Abstract
Giant cell arthritis is a systemic vasculitis. A 51‐year‐old man was presented with sudden onset of right‐side blurred vision, frozen movements, and ptosis in the right eye and left side paresis. The diagnosis of GCA with first manifestations of stroke and carotid dissection may be neglected as an underlying cause.
Keywords: case report, cerebrovascular accident, giant cell arthritis, internal carotid artery dissection, stroke
When diagnosing stroke in elderly patients, based on the symptoms and clinical investigations, we should start treatment immediately before further diagnostic procedures if we suspect GCA, and we have to consider vasculitis in the further diagnosis.
1. INTRODUCTION
Giant cell arthritis (GCA) is the most prevalent systemic arthritis that influences medium‐sized and larger extramural arteries, usually in the fifth decade of life. Intracranial arteries are generally do not get involved. If the ophthalmic artery and its branches are involved, it can lead to irreversible and painless vision loss. 1 , 2
Patients with GCA can present to medical centers with various neurological symptoms such as jaw claudication, headache visual symptoms, or cerebrovascular events. GCA rarely can affect the brain arteries, resulting in ischemic strokes and transient ischemic attacks, whereby the most involved region is vertebrobasilar. 1 , 3 , 4 , 5 As an essential point, in GCA patients with stroke, the overall survival was decreased, with a 10th survival percentile of 4.4 months versus 221.7 months in GCA lonely. 6
We present a middle‐aged patient with sudden visual loss, frozen eye movements, ptosis, and left side paresis with acute ischemic infarction and internal carotid artery dissection in imaging and high erythrocyte sedimentation rate (ESR) level in laboratory tests. The aim of writing this case report is to draw attention to the fact that GCA may develop new and previously unreported ways that require proper diagnosis and prompt treatment to improve the prognosis of the disease.
2. PATIENT INFORMATION
A 51‐year‐old man was presented to the neurology ward of our hospital in April 2021 with sudden onset of right‐side blurred vision, frozen movements, and ptosis in the right eye. He also complained of sudden onset left side paresis. There was a severe recent history of right‐sided frontotemporal headache. There were no symptoms of COVID‐19 (coronavirus disease of 2019) infection, fever, seizure, respiratory problems, and jaw claudication. He also reported smoking, but he quit several years ago and had diabetes mellitus and dyslipidemia.
3. CLINICAL FINDINGS
On examination, the patient was conscious, oriented, obeyed, and vital signs were stable with oxygen saturation via pulse oximetry:94%, there was no tenderness over temporal arteries, and their pulses were normal. In appearance, the right eye had complete ptosis, and in ophthalmoscopy, we detected that the right optic disk was pallor. The right pupil was not responding to light, and the right eye's visual acuity was no light perception. Examination of ocular movements affirms a complete right ophthalmoplegia. Evaluation of the left eye was unremarkable. Also, the force of the left side limbs was reduced to 4/5.
4. DIAGNOSIS ASSESSMENT
The first laboratory tests show white blood cell (WBC) = 6500, hemoglobin (Hb) = 15.1, hematocrit (HCT) = 47.5, platelet count = 245,000, ESR = 90, and c‐reactive protein (CRP) = 15 mg/L. Spiral brain computed tomography (CT) scan and brain magnetic resonance imaging (MRI) showed multifocal diffusion restricting lesions involving all lobes of the right hemisphere (Figure 1) and some involvements in the maxillary sinus that spread to the cavernous sinus. The echocardiography was normal, but the ultrasound of the right carotid artery was completely occluded. Due to the history of diabetes, complete right ophthalmoplegia, right‐side blurred vision, and imaging investigations, we were suspected of mucormycosis.
FIGURE 1.
