Abstract
Objective
To explore the value of uridine diphosphate-glucuronosyltransferase 2B7 (UGT2B7)-161 single nucleotide polymorphism in predicting the occurrence of cardiotoxicity in Chinese human epidermal growth factor 2 (HER-2) positive breast cancer patients who underwent pertuzumab combined with trastuzumab Therapy.
Methods
Fifty patients with HER-2 positive breast cancer who planned to receive trastuzumab and pertuzumab therapy for more than four cycles were enrolled in this study, and blood samples were collected. Thirty healthy volunteers of matching ages were selected as the control group. Myocardial parameters such as global work index, global effective work, and global wasted work were measured before treatment (M0) and at the end of four cycles of treatment month three (M3). Blood samples were collected from patients at the M0 stage, and polymorphisms of the UGT2B7-161 gene were detected to evaluate the predictive ability of different gene phenotypes on the myocardial drug toxicity injury.
Results
There were 35 myocardial work decreased events among 50 patients. There were 24 (47.3%), 15 (40.8%), and 11 (11.8%) patients carrying UGT2B7-161 CC, CT, and TT genotypes, respectively. The occurrence of myocardial work decreased was decreased in UGT2B7-161 TT and CT genotypes (12.5%) compared with CC genotype (41.7%) with statistical significance (P < 0.001). Multivariate logistic regression model analysis exhibited that UGT2B7-161 genotypes, body mass index, and cardiac troponin I were independent factors influencing the risk of cardiotoxicity.
Conclusion
UGT2B7-161 single nucleotide polymorphism is a potential predictive factor for cardiotoxicity in HER-2 positive breast cancer patients receiving trastuzumab and pertuzumab dual-targeted drug therapy.
Keywords: breast cancer, uridine diphosphate-glucuronosyltransferase 2B7, pressure-strain loop, human epidermal growth factor 2, cardiotoxicity, trastuzumab, pertuzumab
Introduction
Human epidermal growth factor 2 (HER-2) positive breast cancer accounts for about 20% of breast cancer cases.1,2 At present, surgical resection is still the preferred treatment for breast cancer with positive HER-2. Postoperative adjuvant chemotherapy has been widely used to consolidate the therapeutic effect and reduce the recurrence rate.3,4 And postoperative trastuzumab combined with pertuzumab therapy are recommended for breast cancer with positive HER-2, which effectively reduces the residual cancer cells, postoperative hematologic metastasis, and the recurrence and mortality of breast cancer.5–7
However, drugs trastuzumab and pertuzumab have strong toxic side effects on the heart, resulting in cardiac damage and affecting prognosis,8 and the cardiotoxicity risk that may be induced by this treatment approach limits its clinical application.9 Therefore, it is of great significance to actively explore novel markers that can predict the risk of cardiotoxicity in patients with HER-2 positive breast cancer undergoing trastuzumab combined with pertuzumab therapy. Uridine diphosphate-glucuronosyltransferase 2B7 (UGT2B7) can inactivate anti-tumor drugs through glycolipidation, and studies have shown that the transformation of UGT2B7-161 SNP C to T allele (UGT2B7-161 C > T) affects the metabolism, toxicity, and efficacy of anthracyclines, and is negatively associated with the risk of cardiotoxicity.10,11 Based on the above evidence, we speculated that UGT2B7-161 gene polymorphism may also be associated with cardiotoxicity caused by trastuzumab combined with pertuzumab therapy.
Pressure-strain loops (PSL) are a new technique for quantifying cardiac function.12 Myocardial function is a set of comprehensive parameters obtained by this technology considering strain and afterload factors, which can better recognize the relationship between left ventricular strain and the increase in afterload pressure under different load conditions, and improve the accuracy and authenticity of myocardial function assessment.13,14 In this study, PSL was used to evaluate the changes of cardiac function parameters in patients with positive HER-2 breast cancer before and after the use of trastuzumab combined with pertuzumab therapy to detect cardiac function damage at an early stage. The purpose of this study was to investigate the value of UGT2B7-161 gene polymorphism in predicting the risk of cardiotoxicity in patients with HER-2 positive breast cancer with undergoing trastuzumab combined with pertuzumab therapy by PSL.
Data and Methods
Subjects
Fifty postoperative breast cancer patients admitted to the breast surgery department of Shenzhen People’s hospital from August 2020 to June 2021 were selected. The patients were 41–58 years old, with an average of 49 ± 7 years old, and planned to receive trastuzumab combined with pertuzumab drug therapy for more than four cycles. Thirty healthy volunteers with matching ages were selected as the control group, aged 40–59 years old, with an average of 49 ± 8 years old.
The inclusion criteria for the study group were: (1) female patients with pathologically confirmed positive HER-2 breast cancer. HER2-positivity was defined as grade 3+ staining intensity by immunohistochemistry (IHC), or confirmation of HER2 gene amplification by fluorescence in situ hybridization (FISH); (2) Patients who will receive trastuzumab combination with pertuzumab in a 21-day cycle and had not received any of these treatments; (3) the patients had no serious heart valvular disease, dilated and hypertrophic cardiomyopathy, coronary heart disease, arrhythmia, structural heart disease, hypertension, endocrine diseases, or thoracic malformation.
