 60% increase in gastrointestinal bleeding in patients using aspirin. 32
Altered GLS was also associated with ID (P = 0.049). Altered GLS is an established independent prognosis marker in CA and is strongly associated with cardiac amyloid burden. 36 , 37 Data regarding a potential link between iron status and LV function are limited. Iron participates in several enzymatic reactions implicated in cellular respiration, oxidative phosphorylation, citric acid cycle, and production of reactive oxygen species. 38 , 39 Indeed, mitochondrial and left ventricular dysfunction have been associated with ID in an animal model. 40 Therefore, ID could be associated with amyloid infiltration and toxicity, leading to cardiomyocyte dysfunction in CA patients.
Iron therapy for HF patients with ID is beneficial, particularly in HFrEF and HFpEF patients. 4 , 41 , 42 , 43 , 44 The benefits include fewer cardiovascular‐related hospitalizations, with improved heart function, exercise capacity, and quality of life. HF patients with anaemia benefit more from intravenous iron therapy than those without anaemia. Intravenous iron therapy reduces heart failure hospitalization but not cardiovascular mortality in HFrEF patients with ID. 15 Considering the prevalence of ID in CA, the benefit of iron therapy in these patients needs to be assessed.
Study limitations
This study has several limitations. Our study was designed as an observational study and not a randomized controlled trial. HF is strongly associated with ID. Thus, in CA patients, the severity of HF may interfere with ID prevalence. Indeed, ID was associated with ATTRv patients that had less severe HF signs compared with AL or ATTRwt patients. Unfortunately, we did not collect data concerning the cardiac hepcidin–ferroportin axis shown to be essential for cardiomyocyte iron homeostasis and heart function. We spared patients from invasive cardiac biopsies that would have allowed us to assess cardiac iron concentration. In HFrEF, cardiac iron concentrations have been shown to be unrelated to biomarkers of systemic iron status or anaemia. 45 However, cardiac iron concentrations are associated with disease severity. It would have been interesting to see if this association also exists in CA.
Our retrospective study design does not allow us to further assess mortality in patients with and without ID, found to be not significantly different. A prospective study is needed to confirm our result. Finally, our study does not address the therapeutic use of iron in CA. The benefit of intravenous iron therapy in CA needs to be evaluated in a randomized, placebo‐controlled trial.
Conclusions
Iron deficiency is frequent in patients with CA and more prevalent in ATTRv compared with AL and ATTRwt amyloidosis. ID was independently associated with female gender, amyloidosis type, diabetes, aspirin treatment, lower haemoglobin levels, and altered left ventricular GLS. The presence of ID does not seem to be associated with increased mortality. Prospective studies are needed to assess whether ID affects morbidity and mortality, as well as the benefit of iron in CA patients.
Conflict of interest
T.D. has received grant and/or consultant fees from Pfizer, Alnylam, Vifor, Akcea, Novartis, Resmed, Bayer, Astra‐Zeneca, Sanofi‐Aventis. A.J.D. has received grant and/or consultant fees from Novartis, Amicus, Sanofi‐Genzyme, Boehringer Ingelheim.
Supporting information
Figure S1: Survival according iron status.
Figure S2: Ferritin levels according to albumin.
Table S1: CRP levels according ID status and CA subtype.
Jobbé‐Duval, A. , Bézard, M. , Moutereau, S. , Kharoubi, M. , Oghina, S. , Zaroui, A. , Galat, A. , Chalard, C. , Hugon‐Vallet, E. , Lemonnier, F. , Eyharts, D. , Poulot, E. , Fanen, P. , Funalot, B. , Molinier‐Frenkel, V. , Audard, V. , Hittinger, L. , Delbarre, M. A. , Teiger, E. , and Damy, T. (2022) Prevalence and determinants of iron deficiency in cardiac amyloidosis. ESC Heart Failure, 9: 1314–1327. 10.1002/ehf2.13818.