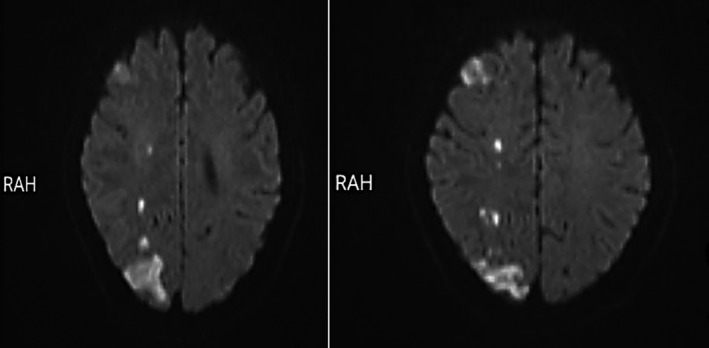
Diffusion‐weighted brain magnetic resonance imaging showing watershed infarct in the right hemisphere
5. THERAPEUTIC INTERVENTION
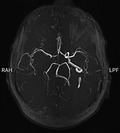
We started routine treatment for cerebrovascular accident (CVA) and Amphotericin B and antibiotics for empirical therapy. We also performed an endoscopic sinus biopsy, which was negative for Mucor, so we stopped the antifungal therapy. Brain magnetic resonance angiography (MRA) demonstrated significant stenosis with a large clot in the right internal carotid artery (ICA) that spreads to the sinus of cavernous (Figure 2). After this report, we promote a CT angiography that shows internal carotid artery dissection (Figure 3). Also, due to the history of ipsilateral frontotemporal headache and the high ESR level, we started pulse of Methylprednisolone 1 gram daily in suspicion of GCA, and temporal artery biopsy was done after 1 week, which was positive for GCA in pathology findings. After 10 days of undergoing corticosteroid therapy, inflammatory markers were reduced (ESR:40). Besides improving headaches, there was no significant change in ptosis and ocular movements, visual acuity, and reduction of left limb's force. After 2 weeks, we discharged the patient with Aspirin 80, clopidogrel 75 miligram, Atorvastatin 40 milligram, and prednisone 50 milligrams daily. Also, he was referred to the rehabilitation center for further treatments.
FIGURE 2.
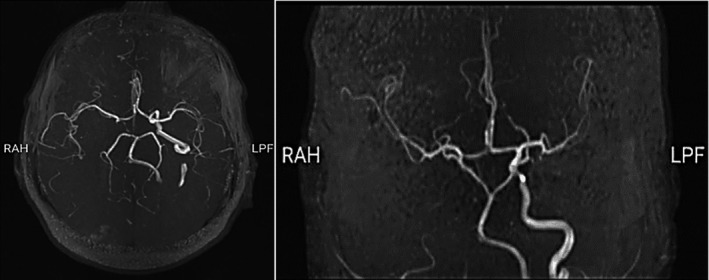
Magnetic resonance angiography showing occluded right proximal internal carotid artery
FIGURE 3.
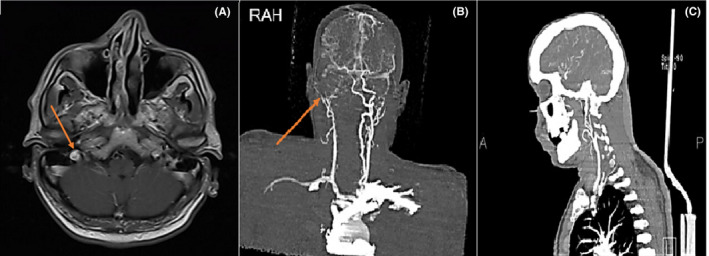
Right carotid artery dissection on contrast‐enhanced MR angiography (CE‐MRA) (a) and CTA (b & c). Carotid artery dissection is characterized by a narrowed eccentric flow void, which is surrounded by a crescent‐shaped, hyperintense mural hematoma expanding the outer vessel diameter
6. FOLLOW‐UP AND OUTCOMES
On the follow‐up, after 4 weeks of rehabilitation treatment, his left limb's force reduction was improved, and his headache was varnished.
