Abstract
Purpose
To investigate the correlation between metabolic syndrome components and chronic kidney disease (CKD) among a community population aged 40 years and older in Southern China.
Patients and Methods
From December 2017 to March 2018, 1969 participants (male n = 715, female n = 1254) aged 40 years and older were recruited in Southern China for a cross-sectional survey. A logistic regression model was established to analyze the correlation between metabolic syndrome components and CKD.
Results
Among the 1969 subjects, 407 (20.7%) were CKD patients, including 152 males (prevalence rate 21.3%) and 255 females (prevalence rate 20.3%). Anthropometric data (waist circumference, age, systolic and diastolic blood pressure), serum/plasma data (serum creatinine, serum uric acid, fasting plasma glucose, C-reactive protein, serum triglyceride), urinary and other findings (body mass index, waist-to-hip and waist-to-height ratios, urinary albumin to creatinine ratio, homeostasis model assessment of insulin resistance) were significantly higher in patients with than without CKD (P < 0.05). Metabolic syndrome and at least some of its components were statistically significant risk factors for CKD in models with and without adjustment for diabetes, obesity and hypertension.
Conclusion
Metabolic syndrome and its single or combined components are independently associated with CKD in community populations aged 40 years and older. The correlation between some components and CKD remained significant in both non-diabetic and non-obese subjects. Correlations between components of metabolic syndrome and CKD show that it is feasible and necessary to carry out targeted screening and intervention tests in people aged 40 and over.
Keywords: metabolic syndrome, chronic kidney disease, risk factors, epidemiological cross-sectional study
Introduction
Chronic kidney disease (CKD) is a public health problem globally.1–5 Metabolic syndrome (MS) is a pathological state in which many risk factors for CKD including obesity, hyperglycemia, hypertension and dyslipidemia (high triglyceride and low HDL-C) co-occur within individuals.6 Studies have shown that MS is a risk factor for a variety of chronic non-communicable diseases including type 2 diabetes mellitus, coronary heart disease, stroke and CKD.7–10 In the past decade, studies on the relationship between MS and CKD have emerged.10–13 The incidence rate of MS is increasing, and in recent years the prevalence of CKD has shown a steady annual increase, presenting a serious threat to life and health, bringing economic burden to society and families, and reducing the quality of life of those affected.1,14 The middle-aged and the elderly are vulnerable to chronic non-communicable diseases, so clinicians need to be alert to MS and CKD in these age groups. Understanding the relationship between MS and CKD and effective intervention measures to control them will help to alleviate the burden imposed by chronic diseases on China’s limited medical resources.
Subjects and Methods
Participants
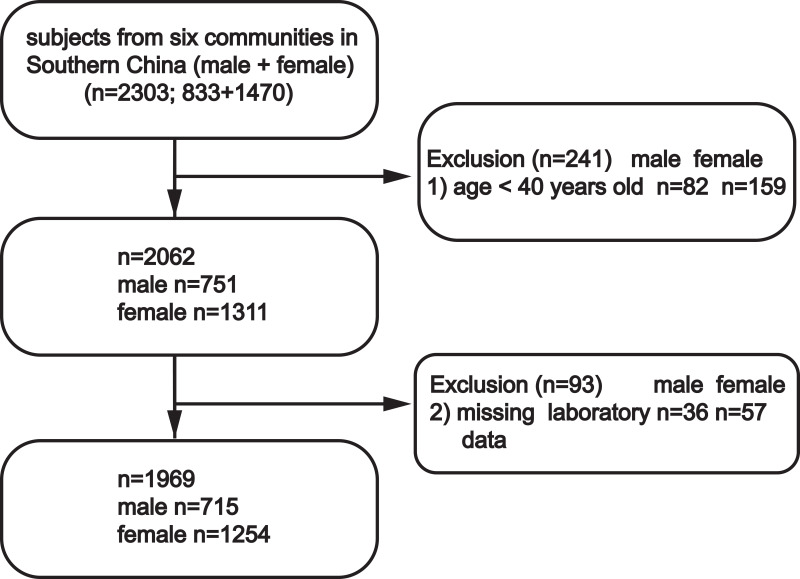
This study was conducted between December 2017 and March 2018. A total of 2303 volunteers residing in six communities of Wanzhai Town, Zhuhai City, Southern China were initially screened. For the cross-sectional analysis, participants aged 40 years and older were screened. Those with missing laboratory data were excluded. A total of 1969 participants were included in the final analysis (Figure 1). All subjects provided written informed consent. The study was approved by the Ethics Committee of the Third Affiliated Hospital of Southern Medical University, Guangzhou and was performed in accordance with the principles stated in the Declaration of Helsinki.
Figure 1.
Study participants were grouped according to sex.
