Abstract
Background
We evaluated the time to progression (TTP) and survival outcomes of second-line therapy for metastatic colorectal cancer among adults aged 70 years and older compared with younger adults following progression on first-line clinical trials.
Methods
Associations between clinical and disease characteristics, time to initial progression, and rate of receipt of second-line therapy were evaluated. TTP and overall survival (OS) were compared between older and younger adults in first- and second-line trials by Cox regression, adjusting for age, sex, Eastern Cooperative Oncology Group Performance Status, number of metastatic sites and presence of metastasis in the lung, liver, or peritoneum. All statistical tests were 2-sided.
Results
Older adults comprised 16.4% of patients on first-line trials (870 total older adults aged >70 years; 4419 total younger adults aged ≤70 years, on first-line trials). Older adults and those with Eastern Cooperative Oncology Group Performance Status >0 were less likely to receive second-line therapy than younger adults. Odds of receiving second-line therapy decreased by 11% for each additional decade of life in multivariable analysis (odds ratio = 1.11, 95% confidence interval = 1.02 to 1.21, P = .01). Older and younger adults enrolled in second-line trials experienced similar median TTP and median OS (median TTP = 5.1 vs 5.2 months, respectively; median OS = 11.6 vs 12.4 months, respectively).
Conclusions
Older adults were less likely to receive second-line therapy for metastatic colorectal cancer, though we did not observe a statistical difference in survival outcomes vs younger adults following second-line therapy. Further study should examine factors affecting decisions to treat older adults with second-line therapy. Inclusion of geriatric assessment may provide better criteria regarding the risks and benefits of second-line therapy.
The increasing prevalence of older adults with cancer in the United States—dubbed the “Silver Tsunami”—accompanies an increase in prevalence of cancer by age in the US population from 216 million in 1975 to an estimated 380 million by 2040 (1). This trend is exemplified in colorectal cancer (CRC), where the highest rates of new cases and deaths occur among adults aged 75-84 years (2). Given that the median age at diagnosis of CRC is between age 69 and 70 years, 70 years is often referenced in the literature as the appropriate age threshold for studying CRC in older adults. Although there has been a modest downward shift in the age at diagnosis in CRC [with incidence expected to increase among those aged <50 years (3-5)], the prevalence of disease remains highest among older adults. According to the National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results Program database, adults aged 65-74 years and 75+ years with CRC make up 1.26% and 2.78% of the US population, respectively, compared with 0.37% of adults aged 40-64 years (2017 age at prevalence estimate). Because older adults are more likely to be diagnosed with metastatic CRC (mCRC), have a higher prevalence of disease (2), and constitute a substantial proportion of the US population, the need to determine best practices for treatment of older adults diagnosed with mCRC based on clinical trials is imperative.
Prior studies evaluating survival outcomes in older adults enrolled in first-line treatment clinical trials for mCRC found no statistically significant difference in overall survival (OS) among older and younger adults in individual trials and pooled trials (6-9). In those studies, the median OS of older adults ranged from 11 to 20 months, with median progression-free survival (PFS) of 5.5 to 9 months. Taken together, the evidence suggests a reasonable survival advantage from initial palliative chemotherapy for older adults meeting enrollment criteria, including being considered fit enough to participate in a therapeutic clinical trial. However, although there are data from individual trials, there are no known data that pool outcomes across trials, inclusive of standard second-line regimens, to aid in discerning the survival benefit for continuing palliative chemotherapy in older adults beyond first progression. Such data could inform patient and physician choices in this setting. The Aide et Recherche en CAncerologie Digestive (ARCAD) is a clinical database of 48 mCRC therapeutic clinical trials that pools individual patient, disease, treatment, and outcome data of 40 016 participants. ARCAD is an international collaborative effort founded as a standing resource to accelerate understanding of mCRC, increase the efficiency of industry-sponsored clinical trials, and improve the efficacy of clinical treatment for patients. Similar pooled analyses of individual data have contributed to our understanding of treatment outcomes for older adults (10) and have been employed by both National Comprehensive Cancer Network (11,12) and European Society for Medical Oncology (13) expert panels to construct recommendations regarding care for this growing subset of patients. Given the current gap in evidence regarding second-line treatment for older adults diagnosed with mCRC, we sought to determine the survival outcomes for older adults participating in first-line trials for mCRC, rate of enrollment on second-line trials, and survival outcomes.