7. DISCUSSION
Diagnosis of GCA is critical because delay can cause irreversible loss of vision in patients. Also, it can be challenging in those without the classic symptoms, such as headaches. The American College of Rheumatology criteria for the classification of GCA are not very specific in diagnosing clinical cases. Therefore, vascular imaging (ultrasonography, computed tomography, magnetic resonance imaging, or positron emission tomography), temporal artery biopsy (TAB), or a combination of these tests help with a definitive diagnosis. 7
GCA‐related stroke is defined as if the stroke showed GCA or occurred between the onset of symptoms and four weeks after the beginning of treatment. A population‐based study proved that GCA‐related stroke mainly affects the vertebrobasilar territory and usually occurs in older men with a history of vascular risk factors. 8 Through cardiovascular risk factors, only having diabetes mellitus was significantly higher in people with stroke during follow‐up. 6 As a point to consider in our case, stroke impacts the internal carotid artery region, and diabetes was the main cardiovascular risk factor. In another study, patients with GCA who experience recent ophthalmic ischemic symptoms and present with low inflammatory variables were more susceptible to stroke. 9 Patients with stroke in vertebrobasilar regions had more commonly irreversible visual loss due to involvement of ophthalmic artery branches derived from the internal carotid artery than the other GCA patients. 10 In confirmation of the previous study, our patient had no significant change in visual acuity after treatment.
The GCA symptoms detected at the time of the CVA were as follows: headaches, signs of polymyalgia rheumatica, and acute anterior neuropathy. Acute‐phase reactants were increased by (83%) at the stroke event. At the time of the stroke, 22% of patients were on antiplatelet therapy. Of the 18 patients, only 5 had magnetic resonance brain imaging that indicated ischemic lesions located in the carotid territories. Vascular stenosis or occlusion was observed in vertebral arteries in 11 individuals, with bilateral involvement in 6 patients; basilar artery in 2 cases, circle of Willis in 2 persons, and internal carotid artery in 1 patient. 6 According to the information mentioned in our case had a severe recent headache and sudden visual loss with moderately elevated CRP and ESR. Also, the brain's parenchymal territory involvement and the kind of vascular stenosis are rare and unusual.
Moghaddasi M et al. reported a 67‐year‐old man who was finally diagnosed with giant cell arthritis. The patient presented with a recently temporal headache, cranial nerves palsy, elevated ESR, and severe internal carotid artery stenosis. However, the biopsy of the temporal artery was normal. 11 A case report presented a 59‐year‐old man with multiple strokes whom the conventional angiogram demonstrated stenosis of bilateral carotid and vertebral vessels. 12 Another case was presented with multiple acute infarctions in the territory of the vertebrobasilar arteries with the left vertebral artery stenosis in the MRI and an increase in inflammatory markers. 13 The cohort of all residents of Olmsted County, Minnesota, in whom GCA was diagnosed and followed up for the duration of 50 years, demonstrated that the incidence of large artery stenosis is 13%. 14 Several studies demonstrated that ischemic cerebrovascular attacks are mainly linked with the occlusion or stenosis of the extramural vessels instead of the intramural vasculitis. 15 , 16 Siemonsen et al. assessed patients suspicious of GCA by using an MRI protocol focused on evaluating the intramural arteries. Ten out of 25 cases presented with vessel wall enhancement (VWE) of the intramural internal carotid arteries (ICA); eventually, all these cases were positive for GCA. Moreover, the involvement of the intramural vessels did not associate with the cerebral ischemic lesions. 17
Few cases report published vertebral and carotid artery dissection and pseudo dissections with severe cerebral ischemic signs and symptoms without GCA. 18 , 19 , 20 , 21 , 22 A case report presented a 56‐year‐old man with arthralgia, weight loss, and progressing minor neurological symptoms over 1 month. Neurosoncological evaluation indicates occlusion in both internal carotid arteries (ICA) and intracranial segments of the left vertebral artery (VA) and the famous hypoechoic halo sign in both superficial temporal arteries. They confirmed GCA diagnosis by inflammatory markers and biopsy. During the treatment, brain MRI suggests watershed infarcts and intracranial dissections of both ICA and left VA. 18 In another report, Zheng X et al. presented a case that ICA dissection was the underlying reason for ION. They recommended that the threshold of suspicion of the ICA problems during ION and initiating treatment should be lowered. 23 As the same in our case, CT angiography indicated right internal carotid artery dissection.