Clinical and Laboratory Indicators
Anthropometric Indices and Socio-Demographic Characteristics
The residents were interviewed, and responses were recorded by medical investigators. Data collected included gender, age, medical history, and current medication. Subjects who completed the interview also underwent physical examination and blood collection. Height, body mass, waist circumference (WC), hip circumference, and blood pressure were measured. Body mass index (BMI) was calculated as body mass (kg)/height (m) 2, waist-to-hip ratio (WHpR) was defined as WC (cm)/hip circumference (cm), and waist-to-height-ratio (WHtR) was defined as WC (cm)/height (cm). Blood pressure (mmHg) was measured by a trained nurse.
Laboratory Examination
After fasting for 12 hours, blood samples were collected from the anterior elbow vein and morning urine was collected. All samples were analyzed in the laboratory of the Third Affiliated Hospital of Southern Medical University. Fasting plasma glucose was determined using standard enzymatic methods (Hitachi 7170, Hitachi, Tokyo, Japan). Insulin was measured by electrochemiluminescence (Roche cobas e601, Roche Diagnostics, Mannheim, Germany). Serum uric acid (UA) levels were measured using colorimetry (Roche cobas 6000, Roche Diagnostics, Mannheim, Germany). Immunoturbidimetry was used to measure C-reactive protein (CRP) (Roche cobas 6000, Orion), and an enzymatic method was used to measure serum creatinine (SCR) (Roche cobas 6000, Roche Diagnostics, Mannheim, Germany). Serum total cholesterol (TC), serum triacylglycerols (TG) and serum high-density lipoprotein cholesterol (HDL-C) were measured using colorimetry (Roche Cobas 6000, Roche Diagnostics, Mannheim, Germany). Serum low-density lipoprotein cholesterol (LDL-C) was estimated using the Friedewald formula. Urinary albumin-to-creatinine ratio (ACR) values were calculated by measuring albumin (Audit Diagnostics, Cork, Ireland; immunoturbidimetric tests) and creatinine (Audit Diagnostics, Cork, Ireland; Jaffe’s kinetic method) concentrations in urine samples. Homeostatic model assessment of insulin resistance (HOMA-IR) is equal to fasting plasma glucose (mmol/L) multiplied by insulin (mu/L) divided by 22.5.
Definition
CKD was defined as estimated glomerular filtration rate (eGFR) <60 mL/(min x 1.73 m2) or ACR ≥30 mg/g.15 eGFR was calculated using the CKD-EPI equation in the Kidney Disease: Improving Global Outcomes guidelines.16,17 Hypertensive disease was defined as SBP ≥140 mmHg or DBP ≥90 mmHg, previously diagnosed with hypertension, or receiving treatment for hypertension. Diabetes was defined as fasting glucose ≥7.0 mmol/L, diagnosed with diabetes, or currently receiving treatment for diabetes. The diagnostic criteria for obesity were BMI ≥28 kg/m2.18 MS was defined according to the 2009 International Diabetes Federation Task Force statement on epidemiology and prevention.6 The criteria for MS were met if any three of the following were true: a. abdominal obesity (AO), male waist circumference ≥85 cm, or female waist circumference ≥80 cm; b. high serum triglyceride (HTG), triacylglycerols ≥ 1.70 mmol/L; c. LHDL-C, low HDL-C at <1.0 mmol/L (males) or HDL-C <1.3 mmol/L (females); d. high blood pressure (HBP), with systolic blood pressure (SBP) ≥130 mmHg, or diastolic blood pressure (DBP) ≥85 mm Hg, or history of hypertension; e. high fasting plasma glucose (HFPG) >5.6 mmol/L or history of diabetes.
Statistical Analyses
All statistical analyses were performed using IBM SPSS version 20 software. Normally distributed data are expressed here as mean ± standard deviation (x ± s), and non-normally distributed data by median and quartiles. The mean differences were compared between the two groups using a t-test or rank sum test. Categorical data are expressed here in absolute numbers and percentages. Categorical data including gender groups were compared using the chi square test. P values <0.05 were considered statistically significant. To investigate the effect of MS and its components on CKD, the latter was considered a dependent variable, while MS and components were independent variables, and univariate logistic regression analysis was carried out. A multiple logistic regression model was used to assess the association between CKD and MS components, and the odds ratio (OR) and 95% CI were determined. Three multivariate logistic regression models were generated: model one was unadjusted, model two was adjusted for age and sex, and model three was adjusted for age, sex, and history of medication for HBP or blood glucose control. A two-sided t-test with a significance level of α=0.05 (P<0.05) was used.
Results
High Detection Rates of CKD and MS and Its Components
The 1969 subjects ranged in age from 40 to 93 years (58.96 ± 10.61 years). Four hundred and seven (20.7%) were CKD patients, including 152 male and 255 female. The levels of BMI, WC, WHpR, WHtR, age, SBP, DBP, SCR, UA, ACR, FPG, CRP, TG and HOMA in the CKD population were significantly higher than those in the non-CKD population (P<0.05; see Table 1). Conversely, the levels of eGFR and HDL-C in the non-CKD population were significantly higher than those in the CKD population. The prevalence of hypertensive disease was 54.6%, but only 31.3% of these patients were receiving antihypertensive treatment. There were significant differences in the classification of hypertension and the distribution of antihypertensive treatment between the non-CKD group and CKD group (P<0.01). The prevalence rates of diabetes, MS and MS components are shown in Table 1.