Methods
First-Line Trials
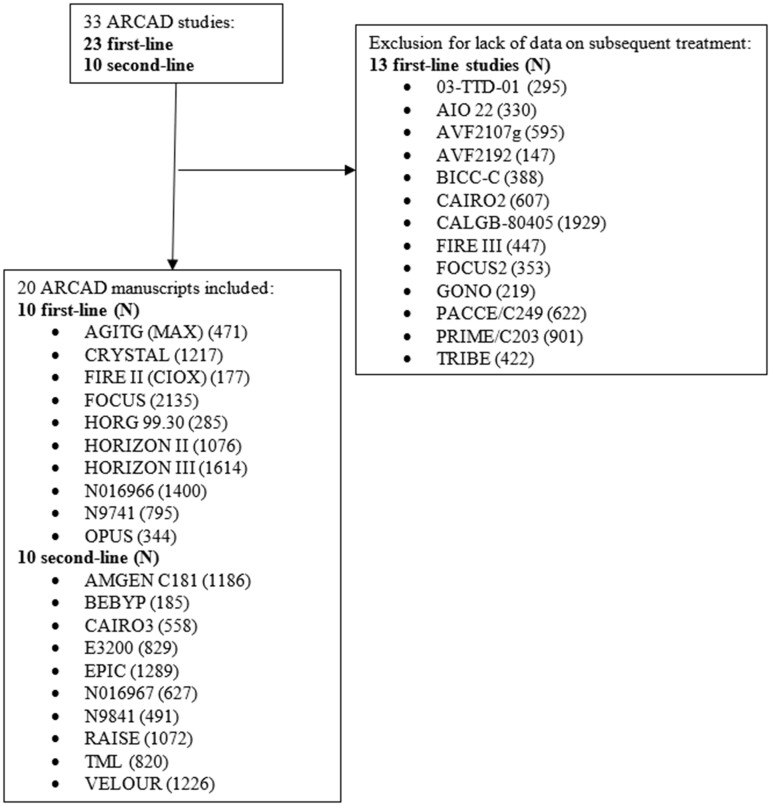
The outcome for first-line trials is time to progression 1 (TTP1), defined as the time from date of random assignment to the date of first disease progression (in which death is censored). For second-line trials, the time to progression 2 (TTP2) outcome is defined as the time from date of random assignment to second-line treatment to the date of first disease progression on second-line treatment. The populations included in first- and second-line trials were unique and did not overlap. We examined OS from initiation of second-line treatment. In sequential trials, this was defined as the time to death since study enrollment. In nonsequential trials, this was defined as time to death since first-line trial enrollment. We also examined age at enrollment using the age threshold of 70 years or younger to indicate younger adults and age older than 70 years to indicate older adults, as per prior analyses (10,14,15). We surveyed a number of clinical trials in the ARCAD database that enrolled participants with mCRC. For first-line trials, we selected those trials that only specified first-line therapy (as opposed to specifying sequential lines of therapy) and that collected data on TTP for subsequent lines of treatment. All studies with subsequent treatment data available were included for analysis. Study and enrollment characteristics for each trial can be found in Table 1. Ten ARCAD trials were included in first-line analysis with available data for subsequent therapy: OPUS, N9741, NO16966, HORIZON III, HORIZON II, HORG 99.30, FOCUS, FIRE II (CIOX), CRYSTAL, and AGITG (MAX) (Figure 1).
Table 1.
Study and enrollment characteristics of ARCAD first- and second-line mCRC clinical trials
| Study | Regimen | Key eligibility | Total accrual (No.) | Agea, No. (%) |
Sex, No. (%) |
ECOG PS, No. (%) |
Metastatic sitesa, No. (%) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <70 y | ≥70 y | Female | Male | 0 | >0 | <2 | ≥2 | ||||
| First-line | |||||||||||
| AGITG (MAX) | C v C + B vs C + B + M |
|
471 | 284 (60.3) | 187 (39.7) | 176 (37.4) | 295 (62.6) | 263 (55.8) | 208 (44.2) | 110 (23.4) | 361 (76.7) |
| CRYSTAL | FOLFIRI vs FOLFIRI + C |
|
1217 | 987 (81.2) | 230 (18.8) | 484 (39.6) | 737 (60.4) | 659 (53.0) | 562 (46.0) | Not available | |
| FIRE II (CIOX) | C + CAPIRI vs C + CAPOX |
|
177 | 150 (84.7) | 27 (15.3) | 51 (28.8) | 126 (71.2) | 123 (69.5) | 54 (30.5) | 76 (42.9) | 101 (57.1) |
| FOCUS | FU ± Ir vs FU ± IrFU/OxFU vs IrFU/OxFU |
|
2135 | 1623 (75.8) | 512 (24.2) | 668 (31.5) | 1450 (68.5) | 877 (41.4) | 1241 (58.6) | 901 (42.5) | 1171 (55.3) |
| HORG 99.30 | FOLFIRI vs FOLFOXIRI |
|
285 | 194 (67.8) | 91 (32.2) | 122 (43.1) | 161 (56.9) | 151 (53.4) | 132 (46.6) | 117 (41.3) | 166 (58.7) |
| HORIZON II | FOLFOX4/mFOLFOX6/CAPOX + Ce 30 mg/d vs FOLFOX4/mFOLFOX6/CAPOX + Ce 20 mg/d vs FOLFOX4/mFOLFOX6/CAPOX + placebo |
|
1076 | 919 (85.4) | 157 (14.6) | 442 (41.1) | 634 (58.9) | 624 (57.0) | 452 (42.0) | 519 (48.2) | 553 (51.4) |
| HORIZON III |
|
|
1614 | 1360 (84.1) | 254 (15.9) | 663 (41.4) | 938 (58.6) | 910 (56.8) | 691 (43.2) | 731 (45.7) | 858 (53.6) |
| N016966 | XELOX + B vs XELOX + Placebo |
|
1400 | 999 (80.3) | 401 (19.7) | 827 (40.7) | 1207 (59.3) | 1146 (56.3) | 888 (43.7) | 831 (40.9) | 1203 (59.1) |
| N9741 | IFL vs FOLFOX vs IROX |
|
795 | 474 (77.3) | 321 (22.7) | 556 (39.3) | 860 (60.7) | 637 (44.0) | 779 (55.0) | 716 (50.6) | 700 (49.4) |
| OPUS | FOLFOX4 vs FOLFOX4 + Ce |
|
344 | 274 (79.7) | 70 (20.3) | 160 (46.5) | 184 (53.5) | 143 (41.6) | 201 (58.4) | 154 (44.8) | 189 (54.9) |
| Second-line | |||||||||||
| AMGEN C181 | FOLFIRI vs FOLFIRI + P |
|
1186 | 928 (78.2) | 258 (21.8) | 464 (39.1) | 722 (60.9) | 574 (48.4) | 612 (51.6) | Not available | |
| BEBYP | mFOLFOX6/FOLFIRI vs mFOLFOX/FOLFIRI + B |
|
185 | 121 (65.2) | 64 (34.8) | 63 (34.2) | 121 (65.8) | 150 (81.5) | 34 (18.5) | 44 (23.9) | 140 (76.1) |
| CAIRO3 | CAPOX-B → control vs C + B |