The control group excluded the history of heart disease, hypertension, diabetes, and abnormal electrocardiogram or echocardiography. All subjects gave informed consent.
The median age of the enrolled patients was 49 years, and the average body mass index (BMI) was 21.8 ± 2.1 kg/m2. There were 3 smokers (21.1%). There was a history of hypertension in 19 cases (25.0%), diabetes in 6 cases (5.3%), hyperlipidemia in 17 cases (23.7%), and hyperuricemia in 8 cases (19.7%). The median level of the baseline left ventricular ejection fraction (LVEF) was 69.0% (P25–P75: 64.3–71.0%), the median level of cardiac Troponin I (cTnI) was 0.040 ng/mL (P25–P75: 0.017–0.078 ng/mL), and the median level of N terminal Pro B type Natriuretic peptide (NT-probNP) was 0.065 ng/mL (P25–P75: 0.053–0.089 ng/mL).
Main Reagents and Instruments
Trastuzumab and pertuzumab were purchased from Shanghai Roche Pharmaceutical Co., Ltd., China. The QIAamp DNA mini kit was purchased from Qiagen, Germany. The polymerase chain reaction (PCR) amplification kit was purchased from TaKaRa, Japan. The centrifuge was purchased from Xiangyi Centrifuge Instrument Co., Ltd., China. The ADVIA Centaur XP immunoluminescence analyzer was purchased from Siemens, Germany. The 7900HT fluorescence quantitative PCR instrument and the ABI 3730XL sequencer were purchased from Applied Biosystems, USA. The GE Vivid E95 is a large-scale ultrasound machine purchased from GE Healthcare. It is equipped with an M5Sc cardiac probe and installed with an EchoPAC 203 software workstation.
Methods to Obtain Cardiac Work Parameters
The GE Vivid E95 M5Sc heart probe was used to obtain cardiac work parameters. Subjects were asked to lie in the left lateral decubitus position to obtain dynamic two-dimensional echocardiography, including the apical left ventricular two-chamber view, the apical left ventricular long axis view, and the apical four-chamber view. The frame frequency and depth were kept the same during the data collection, and the electrocardiogram indexes were recorded synchronously. All measured values were recorded for at least three cardiac cycles and their average values were taken.
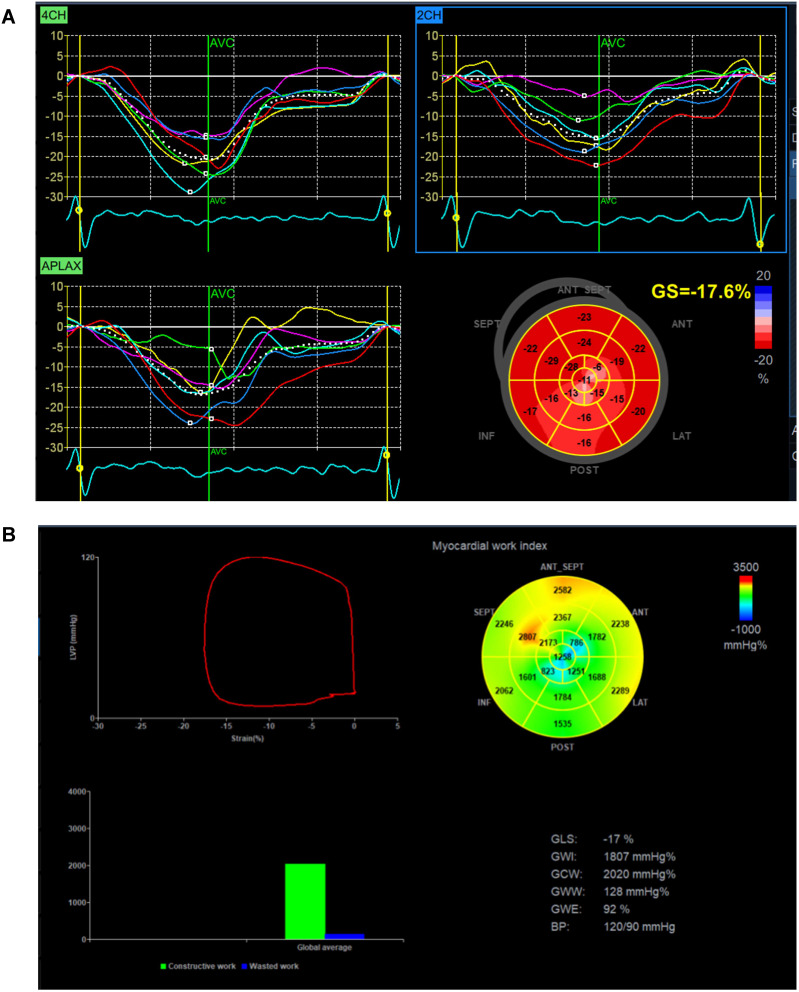
After the data collection, the Digital Imaging and Communication in Medicine images were stored, and the obtained image data were transferred to the EchoPAC 203 workstation. During offline analysis, dynamic images of good quality were clearly visible on both the endocardial and epicardial surfaces. The longitudinal strain and tracking graph of each section were generated. The software automatically calculated the global longitudinal peak strain (GLS) of the left ventricle and the blood pressure input during case collection. After the analysis was completed, the software automatically calculated the global work index (GWI), global effective work (GCW), global wasted work (GWW), and global work efficiency (GWE) (see Figure 1). All images were collected and analyzed by two experienced physicians in three cardiac cycles.