Table 1.
Baseline Characteristics of Subjects in Non-CKD and CKD Groups
| Characteristics | Total | Non-CKD | CKD | P |
|---|---|---|---|---|
| N=1969 | N=1562 | N=407 | ||
| Male (n) | 715(36.3%) | 563 (36.0%) | 152 (37.3%) | 0.626 |
| BMI (kg/m2) | 24.4±3.3 | 24.1± 3.2 | 25.3± 3. 7 | <0.001 |
| WAIST (cm) | 85.4±9.6 | 84.6± 9.4 | 88.5± 9.7 | <0.001 |
| WHpR | 0.89±0.06 | 0.88± 0.06 | 0.91± 0.06 | <0.001 |
| WHtR | 0.54±0.06 | 0.53± 0.06 | 0.57± 0.06 | <0.001 |
| Age (years) | 59.0±10.6 | 57.1± 9. 8 | 66.0± 10.7 | <0.001 |
| SBP (mmHg) | 136.5±19.5 | 133.8± 18.7 | 147.0± 19.1 | <0.001 |
| DBP (mmHg) | 83.3±10.7 | 82.6± 10.5 | 86.0± 10.8 | <0.001 |
| Hypertension classification (%) | ||||
| Normal | 52.8% | 58.5% | 31.0% | |
| 1 | 32.0% | 29.3% | 42.3% | <0.001 |
| 2 | 12.3% | 10.4% | 19.7% | <0.001 |
| 3 | 2.8% | 1.7% | 7.1% | <0.001 |
| SCR (umol/L) | 78.3±24.3 | 74.7± 14.5 | 92.1± 42.7 | <0.001 |
| UA (umol/L) | 350.9±89.2 | 342.0± 84.0 | 385.1± 99.6 | <0.001 |
| eGFR (mL/min/1.73 m2) | 82.0±15.8 | 85.5± 12.4 | 68.8± 19.9 | <0.001 |
| ACR (mg/g) | 11.2 (7.1–0.4) | 9.6 (6.5–14.7) | 44.7 (30.5–87.3) | <0.001 |
| Glu (mmol/L) | 5.4±1.4 | 5.3± 1.1 | 5.9± 2.0 | <0.001 |
| CRP (mg/L) | 1.46 (0.67–2.64) | 1.35 (0.60–2.43) | 1.89 (1.06–3.53) | <0.001 |
| TG (mmol/L) | 1.35 (0.99–1.95) | 1.30 (0.96–1.86) | 1.59 (1.15–2.34) | <0.001 |
| LDL-C (mmol/l) | 3.28±0.95 | 3.26± 0.94 | 3.36± 0.98 | 0.335 |
| HDL-C (mmol/l) | 1.50±0.35 | 1.52±0.35 | 1.44± 0.36 | <0.001 |
| HOMA (mmol/L. mU/L) | 2.13 (1.46–3.32) | 2.01 (1.40–3.03) | 2.72 (1.73–4.15) | <0.001 |
| Hypertension disease (%) | 54.6% | 47.8% | 81.1% | <0.01 |
| Diabetes (%) | 12.8% | 9.5% | 25.6% | <0.001 |
| Metabolic syndrome (%) | 38.5% | 32.5% | 61.7% | <0.001 |
| HBP (%) | 68.3% | 62.4% | 90.9% | <0.001 |
| Elevated systolic blood pressure (%) | 61.0% | 55.5% | 82.3% | <0.001 |
| Elevated diastolic blood pressure (%) | 44.8% | 42.1% | 55.3% | <0.001 |
| AO (%) | 65.5% | 62.0% | 78.6% | <0.01 |
| HTG (%) | 34.0% | 31.0% | 45.2% | <0.01 |
| HFBP (%) | 26.1% | 22.2% | 41.0% | <0.01 |
| LHDL-C (%) | 15.7% | 14.0% | 22.1% | <0.01 |
| Drugs to control blood pressure (%) | 31.3% | 25.3% | 54.5% | <0.01 |
| Drugs to control blood glucose (%) | 11.0% | 9.2% | 18.2% | <0.01 |
Abbreviations: CKD, chronic kidney disease; BMI, body mass index; WHpR, waist-to-hip ratio; WHtR, waist-to-height ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; SCR, serum creatinine; UA, serum uric acid; eGFR, estimated glomerular filtration rate; ACR, urinary albumin to creatinine ratio; Glu, blood glucose; CRP, C-reactive protein; TG, serum triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HOMA, homeostatic model assessment; AO, abdominal obesity; HTG, high triacylglycerols; HFPG, high fasting plasma glucose; LHDL-C, low high-density lipoprotein cholesterol.
Effects of MS and Its Components on CKD
MS and MS components were significantly correlated with the occurrence of CKD and were risk factors for CKD (Table 2).
Table 2.