A combination of corticosteroids and antiplatelets may help prevent stroke occurrence in patients with GCA. However, the combination of anti‐thrombotic and corticosteroid therapy is less effective in GCA patients. The cause of the stroke due to giant cell arthritis is an unusual condition; there are no evidence‐based recommendations or guidelines for the treatment. If GCA is suspected in patients with severe complications, high‐dose glucocorticoids are the mainstay of treatment and should be started immediately. 9 , 24 When we began the corticosteroid pulse, our patient dramatically responded to the treatment in the form of a reduction in the inflammatory markers.
8. PATIENT PERSPECTIVE
The prognosis of GCA was discussed with the patient. It was explained how complicated this situation was. While varnishing headaches, there was no significant change in ptosis and ocular movements, visual acuity, and reduction of left limb's force. Therefore, the patient was referred to the rehabilitation center to continue his treatment.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Leila Hashami, Arsh Haj Mohamad Ebrahim Ketabforoush, and Matineh Nirouei involved in study concept and design, acquisition of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content administrative, technical, and material support. Leila Hashami involved in study supervision.
ETHICAL APPROVAL
None.
CONSENT
Written informed consent was obtained from the patient.
ACKNOWLEDGEMENTS
The authors thank the Clinical Research Development Unit (CRDU) of Shahid Rajaei Hospital, Alborz University of Medical Sciences, Karaj, Iran, for their support, cooperation, and assistance throughout this study. This manuscript has been posted as a preprint in the Research Square (https://doi.org/10.21203/rs.3.rs‐897947/v2).
Hashami L, Haj Mohamad Ebrahim Ketabforoush A, Nirouei M. Giant cell arteritis with rare manifestations of stroke and internal carotid artery dissection: A case study. Clin Case Rep. 2022;10:e05597. doi: 10.1002/ccr3.5597
Funding information
None
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Salvarani C, Giannini C, Miller DV, Hunder G. Giant cell arteritis: involvement of intracranial arteries. Arthritis Rheum. 2006;55(6):985‐989. [DOI] [PubMed] [Google Scholar]
- 2. Borchers AT, Gershwin ME. Giant cell arteritis: a review of classification, pathophysiology, geoepidemiology and treatment. Autoimmun Rev. 2012;11(6):A544‐A554. [DOI] [PubMed] [Google Scholar]
- 3. Lu‐Emerson C, Walker M, Huber BR, Ghodke B, Longstreth WT, Khot SP. Lethal giant cell arteritis with multiple ischemic strokes despite aggressive immunosuppressive therapy. J Neurol Sci. 2010;295(1):120‐124. [DOI] [PubMed] [Google Scholar]
- 4. Zwicker J, Atkins EJ, Lum C, Sharma M. An atypical presentation of giant cell arteritis. Can Med Assoc J. 2011;183(5):E301‐E305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang V, Fantaneanu T, Chakraborty S, Patel V, Dowlatshahi D. Intracranial non‐occlusive thrombus and multiple strokes in giant cell arteritis. Can J Neurol Sci. 2014;39(1):116‐117. [DOI] [PubMed] [Google Scholar]
- 6. Pariente A, Guédon A, Alamowitch S, et al. Ischemic stroke in giant‐cell arteritis: french retrospective study. J Autoimmun. 2019;99:48‐51. [DOI] [PubMed] [Google Scholar]
- 7. Banz Y, Stone JH. Why do temporal arteries go wrong? Principles and pearls from a clinician and a pathologist. Rheumatology. 2018;57(suppl_2):ii3‐ii10. [DOI] [PubMed] [Google Scholar]
- 8. Samson M, Jacquin A, Audia S, et al. stroke associated with giant cell arteritis: a population‐based study. J Neurol Neurosurg Psychiatry. 2015;86(2):216‐221. [DOI] [PubMed] [Google Scholar]