Figure 1.
Cardiac index detection. (A) Left ventricular global strain rate GLS. (B) Left ventricular myocardial work parameters.
Blood Sample Collection and Detection of cTnI and NT-probNP Levels
Whole blood samples were collected with anticoagulant tubes from patients before they received trastuzumab combined with pertuzumab dual-targeted drug therapy (M0) and at the end of four treatment cycles (M3) in the third month after the beginning of chemotherapy. Blood samples collected at M0 were divided into two parts; one part was whole blood reserved for subsequent detection of UGT2B7-161 gene polymorphism. In addition, as with blood samples collected at other follow-up sites, plasma separation was performed within 2 h after collection. The separation process was as follows: The whole blood sample was centrifuged at 1800 g at 4°C for 10 min, then the supernatant was transferred to an Eppendorf tube and centrifuged again at 2500 g at 4°C for 10 min. The plasma was separated and stored in a cryopreserved tube at −80°C for subsequent analysis. The levels of cTnI and NT-probNP in the plasma samples at each follow-up point were detected by the ADVIA Centaur XP immunoluminescence analyzer and supporting reagents.
UGT2B7-161 Gene Polymorphism Detection
Leukocyte DNA was extracted from patients’ M0 blood samples using the QIAamp DNA mini kit. UGT2B7-161 (RS7668258, Genebank Ref mRNA NM_001074) was amplified by the PCR kit and the 7900HT PCR instrument.
The primer sequence upstream was 5 “-TGTCACTGCTACTGTTCTGGA-3” and downstream was 5 “-AGTTGCTGTTCCTTTCTGTCAT-3”.
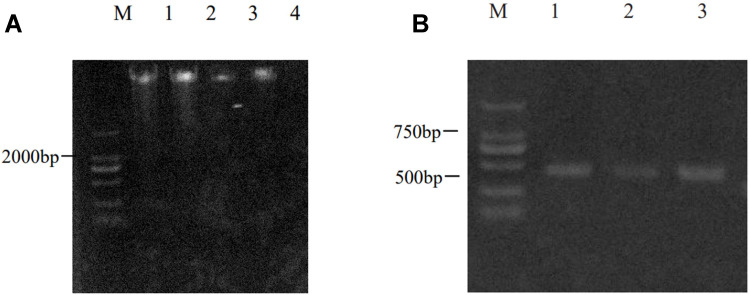
The reaction conditions were pre-denaturation at 95°C for 5 min, followed by 95°C for 15s, 60°C for 30s, 72°C for 30s, for a total of 40 cycles, and finally extended at 72°C for 5 min. The amplified products were sequenced using an ABI 3730XL sequencer, strictly following the instructions (see Figure 2).
Figure 2.
Gene extraction and amplification of UGT2B7-161. (A) Extraction of human genomic DNA of different samples. (B) Electrophoresis of UGT2B7-161 gene amplified by PCR.
Statistical Analysis
SPSS Statistics 24.0 software was used for data processing. Continuous data of a normal distribution were expressed as mean ± standard deviation. When the variance was uniform, one-way analysis of variance (ANOVA) was used for comparison between multiple groups, and the least significant difference method was used for pairwise comparison. The correlation between left ventricle strain and work parameters was determined using Pearson correlation analysis. The measurement data of 10 cases were selected for analysis by another physician, and a repeated test was carried out by the Bland-Altman method. P < 0.05 was considered statistically significant. Continuous data of a skewed distribution were expressed as median (P25–P75), and the classified data was expressed as frequency (percentage). One-way ANOVA was used for measurement data comparison, the Chi-square test was used for counting data comparisons, the Kruskal–Wallis H-test was used for data that did not conform to a normal distribution, and a logistic regression model was used for multivariate analysis.
Results
Occurrence of Myocardial Toxic Events at Different Time Points of Follow-Up
No heart failure, acute coronary syndrome, or serious life-threatening arrhythmias were found at all time points of follow-up. The number of cardiotoxicity events at different follow-up time points was defined as the number of events that occurred between the previous follow-up time point and this follow-up time point. The number of events with ΔLVEF ≥ 10% or LVEF < 53% only or ΔLVEF ≥ 10% and LVEF < 53% were 0, 1 and 2 at M0 and M3, totaling 2 cases of cardiotoxic events (see Table 1).