CKD Risk Factor Correlation Analysis with MS Components
| OR | 95% CI | P | ||
|---|---|---|---|---|
| MS | 3.34 | 2.66–4.19 | <0.01 | |
| MS components | AO | 2.25 | 1.74–2.91 | <0.01 |
| HBP | 6.02 | 4.23–8.57 | <0.01 | |
| HTG | 1.83 | 1.47–2.29 | <0.01 | |
| HFPG | 2.44 | 1.93–3.07 | <0.01 | |
| LHDL-C | 1.74 | 1.32–2.29 | <0.01 | |
Abbreviations: CKD, chronic kidney disease; MS, metabolic syndrome; OR, odds ratio; HBP, high blood pressure; AO, abdominal obesity; HTG, high triacylglycerols; HFPG, high fasting plasma glucose; LHDL-C, low high-density lipoprotein cholesterol.
Unadjusted analysis showed that CKD was associated with HBP, AO, HFPG, and LHDL-C, but not HTG. After adjusting for age and gender, HBP and HFPG were independent risk factors for CKD. After further adjustment for age, sex, hypoglycemic and antihypertensive medication, HBP (P<0.01) and HFPG (P=0.01) remained independent risk factors for CKD (Table 3).
Table 3.
Odds Ratios of Metabolic Syndrome Components for the Prevalence of CKD
| Risk Factors | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95%) CI | P | OR (95%) CI | P | OR (95%) CI | P | |
| HBP | 4.88 (3.40–7.01) | <0.01 | 3.07 (2.10–4.47) | <0.01 | 2.58 (1.75–3.82) | <0.01 |
| AO | 1.37(1.04–1.81) | 0.03 | 1.29 (0.96–1.72) | 0.09 | 1.25 (0.93–1.68) | 0.13 |
| HTG | 1.26(0.98–1.61) | 0.07 | 1.30 (1.00–1.69) | 0.05 | 1.30 (1.00–1.69) | 0.05 |
| HFPG | 1.76 (1.38–2.24) | <0.01 | 1.54 (1.19–1.98) | <0.01 | 1.47 (1.10–1.95) | 0.01 |
| LHDL-C | 1.42 (1.05–1.93) | 0.02 | 1.35 (0.98–1.87) | 0.07 | 1.33 (0.96–1.85) | 0.09 |
Notes: Model 1 unadjusted. Model 2 adjusted for age, sex. Model 3 adjusted for age, sex, and history of taking blood pressure control drugs and blood glucose control drugs.
Abbreviations: CKD, chronic kidney disease; OR, odds ratio; CI, confidence interval; HBP, blood pressure; AO, abdominal obesity; HTG, high triacylglycerols; HFPG, high fasting plasma glucose; LHDL-C, low high-density lipoprotein cholesterol.
Correlation Between MS Components and CKD in Diabetic/Non-Diabetic Participants
HBP was found to be an independent risk factor for CKD in the group with diabetes after adjusting for gender and age (P=0.031). In the uncorrected model in subjects without diabetes, HBP and AO were independent risk factors for CKD (P<0.01 and P=0.05 respectively). After adjusting for sex, age, and medication history, HBP remained an independent risk factor for CKD (P<0.01; Table 4).
Table 4.
Odds Ratios for the Prevalence of CKD in Patients with or without Diabetes
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR (95%) CI | P | OR (95%) CI | P | OR (95%) CI | P | |
| Diabetes (+) | ||||||
| HBP | 3.69 (1.45–9.42) | 0.01 | 2.85 (1.09–7.47) | 0.03 | 2.42 (0.89–6.56) | 0.08 |
| AO | 1.39 (0.66–2.91) | 0.38 | 1.64 (0.76–3.55) | 0.21 | 1.68 (0.78–3.65) | 0.19 |
| HTG | 1.34 (0.78–2.28) | 0.29 | 1.24 (0.71–2.16) | 0.46 | 1.23 (0.70–2.15) | 0.48 |
| LHDL-C | 1.52 (0.81–2.85) | 0.19 | 1.56 (0.80–3.03) | 0.19 | 1.48 (0.75–2.90) | 0.25 |
| Diabetes (-) | ||||||
| HBP | 5.16 (3.48–7.65) | <0.01 | 3.15 (2.08–4.76) | <0.01 | 2.56 (1.67–3.92) | <0.01 |
| AO | 1.36 (1.01–1.84) | 0.05 | 1.23 (0.89–1.68) | 0.21 | 1.17 (0.85–1.61) | 0.34 |
| HTG | 1.26 (0.95–1.66) | 0.11 | 1.34 (0.99–1.80) | 0.06 | 1.34 (0.99–1.81) | 0.05 |
| LHDL-C | 1.41 (0.99–2.01) | 0.05 | 1.33 (0.91–1.93) | 0.14 | 1.31 (0.90–1.91) | 0.16 |
Notes: Model 1 unadjusted. Model 2 adjusted for age, sex. Model 3 adjusted for age, sex, and history of medication for blood pressure or glucose control.