- 9. de Boysson H, Liozon E, Larivière D, et al. Giant cell arteritis–related stroke: a retrospective multicenter case‐control study. J Rheumatol. 2017;44(3):297‐303. [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez‐Gay MA, Vazquez‐Rodriguez TR, Gomez‐Acebo I, et al. Strokes at time of disease diagnosis in a series of 287 patients with biopsy‐proven giant cell arteritis. Medicine. 2009;88(4):227‐235. [DOI] [PubMed] [Google Scholar]
- 11. Moghaddasi M, Benaissa F, Mohebi N, Hashami L, Karimian H, Rast Manesh A. Giant cell arteritis with internal carotid artery stenosis and third nerve palsy: a case report. Glob J Res Anal. 2015;4. https://www.worldwidejournals.com/global‐journal‐for‐research‐analysis‐GJRA/recent_issues_pdf/2015/April/April_2015_1430373180__119.pdf [Google Scholar]
- 12. Cox BC, Fulgham JR, Klaas JP. Recurrent stroke in giant cell arteritis despite immunotherapy. Neurologist. 2019;24(4):139‐141. [DOI] [PubMed] [Google Scholar]
- 13. Elhfnawy AM, Bieber M, Schliesser M, Kraft P. Atypical presentation of giant cell arteritis in a patient with vertebrobasilar stroke: a case report. Medicine. 2019;98(32):e16737‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nuenninghoff DM, Hunder GG, Christianson TJH, McClelland RL, Matteson EL. Incidence and predictors of large‐artery complication (aortic aneurysm, aortic dissection, and/or large‐artery stenosis) in patients with giant cell arteritis: a population‐based study over 50 years. Arthritis Rheum. 2003;48(12):3522‐3531. [DOI] [PubMed] [Google Scholar]
- 15. Wilkinson IMS, Russell RWR. Arteries of the head and neck in giant cell arteritis: a pathological study to show the pattern of arterial involvement. Arch Neurol. 1972;27(5):378‐391. [DOI] [PubMed] [Google Scholar]
- 16. Bogousslavsky J, Deruaz JP, Regli F. Bilateral obstruction of internal carotid artery from giant‐cell arteritis and massive infarction limited to the vertebrobasilar area. Eur Neurol. 1985;24(1):57‐61. [DOI] [PubMed] [Google Scholar]
- 17. Siemonsen S, Brekenfeld C, Holst B, Kaufmann‐Buehler AK, Fiehler J, Bley TA. 3T MRI reveals extra‐ and intracranial involvement in giant cell arteritis. AJNR Am J Neuroradiol. 2015;36(1):91‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parra J, Domingues J, Sargento‐Freitas J, Santana I. Extensive intracranial involvement with multiple dissections in a case of giant cell arteritis. BMJ Case Rep. 2014;2014:bcr2014204130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bajkó Z, Bălaşa R, Moţăţăianu A, et al. Malignant middle cerebral artery infarction secondary to traumatic bilateral internal carotid artery dissection. A case report. J Crit Care Med (Targu Mures). 2016;2(3):135‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Filep RC, Bajko Z, Simu IP, Stoian A. Pseudo‐dissection of the internal carotid artery in acute ischemic stroke. Acta Neurol Belg. 2020;120(2):469‐472. [DOI] [PubMed] [Google Scholar]
- 21. Bajkó Z, Maier S, Moţăţăianu A, et al. Stroke secondary to traumatic carotid artery injury ‐ A case report. J Crit Care Med (Targu Mures). 2018;4(1):23‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Proft F, Czihal M, Remi J, Fesl G, Woischke C, Schulze Koops H. Fatal spontaneous bilateral vertebral artery dissection in giant cell arteritis (GCA). J Vasculitis. 2017;3(2):4‐5. doi: 10.4172/2471-9544.1000123 [DOI] [Google Scholar]
- 23. Zheng X, Wang Y, Chen G, Ma C, Yan W, Chen M. Diagnosis of ischemic optic neuropathy caused by dissection of the internal carotid artery: a case report. Medicine. 2020;99(33):e20034‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bienvenu B, Ly KH, Lambert M, et al. Management of giant cell arteritis: recommendations of the French Study Group for Large Vessel Vasculitis (GEFA). Rev Med Interne. 2016;37(3):154‐165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