|
558 | 421 (75.4) | 137 (24.6) | 196 (35.2) | 361 (64.8) | 345 (61.9) | 212 (38.1) | 238 (42.7) | 305 (54.8) |
| E3200 | FOLFOX vs B vs FOLFOX + B |
|
829 | 644 (77.4) | 185 (22.6) | 326 (39.8) | 494 (60.2) | 407 (49.6) | 413 (50.4) | 251 (30.6) | 569 (69.4) |
| EPIC | Ir vs Ir + Cx |
|
1289 | 989 (76.9) | 300 (23.1) | 482 (37.1) | 816 (62.9) | 664 (51.2) | 634 (48.8) | Not available | |
| N016967 | XELOX vs FOLFOX4 |
|
627 | 507 (80.9) | 120 (19.1) | 242 (38.6) | 385 (61.4) | 295 (47.1) | 332 (52.9) | 208 (33.2) | 419 (66.8) |
| N9841 | Ir vs FOLFOX4 |
|
491 | 367 (74.7) | 124 (25.3) | 204 (41.6) | 287 (58.4) | 0 (0) | 491 (100) | 225 (45.8) | 262 (53.4) |
| RAISE | R → FOLFIRI vs placebo → FOLFIRI |
|
1072 | 847 (79.0) | 225 (21.0) | 457 (42.6) | 615 (57.4) | 522 (48.7) | 550 (51.3) | 364 (33.0) | 702 (65.5) |
| TML | F/C + Ir/Ox vs F/C + Ir/Ox + B |
|
820 | 640 (78.0) | 180 (22.0) | 294 (35.9) | 526 (64.1) | 357 (43.5) | 463 (56.5) | Not available | |
| VELOUR | FOLFIRI + placebo vs FOLIRI + Af |
|
1226 | 1002 (81.7) | 224 (18.3) | 508 (41.4) | 718 (58.6) | 702 (57.3) | 524 (42.7) | Not available | |
Number/percent missing not included in this table. Af = aflibercept; ARCAD = Aide et Recherche en CAncerologie Digestive; B = bevacizumab; C = capecitabine; CAPIRI = irinotecan, capecitabine; CAPOX = oxaliplatin, capecitabine; Ce = cediranib; Cx = cetuximab; ECOG PS = Eastern Cooperative Oncology Group Performance Status; EGFR = epidermal growth factor receptor; F = flourouracil; FOLFIRI = irinotecan, levofolinate, fluorouracil; FOLFOXIRI = irinotecan, levofolinate, fluorouracil, oxaliplatin; FU = levofolinate, fluorouracil, dexamethasone; IFL = irinotecan, fluorouracil; Ir = irinotecan; IrFU = irinotecan, levofolinate, fluorouracil, dexamathasone; IROX = oxaliplatin, irinotecan; KRAS exon 2 mutation = molecular genetic abnormality indicating the presence of a mutation in exon 2 of the KRAS gene; M = mitomycin; mCRC = metastatic colorectal cancer; Ox = Oxaliplatin; OxFU = oxaliplatin, levofolinate, fluorouracil, dexamethasone; P = panitumumab; R = ramucirumab; VEGF = vascular endothelial growth factor; XELOX = oxaliplatin, capecitabine.
Figure 1.
Consort diagram.
Second-Line Trials
Both sequential and nonsequential trials were included in analyses of second-line trials. Sequential trials were defined as second-line trials with predetermined first-line treatment, and nonsequential trials were defined as second-line trials with enrollment not dependent on the regimen and results of a previous trial. By this definition, sequential trial patients were newly diagnosed, and the study protocol had already been defined for first- and second-line treatment. Analyses utilized Cox regressions to adjust for age, sex, Eastern Cooperative Oncology Group Performance Status (ECOG PS), number of metastatic sites, and the presence or absence of metastasis in the lung, liver, or peritoneum. Trials included in this analysis reflect outcomes of patients enrolled in first-line trials who subsequently enrolled in any of the following 10 second-line ARCAD sequential trials: AMGEN C181, BEBYP, CAIRO3, E3200, EPIC, N016967, N9841, RAISE, TML, or VELOUR (Figure 1). Study and enrollment characteristics for each second-line trial can also be found in Table 1. Pooled analysis did not capture the absolute number of individuals enrolled in first-line trials who subsequently enrolled in second-line trials within the ARCAD database; first- and second-line participants reflected in this analysis represent mutually exclusive populations.
All analyses were performed with approval from the local institutional review board in accordance with the precepts of the Helsinki Declaration.
Statistical Analysis
The distributions of time-to-event endpoints were compared by Cox regression models adjusting for covariates. The binary endpoints were compared by log rank test. All statistical tests were 2-sided, and a P value of less than .05 was considered statistically significant.