Abbreviations: CKD, chronic kidney disease; CI, confidence interval; HBP, high blood pressure; AO, abdominal obesity; HTG, high triacylglycerols; LHDL-C, low high-density lipoprotein cholesterol.
Correlation Between MS Components and CKD in Participants with/without Hypertensive Disease
HFPG was an independent risk factor for CKD in the model for patients with hypertension when adjusted for gender, age, and medication history (P=0.02) and in the unadjusted model for patients without hypertension, HFPG (P=0.03), but this relationship was lost after adjustment for gender and age (P=0.42; Table 5).
Table 5.
Odds Ratios for the Prevalence of Chronic Kidney Disease (CKD) in Patients with or without Hypertension
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR (95%) CI | P | OR (95%) CI | P | OR (95%) CI | P | |
| Hypertensive disease (+) | ||||||
| AO | 1.39 (0.99–1.96) | 0.06 | 1.36 (0.95–1.94) | 0.09 | 1.35 (0.94–1.93) | 0.10 |
| HFPG | 1.66 (1.27–2.18) | <0.01 | 1.54 (1.16–2.04) | <0.01 | 1.47 (1.07–2.01) | 0.02 |
| HTG | 1.26 (0.95–1.67) | 0.10 | 1.30 (0.97–1.74) | 0.08 | 1.32 (0.98–1.77) | 0.07 |
| LHDL-C | 1.33 (0.94–1.90) | 0.11 | 1.28 (0.88–1.86) | 0.19 | 1.25 (0.86–1.82) | 0.25 |
| Hypertensive disease (-) | ||||||
| AO | 1.16 (0.70–1.92) | 0.56 | 1.06 (0.62–1.80) | 0.84 | 1.06 (0.62–1.81) | 0.83 |
| HFPG | 1.86 (1.06–3.27) | 0.03 | 1.28 (0.70–2.33) | 0.42 | 1.35 (0.68–2.67) | 0.39 |
| HTG | 1.26 (0.72–2.19) | 0.42 | 1.33 (0.74–2.40) | 0.35 | 1.31 (0.72–2.37) | 0.37 |
| LHDL-C | 1.61 (0.88–2.97) | 0.12 | 1.51 (0.79–2.91) | 0.22 | 1.53 (0.79–2.94) | 0.21 |
Notes: Model 1 unadjusted. Model 2 adjusted for age, sex. Model 3 adjusted for age, sex, and history of taking blood pressure control drugs and blood glucose control drugs.
Abbreviations: CI, confidence interval; AO, abdominal obesity; HTG, high triacylglycerols; HFPG, high fasting plasma glucose; LHDL-C, low high-density lipoprotein cholesterol.
Correlation Between MS Components and CKD in Obese/Non-Obese Population
HBP was an independent risk factor for CKD in the unadjusted model of subjects with obesity and in the model adjusted for gender and age (P<0.01 and P=0.03 respectively). In the unadjusted model in subjects without obesity, HBP, HFPG and LHDL-C were independent risk factors for CKD (P<0.01, P<0.01 and P=0.05 respectively). HBP and HFPG remained independent risk factors after adjustment for gender, age, and medication history (P<0.01 and P=0.02 respectively; Table 6).
Table 6.
Odds Ratios for the Prevalence of Chronic Kidney Disease (CKD) in Patients with or without Obesity
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR (95%) CI | P | OR (95%) CI | P | OR (95%) CI | P | |
| Obesity (+) | ||||||
| HBP | 20.43 (2.74–152.33) | <0.01 | 9.50 (1.23–73.14) | 0.03 | 7.49 (0.96–58.69) | 0.06 |
| HFPG | 1.60 (0.94–2.74) | 0.08 | 1.48 (0.84–2.61) | 0.18 | 1.43 (0.75–2.74) | 0.28 |
| HTG | 1.53 (0.89–2.63) | 0.13 | 1.45 (0.81–2.58) | 0.21 | 1.41 (0.79–2.53) | 0.25 |
| LHDL-C | 1.37 (0.74–2.53) | 0.32 | 1.28 (0.65–2.49) | 0.47 | 1.25 (0.63–2.47) | 0.52 |
| Obesity (-) | ||||||
| HBP | 4.59 (3.17–6.65) | <0.01 | 2.87 (1.95–4.23) | <0.01 | 2.46 (1.65–3.67) | <0.01 |
| HFPG | 1.83 (1.39–2.40) | <0.01 | 1.54 (1.16–2.05) | <0.01 | 1.47 (1.07–2.03) | 0.02 |
| HTG | 1.25 (0.94–1.64) | 0.12 | 1.30 (0.97–1.74) | 0.08 | 1.30 (0.97–1.74) | 0.08 |
| LHDL-C | 1.43 (1.00–2.04) | 0.05 | 1.34 (0.92–1.95) | 0.12 | 1.33 (0.91–1.94) | 0.13 |
Notes: Model 1 unadjusted. Model 2 adjusted for age, sex. Model 3 adjusted for age, sex, and history of taking blood pressure control drugs and blood glucose control drugs.