Table 1.
Occurrence of Myocardial Toxic Events at Different Follow-Up Time Points [Cases (%)]
| Project | M0 | M3 |
|---|---|---|
| Cardiac failure | 0(0) | 0(0) |
| Acute coronary syndrome | 0(0) | 0(0) |
| Life-threatening arrhythmia | 0(0) | 0(0) |
| Only ΔLVEF≥10% | 0(0) | 0(0) |
| Only LVEF<53% | 0(0) | 1(2%) |
| ΔLVEF≥10%and LVEF<53% | 0(0) | 2(4%) |
| Cardiotoxic event | 0(0) | 2(4%) |
Note: Δ LVEF: the change of LVEF relative to M0 at each follow-up time point.
Comparison of Self-Characteristics of Different UGT2B7-161 Genotype HER-2 Positive Breast Cancer
According to UGT2B7-161 gene phenotype, 50 breast cancer patients were divided into a CC group (n = 24), a CT group (n = 15), and a TT group (n = 11). There were no significant differences in age, BMI, smoking history, concomitant diseases, and cardiac function among patients with different genotypes (P > 0.05), suggesting that UGT2B7-161 was not associated with self-characteristics features of breast cancer with positive HER-2 (see Table 2 and Figure 3).
Table 2.
Comparison of Self-Characteristics of Different UGT2B7-161 Genotype HER-2 Positive Breast Cancer
| Parameter | CC (n = 36) | CT (n = 31) | TT (n = 9) | P |
|---|---|---|---|---|
| Median age (years) | 50. 0 | 49. 0 | 54. 0 | 0. 342 |
| BMI(kg/m2) | 21. 7±2. 0 | 23. 9±2. 3 | 24. 4±2. 5 | 0. 543 |
| Smoke* | 1(4. 2) | 0(0) | 2(18. 2) | 0. 528 |
| Chronic disease* | ||||
| Hypertension | 3(12.5) | 5(33.3) | 11(10.0) | 0. 561 |
| Diabetes | 1(4.2) | 1(6.7) | 4(36.4) | 0. 644 |
| Hyperlipaemia | 2(8.3) | 6(40.0) | 9(81.8) | 0. 565 |
| Hyperuricemia | 2(8.3) | 4(26. 7) | 2(18.2) | 0. 454 |
| Cardiac function(M)# | ||||
| LVEF(%) | 67. 5(64. 0–70. 5) | 69. 0(66. 0–73. 0) | 68. 0(64. 5–70. 0) | 0. 254 |
| cTnI(ng/mL) | 0. 041(0. 012–0. 091) | 0. 034(0. 025–0. 055) | 0. 064(0. 041–0. 069) | 0. 334 |
| NT-proBNP(ng/mL) | 0. 067(0. 059–0. 104) | 0. 061(0. 053–0. 087) | 0. 052(0. 049–0. 070) | 0. 273 |
Note: *Examples (%); #median (P25 to P75).
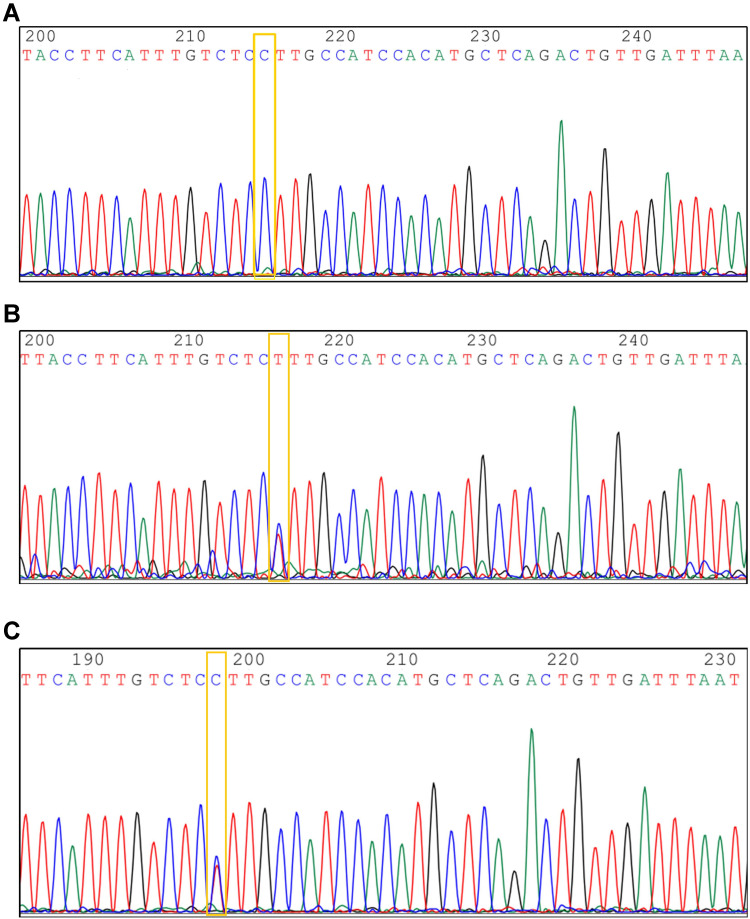
Figure 3.