Results
First-Line Trials
A total of 5289 participants were evaluable for 10 first-line nonsequential studies (Table 1). Older adults comprised 16.4% of patients on first-line trials (870 total older adults aged >70 years; 4419 younger adults aged ≤70 years in first-line trials). Participants in first-line trials were more often younger adults (83.6% were aged ≤70 years), male (62.4%), ECOG PS 0 (55.5%), and had at least 2 metastatic sites (61.8%). In univariate analysis of nonsequential trials of participants with TTP1 failure, older adults and an ECOG PS of at least 1 had statistically significantly lower odds of receiving subsequent treatment (older adults: odds ratio [OR] = 1.24, 95% confidence interval [CI] = 1.01 to 1.53; ECOG PS ≥1: OR = 1.70, 95% CI = 1.44 to 2.02). We observed that a 10-year increase in age was associated with 12% increased odds of no subsequent treatment (OR for age per 10 years = 1.12, 95% CI = 1.04 to 1.21, P = .004).
In a multivariable model, relative to PS = 0, a patient who was ECOG PS of at least 1 had a statistically significantly increased odds of no subsequent treatment (ECOG PS = 1: HR = 1.55, 95% CI = 1.30 to 1.84; ECOG PS >1: HR = 4.07, 95% CI = 2.85 to 5.82) (Table 2).
Table 2.
Odds of no subsequent treatment following participation in first-line trials and survival following participation in second-line trials
| Characteristic | First-line therapy | Second-line therapy | Second-line therapy | ||||
|---|---|---|---|---|---|---|---|
| Odds of no subsequent treatment | Time to progression | Overall survival | |||||
| (n = 5289 [5121 evaluable]) |
(n = 7921 [7408 evaluable]) |
(n = 8280 [7764 evaluable]) |
|||||
| No. (%) | OR (95% CI) | P b | HR (95% CI) | P b | HR (95% CI) | P b | |
| Age at enrollment, per 10 y | 59.9 (10.7)a | 1.11 (1.02 to 1.21) | .01 | 0.97 (0.94 to 0.99) | .005 | 0.99 (0.97 to 1.02) | .62 |
| Age category | |||||||
| ≤70 y | 3889 (88.0) | – | – | – | – | – | – |
| >70 y | 744 (85.5) | – | – | – | – | – | – |
| Sex | |||||||
| Female | 1753 (88.2) | Referent | |||||
| Male | 2880 (87.2) | 1.15 (0.96 to 1.38) | .12 | 0.98 (0.94 to 1.04) | .54 | 0.97 (0.92 to 1.02) | .20 |
| ECOG PS | |||||||
| 0 | 2566 (90.4) | Referent | <.001 | <.001 | <.001 | ||
| 1 | 1815 (85.8) | 1.55 (1.30 to 1.84) | 1.22 (1.16 to 1.28) | 1.51 (1.43 to 1.59) | |||
| >1 | 115 (69.7) | 4.07 (2.85 to 5.82) | 1.59 (1.38 to 1.83) | 3.54 (3.13 to 4.02) | |||
| Metastasis | |||||||
| Lung | 1562 (87.4) | 1.03 (0.86 to 1.23) | .76 | 1.10 (1.04 to 1.18) | .003 | 1.08 (1.01 to 1.16) | .02 |
| Liver | 3421 (88.0) | 0.90 (0.75 to 1.09) | .29 | 1.36 (1.28 to 1.45) | <.001 | 1.62 (1.52 to 1.74) | <.001 |
| Peritoneum | 407 (88.1) | 0.92 (0.68 to 1.24) | .57 | 1.27 (1.03 to 1.57) | .03 | 1.42 (1.15 to 1.75) | .001 |
Values are mean (SD). CI = confidence interval; ECOG PS = Eastern Cooperative Oncology Group Performance Status; HR = hazard ratio; OR = odds ratio.
P values were calculated using a 2-sided Log rank test.
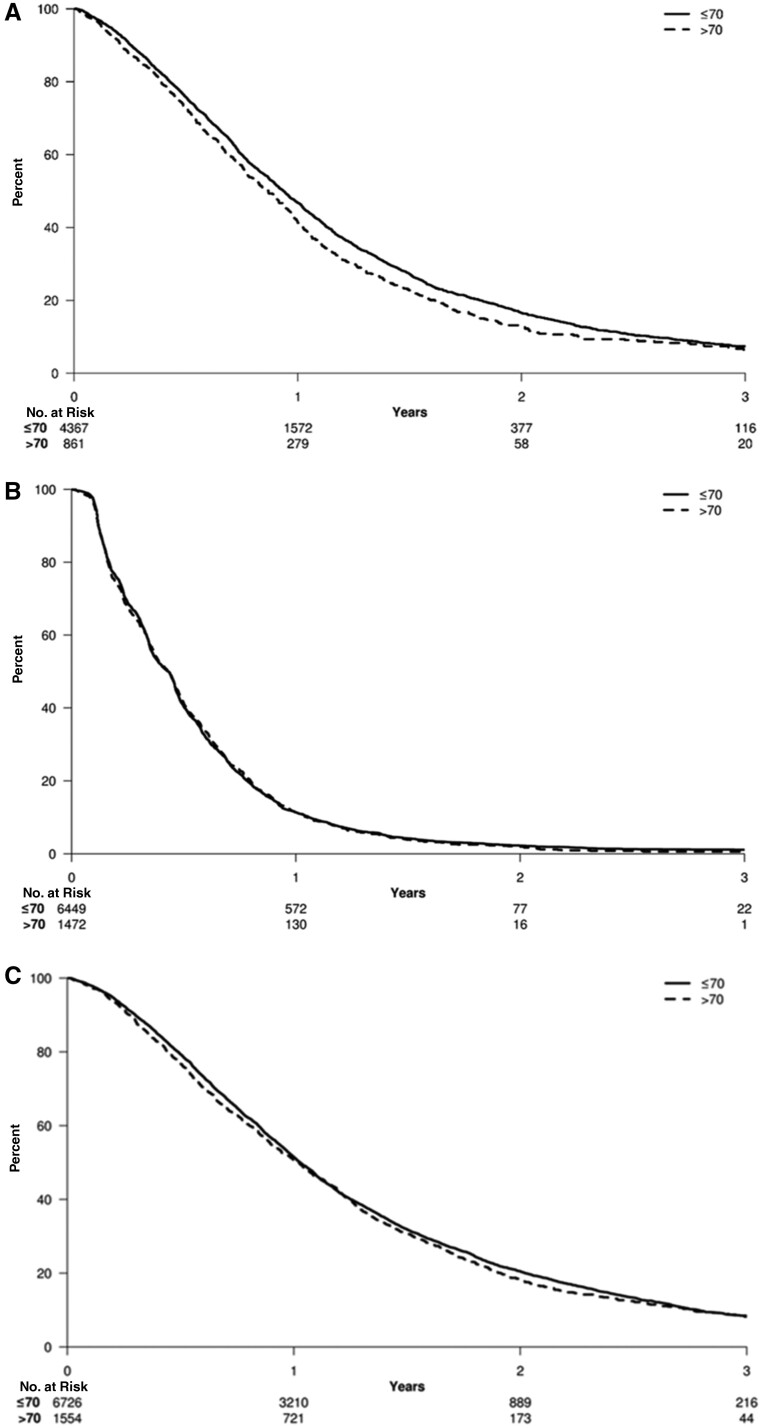
In both univariate and multivariable analysis, loss of each additional month from treatment to TTP1 was statistically significantly associated with shorter OS (HR = 0.95, 95% CI = 0.95 to 0.96, P < .001; and HR = 0.96, 95% CI = 0.95 to 0.96, P < .001) (Figure 2, A). In a univariate model examining age per 10 years, replacing the age category of younger than 70 years or 70 years and older, each additional decade of age was statistically significantly associated with OS (HR = 1.11, 95% CI = 1.02 to 1.21, P = .01).
Figure 2.