Abbreviations: CI, confidence interval; HBP, blood pressure; HTG, high triacylglycerols; HFPG, high fasting plasma glucose; LHDL-C, low high-density lipoprotein cholesterol.
Discussion
In this study including Chinese individuals aged 40 and over we found that MS was associated with CKD. Components of MS, including HTG, HFPG and HBP, AO, and LHDL-C, were found to be associated with CKD, consistent with previous findings.12,19–21 Non-communicable diseases such as obesity,18,22,23 hypertensive disease24–26 diabetes27,28 and hyperuricemia26 are also risk factors for CKD. It is unclear whether insulin resistance is an independent predictor of CKD mortality and cardiovascular complications.29 Our study shows that the level of HOMA-IR in people with CKD is significantly higher than that in the non-CKD population. To date, many studies have evaluated the relationship between MS and CKD. Our survey shows that MS is common, with high occurrence of HBP, AO and HTG (Table 1) and that MS was a risk factor for CKD. A strong unadjusted and adjusted association was observed between MS and its components and CKD.
A previous cross-sectional study of 2310 subjects over 40 years old in China showed that MS was independently related to CKD.19 In that study, prevalence of CKD was significantly higher in subjects with than without MS, and increased with the number of MS components. MS was also associated with CKD in subjects without hypertension or diabetes. These findings are consistent with those of the present study, but prevalence of CKD differed between the two studies, being significantly higher in the present research. One explanation for this difference may be that our definition of CKD included ACR, which increased the detection rate of CKD. The proportion of subjects classified as suffering from CKD, with increased ACR but normal eGFR, was 12.2%. Chen et al20 extracted data from The Third National Health and Nutrition Examination Survey (NHANES III), which contains more than 6000 items of clinical information from the general population in the United States. They found that MS may be an important risk factor for CKD. However, the study included relatively young participants (aged 20 and over) while the present study is more meaningful for formulation of prevention and treatment measures for chronic non-communicable diseases among the middle-aged and elderly in Southern China. In a cross-sectional study of 788 patients diagnosed with hypertension or diabetes in Brazil,12 MS was defined using NCEP-ATPIII standards. The results showed that MS and its single or combined components were independently associated with CKD and were highly consistent with the present results. We extend these findings with a multivariate logistic regression analysis of MS components and CKD after population classification (including diabetes, non-diabetic, hypertensive, non-hypertensive, obese and non-obese populations). Our data allow the following conclusions to be drawn. In the fully corrected models (Table 4), none of the MS components showed an independent association with CKD in patients with diabetes, while in those without diabetes, HBP was a single independent contributor. Similar results were obtained if analyses were performed in groups according to the presence of obesity: HFPG was the sole component of MS significantly affecting the odds of CKD in lean individuals only (Table 6). Similarly, in patients with hypertension (Table 5), only those with HFPG had increased odds of CKD, and neither component showed a significant impact in normotensive subjects.
In exploring the relationship between the components of MS and the prevalence of CKD, we found that in addition to hypertension and diabetes (which have long been known as major risk factors for CKD progression), HTG is associated with CKD. The mechanism by which lipid metabolism disorder affects renal function is not fully understood but includes oxidative stress, chronic inflammation, hemodynamic changes, atherosclerosis, and endothelial dysfunction.30 Studies have reported that high plasma triglyceride level is an independent risk factor for the occurrence and development of human glomerular disease. Although the data suggest that oxidative stress and insulin resistance mediate lipid kidney injury, its pathophysiological mechanism has not been fully understood.30–32 In addition to the increase in TG, various types of dyslipidemia are also related to CKD.31,33 Further studies are needed to explain the mechanism of the effect of elevated TG on renal function.
In this study, the rate of antihypertensive medication in participants with hypertension (31.3%) was significantly lower than the prevalence of hypertension (54.6%). This discrepancy was related to the following factors. First, 32.7% of participants were known to have hypertension, but 1.4% of these did not take blood pressure regulating drugs due to difficulties following medical advice or ability to temporarily control blood pressure by lifestyle changes without medication. Second, 21.9% of the participants did not know they were suffering from hypertension, were informed of their hypertension for the first time while participating in this study, and were not treated during the study period.
In the unadjusted model of non-diabetic subjects, the waist circumference was correlated with CKD (OR=1.36), but the correlation was not significant after adjustment for gender and age (Table 4). These results indicate that an association between AO and CKD in populations without diabetes is highly dependent on gender and age. A correlation was found between HDL-C and CKD in the unadjusted model of non-obese subjects (OR = 1.43) but after adjusting for gender and age, this correlation was not significant (P > 0.05). An association between HDL-C and CKD in non-obese people was also dependent on gender and age (Table 6). From an epidemiological point of view, this study provides evidence for associations between MS components and CKD in middle and old-aged populations without diabetes/obesity in Asia. Further research will enrich the data on correlations between AO and CKD in subjects without diabetes, or LHDL-C in non-obese populations, and may provide guidance for the prevention and treatment of chronic diseases including government policy.
