Abstract
Hepatitis B virus is a known carcinogen for hepatocellular carcinoma, which is rare in the pediatric population. We report a 13‐year‐old patient with hepatitis B surface antigen‐positive multifocal hepatocellular carcinoma in a noncirrhotic liver. Her APRI score was 0.24. Her BCLC stage was C, and her caregiver opted for palliative care.
Keywords: gastroenterology and hepatology, oncology, pediatrics and adolescent medicine
Hepatitis B virus is a known carcinogen for hepatocellular carcinoma, which is rare in the pediatric population. We report a 13‐year‐old patient with hepatitis B surface antigen‐positive multifocal hepatocellular carcinoma in a noncirrhotic liver. Her APRI score was 0.24. Her BCLC stage was C, and her caregiver opted for palliative care.
1. INTRODUCTION
Hepatocellular carcinoma is the second most common pediatric primary hepatic malignancy following hepatoblastoma. Hepatitis virus‐associated HCC in children is mainly HBV related, and most children with HCC are HBsAg seropositive. 1 Hepatitis B virus (HBV) is directly oncogenic by incorporating into host genetic material. 2 , 3 Pediatric HCC is typically diagnosed in older children and adolescents. The majority of pediatric HCC (70%) arise on a normal liver, with the remaining 30% occurring in the background of chronic liver diseases such as cirrhosis, metabolic disorders, chronic cholestasis, and chronic viral hepatitis. 4
2. CASE REPORT
A 13‐year‐old adolescent girl presented to the emergency unit of Cape Coast Teaching Hospital, Ghana, with a 1‐month history of right upper quadrant pain and abdominal distension. This was associated with fever, dark stool, weight loss, and jaundiced sclera but no bone pain, vomiting, seizure, or cough. There was no prior episode of jaundice or a history of blood transfusion. She was said to have received all her immunizations; unfortunately, her child health record book was not available for confirmation. The antenatal record book of her mother was also unavailable to determine her hepatitis B status during pregnancy. Her primary caregiver was her mother, a 30‐year‐old trader. Her ECOG clinical performance status was grade 2. Her parents were divorced, so additional first‐hand information could not be obtained about her father.
She was febrile (39.0°C), tachycardic (134 bpm), had a tinge of scleral jaundice, was mildly pale, and wasted. Abdominal examination revealed a tender, firm, and nodular liver, which measured 23 cm below the right subcostal margin. No other abdominal mass was palpated. There were no peripheral stigmata of chronic liver disease such as finger clubbing, palmar erythema, and leukonychia. Other systemic examinations were grossly intact.
Abdominal ultrasound showed a grossly enlarged liver measuring 20–23 cm with the presence of multiple solid echogenic nodules of varying sizes distributed throughout both lobes of the liver. Two of the largest nodules measured were (6.99 × 5.4) cm and (5.34 × 6.12) cm. The spleen and all other organs were sonographically normal. The radiological diagnosis listed was a primary hepatic malignancy to query metastatic lesions. Chest X‐ray was normal. A complete blood count showed an elevated WBC of 20.9 × 109/L with an accompanying increase in granulocytes 14.2 × 109/L, low hemoglobin 8.2 g/dl, elevated platelets 644/µl, and an elevated ESR of 136 mm/hr. Blood and urine culture with sensitivity tests yielded no bacterial growth. The peripheral blood film comment was insignificant. Liver chemistries were as follows: aspartate aminotransferase (AST) 55 U/L, alkaline phosphatase (ALP) 448 U/L, and gamma‐glutamyl transferase 170 U/L. ALT and albumin were within the normal range. The other parameters of the liver chemistries were essentially normal. The clotting profile revealed an elevated INR and aPTT of 1.89 and 67.7, respectively. Her Child‐Pugh class was B. Lactate dehydrogenase was elevated 341 IU/L. Serum alpha‐fetoprotein was as high as 1677.1 IU/ml. Quantitative human chorionic gonadotropin was normal. Renal function tests with electrolytes were all within normal ranges.
An initial viral screen was positive for HBsAg and negative for hepatitis C and HIV. A follow‐up viral profile depicted a chronic infection with high infectivity evidenced by the presence of the surface antigen (HBsAg), core antibody (cAb), and e antigen (HBeAg) with the absence of the core IgM (HBcIgM) and surface antibody (HBsAb). Her viral load was 2053 copies/ml.