Time to progression and overall survival (OS) analyses for first- and second-line clinical trials in older adults vs younger adults. Kaplan-Meier curves are shown for (A) first-line OS (hazard ratio [HR] = 1.11, 95% confidence interval [CI] = 1.02 to 1.21, P = .01), (B) second-line time to progression 2 [TTP2] (HR = 1.00, 95% CI = 0.94 to 1.06, P = .97), and (C) second-line OS (HR = 1.05, 95% CI = 0.99 to 1.12, P = .11). P values were calculated using a 2-sided Log rank test.
Second-Line Trials
A total of 7921 participants were evaluable for TTP2 in the 10 second-line sequential and nonsequential studies (Table 1). Older and younger adults enrolled in second-line trials experienced similar median TTP and median OS (median TTP = 5.1 vs 5.2 months, respectively; median OS = 11.6 vs 12.4 months, respectively). Participants in second-line trials were often younger (≤70 years) adults (78.3%), male (61.1%), ECOG PS 0 (52.7%), and had at least 2 metastatic sites (74.3%). In multivariable analysis, ECOG PS of at least 1, presence of liver metastasis, and number of metastatic sites of at least 2 were statistically significantly associated with shorter TTP2 and OS (Table 2). Median follow-up for TTP2 for both sequential and nonsequential trials was 13.7 months. Regarding patient age, univariate analysis showed that each subsequent decade was associated with slightly reduced risk of TTP2 (HR = 0.98, 95% CI = 0.95 to 1.00), indicating similar TTP regardless of increasing age. Further, in univariate analysis, there was no statistically significant difference in TTP2 during second-line clinical trials for older adults and younger adults (HR = 1.00, 95% CI = 0.94 to 1.06, P = .97) (Figure 2, B).
A total of 8280 participants were evaluable for OS in the 10 second-line sequential and nonsequential studies (Table 1). Unique participants in second-line trials were more likely to be younger (≤70 years) adults (78.0%), male (60.9%), ECOG PS 0 (51.7%), and have at least 2 metastatic sites (74.3%). Median follow-up for OS for both sequential and nonsequential trials was 30.4 months.
Median OS was 12.4 and 12.3 months for older adults and younger adults, respectively. In multivariable analysis, ECOG PS of at least 1, presence of liver metastasis, peritoneal metastases, and 2 or more metastatic sites were statistically significant associated with shorter OS (Table 2). Statistically significantly shorter OS was observed for ECOG PS of at least 1 and liver and/or peritoneal metastases in multivariable analysis, with lung metastases emerging as another statistically significant risk factor for shorter OS. Unlike analysis for TTP1 for first-line trials and TTP2 for second-line trials, cohorts separated by each additional decade of age did not show any statistically significant association by cohort with OS (P = .62) (Figure 2). There was no statistically significant difference in OS during second-line clinical trials for older adults and younger adults (OS: HR = 1.05, 95% CI = 0.99 to 1.12, P = .11) (Figure 2, C).
Discussion
Older adults remain underrepresented as a proportion of the population of patients with mCRC in clinical trials of both first- and second-line treatments. Despite the fact that older adults are more likely to develop CRC, analysis of the ARCAD database clearly shows that there are statistically significantly fewer older adults enrolled in both first-line and second-line clinical trials. Beyond this, our data show that the chance of receiving second-line therapy decreased by 11% for each additional decade of life in multivariable analysis (P = .01). The a priori age threshold of older than 70 years did not predict progression or OS on first- or second-line trials.