Identification of genotypes of UGT2B7-161. (A) UGT2B7-161 sample gene was CC type. (B) UGT2B7-161 sample gene was TT type. (C) UGT2B7-161 sample gene was CT type.
Comparison of General Data
There were no significant differences in age and systolic blood pressure among the three groups (P > 0.05), or in the routine echocardiography parameters for left ventricular end-diastolic diameter, end-diastolic volume, end-systolic diameter, end-systolic volume, LVEF, and fractional shortening among the three groups (all P > 0.05; see Table 3).
Table 3.
Comparison of General Data and Left Ventricular Two-Dimensional Echocardiography Parameters Between Breast Cancer Patients Before and After Chemotherapy and the Control Group (X ± S)
| Group | No. | Age (Years) | Systolic Pressure (mmHg) | LVEDs (mm) | LVESV (mL) | LVEDd (mm) | LVEDV (mL) | LVEF (%) | FS (%) |
|---|---|---|---|---|---|---|---|---|---|
| Control group | 30 | 49±8 | 118±5 | 30.8±1.3 | 38±3 | 47.4±2.7 | 107±12 | 63.0±5.2 | 33.2±1.8 |
| M0 group | 50 | 49±7 | 121±6 | 29.2±2.5 | 34±5 | 47.0±1.4 | 104±6 | 62.2±5.9 | 32.0±3.2 |
| M3 group | 50 | 49±7 | 120±5 | 29.0±3.1 | 34±8 | 47.2±1.8 | 105±9 | 60.4±4.8 | 30.8±1.3 |
| F | 0.263 | 0.044 | 0.839 | 0.810 | 0.048 | 8.114 | 2.046 | 0.980 | |
| P | 0.610 | 0.985 | 0.456 | 0.468 | 0.953 | 0.893 | 0.172 | 0.403 |
Notes: LVEDd is left ventricular end-diastolic diameter, LVEDV is left ventricular end-diastolic volume, LVEDs is left ventricular end-systolic diameter, LVESV is left ventricular end-systolic volume, LVEF is left ventricular ejection fraction, FS is short axis shortening rate.
Comparison with the Control Group
Compared with the control group, the GLS, GWI, GCW, GWW, and GWE of the left ventricle in the breast cancer group before chemotherapy had no statistically significant difference (P > 0.05). After therapy, compared with before, there were statistically significant differences in the left ventricular GLS, GWI, GCW, and GWE (P < 0.05), and GWW increased (P < 0.05) (see Table 4 and Figure 4). The Bland-Altman test showed good repeatability between and within observers.
Table 4.
Comparison of Parameters of Long Axial Strain and Left Ventricular Myocardial Work in Each Group (x ± S)
| Group | No. | GLS (%) | GWI (mmHg%) | GCW (mmHg%) | GWW (mmHg%) | GWE (%) |
|---|---|---|---|---|---|---|
| Control group | 30 | −21.0±1.6 | 2040±193 | 2359±194 | 64±28 | 96.5±1.1 |
| M0 group | 50 | −20.1±1.4 | 2029±165 | 2339±207 | 67±22 | 96.7±0.9 |
| M3 group | 50 | −17.5±2.8 | 1831±301 | 1962±340 | 84±26 | 93.4±2.1 |
| P* | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | |
| P# | <0.001 | <0.001 | <0.001 | 0.450 | <0.001 |
Notes: GLS is the overall longitudinal peak strain, GWI is the overall work index, GCW is the overall effective work, GWW is the overall ineffective work, GWE is the overall work efficiency; GLS minus sign indicates direction; Compared with control group, *P<0.05; Compared with the group before chemotherapy, #P<0.05.
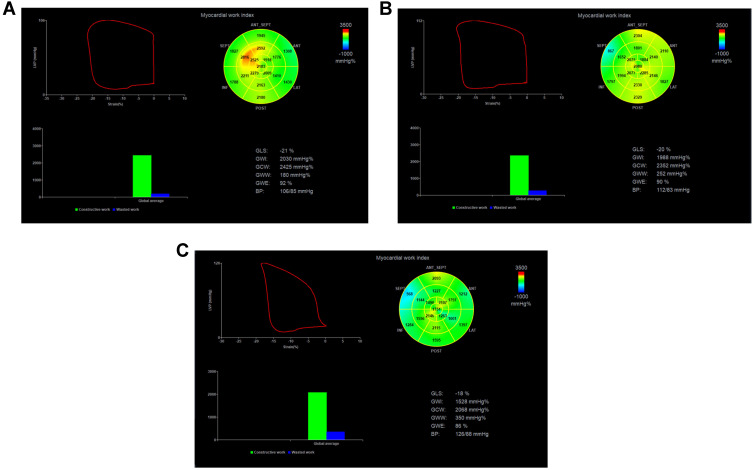
Figure 4.