Computed tomography of the abdomen and pelvis with contrast showed a markedly enlarged liver with a nodular surface. There were multiple, ill‐defined, hypo‐, and hyperdense lesions predominantly in the right lobe (Figure 1). The largest lesion measuring 11.1 × 9.6 cm was noted in segment 5. The lesions showed arterial phase enhancement and minimal washout in the portovenous phase. The lesions showed areas of nonenhancement suggestive of necrosis or hemorrhage. Enlarged lymph nodes were noted along the superior mesenteric artery and coeliac trunk. Mild ascites were noted. The portal vein was dilated with a filling defect indicative of tumor thrombosis. The lesions compressed the intra‐ and infrahepatic inferior vena cava. The other abdominal organs were normal. The differential diagnoses listed by the radiologist were multicentric hepatocellular carcinoma and hepatoblastoma. The pelvic findings were bilateral ureteric and urinary bladder wall calcifications. GeneXpert test for MTB on sputum sample was negative.
FIGURE 1.

(A–D) shows triple‐phase CT scan of the liver. (A) Noncontrast image of the liver showing isodense lobulated extracapsular masses in segments V and VI. (B) Arterial phase image shows heterogeneous contrast enhancement of the masses. The nonenhancing foci are suggestive of necrosis or hemorrhage. (C) Portovenous phase shows washout of contrast medium becoming hypoattenuating relative to the normal liver parenchyma. A large minimally enhancing hypodense filling defect is noted in the portal vein indicative of tumor thrombus. Enlarged lymph nodes were noted along the superior mesenteric artery and coeliac trunk (not shown in these images). (D) Delayed phase with evidence of capsule formation. Mild ascites
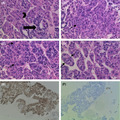
Histological examination of the lesion with routine hematoxylin and eosin staining showed disorganized architecture by mildly pleomorphic dysplastic hepatocytes, and some show intranuclear pseudoinclusions. The lesional cells form pseudoglands and microtrabeculae (thickened plates). The trabeculae are wrapped by endothelial cells (Figure 2D). The architecture and cytomorphology suggested a moderately differentiated hepatocellular carcinoma, which was confirmed by immunohistochemical staining with hep PAR‐1 showing diffuse strong cytoplasmic staining (Figure 2E) and negative pan‐cytokeratin staining (Figure 2F).
FIGURE 2.

Shows (×400) (A) pseudoglandular formation (black arrow) and trabeculae (black arrowhead). (B) Pleomorphic hepatocytes (black arrow). (C) Pleomorphic dysplastic hepatocytes with intranuclear pseudo inclusions (black arrows). (D) microtrabeculae wrapped by endothelial cells (two black arrows). (E) Shows (×100) diffuse strong cytoplasmic staining with hepPAR‐1. (F) Shows negative staining with pan‐cytokeratin
The provisional diagnosis was an abdominal malignancy complicated by anemia and sepsis. The final diagnosis was hepatitis B surface antigen‐positive multifocal hepatocellular carcinoma in a noncirrhotic liver with BCLC stage C.
She was started on intravenous cefotaxime 2 g daily and metronidazole 260 mg three times daily. She was transfused with a unit of blood. Paracetamol and morphine were added for pain control. She was administered intramuscular vitamin K 10 mg three times daily for 24 h.
Fever settled on day 4 of intravenous antibiotics, and the elevated INR and aPTT corrected after the administration of parenteral vitamin K. She spent a total of 21 days on admission and was discharged home on analgesics.
The mother was counseled on the prognosis of the child's condition. She did not consent to any further medical care and opted for home palliative care.
3. DISCUSSION
Hepatitis B virus has been well established as a substantial carcinogen. It is well documented that the virus can be integrated into the host genome, leading to changes in genomic function or chromosomal instability. 5 A study performed by Chang et al. 6 in Taiwan showed integration of hepatitis B viral DNA into the host cellular DNA sequences upon analyzing hepatocellular carcinoma tissues of eight children. A pattern of single‐site integration in four children and a multiple‐site integration pattern in one child was demonstrated. In the remaining three children, however, viral DNA could not be demonstrated in the tumor tissues.