Despite the fact that fewer older adults participated in first- and second-line clinical trials, older adults with CRC had similar outcomes to younger adults enrolled in CRC clinical trials. On both first-line and second-line trials, there was no difference in TTP or OS between older and younger adults with CRC.
Prior studies have established similar survival rates among older adults and younger adults in the palliative treatment setting. Folprecht and colleagues noted a similar median overall survival of 11 months and slightly better median PFS of 5.5 months in pooled analysis of 22 trials of fluorouracil for older adults vs younger adults (median OS = 10.8 vs 11.3 months, P value not statistically significant; median PFS 5.5 vs 5.3 months, P = .01 for older adults aged ≥70 years vs younger adults aged <70 years) (16). Similar survival was also noted in studies of oxaliplatin-based (17-19) and irinotecan-based (20) regimens. Studies of regimens that included targeted therapies have not included specific age analyses (21-27). Age-specific analyses of immunotherapy trials are forthcoming but not applicable to the current analysis, which lacked studies including immunotherapy for mCRC (28).
Understanding treatment patterns for older adults beyond first-line therapy provides useful insights into the dissemination and uptake of treatment recommendations. On the whole, older adults are less likely to be referred for oncology subspecialty care and, when referred, have lower rates of both routine- and clinical trial-based therapy for advanced disease (29,30). We have now established similarly low rates of enrollment in clinical trials beyond first-line therapy. Several factors may explain this difference in clinical trial enrollment by age, including patient preference, provider bias, presence of limiting concurrent medical conditions (frequently leading to reduced eligibility for trials), or difficulties in access to treatment (31-33). Understanding that TTP2 is comparable regardless of age, we must question whether the observed differences in trial enrollment rates are (1) comparable with the proportion of older adults receiving standard therapy outside of clinical trials, and (2) whether this difference is due to objective selection criteria.
Knowing that standards for cancer care emerge from results of clinical trials, it is imperative that we enable generalization of findings in both structure and execution of clinical trials for patients with mCRC. How can we increase cancer care parity for older adults? In addition to increasing enrollment in first-line trials, we also need to design studies such that we can learn as much as possible about the outcomes of older adults. Previous work has demonstrated that validated measures of frailty are an important metric in assessing the benefit of a therapeutic intervention in the elderly population. The Geriatric Assessment has been successfully embedded within cooperative group clinical trials (34), and the Cancer in Aging Research Group Toxicity Score can identify subsets of older adults at higher risk of symptomatic adverse events or death (35). Clinical trial endpoints should include those outcomes that mirror the values and preferences of older adults (33,36,37). Specifically, measures of quality of survival rather than TTP or OS may provide insights into preservation or recovery of function, active life expectancy, and disability-free survival. Increased inclusion of patient-reported outcomes is encouraged by the US Food and Drug Administration to enhance the drug approval process with the added insight of the patient experience (38). This is supported by the subsequent NCI development, validation, and improved survival outcome measures associated with systematic documentation of patient-reported outcomes in clinical trials and routine care (39-41).
Secondly, we must expand clinical trial methodology to include key patient characteristics pertinent to older adults. Specific recommendations have been published in detail elsewhere and are summarized here (42,43). ARCAD investigators have previously called for standardization of baseline characteristics to report in Phase 3 trials investigating systemic treatment of mCRC. Among recommendations for standard inclusion of demographic, cancer characteristics (stage, differentiation, metastases, potential for resection, etc), and laboratory values, recommendations include consideration of comorbidity or evaluation of frailty defined as the accumulation of biologic deficits or disability associated with reduced quality and duration of life (44).
Although the strength of this trial is that it evaluated outcomes from 20 trials and 13 149 patients, the current analysis included only patients enrolled in clinical trials and as such is limited by lack of data regarding the toxicity among older and younger adults, concurrent medical conditions, dose modifications, genomic data, sidedness, patient and family preferences, provider bias, or comparison of patient characteristics among those patients not referred to an oncology subspecialist and those who were referred but either were not offered or chose not to enroll in these clinical trials. Adverse event data were not collected consistently across all studies to permit robust comparison by age. It is unclear whether the addition of that data would statistically significantly affect PFS and OS, although death from other conditions certainly contributes to observed OS outcomes.
This ARCAD analysis provides the largest analysis to date evaluating enrollment and survival outcomes of older adults accrued to pivotal second-line mCRC clinical trials, providing insights into factors associated with enrollment and outcome disparities by age. Age should be considered less of an enrollment criterion for clinical trials. Although the age threshold selected aligns with prior published studies, the examination of cohorts of patients with increasing age by decade likely reflects the competing risk of older adult death from noncancer causes. The analyses are limited by rates of enrollment for older adults in individual studies with limited accrual of the oldest subsets of older adults, for example, those aged 80 years or older. Yet, the findings support consideration of enrollment of older adults in second-line trials to understand how the treatments under study and treatment dose intensity influence their PFS and OS. Further prospective studies are needed to understand the impact of concurrent medical conditions, polypharmacy, and sociodemographic factors beyond age on clinical trial offerings by providers and on preferences of patients. Further consideration should be given to the intersection of age with these factors and inclusion of objective assessment of fitness for clinical trials to enhance offerings for older adults.