References
- 1. Camaschella C. Iron deficiency. Blood 2019; 133: 30–39. [DOI] [PubMed] [Google Scholar]
- 2. Klip IT, Comin‐Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, Van Veldhuisen DJ. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165: 575–582.e3. [DOI] [PubMed] [Google Scholar]
- 3. Macdougall IC, Bock AH, Carrera F, Eckardt KU, Gaillard C, van Wyck D, Roubert B, Nolen JG, Roger SD, FIND‐CKD Study Investigators . FIND‐CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant 2014; 29: 2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole‐Wilson PA, Ponikowski P. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448. [DOI] [PubMed] [Google Scholar]
- 5. Authors/Task Force Members , McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GYH, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, ESC Committee for Practice Guidelines (CPG) , Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Ž, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Document Reviewers , McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Ørn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012; 14: 803–869. [DOI] [PubMed] [Google Scholar]
- 6. Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation 2018; 138: 80–98. [DOI] [PubMed] [Google Scholar]
- 7. Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013; 34: 816–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yeo TJ, Yeo PSD, Ching‐Chiew Wong R, Ong HY, Leong KTG, Jaufeerally F, Sim D, Santhanakrishnan R, Lim SL, M.Y. Chan M, Chai P, Low AF, Ling LH, Ng TP, Richards AM, Lam CSP. Iron deficiency in a multi‐ethnic Asian population with and without heart failure: prevalence, clinical correlates, functional significance and prognosis: iron deficiency in Asian heart failure. Eur J Heart Fail 2014; 16: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 9. Martens P, Nijst P, Verbrugge FH, Smeets K, Dupont M, Mullens W. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid‐range and preserved ejection fraction. Acta Cardiol 2018; 73: 115–123. [DOI] [PubMed] [Google Scholar]
- 10. Bekfani T, Pellicori P, Morris D, Ebner N, Valentova M, Sandek A, Doehner W, Cleland JG, Lainscak M, Schulze PC, Anker SD, von Haehling S. Iron deficiency in patients with heart failure with preserved ejection fraction and its association with reduced exercise capacity, muscle strength and quality of life. Clin Res Cardiol 2019; 108: 203–211. [DOI] [PubMed] [Google Scholar]
- 11. Okonko DO, Grzeslo A, Witkowski T, Mandal AKJ, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole‐Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency. J Am Coll Cardiol 2008; 51: 103–112. [DOI] [PubMed] [Google Scholar]
- 12. Toblli JE, Lombraña A, Duarte P, di Gennaro F. Intravenous iron reduces NT‐pro‐brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol 2007; 50: 1657–1665. [DOI] [PubMed] [Google Scholar]
- 13. Ponikowski P, Kirwan BA, Anker SD, McDonagh T, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Göhring UM, Keren A, Khintibidze I, Kragten H, Martinez FA, Metra M, Milicic D, Nicolau JC, Ohlsson M, Parkhomenko A, Pascual‐Figal DA, Ruschitzka F, Sim D, Skouri H, van der Meer P, Lewis BS, Comin‐Colet J, von Haehling S, Cohen‐Solal A, Danchin N, Doehner W, Dargie HJ, Motro M, Butler J, Friede T, Jensen KH, Pocock S, Jankowska EA, Azize G, Fernandez A, Zapata GO, Garcia Pacho P, Glenny A, Ferre Pacora F, Parody ML, Bono J, Beltrano C, Hershson A, Vita N, Luquez HA, Cestari HG, Fernandez H, Prado A, Berli M, García Durán R, Thierer J, Diez M, Lobo Marquez L, Borelli RR, Hominal MÁ, Metra M, Ameri P, Agostoni P, Salvioni A, Fattore L, Gronda E, Ghio S, Turrini F, Uguccioni M, di Biase M, Piepoli M, Savonitto S, Mortara A, Terrosu P, Fucili A, Boriani G, Midi P, Passamonti E, Cosmi F, van der Meer P, van Bergen P, van de Wetering