This study has several limitations. First, it is a cross-sectional study with a relatively small sample which was not randomly selected, so selection bias may have occurred. For example, in this study, the average age was high (59.0 ± 10.6 years) and only 36% of the subjects were men. Second, creatinine is the product of muscle metabolism. Creatinine-based eGFR may not allow accurate assessment of renal function in middle-aged and elderly people. Third, medical staff completed the questionnaires which may have led to inaccurate reporting of the participants” medication and other history.
In 2017, the total medical expenditure on CKD and end stage renal disease (ESRD) in the United States exceeded 120 billion US dollars, and the total expenditure on patients with this disease was 35.9 billion US dollars, accounting for 7.2% of the national medical and health budget. The incidence rate of ESRD continues to rise, reached 746,557 cases in 2017 and it has become a burden for society and families.14,34 According to the 2016 data report of the China kidney disease network, the total medical expenditure of all CKD patients included in the 2016 analysis was almost 4 billion US dollars, there were close to one million patients with CKD among the population over 18 years old in China, many with ESRD, and these numbers show large regional variations so the renal treatment burden may be underestimated.1 To control the increased use of renal replacement therapy, it is important to understand the relationship between metabolic diseases and CKD. Effective interventions to control MS and CKD will help to alleviate the burden of chronic diseases related to limited medical resources. Early identification of individuals with obesity, diabetes, and hypertension (risk factors for many diseases including MS and CKD) and the provision of education about their condition will help prevent progression to cardiovascular disease or ESRD.
Conclusion
In the fully adjusted models, patients with diabetes/obesity who manifest MS components do not have elevated odds for CKD. Similar results were obtained in normotensive subjects. MS is common in the middle-aged and elderly, is a modifiable risk factor and is easy to monitor. Therefore, identification of strategies to reduce its prevalence and severity through intervention is the goal of CKD prevention and treatment, and needs further research. A prospective study with longer follow-up is warranted.
Funding Statement
This study was supported by: Risk factors and prediction model of chronic kidney disease caused by metabolic syndrome: A multicentric prospective cohort study Clinical trial training project of Southern Medical University (LC2016PY047, 2016); Science and Technique Program of Guangzhou (201604020015, 2015); South Wisdom Valley Innovative Research Team Program (CXTD-004, 2014); Shenzhen Fund for Guangdong Provincial High- level Clinical Key Specialties (NO. SZGSP001); and Shenzhen Governmental Sustainable Development Fund (KCXFZ20201221173612034).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zhang L, Zhao MH, Zuo L, et al. China Kidney Disease Network (CK-NET) 2016 annual data report. Kidney Int Suppl. 2020;10(2):e97–e185. doi: 10.1016/j.kisu.2020.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy D, McCulloch CE, Lin F, et al. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med. 2016;165(7):473–481. doi: 10.7326/M16-0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonsson AJ, Lund SH, Eriksen BO, Palsson R, Indridason OS. The prevalence of chronic kidney disease in Iceland according to KDIGO criteria and age-adapted estimated glomerular filtration rate thresholds. Kidney Int. 2020;98(5):1286–1295. doi: 10.1016/j.kint.2020.06.017 [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822. doi: 10.1016/s0140-6736(12)60033-6 [DOI] [PubMed] [Google Scholar]
- 5.Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol. 2017;13(2):104–114. doi: 10.1038/nrneph.2016.163 [DOI] [PubMed] [Google Scholar]
- 6.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Lerman LO. The metabolic syndrome and chronic kidney disease. Transl Res. 2017;183:14–25. doi: 10.1016/j.trsl.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho JS, Cannaday JJ, Barlow CE, Mitchell TL, Cooper KH, FitzGerald SJ. Relation of the number of metabolic syndrome risk factors with all-cause and cardiovascular mortality. Am J Cardiol. 2008;102(6):689–692. doi: 10.1016/j.amjcard.2008.05.010 [DOI] [PubMed] [Google Scholar]
- 9.Xu H, Kuja-Halkola R, Chen X, Magnusson PKE, Svensson P, Carrero JJ. Higher body mass index is associated with incident diabetes and chronic kidney disease independent of genetic confounding. Kidney Int. 2019;95(5):1225–1233. doi: 10.1016/j.kint.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 10.Pammer LM, Lamina C, Schultheiss UT, et al. Association of the metabolic syndrome with mortality and major adverse cardiac events: a large chronic kidney disease cohort. J Intern Med. 2021;290(6):1219–1232. doi: 10.1111/joim.13355 [DOI] [PubMed] [Google Scholar]