Funding
The National Cancer Institute Gastrointestinal Cancer Center Specialized Programs of Research Excellence (SPORE) Career Development Award (5P50CA127003-08) funded Dr McCleary’s effort. The ARCAD Foundation funded data collection and analysis.
Notes
Role of the funder: The ARCAD Foundation supported the data analysis and manuscript preparation for this study. The NCI GCC SPORE Career Development Award (5P50CA127003-08) funded Dr McCleary’s data interpretation and authorship.
Disclosures: AFS served on advisory boards and as a speaker at satellite symposia for Merck, Roche, Servier, Incyten, Sanofi, Bayer, Bristol Myers Squibb, Amgen, Eli Lilly, and Pierre Fabre. JMC received research funding to his institution from Abbvie, Merus, Roche, and Bristol Myers Squibb. He received research funding from Merck, Astrazeneca, Esperas Pharma, and Tesaro, received consulting fees from Bristol Myers Squibb, and received travel funding from Bristol Myers Squib. VH served as honoraria for Merck, Amgen, Roche, Sanofi, Sirtex, Servier, Pfizer. He served as a consultant or Merck, Amgen, Roche, Sanofi, Sirtex, and Bristol Myers Squibb. He served on an advisory board for Merck, Novartis, Boehringer Ingelheim, Servier, Pierre-Fabre, Celgene, and Terumo. He received research funding to his institution from Merck, Amgen, Roche, Sanofi, Pfizer, Boehringer-Ingelheim, Sirtex, Bayer, and Servier. He received travel funding from Merck, Roche, Amgen, Sirtex, Bayer, and Servier. SML is supported by the NCI Cancer Center Support Grant (P30CA008748). No potential competing interest was reported by the remaining authors.
Author contributions: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization: NJM, EH, QS, WSH. Writing—original draft: NJM, EH. Writing—review and edits: NJM, WSH, EH, JMC, JAM, JZ, RA, AG, AFS, EVC, RMG, MP, JT, MS, LBS, BJG, DA, MLR, MK, HJS, HCP, PMH, NT, GM, JS, CB, VH, TY, BC, AG, QS, SML.
Prior presentations: Findings from this study were presented at the Gastrointestinal American Society of Clinical Oncology (GI ASCO) January 2019 by co-author James Cleary, MD PhD. Findings from this study were also presented at the American Society of Clinical Oncology 2020 Annual Meeting.
Acknowledgements: Dr McCleary’s effort was funded by the NCI GCC SPORE Career Development Award (5P50CA127003-08). Data collection was funded by the ARCAD Foundation.
This work is dedicated to the memory of Daniel J. Sargent. Dan was one of the world’s foremost experts in biostatistics and oncology who brought together disparate investigators and established data sharing across academia and industry internationally. His groundbreaking initiatives of integrating large collections of databases enabled research to answer questions otherwise beyond statistical possibility, to design important new clinical studies, to make regulatory observations, and to set new standards. He pushed these innovations farther to prospectively plan internationally combined analyses that answered questions previously believed to be impossible. The world of oncology statistics and analysis will not be the same without him, but his legacy continues.
Data Availability
The data sharing of individual patient data from each participating trial will be subject to the policy and procedures of the institutions and groups who conducted the original study. Please contact Nadine Jackson McCleary, MD MPH at 450 Brookline Ave, Boston, MA 02215. Email address: nj_mccleary@dfci.harvard.edu.
References
- 1. Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “silver tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev.2016;25(7):1029–1036. [DOI] [PMC free article] [PubMed]
- 2. National Cancer Institute Division of Cancer Control and Population Sciences. Cancer Stat Facts: Colorectal Cancer. Bethesda, MD: National Cancer Institute; 2020. [Google Scholar]
- 3. Bailey CE, Hu C-Y, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang L, Xiong Z, He W, et al. Proximal shift of colorectal cancer with increasing age in different ethnicities. CMAR. 2018;10:2663–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Cancer Society. Cancer Facts and Figures 2020. Atlanta, GA: American Cancer Society; 2020 [Google Scholar]
- 6. Sanoff HK, Sargent DJ, Campbell ME, et al. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol. 2008;26(35):5721–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jeffrey A, Meyerhardt LL, Sanoff HK, Carpenter W IV, Schrag D.. Effectiveness of bevacizumab with first-line combination chemotherapy for Medicare patients with stage IV colorectal cancer. J Clin Oncol. 2012;30(6):608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanoff HK, Goldberg RM, Ivanova A, et al. Multicenter, randomized, double-blind phase 2 trial of FOLFIRI with regorafenib or placebo as second-line therapy for metastatic colorectal cancer. Cancer. 2018;124:(15):3118–3126. [DOI] [PubMed] [Google Scholar]
- 9. Jean Yves Douillard TM, Stephens R, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol. 2008;26:(9):1443–1451. [DOI] [PubMed] [Google Scholar]
- 10. Jackson McCleary N, Meyerhardt J, Green E, et al. ; The ACCENT Collaborative Group. Impact of older age on the efficacy of newer adjuvant therapies in> 12,500 patients (pts) with stage II/III colon cancer: findings from the ACCENT Database. J Clin Oncol. 2009;27(suppl 15):4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Colon Cancer. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2020. [Google Scholar]
- 12. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Rectal Cancer. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2020. [Google Scholar]
- 13. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. [DOI] [PubMed] [Google Scholar]
- 14. De Gramont A, Boni C, Navarro M, et al. Oxaliplatin/5FU/LV in adjuvant colon cancer: updated efficacy results of the MOSAIC trial, including survival, with a median follow-up of six years. J Clin Oncol. 2007;25(suppl 18):4007. [Google Scholar]