M, Al‐Windy NYY, Tanis W, Meijs M, Groutars RGEJ, The HKS, Kietselaer B, van Kesteren H, Beelen DPW, Heymeriks J, van de Wal R, Schaap J, Emans M, Westendorp P, Nierop PR, Nijmeijer R, Manintveld OC, Dorobantu M, Darabantiu DA, Zdrenghea D, Toader DM, Petrescu L, Militaru C, Crisu D, Tomescu MC, Stanciulescu G, Rodica Dan A, Iosipescu LC, Serban DL, Drozdz J, Szachniewicz J, Bronisz M, Tycińska A, Wozakowska‐Kaplon B, Mirek‐Bryniarska E, Gruchała M, Nessler J, Straburzyńska‐Migaj E, Mizia‐Stec K, Szelemej R, Gil R, Gąsior M, Gotsman I, Halabi M, Shochat M, Shechter M, Witzling V, Zukermann R, Arbel Y, Flugelman M, Ben‐Gal T, Zvi V, Kinany W, Weinstein JM, Atar S, Goland S, Milicic D, Horvat D, Tušek S, Udovicic M, Šutalo K, Samodol A, Pesek K, Artuković M, Ružić A, Šikić J, McDonagh T, Trevelyan J, Wong YK, Gorog D, Ray R, Pettit S, Sharma S, Kabir A, Hamdan H, Tilling L, Baracioli L, Nigro Maia L, Dutra O, Reis G, Pimentel Filho P, Saraiva JF, Kormann A, dos Santos F, Bodanese L, Almeida D, Precoma D, Rassi S, Costa F, Kabbani S, Abdelbaki K, Abdallah C, Arnaout MS, Azar R, Chaaban S, Raed O, Kiwan G, Hassouna B, Bardaji A, Zamorano J, del Prado S, Gonzalez Juanatey JR, Ga Bosa Ojeda FI, Gomez Bueno M, Molina BD, Pascual Figal DA, Sim D, Yeo TJ, Loh SY, Soon D, Ohlsson M, Smith JG, Gerward S, Khintibidze I, Lominadze Z, Chapidze G, Emukhvari N, Khabeishvili G, Chumburidze V, Paposhvili K, Shaburishvili T, Khabeishvili G, Parhomenko O, Kraiz I, Koval O, Zolotaikina V, Malynovsky Y, Vakaliuk I, Rudenko L, Tseluyko V, Stanislavchuk M. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double‐blind, randomised, controlled trial. Lancet 2020; 396: 1895–1904. [DOI] [PubMed] [Google Scholar]
- 14. Bolger AP, Bartlett FR, Penston HS, O'Leary J, Pollock N, Kaprielian R, Chapman CM. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol 2006; 48: 1225–1227. [DOI] [PubMed] [Google Scholar]
- 15. Mohammed SF, Mirzoyev SA, Edwards WD, Dogan A, Grogan DR, Dunlay SM, Roger VL, Gertz MA, Dispenzieri A, Zeldenrust SR, Redfield MM. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2014; 2: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. González‐López E, Gallego‐Delgado M, Guzzo‐Merello G, de Haro‐del Moral FJ, Cobo‐Marcos M, Robles C, Bornstein B, Salas C, Lara‐Pezzi E, Alonso‐Pulpon L, Garcia‐Pavia P. Wild‐type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 2015; 36: 2585–2594. [DOI] [PubMed] [Google Scholar]
- 17. Lindmark K, Pilebro B, Sundström T, Lindqvist P. Prevalence of wild type transtyrethin cardiac amyloidosis in a heart failure clinic. ESC Heart Fail 2020; 8: ehf2.13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Damy T, Maurer MS, Rapezzi C, Planté‐Bordeneuve V, Karayal ON, Mundayat R, Suhr OB, Kristen AV. Clinical, ECG and echocardiographic clues to the diagnosis of TTR‐related cardiomyopathy. Open Heart 2016; 3: e000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Damy T, Jaccard A, Guellich A, Lavergne D, Galat A, Deux JF, Hittinger L, Dupuis J, Frenkel V, Rigaud C, Plante‐Bordeneuve V, Bodez D, Mohty D. Identification of prognostic markers in transthyretin and AL cardiac amyloidosis*. Amyloid 2016; 23: 194–202. [DOI] [PubMed] [Google Scholar]
- 20. Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ, Bokhari S, Castano A, Dorbala S, Johnson GB, Glaudemans AWJM, Rezk T, Fontana M, Palladini G, Milani P, Guidalotti PL, Flatman K, Lane T, Vonberg FW, Whelan CJ, Moon JC, Ruberg FL, Miller EJ, Hutt DF, Hazenberg BP, Rapezzi C, Hawkins PN. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016; 133: 2404–2412. [DOI] [PubMed] [Google Scholar]