Bull’s eye of 17 segments of left ventricular myocardium. (A–C) are control group and before and after targeted therapy in breast cancer group, respectively.
Relationship Between UGT2B7-161 Gene Polymorphism and Cardiotoxicity
Myocardial work decreased events occurred in 35 (70.0%) of the 50 patients receiving trastuzumab combined with pertuzumab dual-targeted drug therapy, 19 (79.2%) of 24 UGT2B7-161 CC patients, and 16 (61.5%) of 26 TT+CT patients. Among the 15 patients without myocardial toxicity, 5 (33.3%) had CC type and 10 (66.7%) had TT+CT type. Patients with the TT+CT genotype had a lower incidence of myocardial work decreased events than those with the CC genotype (P = 0.004).
Logistic Univariate Regression Analysis of the Risk of Cardiotoxicity
Logistic univariate regression analysis showed that the UGT2B7-161 gene phenotype, BMI, cTnI, and hyperuricemia were associated with the risk of myocardial toxicity (P < 0. 05). Age, smoking, hypertension, diabetes, hyperlipidemia, chronic kidney disease, and NT-probNP were not associated with the risk of myocardial toxicity (P > 0.05). See Table 5.
Table 5.
Logistic Univariate Analysis of Influencing Changes in Myocardial Work
| Clinicopathological Parameter | Case No. | Cardiotoxicity | p | |
|---|---|---|---|---|
| Occurrence | Nonevent | |||
| UGT2B7-161 Genetic phenotypes | 0.004 | |||
| CC | 24 | 19 | 5 | |
| CT+TT | 26 | 16 | 10 | |
| Age (years) | 0.544 | |||
| ≥50 | 27 | 16 | 11 | |
| <50 | 23 | 19 | 14 | |
| BMI (kg /m2) | 0.016 | |||
| ≥25. 0 | 8 | 5 | 3 | |
| <25. 0 | 42 | 30 | 12 | |
| Smoke | 0.210 | |||
| Exist | 3 | 2 | 1 | |
| None | 47 | 33 | 14 | |
| Hypertension | 0.073 | |||
| Exist | 19 | 13 | 6 | |
| None | 31 | 22 | 9 | |
| Diabetes | 0.136 | |||
| Exist | 6 | 4 | 2 | |
| None | 44 | 31 | 13 | |
| Hyperlipemia | 0.188 | |||
| Exist | 17 | 12 | 5 | |
| None | 33 | 23 | 10 | |
| Hyperuricemia | 0.026 | |||
| Exist | 8 | 6 | 2 | |
| None | 42 | 29 | 13 | |
| cTnI (ng/mL) | 0.001 | |||
| ≥0. 040 | 27 | 15 | 12 | |
| <0. 040 | 23 | 9 | 14 | |
| NT-proBNP (ng/mL) | 0.068 | |||
| ≥0. 065 | 21 | 16 | 5 | |
| <0. 065 | 29 | 19 | 10 | |
Logistic Multivariate Regression Analysis of the Risk of Cardiotoxicity
The UGT2B7-161 gene phenotype, BMI, cTnI and hyperuricemia were included in the logistic multivariate regression model. The results showed that the UGT2B7-161 gene phenotype, BMI, and cTnI were independent factors affecting the occurrence of myocardial toxicity (P < 0.05). Hyperuricemia was not associated with myocardial toxicity (P = 0.226). See Table 6.
Table 6.