Fibrogenesis represents a major driving force in the development of liver fibrosis with progression to cirrhosis. Liver fibrosis leads to cirrhosis and all the complications of end‐stage liver disease including portal hypertension, ascites, encephalopathy, synthesis dysfunction, and impaired metabolic capability. Identifying liver fibrosis is, therefore, important to evaluate the severity of liver damage and to establish a prognosis. Currently, the gold standard in the assessment for liver fibrosis is a biopsy. 7 Due to the invasiveness of liver biopsy, noninvasive alternatives such as aspartate transaminase‐to‐platelet ratio (APRI) 8 among others have been developed for measuring the degree of fibrosis. APRI score is advantageous as it incorporates laboratory data that are routinely obtained as a standard of care into a simple biomarker formula that is easy to perform and reproduce. The formula for APRI as described in the original article 8 is APRI:(AST/[ULN]/PLT [109/L]) × 100. Its utility has been proved in adults but with few reports in children. 9 A study performed by Zhijian et al. 10 to investigate the diagnostic accuracy of APRI for the prediction of the fibrosis stage of adolescents with HBV‐related fibrosis and inflammation showed that the score (0.86 ± 0.58) for patients with mild liver fibrosis was significantly lower than the patients with significant fibrosis (1.39 ± 0.95; p < 0.01). Flores‐Calderón et al 11 also evaluated the utility of Fibrotest® score and APRI in the liver biopsies of 68 Mexican children with CLD. The study aimed to identify the suitability of these two methods in identifying children with liver fibrosis. The outcome, with respect to APRI in differentiating between advanced fibrosis (METAVIR > F3) and no fibrosis (METAVIR F0) was AUROC = 0.97 (95% CI 0.92–1.00) (cutoff value 0.82), sensitivity 88% (95% CI 68–96) and specificity = 100% (95% CI 46–100). Similar results were found in other studies. 12 , 13 These findings indicate that the APRI score performed reasonably well in fibrosis assessment as they could differentiate between no or insignificant fibrosis and significant fibrosis in children. Therefore, an APRI score of 0.24 in our case can indicate the lack of significant liver fibrosis. An AST value of 35 was used as the upper limit of normal (ULN). Although the abdominal ultrasonographic images of our patient could not be retrieved, there was no sonographic evidence of liver cirrhosis from the formal report. Second, normal liver parenchyma was seen on the abdominal CT scan. Splenomegaly is often used radiologically as an indicator of cirrhosis. The clinical syndrome whereby hematological changes of thrombocytopenia and neutropenia occur, in association with cirrhosis and splenomegaly, is known as hypersplenism. 14 None of these were observed in our patient. Hence, the mild ascites noted on the abdominal CT could be attributed to HCC‐induced portal vein thrombosis and not portal hypertension due to the absence of both peripheral stigmata of CLD and hypersplenism.
With respect to hepatocellular function, two markers that can be looked at are albumin and PT time. 15 The INR is commonly used as a surrogate for the PT value. Her serum albumin level (40 g/dl) was normal. She, however, had a prolonged INR, which was corrected by the administration of parenteral vitamin K. Gamma carboxylation is markedly affected in a nonfunctional or a poorly functioning liver, irrespective of the presence of vitamin K. This is the basis for parenteral administration of vitamin K to patients who have elevated PT values. Correction of the PT value informs that the synthetic function of the liver is probably still within normal limits, and the deficiency most likely is due to obstruction. 16 It is, therefore, useful to recheck the INR value after administration of parenteral vitamin K, which we did in our case. On the contrary, the administration of vitamin K in patients with cirrhosis does not affect INR changes. 17
AFP is considered to be the most useful biomarker for HCC evaluation, and it is seen to be raised in more than half of patients. 18