- 11.Lea JP. Metabolic syndrome, CKD progression, and death: the good, the bad, and the ugly. Clin J Am Soc Nephro. 2013;8(6):893–895. doi: 10.2215/CJN.03960413 [DOI] [PubMed] [Google Scholar]
- 12.Comini LO, de Oliveira LC, Borges LD, et al. Individual and combined components of metabolic syndrome with chronic kidney disease in individuals with hypertension and/or diabetes mellitus accompanied by primary health care. Diabetes Metabol Syndr Obes. 2020;13:71–80. doi: 10.2147/DMSO.S223929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yun HR, Kim H, Park JT, et al. Obesity, metabolic abnormality, and progression of CKD. Am J Kidney Dis. 2018;72(3):400–410. doi: 10.1053/j.ajkd.2018.02.362 [DOI] [PubMed] [Google Scholar]
- 14.Saran R, Robinson B, Abbott KC, et al. US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75(1Suppl 1):A6–A7. doi: 10.1053/j.ajkd.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 15.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO controversies conference report. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483 [DOI] [PubMed] [Google Scholar]
- 18.Cao X, Zhou J, Yuan H, Wu L, Chen Z. Chronic kidney disease among overweight and obesity with and without metabolic syndrome in an urban Chinese cohort. BMC Nephrol. 2015;16:85. doi: 10.1186/s12882-015-0083-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Zuo L, Wang F, et al. Metabolic syndrome and chronic kidney disease in a Chinese population aged 40 years and older. Mayo Clin Proc. 2007;82(7):822–827. doi: 10.4065/82.7.822 [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140(3):167–174. doi: 10.7326/0003-4819-140-3-200402030-00007 [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Sun B, Sheng LT, et al. Association between weight status, metabolic syndrome, and chronic kidney disease among middle-aged and elderly Chinese. Nutr Metab Cardiovasc Dis. 2020;30(11):2017–2026. doi: 10.1016/j.numecd.2020.06.025 [DOI] [PubMed] [Google Scholar]
- 22.Evangelista LS, Cho WK, Kim Y. Obesity and chronic kidney disease: a population-based study among South Koreans. PLoS One. 2018;13(2):e0193559. doi: 10.1371/journal.pone.0193559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garofalo C, Borrelli S, Minutolo R, Chiodini P, De Nicola L, Conte G. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 2017;91(5):1224–1235. doi: 10.1016/j.kint.2016.12.013 [DOI] [PubMed] [Google Scholar]
- 24.Xie K, Bao L, Jiang X, et al. The association of metabolic syndrome components and chronic kidney disease in patients with hypertension. Lipids Health Dis. 2019;18(1):229. doi: 10.1186/s12944-019-1121-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeda T, Yoshimura C, Takahashi K, et al. Usefulness of the blood pressure classification in the new 2017 ACC/AHA hypertension guidelines for the prediction of new-onset chronic kidney disease. J Hum Hypertens. 2019;33(12):873–878. doi: 10.1038/s41371-019-0198-7 [DOI] [PubMed] [Google Scholar]
- 26.Shi W, Wang H, Zhou Y, Sun Y, Chen Y. Synergistic interaction of hyperuricemia and hypertension on reduced eGFR: insights from a general Chinese population. Postgrad Med. 2020;132(3):263–269. doi: 10.1080/00325481.2020.1718387 [DOI] [PubMed] [Google Scholar]
- 27.Lin L, Lu J, Chen L, et al. Glycemic status and chronic kidney disease in Chinese adults: findings from the REACTION study. J Diabetes. 2017;9(9):837–845. doi: 10.1111/1753-0407.12490 [DOI] [PubMed] [Google Scholar]
- 28.Duan J, Wang C, Liu D, et al. Prevalence and risk factors of chronic kidney disease and diabetic kidney disease in Chinese rural residents: a cross-sectional survey. Sci Rep. 2019;9(1):10408. doi: 10.1038/s41598-019-46857-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol. 2016;311(6):F1087–F1108. doi: 10.1152/ajprenal.00340.2016 [DOI] [PubMed] [Google Scholar]
- 30.Jabarpour M, Rashtchizadeh N, Argani H, et al. The impact of dyslipidemia and oxidative stress on vasoactive mediators in patients with renal dysfunction. Int Neurol Nephrol. 2019;51(12):2235–2242. doi: 10.1007/s11255-019-02319-7 [DOI] [PubMed] [Google Scholar]
- 31.Bulbul MC, Dagel T, Afsar B, et al. Disorders of lipid metabolism in chronic kidney disease. Blood Purif. 2018;46(2):144–152. doi: 10.1159/000488816 [DOI] [PubMed] [Google Scholar]
- 32.Trevisan R, Dodesini AR, Lepore G. Lipids and renal disease. J Am Soc Nephrol. 2006;17(4 Suppl 2):S145–7. doi: 10.1681/ASN.2005121320 [DOI] [PubMed] [Google Scholar]
- 33.Miljkovic M, Stefanovic A, Simic-Ogrizovic S, et al. Association of dyslipidemia, oxidative stress, and inflammation with redox status in VLDL, LDL, and HDL lipoproteins in patients with renal disease. Angiology. 2018;69(10):861–870. doi: 10.1177/0003319718780041 [DOI] [PubMed] [Google Scholar]
- 34.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72(3):247–259. doi: 10.1038/sj.ki.5002343 [DOI] [PubMed] [Google Scholar]