Logistic Multivariate Analysis of Influencing Risk of Myocardial Toxicity
| Factor | OR (95% CI) | P |
|---|---|---|
|
UGT2B7-161 Genetic phenotypes CT+TT vs CC |
0. 107 (0. 023–0. 490) | <0. 001 |
| BMI (≥24. 0 kg /m2 vs <24. 0 kg /m2) | 6. 134 (1. 120–33. 589) | 0. 037 |
| Genetic phenotypes (exist vs none) | 2. 550 (0. 559–11. 626) | 0. 226 |
| cTnI (≥0.040 ng/mL vs <0.040 ng/mL) | 9. 183 (2. 046–41. 218) | 0. 004 |
Discussion
The traditional method of evaluating the systolic function of the left ventricle is usually to measure the indicators of myocardial fiber systolic shortening, including the overall indicators such as LVEF and local indicators such as the ventricular wall thickening rate, but the shortening index cannot reflect the work and oxygen consumption of myocardium.15 Although LVEF is still the most commonly used cardiac function parameter Clinically, once its clinical significance changes, it often indicates irreversible damage to the myocardia. Its low sensitivity to myocardial injury means that drug-related myocardial damage cannot be found in the subclinical stage.13
In this study, conventional two-dimensional echocardiography left ventricular systolic function parameters did not show myocardial impairment, while GWI, GCW, GWE, and GLS showed a decreasing trend compared with their levels before the therapy (P < 0.05). Conventional echocardiography left ventricular systolic parameters could not detect the toxicity of trastuzumab and pertuzumab drugs in myocardium in a timely and sensitive way. The results of this study showed that the myocardial cell toxicity caused by trastuzumab combined with pertuzumab therapy reduced the GLS capacity of the left ventricle, weakened the contractility of the left ventricle, and both GWI and GCW of the left ventricle showed a downward trend. The abnormal electromechanical activity of the myocardium caused by the cardiotoxicity of the therapy drugs and the unsynchronized movement of the ventricular wall affected the ventricular systolic function. This reduced the effectiveness of the myocardium and its ability to pump blood effectively, thereby increasing the GWW of the myocardium, which is consistent with the research results of Russell et al.16 The increase of useless work further affects the efficiency of the left ventricular myocardium. In addition, GLS decreased after trastuzumab combined with pertuzumab drug therapy compared with before, and GLS was positively correlated with the decrease of left ventricular work parameters GWI and GCW, indicating that the longitudinal strain capacity of the myocardium decreased, resulting in the decrease of myocardial contractility, which directly affected the ability of the heart to do work. Using GWI, GCW, and GWE parameters, this technique can intuitively quantify the myocardial working capacity and provide objective reference for clinical diagnosis and treatment. And 35 patients who occurred myocardial work decreased, were reduced targeted drug dosage or extended treatment interval, and/or added cardioprotective drugs to preintervene the occurrence of myocardial drug toxicity events. We supposed that the decrease in cardiac work parameters could be corrected in this way.
Previous studies17 have shown that there is no difference in epirubicin metabolism between histidine and tyrosine variants of UGT2B7, whereas SNP at position 161 is associated with the efficacy of morphine glucose-aldehyde acidification. A study of 99 patients who received morphine analgesia found that the glucose-aldehyde acidification of 161-base CC homozygous was significantly lower than that of CT heterozygous or TT homozygous.18 Because of these findings, we hypothesized that CC homozygous patients would have lower clearance rates and higher toxicity rates compared to CT and TT patients. In contrast, we hypothesized that TT homozygous patients would have a faster clearance rate, reduced drug efficacy, and poorer prognosis than patients with CC or CT variants. In this study, the incidence of drug cardiotoxicity in 161-base CC homozygous was significantly higher than that in the TT+CT group, which was consistent with the expected result. However, the expected result that drug cardiotoxicity in 161-base TT homozygous was lower than that in CT heterozygous has not been obtained, which may be related to the limitations of this study.
The GWI, GCW, GWW, and GWE of the myocardia had changed when the LVEF did not change significantly. However, differences in UGT2B7-161 gene polymorphism (CC, TT, CT) were statistically significant in patients who underwent trastuzumab combined with pertuzumab therapy had changes in myocardial work parameters. It can be used as a predictor of the occurrence of cardiotoxicity in breast cancer patients receiving targeted drug therapy HER-2.
The limitations of this study are as follows: (1) This study was a single-center cohort study, with a single source of HER-2 positive breast cancer patients, which may lead to selection bias and geographical limitation of the study; (2) The follow-up period of this study was short, and the UGT2B7-161 gene TT+CT type was not studied for evaluating the long-term efficacy of cardiotoxicity; (3) This study did not involve the underlying mechanism of UGT2B7-161 gene polymorphism and cardiotoxicity, which needs further investigation; (4) The sample size was small, and only before and after therapy was compared; (5) The cardiac muscle work capacity of PSL was mainly discussed. Although the blood pressure values of the included patients were selected within the normal range, there were still some variable differences, and the work difference caused by the fluctuation of blood pressure within the normal range could not be excluded;13 (6) PSL does not consider curvature myocardium thickness and only represents the indicator of work rather than directly measuring work value.3 (7) Whether the change of myocardial parameters is equivalent to the occurrence of myocardial toxic events has not been discussed and proved in detail in this study, which needs to be verified in a large number of cases.
Conclusion
In this study, we found that Ultrasound pressure-strain loop (PSL) is more objective and effective than strain in evaluating myocardial function, and its myocardial work parameters can be used for early assessment of subtle changes in myocardial systolic function and motor coordination.
In conclusion, prior to the occurrence of drug-induced myocardial damage in patients with HER-2 positive breast cancer receiving trastuzumab combined with pertuzumab dual-targeted therapy, planning myocardial protection measures in advance will be of great help to improve the prognosis of patients.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding Statement
Sanming Cultivation Project of Medicine in Shenzhen (grant number SYLY201903).