In Ghana, HBV vaccination under the EPI was started in 2002 comprising of three doses of diphtheria, pertussis, tetanus, hemophilus influenzae type B, and hepatitis B (DPT/HiB/hepB) 5 in 1 vaccine at 6, 10, and 14 weeks of age. 19 This timeline qualifies our patient to have been immunized since she was born after this initiative. Although her immunization records were unavailable to ascertain her definite and full vaccination history, a negative surface antibody (HBsAb) indicates a lack of immunity. 20 On the other hand, interpretation of her full viral profile depicted a chronic infection with high infectivity evidenced by the presence of the surface antigen (HBsAg), core antibody (cAb), and e antigen (HBeAg) with the absence of the core IgM (HBcIgM) and surface antibody (HBsAb). 21 Again, due to the unavailability of her vaccination records, the timeline to this past infection was impossible to establish. A positive HBeAg in our patient is also a factor that increases her risk of developing hepatocellular carcinoma. 22 A viral load above 2000 IU/ml as identified in our patient is a significant predictor of hepatocarcinogenesis. 23
The presence of disorganized architecture with thickened plates (trabeculae), nuclear pleomorphism, nuclear pseudoinclusions, and endothelial cell wrapping of tumor cells and immunoreactivity for hep PAR‐1 is typical of hepatocellular carcinoma. 24
Owing to the majority (70%) of pediatric HCC occurring in normal liver, the absence of accompanying liver cirrhosis should theoretically permit significant anatomic resection thanks to hepatic reserve while reserving liver transplantation for unresectable tumors. However, hepatocellular carcinoma is different from other malignancies because the prognosis is not only dependent upon the tumor stage but also on the liver function impairment due to accompanying liver cirrhosis. The Barcelona Clinic Liver Cancer (BCLC) staging system is the commonly used classification system to guide the clinical management of HCC. 25 The three components are the tumor status (defined by tumor size and number, the presence of vascular invasion, and extrahepatic spread), liver function (defined by the Child‐Pugh class), and clinical performance status of the patient (defined by the ECOG classification). Our patient had a portal vein invasion, Child‐Pugh class B, and clinical performance status of 2. These parameters placed her at an advanced stage (BCLC C). Patients at this stage are recommended to be evaluated for systemic therapy. 26 However, the child's mother refused further medical care and opted for home palliative care. Despite the mother's refusal of further medical care, the prognosis was poor as the overall survival at this stage is 11 months. 25
One other limitation was the unavailability of the mother's antenatal record, without which we could not establish any possibility of vertical transmission.
4. CONCLUSIONS
Africa is an endemic region for HBV, and although hepatocellular carcinoma is rare in the pediatric population, it should be considered a differential diagnosis in a child presenting with an abdominal mass and HBsAg positive. Second, regular ultrasound surveillance for chronic HBsAg seropositive children may be helpful in early identification of hepatic lesions.
CONFLICT OF INTEREST
The authors declare no competing interest.
AUTHOR CONTRIBUTIONS
K. Danso, R. Akuaku, R. Taylor, E. Amoako, and L. Tagoe were involved in the clinical management of the patient. K. Danso and R. Akuaku wrote the manuscript. E. Amoako critically reviewed and revised the manuscript. K. Ulzen‐Appiah was the pathologist who performed the histologic examination, immunohistochemistry, and authored the corresponding pathological findings. B. Jimah was the radiologist who performed the abdominal computed tomography, ultrasound‐guided liver biopsy, and authored the corresponding radiological findings. All authors read and approved the final version of the manuscript.
CONSENT
A consent form was thumb printed by the mother of the child to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGEMENTS
We would like to thank Mr. Martin Ampofo (Medical Laboratory, Cape Coast Teaching Hospital, Cape Coast, Ghana) for his contribution to the preparation of this article.