- 21. Damy T, Kristen AV, Suhr OB, Maurer MS, Planté‐Bordeneuve V, Yu CR, Ong ML, Coelho T, Rapezzi C, THAOS Investigators . Transthyretin cardiac amyloidosis in continental Western Europe: an insight through the transthyretin amyloidosis outcomes survey (THAOS). Eur Heart J 2019: ehz173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mohty D, Damy T, Cosnay P, Echahidi N, Casset‐Senon D, Virot P, Jaccard A. Cardiac amyloidosis: updates in diagnosis and management. Arch Cardiovasc Dis 2013; 106: 528–540. [DOI] [PubMed] [Google Scholar]
- 23. Obici L, Suhr OB. Diagnosis and treatment of gastrointestinal dysfunction in hereditary TTR amyloidosis. Clin Auton Res 2019; 29: 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iida T, Yamano H, Nakase H. Systemic amyloidosis with gastrointestinal involvement: diagnosis from endoscopic and histological views: endoscopic views of systemic amyloidosis. J Gastroenterol Hepatol 2018; 33: 583–590. [DOI] [PubMed] [Google Scholar]
- 25. Dispenzieri A, Merlini G, Comenzo RL. Amyloidosis: 2008 BMT Tandem Meetings (February 13–17, San Diego). Biol Blood Marrow Transplant 2008; 14: 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Béquignon E, Guellich A, Bartier S, Raynal M, Prulière‐Escabasse V, Canouï‐Poitrine F, Coste A, Damy T. How your ears can tell what is hidden in your heart: wild‐type transthyretin amyloidosis as potential cause of sensorineural hearing loss inelderly‐AmyloDEAFNESS pilot study. Amyloid 2017; 24: 96–100. [DOI] [PubMed] [Google Scholar]
- 27. Manito N, Cerqueiro JM, Comín‐Colet J, García‐Pinilla JM, González‐Franco A, Grau‐Amorós J, Peraira JR, Manzano L. Documento de consenso de la Sociedad Española de Cardiología y la Sociedad Española de Medicina Interna sobre el diagnóstico y tratamiento del déficit de hierro en la insuficiencia cardíaca. Rev Clin Esp 2017; 217: 35–45. [DOI] [PubMed] [Google Scholar]
- 28. McDonagh T, Damy T, Doehner W, Lam CSP, Sindone A, van der Meer P, Cohen‐Solal A, Kindermann I, Manito N, Pfister O, Pohjantähti‐Maaroos H, Taylor J, Comin‐Colet J. Screening, diagnosis and treatment of iron deficiency in chronic heart failure: putting the 2016 European Society of Cardiology heart failure guidelines into clinical practice. Eur J Heart Fail 2018; 20: 1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Markousis‐Mavrogenis G, Tromp J, Ouwerkerk W, Devalaraja M, Anker SD, Cleland JG, Dickstein K, Filippatos GS, Harst P, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, Zannad F, Zwinderman AH, Hillege HL, Veldhuisen DJ, Kakkar R, Voors AA, Meer P. The clinical significance of interleukin‐6 in heart failure: results from the BIOSTAT‐CHF study. Eur J Heart Fail 2019; 21: 965–973. [DOI] [PubMed] [Google Scholar]
- 30. Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber‐Eibel J, von Haehling S, Schroedl W, Karhausen T, Doehner W, Rauchhaus M, Poole‐Wilson P. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol 2007; 50: 1561–1569. [DOI] [PubMed] [Google Scholar]
- 31. Cowan AJ, Skinner M, Seldin DC, Berk JL, Lichtenstein DR, O'Hara CJ, Doros G, Sanchorawala V. Amyloidosis of the gastrointestinal tract: a 13‐year, single‐center, referral experience. Haematologica 2013; 98: 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mahady SE, Margolis KL, Chan A, Polekhina G, Woods RL, Wolfe R, Nelson MR, Lockery JE, Wood EM, Reid C, Ernst ME, Murray A, Thao LTP, McNeil JJ. Major GI bleeding in older persons using aspirin: incidence and risk factors in the ASPREE randomised controlled trial. Gut 2021; 70: 717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abe R, Katoh N, Takahashi Y, Takasone K, Yoshinaga T, Yazaki M, Kametani F, Sekijima Y. Distribution of amyloidosis subtypes based on tissue biopsy site—consecutive analysis of 729 patients at a single amyloidosis center in Japan. Pathol Int 2020; 71: pin.13041. [DOI] [PubMed] [Google Scholar]