- 15. Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29(28):3768–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Folprecht G, Cunningham D, Ross P, et al. Efficacy of 5-fluorouracil-based chemotherapy in elderly patients with metastatic colorectal cancer: a pooled analysis of clinical trials. Ann Oncol. 2004;15(9):1330–1338. [DOI] [PubMed] [Google Scholar]
- 17. Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24(25):4085–4091. [DOI] [PubMed] [Google Scholar]
- 18. Figer A, Perez‐Staub N, Carola E, et al. FOLFOX in patients aged between 76 and 80 years with metastatic colorectal cancer: an exploratory cohort of the OPTIMOX1 study. Cancer. 2007;110(12):2666–2671. [DOI] [PubMed] [Google Scholar]
- 19. Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet. 2011;377(9779):1749–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Folprecht G, Seymour MT, Saltz L, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol. 2008;26(9):1443–1451. [DOI] [PubMed] [Google Scholar]
- 21. Van Cutsem E, Rivera F, Berry S, et al. ; First BEAT investigators. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20(11):1842–1847. [DOI] [PubMed] [Google Scholar]
- 22. Kozloff M, Sugrue M, Chiruvolu P, et al. Safety and effectiveness of bevacizumab (BV) and chemotherapy (CT) in elderly patients with metastatic colorectal cancer (mCRC): results from the BRiTE observational cohort study. Ann Oncol. 2008;19:26. [Google Scholar]
- 23. Douillard J-Y, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J Clin Oncol. 2010;28(31):4697–4705. [DOI] [PubMed] [Google Scholar]
- 24. Jehn C, Böning L, Kröning H, et al. Cetuximab-based therapy in elderly comorbid patients with metastatic colorectal cancer. Br J Cancer. 2012;106(2):274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abdelwahab S, Azmy A, Abdel-Aziz H, et al. Anti-EGFR (cetuximab) combined with irinotecan for treatment of elderly patients with metastatic colorectal cancer (mCRC). J Cancer Res Clin Oncol. 2012;138(9):1487–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sastre J, Aranda E, Grávalos C, et al. First-line single-agent cetuximab in elderly patients with metastatic colorectal cancer. A phase II clinical and molecular study of the Spanish group for digestive tumor therapy (TTD). Crit Rev Oncol Hematol. 2011;77(1):78–84. [DOI] [PubMed] [Google Scholar]
- 27. Sastre J, Grávalos C, Rivera F, et al. First-line cetuximab plus capecitabine in elderly patients with advanced colorectal cancer: clinical outcome and subgroup analysis according to KRAS status from a Spanish TTD Group Study. Oncologist. 2012;17(3):339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nishijima TF, Muss HB, Shachar SS, Moschos SJ.. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: a systematic review and meta-analysis. Cancer Treat Rev. 2016;45:30–37. [DOI] [PubMed] [Google Scholar]
- 29. Murthy VH, Krumholz HM, Gross CP.. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. [DOI] [PubMed] [Google Scholar]
- 30. Aapro MS, Köhne C-H, Cohen HJ, Extermann M.. Never too old? Age should not be a barrier to enrollment in cancer clinical trials. Oncologist. 2005;10(3):198–204. [DOI] [PubMed] [Google Scholar]
- 31. McCleary NJ, Dotan E, Browner I.. Refining the chemotherapy approach for older patients with colon cancer. J Clin Oncol. 2014;32(24):2570–2580. [DOI] [PubMed] [Google Scholar]
- 32. Shahrokni A, Wu AJ, Carter J, Lichtman SM.. Long term toxicity of cancer treatment in older patients. Clin Geriatr Med. 2016;32(1):63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Extermann M, Hurria A.. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25(14):1824–1831. [DOI] [PubMed] [Google Scholar]
- 34. Hurria A, Cirrincione C, Muss H, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. 2011;29:1290-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ostwal V, Ramaswamy A, Bhargava P, et al. Cancer Aging Research Group (CARG) score in older adults undergoing curative intent chemotherapy: a prospective cohort study. BMJ Open 2021;11:e047376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mahmoudzadeh S, Shahrokni A.. Cancer and aging; preparing for silver tsunami. J Geriatr Palliat Care. 2015;3:7. [Google Scholar]
- 37. Quach C, Sanoff HK, Williams GR, Lyons JC, Reeve BB.. Impact of colorectal cancer diagnosis and treatment on health‐related quality of life among older Americans: a population‐based, case‐control study. Cancer. 2015;121(6):943–950. [DOI] [PubMed] [Google Scholar]
- 38. Food and Drug Administration. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Silver Spring, MD: Food and Drug Administration; 2009. [Google Scholar]
- 39. Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015;1(8):1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. National Cancer Institute Division of Cancer Control and Population Sciences. Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). National Cancer Institute; 2020.
- 42. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goey KK, Sørbye H, Glimelius B, et al. Consensus statement on essential patient characteristics in systemic treatment trials for metastatic colorectal cancer: supported by the ARCAD Group. Eur J Cancer. 2018;100:35–45. [DOI] [PubMed] [Google Scholar]
- 44. Lachmann R, Stelmach-Mardas M, Bergmann MM, et al. The accumulation of deficits approach to describe frailty. PLoS One. 2019;14(10):e0223449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sharing of individual patient data from each participating trial will be subject to the policy and procedures of the institutions and groups who conducted the original study. Please contact Nadine Jackson McCleary, MD MPH at 450 Brookline Ave, Boston, MA 02215. Email address: nj_mccleary@dfci.harvard.edu.