Danso KA, Akuaku RS, Taylor RR, et al. A case report of a teenager with hepatitis B surface antigen‐positive multifocal hepatocellular carcinoma in a noncirrhotic liver. Clin Case Rep. 2022;10:e05622. doi: 10.1002/ccr3.5622
Funding information
The authors received funding from the World Child Cancer Organization in performing most of the diagnostic investigations
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Chang M‐H. Decreasing incidence of hepatocellular carcinoma among children following universal hepatitis B immunization. Liver Int. 2003;23(5):309‐314. [DOI] [PubMed] [Google Scholar]
- 2. Bisceglie AMD. Hepatitis B and hepatocellular carcinoma. Hepatology. 2009;49(S5):S56‐S60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruix J, Llovet JM. Hepatitis B virus and hepatocellular carcinoma. J Hepatol. 2003;39:59‐63. [DOI] [PubMed] [Google Scholar]
- 4. Angelico R, Grimaldi C, Saffioti MC, Castellano A, Spada M. Hepatocellular carcinoma in children: hepatic resection and liver transplantation. Transl Gastroenterol Hepatol. 2018;3:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fung J, Lai C‐L, Yuen M‐F. Hepatitis B and C virus‐related carcinogenesis. Clin Microbiol Infect. 2009;15(11):964‐970. [DOI] [PubMed] [Google Scholar]
- 6. Chang M‐H, Chen P‐J, Chen J‐Y, et al. Hepatitis B virus integration in hepatitis B virus‐related hepatocellular carcinoma in childhood. Hepatology. 1991;13(2):316‐320. [PubMed] [Google Scholar]
- 7. Kim WR, Berg T, Asselah T, et al. Evaluation of APRI and FIB‐4 scoring systems for non‐invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016;64(4):773‐780. [DOI] [PubMed] [Google Scholar]
- 8. Loaeza‐del‐Castillo A, Paz‐Pineda F, Oviedo‐Cárdenas E, Sánchez‐Avila F, Vargas‐Vorácková F. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann Hepatol. 2008;7(4):350‐357. [PubMed] [Google Scholar]
- 9. Yang HR, Kim HR, Kim MJ, Ko JS, Seo JK. Noninvasive parameters and hepatic fibrosis scores in children with nonalcoholic fatty liver disease. World J Gastroenterol. 2012;18(13):1525‐1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhijian Y, Hui L, Weiming Y, et al. Role of the aspartate transaminase and platelet ratio index in assessing hepatic fibrosis and liver inflammation in adolescent patients with HBeAg‐positive chronic hepatitis B. Gastroenterol Res Pract. 2015;2015:e906026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flores‐Calderón J, Moran‐Villota S, Ramón‐García G, et al. Non‐invasive markers of liver fibrosis in chronic liver disease in a group of Mexican children. A multicenter study. Ann Hepatol. 2012;11(3):364‐368. [PubMed] [Google Scholar]
- 12. Lebensztejn DM, Skiba E, Sobaniec‐Lotowska M, Kaczmarski M. A simple noninvasive index (APRI) predicts advanced liver fibrosis in children with chronic hepatitis B. Hepatology. 2005;41(6):1434‐1435. [DOI] [PubMed] [Google Scholar]
- 13. Wai C‐T, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518‐526. [DOI] [PubMed] [Google Scholar]
- 14. Kakehashi R, Watanabe S, Ikoma J, Suzuki S. Blood chemistry, hematology of patients with liver cirrhosis. Nihon Rinsho. 1994;52(1):45‐49. [PubMed] [Google Scholar]
- 15. Wolf PL. Biochemical diagnosis of liver disease. Indian J Clin Biochem. 1999;14(1):59‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D'Agata ID, Balistreri WF. Evaluation of liver disease in the pediatric patient. Pediatr Rev. 1999;20(11):376‐390. [PubMed] [Google Scholar]
- 17. Meyer AV, Green M, Pautler HM, Korenblat K, Deal EN, Thoelke MS. Impact of vitamin K administration on INR changes and bleeding events among patients with cirrhosis. Ann Pharmacother. 2016;50(2):113‐117. [DOI] [PubMed] [Google Scholar]
- 18. Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015;21(37):10573‐10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Awuku YA, Yeboah‐Afihene M. Hepatitis B at‐birth dose vaccine: an urgent call for implementation in Ghana. Vaccines. 2018;6(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Towell V, Cowie B. Hepatitis B serology. Aust Fam Physician. 2012;1(41):212‐214. [PubMed] [Google Scholar]
- 21. Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev. 2006;28(1):112‐125. [DOI] [PubMed] [Google Scholar]
- 22. Yang H‐I, Lu S‐N, Liaw Y‐F, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347(3):168‐174. [DOI] [PubMed] [Google Scholar]
- 23. Kawanaka M, Nishino K, Nakamura J, et al. Quantitative levels of hepatitis B virus DNA and surface antigen and the risk of hepatocellular carcinoma in patients with hepatitis B receiving long‐term nucleos(t)ide analogue therapy. Liver Cancer. 2014;3(1):41‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schlageter M, Terracciano LM, D'Angelo S, Sorrentino P. Histopathology of hepatocellular carcinoma. World J Gastroenterol. 2014;20(43):15955‐15964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schütte K, Schulz C, Malfertheiner P. Hepatocellular carcinoma: current concepts in diagnosis, staging and treatment. GAT. 2014;1(2):84‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation Barcelona Clinic Liver Cancer (BCLC) staging system. The 2022 update. J Hepatol. 2021;76(3):681‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