- 34. Rosenzweig M, Comenzo RL. Liver and gastrointestinal involvement. Hematol Oncol Clin North Am 2020; 34: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 35. Praveen M, Jain N, Raizada N, Sharma S, Narang S, Madhu SV. Anaemia in patients with type 2 diabetes mellitus without nephropathy is related to iron deficiency. Diabetes Metab Syndr Clin Res Rev 2020; 14: 1837–1840. [DOI] [PubMed] [Google Scholar]
- 36. Quarta CC, Solomon SD, Uraizee I, Kruger J, Longhi S, Ferlito M, Gagliardi C, Milandri A, Rapezzi C, Falk RH. Left ventricular structure and function in transthyretin‐related versus light‐chain cardiac amyloidosis. Circulation 2014; 129: 1840–1849. [DOI] [PubMed] [Google Scholar]
- 37. Ternacle J, Bodez D, Guellich A, Audureau E, Rappeneau S, Lim P, Radu C, Guendouz S, Couetil JP, Benhaiem N, Hittinger L, Dubois‐Randé JL, Plante‐Bordeneuve V, Mohty D, Deux JF, Damy T. Causes and consequences of longitudinal LV dysfunction assessed by 2D strain echocardiography in cardiac amyloidosis. JACC Cardiovasc Imaging 2016; 9: 126–138. [DOI] [PubMed] [Google Scholar]
- 38. Lakhal‐Littleton S. Mechanisms of cardiac iron homeostasis and their importance to heart function. Free Radic Biol Med 2019; 133: 234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jankowska EA, Ponikowski P. Molecular changes in myocardium in the course of anemia or iron deficiency. Heart Fail Clin 2010; 6: 295–304. [DOI] [PubMed] [Google Scholar]
- 40. Dong F, Zhang X, Culver B, Chew HG Jr, Kelley RO, Ren J. Dietary iron deficiency induces ventricular dilation, mitochondrial ultrastructural aberrations and cytochrome c release: involvement of nitric oxide synthase and protein tyrosine nitration. Clin Sci 2005; 109: 277–286. [DOI] [PubMed] [Google Scholar]
- 41. Zhang J, Hu S, Jiang Y, Zhou Y. Efficacy and safety of iron therapy in patients with chronic heart failure and iron deficiency: a systematic review and meta‐analysis based on 15 randomised controlled trials. Postgrad Med J 2020: postgradmedj‐2019–137342. [DOI] [PubMed] [Google Scholar]
- 42. Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin‐Colet J, Ruschitzka F, Lüscher TF, Arutyunov GP, Motro M, Mori C, Roubert B. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron‐deficient heart failure patients: an individual patient data meta‐analysis. Eur J Heart Fail 2018; 20: 125–133. [DOI] [PubMed] [Google Scholar]
- 43. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 44. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group , de Boer RA, Christian Schulze P, Abdelhamid M, Aboyans V, Adamopoulos S, Anker SD, Arbelo E, Asteggiano R, Bauersachs J, Bayes‐Genis A, Borger MA, Budts W, Cikes M, Damman K, Delgado V, Dendale P, Dilaveris P, Drexel H, Ezekowitz J, Falk V, Fauchier L, Filippatos G, Fraser A, Frey N, Gale CP, Gustafsson F, Harris J, Iung B, Janssens S, Jessup M, Konradi A, Kotecha D, Lambrinou E, Lancellotti P, Landmesser U, Leclercq C, Lewis BS, Leyva F, Linhart A, Løchen ML, Lund LH, Mancini D, Masip J, Milicic D, Mueller C, Nef H, Nielsen JC, Neubeck L, Noutsias M, Petersen SE, Sonia Petronio A, Ponikowski P, Prescott E, Rakisheva A, Richter DJ, Schlyakhto E, Seferovic P, Senni M, Sitges M, Sousa‐Uva M, Tocchetti CG, Touyz RM, Tschoepe C, Waltenberger J, Adamo M, Baumbach A, Böhm M, Burri H, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gardner RS, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 45. Hirsch VG, Tongers J, Bode J, Berliner D, Widder JD, Escher F, Mutsenko V, Chung B, Rostami F, Guba‐Quint A, Giannitsis E, Schultheiss HP, Vogt C, Bauersachs J, Wollert KC, Kempf T. Cardiac iron concentration in relation to systemic iron status and disease severity in non‐ischaemic heart failure with reduced ejection fraction. Eur J Heart Fail 2020; 22: 2038–2046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Survival according iron status.
Figure S2: Ferritin levels according to albumin.
Table S1: CRP levels according ID status and CA subtype.