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of Shenzhen People’s Hospital. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389(1):1134–1150. doi: 10.1016/S0140-6736(16)31891-8 [DOI] [PubMed] [Google Scholar]
- 2.Labidi S, Mejri N, Lagha A, et al. Targeted therapies in HER 2-overexpressing metastatic breast cancer. Breast Care (Basel). 2016;11(6):418–422. doi: 10.1159/000452194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S, Wei W, Jiang Y, et al. Comparison of the efficacy and survival analysis of neoadjuvant chemotherapy for HER-2-positive breast cancer. Drug Des Devel Ther. 2018;12:3085–3093. doi: 10.2147/DDDT.S171534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Liang Y, Feng Z, et al. Efficacy and safety of HER 2 inhibitors in combination with or without pertuzumab for HER 2-positive breast cancer: a systematic review and meta-analysis. BMC Cancer. 2019;19(1):973. doi: 10.1186/s12885-019-6132-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escrivá-de-romaní S, Arumí M, Bellet M, et al. HER 2-positive breast cancer: current and new therapeutic strategies. Breast. 2018;39:80–88. doi: 10.1016/j.breast.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 6.Shao Z, Pang D, Yang H, et al. Efficacy, safety, and tolerability of pertuzumab, trastuzumab, and docetaxel for patients with early or locally advanced ERBB2-positive breast cancer in Asia: the PEONY phase 3 randomized clinical trial. JAMA Oncol. 2019. doi: 10.1001/jamaoncol.2019.3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denduluri N, Somerfield MR, Giordano SH. Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: ASCO clinical practice guideline focused update summary. J Oncol Pract. 2018;14(8):508–510. doi: 10.1200/JOP.18.00207 [DOI] [PubMed] [Google Scholar]
- 8.Kun C, Diansa G. Cardiotoxicity management of breast cancer therapy in the holistic management of breast cancer comorbidities. Chin J Clin Oncol. 2019;12(2):125–129. [Google Scholar]
- 9.Dent SF, Kikuchi R, Kondapalli L, et al. Optimizing cardiovascular health in patients with cancer: a practical review of risk assessment, monitoring, and prevention of cancer treatment–related cardiovascular toxicity. Am Soc Clin Oncol Educ Book. 2020;3(40):1–15. [DOI] [PubMed] [Google Scholar]
- 10.Sawyer MB, Innocenti F, Das S, et al. A pharmacogenetic study of uridine diphosphate-glucuronosyltransferase 2B7 in patients receiving morphine. Clin Pharmacol Ther. 2003;73(6):566–574. doi: 10.1016/S0009-9236(03)00053-5 [DOI] [PubMed] [Google Scholar]
- 11.Al-Eitan LN, Rababa’h DM, Alghamdi MA, Khasawneh RH. Association between ESR1, ESR2, HER 2, UGT1A4, and UGT2B7 polymorphisms and breast Cancer in Jordan: a case-control study. BMC Cancer. 2019;19:1257. doi: 10.1186/s12885-019-6490-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Mahdiui M, van der Bijl P, Abou R, et al. Global left ventricular myocardial work efficiency in healthy individuals and patients with cardiovascular disease. J Am Soc Echocardiogr. 2019;32(9):1120–1127. doi: 10.1016/j.echo.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 13.Clemmensen TS, Eiskjær H, Mikkelsen F, et al. Left ventricular pressure-strain-derived myocardial work at rest and during exercise in patients with cardiac amyloidosis. J Am Soc Echocardiogr. 2020;33(5):573–582. doi: 10.1016/j.echo.2019.11.018 [DOI] [PubMed] [Google Scholar]
- 14.Chan J, Edwards NFA, Khandheria BK, et al. A new approach to assess myocardial work by non-invasive left ventricular pressure strain relations in hypertension and dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2019;20(1):31–39. doi: 10.1093/ehjci/jey131 [DOI] [PubMed] [Google Scholar]
- 15.Boe E, Skulstad H, Smiseth OA. Myocardial work by echocardiography: a novel method ready for clinical testing. Eur Heart J Cardiovasc Imaging. 2019;20(1):18–20. doi: 10.1093/ehjci/jey156 [DOI] [PubMed] [Google Scholar]
- 16.Russell K, Eriksen M, Aaberge L, et al. Assessment of wasted myocardial work: a novel method to quantify energy loss due to uncoordinated left ventricular contractions. Am J Physiol Heart Circ Physiol. 2013;305(7):H996–1003. doi: 10.1152/ajpheart.00191.2013 [DOI] [PubMed] [Google Scholar]
- 17.Sawyer MB, Pituskin E, Damaraju S, et al. A uridine glucurono- syltransferase 2B7 polymorphism predicts epirubicin clearance and outcomes in early-stage breast cancer. Clin Breast Cancer. 2016;16:139–144.e3. doi: 10.1016/j.clbc.2015.09.006 [DOI] [PubMed] [Google Scholar]
- 18.Agha A, Zarifa A, Kim P, et al. The role of cardiovascular imaging and serum biomarkers in identifying cardiotoxicity related to cancer therapeutics. Method DeBakey Cardiovasc. 2019;15(4):258–266. doi: 10.14797/mdcj-15-4-258 [DOI] [PMC free article] [PubMed] [Google Scholar]